Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Patient samples
2.2. Neutrophil isolation
2.3. Neutrophil co-culturing and stimulation
2.4. Immunofluorescence staining
2.5. Statistical analysis
3. Results
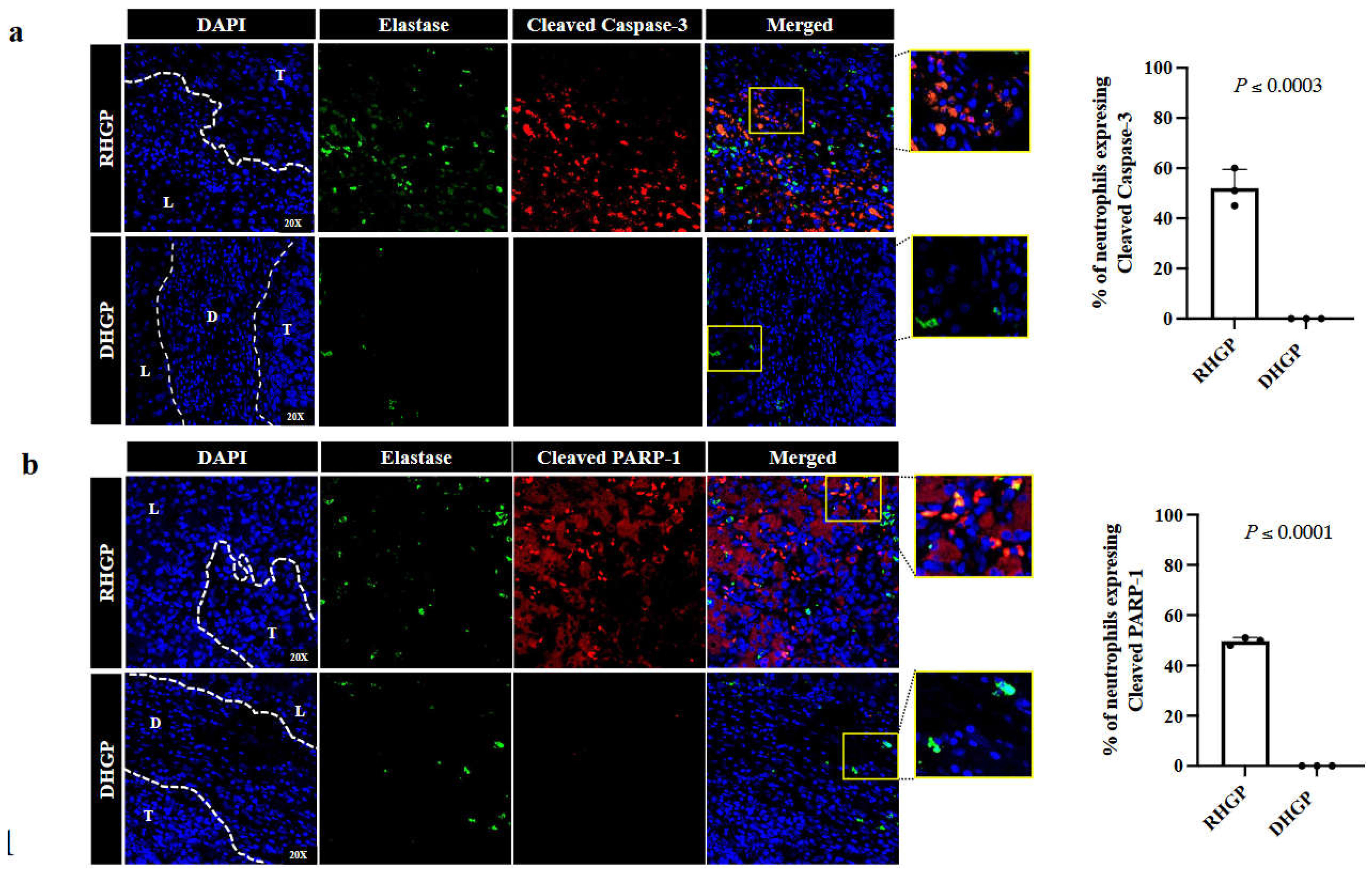
3.2. Vessel co-opting tumours comprise a higher number of apoptotic neutrophils compared to angiogenic lesions
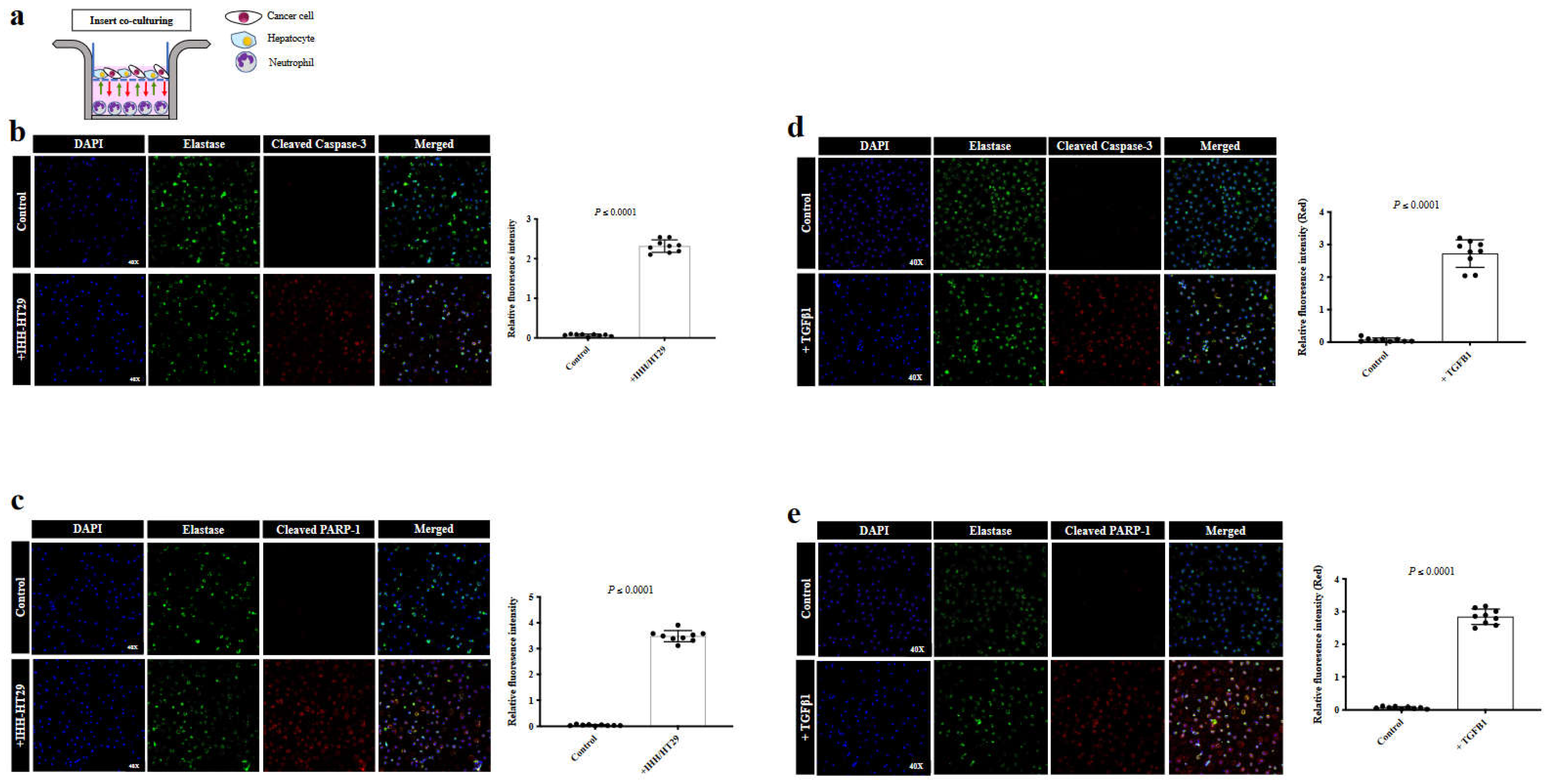
3.3. TGFβ1 mediates apoptosis in CRCLM tumours
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ibrahim, N.; Lazaris, A.; Rada, M.; Petrillo, S.; Huck, L.; Hussain, S.; Ouladan, S.; Gao, Z.; Gregorieff, A.; Essalmani, R.; et al. Angiopoietin1 Deficiency in Hepatocytes A Ff Ects the Growth of Colorectal Cancer Liver. Cancers (Basel) 2020, 12, 1–18. [Google Scholar]
- Frentzas, S.; Simoneau, E.; Bridgeman, V.L.; Vermeulen, P.B.; Foo, S.; Kostaras, E.; Nathan, M.R.; Wotherspoon, A.; Gao, Z.H.; Shi, Y.; et al. Vessel Co-Option Mediates Resistance to Anti-Angiogenic Therapy in Liver Metastases. Nat Med 2016, 22, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- Mayer, T.Z.; Kim, D.H.; Rada, M.; Petrillo, S.; Lazaris, A.; Metrakos, P. Role of Innate Immune Cells in the Development of Vessel Co-Opting CRC Liver Metastases. Cancer Res 2020, 80. [Google Scholar] [CrossRef]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel Co-Option in Cancer. Nat Rev Clin Oncol 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Kapelanski-Lamoureux, A.; Tsamchoe, M.; Petrillo, S.; Lazaris, A.; Metrakos, P. Angiopoietin-1 Upregulates Cancer Cell Motility in Colorectal Cancer Liver Metastases through Actin-Related Protein 2/3. Cancers (Basel) 2022, 14, 2540. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front Immunol 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in Cancer Carcinogenesis and Metastasis. J Hematol Oncol 2021, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat Rev Immunol 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Mollinedo, F. Neutrophil Degranulation, Plasticity, and Cancer Metastasis. Trends Immunol 2019, 40, 228–242. [Google Scholar] [CrossRef]
- Palmieri, V.; Lazaris, A.; Mayer, T.Z.; Petrillo, S.K.; Alamri, H.; Rada, M.; Jarrouj, G.; Park, W.; Gao, Z.; McDonald, P.P.; et al. Neutrophils Expressing Lysyl Oxidase-like 4 Protein Are Present in Colorectal Cancer Liver Metastases Resistant to Anti-angiogenic Therapy. J Pathol 2020, 251, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Hassan, N.; Lazaris, A.; Metrakos, P. The Molecular Mechanisms Underlying Neutrophil Infiltration in Vessel Co-Opting Colorectal Cancer Liver Metastases. Front Oncol 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Zhang, X.; Feng, M.; Ma, J.; Li, J.; Yang, X.; Fang, F. Exosome-Mediated Secretion of LOXL4 Promotes Hepatocellular Carcinoma Cell Invasion and Metastasis. Mol Cancer 2019, 18, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat Rev Dis Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Yao, F.; Huang, C. Lysyl Oxidase Family Proteins: Prospective Therapeutic Targets in Cancer. Int J Mol Sci 2022, 23, 12270. [Google Scholar] [CrossRef]
- Sun, C.; Ma, S.; Chen, Y.; Kim, N.H.; Kailas, S.; Wang, Y.; Gu, W.; Chen, Y.; Tuason, J.P.W.; Bhan, C.; et al. Diagnostic Value, Prognostic Value, and Immune Infiltration of LOX Family Members in Liver Cancer: Bioinformatic Analysis. Front Oncol 2022, 12. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Zhang, C.; Chan, Y.-T.; Yuen, M.-F.; Feng, Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021, 73, 2326–2341. [Google Scholar] [CrossRef]
- Görögh, T.; Quabius, E.S.; Heidebrecht, H.; Nagy, A.; Muffels, T.; Haag, J.; Ambrosch, P.; Hoffmann, M. Lysyl Oxidase Like-4 Monoclonal Antibody Demonstrates Therapeutic Effect against Head and Neck Squamous Cell Carcinoma Cells and Xenografts. Int J Cancer 2016, 138, 2529–2538. [Google Scholar] [CrossRef]
- Jiang, W.-P.; Sima, Z.-H.; Wang, H.-C.; Zhang, J.-Y.; Sun, L.-S.; Chen, F.; Li, T.-J. Identification of the Involvement of LOXL4 in Generation of Keratocystic Odontogenic Tumors by RNA-Seq Analysis. Int J Oral Sci 2014, 6, 31–38. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J Innate Immun 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Brostjan, C.; Oehler, R. The Role of Neutrophil Death in Chronic Inflammation and Cancer. Cell Death Discov 2020, 6. [Google Scholar] [CrossRef]
- Rada, M.; Tsamchoe, M.; Kapelanski-lamoureux, A.; Hassan, N.; Bloom, J.; Petrillo, S.; Kim, D.H.; Lazaris, A.; Metrakos, P. Cancer Cells Promote Phenotypic Alterations in Hepatocytes at the Edge of Cancer Cell Nests to Facilitate Vessel Co-Option Establishment in Colorectal Cancer Liver Metastases. 2022, 1–19.
- Althubiti, M.; Rada, M.; Samuel, J.; Escorsa, J.M.; Najeeb, H.; Lee, K.-G.; Lam, K.-P.; Jones, G.D.D.; Barlev, N.A.; Macip, S. BTK Modulates P53 Activity to Enhance Apoptotic and Senescent Responses. Cancer Res 2016, 76. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front Oncol 2019, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Andzinski, L.; Wu, C.F.; Lienenklaus, S.; Kröger, A.; Weiss, S.; Jablonska, J. Delayed Apoptosis of Tumor Associated Neutrophils in the Absence of Endogenous IFN-β. Int J Cancer 2015, 136, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Lazaris, A.; Kapelanski-Lamoureux, A.; Mayer, T.Z.; Metrakos, P. Tumor Microenvironment Conditions That Favor Vessel Co-Option in Colorectal Cancer Liver Metastases: A Theoretical Model. Semin Cancer Biol 2021, 71, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Rada, M.; Kapelanski-Lamoureux, A.; Petrillo, S.; Tabariès, S.; Siegel, P.; Reynolds, A.R.; Lazaris, A.; Metrakos, P. Runt Related Transcription Factor-1 Plays a Central Role in Vessel Co-Option of Colorectal Cancer Liver Metastases. Commun Biol 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Rada, M.; Tsamchoe, M.; Kapelanski-lamoureux, A.; Hassan, N.; Bloom, J.; Petrillo, S.; Kim, D.H.; Lazaris, A.; Metrakos, P. Cancer Cells Promote Phenotypic Alterations in Hepatocytes at the Edge of Cancer Cell Nests to Facilitate Vessel Co-Option Establishment in Colorectal Cancer Liver Metastases. 2022, 1–19.
- Uribe-Querol, E.; Rosales, C. Neutrophils in Cancer: Two Sides of the Same Coin. J Immunol Res 2015, 2015. [Google Scholar] [CrossRef]
- Granot, Z. Neutrophils as a Therapeutic Target in Cancer. Front Immunol 2019, 10, 1710. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenvironment 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis. PLoS One 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S. v.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The Prognostic Landscape of Genes and Infiltrating Immune Cells across Human Cancers. Nat Med 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Shitara, K.; Matsuo, K.; Oze, I.; Mizota, A.; Kondo, C.; Nomura, M.; Yokota, T.; Takahari, D.; Ura, T.; Muro, K. Meta-Analysis of Neutropenia or Leukopenia as a Prognostic Factor in Patients with Malignant Disease Undergoing Chemotherapy. Cancer Chemother Pharmacol 2011, 68, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, X.H.F. Tumor-Associated Neutrophils and Macrophages—Heterogenous but Not Chaotic. Front Immunol 2020, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-Infiltrating Neutrophils Constitute the Major In Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment. Neoplasia (United States) 2014, 16, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Grecian, R.; Whyte, M.K.B.; Walmsley, S.R. The Role of Neutrophils in Cancer. Br Med Bull 2018, 128, 5–14. [Google Scholar] [CrossRef]
- Loffredo, S.; Borriello, F.; Iannone, R.; Ferrara, A.L.; Galdiero, M.R.; Gigantino, V.; Esposito, P.; Varricchi, G.; Lambeau, G.; Cassatella, M.A.; et al. Group V Secreted Phospholipase A2 Induces the Release of Proangiogenic and Antiangiogenic Factors by Human Neutrophils. Front Immunol 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Rada, M.; Kapelanski-Lamoureux, A.; Zlotnik, O.; Petrillo, S.; Lazaris, A.; Metrakos, P. Disruption of Integrin Alpha-5/Beta-1-Dependent Transforming Growth Factor Beta-1 Signaling Pathway Attenuates Vessel Co-Option in Colorectal Cancer Liver Metastases. bioRxiv 2022, 2003–2005. [Google Scholar] [CrossRef]
- Ganeshan, K.; Johnston, L.K.; Bryce, P.J. TGF-Β1 Limits the Onset of Innate Lung Inflammation by Promoting Mast Cell–Derived IL-6. The Journal of Immunology 2013, 190, 5731–5738. [Google Scholar] [CrossRef]
- Setargew, Y.F.I.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-linking as an Anti-stromal Therapy in Solid Tumours. Cancers (Basel) 2021, 13, 1–26. [Google Scholar] [CrossRef]
- Lin, H.Y.; Li, C.J.; Yang, Y.L.; Huang, Y.H.; Hsiau, Y.T.; Chu, P.Y. Roles of Lysyl Oxidase Family Members in the Tumor Microenvironment and Progression of Liver Cancer. Int J Mol Sci 2020, 21, 1–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




