1. Introduction
Human health is under constant threat by various factors, most notably disease [
1,
2]. Mild diseases cause minor discomfort, whereas serious conditions, such as cancer, often lead to death. In light of the coronavirus disease 2019 (COVID-19) pandemic, the threat imposed by viral disease considerably increased following late 2019 [
3,
4,
5]. Various effective therapeutics have been developed in a bid to prevent or cure disease. However, not all treatments are curative, highlighting the importance of early diagnosis for effective disease management and patient survival. This has given rise to a plethora of diagnostic approaches [
6].
Diagnostics are usually based on the detection of a causative pathogen or biomarker [
7,
8]. Enzyme-linked immunosorbent assay (ELISA) is frequently used for biomarker detection [
9,
10]. It has several advantages, such as quantification, low limit of detection (LOD), as well as high selectivity, accuracy, and reproducibility. Despite these merits, ELISA-based diagnostics are often limited by a long reaction time (4–6 h) and the need for specialized laboratory equipment and personnel [
11]. Recently, lateral flow immunoassay (LFIA) has gained attention as an alternative to ELISA because of its low cost, short reaction time, and ease of use [
12,
13].
Probe selection is essential for the efficient detection or quantification of target biomarkers using LFIA [
14]. In the early stages of LFIA development, organic dyes such as fluorescein isothiocyanate were used as probes. These dyes generate strong signals; however, they are also associated with photobleaching [
15,
16]. Researchers have therefore sought alternative probe materials, identifying nanomaterials as adequate candidates. As a result, the efficiency and convenience of LFIA diagnosis have been enhanced using various optical metal nanoparticles (NPs) such as gold NPs (Au NPs), quantum dots (QDs), and upconversion NPs (UCNPs). This review discusses recent trends in LFIA using optical NPs as probes for biomarker detection.
2. LFIA Assays with Optical NPs
2.1. Principle of LFIA
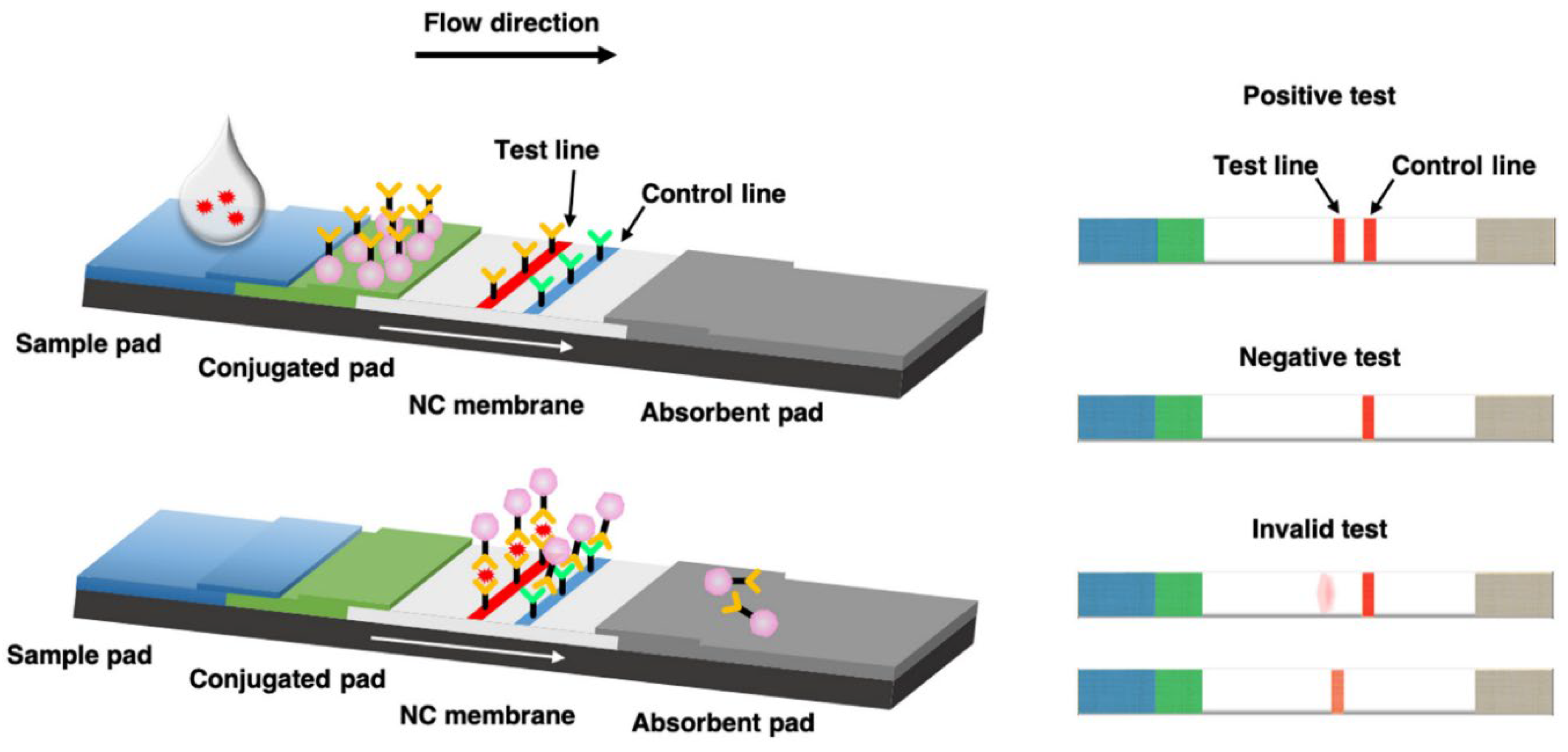
The LFIA kit consists of sample, conjugate, and absorbent pads, which are attached to a nitrocellulose membrane backbone (
Figure 1) [
17,
18,
19]. A liquid sample to be diagnosed is loaded onto the sample pad, and the loaded sample is developed toward the absorbent pad. When the developed sample reaches the conjugate pad, it is mixed with probes preloaded onto the conjugate pad. Subsequently, samples and probes are developed together. Targets in the sample are bound and complexed with probes as antibodies capture the targets, immobilizing them onto the probes while developing together. Upon reaching the test line where target-capturing antibodies are immobilized, the target–probe complexes are captured and cannot be developed further. Other components in the samples and non-complex probes pass the test line, with non-complex probes captured at the control line by antibodies immobilized on the control line. The captured probes exhibit their characteristic optical signal at the corresponding lines, with the type of optical signal and signal intensities varying based on probe type and optical properties. We thus reviewed papers on LFIA after classifying them based on the type of optical NPs used as probes in LFIA.
2.2. LFIAs with Au NPs
Au NPs are most widely used optical nanomaterials in LFIA due to excellent optical property. Au NPs are fabricated via a seed-mediated growth method, wherein Au seeds act as the core of Au NPs. Au seeds are usually fabricated using Turkevich’s method (approximately 15 nm) or Martin’s method (3 to 5 nm) [
20,
21]. Different from the bulk form, Au NPs have unique optical properties and have been applied in bioimaging and biosensing [
22,
23]. Au NPs can be used as substrates for surface-enhanced Raman scattering and emit the characteristic Raman signal of the target compound. In addition, well-dispersed Au NPs with a size of 15 to 150 nm exhibited a red color in solution based on the localized surface plasmon resonance (LSPR) effect. The color of Au NPs can be controlled by adjusting their size via Au NP growth.
Khlebtsov et al. quantified and revealed the relationship between Au NP size and LOD [
24]. They prepared spherical and monodisperse Au NPs of various sizes (from 16 to 115 nm) and characterized them. The Au NPs were dropped onto membranes with serial dilution, and the signal intensity of each spot was measured. The LOD for each Au NP was inversely proportional to 3.1 power of particle size of particles per spot area.
One limitation of Au NPs is the weak signal causes inadequate sensitivity. For this reason, efforts for signal enhancement have been reported. For example, the accumulation of more Au NPs at the test line is the simplest approach for signal enhancement. Shen et al. utilized polyamidoamine dendrimer (PAMAM) to induce the aggregation of Au NPs [
25]. PAMAM, highly branched, tree-like, water-soluble, multifunctional macromolecules, are attached to the surface of the Au NPs via electrostatic interaction; so, the authors dispensed and ran the PAMAM-Au NPs-antibody conjugate including multiple Au NPs. Their concepts are based on the fact that the signal produced by an aggregate of smaller Au NPs can be stronger than the signal produced by a larger individual Au NP. The assay results show that 0.1 ng/mL of LOD and is 20-fold more sensitive than the conventional LFIA method using individual Au NP at the identical condition.
However, the Au NP assemblages are usually hard to control precisely in terms of number and final size, and Au NPs themselves tend to be naturally aggregated according to the environment. Therefore, other research groups suggested a method that sequentially ran two kinds of Au NP conjugates. Shen et al. described dual Au NP conjugates-based LFIA using oligonucleotides [
26]. The first Au NP (30 nm) was modified with DNA1, and the second Au NP (16 nm) was modified with DNA2 and aptamer. Therefore, they were bound specifically via hybridization between DNA1 and DNA2. This system was verified using thrombin as a potential biomarker, and the LOD was 0.25 nM with a linear range of 0.5 to 120 nM. Similarly, Shen et al. developed the signal amplification method using dual gold Au NPs [
27]. They separately prepared two Au NP conjugates. The first one was Au NPs modified with biotin and antibody, and the second one was Au NPs modified with streptavidin. In the one-step process using two conjugation pads, they conducted an assay without the additional steps. They proved a concept to detect hepatitis B surface antigen (HBsAg), a biomarker of hepatitis B virus infection. The LOD of the system was 0.06 ng/mL with 30-fold enhancement compared to the conventional LFIA method.
Although the post-assay growth of the Au NPs was once tested as a signal enhancement method, its additional procedure for loading gold enhancers was laborious and time-consuming [
28,
29]. Panraksa et al. reported one-step Au NP enhancement via sequential flow LFIA [
30]. The authors designed the patterned nitrocellulose membrane consisting of two channels. Thus, all immunoreagents and gold enhancer solutions were delivered sequentially. With the gold enhancement system, the LOD for C-reactive protein (CRP) visualized by the naked eye was 0.1 μg/mL, and the calculated LOD was found to be 0.001 μg/mL with a linear range in a range of 0.1 to 5 μg/mL.
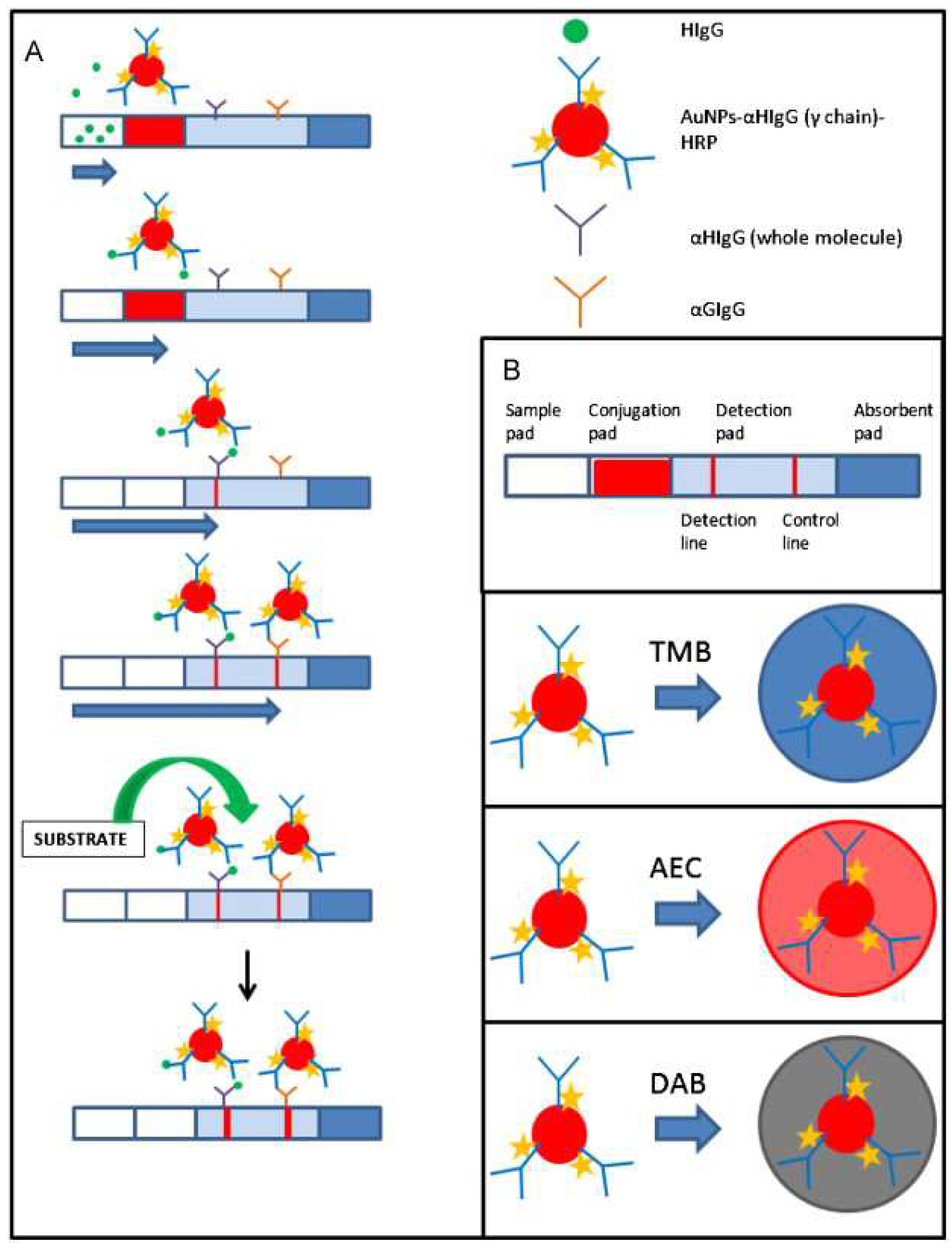
Meanwhile, there also was an enzymatic amplification inspired by traditional bioassays. Parolo et al. proposed a method for enhancing sensitivity by introducing horseradish peroxidase (HRP) onto the Au NPs (
Figure 2) [
17]. HRP was introduced onto Au NPs after conjugation to an antibody, whereafter the Au NPs were developed. After development, the fabricated particles were red, which originated from the SPR effect, similar to the Au NPs without HRP. The developed LFIA strips were dipped into three different HRP substrate solutions (3,3’,5,5’-tetramethylbenzidine (TMB), 3-amino-9-ethylcarbazole (AEC), and 3,3’-diaminobenzidinetetrahydrochloride (DAB)), and the signal intensity of each LFIA strip was compared. The strips dipped in the TMB and AEC solutions showed a deeper color at the test line, and this difference could be confirmed with the naked eye. In the case of the TMB-treated strip, the LOD was approximately 10-fold lower than that of Au NPs without HRP.
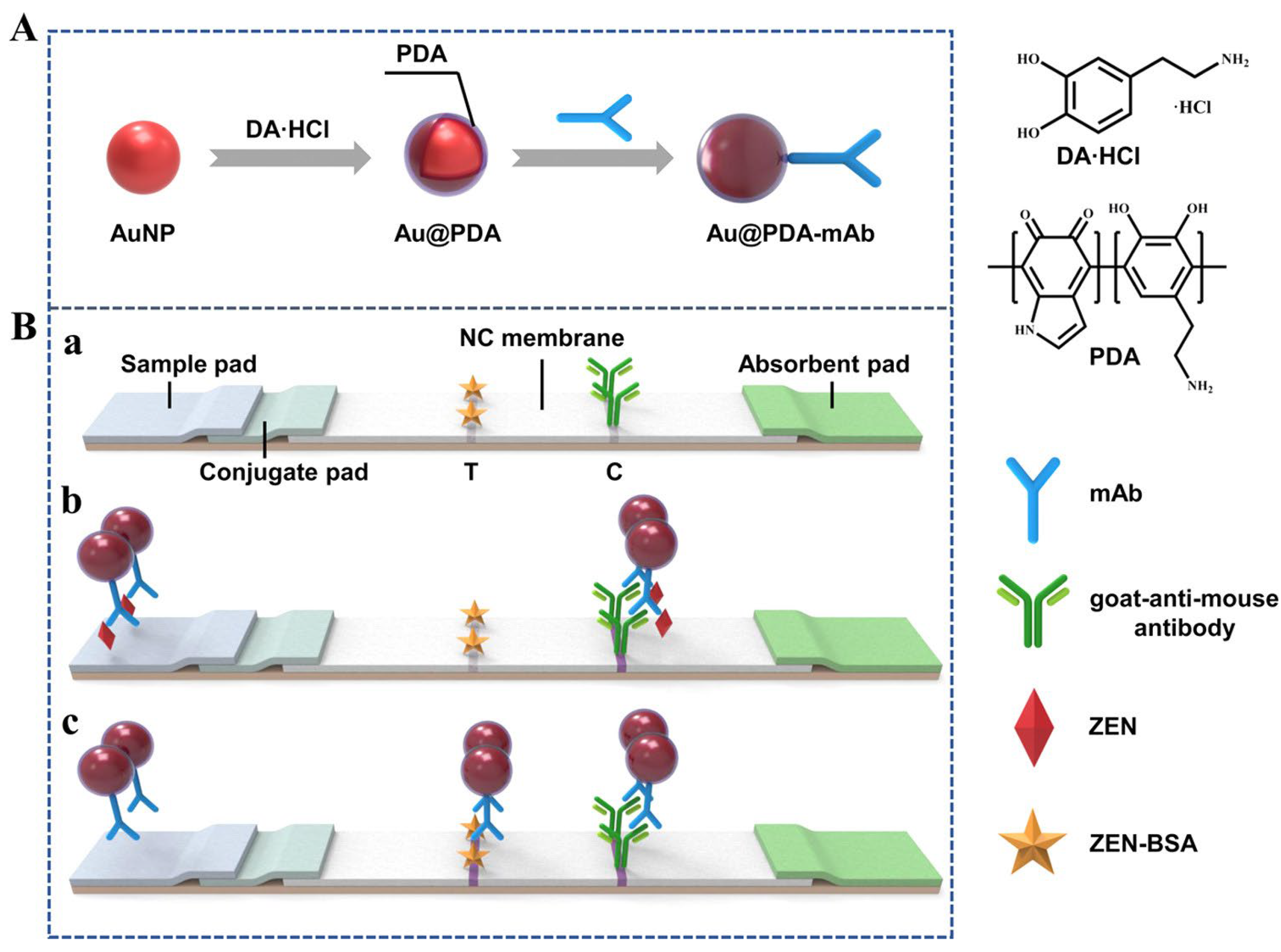
Another approach for enhancing sensitivity by coating Au NPs with polydopamine was reported by Xu et al. (
Figure 3) [
31]. Polydopamine can easily adhere to the surface of Au NPs, with the encapsulation enhancing their biostability and biocompatibility. Furthermore, polydopamine-coated Au NPs (Au@PDA-10) exhibited a deep purple color, which was stable even under basic conditions or high-salt concentration solutions, with the red color of uncoated Au NPs being fainter. Based on this optical property, the LFIA LOD for zearalenone, which is based on Au@PDA-10, was 10-fold lower than that based on traditional Au NPs (7.4 pg/mL and 76.1 pg/mL, respectively).
The effect that antibody introduction on Au NPs has on the LOD was also studied previously [
32]. Nardo et al. fabricated Au NPs, and introduced an anti-cortisol antibody onto the surface of Au NPs via three ways; adsorption, covalent conjugation, and protein-protein interactions. Each Au NP was used for the detection of cortisol using LFIA. The result revealed that the LOD was unrelated to the method of antibody introduction but depended only on the amount of introduced antibodies.
2.3. LFIAs with QDs
QDs are a type of semiconductor NPs that can emit fluorescence signals under ultraviolet light (UV) irradiation, with a high quantum yield [
33,
34,
35]. Based on quantum confinement effects, the wavelength of emitted light is determined by the size of QDs, and the bandwidth of the emitted light spectra is narrower than that of fluorescent organic dyes, such as fluorescence I or rhodamine B [
15]. Their excellence in brightness and stability offers an opportunity for sensitive and quantitative detection, so they are considered a very promising probe in biosensing research. Meanwhile, there also was an enzymatic amplification inspired by traditional bioassays. Furthermore, the color of emitted light can be varied by adjusting the size of particles, providing the possibility of multiplex analysis with QDs [
36,
37].
Considering the above-described advantages, many research groups have developed LFIA systems using QDs. Bock et al. fabricated silica-coated CdSe@ZnS QDs via reverse microemulsion for the detection of prostate-specific antigen (PSA) via LFIA [
38]. An anti-PSA antibody was then conjugated onto the surface of silica-coated CdSe@ZnS NPs via chemical conjugation. The LOD of PSA with this LFIA system was calculated to be 1.0754 ng/mL, which is lower than that of the gray zone for prostate cancer (4–10 ng/mL).
The characteristics of QDs are highly suitable for multiplexed detection, which is an important objective of LFIA. The importance of multiplexed detection is getting more and more emphasized in clinical diagnosis since there often is a need to detect one or more related biomarkers from identical samples to generate more conclusive information [
39]. Wang et al. demonstrated a QD-based LFIA for simultaneous quantitative detection of multiple tumor markers, alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) [
40]. The authors introduced two types of CdSe/ZnS core-shell QDs (546 nm and 620 nm) for the anti-AFP antibody and anti-CEA antibody, respectively, and there was single test line, which consisted of a mixture of mouse anti-AFP McAb and mouse anti-CEA McAb, and one control line. The LOD was 3 ng/mL and 2 ng/mL for two model biomarkers without obvious cross-reactivity. The authors validated the tests using 130 clinical samples, and the results exhibited high sensitivity (93% for AFP and 87% for CEA) and specificity (94% for AFP and 97% for CEA).
Wu et al. developed a QD-based LFIA for the simultaneous detection of two subtypes of influenza A (H5 and H9) [
41]. The authors synthesized CdSe/ZnS core-shell QDs (620 nm) and functionalized them with Influenza A virus subtype H5 and H9 antibodies, respectively. Two test line for subtype H5 and H9 was separately fabricated on the strip, and the captured QD-Antiobody complex produced a bright fluorescent band in response to UV excitation at 365 nm. The authors acquire images using three kinds of samples with various dilution factors, containing subtypes H5, H9, and a mixture of H5 and H9. The LOD of both virus subtypes was 0.016 HAU and 0.25 HAU, respectively. The specificity of the test was evaluated using other subtypes of type A influenza viruses (H1, H3, H5N1 re-4/6, H7N9, H9N2 re-2, and H9 SD696) and other viral antigens. They validated the assay with 47 clinical samples and reported a 100% match with real-time PCR assay.
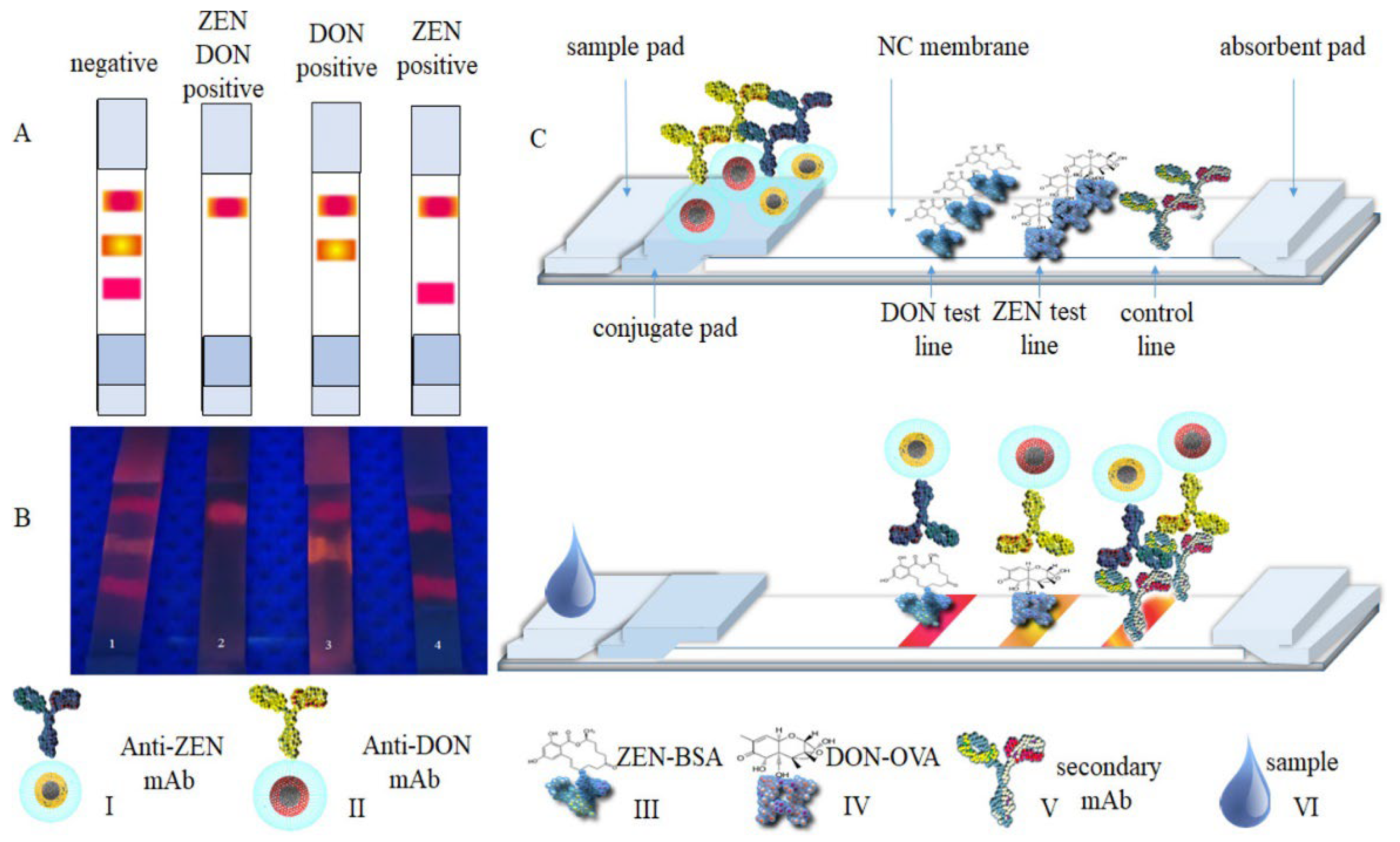
Goryacheva et al. fabricated two types of CdSe-based core-shell type QDs that emit orange and red colors, coating them with silica for application (
Figure 4) [
42]. An anti-deoxynivalenol antibody (Anti-DON) and an anti-zearalenone antibody (Anti-ZEN) were conjugated onto the surface of QD type via chemical conjugation and developed with samples. When the corresponding targets were present in the sample, QD types were selectively immobilized onto each test line to generate the fluorescence signal. The cutoff values for zearalenone and deoxynivalenol in this LFIA system were 40 and 400 μg/kg, respectively.
Despite the excellence of QDs as a reporter, their toxicity often hinders their practical applicability. Wang et al. synthesized eco-friendly Cu:Zn−In−S/ZnS QDs for the detection of tetanus antibodies [
43]. The tetanus antigen and standard human IgG were modified on the test line and control line, respectively, and then QD-goat anti-human IgG (Fc) was loaded on the conjugation pad. The LoD was 0.001 IU/mL, which is much lower than both the minimum required level for protection (0.01 IU/mL) and the Au NP-based LFIA.
Shen et al. proposed LFIA using QD beads which consisted of CdSe/ZnS QDs as amplification probes [
44]. The size of the QD beads was estimated to be 247 13 nm, and they are conjugated with the anti-HBsAg monoclonal antibody. On the LFIA strip, goat anti-HBsAg polyclonal antibody and donkey anti-mouse polyclonal antibody were coated on the test line and control line. The LOD of the assay was 75 pg/mL, which is much higher than that of the routinely-used Au NP-based LFIA. The authors also evaluated 96 clinical serum samples and compared the results with the commercial HBsAg ELSA kits. No false negative results were detected, and the quantified results from the positive sample were comparable to the commercial HBsAg CLIA kits.
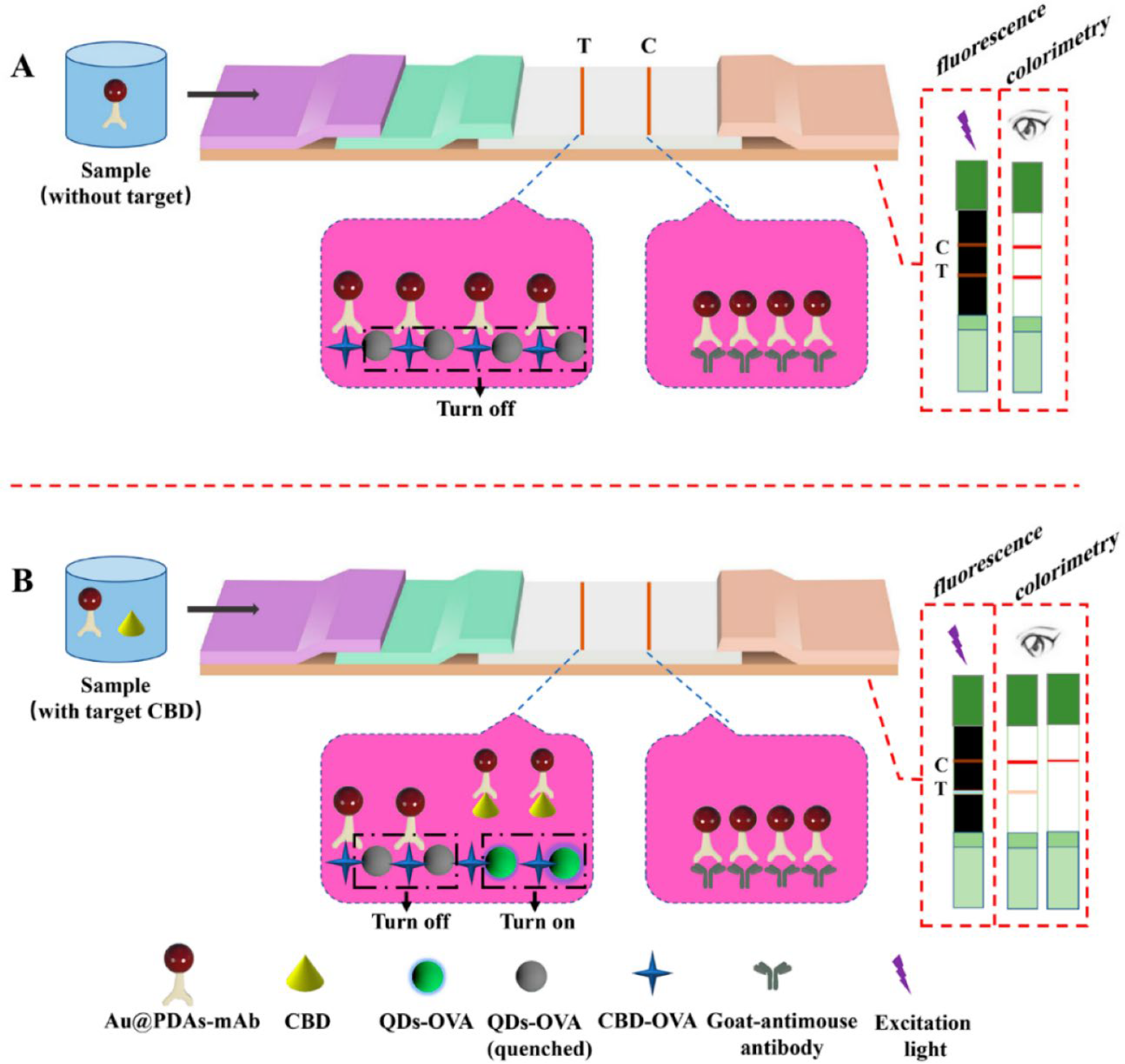
Mao et al. reported an LFIA method in which QDs were not loaded onto the sample pad but were immobilized onto the test line (
Figure 5) [
45]. They immobilized QDs and carbendazim, as the target compound, onto the test line, coating with ovalbumin. Anti-carbendazim antibody-conjugated Au NPs (Au@PDA-mAb) were prepared and developed from a sample pad. In the absence of carbendazim in the sample, the carbendazim immobilized on the test line captured as much Au@PDAs-mAb as possible. Because of the captured Au@PDAs-mAb, the fluorescence signal of the test line was quenched, and a deep red line was observed. In contrast, the amount of captured Au@PDAs-mAb on the test line was reduced when carbendazim was present in the sample because Au@PDAs-mAb was already bound to carbendazim before development. Thus, the color of the test line was fainter, and the fluorescence signal intensity increased, similar to the number of unquenched QDs. The cutoff value for carbendazim was 0.0156 μg/mL in fluorescence analysis and 0.5 μg/mL in colorimetric analysis.
The attention to QD-based LFIA has been continued during the COVID-19 pandemic. Li et al. developed a QD-based LFIA for the detection of SARS-CoV-2-specific antibodies [
46]. For the probe preparation, the authors synthesized ZnCdSe/ZnS QDs and conjugated them with SARS-CoV-2 nucleocapsid (N) proteins. Staphylococcus aureus protein A (SPA) and mAb 4B8 antibody were coated on the test line and control line, respectively. The SPA is utilized as a capture molecule, targeting the Fc segment of SARS-CoV-2-specific antibodies. The LOD was 1:1.024 ⅹ10
5 with an IgG concentration of 48.84 ng/mL. No cross-reactivity was observed against the antibodies of other relevant coronaviruses and respiratory infection-related viruses. Zhou et al. also prepared highly luminescent QD nanobeads embodying CdSe/ZnS QDs into the polymeric matrix for the detection of SARS-CoV-2 total antibodies [
47]. In order to employ a double antigen assay, the authors conjugated QD nanobeads with SARS-CoV-2 spike proteins and also separately prepared QD nanobeads labeled with mouse anti-digoxin antibodies. The test line and control line were coated with spike protein and mouse anti-digoxin antibodies, respectively. They confirmed the performance of the assay using 122 serum samples, including 69 positive and 53 negative samples validated with RT-PCR.
2.4. LFIAs with UCNPs
Organic dyes or QDs, both of which are widely used as fluorescence probes in bioimaging, emit distinct fluorescent light under irradiation with short-wavelength light, such as UV light. Although fluorescence imaging with UV light is useful, UV light can damage samples and induce autofluorescence owing to its absorption by biological samples. To overcome this problem, UCNPs, which can be excited by irradiation with long-wavelength light, have been developed [
48]. Most UCNPs absorb near-infrared (NIR) light, which does not interfere with biological samples, and emit fluorescence. In addition, UCNPs have various optical properties similar to QDs, such as a narrow emission bandwidth, high photostability, long lifetime, and tunable emission [
49,
50].
Jin et al. optimized UCNP-Antibody conjugates in terms of particle size and probe density through the systemic study [
51]. For the optimization, the authors chose C-reactive protein (CRP) as an example of LFIA. They first synthesized UCNPs (NaYF
4: Yb
3+, Er
3+) having various diameters (50 nm, 100 nm, 200 nm, and 500 nm) and conjugated with anti-CRP Ab#8 antibodies with various densities (from 90% to 10%). The LFIA strip was prepared with anti-CRP Ab7# antibody for the test line and goat anti-mouse IgG antibody for the control line. In this investigation, they found the fact that LOD decreased with the particle size and increasing concentration of the probe. At an optimum condition, LOD was 0.046 ng/mL with a broad detection range between 0.2 and 300 ng/mL. Lastly, they validated the assay using 12 clinical samples, and the detection results are consistent with the chemiluminescent immunoassay method.
Liu et al. reported a detection method for cephalexin with luminescent UCNP-based LFIA [
52]. They fabricated core-shell type NaGdF
4:Yb,Er@NaGdF
4 UCNPs via a seed-mediated method and conjugated an anti-cephalexin monoclonal antibody using click chemistry. The visual detection limit of CEX with the fabricated UCNPs was 10 ng/mL, which is similar to that of Au NPs. Detection of CEX with fabricated UCNPs showed linearity in the 0.5 to 100 ng/mL range, and the LOD of CEX with UCNPs was determined to be 0.6 ng/mL. Because this LOD was similar to that of Au NPs, the authors insisted that the fabricated core-shell-type UCNPs could be used as an alternative to Au NPs.
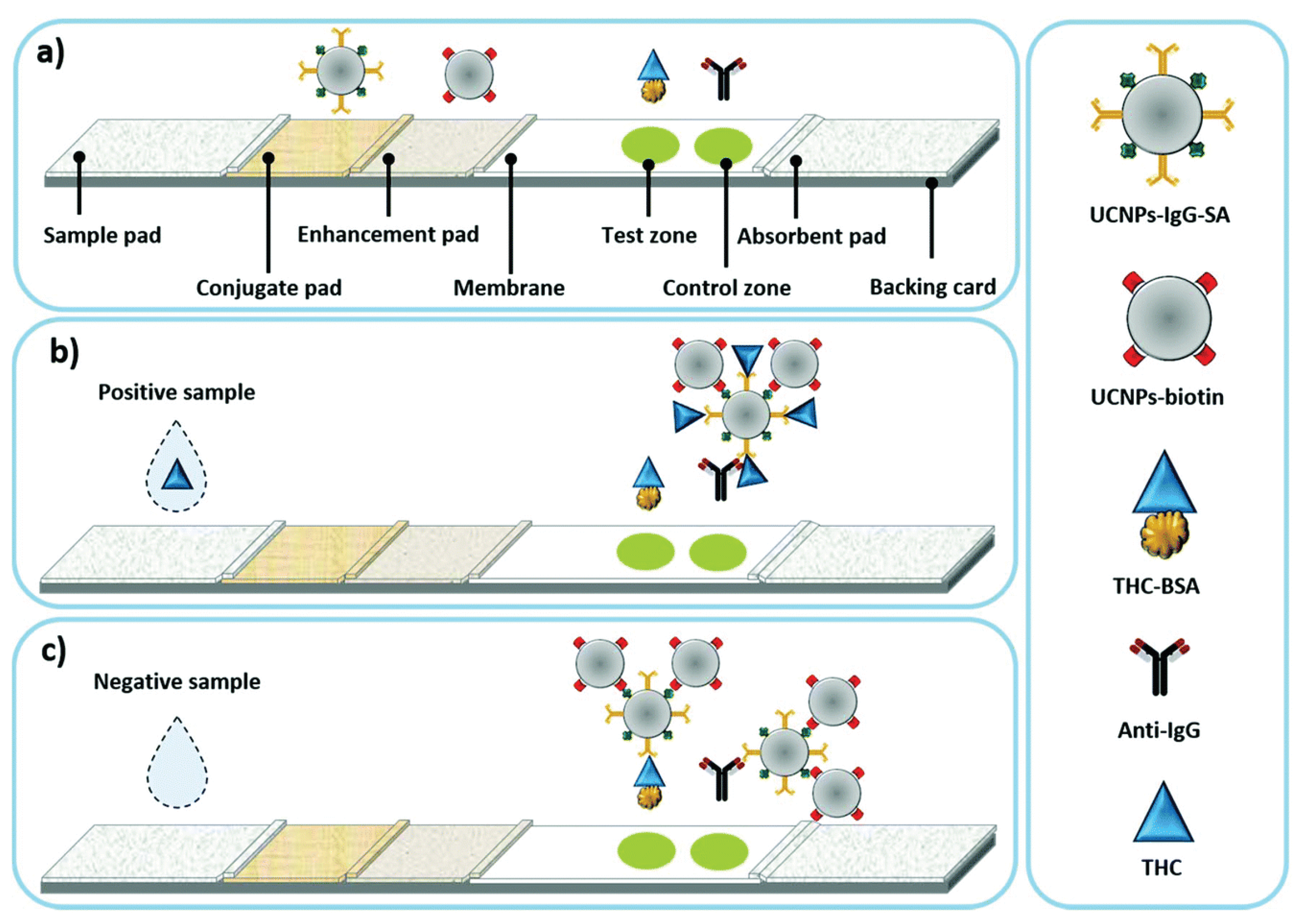
Another application of UCNPs in LFIA analysis was reported by Chand et al. (
Figure 6) [
53]. For the detection of tetrahydrocannabinol (THC), which is a biomarker for cannabis detection, IgG and streptavidin-conjugated UCNPs (UCNPs-IgG-SA) were prepared and loaded onto the conjugate pad of LFIA. Biotin-conjugated UCNPs (UCNP-biotin) were also prepared and loaded onto an enhancement pad located next to the conjugate pad to enhance the fluorescence signal. In the presence of THC in the sample, UCNPs-IgG-SA sequentially bound to THC and UCNP-biotin during development. As the IgG of the UCNPs complex was already bound to THC, the THC-bound UCNP complex was rarely immobilized on the test line coated with THC. Meanwhile, when THC was absent from the sample, the UCNPs complex could be immobilized on the test line and showed a fluorescence signal. This LFIA system for THC detection showed a 20% increase in intensity compared to the standard LFIA, with an LOD for THC at 2 ng/mL.
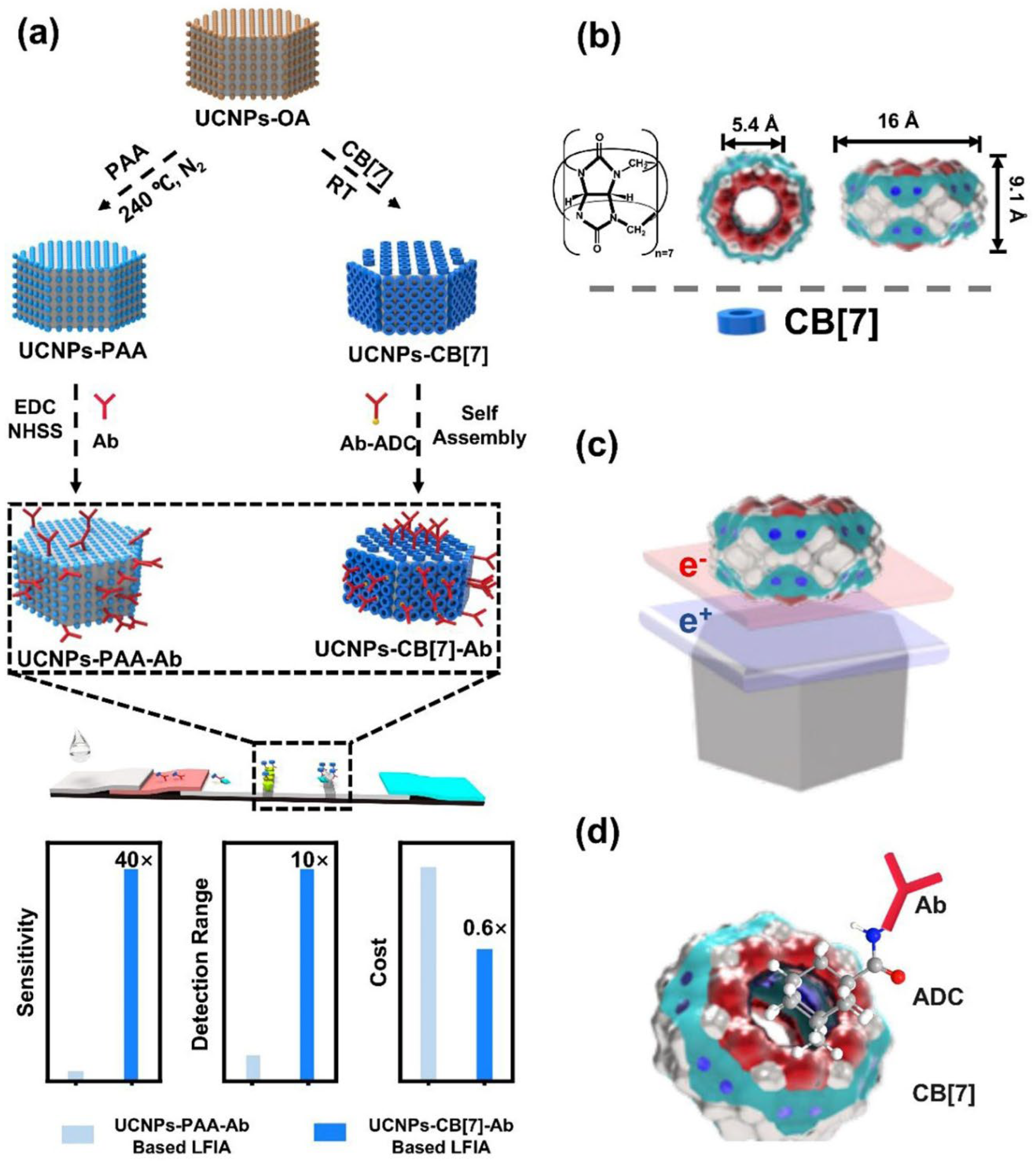
To improve the photoluminescence efficiency of UCNPs used in LFIA, Huang et al. modified the surface of UCNPs using a supramolecular self-assembly strategy (
Figure 7) [
54]. UCNPs were coated with cucurbit [
7] uril (CB[
7]) and bound antibodies for target recognition via host–guest interactions between CB[
7] and 1-adamantanecarboxyl conjugated with antibodies. The prepared UCNPs showed high PL intensity and stability as well as high binding affinity for targets such as
Escherichia coli O157:h7 or danofloxacin, even though less antibody was consumed during modification. Compared to UCNPs coated with polyacrylic acid, CB[
7]-coated UCNPs showed a 40-fold higher sensitivity and a 10-fold higher detection range in LFIA.
Thanks to the unique characteristics of UCNPs, the improvement of assay design has also been achieved. For example, Guo et al. developed an LFIA assay using orthogonal emissive UCNPs to suggest a solution for the double-line issue in LFIA [
55]. The authors utilized Lanthanide-ion-doped UCNPs, which possessed ladder-like energy levels, as signal reporters for both reporting signal and calibrating signals. They designed dumbbell-shaped UCNP displayed red and green emissions from the same activator Er
3+ ion; so, the energy migration pathways can be manipulated under 980 nm and 808 nm excitations, and thus, the assay did not require a separated control line and acquire the results from the integrated test line. They took aflatoxin B1, a grain toxin, as a model target for rapid and quantitative detections. The LOD was 25 ng/mL with a linear range from 20 to 500 μg/mL. Also, they also demonstrated an assay using a ring-assembled strip based on the single-line assay for the improvement of detection capacity.
Even though a UCNP-based LFIA for the detection of SARS-CoV-2 has not yet been reported, UCNPs are attractive probes in the detection of infectious viruses. For example, Martiskainen et al. developed an assay for the detection of human immunodeficiency viruses 1 (HIV-1) and 2 (HIV-2) antibodies [
56]. Because anti-HIV antibodies indicate the body’s immune response to HIV infection, there are various commercial kits detecting those antibodies in the market. The authors conjugated UCNPs with the HIV-1 and HIV-1 recombinant antigens. Those antigens were also coated on the test line, while HIV-1 gp41 rabbit polyclonal serum was printed on the control line. Martiskainen et al. also developed a UCNP-based LFIA for the detection of HBsAg [
57]. They conjugated UCNPs with mouse monoclonal anti-HBsAg antibody, which is in-house produced by hybridoma technology. The mixture of three kinds of anti-HBsAg antibodies, including two in-house and one commercial one, was coated on the test line, and rabbit anti-mouse IgG was coated on the control line. The LOD of the assay was calculated to be 0.1 IU/ml in the spiked serum, and it is comparable to the LOD of commercial LFIA kits. Finally, they compared their UCNP-based LFIA with commercial chemiluminescent immunoassay.
2.5. LFIAs with Silica Template Based NPs
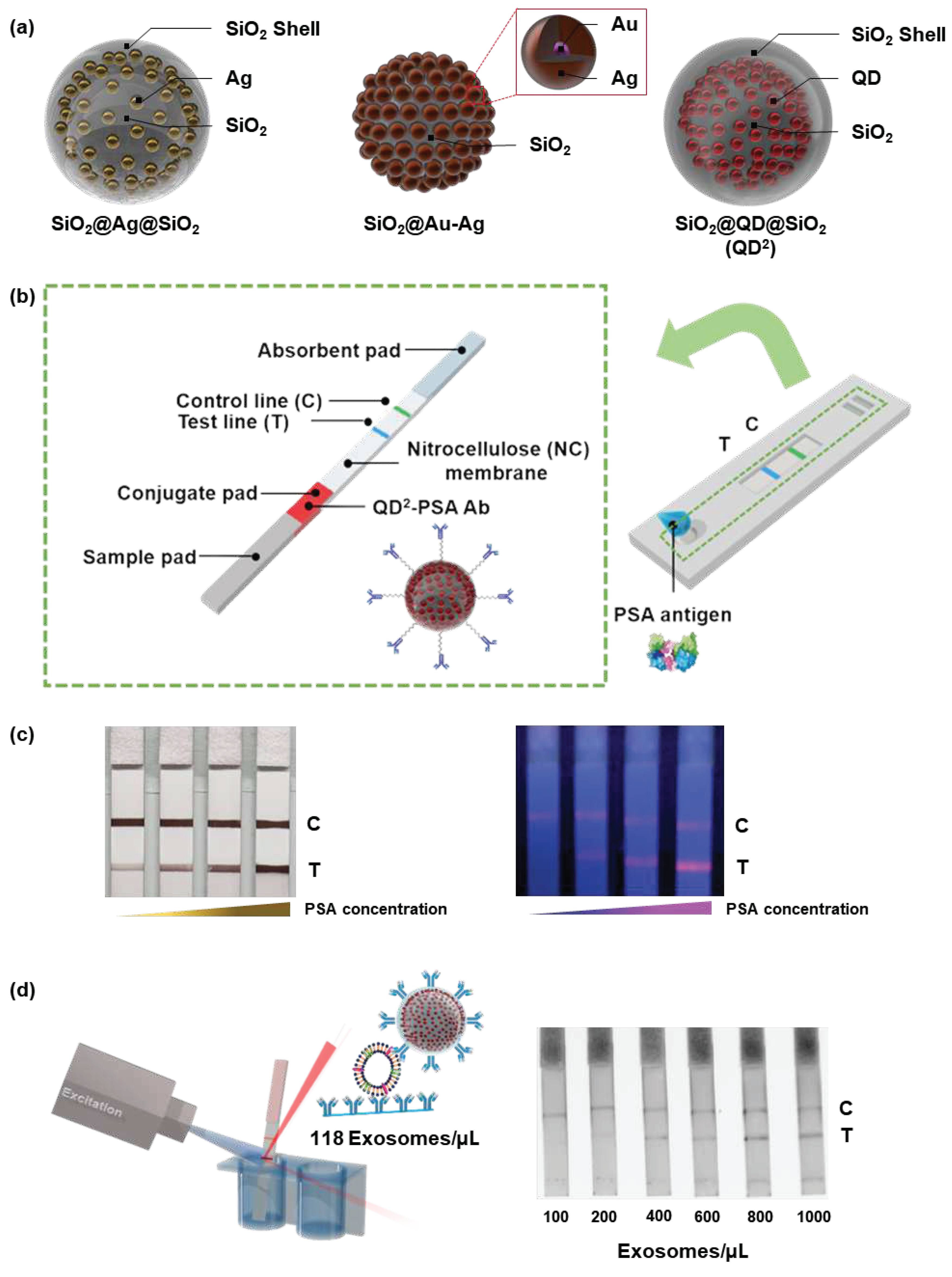
Despite their utility, NPs of only a few nanometers in size are difficult to handle during surface modification. In addition, enhancement of the signal intensity is necessary for sensitive detection because of the weak signal intensity of individual NPs. To overcome these problems, our group developed a system in which metal NPs, such as silver, QDs, or Au/silver alloys, were embedded onto a silica NP template [
58,
59,
60]. The signal intensity of fabricated individual fabricated NPs was markedly enhanced because of the numerous embedded metal particles compared with that of single metal NPs.
Kim et al. prepared silica-encapsulated silver-assembled silica NPs (SiO
2@Ag@SiO
2 NPs) and detected PSA [
58]. Thiol-modified silica NPs, approximately 150 nm in size, were used as templates. Finally, SiO
2@Ag@SiO
2 NPs were prepared by covering the fabricated NPs with a silica shell to prevent contact with the silver NPs. During the formation of silver NPs, the concentration of the silver precursor was varied to control the amount and size of the embedded silver NPs. For SiO
2@Ag@SiO
2 NP
2.6, the highest concentration of the silver precursor exhibited a light gray color when dispersed in ethanol, and a light pink or yellow color was seen when the concentration of the silver precursor was low. Based on their enhanced LSPR effect, which originated from the narrow gap between the embedded silver NPs, SiO
2@Ag@SiO
2 NP
2.6 also showed a strong optical signal intensity when dropped onto the nitrocellulose membrane. With SiO
2@Ag@SiO
2 NP
2.6, PSA could be detected within 10 min with an LOD of 1.1 ng/mL.
Au-silver-assembled NPs have also been embedded onto silica templates for the fabrication of optical nanoprobes [
59]. Pre-synthesized Au NPs (7 nm in size) were attached to the surface of silica NPs, and silver NPs were added around the attached Au NPs. These NPs (SiO
2@Ag-Au NPs) exhibited a deep gray color when dispersed in ethanol and had stronger optical intensity than Au NPs when the same concentration of particles was dropped onto the nitrocellulose membrane. SiO
2@Au-Ag NPs enabled the visual detection of PSA in clinical samples via LFIA, and a semi-quantitative analysis was also possible.
QD-embedded silica-encapsulated NPs (SiO
2@QD@SiO
2; QD
2) were also used as optical nanoprobes in LFIA [
60,
61]. QDs
2 were prepared by attaching QDs onto thiolated silica NPs and silica encapsulation [
62]. QD
2 showed approximately 200-fold stronger photoluminescence (PL) intensity than single QDs because many QDs were embedded onto silica templates. Bock et al. conjugated anti-PSA antibodies onto the surface of QDs
2 and used them to detect PSA via LFIA [
61]. After the development of samples, a photograph of LFIA strips was captured under a 365 nm UV lamp, and the signal intensities were evaluated using the ImageJ program. The LOD of LFIA for PSA was calculated as 0.138 ng/mL, which is far lower than the ”safe zone” threshold for prostate cancer (2.5 ng/mL) [
63]. In addition to good sensitivity, the fabricated LFIA kit showed high selectivity such that the kit did not exhibit a signal at the test line with alpha-fetoprotein or newborn calf serum. With this superior performance, 47 clinical samples were analyzed using the LFIA kit. The resulting area under the curve was 0.852, and there were no false-negative results. Kim et al. applied QDs
2 to LFIA for the detection of exosomes [
61]. Anti-CD63 antibodies, which can bind to exosomal CD63, were immobilized onto the surface of QDs
2 to capture exosomes in the sample. The calculated LOD was 118 exosomes/μL, which was approximately 11 times lower than previous results for the detection of PSA with LFIA using Au NPs [
64], double Au NP conjugates [
65], and Au-palladium NPs [
66].
Other research groups also reported silica template-based methods for the fabrication of LFIA probes. Lu et al. fabricated spherical core-shell gold-silica nanoparticles (AuNP@SiO
2 NPs) by silylation of surfactant-stabilized AuNPs [
67]. The authors utilized AFP as a model biomarker. Based on the highly stable NP, the LOD of the assay was down to 300 pg/mL, and it is comparable level to the performance of ELISA.
During the COVID-19 pandemic, the LFIA development using a silica template-based method has also been achieved. Wang et al. developed colorimetric-fluorescent dual-mode LFIA for the detection of IgM and IgG in human serum [
68]. The authors decorated both Au NPs and CdSe/ZnS QDs on the SiO
2 NPs to fabricate SiO
2@Au@QD nanobeads with the help of polyethylenimine (PEI) layers. Thereafter, SARS-CoV-2 spike (S) proteins are conjugated on the surface of the SiO
2@Au@QD nanobeads. The anti-human IgG and anti-human IgM antibodies were separately coated on two test lines, whereas the anti-S antibody was coated on the control line. In the fluorescence signal, the assay was 100 times more sensitive than commonly used Au-based LFIA for SARV-CoV-2-specific IgM/IgG detection. The colorimetric-fluorescent dual-mode detection was the useful ability to rapid detection (colorimetric) and more accurate and quantitative detection (fluorescent). The authors validated 57 clinical samples (16 positives and 41 negatives) and identified 100% of patients with 100% specificity. Since IgG level, IgM level, and IgG/IgM ratio is a valuable indicators of the patient’s infection history, the simultaneous detection of IgG and IgM can not only contribute accurate identification of a SARS-CoV-2-infected person but also monitor the progress of the disease.
Figure 8.
Figure 8. (a) Illustration of silica NPs [
58,
59,
60]. (b) Schematic illustration of a LFIA system for PSA detection with silica NPs [
60]. (c) Detection of PSA via LFIA with SiO
2@Au-Ag (left) and QD
2 (right) [
59,
60]. (d) Schematic illustration of a LFIA system for exosome detection with QD
2 (left) and results from exosome detection with the LFIA system (right) [
61].
Figure 8.
Figure 8. (a) Illustration of silica NPs [
58,
59,
60]. (b) Schematic illustration of a LFIA system for PSA detection with silica NPs [
60]. (c) Detection of PSA via LFIA with SiO
2@Au-Ag (left) and QD
2 (right) [
59,
60]. (d) Schematic illustration of a LFIA system for exosome detection with QD
2 (left) and results from exosome detection with the LFIA system (right) [
61].
3. Conclusions and Prospective
The LFIA is considered the most promising diagnostic tool because it is simple, rapid, and low-cost. Compared with other diagnostic methods, such as ELISA, LFIA can be used without the need for expensive or uncommon instruments. Furthermore, quantitative/qualitative analysis of target biomarkers is enabled by the use of photographic images. However, its application is often limited by low sensitivity as well as low reproducibility. The recent advances based on the engineering of optical NPs lay the foundations for more sensitive and accurate LFIA.
In this review article, we have discussed LFIA systems with various types of optical NPs, including Au NPs, QDs, UCNPs, and silica template-based NPs. These systems have successively been developed and have shown potential for the detection of clinically relevant targets, such as cancer biomarkers like AFP, CEA, and PSA. Researchers have highlighted the potential of LFIA for biomarker detection at an early disease stage, even when a little amount of sample is analyzed. Enhanced LFIA is to be of particular value in diseases where early detection is essential, such as pancreatic cancer. We, therefore, expect that the rapid, sensitive, convenient, and accurate diagnosis of diseases can be achieved when optical NPs are optimized for a given target.
In addition, the present COVID-19 pandemic highlights the important role of LFIA once again. In this urgent situation evolved by a highly contagious virus, the short sample-to-result time and user-friendly procedure of LFIA enable us to conduct mass-scale self-testing. Although the sensitivity of these commercial kits was somewhat unsatisfactory, they have contributed to preventing the spreading of SARS-CoV-2. There will be an increasing demand for rapid and convenient diagnostics in the future. Therefore, it is essential that research on the enhancement of optical NPs used in LFIA be continued.
Author Contributions
Conceptualization, J.K., D.-E.K., and B.-.H.J.; writing-original draft preparation, J.K., M.-S.S., and H.-M.K.; writing-review and editing, J.-H.S., X.-H.P., and S.-M.P.; Supervisor, B.-H.J.
Funding
This paper was funded by the Ministry of Science and ICT (NRF-2022R1A2C2012883), by the Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20018608), and by Konkuk University Researcher Fund in 2021 (2021-A019-0261).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This study was funded by the Ministry of Science and ICT (NRF-2022R1A2C2012883); Ministry of Trade, Industry & Energy (MOTIE) of the Republic of Korea (No. 20018608); and the Konkuk University Researcher Fund in 2021 (2021-A019-0261).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ezzati, M.; Lopez, A.D.; Rodgers, A.; Vander Hoorn, S.; Murray, C.J. Selected Major Risk Factors and Global and Regional Burden of Disease. The Lancet 2002, 360, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. The Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, O.; Martiny, D.; Rochas, O.; Van Belkum, A.; Kozlakidis, Z. Considerations for Diagnostic COVID-19 Tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Newbigging, A.M.; Le, C.; Pang, B.; Peng, H.; Cao, Y.; Wu, J.; Abbas, G.; Song, J.; Wang, D.-B.; et al. Molecular Diagnosis of COVID-19: Challenges and Research Needs. Anal. Chem. 2020, 92, 10196–10209. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.-P.; Hussell, T.; et al. COVID-19 Therapeutics: Challenges and Directions for the Future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. Biomarkers and Surrogate Endpoints. Br. J. Clin. Pharmacol. 2005, 59, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Sonawane, M.D.; Song, K.-S.; Kim, T. Biomarker Detection Technologies and Future Directions. Analyst 2016, 141, 740–755. [Google Scholar] [CrossRef]
- Jahrling, PeterB. ; Niklasson, BoS.; Mccormick, JosephB. EARLY DIAGNOSIS OF HUMAN LASSA FEVER BY ELISA DETECTION OF ANTIGEN AND ANTIBODY. The Lancet 1985, 325, 250–252. [Google Scholar] [CrossRef]
- Guimarães, A.J.; Pizzini, C.V.; De Matos Guedes, H.L.; Albuquerque, P.C.; Peralta, J.M.; Hamilton, A.J.; Zancopé-Oliveira, R.M. ELISA for Early Diagnosis of Histoplasmosis. J. Med. Microbiol. 2004, 53, 509–514. [Google Scholar] [CrossRef]
- Cheng, C.-M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-Based ELISA. Angew. Chem. 2010, 122, 4881–4884. [Google Scholar] [CrossRef]
- Andryukov, B. Somov Research Institute of Epidemiology and Microbiology, Vladivostok, Russian Federation; 2 Far Eastern Federal University (FEFU), Vladivostok, Russian Federation Six Decades of Lateral Flow Immunoassay: From Determining Metabolic Markers to Diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Gumus, E.; Bingol, H.; Zor, E. Lateral Flow Assays for Detection of Disease Biomarkers. J. Pharm. Biomed. Anal. 2023, 225, 115206. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ding, L.; Huang, X.; Xiong, Y. Tailoring Noble Metal Nanoparticle Designs to Enable Sensitive Lateral Flow Immunoassay. Theranostics 2022, 12, 574–602. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Kang, K.; Li, Q.; Wang, Y.; He, X. Rapid and Sensitive Detection of Cardiac Troponin I for Point-of-Care Tests Based on Red Fluorescent Microspheres. Molecules 2018, 23, 1102. [Google Scholar] [CrossRef]
- Parolo, C.; De La Escosura-Muñiz, A.; Merkoçi, A. Enhanced Lateral Flow Immunoassay Using Gold Nanoparticles Loaded with Enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Hsiao, W.W.-W.; Le, T.-N.; Pham, D.M.; Ko, H.-H.; Chang, H.-C.; Lee, C.-C.; Sharma, N.; Lee, C.-K.; Chiang, W.-H. Recent Advances in Novel Lateral Flow Technologies for Detection of COVID-19. Biosensors 2021, 11, 295. [Google Scholar] [CrossRef]
- Turkevich, J. Colloidal Gold. Part II: Colour, Coagulation, Adhesion, Alloying and Catalytic Properties. Gold. Bull. 1985, 18, 125–131. [Google Scholar] [CrossRef]
- Martin, M.N.; Basham, J.I.; Chando, P.; Eah, S.-K. Charged Gold Nanoparticles in Non-Polar Solvents: 10-Min Synthesis and 2D Self-Assembly. Langmuir 2010, 26, 7410–7417. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Cho, H.-Y.; Choi, H.; Lee, J.-Y.; Choi, J.-W. Application of Gold Nanoparticle to Plasmonic Biosensors. IJMS 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]
- Amri, C.; Shukla, A.K.; Lee, J.-H. Recent Advancements in Nanoparticle-Based Optical Biosensors for Circulating Cancer Biomarkers. Materials 2021, 14, 1339. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Tumskiy, R.S.; Burov, A.M.; Pylaev, T.E.; Khlebtsov, N.G. Quantifying the Numbers of Gold Nanoparticles in the Test Zone of Lateral Flow Immunoassay Strips. ACS Appl. Nano Mater. 2019, 2, 5020–5028. [Google Scholar] [CrossRef]
- Shen, G.; Xu, H.; Gurung, A.S.; Yang, Y.; Liu, G. Lateral Flow Immunoassay with the Signal Enhanced by Gold Nanoparticle Aggregates Based on Polyamidoamine Dendrimer. Anal. Sci. 2013, 29, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhang, S.; Hu, X. Signal Enhancement in a Lateral Flow Immunoassay Based on Dual Gold Nanoparticle Conjugates. Clin. Biochem. 2013, 46, 1734–1738. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, G. Signal-Enhanced Lateral Flow Immunoassay with Dual Gold Nanoparticle Conjugates for the Detection of Hepatitis B Surface Antigen. ACS Omega 2019, 4, 5083–5087. [Google Scholar] [CrossRef]
- Panferov, V.G.; Safenkova, I.V.; Zherdev, A.V.; Dzantiev, B.B. Post-Assay Growth of Gold Nanoparticles as a Tool for Highly Sensitive Lateral Flow Immunoassay. Application to the Detection of Potato Virus X. Microchim. Acta 2018, 185, 506. [Google Scholar] [CrossRef]
- Razo, S.C.; Panferova, N.A.; Panferov, V.G.; Safenkova, I.V.; Drenova, N.V.; Varitsev, Y.A.; Zherdev, A.V.; Pakina, E.N.; Dzantiev, B.B. Enlargement of Gold Nanoparticles for Sensitive Immunochromatographic Diagnostics of Potato Brown Rot. Sensors 2019, 19, 153. [Google Scholar] [CrossRef]
- Panraksa, Y.; Apilux, A.; Jampasa, S.; Puthong, S.; Henry, C.S.; Rengpipat, S.; Chailapakul, O. A Facile One-Step Gold Nanoparticles Enhancement Based on Sequential Patterned Lateral Flow Immunoassay Device for C-Reactive Protein Detection. Sens. Actuators B Chem. 2021, 329, 129241. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, G.; Fang, B.; Xiong, Q.; Duan, H.; Lai, W. Lateral Flow Immunoassay Based on Polydopamine-Coated Gold Nanoparticles for the Sensitive Detection of Zearalenone in Maize. ACS Appl. Mater. Interfaces 2019, 11, 31283–31290. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Cavalera, S.; Baggiani, C.; Giovannoli, C.; Anfossi, L. Direct vs Mediated Coupling of Antibodies to Gold Nanoparticles: The Case of Salivary Cortisol Detection by Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2019, 11, 32758–32768. [Google Scholar] [CrossRef] [PubMed]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C.; Cárdenas, S.; Simonet, B.M.; Valcárcel, M. Quantum Dots Luminescence Enhancement Due to Illumination with UV/Vis Light. Chem. Commun. 2009, 5214. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Tavares, A.J.; Krull, U.J. Beyond Labels: A Review of the Application of Quantum Dots as Integrated Components of Assays, Bioprobes, and Biosensors Utilizing Optical Transduction. Anal. Chim. Acta 2010, 673, 1–25. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent Quantum Dots for Multiplexed Biological Detection and Imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum Dot Bioconjugates for Imaging, Labelling and Sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Bock, S.; An, J.; Kim, H.; Kim, J.; Jung, H.; Pham, X.; Rho, W.; Jun, B. A Lateral Flow Immunoassay for Prostate-Specific Antigen Detection Using Silica-Coated CdSe @ ZnS Quantum Dots. Bull. Korean Chem. Soc. 2020, 41, 989–993. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J. Multiplexed Lateral Flow Biosensors: Technological Advances for Radically Improving Point-of-Care Diagnoses. Biosens. Bioelectron. 2016, 83, 177–192. [Google Scholar] [CrossRef]
- Wang, C.; Hou, F.; Ma, Y. Simultaneous Quantitative Detection of Multiple Tumor Markers with a Rapid and Sensitive Multicolor Quantum Dots Based Immunochromatographic Test Strip. Biosens. Bioelectron. 2015, 68, 156–162. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.; Zhou, C.; Mao, M.; Liu, Q.; Shen, H.; Cen, Y.; Qin, Z.; Ma, L.; Song Li, L. Multiplexed Detection of Influenza A Virus Subtype H5 and H9 via Quantum Dot-Based Immunoassay. Biosens. Bioelectron. 2016, 77, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Goryacheva, I.Yu.; De Saeger, S.; Gaponik, N. Silanized Luminescent Quantum Dots for the Simultaneous Multicolor Lateral Flow Immunoassay of Two Mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, H.-M.; Chen, J.; Liu, J.; Zhang, L.; Qu, L.; Li, Z.; Lin, Y. Quantum Dot-Based Lateral Flow Test Strips for Highly Sensitive Detection of the Tetanus Antibody. ACS Omega 2019, 4, 6789–6795. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, Y.; Fu, F.; Xu, H.; Lv, J.; Xiong, Y.; Wang, A. Immunochromatographic Assay for Quantitative and Sensitive Detection of Hepatitis B Virus Surface Antigen Using Highly Luminescent Quantum Dot-Beads. Talanta 2015, 142, 145–149. [Google Scholar] [CrossRef]
- Mao, X.; Wang, Y.; Jiang, L.; Zhang, H.; Zhao, Y.; Liu, P.; Liu, J.; Hammock, B.D.; Zhang, C. A Polydopamine-Coated Gold Nanoparticles Quenching Quantum Dots-Based Dual-Readout Lateral Flow Immunoassay for Sensitive Detection of Carbendazim in Agriproducts. Biosensors 2022, 12, 83. [Google Scholar] [CrossRef]
- Li, Z.; Wang, A.; Zhou, J.; Chen, Y.; Liu, H.; Liu, Y.; Zhang, Y.; Ding, P.; Zhu, X.; Liang, C.; et al. A Universal Fluorescent Immunochromatography Assay Based on Quantum Dot Nanoparticles for the Rapid Detection of Specific Antibodies against SARS-CoV-2 Nucleocapsid Protein. IJMS 2022, 23, 6225. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Liu, W.; Fang, H.; Li, X.; Hou, L.; Liu, Y.; Lai, W.; Huang, X.; Xiong, Y. Development of a Rapid and Sensitive Quantum Dot Nanobead-Based Double-Antigen Sandwich Lateral Flow Immunoassay and Its Clinical Performance for the Detection of SARS-CoV-2 Total Antibodies. Sens. Actuators B Chem. 2021, 343, 130139. [Google Scholar] [CrossRef]
- Brandmeier, J.C.; Jurga, N.; Grzyb, T.; Hlaváček, A.; Obořilová, R.; Skládal, P.; Farka, Z.; Gorris, H.H. Digital and Analog Detection of SARS-CoV-2 Nucleocapsid Protein via an Upconversion-Linked Immunosorbent Assay. Anal. Chem. 2023, 95, 4753–4759. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. A General Introduction to Luminescent Materials. In Luminescent Materials; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1994; pp. 1–9. ISBN 978-3-540-58019-5. [Google Scholar]
- Yen, W.M.; Weber, M.J. (Eds.) Inorganic Phosphors; CRC Press, 2004; ISBN 978-0-203-50632-5.
- Jin, B.; Du, Z.; Ji, J.; Bai, Y.; Tang, D.; Qiao, L.; Lou, J.; Hu, J.; Li, Z. Regulation of Probe Density on Upconversion Nanoparticles Enabling High-Performance Lateral Flow Assays. Talanta 2023, 256, 124327. [Google Scholar] [CrossRef]
- Liu, C.; Ma, W.; Gao, Z.; Huang, J.; Hou, Y.; Xu, C.; Yang, W.; Gao, M. Upconversion Luminescence Nanoparticles-Based Lateral Flow Immunochromatographic Assay for Cephalexin Detection. J. Mater. Chem. C 2014, 2, 9637–9642. [Google Scholar] [CrossRef]
- Chand, R.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Upconverting Nanoparticle Clustering Based Rapid Quantitative Detection of Tetrahydrocannabinol (THC) on Lateral-Flow Immunoassay. Analyst 2021, 146, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Chen, Y.; Xiong, Q.; Wang, Y.; Duan, H.; Lai, W. Improving the Performance of Upconversion Nanoprobe-Based Lateral Flow Immunoassays by Supramolecular Self-Assembly Core/Shell Strategies. Sens. Actuators B Chem. 2020, 318, 128233. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, Y.; Liu, J.; Fu, S.; Zhang, J.; Mei, Q.; Zhang, Y. Single-Line Flow Assay Platform Based on Orthogonal Emissive Upconversion Nanoparticles. Anal. Chem. 2021, 93, 3010–3017. [Google Scholar] [CrossRef] [PubMed]
- Martiskainen, I.; Juntunen, E.; Salminen, T.; Vuorenpää, K.; Bayoumy, S.; Vuorinen, T.; Khanna, N.; Pettersson, K.; Batra, G.; Talha, S.M. Double-Antigen Lateral Flow Immunoassay for the Detection of Anti-HIV-1 and -2 Antibodies Using Upconverting Nanoparticle Reporters. Sensors 2021, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Martiskainen, I.; Talha, S.M.; Vuorenpää, K.; Salminen, T.; Juntunen, E.; Chattopadhyay, S.; Kumar, D.; Vuorinen, T.; Pettersson, K.; Khanna, N.; et al. Upconverting Nanoparticle Reporter–Based Highly Sensitive Rapid Lateral Flow Immunoassay for Hepatitis B Virus Surface Antigen. Anal. Bioanal. Chem. 2021, 413, 967–978. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.; Bock, S.; An, J.; Choi, Y.-S.; Pham, X.-H.; Cha, M.G.; Seong, B.; Kim, W.; Kim, Y.-H.; et al. Silver-Assembled Silica Nanoparticles in Lateral Flow Immunoassay for Visual Inspection of Prostate-Specific Antigen. Sensors 2021, 21, 4099. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, J.; An, J.; Bock, S.; Pham, X.-H.; Huynh, K.-H.; Choi, Y.; Hahm, E.; Song, H.; Kim, J.-W.; et al. Au–Ag Assembled on Silica Nanoprobes for Visual Semiquantitative Detection of Prostate-Specific Antigen. J. Nanobiotechnol. 2021, 19, 73. [Google Scholar] [CrossRef]
- Bock, S.; Kim, H.-M.; Kim, J.; An, J.; Choi, Y.-S.; Pham, X.-H.; Jo, A.; Ham, K.; Song, H.; Kim, J.-W.; et al. Lateral Flow Immunoassay with Quantum-Dot-Embedded Silica Nanoparticles for Prostate-Specific Antigen Detection. Nanomaterials 2021, 12, 33. [Google Scholar] [CrossRef]
- Kim, H.-M.; Oh, C.; An, J.; Baek, S.; Bock, S.; Kim, J.; Jung, H.-S.; Song, H.; Kim, J.-W.; Jo, A.; et al. Multi-Quantum Dots-Embedded Silica-Encapsulated Nanoparticle-Based Lateral Flow Assay for Highly Sensitive Exosome Detection. Nanomaterials 2021, 11, 768. [Google Scholar] [CrossRef]
- Jun, B.-H.; Hwang, D.W.; Jung, H.S.; Jang, J.; Kim, H.; Kang, H.; Kang, T.; Kyeong, S.; Lee, H.; Jeong, D.H.; et al. Ultrasensitive, Biocompatible, Quantum-Dot-Embedded Silica Nanoparticles for Bioimaging. Adv. Funct. Mater. 2012, 22, 1843–1849. [Google Scholar] [CrossRef]
- Barry, M.J. Prostate-Specific–Antigen Testing for Early Diagnosis of Prostate Cancer. N. Engl. J. Med. 2001, 344, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Rodríguez, M.; López-Cobo, S.; Reyburn, H.T.; Costa-García, A.; López-Martín, S.; Yáñez-Mó, M.; Cernuda-Morollón, E.; Paschen, A.; Valés-Gómez, M.; Blanco-López, M.C. Development of a Rapid Lateral Flow Immunoassay Test for Detection of Exosomes Previously Enriched from Cell Culture Medium and Body Fluids. J. Extracell. Vesicles 2016, 5, 31803. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, Y.; Cao, Y.; Huang, Y.; Xu, L.-P.; Zhang, X.; Wang, S. Enhanced Lateral Flow Assay with Double Conjugates for the Detection of Exosomes. Sci. China Chem. 2018, 61, 1423–1429. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Shi, Q.; Du, D.; Liu, D.; Luo, Y.; Xu, W.; Lin, Y. Au@Pd Nanopopcorn and Aptamer Nanoflower Assisted Lateral Flow Strip for Thermal Detection of Exosomes. Anal. Chem. 2019, 91, 13986–13993. [Google Scholar] [CrossRef]
- Lu, X.; Mei, T.; Guo, Q.; Zhou, W.; Li, X.; Chen, J.; Zhou, X.; Sun, N.; Fang, Z. Improved Performance of Lateral Flow Immunoassays for Alpha-Fetoprotein and Vanillin by Using Silica Shell-Stabilized Gold Nanoparticles. Microchim. Acta 2019, 186, 2. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.; Gu, B.; Liu, H.; Zhou, Z.; Shi, L.; Cheng, X.; Wang, S. Sensitive and Simultaneous Detection of SARS-CoV-2-Specific IgM/IgG Using Lateral Flow Immunoassay Based on Dual-Mode Quantum Dot Nanobeads. Anal. Chem. 2020, 92, 15542–15549. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).