1. Introduction
Prepulse inhibition (PPI) refers to the phenomenon in which an animal or human’s startle response to a strong stimulus is reduced when preceded by a weak prestimulus of the same modality [
1,
2,
3]. The degree of this reduction (PPI) is calculated as a ratio of the response intensity to the paired weak prestimulus and strong stimulus, to the intensity of the response to a single strong stimulus [
3,
4,
5]. The PPI phenomenon is based on the sensorimotor gating mechanism and involves brainstem structures, as well as some diencephalon and the basal ganglia areas [
3,
6]. It is clinically significant due to the observed PPI changes in patients with schizophrenia, schizotypal personality disorder, obsessive-compulsive disorder, Huntington’s disease, and other psychiatric and neurological disorders [
7]. PPI is commonly performed in animal models of human disease to investigate mechanisms of impairment and drug testing. [
4,
8,
9,
10]. Various types of startle stimuli are applied, including acoustic, visual, and tactile [
3,
4].
Commercial acoustic startle response (ASR) and PPI testing systems are designed for both rats and mice. These systems present experimental animals with startle stimuli, detect startle responses, and provide sound isolation. Examples of these commercial systems include SR-LAB (San Diego Instruments, San Diego, CA, USA), MED-ASR-PRO1 (Med Associates, Fairfax, VT, USA), StartFear (Panlab, Barcelona, Spain), Startle Reflex apparatus (Imetronic, Marcheprime, France), and Startle Reflex system (Kinder Scientific, Chula Vista, CA, USA). The provided software for these setups allows the experimenter to define the number of stimuli of each of the three types (weak prepulse, strong pulse, and paired prepulse-pulse stimulus), their volume, and the time between stimuli. After all the parameters are set, the system executes the program automatically without any further experimenter’s participation. Although commercial systems provide all the necessary features for the PPI procedure, their high cost might be a barrier to using them. Also, it is not always possible to buy expensive equipment for just one series of experiments. Another downside of the commercial systems is the closed source code, which affects their transparency and result reproducibility thus making it impossible to upgrade or repurpose setups [
11].
The simplicity of all the details necessary for the system without the need to buy specialized devices makes the PPI method even more attractive for researchers.
PPI does not require any complicated hardware, and its setup can be assembled by researchers themselves using the available equipment already present in the laboratory. The simplicity of all the details necessary for the system without the need to buy specialized devices makes the PPI method even more attractive for researchers. Several scientific groups have already developed the custom systems for ASR and PPI protocols. Gerum et al. [
12] suggested a Python-based setup for PPI and gap-prepulse inhibition of the acoustic startle reflex (GPIAS) protocols [
13], which requires an additional soundcard for sound presentation and a data acquisition card for recording and digitization of analog signal from acceleration sensor. Virag et al. [
11] repurposed a kitchen scale for startle response measurement. In this open-source solution the hardware is controlled by Arduino boards, while the graphical user interface for PC is written in Python, and data analysis is performed using R. Although these developments solve the problem of expensive and narrow-purpose commercial setups, they still require some additional components and expertise in the scripting languages used. A possible further step would be to implement the PPI protocol on hardware already present in laboratories, familiar to researchers, and therefore allowing for simultaneous conduction of electrophysiological experiments.
CED Power1401, produced by Cambridge Electronic Design Ltd. in Cambridge, UK, is a data acquisition and control interface widely used in neuroscience research. The interface operates with Spike2 software and allows installing custom scripts in Spike2 scripting language. Electrophysiological registration can also be performed in conjunction with startle response tests. High precision and accuracy of the CED Power1401 make it suitable for experimental protocols that require high temporal resolution. Furthermore, it allows for both analog-digital input and digital-analog output, making it ideal for the PPI procedure.
Setting the parameters of the acoustic signals to align with the research objectives is the primary challenge in implementing the PPI paradigm. The PPI paradigm requires an adjustment of various parameters and specifications to fit the experimental design [
4]:
Length and amplitude of acoustic pulses;
Intervals between prestimulus and stimulus in paired pulses (interstimulus interval, ISI);
Intervals between consecutive stimuli presentations (intertrial interval, ITI);
Variability of presentation frequency to prevent the development of reflexes for the next stimulus;
Number of stimuli of each type and their presentation order;
Synchronization of ASR amplitude registration and the timing of stimuli for further analysis;
Calibration of output voltage to the sound volume of the existing equipment.
While there is a Spike2 script available for generating paired pulses and analyzing results (PPulse.s2s, published on the official CED website [
14]), it lacks some of the parameters typically required for the PPI protocol, such as sound and white noise generation, sound volume settings, and presentation order of different types of stimuli. Although CED Power1401 and Spike2 have been used for stimulus presentation in the PPI procedure before [
2], no software or setup description has been published.
This article proposes a low-cost and flexible solution for acoustic startle response tests that meets all of the aforementioned requirements. It is simple to set up and operate, and can be used in any laboratory with CED Power1401 or a similar Spike2-based interface.
The setup has been successfully used in our previous studies, and the results have been published earlier [
9,
10]. The current paper provides an example of using Spike2-based data acquisition interface to assess PPI in wild-type and knockout rats.
2. Materials and Methods
2.1. Description of the script interface and system components
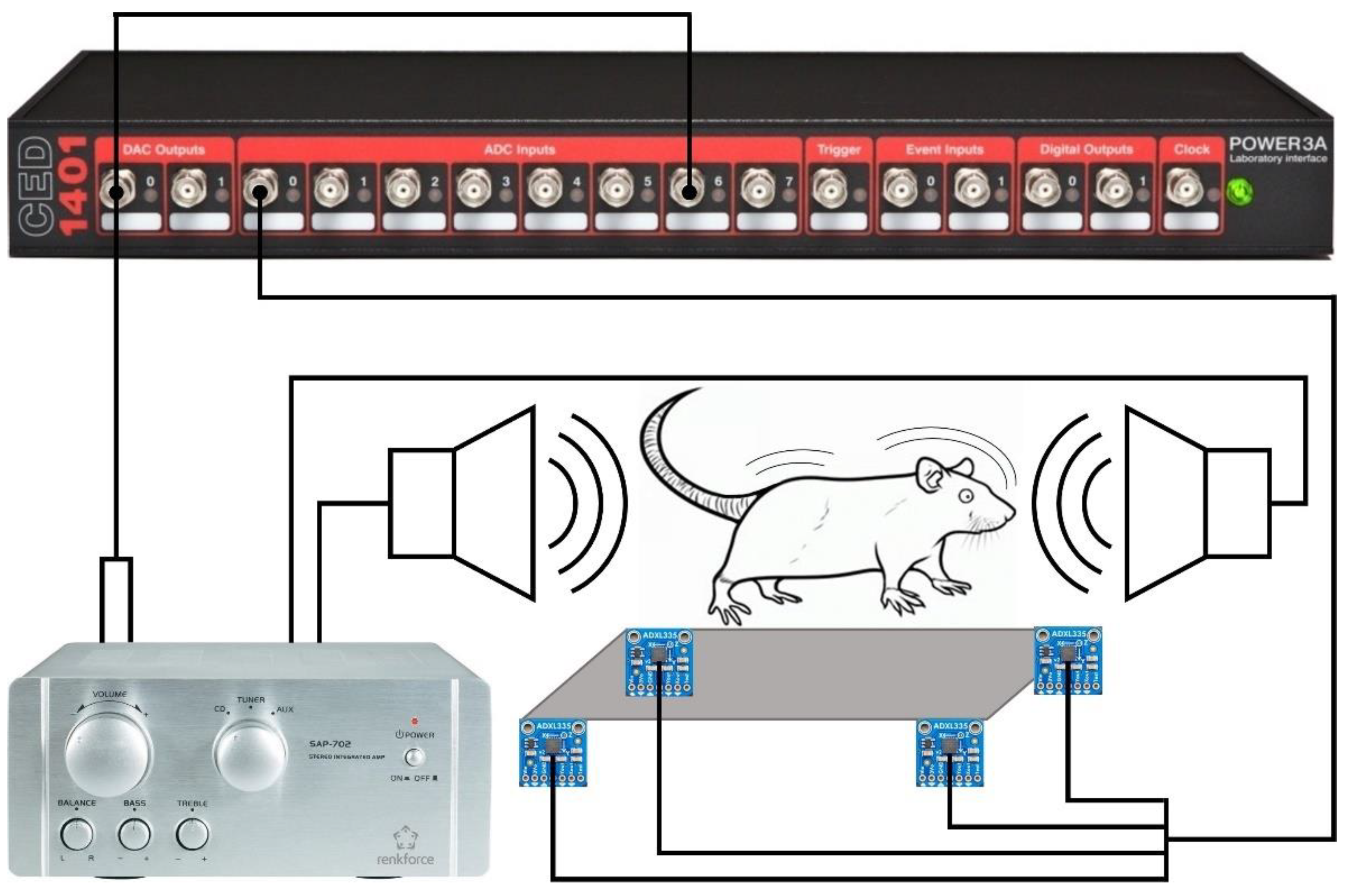
The procedure requires several pieces of equipment, including a data acquisition and control interface CED Power1401 (CED Power1401-3A, Cambridge Electronic Design, Cambridge, UK), a sound amplifier (Renkforce SAP-702, Conrad Electronic SE, Hirschau, Germany), a cage with two loudspeakers installed in the wall to present acoustic stimuli (Speaka Special DL-1152BK4, Conrad Electronic SE, Hirschau, Germany), and a platform with four vibration sensors (ADXL335 accelerometer, Adafruit Industries, New York City, NY, US) to capture the animal's startle response. A block diagram of the system is shown in the
Figure 1. Three channels of the data acquisition and control interface are used for this procedure. The first channel is a digital-analog output channel (DAC output 0, Fig. 1), which controls the acoustic signal on the loudspeakers. The same channel also sends its output to an analog-digital input channel (ADC input 6, Fig. 1) to record the output and to mark the precise moment of signal presentation for further analysis. The third channel (ADC input 0,
Figure 1) receives the signal from the vibration sensors on the platform and obtains information about the animal's movements.
Calibration of the sound volume is necessary to match specific hardware, including sound amplifier settings, loudspeakers, and cage acoustics. The Db2Vol function (
Figure 2) converts custom sound volume settings in decibels to the linear range of 1-100. The function will further convert this value into the amplitude of the output voltage on the digital-analog channel. The variable “volume” is defined as percentage of the maximal output voltage on the given CED Power1401 apparatus that may be changed. During the calibration, measure the actual sound volume inside the cage using a sound level meter (DT-8820, CEM, Shenzhen, China). The calibration steps are as follows:
Assign “volume” directly to the “db” variable as shown in the
Figure 2;
In the Settings dialog box (
Figure 3), set "Background volume" to 100, which sends the maximal possible voltage on the output channel, and run the script. Set the sound amplifier's volume to the lowest level that yields the maximum sound volume necessary for the experiment (e.g., 120 dB) or possible, as measured with a sound level meter;
Set "Background volume" in the range of 1-10 with a step of 1, and in the range of 10-100 with a step of 5 or 10. For each value measure the corresponding sound level in the cage. Plot the sound level as a function of the "Background volume" in the range of 1-100 and make a logarithmic approximation;
Assign the “a” and “b” variables in the “Db2Vol” function (
Figure 2) to the corresponding values of the resulting equation of the logarithmic approximation:
Figure 3.
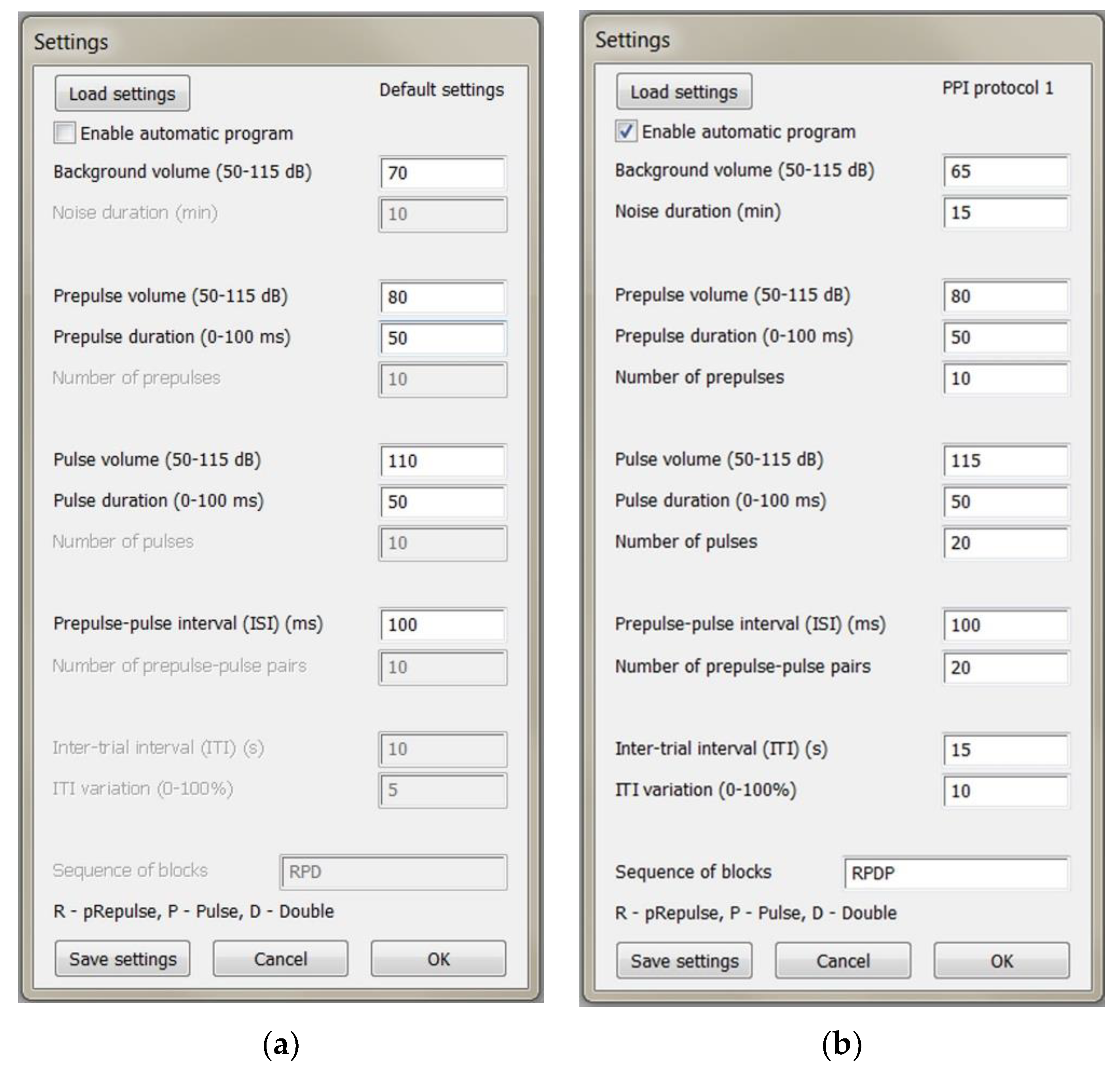
The script interface called in the Spike2 program: (a) Manual mode; (b) Automatic mode.
Figure 3.
The script interface called in the Spike2 program: (a) Manual mode; (b) Automatic mode.
Throughout the experiment and between stimuli, white noise should be present in the background. Pulse signals also contain white noise with a higher amplitude. Signals can be presented either manually by pressing keys on the keyboard or automatically using a predefined program. In manual mode (
Figure 3a), the experimenter can set the volume of the background noise, prepulse and pulse volume and duration, and the duration of the interstimulus interval (ISI) between the prepulse and pulse. The volume range indicated in the interface (
Figure 3) is specific to laboratory conditions and is not strictly limited. The lower limit is determined by environmental noise and can be significantly reduced if an anechoic chamber is used. The upper limit depends on the maximum volume of the loudspeakers. If the volume of the prepulse is set below the level of the background noise, a gap-prepulse protocol can also be implemented [
13]. To evoke each of the three types of signals, the corresponding keys on the keyboard are pressed: “r” for prepulse, “p” for pulse, and “d” for a double stimulus of prepulse and pulse. It is essential that the keyboard layout is set to English, and the current active window is the recording window in the Spike2 program. In addition to these settings, in automatic mode (
Figure 3b), the experimenter can set the duration of habituation with background noise at the beginning of the experiment and the number of stimuli of each of the three types. The intertrial interval (ITI), which is the time between repetitive presentations of signals, together with its variation as a percentage of the initial ITI, can also be customized.
The experimenter has the option to load and save specific protocol settings. The current protocol's name is displayed in the upper-right corner of the Settings dialog. Additionally, a default settings file can be set by including its path at the start of the script.
The custom Spike2 script for the setup is published in open access on GitHub [
15]. It consists of a single PPI.s2s file and does not require any additional files. To add this script to the Spike2 software, it should be opened through the "Scripts" section in the interface.
2.2. An example of using the script in testing WT and DAT-KO rats
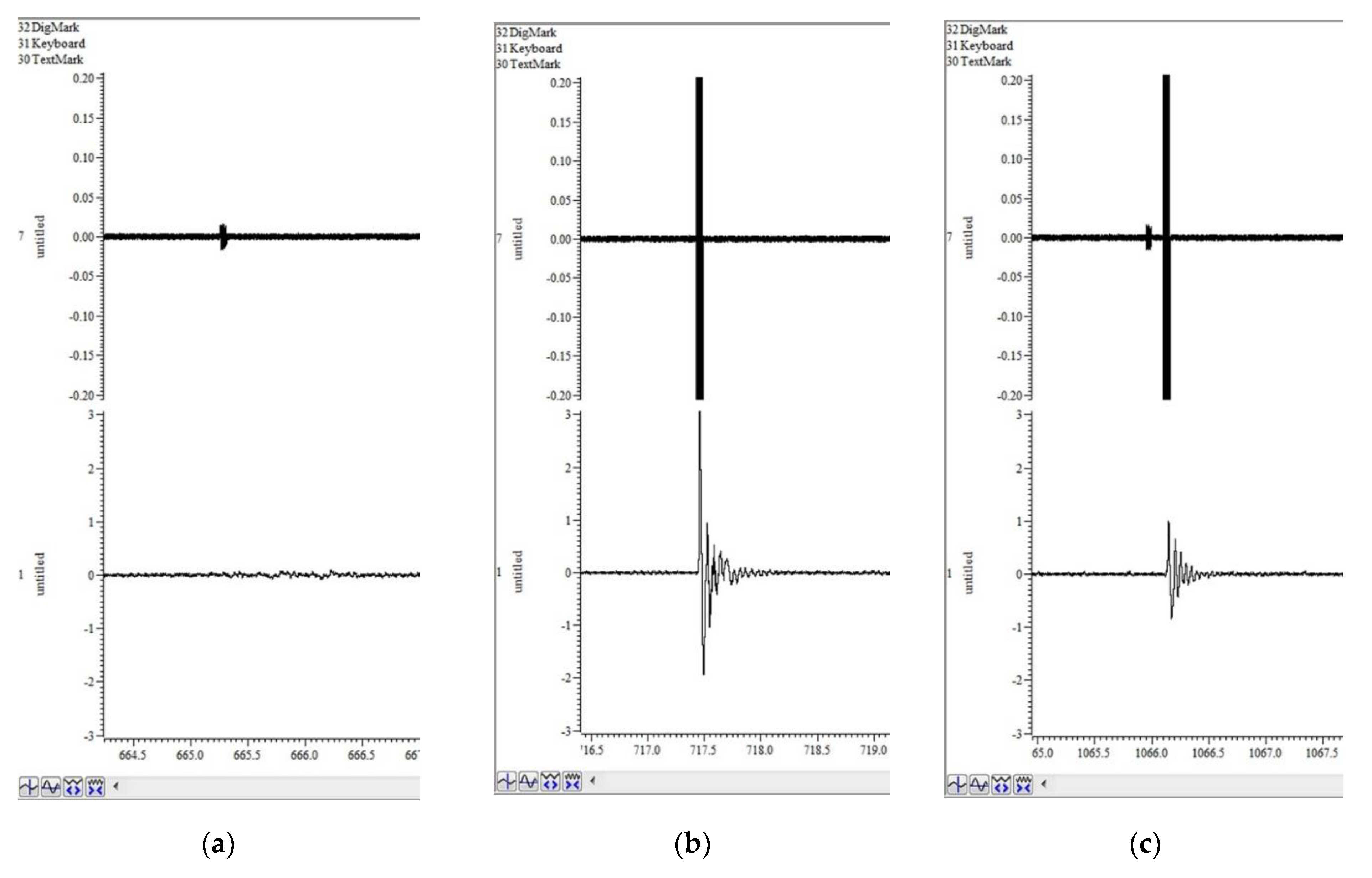
Screenshots of the working window of the Spike2 program can be seen in
Figure 4. The upper channel registers DAC output, which controls the volume of the loudspeakers. It shows the background white noise, as well as pulses of different types, consisting of white noise of greater amplitude. The lower channel is the ADC input from vibration sensors on the platform with the animal. Between pulses, it records the animal's background activity. There is no significant reaction to a single prepulse (
Figure 4a), which means that the amplitude of the prepulse is appropriately chosen.
Figure 4b shows a strong startle response to a single pulse. However, the response to a double pulse is apparently lower (
Figure 4c) and reflects the PPI effect.
The ASR value was taken as the potential difference (in mV) between the minimum and maximum deviations observed no further than 200 ms from the moment of stimulus presentation. To calculate the PPI, the following formula was used:
As an example of the use of the script we developed, a series of experiments using the PPI registration system was conducted in order to assess attention impairment in dopamine knockout (DAT-KO) rats compared with wild-type (WT) rats.
We used 10 WT and 10 DAT-KO female rats of the same age (4 months). The protocol of the animal study was approved by the Ethics Committee for Animal Research of Saint Petersburg State University, St. Petersburg, Russia No. 131-03-10 of 22 November 2021. A day before the experiments, each rat was placed in the apparatus upon presentation of "white noise" with an intensity of 74 dB for 20 minutes to habituate to the experimental conditions. On the day of the experiment, each animal was presented with "white noise" with an intensity of 74 dB for 10 minutes, followed by ten acoustic stimuli with an intensity of 78 dB and a duration of 50 ms (prepulse). The intensity was selected based on the absence of a motor response in animals. This was followed by 20 acoustic stimuli with an intensity of 100 dB and a duration of 50 ms (pulse), causing a pronounced startle response, and 20 combinations of acoustic stimuli (prepulse + pulse) to register changes in the startle amplitude and calculate prepulse inhibition. The interval between prepulse and pulse stimuli was 100 ms. The interval between presentations of stimuli or paired stimuli varied between 10 to 14 s. Signals were recorded from the platform with vibration sensors using the Spike2 program with a CED Power1401 analog-to-digital converter (Cambridge Electronic Design) synchronized with the program for delivering acoustic stimuli.
3. Results
As an example, we measured the PPI in DAT-KO and DAT-WT female rats who lack a dopamine transporter and are characterized by a significant increase in the level of extracellular dopamine in the striatum [
16]. The hyperactivity of these animals allows using them in model experiments for the ADHD syndrome investigations. During the experiments, we encountered difficulties in recording the reactions of DAT-KO rats, which are characterized by hyperactivity and an increased level of motor activity. This made it difficult to distinguish the motor reactions of animals from the background level of spontaneous movements. Mechanical restraint of DAT-KO rats causes significant stress, which could affect the results of the experiment. This problem was solved by installing a video camera synchronized with the recording of the animal's motor reactions and the moments of presentation of sound signals, which made it possible to avoid the superposition of the recorded motor reactions on such behavioral patterns of rats as rapid movements around the cage, grooming, and rearing.
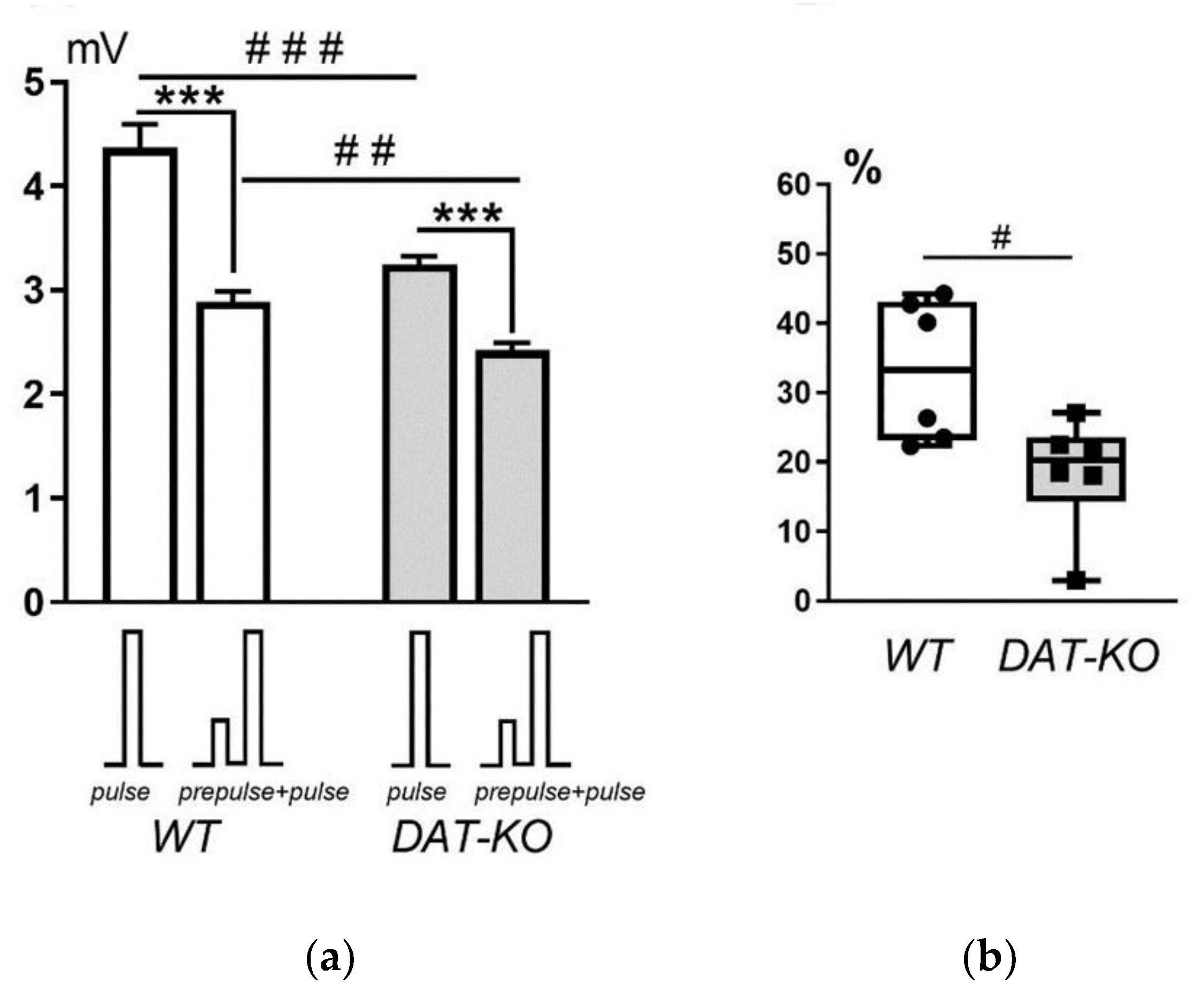
The analysis of the results of testing DAT-KO and WT rats in the PPI setup showed that the amplitude of the startle response (
Figure 5a) in WT animals was significantly greater than that of DAT-KO rats in response to a single sound signal (pulse, 4.4 ± 0.2 mV vs. 3.2 ± 0.1 mV; p < 0.0001) and to the combination of two sound stimuli (prepulse+pulse, 2.9 ± 0.1 mV vs. 2.4 ± 0.1 mV; p = 0.0006). In both groups of animals (WT and DAT-KO), a significant decrease in startle amplitude was observed upon presentation of two sound stimuli compared to the response to a single stimulus (p < 0.0001).
Analysis of the magnitude of PPI showed that this parameter was significantly lower (p < 0.01) in knockout rats compared to the wild-type rats (
Figure 5b). These observations indicate a reduced level of attention in DAT-KO rats, which are a model of ADHD. Therefore, we can conclude that the PPI registration system developed in this study provides a reliable and valid method for assessing attention impairment in rodents.
4. Discussion
The presented experimental setup offers an affordable and straightforward approach for performing experiments with PPI of acoustic startle response. Similarly, to the earlier articles [
9,
10] this study showed high effectiveness and valid output results.
One of the primary advantages of the proposed setup is its low cost. Researchers who already have a CED Power1401 and Spike2 software can perform PPI experiments without any additional investments, thus making the method more accessible to scientists with limited budgets. Furthermore, the custom script can be modified and repurposed for various experimental designs and adapted to suit specific research needs. The custom script also offers flexibility in selecting the acoustic stimuli, volumes, and intervals between the stimuli, enabling researchers to perform more complex and detailed experiments.
Another significant advantage of the setup is the possibility to perform simultaneous electrophysiological recordings during the PPI experiments. This advantage allows researchers to conduct electrophysiological experiments that require high temporal resolution, such as single-unit recordings, local field potentials (LFPs), and electroencephalogram (EEG) recordings, in conjunction with the PPI experiments. Such multimodal recordings enable the correlation of neural activity with behavioral responses, which can provide deeper insights into the underlying neural mechanisms of PPI.
Although the proposed experimental setup requires experience with the Spike2 software and the CED Power1401 interface, it does not require a deep understanding of the Spike2 scripting language. A user-friendly graphical interface enables researchers to perform the experiments without extensive knowledge of the language and automates most of the experimental steps. The Spike2 software also has an in-built synchronization with video tracking, which improves the accuracy of the results and reduces the need for manual intervention.
However, the proposed experimental setup has some limitations that need to be addressed. It relies on the quality and calibration of the existing equipment, including the sound equipment, which may affect the results accuracy and reproducibility. Additionally, while the presented custom script appears is flexible in adjusting various parameters, it lacks some features offered by commercial systems, such as the presentation of pure tones and automatic data analysis. Manual acquisition of the amplitudes of the startle responses may be significantly time-consuming and prone to errors. Moreover, in animals with increased motor activity, spontaneous movements may mask motor reactions in response to auditory stimuli. As a consequence, synchronous video recording of motor activity can be very useful for such control.
Future developments should aim to address the limitations of the proposed setup and enhance its capabilities. One potential improvement would be to develop an algorithm for automatic analysis of the results. Another possible improvement would be to incorporate new types of stimuli, in addition to white noise, and increase flexibility in the number of consecutive presentations. To minimize the impact of spontaneous movements and ensure accurate data acquisition, mechanical constraints should be used to restrict the animal's locomotion without causing excessive stress.
Most behavioral experiments are performed on males, but data obtained on females are also important for understanding the mechanisms of behavioral reactions and for adequate interpretation of drug testing results. According to the results of experiments on mice (males and females), DAT-KO animals demonstrate reduced prepulse inhibition, compared to WT mice [
8]. According to the results of our previous experiments on DAT knockout rat males [
9,
10], ASR in response to single pulses was generally higher than to prepulse+pulse, and PPI in DAT-KO rats male was significantly lower than in WT males. In spite of some differences compared to the data obtained on males [
9,
10], the data obtained in female WT and DAT-KO rats show the same tendency: ASR on single pulse is higher than ASR on prepulse+pulse, and PPI in DAT-KO rats is reduced compared to that in WT.
In summary, the proposed experimental setup offers a cost-effective and flexible approach to performing PPI experiments with acoustic startle response. While the setup has some limitations, it also has great potential for multimodal recordings and complex experimental designs. Future studies should aim at addressing the limitations of the proposed setup and at enhancing its capabilities, which would make the method more accessible and efficient for researchers.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Script for acoustic startle response setup.
Author Contributions
Conceptualization, A.P., N.K. and A.V.; software, A.P.; investigation, A.P., V.Z. and A.V.; resources, V.Z.; writing—original draft preparation, A.P. and A.V.; writing—review and editing, N.K. and A.V..; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation grant number 21-75-20069. V.Z. were supported by the project ID: 94030300 of the St. Petersburg State University, St. Petersburg, Russia.
Institutional Review Board Statement
Ethics committee of Saint Petersburg State University, St. Petersburg, Russia, resolution No. 131-03-10 of 22 November 2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data used in this study are available on request from the corresponding author.
Acknowledgments
Breeding and genotyping of knockout animals were performed at the Research Resource Center “Vivarium” of the Research Park of the Saint Petersburg State University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graham, F.K. The More or Less Startling Effects of Weak Prestimulation. Psychophysiology 1975, 12, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Logothetis, N.K.; Eschenko, O. Phasic Activation of the Locus Coeruleus Attenuates the Acoustic Startle Response by Increasing Cortical Arousal. Sci Rep 2021, 11, 1409. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Nieto, R.; Hormigo, S.; López, D.E. Prepulse Inhibition of the Auditory Startle Reflex Assessment as a Hallmark of Brainstem Sensorimotor Gating Mechanisms. Brain Sci 2020, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, C.; Marsicano, G.; Busquets-Garcia, A. Assessing Prepulse Inhibition of Startle in Mice. Bio Protoc 2018, 8. [Google Scholar] [CrossRef]
- Miller, E.A.; Kastner, D.B.; Grzybowski, M.N.; Dwinell, M.R.; Geurts, A.M.; Frank, L.M. Robust and Replicable Measurement for Prepulse Inhibition of the Acoustic Startle Response. Mol Psychiatry 2021, 26, 1909–1927. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.; Geyer, M.; Braff, D. Neural Circuit Regulation of Prepulse Inhibition of Startle in the Rat: Current Knowledge and Future Challenges. Psychopharmacology (Berl) 2001, 156, 194–215. [Google Scholar] [CrossRef] [PubMed]
- Braff, D.L.; Geyer, M.A.; Swerdlow, N.R. Human Studies of Prepulse Inhibition of Startle: Normal Subjects, Patient Groups, and Pharmacological Studies. Psychopharmacology (Berl) 2001, 156, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Ralph, R.J.; Paulus, M.P.; Fumagalli, F.; Caron, M.G.; Geyer, M.A. Prepulse Inhibition Deficits and Perseverative Motor Patterns in Dopamine Transporter Knock-Out Mice: Differential Effects of D1 and D2 Receptor Antagonists. The Journal of Neuroscience 2001, 21, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Volnova, A.; Kurzina, N.; Belskaya, A.; Gromova, A.; Pelevin, A.; Ptukha, M.; Fesenko, Z.; Ignashchenkova, A.; Gainetdinov, R.R. Noradrenergic Modulation of Learned and Innate Behaviors in Dopamine Transporter Knockout Rats by Guanfacine. Biomedicines 2023, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Ptukha, M.; Fesenko, Z.; Belskaya, A.; Gromova, A.; Pelevin, A.; Kurzina, N.; Gainetdinov, R.R.; Volnova, A. Effects of Atomoxetine on Motor and Cognitive Behaviors and Brain Electrophysiological Activity of Dopamine Transporter Knockout Rats. Biomolecules 2022, 12, 1484. [Google Scholar] [CrossRef]
- Virag, D.; Homolak, J.; Kodvanj, I.; Babic Perhoc, A.; Knezovic, A.; Osmanovic Barilar, J.; Salkovic-Petrisic, M. Repurposing a Digital Kitchen Scale for Neuroscience Research: A Complete Hardware and Software Cookbook for PASTA. Sci Rep 2021, 11, 2963. [Google Scholar] [CrossRef] [PubMed]
- Gerum, R.; Rahlfs, H.; Streb, M.; Krauss, P.; Grimm, J.; Metzner, C.; Tziridis, K.; Günther, M.; Schulze, H.; Kellermann, W.; et al. Open(G)PIAS: An Open-Source Solution for the Construction of a High-Precision Acoustic Startle Response Setup for Tinnitus Screening and Threshold Estimation in Rodents. Front Behav Neurosci 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; Brozoski, T.J.; Bauer, C.A.; Parrish, J.L.; Myers, K.; Hughes, L.F.; Caspary, D.M. Gap Detection Deficits in Rats with Tinnitus: A Potential Novel Screening Tool. Behavioral Neuroscience 2006, 120, 188–195. [Google Scholar] [CrossRef] [PubMed]
- CED Cambridge Electronic Design Limited. Spike2 Scripts. Available online: https://ced.co.uk/downloads/scriptspkcont (accessed on 27 February 2023).
- Pelevin, Arseniy. Spike2-Based Setup for Acoustic Startle Response (ASR) and Prepulse Inhibition (PPI) Protocols. Available online: https://github.com/ArseniyPelevin/ASR-PPI-Spike2 (accessed on 19 April 2023).
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. The Journal of Neuroscience 2018, 38, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).