Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Data and Methods
2.1. Data
2.1.1. Spectra from the USGS Library
2.1.2. Synthetic Data
2.1.3. Hyperspectral Data
- (1)
- Cuprite image data
- (2)
- Hyperion image data
2.2. Methods
2.2.1. Linear Mixing Model
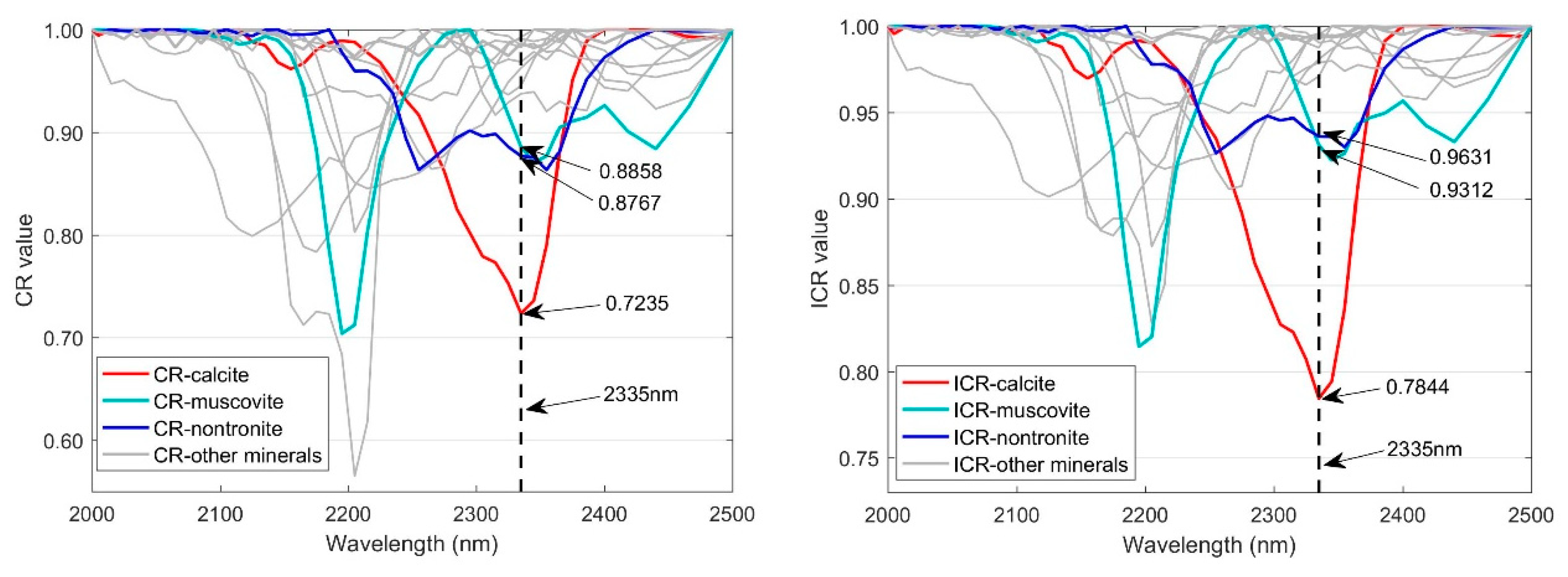
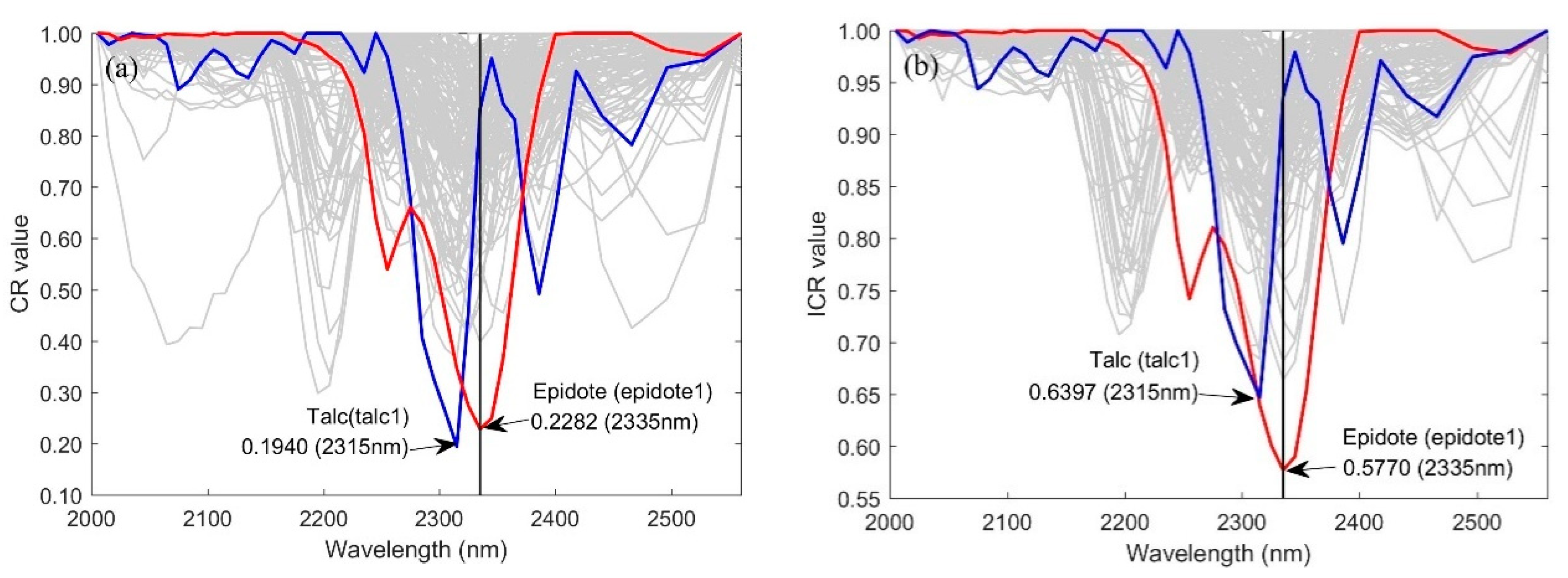
2.2.2. Construction of Improved Continuum Removal Algorithm
2.2.3. Abundance Normalization
- (1)
- Sum abundance normalization
- (2)
- Ratio abundance normalization
2.2.4. Evaluation
3. CRBD/Improved CRBD Varies with Carbonate Abundance
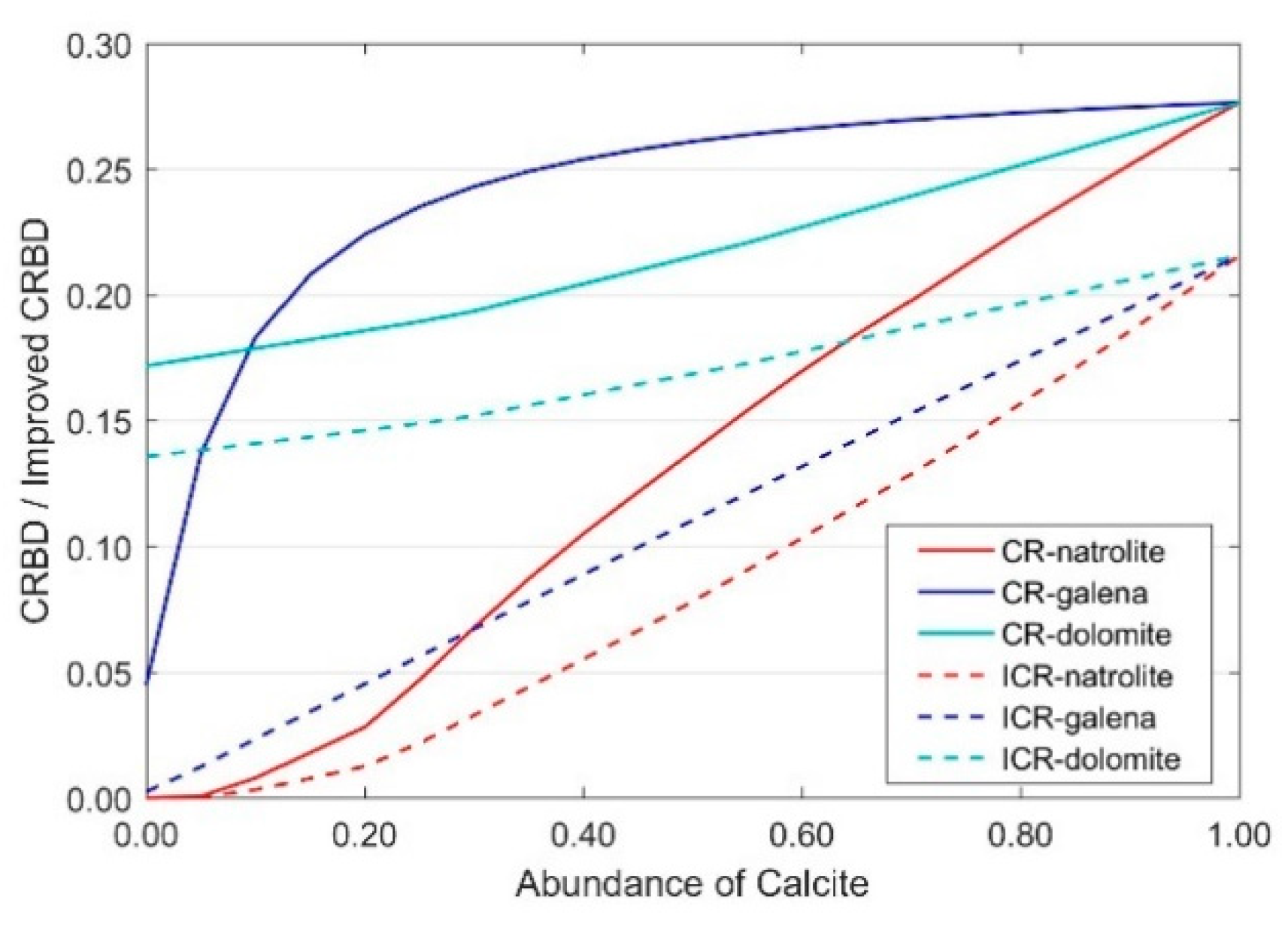
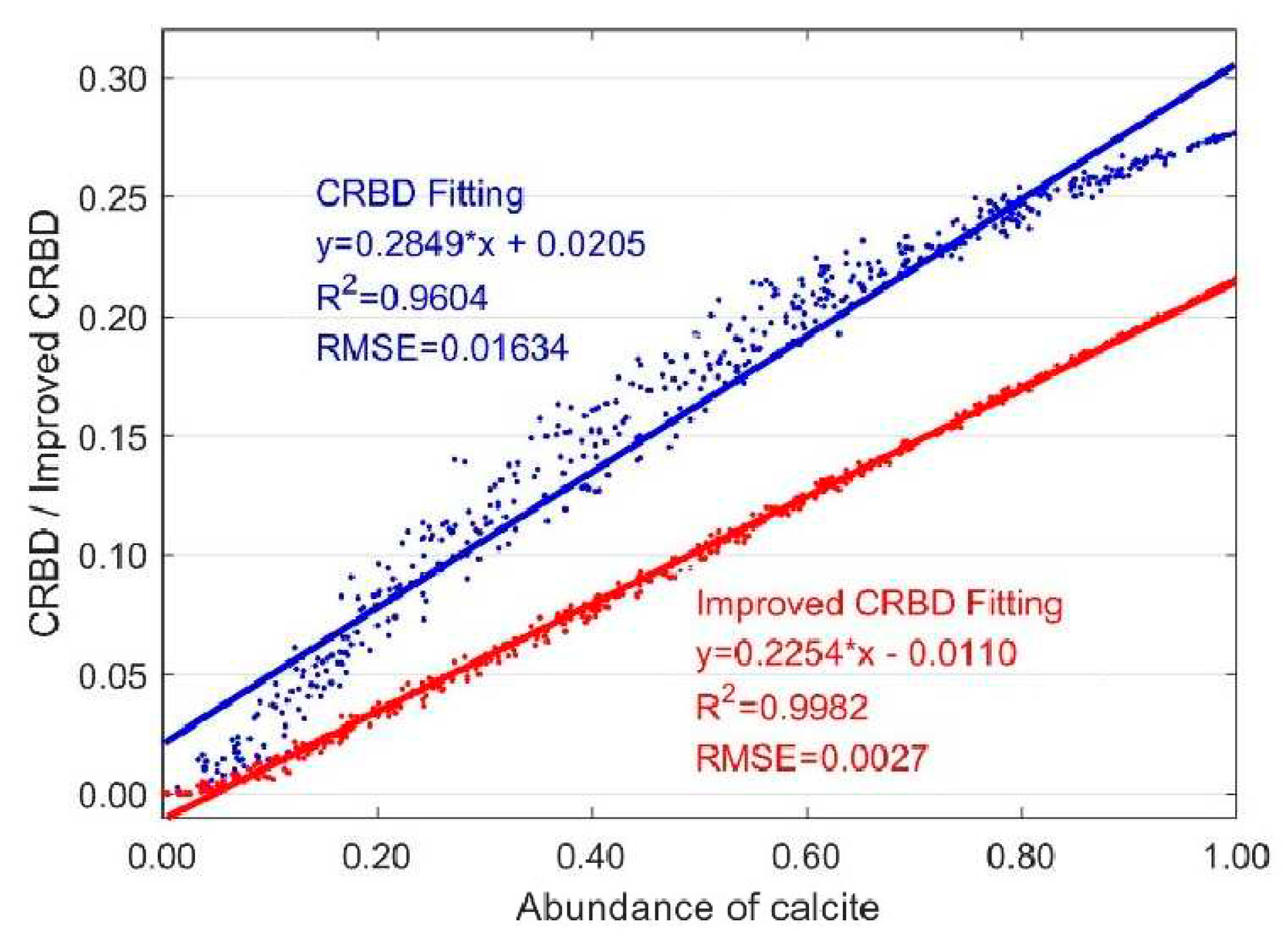
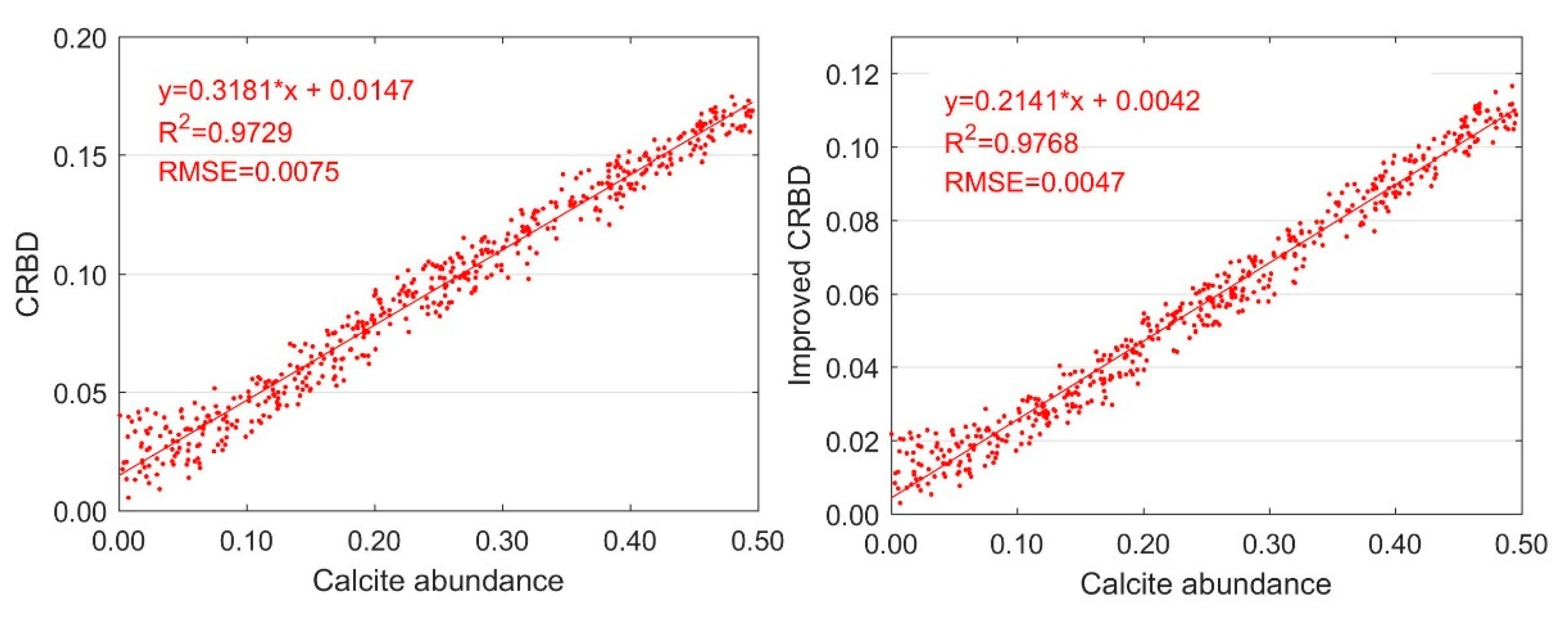
3.1. Variation with One Carbonate Mineral
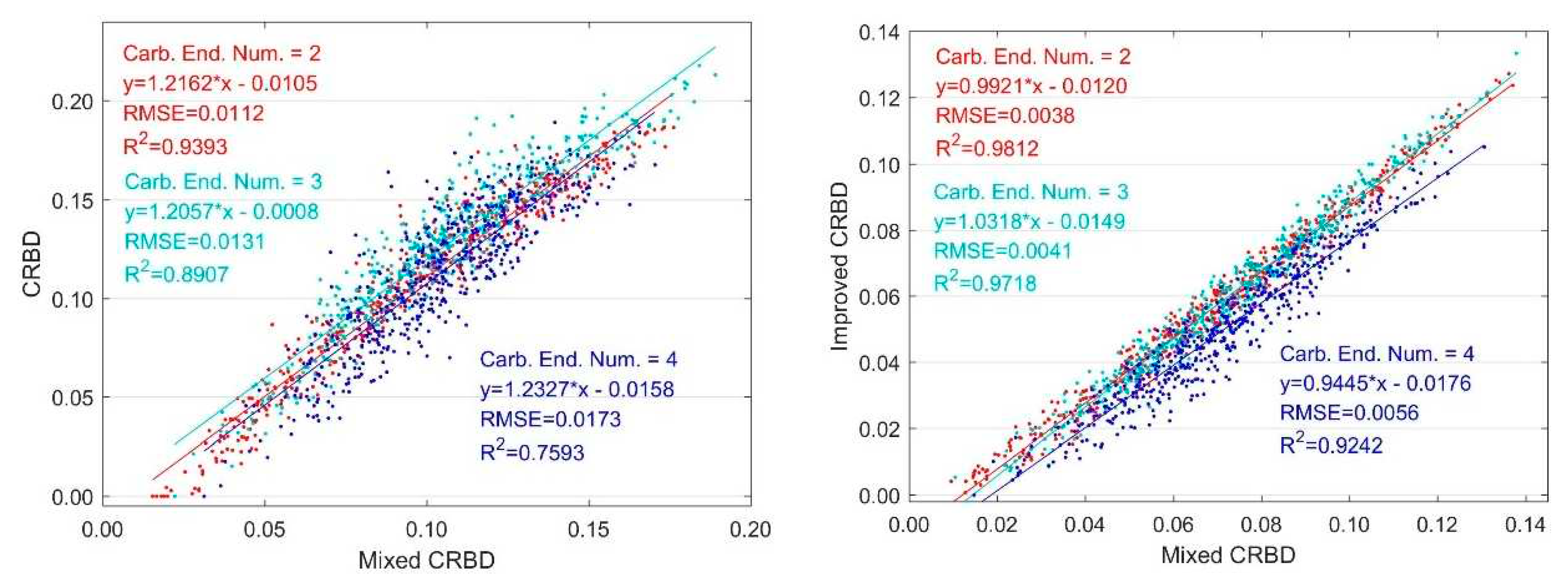
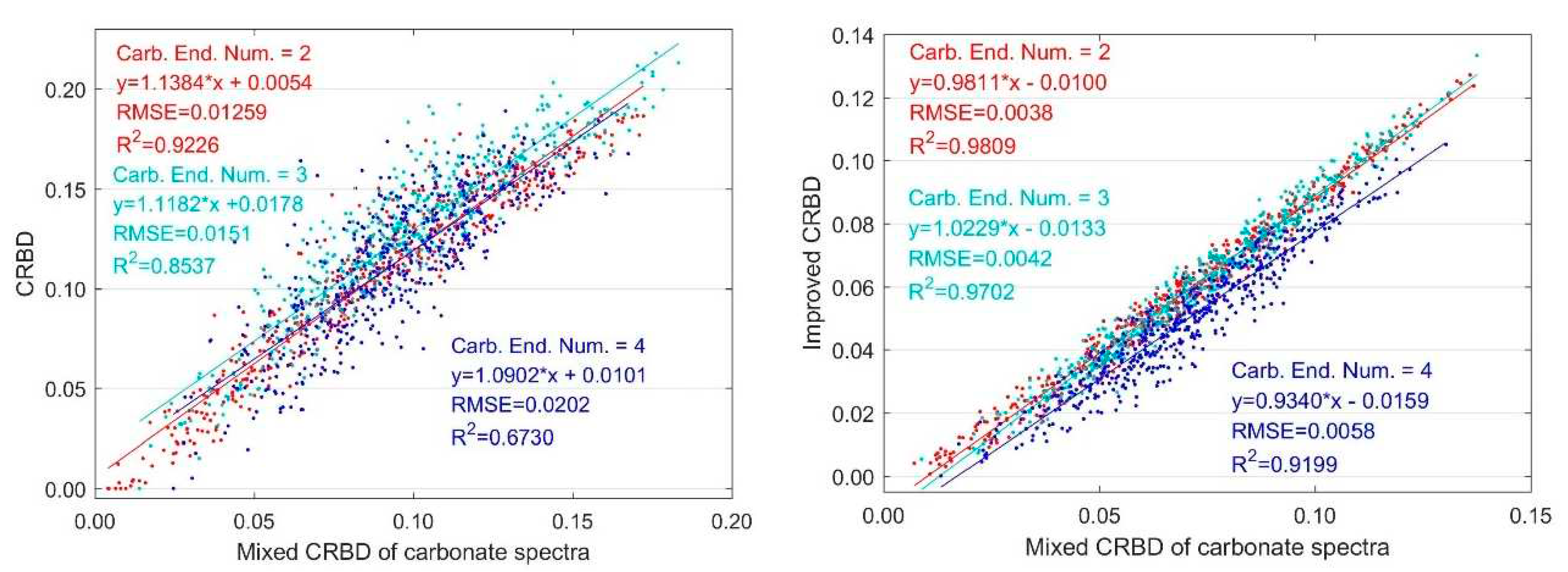
3.1.1. Variation with Two Endmembers Mixing
3.1.2. Variation with Multiple Endmember Mixing
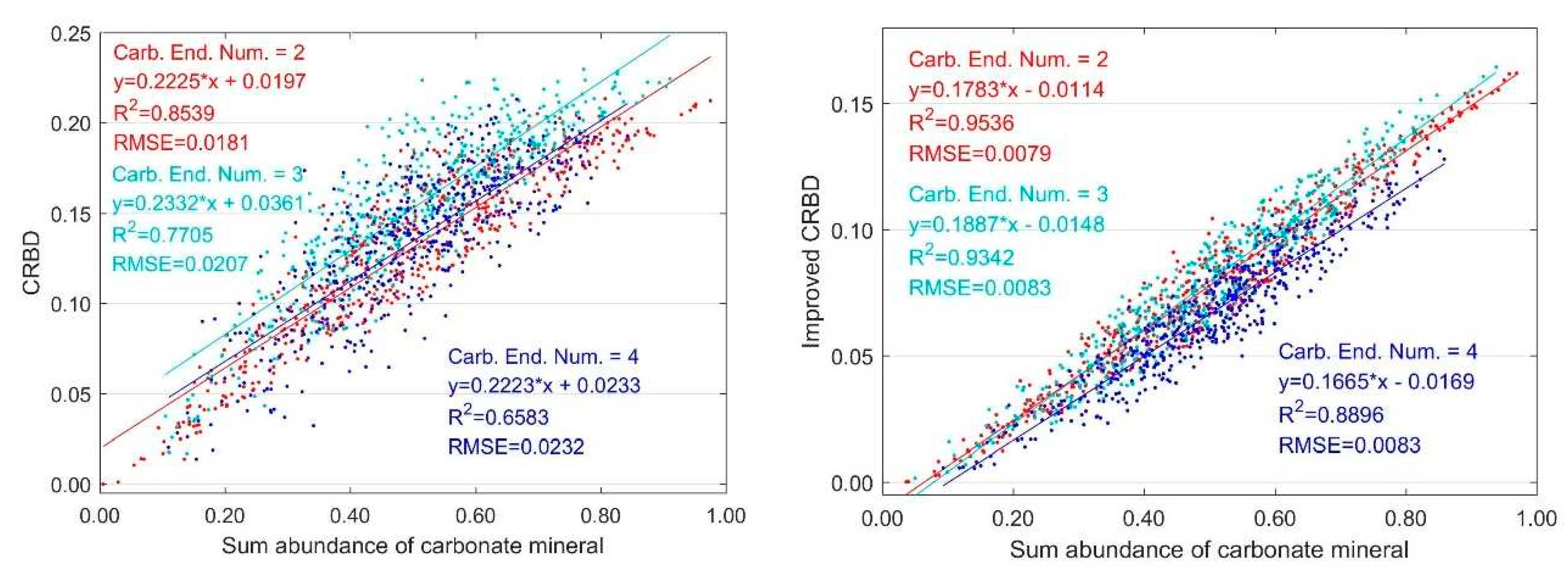
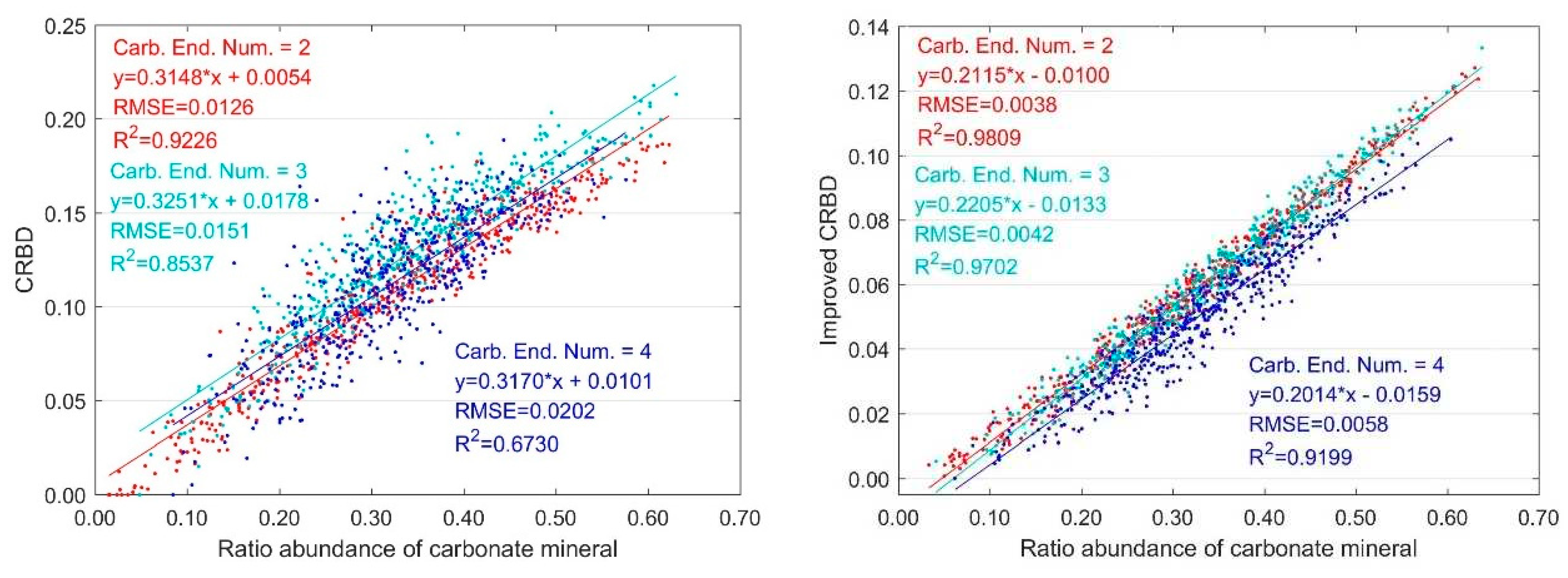
3.2. Variation with Multiple Carbonate Minerals and Normalized Abundance
3.2.1. Variation with Sum Abundance
3.2.2. Variation with Ratio Abundance
4. Carbonate Mineral Abundance Inversion
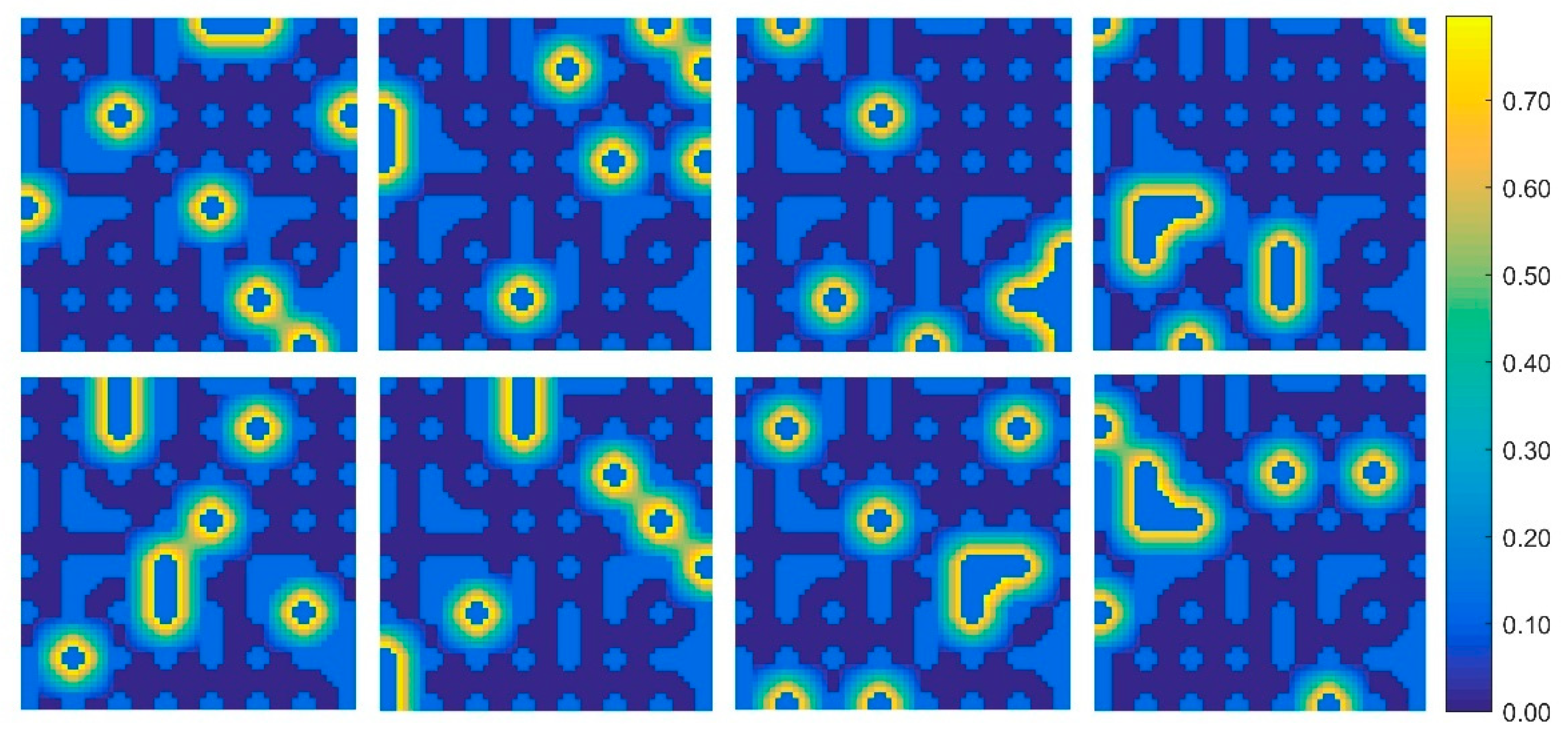
4.1. Abundance Inversion of Synthetic Image Data
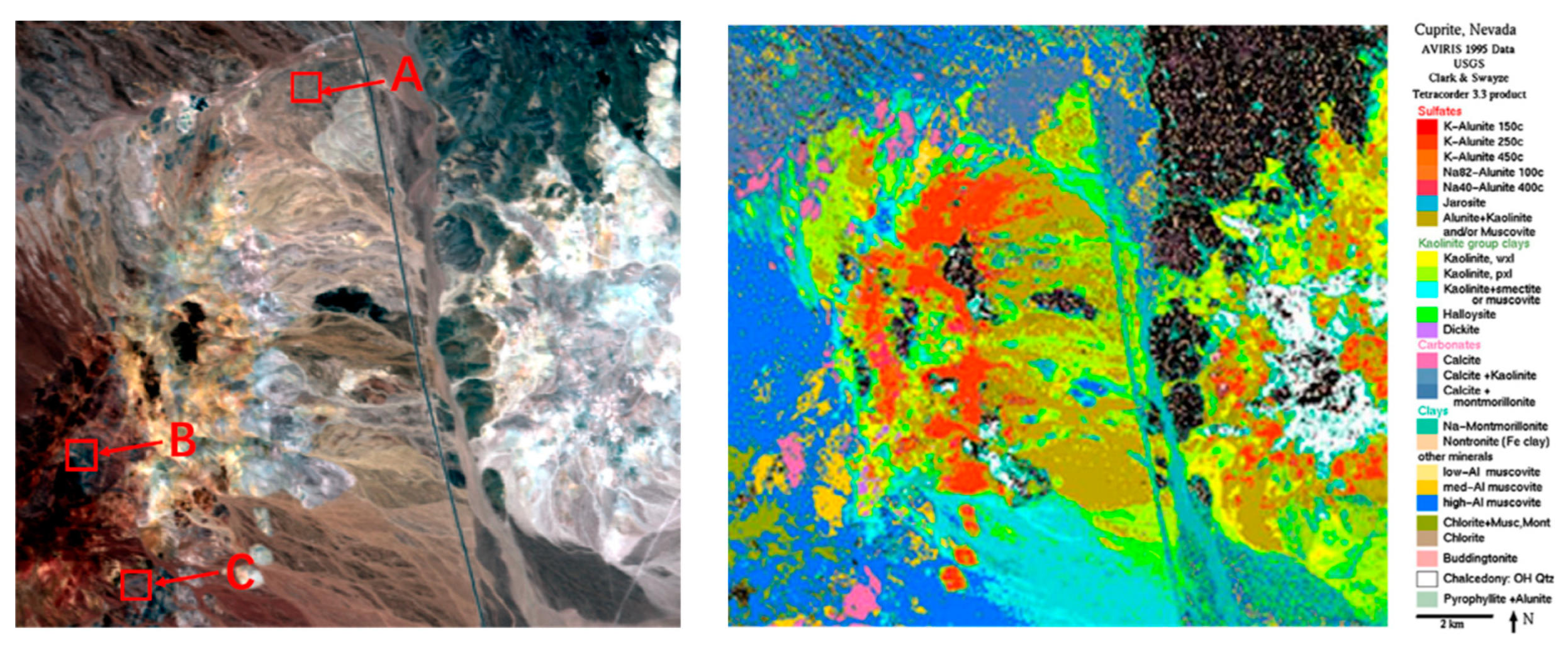
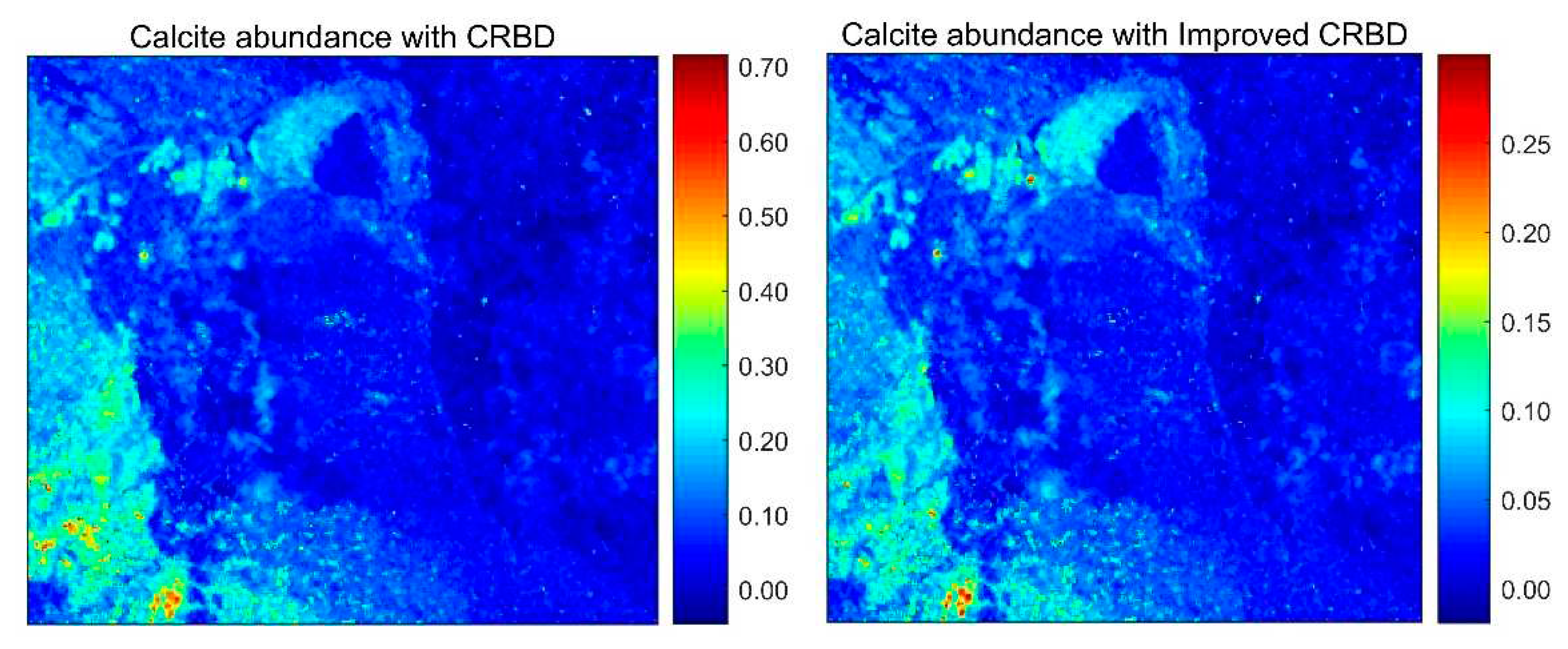
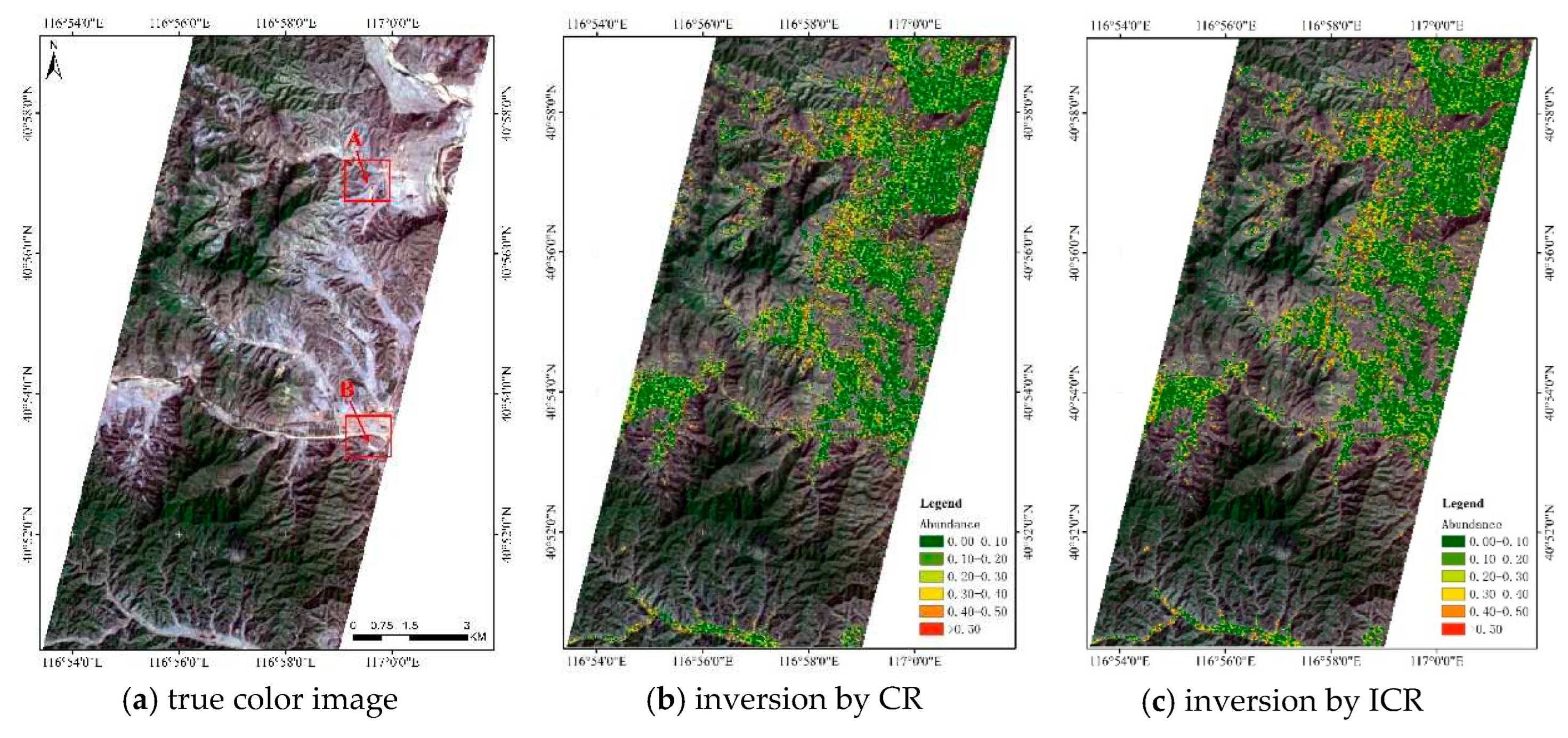
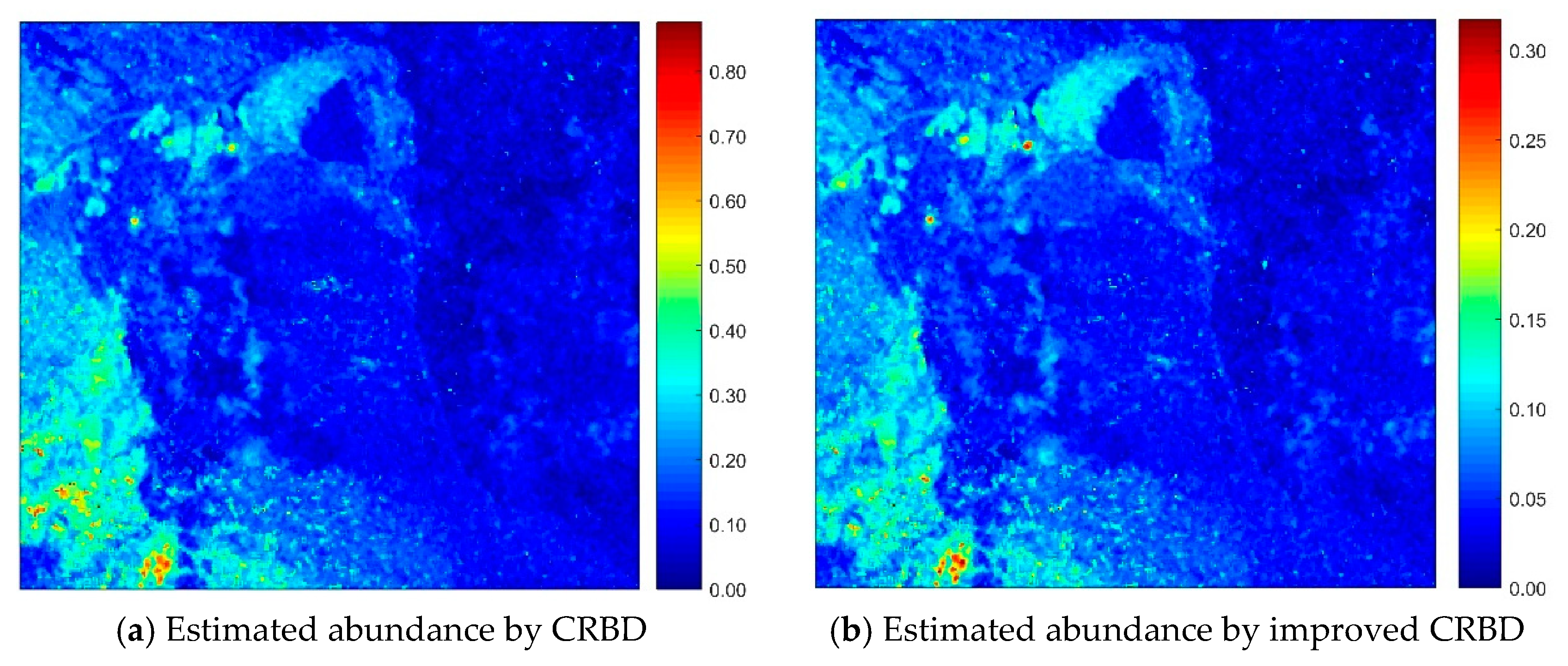
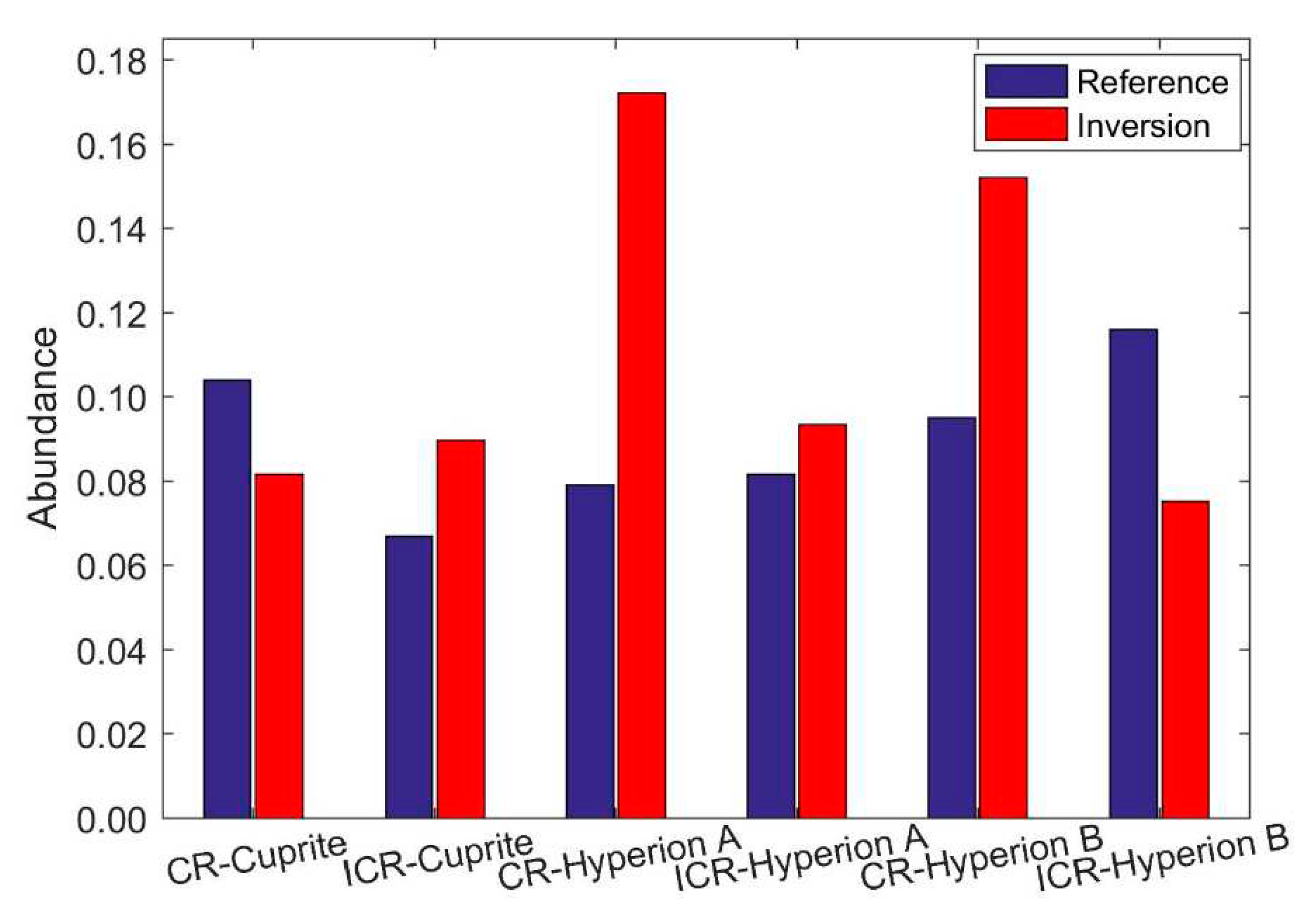
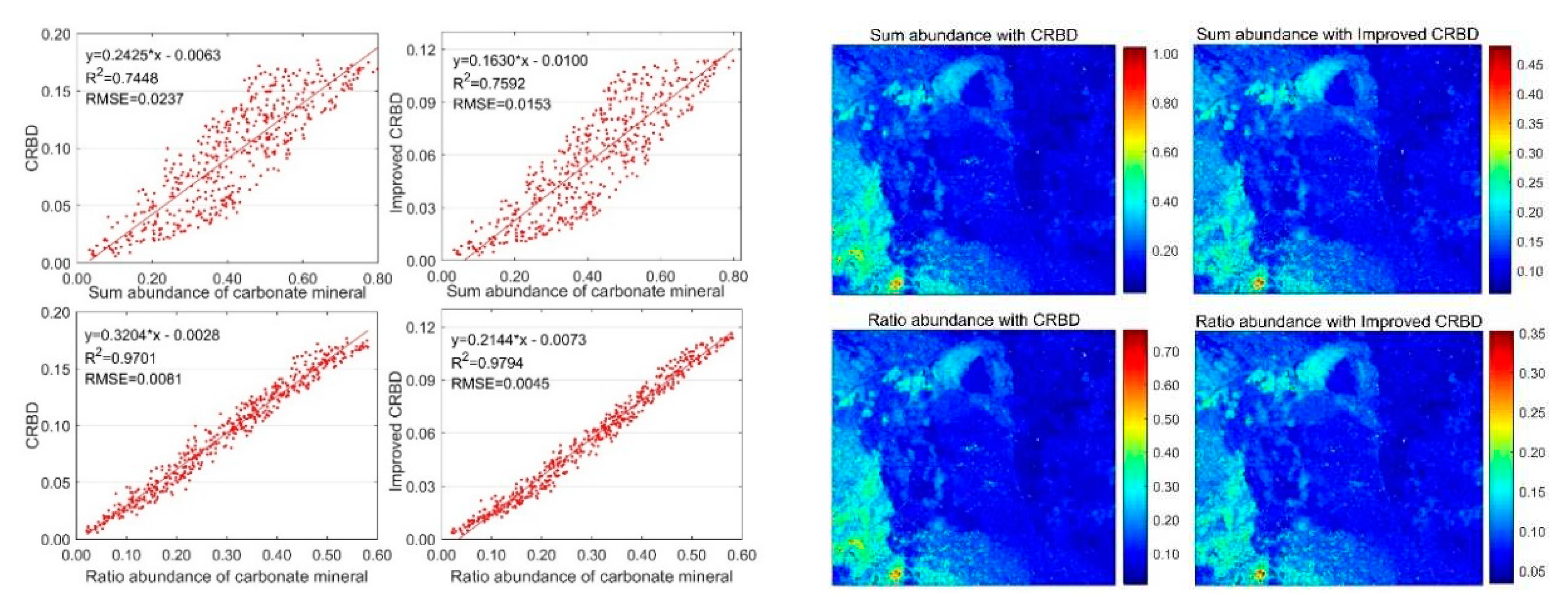
4.2. Abundance Inversion of Real Image Data, Cuprite Dataset
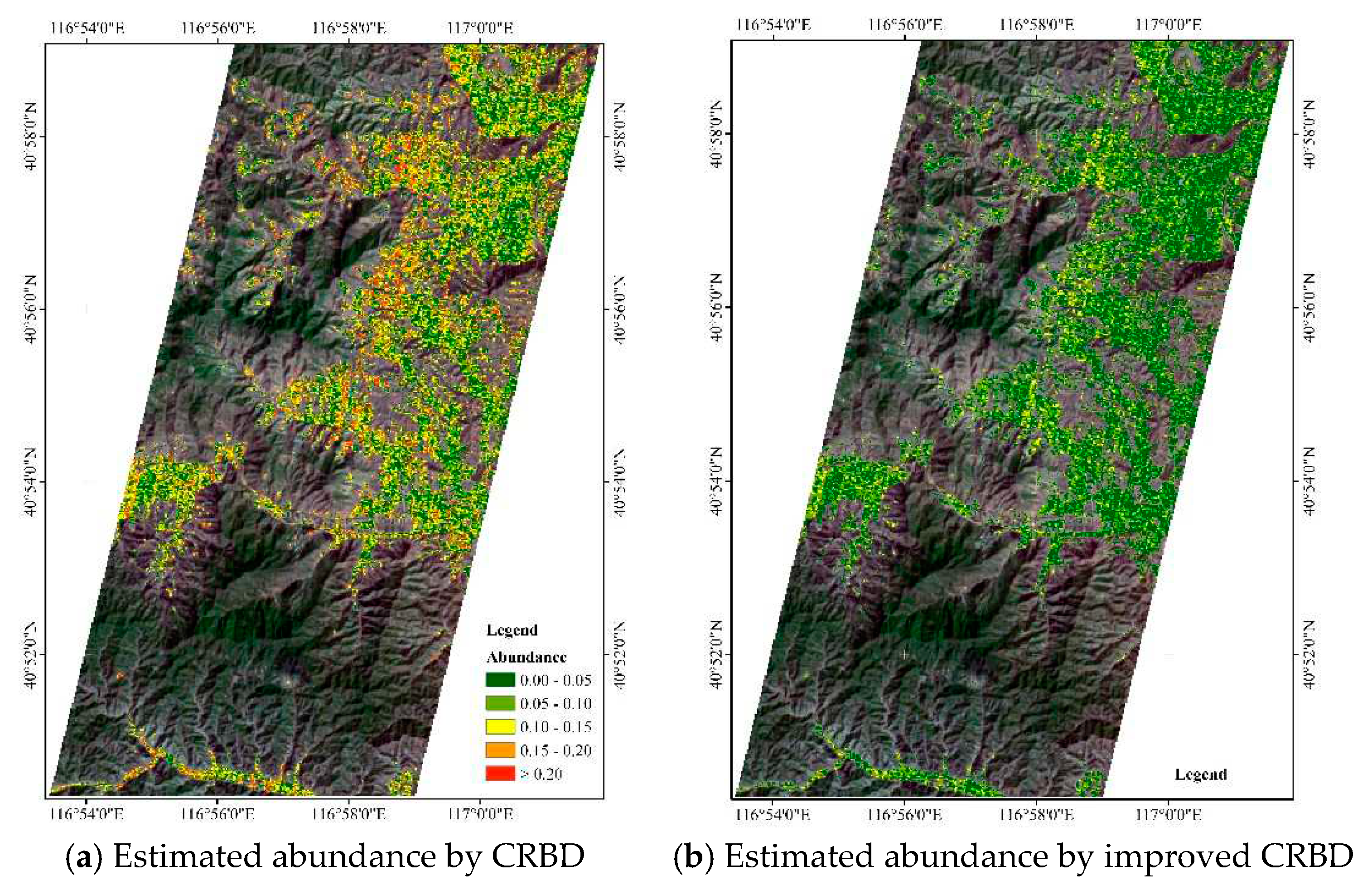
4.3. Abundance Inversion of Real Image Data, Hyperion Image Dataset
5. Discussions and Future Works
5.1. Simplified Abundance Inversion Model
5.1.1. Simplified Processing
5.1.2. Applications by Simplified Inversion Model
5.2. General Abundance Inversion Model
5.2.1. General Abundance Inversion Model by USGS Library
5.2.2. Abundance Inversion without Ground Samples
5.3. Considering the Absorption Influence of Non-Carbonate
5.3.1. Influence on Cuprite Image Data
5.3.2. Influence on Hyperion Image Data
5.4. Future Works
5.4.1. Relationship between Spectral Absorption and Carbonate Ion Concentration
5.4.2. Effects by Particle Size, Cation Types, and Other Factors
6. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altamimi, Amal, and Belgacem Ben Youssef. “A Systematic Review of Hardware-Accelerated Compression of Remotely Sensed Hyperspectral Images.” Sensors 22, no. 1 (2021): 263. [CrossRef]
- Heylen, Rob, Mario Parente, and Paul Gader. “A Review of Nonlinear Hyperspectral Unmixing Methods.” IEEE Journal of Selected Topics in Applied Earth Observations & Remote Sensing 7, no. 6 (2014): 1844-68. [CrossRef]
- Zastrow, Allison, and D. Timothy Glotch. “Distinct Carbonate Lithologies in Jezero Crater, Mars.” Geophysical Research Letters 48, no. 9 (2021): 1-10. [CrossRef]
- Kumaresan, P. R., J. Saravanavel, and P. Kathiresan. “Lithological Mapping of Eratosthenes Crater Region Using Moon Mineralogy Mapper of Chandrayaan-1.” Planetary and space science 182 (2020): 104817. [CrossRef]
- Bishop, J. L., R. T. Schelble, C. P. Mckay, A. J. Brown, and K. A. Perry. “Carbonate Rocks in the Mojave Desert as an Analogue for Martian Carbonates.” International Journal of Astrobiology 10, no. 04 (2011): 349-58. [CrossRef]
- Marinoni, Andrea, and Harold Clenet. “Higher Order Nonlinear Hyperspectral Unmixing for Mineralogical Analysis over Extraterrestrial Bodies.” IEEE Journal of Selected Topics in Applied Earth Observations & Remote Sensing 10, no. 8 (2017): 3722-33. [CrossRef]
- Mahmud, M. S., J. Z. Huang, and X. Fu. “Variational Autoencoder-Based Dimensionality Reduction for High-Dimensional Small-Sample Data Classification.” International Journal of Computational Intelligence and Applications 19, no. 1 (2020): 2050002. [CrossRef]
- Green, A. A., M. Berman, P. Switzer, and M. D. Craig. “A Transformation for Ordering Multispectral Data in Terms of Image Quality with Implications for Noise Removal.” Ieee Transactions on Geoscience and Remote Sensing 26, no. 1 (1988): 65-74. [CrossRef]
- Clark, R. N., and T. L. Roush. “Reflectance Spectroscopy: Quantitative Analysis Techniques for Remote Sensing Applications.” Journal of Geophysical Research 89, no. B7 (1984): 6329-40. [CrossRef]
- Boardman, J. W., and F. A. Kruse. “Automated Spectral Analysis: A Geologic Example Using Aviris Data, North Grapevine Mountains, Nevada.” Paper presented at the In Proceedings, ERIM Tenth Thematic Conference on Geologic Remote Sensing, Ann Arbor, MI: Environmental Research Institute of Michigan 1994.
- Zhao, Chunhui, Chuang Li, and Shou Feng. “A Spectral–Spatial Method Based on Fractional Fourier Transform and Collaborative Representation for Hyperspectral Anomaly Detection.” Ieee Geoscience and Remote Sensing Letters 18, no. 7 (2020): 1259-63. [CrossRef]
- Clark, R. N. “Spectroscopy of Rocks and Minerals, and Principles of Spectroscopy.” In In Manual of Remote Sensing, edited by A. N. Rencz, 3-58: New York: John Wiley and Sons, 1999.
- Rodger, Andrew, and Thomas Cudahy. “Vegetation Corrected Continuum Depths at 2.20 µm: An Approach for Hyperspectral Sensors.” Remote Sensing of Environment 113, no. 10 (2009): 2243-57.
- Murphy, R. J. “The Effects of Surficial Vegetation Cover on Mineral Absorption Feature Parameters.” International Journal of Remote Sensing 16, no. 12 (1995): 2153-64. [CrossRef]
- Rodger, A., and T. Cudahy. “Vegetation Corrected Continuum Depths at 2.20 M: An Approach for Hyperspectral Sensors.” Remote Sensing of Environment 113, no. 10 (2009): 2243-57.
- Miao, L. D., and H. R. Qi. “Endmember Extraction from Highly Mixed Data Using Minimum Volume Constrained Nonnegative Matrix Factorization.” Ieee Transactions on Geoscience and Remote Sensing 45, no. 3 (2007): 765-77. [CrossRef]
- Chen, Lei, Shengbo Chen, and Xulin Guo. “Multilayer Nmf for Blind Unmixing of Hyperspectral Imagery with Additional Constraints.” Photogrammetric Engineering & Remote Sensing 83, no. 4 (2017): 307-16. [CrossRef]
- Rajabi, Roozbeh, and Hassan Ghassemian. “Spectral Unmixing of Hyperspectral Imagery Using Multilayer Nmf.” Geoscience and Remote Sensing Letters, IEEE 12, no. 1 (2015): 38-42. [CrossRef]
- Wang, N., B. Du, L. P. Zhang, and L. F. Zhang. “An Abundance Characteristic-Based Independent Component Analysis for Hyperspectral Unmixing.” Ieee Transactions on Geoscience and Remote Sensing 53, no. 1 (2015): 416-28. [CrossRef]
- Chen, Shengbo, Yijing Cao, Lei Chen, and Xulin Guo. “Geometrical Constrained Independent Component Analysis for Hyperspectral Unmixing.” International Journal of Remote Sensing 41, no. 17 (2020): 6783-804. [CrossRef]
- Jain, R., and R. U. Sharma. “Airborne Hyperspectral Data for Mineral Mapping in Southeastern Rajasthan, India.” International Journal of Applied Earth Observation and Geoinformation 81 (2019): 137-45. [CrossRef]
- Wei, Jing, Yanfang Ming, Qiang Jia, and Dongxu Yang. “Simple Mineral Mapping Algorithm Based on Multitype Spectral Diagnostic Absorption Features: A Case Study at Cuprite, Nevada.” Journal of Applied Remote Sensing 11 (2017): 026015. [CrossRef]
- Rodger, Andrew, Carsten Laukamp, Maarten Haest, and Thomas Cudahy. “A Simple Quadratic Method of Absorption Feature Wavelength Estimation in Continuum Removed Spectra.” Remote Sensing of Environment 118 (2012): 273–83. [CrossRef]
- Kim, Y., M. C. Caumon, O. Barres, A. Sall, and J. Cauzid. “Identification and Composition of Carbonate Minerals of the Calcite Structure by Raman and Infrared Spectroscopies Using Portable Devices.” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 261 (2021): 119980. [CrossRef]
- Gomez, C., P. Lagacherie, and G. Coulouma. “Continuum Removal Versus Plsr Method for Clay and Calcium Carbonate Content Estimation from Laboratory and Airborne Hyperspectral Measurements.” Geoderma 148, no. 2 (2008): 141-48. [CrossRef]
- Zhang, Shihong, Keyan Xiao, Jianping Chen, Jie Xiang, Ning Cui, and Xiaonan Wang. “Development and Future Prospects of Quantitative Mineral Assessment in China.” China Geology 2, no. 2 (2019): 198-210. [CrossRef]
- Datta, S., B. K. Sinha, S. Bhattacharjee, and T. Seal. “Nutritional Composition, Mineral Content, Antioxidant Activity and Quantitative Estimation of Water Soluble Vitamins and Phenolics by Rp-Hplc in Some Lesser Used Wild Edible Plants.” Heliyon 5, no. 2019 (2019): e01431. [CrossRef]
- Noda, Shuho, Yamaguchi, and Yasushi. “Estimation of Surface Iron Oxide Abundance with Suppression of Grain Size and Topography Effects.” Ore Geology Reviews 83 (2017): 312-20. [CrossRef]
- Pilorget, C. , and J. Fernando. “Quantifying the Minerals Abundances on Planetary Surfaces Using Vis-Nir Spectroscopy, What Uncertainties Should We Expect? General Results and Application to the Case of Phyllosilicates and Carbonates on Mars.” Icarus 365, no. 2021 (2021): 114498. [CrossRef]
- Lin, Honglei, John F. Mustard, and X. Zhang. “A Methodology for Quantitative Analysis of Hydrated Minerals on Mars with Large Endmember Library Using Crism near-Infrared Data.” Planetary and space science 165 (2019): 124-36. [CrossRef]
- Chen, Shengbo, Lei Chen, Yanli Liu, and Xinlong Li. “Experimental Simulation on Mixed Spectra of Leaves and Calcite for Inversion of Carbonate Minerals from Eo-1 Hyperion Data.” Giscience & Remote Sensing 50, no. 6 (2013): 690-703. [CrossRef]
- Zhao, Hengqian, and Xuesheng Zhao. “Nonlinear Unmixing of Minerals Based on the Log and Continuum Removal Model.” European Journal of Remote Sensing 52, no. 1 (2019): 277-93. [CrossRef]
- Yan, S., B. Zhang, Y. Zhao, L. Zheng, Q. Tong, and K. Yang. “Summarizing the Vis-Nir Spectra of Minerals and Rocks.” remote sensing technology and application 18, no. 4 (2003): 191-201.
- Zhai, Wenyu, Lei Chen, Yixuan Xu, and Xiangyu Kong. “Analysis of Impact Factors and Applications by Using Spectral Absorption Depth for Quantitative Inversion of Carbonate Mineral.” Spectroscopy & Spectral Analysis 41, no. 7 (2021): 2226-32. [CrossRef]
- Li, Hengchao, Shuang Liu, Xinru Feng, and Shaoquan Zhang. “Sparsity-Constrained Coupled Nonnegative Matrix-Tensor Factorization for Hyperspectral Unmixing.” IEEE Journal of Selected Topics in Applied Earth Observations & Remote Sensing 13 (2020): 5061-73. [CrossRef]
- Gaffey, Susan J. “Spectral Reflectance of Carbonate Minerals in the Visible and near Infrared (0.35–2.55 Um): Anhydrous Carbonate Minerals.” Journal of Geophysical Research 92, no. B2 (1987): 1429-40. [CrossRef]
- Hunt, G. R., and J. W. Salisbury. “Visible and near Infrared Spectra of Minerals and Rocks. Ii. Carbonate.” Modern Geology 1971, no. 2 (1971): 23-30.
- Kurz, Tobias H., Julie Dewit, Simon J. Buckley, John B. Thurmond, David W. Hunt, and Rudy Swennen. “Hyperspectral Image Analysis of Different Carbonate Lithologies (Limestone, Karst and Hydrothermal Dolomites): The Pozalagua Quarry Case Study (Cantabria, North-West Spain).” Sedimentology 2012, no. 59 (2012): 623-45. [CrossRef]
- Crowley, James K. “Visible and near-Infrared Spectra of Carbonate Rocks: Reflectance Variations Related to Petrographic Texture and Impurities.” Journal of Geophysical Research 91, no. B5 (1986): 5001. [CrossRef]
- Van der Meer, Freek “Spectral Reflectance of Carbonate Mineral Mixtures and Bidirectional Reflectance Theory: Quantitative Analysis Techniques for Application in Remote Sensing.” Remote Sensing Reviews 13, no. 1-2 (1995): 67-94. [CrossRef]
- Kopačková, Veronika, and Lucie Koucká. “Integration of Absorption Feature Information from Visible to Longwave Infrared Spectral Ranges for Mineral Mapping.” Remote Sensing 9, no. 10 (2017): 1006. [CrossRef]
- Brown, A. J., S. J. Hook, A. M. Baldridge, J. K. Crowley, N. T. Bridges, B. J. Thomson, G. M. Marion, Carlos Roberto De Souza Filho, and J. L. Bishop. “Hydrothermal Formation of Clay-Carbonate Alteration Assemblages in the Nili Fossae Region of Mars.” Earth & Planetary Science Letters 297, no. 2010 (2010): 174-82. [CrossRef]


| Carbonate Minerals Group1 (Absorption) |
Other Minerals | |
|---|---|---|
| Group 2 (Flat) | Group 3 (Reflected Peak) | |
| Calcite(Ca[CO3]) Dolomite ((Ca, Mg)[CO3]2) Rhodochrosite (Mn[CO3]) Strontianite (Sr[CO3]) Witherite (Ba[CO3]) Magnesite(Mg[CO3]) |
Chalcopyrite (CuFeH4S2) Galena (PbS) Grossular (Ca3Al2[SiO4]3) Hematite (Fe₂O₃) Hypersthene ((Mg, Fe)[SiO3]) Microcline (K[AlSi3O8]) Olivine((Mg, Fe)2[SiO4]) Quartz (SiO2) Anorthite (Ca[Al₂Si₂O₈]) |
Heulandite (Ca[Al2Si7O18]·6H2O) Natrolite (Na2[Al2Si3O10]·2H2O) Kaolinite(Al4[Si4O10](OH)8) Montmorillonite((Na,Ca)0.33(Al,Mg)2 [Si4O10](OH)2·nH2O ) Jarosite(KFe3[SO4]2(OH)6) Goethite (FeO(OH)) Buddingtonite ((NH4)[AlSi3O8]) Hypersthene ((Mg,Fe)2[Si2O6]) Chabazite((Ca, K2, Na2)2 [Al2Si4O12]2·12H2O) |
| Minerals with Absorption Group 1 (Absorption Valley) |
Other Minerals | |
|---|---|---|
| Group 2 (Flat Spectra) | Group 3 (Reflected Peak) | |
| Calcite (Ca[CO3]), Muscovite (KAl2[AlSi3O10](OH)2), Nontronite (Na0.33Fe23+(Al,Si)4O10(OH)2·nH2O) |
Pyrope(Mg3Al2[SiO4]3), Dumortierite ((Al,Fe3+)7BO3[SiO4]3O3), Sphene (CaTi[SiO4](O,OH,Cl,F)), Desert varnish |
Alunite (KAl(SO4)2·12H2O), Buddingtonite ((NH4)AlSi3O8·nH2O), Kaolinite (Al4[Si4O10](OH)8), Jarosite (KFe3[SO4]2(OH)6), Chalcedony (SiO2), Andradite (Ca3Fe2[SiO4]3), Montmorillonite ((Na,Ca)0.33(Al,Mg)2 [Si4O10](OH)2·nH2O ) |
| Inversion Methods | Carbonate Endmember Number | Mean RMSE | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Sum abundance with FCLS | 0.0000 | 0.1682 | 0.1472 | 0.1487 | 0.1160 |
| Ratio abundance with FCLS | 0.0000 | 0.0598 | 0.0464 | 0.0923 | 0.0496 |
| Sum abundance with MVCNMF | 0.0825 | 0.2052 | 0.4177 | 0.4251 | 0.2826 |
| Ratio abundance with MVCNMF | 0.0389 | 0.1103 | 0.3207 | 0.1573 | 0.1568 |
| Sum abundance with CMLNMF | 0.0177 | 0.2264 | 0.2252 | 0.4800 | 0.2373 |
| Ratio abundance with CMLNMF | 0.0123 | 0.1433 | 0.2121 | 0.1712 | 0.1347 |
| Sum abundance with GCICA | 0.2116 | 0.3447 | 0.4395 | 0.2813 | 0.3193 |
| Ratio abundance with GCICA | 0.1758 | 0.2722 | 0.3568 | 0.1877 | 0.2481 |
| Sum abundance with ACICA | 0.1690 | 0.2552 | 0.3137 | 0.3953 | 0.2833 |
| Ratio abundance with ACICA | 0.1690 | 0.1848 | 0.2513 | 0.2108 | 0.2040 |
| Sum abundance with CR | 0.0639 | 0.1195 | 0.1365 | 0.1888 | 0.1272 |
| Ratio abundance with CR | 0.0639 | 0.0808 | 0.1006 | 0.1264 | 0.0929 |
| Sum abundance with ICR | 0.0348 | 0.0699 | 0.0727 | 0.1011 | 0.0696 |
| Ratio abundance with ICR | 0.0348 | 0.0379 | 0.0336 | 0.0536 | 0.0400 |
| Algorithm | Calcite abundance | |
| FCLS | 0.1395 | |
| MVCNMF | 0.1250 | |
| CMLNMF | 0.3150 | |
| GCICA | 0.1754 | |
| ACICA | 0.2832 | |
| Inversion | 0.2938(CR) | 0.1268 (ICR) |
| RE | 41.52% | 38.92% |
| Algorithm | Parameter of fitting equation | R2 | RMSE | |
| Slope | y-intercept | |||
| Calcite abundance with CR | 0.2778 | 0.0440 | 0.9393 | 0.0099 |
| Calcite abundance with ICR | 0.1918 | 0.0135 | 0.9366 | 0.0070 |
| Inversion Method | Inverted Abundance | RE | |
|---|---|---|---|
| Position A | Position B | ||
| FCLS | / | 0.0747 | -25.30% |
| MVCNMF | / | 0.1334 | 33.40% |
| CMLNMF | / | 0.1880 | 88.00% |
| GCICA | / | 0.4119 | 311.90% |
| ACICA | / | 0.1429 | 42.90% |
| CR | / | 0.3314 | 231.40 |
| ICR | / | 0.1296 | 29.60% |
| Carbonate endmember number | Sum abundance with CR | Sum abundance with ICR | Ratio abundance with CR | Ratio abundance with ICR |
| 1 | 0.1136 | 0.0410 | 0.1136 | 0.0410 |
| 2 | 0.3267 | 0.3152 | 0.2829 | 0.2558 |
| 3 | 0.4603 | 0.4494 | 0.3909 | 0.3684 |
| 4 | 0.5292 | 0.5454 | 0.36480 | 0.3605 |
| Algorithm | Sum Abundance | Ratio Abundance | ||
|---|---|---|---|---|
| FCLS | 0.2736 | 0.1885 | ||
| MVCNMF | 0.3993 | 0.2971 | ||
| CMLNMF | 0.2795 | 0.2565 | ||
| GCICA | 0.2222 | 0.1566 | ||
| ACICA | 0.2500 | 0.1762 | ||
| Inversion | 0.4719(CR) | 0.2535 (ICR) | 0.3463(CR) | 0.1802(ICR) |
| RE | 65.63% | -11.03% | 61.08% | -16.18% |
| Algorithm | Parameter of Fitting Equation | R2 | RMSE | |
|---|---|---|---|---|
| Slope | y-Intercept | |||
| Sum abundance with CR | 0.1634 | 0.0317 | 0.6097 | 0.0270 |
| Sum abundance with ICR | 0.1202 | 0.0019 | 0.7090 | 0.0157 |
| Ratio abundance with CR | 0.2657 | 0.0254 | 0.9226 | 0.0119 |
| Ratio abundance with ICR | 0.1861 | 0.0003 | 0.9731 | 0.0048 |
| Inversion method | RE | Mean RE | |
|---|---|---|---|
| Position A | Position B | ||
| Sum abundance with FCLS | -8.83% | -24.76% | 16.79% |
| Ratio abundance with FCLS | 20.98% | 21.77% | 21.38% |
| Sum abundance with MVCNMF | -5.34% | -75.68% | 21.45% |
| Ratio abundance with MVCNMF | 41.53% | -55.96% | 33.96% |
| Sum abundance with CMLNMF | -2.29% | -36.08% | 19.18% |
| Ratio abundance with CMLNMF | -7.40% | 32.46% | 19.93% |
| Sum abundance with GCICA | -34.89% | 8.02% | 21.45% |
| Ratio abundance with GCICA | -55.59% | -12.33% | 33.96% |
| Sum abundance with ACICA | -59.17% | -71.42% | 65.30% |
| Ratio abundance with ACICA | -72.15% | -76.81% | 74.48% |
| Sum abundance with CR | 41.29% | -3.16% | 22.22% |
| Ratio abundance with CR | 111.77% | 89.58% | 100.67% |
| Sum abundance with ICR | -26.23% | -31.74% | 28.98% |
| Ratio abundance with ICR | 7.80% | -5.80% | 6.80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





