1. Introduction
Almost 615 million cases of the coronavirus disease from 2019 (COVID-19) have been recorded at the time of writing this manuscript. In about 6.5 million people, COVID-19 led to death [
1]. From the beginning of the pandemic, it was known that the virus named SARS-CoV-2, which is responsible for this disease, spreads by droplet transmission and has a reservoir in the upper aerodigestive tract [
2]. The nasopharynx is almost universally targeted for swab collection in the diagnosis of COVID-19 because of the high viral load there. Swabs from the nasopharynx followed by detection of the viral genome using reverse transcription polymerase chain reaction (PCR) are therefore considered to be the gold standard for SARS-CoV-2 detection [
3] Due to the anatomical connection between the nasopharynx and the middle ear through the Eustachian tube, it is assumed that the middle ear and the mastoid air cell system may become contaminated with SARS-CoV-2 during COVID-19 [
4]. Therefore, the middle ear and the mastoid are considered to be potentially infectious spaces. Especially ear surgery of long duration, or the spreading of droplets and aerosols when drilling or suctioning seems to pose a potential risk for the operating room personnel. Therefore, ENT doctors have been warned about ear surgery in patients with unknown SARS-CoV-2 status [
4].
To date, SARS-CoV-2 was found post mortem in the middle ear of some, but not all patients with COVID-19 [
5,
6,
7,
8]. It is not clear whether SARS-CoV-2 penetrated the ear passively post mortem or existed in the middle ear before death.
The aim of our study was to investigate the presence of SARS-CoV-2 in the middle ear of living patients. If SARS-CoV-2 is found in the middle ear of living patients, even more serious questions arise: 1. Is it present in patients with a severe course of disease only, or in asymptomatic patients, too? 2. Can SARS-CoV-2 be found in the middle ear of vaccinated patients? 3. Does the middle ear constitute a reservoir for SARS-CoV-2? 4. Is SARS-CoV-2 in the middle ear a threat to the ear-surgeon and operating room staff?
2. Materials and Methods
2.1. Study Design, Inclusion Criteria and Ethics Approval
The present monocentric, prospective study was performed between March 2021 and March 2022. Patients older than 1 year who underwent middle ear surgery in our department of otorhinolaryngology (ORL) and agreed to participate in the study were included. We took into account both elective and acute ear surgeries with opening of the middle ear. Paracentesis (PC), tympanic drainage (TD), tympanoplasty with and without mastoidectomy, stapesplasty, cochlear and vibrant soundbridge implantation were taken into account. All indications and operations were conducted by three experienced ORL doctors. Exclusion criteria were: age less than 1 year and no informed consent to participate in the study. The study protocol was approved by Johannes Kepler University Ethics Committee in accordance with the Declaration of Helsinki (EK Nr: 1026/2021). Written consent was obtained from all participants.
2.2. Material
2.2.1. Clinical data
Age, weight, sex, indication and type of surgery, previous illnesses, history of vaccination and any contact with people who tested positive for SARS-CoV-2 were recorded preoperatively. At the follow up visit 3 months after surgery, patients were additionally asked about SARS-CoV-2 infections in the postoperative period. We also recorded the number of all middle ear operations in our ENT department separately for children and adults and the number of SARS-CoV-2 positive patients in our country and our region during the study period [
9].
2.3. Sample Collection
In each patient, a swab from the nasopharynx as well as secretion from the middle ear were collected during surgery. After the surgery, another swab was taken from the filter between the tracheal tube and respirator.
In children who underwent tonsillectomies, samples from the nasopharynx were collected through the mouth under direct vision using a mirror to avoid contamination. In adults and children who underwent isolated ear surgery, swabs from the nasopharynx were taken through the nose during surgery. The virus stabilization Vacuette Tube 3ml (Greiner Bio-One) was used, with specimen collection using nasopharyngeal flocked swabs (iClean®).
Middle ear secretion was collected immediately after opening the middle ear. If there was no accumulation of fluid, the middle ear was irrigated using 1 ml of sterile normal saline and the resultant specimen was collected. Afterwards, 1 ml of saline was used to rinse the area. In bilateral operations, material was obtained from both ears, mixed in a sample container and analyzed together. A sterile tracheal suction trap (Primed®) combined with a conventional suction system was used to collect ear secretion. Biological material was sent to the laboratory immediately after the operation.
2.4. Laboratory Procedures
For molecular analysis, aliquots were frozen at minus 80°C to be batch processed later for nucleic acid extraction and analysis. QIASymphony Virus/Pathogen Midi Kit (Co. Qiagen) was used for nucleic acid extraction, whereas for detection the Real-Time PCR SARS-CoV-2 Winterplex assay (genesig®) was utilized in conjunction with the Cobas z480 Real-Time Analyzer.
2.5. Statistical Analysis
Descriptive statistics are provided for all observations with the groups of children and adults shown separately. For nominal variables, absolute and relative frequencies were computed. Non-normal metric and ordinal variables are described with median and interquartile range (median (IQR)). Normality of metric variables was tested using the Shapiro-Wilk test (SW). Associations between nominal variables or ordinal variables with few observations were analyzed using Fisher's exact test based on 100 000 Monte Carlo simulations. For metric and ordinal variables, the Mann-Whitney U test was used to test differences across the study groups. The level of significance was set at 0.05 and tests were conducted two-sided. Statistical analyses were conducted using the statistical software package R [
10].
3. Results
3.1. Demographics
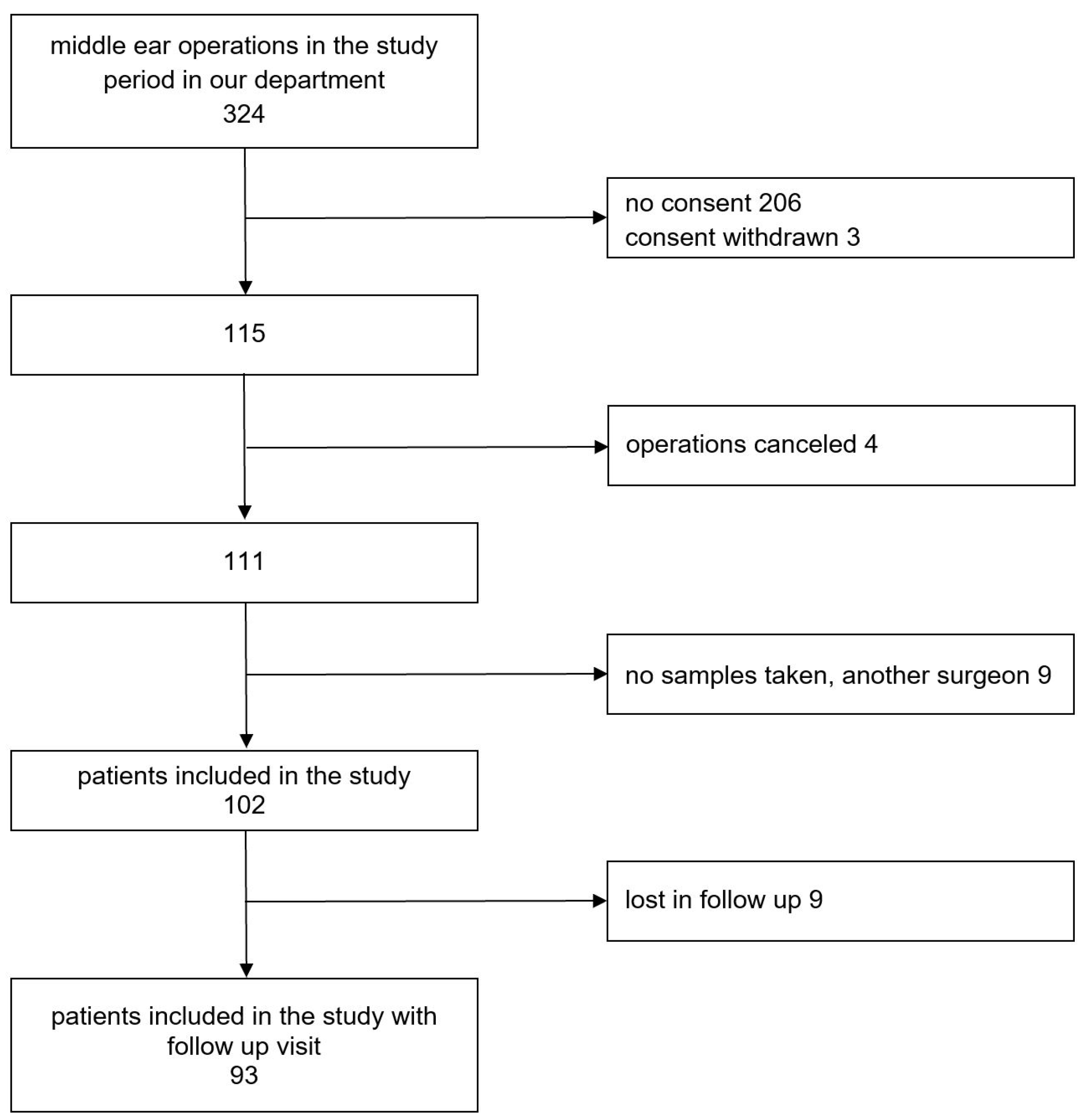
From 324 patients who underwent middle ear surgery in our department, 115 signed the information sheet and were enrolled in our study. Operations were canceled in 4 patients. Another 9 patients were excluded from the study because the surgery was performed by a surgeon who was not part of the research team, or due to failure in sample collection,
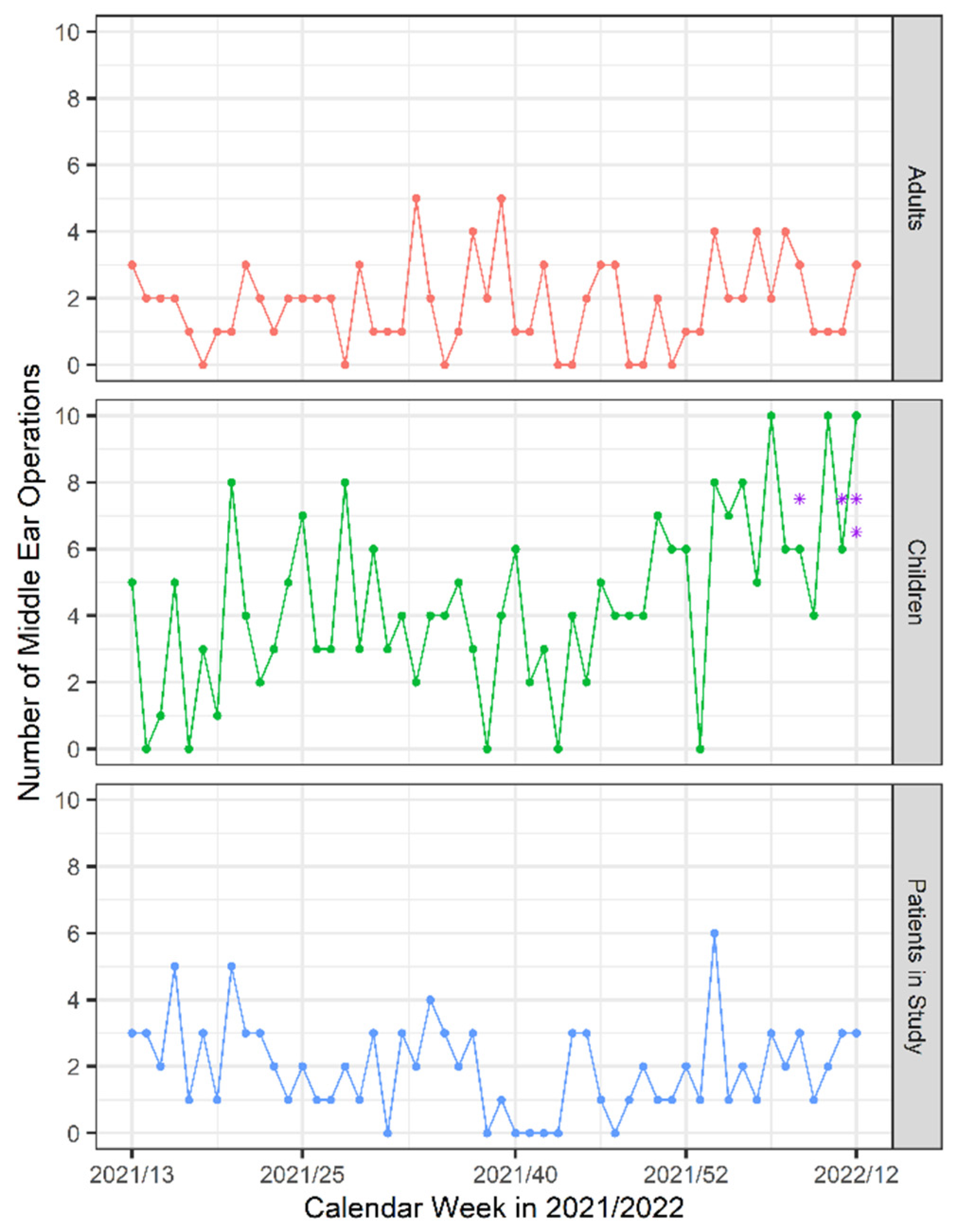
Figure 1. The number of all middle ear operations in our ENT department in children and adults during the study period and number of patients enrolled in the study are shown in
Figure 2.
Group characteristics of the 102 patients who were ultimately included in the study are listed in
Table 1. A follow-up visit was reported three months postoperatively for 91.18% of the study participants. The median age at surgery was 5.5 years (range, 1 - 94 yrs, IQR of 43 yrs), 63 participants (61.76%) were children and 57 (55.88%) male.
To better understand whether there are some factors that affect colonization of the mastoid and middle ear with SARS CoV-2 chronic diseases, disorders and the American Society of Anaesthesiologists (ASA) classification of Physical Health are shown in
Table 2. ASA Score was grade I in 70 patients (68.63%) and grade II in 29 patients (28.43%). Scores IV to VI were not recorded at all. Statistically significant differences between the group of children and adults were found regarding the occurrence of cardiovascular disease, respiratory disease, gastroesophageal reflux, and regarding the ASA-Score.
3.2. SARS-CoV-2
Epidemiological data is presented in
Table 3. In our group, 25 patients (24.51% of all patients) were vaccinated against SARS-CoV-2, including two children (1.96%). Thirteen (12.75%) patients had had COVID-19 and 34 (33.33%) had proven contact with a SARS-CoV-2 positive person at any time prior to enrolment in the study. The number of unvaccinated patients in these groups was 11 (10.78%) and 28 (27.45%), respectively.
From 102 study participants, two (1.96%) had positive SARS-CoV-2 PCR tests in both the middle ear and the nasopharynx. Two (1.96%) further participants had positive SARS-CoV-2 PCR tests in the nasopharynx only, but not in the middle ear.
Ten of the intraoperatively negative patients had had COVID-19 preoperatively: a minimum of 8 days and a maximum of 495 days were counted from infection to surgery, the median time was 166.5 days. Proven contact with SARS-CoV-2-positive persons was reported in 32 patients before operation: the minimum was 9 days, the maximum 667 days and the median time was 129 days.
All SARS-CoV-2-positive patients were children, who had an elective operation. Characteristics of SARS-CoV-2-positive patients are presented in
Table 4. They had no chronic diseases or disorders. Health status of all of these patients was assessed as ASA-score I. None of the four children was vaccinated against SARS-CoV-2. Three of them had COVID-19 preoperatively occurring 7, 50 and 51 days before surgery. Moreover, two of them had contact with SARS-CoV-2-positive people preoperatively, 15 and 68 days before surgery. None of the 4 children had COVID-19 symptoms before, during or after the operation. Cycle threshold (ct) values of the PCR test were between 25.94 and 37.06.
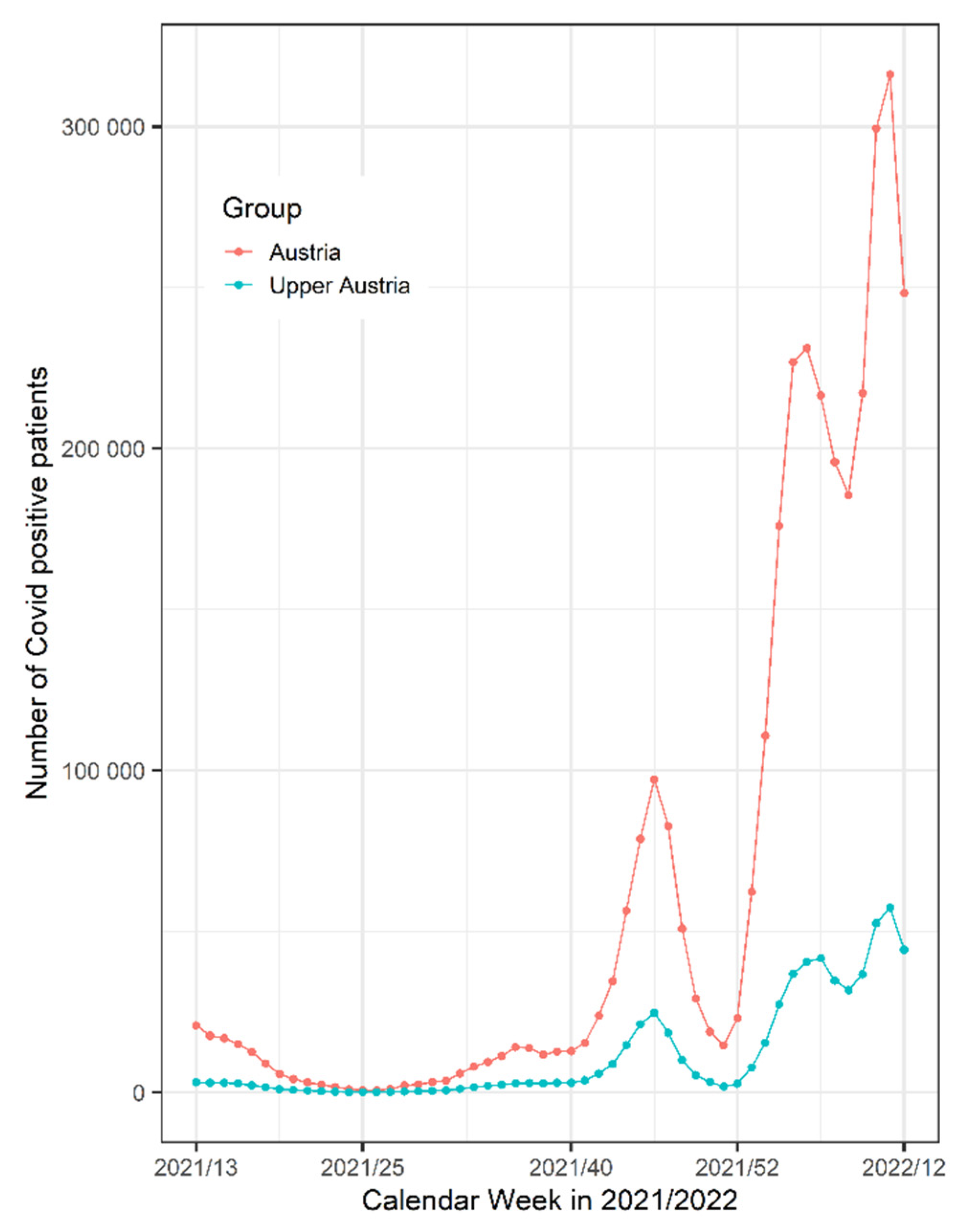
Figure 3 shows the number of SARS-CoV2 positive patients in Austria and in our region of Upper Austria during the study period.
4. Discussion
The connection between the upper respiratory tract and the middle ear via the eustachian tube poses the possibility for viruses to enter the middle ear spaces. Three post mortem studies confirmed the presence of SARS-CoV-2 in biopsies from the middle ear [
5,
6,
7]. Furthermore, physiological research indicates that the mucosal lining of the middle ear can be affected by SARS-CoV-2. Both middle ear and nasal epithelial cells show relatively high expression of angiotensin converting enzyme 2 (ACE2), required for SARS-CoV-2 entry [
6,
11]. In particular cases, attempts were made to examine the middle ear and mastoid in living patients, but no SARS-CoV-2 could be detected [
12]. It was not clear whether SARS-CoV-2 penetrated the ear passively post mortem or existed in the middle ear before death. Three post mortem studies were conducted in corpses who were SARS-CoV-2 positive and met COVID-19 criteria before death. Middle ear samples were positive in 67% (n=2/3) and 50% (n=3/6 and n=4/8) of these cases [
5,
6,
7]. Additionally, two of these studies performed nasal swabs, which were positive in 50% and 75% of cases, respectively [
6,
7]. All cases with positive results from the ear also had positive results from the nasal swabs. Frazier et al. investigated SARS-CoV-2 presence in the middle ears and mastoids separately in three corpses [
5]. In one corpse SARS-CoV-2 was found only in one middle ear, whereas the other middle ear and both of the mastoids were virus free. The second corpse was positive for SARS-CoV-2 in both middle ears and both mastoids. The third corpse had no virus in either the middle ears or mastoids.
The CovEar study is the first pilot study to examine and confirm the presence of SARS-CoV-2 in the middle ear of living patients. None of the CovEar study participants had infection symptoms before, during or after the operation. Although the study group included people without an upper age limit, the virus was detected only in children at the age of 4 to 8 years in the middle ear or the nasopharynx. The studies conducted thus far were performed in adults only [
5,
6,
7].
All the children who tested positive in the CovEar study, either in the middle ear or the nasopharynx, had an asymptomatic course of disease at the time of operation and tested negatively. We analyzed the SARS-CoV-2-positive cases separately. One child with SARS-CoV-2 in the middle ear had proven contact with an infected person 15 days before operation. The other one had a positive test result 7 days before operation and a negative result directly before the surgery. The parents of this child did not inform the doctor about the recent infection until the follow-up visit. The two children with positive samples from the nasopharynx only had COVID-19 50 and 51 days before operation. Postoperative infection signs were denied in the follow-up visit 3 months postoperatively by all four positive patients.
In post mortem studies, the viral load detected in the middle ear was significantly lower than in the nasal cavity in two of three cases. In the third case, it was higher but not significant [
6]. Another post mortem study confirmed that the virus concentration is highest in the nasopharynx and lowest in the mastoid in three of four cases [
5,
7]. In our study, the viral load at the time of operation was low in all cases. There was also no consistency when comparing the ct-value in the middle ear and the nasopharynx. In one case the ct-value in the middle ear was higher (29.77) than in the nasopharynx (25.94), and in the other case the ct-value in the nasopharynx (32.15) was higher than in the middle ear (30.03). Our question was whether it is possible for SARS-CoV-2 to exist as a reservoir in the middle ear of SARS-CoV-2 negative individuals. In our group, persons SARS-CoV-2-positive in the middle ear were also positive in nasopharynx.
It is not clear why some people who had COVID-19 preoperatively, or had proven contact with SARS-CoV-2-positive people, were positive during operation whereas others were not. In some intraoperative negative cases, the time between proven contact with SARS-CoV-2-positive persons or having COVID-19 and the operation was similar, or even lower than in cases of intraoperative SARS-CoV-2-positive individuals. It was proposed that comorbidities may affect middle ear colonization [
5]. In the data from the CovEar study, we could not find any indications that additional diseases influence the colonization of the middle ear. Indeed none of the intraoperatively positive individuals were vaccinated, but still the number of individuals was too low to propose that the vaccination might influence the result. All positive SARS-CoV-2 cases were diagnosed during the period of time when the number of SARS-CoV-2 positive patients in Austria and in our region of Upper Austria was very high (
Figure 3).
In the beginning of the pandemic, many preventive measures and regulations, especially during ear surgery, were introduced to avoid virus transmission. Presence of SARS-CoV-2 in the middle ear was presumed. The drilling and suctioning usually performed during ear surgery can cause droplets and aerosols that spread in the operating room [
4,
13,
14,
15]. Consequently, there was a warning about performing ear surgery in patients with unknown status or after COVID-19. The evidence from our study points to the fact that there may be patients in the operating theatre with the virus in the middle ear. Moreover, the confirmation of the presence of SARS-CoV-2 in the middle ear requires more attention to be paid to precautionary measures before procedures on the middle ear are carried out. Due to the direct proximity of the structures of the middle and inner ear, confirmation of the presence of the virus in the middle ear also gives rise to concerns about the penetration of the virus into the structures of the inner ear. Kurabi et al. proposed that SARS-CoV-2, if present in the middle ear, could lead to direct inner ear SARS-COV-2 infection [
6]. During the pandemic, reports about the possible effects of SARS-CoV-2 on various systems of the human body began to appear [
16]. However, the reports very rarely indicated effects of the virus on the audio-vestibular system. It is suspected that these symptoms were not reported because attention was primarily focused on life-threatening symptoms [
17]. After one year into the pandemic, the number of studies reporting audio-vestibular symptoms after COVID-19 increased [
17,
18,
19,
20,
21]. It was estimated that the prevalence of vertigo, hearing loss and tinnitus after SARS-CoV-2 infection was 7.2; 7.6 and 14.8% respectively [
21]. However, these symptoms can not only be induced by COVID-19, but also potentially by antiviral medication as well [
17,
20]. Moreover, the viral load detected in symptomatic and asymptomatic patients is similar [
22]. Nonetheless, it still remains unclear whether asymptomatic SARS-CoV-2 infections can provoke audio-vestibular symptoms that cannot be subsequently linked with COVID-19. Further investigation is needed in this area.
The main limitation of our study is the relatively small number of cases and their heterogeneity. A further limitation is the small volume of liquid collected in the middle ear. Due to the small amount of test material, it was not possible to repeat the measures. We tried to increase the volume by using fluid from both ears in patients who were operated on both sides.
5. Conclusions
The findings of this study show that SARS-CoV-2 penetrates into the middle ear of living patients, including asymptomatic individuals. The presence of SARS-CoV-2 in the middle ear can have implications for ear surgery and can pose a risk of infection for operating room staff. It may also directly affect the audio-vestibular system.
Author Contributions
Conceptualization, N.R, N P-F. and P.M.Z; methodology, N.R, N.P-F, S.R. and P.H.; formal analysis, N.R, S.R. and P.H.; investigation, N.R, N.P-F., P.M.Z, C.P. and M.W-Z; resources, P.M.Z., C.P. and M.W-Z.; writing—original draft preparation, N.R. and N.P-F.; writing—review and editing, P.M.Z., C.P., M.W-Z., S.R, and P.H; visualization, N.R, N.P-F., S.R. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Johannes Kepler University Ethics Committee, EK Nr: 1026/2021, date of approval 24.03.2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors declare that the data of this research is available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- COVID live - Coronavirus statistics - worldometer. Available online: https://www.worldometers.info/coronavirus/. (accessed on 15 Sep 2022).
- Liaw, J.; Saadi, R.; Patel, V.A.; Isildak, H. Middle Ear Viral Load Considerations in the COVID-19 Era: A Systematic Review. Otol Neurotol 2020, 42, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Deitmer, T.; Dietz, A.; Chaberny, I.F.; Pietsch, C. [The nasal and pharyngeal swab techniques during the COVID-19-pandemic - the ENT-perspective - SARS-CoV-2, Coronavirus, nasal swab, pharyngeal swab, complications]. Laryngorhinootologie 2021, 100, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, E.A. COVID-19 Pandemic and Otologic Surgery. J Craniofac Surg 2020, 31, e651–e652. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.M.; Hooper, J.E.; Mostafa, H.H.; Stewart, C.M. SARS-CoV-2 Virus Isolated From the Mastoid and Middle Ear: Implications for COVID-19 Precautions During Ear Surgery. JAMA Otolaryngol Head Neck Surg. 2020, 146, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Kurabi, A.; Pak, K.; DeConde, A.S.; Ryan, A.F.; Yan, C.H. Immunohistochemical and qPCR Detection of SARS-CoV-2 in the Human Middle Ear Versus the Nasal Cavity: Case Series. Head Neck Pathol 2022, 16, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Jeican, I.I.; Aluaș, M.; Lazăr, M.; Barbu-Tudoran, L.; Gheban, D.; Inișca, P.; Albu, C.; Tripon, S.; Albu, S.; Siserman, C.; Vica, M.L.; Muntean, M.; Opincariu, I.; Junie, L.M. Evidence of SARS-CoV-2 Virus in the Middle Ear of Deceased COVID-19 Patients. Diagnostics (Basel) 2021, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Kesser, B.W. News Flash!—SARS-CoV-2 Isolated From the Middle Ear and Mastoid. JAMA Otolaryngol Head Neck Surg 2020, 146, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Austrian Agency for Health and Food Safety, (AGES Österreichische Agentur für Gesundheit und Ernährungssicherheit). Available online: https://www.ages.at/en/ (accessed on 01 Sep 2022).
- Ripley, B.D. The R project in statistical computing. MSOR connect 2001, 1, 23–25. [Google Scholar] [CrossRef]
- Sigrist, C.J.; Bridge, A.; Le Mercier, P. A potential role for integrins in host cell entry by SARS-CoV-2. Antiviral Res 2020, 177, 104759. [Google Scholar] [CrossRef] [PubMed]
- Wanna, G.B.; Schwam, Z.G.; Kaul, V.F.; Cosetti, M.K.; Perez, E.; Filip, P.; Javaid, W.; Kandel, A.; Paniz-Mondolfi, A.; Govindaraj, S.; Genden, E.M. COVID-19 sampling from the middle ear and mastoid: A case report. Am J Otolaryngol 2020, 41, 102577. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.; Kutz, W.; Isaacson, B.; Badr-El-Dine, M.; Nogueira, J.F.; Marchioni, D.; Presutti, L. COVID-19 and ear endoscopy in otologic practices. Eur Arch Otorhinolaryngol 2021, 278, 2133–2135. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Sharma, A.; Singhal, B. Covid-19 and Ear Surgery: Treatment Strategies and Triage during the Post-lockdown Period. Indian J Otolaryngol Head Neck Surg 2021, 73, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Couloigner, V.; Schmerber, S.; Nicollas, R.; Coste, A.; Barry, B.; Makeieff, M.; Boudard, P.; Bequignon, E.; Morel, N.; Lescanne, E. COVID-19 and ENT Surgery. Eur Ann Otorhinolaryngol Head Neck Dis 2020, 137, 161–166. [Google Scholar] [CrossRef] [PubMed]
- El-Anwar, M.W.; Elzayat, S.; Fouad, Y.A. ENT manifestation in COVID-19 patients. Auris Nasus Larynx 2020, 47, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Almufarrij, I.; Uus, K.; Munro, K.J. Does coronavirus affect the audio-vestibular system? A rapid systematic review. Int J Audiol 2020, 59, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Munro, K.J.; Uus, K.; Almufarrij, I.; Manchaiah, V. Persistent self-reported changes in hearing and tinnitus in post-hospitalisation COVID-19 cases. Int J Audiol 2020, 59, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol 2020, 41, 102483. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, S.; Bello Alvarez, M.; Mungul, S.; Hari, K. Otologic dysfunction in patients with COVID-19: A systematic review. Laryngoscope Investig Otolaryngol 2020, 5, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Almufarrij, I.; Munro, K.J. One year on: an updated systematic review of SARS-CoV-2, COVID-19 and audio-vestibular symptoms. Int J Audiol 2020, 60, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; Guo, Q.; Song, T.; He, J.; Yen, H.-L.; Peiris, M.; Wu, J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).