1. Introduction
The brain is an important organ which is fundamental to regulate all biological and physiological functions. It is divided into several regions which may encounter changes during development, maturation, normal aging, neurological disorders, and trauma. Together with the spinal cord, it forms the central nervous system. It is made of neurons, which store information and send impulses, and glial cells, astrocytes and oligodendrocytes, which support, insulate, and protect neurons. Neurons are very sensitive cells with high demand for oxygen, glucose and other molecules. In order to work properly, neurons activity is based on the production of action potentials and the formation of ion gradients. Neurons use electrical impulses and chemical signals to send information to different areas of the brain and between the brain and the rest of the nervous system. The transmission of the signal occurs at synapses, the connection between axons and dendrites of the adjacent neuron, with the release of neurotransmitters. Glial cells normally outnumber the neurons and in the mature CNS consists in astrocytes, oligodendrocytes and microglial cells. In the PNS they are named Schwann cells, enteric glial cells and satellites cells [
1].
The neurotransmitter release from presynaptic neurons is one of the main events in synaptic transmission. The neurotransmitter moves towards the synaptic cleft and binds specific receptors on the plasma membrane of postsynaptic neurons. The transmission of the information is based on the release of specific molecules through different systems [
2]. This can be either by spontaneous vesicle fusion, which is independent of presynaptic action potentials, or by evoking the neurotransmitter release [
3]. Molecular machinery triggering spontaneous vesicle fusion may differ from that underlying evoked release and may be one of the sources of heterogeneity in release mechanisms. The transmission of the signal is dependent on the myelin sheath. Myelin consists of multiple layers of specialized cell membrane that surround the axons larger than 1 µm in diameter. The membranes are opposed and form the intraperiod line (IPL), a thin space that separates the membranes and which is contiguous with the extracellular space. On the contrary, the major dense line (MDL) is a 3 nm compartment between two cytoplasmic leaflets of stacked myelin membranes, where myelin basic protein (MBP) is abundant. The myelin sheath covers the axons with the exclusion of the nodes of Ranvier, which are small gaps at the synapses, and affects the capacitance/resistance and current flow. The myelin sheath avoids the loss of signal and it is slightly different in CNS compared to the one in PNS. Among the shared aspects, high concentrations of voltage-dependent sodium channels at the nodes of Ranvier are linked to the cytoskeleton by ankyrin G while the presence of Caspr (contactin-associated protein)/paranodin (a glycoprotein of neuronal paranodal membranes), the potassium channels Kv1.1 and Kv1.2, the associated β2 subunit, and Caspr2, characterize both the myelin sheaths. Among the differences, there are the cellular origins, the anatomic details and the molecular constituents.
Despite of being similar in the structure, specific cell types are responsible for the formation of myelin, the oligodendrocytes and the Schwann cells in the CNS and PNS, respectively.
Oligodendrocytes are responsible for the formation of up to 80 different layers of myelin membrane around the axons. The aim of the synthesis and maturation of these sheaths is a fast and efficient nerve conduction, while the maintenance and the remodelling of myelin are linked to the interaction between oligodendrocytes and glial and neuronal cells and its role in providing metabolic support to neurons, ion and water homeostasis, and adapting to activity-dependent neuronal signals [
4].
The CNS myelin has a high lipid content (about 70%) made of galactosphingolipids, sphingomyelin and cholesterol. On the contrary only few proteins, specific for the CNS, compose the myelin sheath but with high density, among these proteolipid protein (PLP), myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) [
5,
6]. PLP is the most abundant transmembrane protein and a suggested explanation for the tight apposition of membrane sheaths due to the hydrophilic extracellular domains. MBP is an unstructured polypeptide chain which binds to the membrane where is necessary for the compaction of the two adjacent cytoplasmic membrane surfaces into the MDL. Another important protein is claudin-11 which is a tight junction protein and connects the outer leaflets of the myelin lamellae. CNS myelin does contain tight juctions, which enhance the insulative properties of myelin, but lacks the components of adherens and gap junctions [
4].
During the synthesis of myelin, lipids preassemble together with PLP and their transport to the myelin sheath is mediated by vesicles and motor proteins. Small-diameter axons have a high number of internodes ranging from 20 to 200 μm in length, while the oligodendrocytes that myelinate larger diameter axons have fewer processes, but longer internodes and thicker myelin sheaths Hildebrand [
7].
The myelin membrane is defined “compacted” when the extracellular and cytoplasmic leaflets of the adjacent myelin lamellae connect very tightly and most of the water is removed. Consequently, electron-dense lines can be seen by electron microscopy [
4].
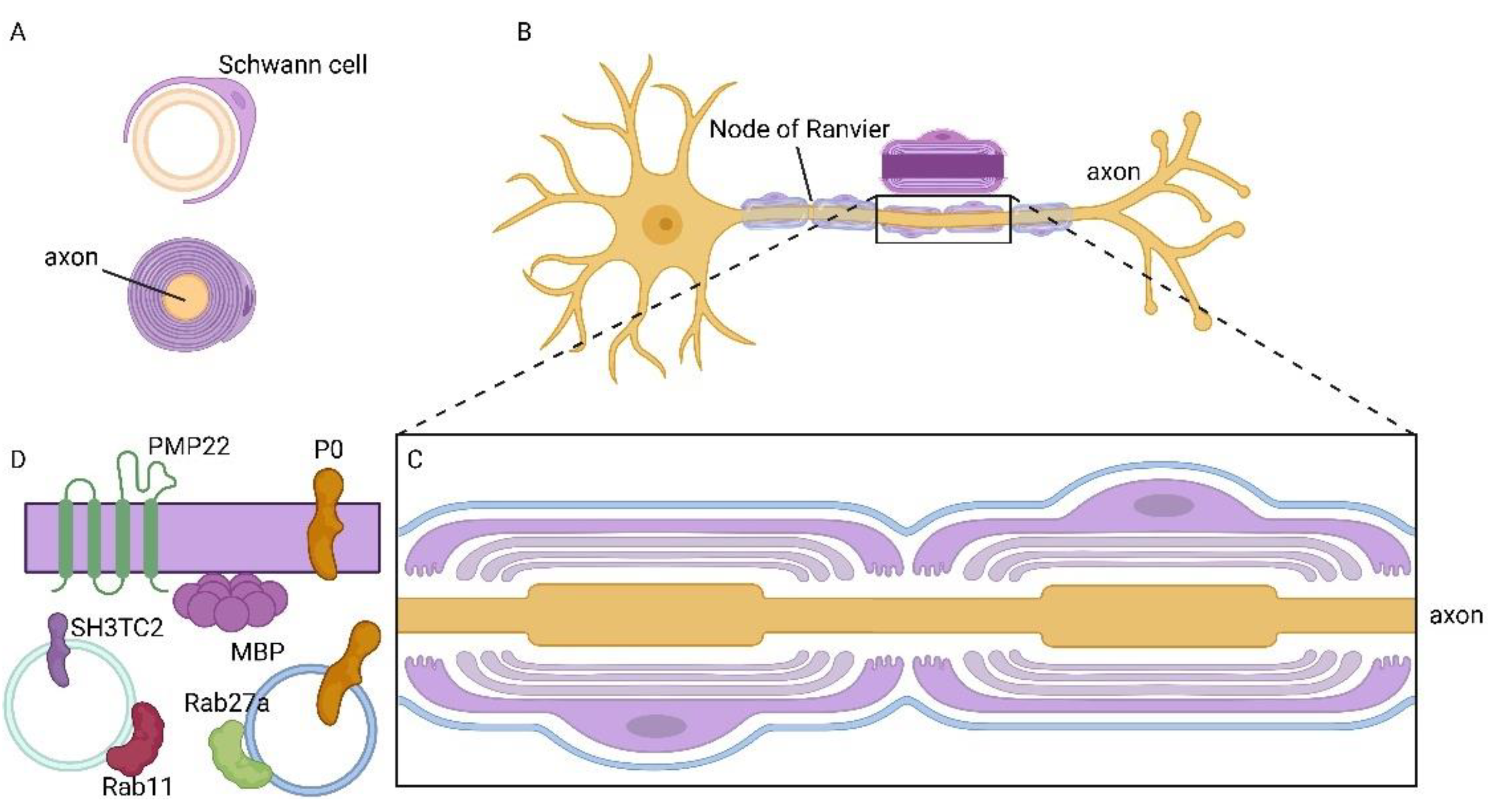
The PNS myelin is dependent on Schwann cells which present a basal lamina and microvilli containing F-actin and potassium channels fundamental for axonal activity and linked to the nodal axolemma. On the contrary of oligodendrocytes, schwann cells make a single internodal region of myelin [
6]. In these cells, the endoplasmic reticulum and Golgi apparatus are found in a perinuclear position, while most of the cytoplasm is external to the main part of the cell. Moreover, Schwann cells have myelin incisures, also known as Schmidt-Lanterman incisures, which are small pockets of residual cytoplasm histologically visible that are produced during the myelination process in the inner layer of the myelin sheath [
8].
The “compact” myelin sheath forms the bulk of the myelin sheath and the distance between two layers of cell membranes is lowered, while the lateral border of the sheath and the Schmidt-Lanterman incisures constitute the “non-compact” myelin sheath. Both the components contain a non-overlapping set of proteins.
Compact myelin in the PNS is largely composed of lipids, mainly cholesterol and sphingolipids, including galactocerebroside and sulfatide. Interestingly, myelin protein 0 (P0) is the major protein component together with Po-like proteins and peripheral myelin protein 22 (PMP22). P0 is a single membrane glycoprotein and it is the most abundant protein in myelin. P0 has a structural role and it is required for the proper formation and maintenance of myelin, similarly to the transmembrane protein PMP22.
The schwann cell basal lamina is composed of laminin 2 (comprised of α2/merosin, β1, and γ1 laminin chains), type IV collagens, entactin/nidogen, fibronectin, N-syndecan and glypican. Furthermore, it contains the integrin α6β4 and dystroglycan. The apical surface is highly enriched in myelin-associated glycoprotein (MAG) [
8]. MAG is a type 1 transmembrane glycoprotein which function in the glia-axon interaction and in the maintenance of myelinated axons. In the PNS, the non-compact myelin contains E-cadherin, MAG, and connexin32 (Cx32). Moreover, adherents and gap junctions are located at the plasma membrane.
The myelin sheath is fundamental for the transmission of signal and the proper functionality of the nervous system, therefore its alteration determines diseases named demyelinating and dysmyelinating [
9]. In the first case, neurological disorders divided into primary demyelination, where myelin and supporting cells are damaged or degraded, or secondary demyelination, where axonal damage determined the interruption of the axon-glial interactions necessary for the maintenance of the myelin sheats, occur. If the onset of the pathology is during development, the myelin is not formed and metabolic disturbancies affect myelin synthesis or degradation (dysmyelinating disease). For instance, specific mutations in the Po gene cause CMT (Charcot-Marie-Tooth) type 1B due to the alterations in the amount of Po protein. An extra copy of PMP22 gene has been associated with CMT1A, presenting an increase in the amount of PMP22 in compact myelin. Other diseases due to an alteration of this gene or CX32 are hereditary neuropathy with liability to pressure palsies (HNPP), X-linked Charcot-Marie-Tooth disease (CMTX) [
8] or multiple sclerosis which is a CNS demyelinating disorder [
6,
10]. Besides the above-mentioned neurological pathologies, other diseases involving myelinated axons may occur, such as acquired inflammatory and infectious diseases of myelin, acquired toxic-metabolic diseases, nutritional diseases, hereditary metabolic diseases, and diseases of myelin due to physical agents [
11].
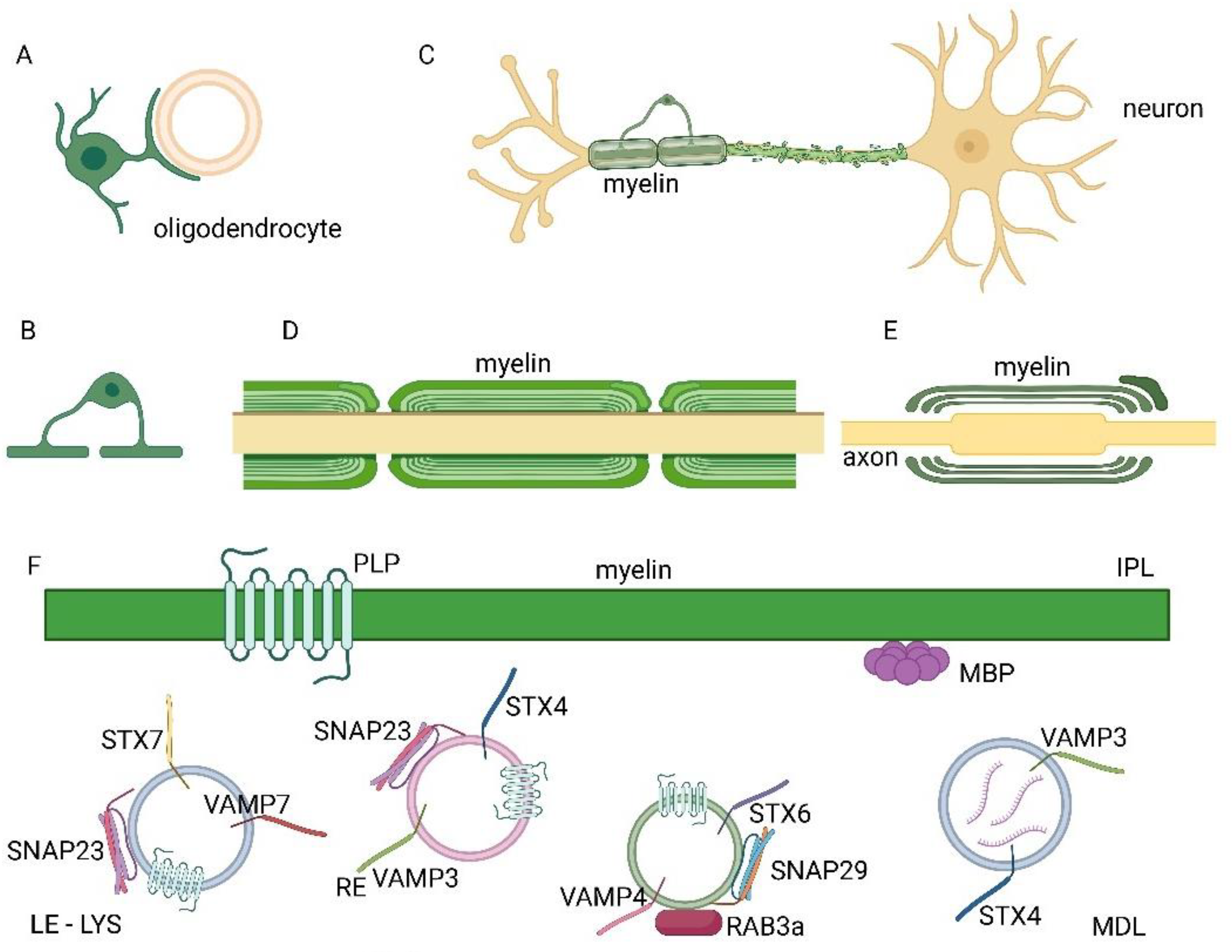
2. SNAREs
The synthesis of myelin sheath and the assembly of myelin proteins are regulated by neuronal signals. Interestingly, some soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) are an important component of the fusion machinery and are involved in delivering molecules to the myelin sheath. SNAREs are involved in the regulation of vesicle fusion, they are normally membrane proteins and are involved in the docking and fusion between two vesicles or a vesicle and a specific compartment. SNARE proteins are divided into v-SNAREs (vesicle-SNARE) and t- SNAREs (target-SNARE) which locate on the two different membranes and that assemble in one unique complex when the fusion process is complete. Ternary or quaternary complexes are located at different compartments and are grouped in R-, Qa-, Qb-, Qb-c-, or Qc- SNAREs depending on the aminoacidic residue that it is exposed in the formation of the zero ionic layer in the assembled core SNARE complex. For quaternary complexes, a Qa-, a Qb-, a Qc- and a R- SNARE are required. Ternary complexes are made only of a Qb-c, a Qa-, and a R- SNARE. Several combinations of R- and Q-SNARE take part to specific functions [
12].
For the formation of the myelin sheath, some important events occur, such as the MBP transport and the exocytosis of PLP. It has been demonstrated the role of several SNARE proteins in this process. In the work of Feldmann and colleagues, it has been proved that the transport of PLP is regulated by two different R-SNARE vamp proteins, vamp3 (or cellubrevin) and vamp7 (also named TI-VAMP). Both of them colocalized with PLP in different compartments, recycling endosomes (RE) in the case of vamp3 and late endosomes and lysosomes for vamp7. In particular, immunoelectronmicroscopy on primary cultures has proved colocalization of vamp3 and PLP on RE on the ultrastructural level. Putative components of the complex including this SNARE protein were syntaxin 4 and snap23. It has been demonstrated a role for vamp3 in PLP transport in the secretory pathway as it mediated the fusion of RE-derived vesicles with the plasma membrane of oligodendrocytes. Interestingly, vamp3 mRNA expression and protein levels were upregulated during the differentiation process. Nevertheless, the study of VAMP3-deficient mice did not show any myelination defects.
Similarly, also vamp7 localization and function determined an enrichment of PLP in the myelin membrane during myelination as it controls exocytosis of PLP from late endosomes/lysosomes in a transcytosis pathway. Syntaxin 3 and snap23 have been identified as prime acceptor of vamp7. Interestingly, functional inactivation of vamp7 decreased PLP surface transport. Therefore, the role of VAMP7 is linked to the exocytosis of lysosome-related organelles and the transport of cargo to the myelin membrane in order to promote myelin biogenesis. PLP was enriched in the myelin membrane during myelination. In order to study the role of vamp7 in vivo, the authors used mice deficient for a subunit of adaptor protein 3 (AP-3) which determined a lack of functional AP-3 and the mislocalization of vamp7 to early endosomes and altered secretion. Consequently, immunohistochemical staining of PLP showed a lower signal in the striatum and the hippocampus where the signal of myelinated fine fibers was extremely low.
Interestingly, silencing of vamp3 or vamp7 in primary oligodendrocytes determined a lower amount of PLP on the plasma membrane and they have been found able to have a synergistic effect [
13].
Moreover, another Q-SNARE, named SNAP-29, has been associated in complex with syntaxin 6 and vamp4 to the surface transport of PLP in oligodendrocytes through the interaction with Rab3a. SNAP-29 is abundant in oligodendrocytes during myelination and in noncompact myelin of the peripheral nervous system. Moreover, the two proteins overlapped in glia and neurons and were recruited together from the periphery of the cell where SNAP29 is normally located toward perinuclear membrane localization when active Rab3a was present, therefore SNAP29 was considered to be an effector of the GTPase protein. Both Rab3a and SNAP-29 enhanced the trafficking of PLP to the plasma membrane. SNAP-29 and rab3a expression were linked to the remyelination process as it has been proved that in case of pathologies, such as sciatic nerve crush and Charcot Marie Tooth 1A (CMT1A), the abundance of SNAP29 and Rab3a followed the expected reduced and restored abundance during demyelination and remyelination that would occur to proteins involved in myelin formation. Besides to Rab3A, SNAP-29 interacts also with rab24 and sept4 [
14].
It has been demonstrated that syntaxin 4 is fundamental for the transcriptional expression of MBP. This protein localizes at the cytoplasmic surface of myelin membranes where the SNARE protein is located, while syntaxin 3 is localized mainly in the cell body. MBP is a basic and membrane-associated adhesive protein and it is transported to the myelin sheath in a syntaxin 4-dependent mechanism in its mRNA form, which should avoid premature adhesion of membranes. Subsequently, MBP mRNA is assembled in nonmembranous granules. Syntaxin 4-mediated autocrine signaling was necessary for initiating MBP mRNA transcription. Syntaxin 4 is upregulated during the differentiation of oligodendrocytes, while its downregulation determined the block of MBP mRNA transcription which was rescued by conditioned medium from developing oligodendrocytes. Also in this case, syntaxin 4 formed a complex with vamp3. Interestingly, downregulation of vamp3 determined a reduction of MBP expression levels and a slight increase in the amount of syntaxin 4. The downregulation of syntaxin 3 did not affect the expression of MBP, but it mislocalized PLP to cell body [
15].
Interestingly in a SNAP25 knock-out mouse, the inhibition of regulated vesicular trafficking in layer VI cortical projection neurons decreased MBP and the amount of myelinated projections at postnatal day (P14) but it did not affect the initial timing of onset of myelination in the brain (P7/P8). Oligodendrocytes maturation was therefore affected [
16].
Besides PLP and MBP, other proteins and lipids are necessary for myelin sheath formation. They are synthetized, directed and trafficked toward myelin and membrane expansion within myelin sheaths can occur through several mechanisms, such as vesicular trafficking to the cell surface, non-vesicular lipid transport, or membrane incorporation of lipoproteins. Lam and colleagues have demonstrated that oligodendrocytes may add new membrane through the action of vamp2 and vamp3 SNAREs by initiating wrapping and power sheath elongation and that these SNAREs were required for node of Ranvier assembly. Moreover, membrane expansion has been associated with axon-myelin adhesion proteins transport by vamp2 and vamp3 to the inner tongue and paranodes to assemble nodes of Ranvier. Among the proteins that might be affected by vamp2 and vamp3 there were contactin-1 (Cntn1), neurofascin (Nfasc), and myelin associated glycoprotein (MAG). The first two were necessary for the establishment of axon myelin junctions at paranodes, whereas MAG maintains axon-myelin interactions at internodes. Furthermore, it has been demonstrated that genetic inactivation of vamp2 and vamp3 in myelinating oligodendrocytes was responsible of severe hypomyelination and premature death without oligodendrocyte loss [
17].
Taking all this into consideration, the expression of some SNAREs, such as syntaxin 3, syntaxin 4, and VAMP3, is linked to that of myelin markers and myelination. While their abundance increases, the one of SNAP29 decreases. Other affected proteins are vamp4, syntaxin 2, syntaxin 8, and SNAP23. Furthermore, the maturation and the accomplishment of myelinated axons is associated with syntaxin 3, syntaxin 4, SNAP-23 and vamp3. Moreover, syntaxin 3, syntaxin 4, and VAMP7/tetanustoxin-insensitive VAMP accumulated in myelin during development, suggesting a role in myelin membrane fusion, while the increase in expression of VAMP2 with maturation is most likely due to its abundance in synapses [
13] (
Figure 1).
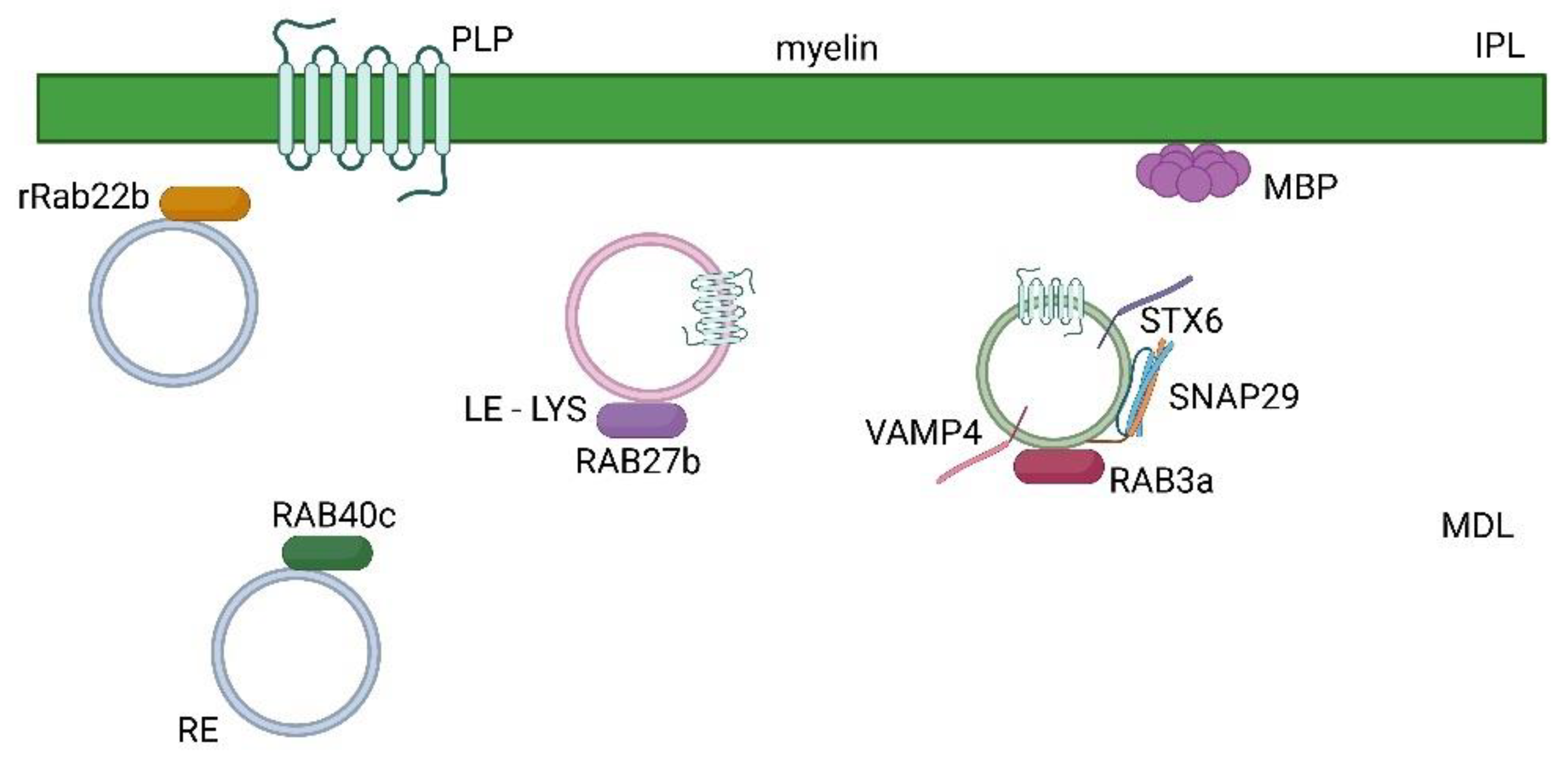
3. RABs
The synthesis of myelin sheath and the assembly of myelin proteins are regulated by rab (Ras-related proteins in brain) proteins through the modulation of the intracellular trafficking and fusion of vesicles. Several rabs have been discovered to affect important processes for myelin growth (
Figure 2). Rabs are small GTPases belonging to the Ras superfamily and are involved in the regulation of the intracellular trafficking and the fusion of vesicles between different compartments [
18]. Rab GTPases are molecular switches cycling between an active GTP-, membrane-bound form, and an inactive, cytosolic, GDP-bound form. Rabs exert their functions by interacting with specific effectors [
19].
In oligodendrocytes the following rab proteins are expressed: rab1, rab1b and rab2 in the early secretory pathway, rab8 and rab12 in the late secretory pathway and rab3a and rab3b in the regulated exocytic pathway. Furthermore, rrab22b regulates the transport from trans Golgi network to endosomes and rab5a, rab5b, rab5c, rab7 and rab9 are involved in the endocytic process. Rab14, rab18, rab23, rab24, rab26, rab40c and rab28 are also expressed in oligodendrocytes [
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
Interestingly, SNAP-29, a SNARE abundant in oligodendrocytes during myelination and in Schwann cells, interacts with different rab proteins, such as rab3a and rab24, with its N-terminal domain in a GTP-dependent or GTP-independent manner. As previously mentioned, rab3a and SNAP-29 colocalized both at the synapses and in the myelinating glia, and their expression enhanced the surface-directed trafficking of myelin proteolipid protein [
14].
Rab40c is a small GTPase that localized in the perinuclear recycling compartment (PRC), and it has been linked to endocytic events such as receptor recycling and myelin formation as both rab40c mRNA and protein increase as oligodendrocytes differentiate. Interestingly, Rab40c mRNA expression was identified in primary cultures of oligodendrocytes, oligodendrocyte progenitors, microglia, and astrocytes [
29].
Other rabs that are involved in myelin biogenesis and maintenance and oligodendrocytes differentiation are rab27b, rab35 and rrab22b. Rab35 and its effector, ACAP2, are downregulated during differentiation of oligodendrocytes, affect arf6 activity and promote myelination. This event is mediated also by the role of cytohesin-2 [
30]. The transport of certain proteins from the trans Golgi to myelin was mediated by small tubule vesicular organelles containing the rat Rab protein rRab22b. These vesicles have been associated with areas close to the axons where active formation of myelin occurred [
27].
One of the rab proteins which has a role in PLP transport is rab27b. Rab27b colocalized with PLP in late endosomes/lysosomes of mature oligodendrocytes and it regulated its surface transport and exocytosis. In particular, silencing of rab27b determined the reduction of lysosomal exocytosis and therefore regulates the surface expression of myelin protein in oligodendrocytes and its release from myelin like membrane (MLM) formation in oligodendrocytes-neuron co-cultures [
31].
Interestingly, it has been demonstrated that rab proteins can regulate the demyelination of Schwann cells (
Figure 3). Indeed, altered rab11-dependent endocytotic trafficking of the laminin receptor SH3TC2 downregulated myelination, determined denervation and affected its maintenance. The myelination process is dependent on the expression of specific isoforms of the integrin receptors. The most important ones are α6β1 and α6β4, which are related to the early step of the myelination process and the structural stability of the mature myelin sheath. The endocytic recycling of the α6β4 complex regulates its surface expression [
32].
Similarly, downregulation of rab27a, which regulates the trafficking of the secretory lysosomes to the plasma membrane, inhibited the lysosomal exocytosis in Schwann cells and reduced the remyelination of regenerated sciatic nerve by modulating the trafficking of the myelin protein P0 in late endosomes and lysosomes [
33].
Rab35 modulates endosomal trafficking, the secretion of extracellular vesicles, actin dynamics, cell migration and cell signaling. Moreover, rab35 controls myelin sheath synthesis by forming a complex with the myotubularin-related phosphatydilinositol (PI) 3-phosphatase MTMR13 and MTMR2, whose mutated forms have been associated with CMT4B2 and B1 in humans. In particular, Rab35-GTP recruited MTMR13-based lipid phosphatase complexes. Rab35 was able to down-regulate lipid-mediated mTORC1 activation and alter myelin biogenesis. Disruption of rab35 activity determined the hyperactivation of mTORC1 signaling due to elevated levels of PI 3-phosphates and hypermyelination and loss of rab35 in Schwann cells. Therefore, the synthesis of myelin sheath was altered [
34].