Submitted:
05 May 2023
Posted:
06 May 2023
You are already at the latest version
Abstract
Keywords:
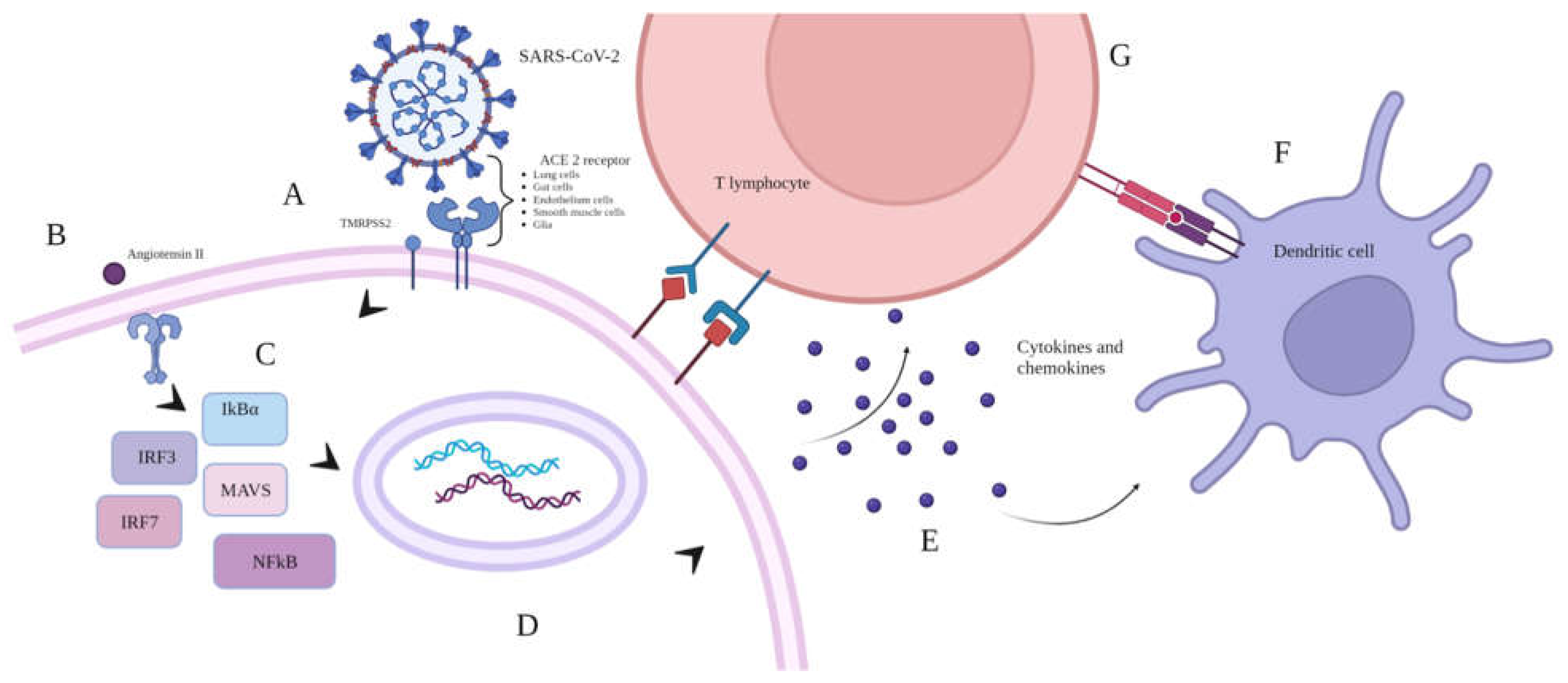
1. Introduction
2.1. Immunology of macrophage derived chemokine
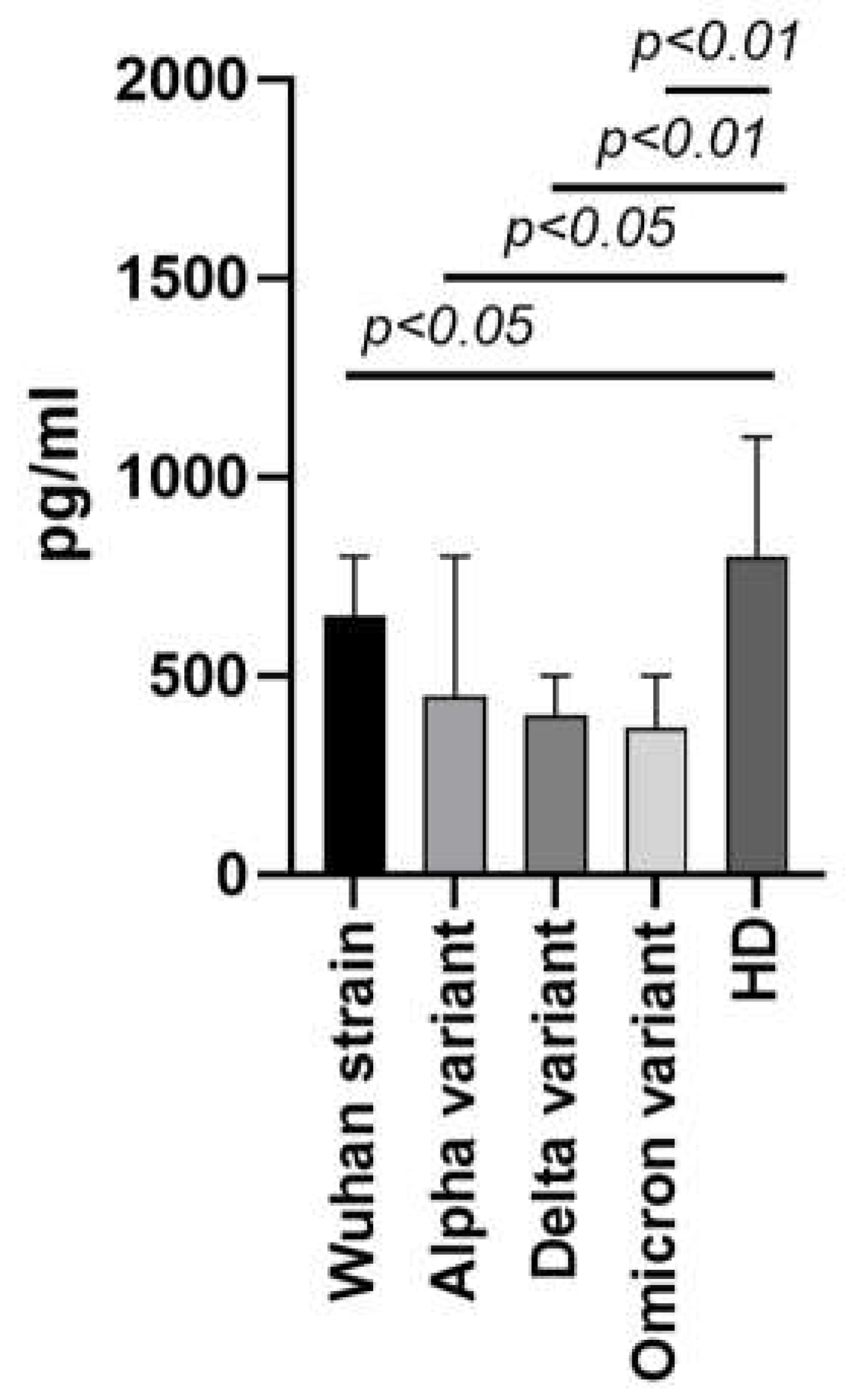
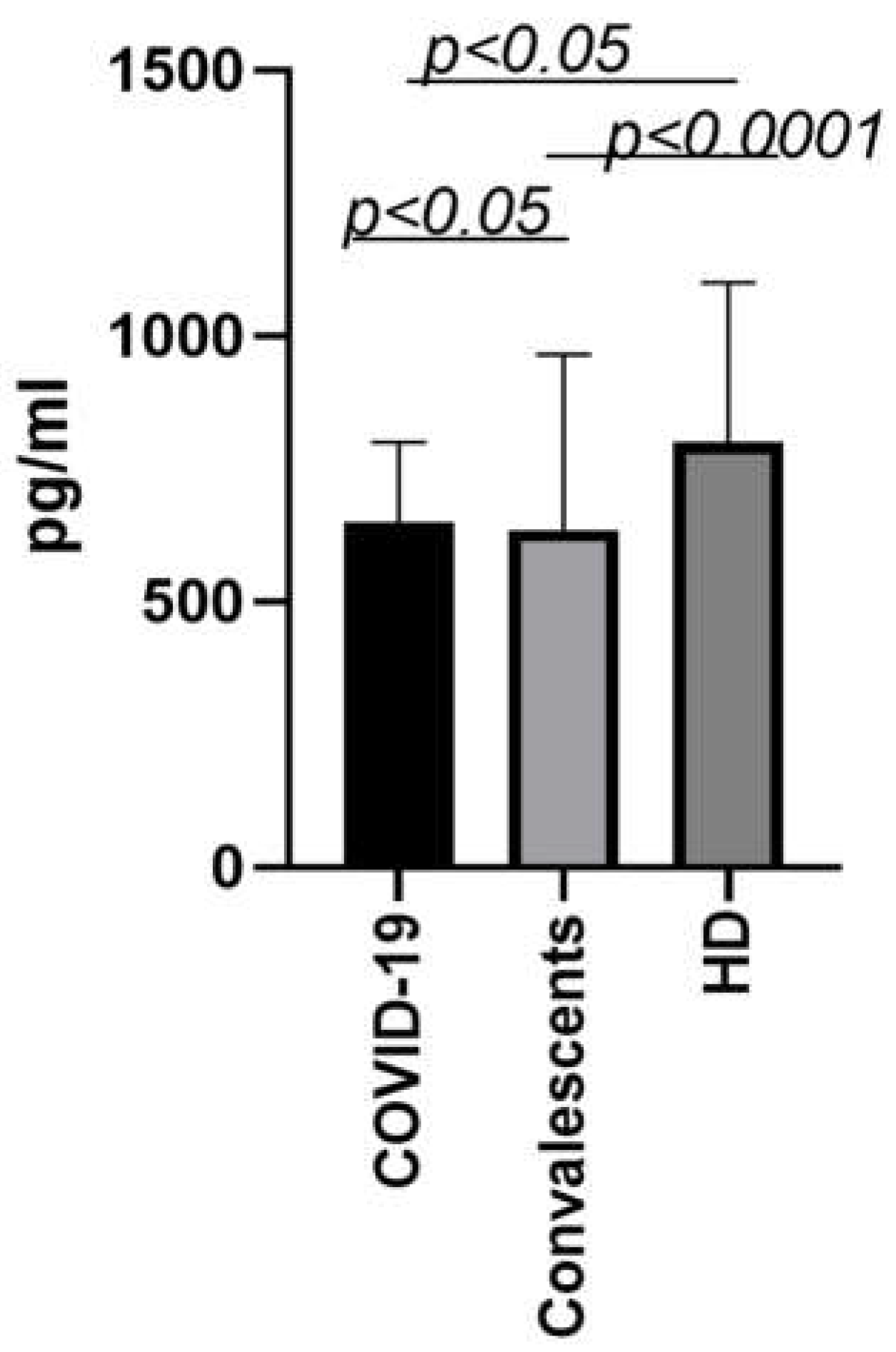
2.2. Changes in MDC/CCL22 concentrations in COVID-19 in vitro and in vivo
2.3. Macrophage-derived chemokine MDC/CCL22 in various pathologies
2.3.1. MDC/CCL22 in oncology of non-respiratory organs
2.3.2. Macrophage-derived chemokine MDC/CCL22 in autoimmunity and atopic diseases
2.3.3. Macrophage-derived chemokine MDC/CCL22 in respiratory disease
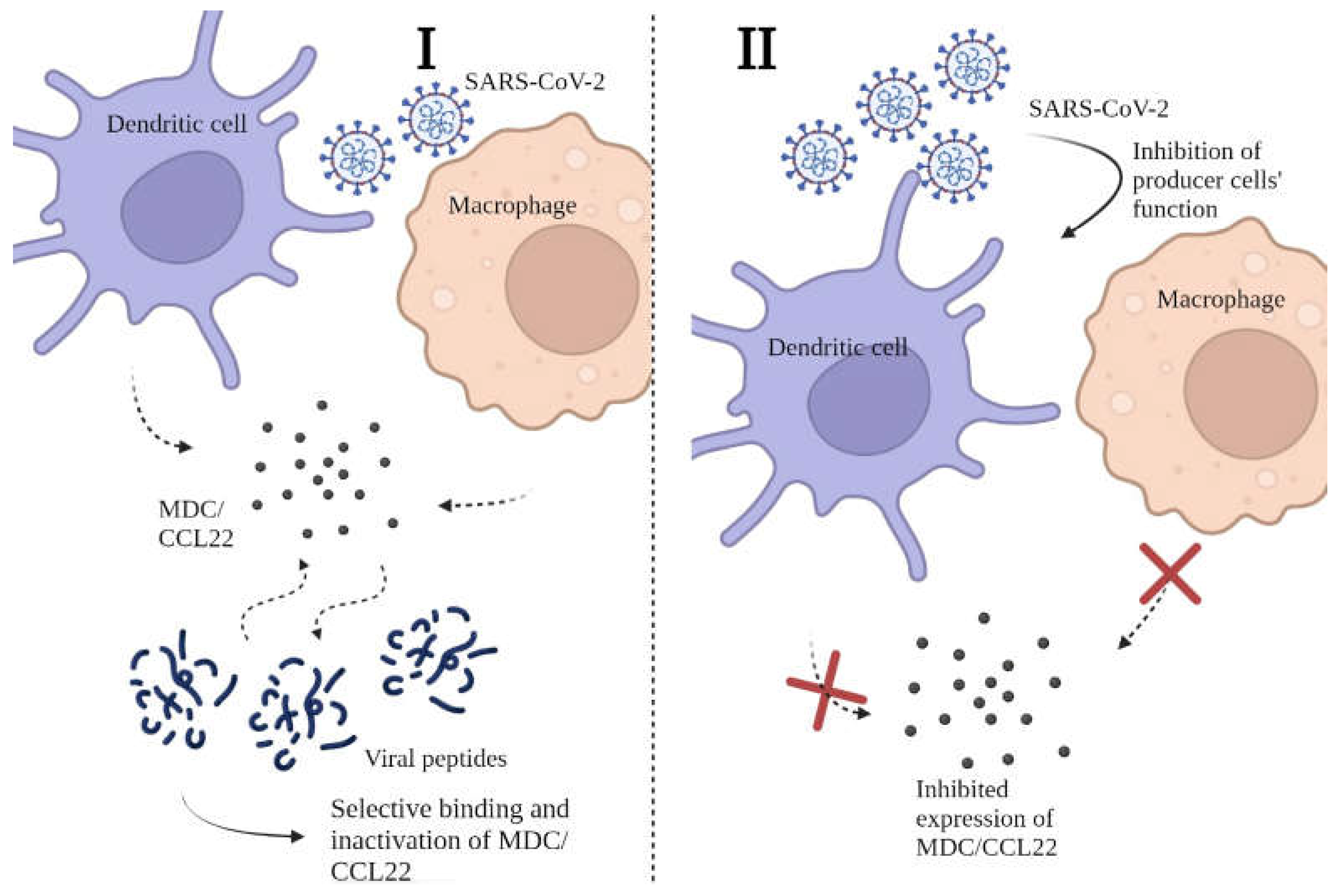
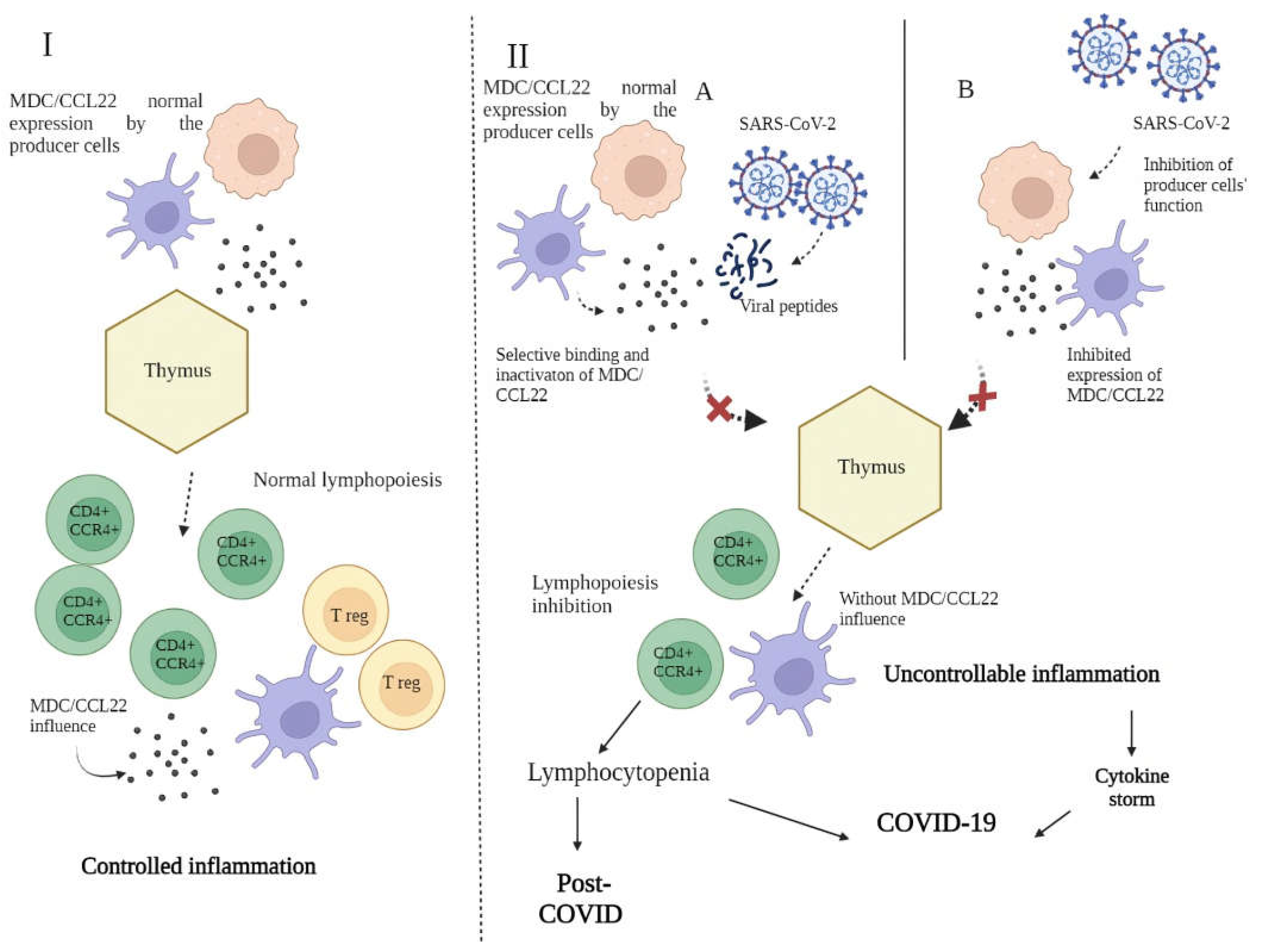
2.6. Possible mechanisms behind the decrease in MDC/CCL22 concentrations in COVID-19
2.7. Conclusions
Acknowledgements
Conflicts of Interest
References
- Liu YC, Kuo RL, Shih SR. COVID-19: The first documented coronavirus pandemic in history. Biomed J. 2020 Aug;43(4):328-333. https://doi.org/10.1016/j.bj.2020.04.007. Epub 2020 May 5. PMID: 32387617; PMCID: PMC7199674. [CrossRef]
- Borczuk, A.C., Yantiss, R.K. The pathogenesis of coronavirus-19 disease. J Biomed Sci 29, 87 (2022). https://doi.org/10.1186/s12929-022-00872-53. [CrossRef]
- Boechat JL, Chora I, Morais A, Delgado L. The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives. Pulmonology. 2021 Sep-Oct;27(5):423-437. https://doi.org/10.1016/j.pulmoe.2021.03.008. Epub 2021 Apr 9. PMID: 33867315; PMCID: PMC8040543. [CrossRef]
- Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022 Mar 11;375(6585):1122-1127. https://doi.org/10.1126/science.abm8108. Epub 2022 Mar 10. PMID: 35271343. [CrossRef]
- Salamanna F, Maglio M, Landini MP, Fini M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front Med (Lausanne). 2020 Dec 3;7:594495. https://doi.org/10.3389/fmed.2020.594495. PMID: 33344479; PMCID: PMC7744810. [CrossRef]
- Shirbhate E, Pandey J, Patel VK, Kamal M, Jawaid T, Gorain B, Kesharwani P, Rajak H. Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacol Rep. 2021 Dec;73(6):1539-1550. https://doi.org/10.1007/s43440-021-00303-6. Epub 2021 Jun 27. PMID: 34176080; PMCID: PMC8236094. [CrossRef]
- Xu J, Lazartigues E. Expression of ACE2 in Human Neurons Supports the Neuro-Invasive Potential of COVID-19 Virus. Cell Mol Neurobiol. 2022 Jan;42(1):305-309. https://doi.org/10.1007/s10571-020-00915-1. Epub 2020 Jul 4. PMID: 32623546; PMCID: PMC7334623. [CrossRef]
- Boechat JL, Chora I, Morais A, Delgado L. The immune response to SARS-CoV-2 and COVID-19 immunopathology - Current perspectives. Pulmonology. 2021 Sep-Oct;27(5):423-437. https://doi.org/10.1016/j.pulmoe.2021.03.008. Epub 2021 Apr 9. PMID: 33867315; PMCID: PMC8040543. [CrossRef]
- Rodriguez L., Brodin P. Unraveling the immune response in severe COVID-19. J. Clin. Immunol., 2020, Vol. 40, no. 7, pp. 958-959. [CrossRef]
- Wiech M, Chroscicki P, Swatler J, Stepnik D, De Biasi S, Hampel M, Brewinska-Olchowik M, Maliszewska A, Sklinda K, Durlik M, Wierzba W, Cossarizza A, Piwocka K. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front Immunol. 2022 Jun 10;13:886431. https://doi.org/10.3389/fimmu.2022.886431. PMID: 35757700; PMCID: PMC9226563. [CrossRef]
- Wallet MA, Sen P, Tisch R. Immunoregulation of dendritic cells. Clin Med Res. 2005 Aug;3(3):166-75. https://doi.org/10.3121/cmr.3.3.166. PMID: 16160071; PMCID: PMC1237158. [CrossRef]
- Godiska R, Chantry D, Raport CJ, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray PW. Human macrophage-derived chemokine (MDC), a novel chemoattractant for monocytes, monocyte-derived dendritic cells, and natural killer cells. J Exp Med. 1997; 185:1595–1604. [PubMed: 9151897]. [CrossRef]
- D. Chantry, P. Romagnani, C.J. Raport, C.L. Wood, A. Epp, P.W. Gray Macrophage-derived chemokine is localized to thymic medullary epithelial cells and is a chemoattractant for CD3(+), CD4(+), CD8(low) thymocytes Blood, 94 (1999), pp. 1890-1898.
- Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG 3rd. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998 Nov 1;161(9):5027-38. PMID: 9794440.
- Imai T, Chantry D, Raport CJ, Wood CL, Nishimura M, Godiska R, Yoshie O, Gray PW. Macrophage-derived chemokine is a functional ligand for the CC chemokine receptor 4. J Biol Chem. 1998 Jan 16;273(3):1764-8. https://doi.org/10.1074/jbc.273.3.1764. PMID: 9430724. [CrossRef]
- Arsentieva N.A., Liubimova N.E., Batsunov O.K., Korobova Z.R., Stanevich O.V., Lebedeva A.A., Vorobyov E.A., Vorobyova S.V., Kulikov A.N., Lioznov D.A., Sharapova M.A., Pevtcov D.E., Totolian A.A. Plasma cytokines in patients with COVID-19 during acute phase of the disease and following complete recovery. Medical Immunology (Russia). 2021;23(2):311-326. (In Russ.) https://doi.org/10.15789/1563-0625-PCI-2312. [CrossRef]
- Arsentieva N.A., Liubimova N.E., Batsunov O.K., et al. Predictive value of specific cytokines for lethal COVID-19 outcome // Russian Journal of Infection and Immunity. - 2022. - Vol. 12. - N. 5. - P. 859-868. https://doi.org/10.15789/2220-7619-PVO-2043. [CrossRef]
- Korobova ZR, Arsentieva NA, Liubimova NE, Batsunov OK, Dedkov VG, Gladkikh AS, Sharova AA, Adish Z, Chernykh EI, Kaschenko VA, Ratnikov VA, Gorelov VP, Stanevich OV, Kulikov AN, Pevtsov DE, Totolian AA. Cytokine Profiling in Different SARS-CoV-2 Genetic Variants. Int J Mol Sci. 2022 Nov 16;23(22):14146. https://doi.org/10.3390/ijms232214146. PMID: 36430621; PMCID: PMC9692520. [CrossRef]
- Fergie J, Srivastava A. Immunity to SARS-CoV-2: Lessons Learned. Front Immunol. 2021 Mar 19;12:654165. https://doi.org/10.3389/fimmu.2021.654165. PMID: 33815415; PMCID: PMC8018176. [CrossRef]
- Yang B, Yang J, Zhou L, Xue C, Li H, Hu W, Liu N. Inflammatory cytokine depletion in severe coronavirus disease 2019 infectious pneumonia: A case report. Medicine (Baltimore). 2020 Dec 4;99(49):e23449. https://doi.org/10.1097/MD.0000000000023449. PMID: 33285740; PMCID: PMC7717847. [CrossRef]
- Kudryavtsev IV, Arsentieva NA, Korobova ZR, Isakov DV, Rubinstein AA, Batsunov OK, Khamitova IV, Kuznetsova RN, Savin TV, Akisheva TV, Stanevich OV, Lebedeva AA, Vorobyov EA, Vorobyova SV, Kulikov AN, Sharapova MA, Pevtsov DE, Totolian AA. Heterogenous CD8+ T Cell Maturation and 'Polarization' in Acute and Convalescent COVID-19 Patients. Viruses. 2022 Aug 28;14(9):1906. https://doi.org/10.3390/v14091906. PMID: 36146713; PMCID: PMC9504186. [CrossRef]
- Wiech M, Chroscicki P, Swatler J, Stepnik D, De Biasi S, Hampel M, Brewinska-Olchowik M, Maliszewska A, Sklinda K, Durlik M, Wierzba W, Cossarizza A, Piwocka K. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front Immunol. 2022 Jun 10;13:886431. https://doi.org/10.3389/fimmu.2022.886431. PMID: 35757700; PMCID: PMC9226563. [CrossRef]
- Tufa A, Gebremariam TH, Manyazewal T, Getinet T, Webb DL, Hellström PM, Genet S. Inflammatory mediators profile in patients hospitalized with COVID-19: A comparative study. Front Immunol. 2022 Jul 25;13:964179. https://doi.org/10.3389/fimmu.2022.964179. PMID: 35958594; PMCID: PMC9359079. [CrossRef]
- Ling L, Chen Z, Lui G, Wong CK, Wong WT, Ng RWY, Tso EYK, Fung KSC, Chan V, Yeung ACM, Hui DSC, Chan PKS. Longitudinal Cytokine Profile in Patients With Mild to Critical COVID-19. Front Immunol. 2021 Dec 6;12:763292. https://doi.org/10.3389/fimmu.2021.763292. PMID: 34938289; PMCID: PMC8685399. [CrossRef]
- Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, Tseng J, Spellberg JP, Elias CG 3rd. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998 Nov 1;161(9):5027-38. PMID: 9794440.
- Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D'Amico G, Struyf S, Transidico P, Bonecchi R, Del Prete A, Allavena P, Ruco LP, Chiabrando C, Girolomoni G, Mantovani A, Sozzani S. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001 Mar;31(3):812-2.
- Oronsky B, Larson C, Hammond TC, Oronsky A, Kesari S, Lybeck M, Reid TR. A Review of Persistent Post-COVID Syndrome (PPCS). Clin Rev Allergy Immunol. 2021 Feb 20:1–9. https://doi.org/10.1007/s12016-021-08848-3. Epub ahead of print. PMID: 33609255; PMCID: PMC7896544. [CrossRef]
- H.L. Tang, J.G. Cyster Chemokine up-regulation and activated T cell attraction by maturing dendritic cells Science, 284 (1999), pp. 819-822.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. https://doi.org/10.1038/nm1093. [CrossRef]
- Klarquist J, Tobin K, Farhangi Oskuei P, Henning SW, Fernandez MF, Dellacecca ER, Navarro FC, Eby JM, Chatterjee S, Mehrotra S, et al. Ccl22 diverts T regulatory cells and controls the growth of melanoma. Cancer Res. 2016;76(21):6230–6240. https://doi.org/10.1158/0008-5472.CAN-16-0618. [CrossRef]
- Zhou M, Bracci PM, McCoy LS, Hsuang G, Wiemels JL, Rice T, Zheng S, Kelsey KT, Wrensch MR, Wiencke JK. Serum macrophage-derived chemokine/CCL22 levels are associated with glioma risk, CD4 T cell lymphopenia and survival time. Int J Cancer. 2015 Aug 15;137(4):826-36. https://doi.org/10.1002/ijc.29441. Epub 2015 Feb 2. PMID: 25604093; PMCID: PMC4478165. [CrossRef]
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res. 2009;69(5):2000–2009. https://doi.org/10.1158/0008-5472.CAN-08-2360. [CrossRef]
- Furue M, Ulzii D, Vu YH, Tsuji G, Kido-Nakahara M, Nakahara T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran J Immunol. 2019 Jun;16(2):97-107. https://doi.org/10.22034/IJI.2019.80253. PMID: 31182684. [CrossRef]
- Ushio A, Arakaki R, Otsuka K, Yamada A, Tsunematsu T, Kudo Y, Aota K, Azuma M, Ishimaru N. CCL22-Producing Resident Macrophages Enhance T Cell Response in Sjögren's Syndrome. Front Immunol. 2018 Nov 8;9:2594. https://doi.org/10.3389/fimmu.2018.02594. PMID: 30467506; PMCID: PMC6236111. [CrossRef]
- Columba-Cabezas S, Serafini B, Ambrosini E, Sanchez M, Penna G, Adorini L, Aloisi F. Induction of macrophage-derived chemokine/CCL22 expression in experimental autoimmune encephalomyelitis and cultured microglia: implications for disease regulation. J Neuroimmunol. 2002 Sep;130(1-2):10-21. https://doi.org/10.1016/s0165-5728(02)00170-4. PMID: 12225884. [CrossRef]
- Fox KA, Kirwan DE, Whittington AM, Krishnan N, Robertson BD, Gilman RH, López JW, Singh S, Porter JC, Friedland JS. Platelets Regulate Pulmonary Inflammation and Tissue Destruction in Tuberculosis. Am J Respir Crit Care Med. 2018 Jul 15;198(2):245-255. https://doi.org/10.1164/rccm.201710-2102OC. PMID: 29420060; PMCID: PMC6058979. [CrossRef]
- Nakanishi, T., Imaizumi, K., Hasegawa, Y. et al. Expression of macrophage-derived chemokine (MDC)/CCL22 in human lung cancer. Cancer Immunol Immunother 55, 1320–1329 (2006). https://doi.org/10.1007/s00262-006-0133-y. [CrossRef]
- Richter JR, Sutton JM, Belizaire RM, Friend LA, Schuster RM, Johannigman TA, Miller SG, Lentsch AB, Pritts TA. Macrophage-derived chemokine (CCL22) is a novel mediator of lung inflammation following hemorrhage and resuscitation. Shock. 2014 Dec;42(6):525-31. https://doi.org/10.1097/SHK.0000000000000253. PMID: 25136780; PMCID: PMC4236272. [CrossRef]
- Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001 Jun;280(6):G1217-26. https://doi.org/10.1152/ajpgi.2001.280.6.G1217. PMID: 11352815. [CrossRef]
- Mantovani A, Gray PA, Van Damme J, Sozzani S. Macrophage-derived chemokine (MDC). J Leukoc Biol. 2000 Sep;68(3):400-4. PMID: 10985257.
- Alcami A, Saraiva M. Chemokine Binding Proteins Encoded by Pathogens. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK5966/.
- Banu S, Nagaraj R, Idris MM. A proteomic perspective and involvement of cytokines in SARS-CoV-2 infection. PLoS One. 2023 Jan 6;18(1):e0279998. https://doi.org/10.1371/journal.pone.0279998. PMID: 36608055; PMCID: PMC9821788. [CrossRef]
- Hamdorf, M., Imhof, T., Bailey-Elkin, B., Betz, J., Theobald, S. J., Simonis, A., Cristanziano, V. D., Gieselmann, L., Dewald, F., Lehmann, C., Augustin, M., Klein, F., Alcazar, M. A., Rongisch, R., Fabri, M., Rybniker, J., Goebel, H., Stetefeld, J., Brachvogel, B., … Bock, F. (2022). The unique ORF8 protein from SARS-COV-2 binds to human dendritic cells and induces a hyper-inflammatory cytokine storm (Preprint). https://doi.org/10.1101/2022.06.06.494969. [CrossRef]
- Shahanshah Khan, Mahnoush S Shafiei, Christopher Longoria, John W Schoggins, Rashmin C Savani, Hasan Zaki (2021) SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway eLife 10:e68563 https://doi.org/10.7554/eLife.68563. [CrossRef]
- Wu, W., Cheng, Y., Zhou, H. et al. The SARS-CoV-2 nucleocapsid protein: its role in the viral life cycle, structure and functions, and use as a potential target in the development of vaccines and diagnostics. Virol J 20, 6 (2023). https://doi.org/10.1186/s12985-023-01968-6. [CrossRef]
- López-Muñoz AD, Kosik I, Holly J, Yewdell JW. Cell surface SARS-CoV-2 nucleocapsid protein modulates innate and adaptive immunity. Sci Adv. 2022 Aug 5;8(31):eabp9770. https://doi.org/10.1126/sciadv.abp9770. Epub 2022 Aug 3. PMID: 35921414; PMCID: PMC9348789. [CrossRef]
- González-Motos V, Kropp KA, Viejo-Borbolla A. Chemokine binding proteins: An immunomodulatory strategy going viral. Cytokine Growth Factor Rev. 2016 Aug;30:71-80. https://doi.org/10.1016/j.cytogfr.2016.02.007. Epub 2016 Mar 4. PMID: 26987612. [CrossRef]
- Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: What it shows and what can be learned. Immunol Lett. 2020 Sep;225:31-32. https://doi.org/10.1016/j.imlet.2020.06.013. Epub 2020 Jun 20. PMID: 32569607; PMCID: PMC7305732. [CrossRef]
- Ho CY, Wong CK, Li EK, Tam LS, Lam CW. Suppressive effect of combination treatment of leflunomide and methotrexate on chemokine expression in patients with rheumatoid arthritis. Clin Exp Immunol. 2003 Jul;133(1):132-8. https://doi.org/10.1046/j.1365-2249.2003.02192.x. PMID: 12823287; PMCID: PMC1808740. [CrossRef]
- Pérez-Gómez, A., Vitallé, J., Gasca-Capote, C. et al. Dendritic cell deficiencies persist seven months after SARS-CoV-2 infection. Cell Mol Immunol 18, 2128–2139 (2021). https://doi.org/10.1038/s41423-021-00728-2. [CrossRef]
- Winheim E, Rinke L, Lutz K, Reischer A, Leutbecher A, Wolfram L, Rausch L, Kranich J, Wratil PR, Huber JE, Baumjohann D, Rothenfusser S, Schubert B, Hilgendorff A, Hellmuth JC, Scherer C, Muenchhoff M, von Bergwelt-Baildon M, Stark K, Straub T, Brocker T, Keppler OT, Subklewe M, Krug AB. Impaired function and delayed regeneration of dendritic cells in COVID-19. PLoS Pathog. 2021 Oct 6;17(10):e1009742. https://doi.org/10.1371/journal.ppat.1009742. PMID: 34614036; PMCID: PMC8523079. [CrossRef]
- Chang T, Yang J, Deng H, Chen D, Yang X, Tang ZH. Depletion and Dysfunction of Dendritic Cells: Understanding SARS-CoV-2 Infection. Front Immunol. 2022 Feb 21;13:843342. https://doi.org/10.3389/fimmu.2022.843342. PMID: 35265087; PMCID: PMC8898834. [CrossRef]
- Montazersaheb, S., Hosseiniyan Khatibi, S.M., Hejazi, M.S. et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J 19, 92 (2022). https://doi.org/10.1186/s12985-022-01814-1. [CrossRef]
- Alamri, A.; Fisk, D.; Upreti, D.; Kung, S.K.P. A Missing Link: Engagements of Dendritic Cells in the Pathogenesis of SARS-CoV-2 Infections. Int. J. Mol. Sci. 2021, 22, 1118. https://doi.org/10.3390/ijms22031118. [CrossRef]
- Chu H, Zhou J, Wong BH, Li C, Cheng ZS, Lin X, Poon VK, Sun T, Lau CC, Chan JF, To KK, Chan KH, Lu L, Zheng BJ, Yuen KY. Productive replication of Middle East respiratory syndrome coronavirus in monocyte-derived dendritic cells modulates innate immune response. Virology. 2014 Apr;454-455:197-205. https://doi.org/10.1016/j.virol.2014.02.018. Epub 2014 Mar 7. PMID: 24725946; PMCID: PMC7111975. [CrossRef]
- Onodi F, Bonnet-Madin L, Meertens L, Karpf L, Poirot J, Zhang SY, Picard C, Puel A, Jouanguy E, Zhang Q, Le Goff J, Molina JM, Delaugerre C, Casanova JL, Amara A, Soumelis V. SARS-CoV-2 induces human plasmacytoid pre-dendritic cell diversification via UNC93B and IRAK4. bioRxiv [Preprint]. 2021 Jan 8:2020.07.10.197343. https://doi.org/10.1101/2020.07.10.197343. Update in: J Exp Med. 2021 Apr 5;218(4): PMID: 33442685; PMCID: PMC7805442. [CrossRef]
- Cai G, Du M, Bossé Y, Albrecht H, Qin F, Luo X, Androulakis XM, Cheng C, Nagarkatti M, Nagarkatti P, Christiani DC, Whitfield ML, Amos CI, Xiao F. SARS-CoV-2 Impairs Dendritic Cells and Regulates DC-SIGN Gene Expression in Tissues. Int J Mol Sci. 2021 Aug 26;22(17):9228. https://doi.org/10.3390/ijms22179228. PMID: 34502134; PMCID: PMC8431536. [CrossRef]
- Galati D, Zanotta S, Capitelli L, Bocchino M. A bird's eye view on the role of dendritic cells in SARS-CoV-2 infection: Perspectives for immune-based vaccines. Allergy. 2022 Jan;77(1):100-110. https://doi.org/10.1111/all.15004. Epub 2021 Jul 24. PMID: 34245591; PMCID: PMC8441836. [CrossRef]
- Venet, M., Ribeiro, M.S., Décembre, E. et al. Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2. Nat Commun 14, 694 (2023). https://doi.org/10.1038/s41467-023-36140-9. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




