Preface
Atherosclerosis is a dynamic, chronic and progressive process that involves the vascular bed (coronary and peripheral) [
1,
2]. Risk factors are associated with the progression rate and with the evolution of atherosclerosis. The traditional risk factors include familial genetic pattern, old age, postmenopausal women, gender (males are affected more at a younger age), smoking (all types of tobacco smoking), Type 1 and Type 2 Diabetes Mellitus, hypercholesterolemia, hypertriglyceridemia, hyperuricemia, hyper-Lp (a) levels, hyperhomocyeteinemia, sedentary life style, and unhealthy diet. The non-traditional risk factors include viral infections (CMV, EBV, HIV), bacterial infections (helicobacter pylori), periodontitis, gingivitis, air pollution, mental stress, depression, chronic systemic inflammatory diseases (SLE, RA, Psoriasis), and sleep disorders.
Endothelial dysfunction: The endothelium forms a barrier between blood cells and coagulation proteins, proatherogenic and prothrombotic peptides and the layers covered by the endothelium. The endothelium is responsible to adjust the vascular tone and hemodynamic balance of the vascular system. Endothelial dysfunction plays a key factor in atherosclerosis, caused by lack of local nitric oxide [NO] [
3]. It accompanies hypercholesterolemia, diabetes, hypertension, cigarette smoking – leading to the development of atherosclerosis [
4]. Endothelial dysfunction can be reversed by treating hyperlipidemia and other damaging processes, and the mechanism is believed to be associated with an increase in vascular endogenous NO [
5,
6,
7,
8,
9].
Diabetes: Atherosclerosis and type 2 diabetes mellitus [T2DM] have common pathological pathways, i.e., elevation of pro-inflammatory cytokines [
10]. In a database of 3.5 million self-referred subjects, T2DM was a shown to be a risk equivalent for coronary artery disease (CAD), peripheral artery disease (PAD) and carotid artery stenosis (CAS). Subjects who suffered from T2DM and CAD had an especially robust risk to develop PAD and CAS [
11]. Treating T2DM with Insulin may also increase the vascular inflammatory response with macrophages formation, increased expression of CD36 [
12]. The increased cardiovascular disease (CVD) risk in patients with T2DM is due to accelerated atherosclerosis [
12]. Endothelial dysfunction is a common phenomenon among all diabetic patients without any relation to the degree of retinopathy [
13]. Vascular cell adhesion molecule (VCAM-1) and endothelial selectin adhesion molecule (E-selectin) increase in parallel with the severity of diabetic retinopathy, with a significant increase in markers of inflammation in more severe stages of retinopathy [
14]. Diabetic retinopathy is a risk factor for increased cardiovascular death. Endothelial progenitor cells (EPCs) are inhibited in patients with T2DM. EPCs could serve as a marker for CVD risk [
15]. Micro RNA 423 (miR-423) levels decreased in diabetics with proliferative diabetic retinopathy compared to controls. More than that, it was found that NO was increased in diabetics with severe retinopathy, while VEGF was inhibited. It may suggest an effect of miR-423 on VEGF, with an indirect inhibition of eNOS [
16].
What do we know about peripheral artery disease (PAD)?
The term PAD describes a family of diseases of blood vessels, atherosclerotic disease of peripheral arteries, vasculitis, deep and superficial vein insufficiency and thrombosis.
Lower Leg PAD: PAD is caused by partial or complete blockage of blood supply to the legs [
17]. The most common cause is atherosclerosis, but it may result also secondary to thrombus formation in the blood vessels, emboli or vasculitis. Major cardiovascular events usually accompany PAD, while intermittent claudication and ischemic leg pain jeopardize quality of life [
18].
Aging and PAD: Old age is a significant risk factor to atherosclerosis and CVD. The mechanism is not completely clear, but alteration in mitochondrial function may play a role in this process. Atherosclerosis begins in adulthood with deposition of fatty streaks [
19]. Ankle brachial index (ABI) is the systolic blood pressure measured in the leg divided by the blood pressure in the arm. ABI may discover PAD even at a pre-clinical stage. Using this method estimated that 4% of the healthy subjects under 40 years of age, and 15%-20% of the healthy subjects aged 65 years of age and older have PAD. [
20].
Intermittent claudication and rest pain are the main symptoms of patients with peripheral arterial disease. Pain or a sense of fatigue and discomfort during exercise which is relieved at rest. Because of pain during exercise patients tend to walk slowly and walk to a shorter distance than before [
21,
22]. Patients with PAD have a higher rate of CVD events and limb loss. They have more CAD and cerebrovascular events [
23]. Patients with an abnormal ABI are expected to experience CVD events in the future. Patients with PAD have a 2.5- to 6-fold increased rate pf death from CVD with a mortality rate of 4.3%-4.9% per year. Patients with severe PAD have the highest likelihood to die, and it is estimated that about 25% of the patients with severe PAD die within 1 year. Those who underwent amputation have the highest death rate, as high as 45% within a year [
23,
24,
25].
Aims
The present management: Patients with PAD, especially patients with DM who suffer from below the knee arterial disease, suffer from ischemic leg pain during exercise or at rest, ischemic leg ulcers, and eventually are prone to a major leg amputation and death within the 2-3 years. The regular management today involves life style modification, exercise, medications (anti-inflammatory and anti-platelet / anti-coagulant medications and endovascular intervention with balloon angioplasty, stent insertion or atherectomy. Balloon drug coated angioplasty is now more popular, as well as drug eluting stent intervention, but neither approach has a real beneficial cure for those patients who suffer chronic leg ischemia with below the knee arterial disease.
Our aim is to offer a new approach, the opposite approach that has not been applied so far, and that is the "primum non nocre" approach (first do no harm), using a regenerative stem cells' technology to enhance native collateral blood vessels, to enhance angiogenesis and vasculogenesis. Those stem cells that we will use will be delivered locally proximal to the area of need, and will be secreted over a period of time, with a top-up option to deliver more stem cells in the future periodically as needed.
Current Approach to PAD
A. Medical management
Risk Factor Modification: Statins reduce CVD events. The Heart Protection Study found that lipid-lowering therapy with simvastatin reduced the adverse CVD events, including more than 6700 patients with PAD. Pooled results from 17 trials found that Statins reduced CVD in PAD patients by 26%. Pro-protein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have shown promising preliminary results in reducing CV risk in patients with atherosclerosis and are under investigation in large CV outcomes trials that include patients with symptomatic PAD [
26].
Smoking Cessation: Cigarette smoking increase atherosclerosis. Nonsmokers with PAD have less MI and mortality than those who smoke, and PAD patients who stopped smoking have a 2-fold survival rate in 5 years compared to smokers [
27,
28].
Controlling Diabetes Mellitus (DM): Intense glucose lowering and maintenance of normal glucose level of diabetic patients decrease microangiopathic complications, but most classes of glucose-lowering drugs have not shown a reduction of macro vascular events. In several trials, intensive glucose control versus standard therapy has not reduced ischemic risk associated with increased mortality.
Follow-up of patients who took part in the United Kingdom Prospective Diabetes Study (UKPDS) did find that intensive glucose lowering and maintenance lead to a 15% decrease in MI rate, suggesting a positive glycemic legacy in patients with new onset T2DM and without prior CV events. Recent clinical trials have shown that certain glucose-lowering agents decreased CVD. For example, the EMPA-REG trial empagliflozin decreased all-cause mortality by 32% in diabetic patients. The observations of increased rates of leg and foot amputation in ongoing studies with a related agent, however, indicate caution and warrant further study for limb risk in patients with PAD. The glucagon-like protein (GLP)-1 agonists liragulitide and semaglutide improved macro vascular outcomes in patients with T2DM [
29,
30,
31,
32,
33,
34]. Blood Pressure Control: Treating hypertension reduce stroke events and CVD. In patients with PAD, the intensity of antihypertensive treatment must take into consideration benefits of reduced risk of CV events and the potential to exacerbate limb symptoms. Although several studies have suggested that intensive BP control (versus moderate BP control) reduce CV events in diabetic patients with PAD, data with regard to specific targets are mixed. There are no comparative studies of antihypertensive medications in PAD patients, but findings from several clinical trials show that angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with atherosclerosis, including those with PAD, are effective and beneficial. In the Heart Outcomes Prevention Evaluation study (HOPE), the ACE inhibitor ramipril lowered CV death by 22% (44% of them had PAD) [
35,
36,
37].
Antiplatelet Therapy: Antiplatelet reduce the CVD risk in patients with CAD. The Antithrombotic Trialists' (ATT) Collaboration Meta-analysis of more than 9000 symptomatic PAD patients demonstrated a significant reduction in CVD death with antiplatelet monotherapy. In the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin trial (PEGASUS-TIMI 54 trial), adding ticagrelor 60 mg to aspirin resulted in a 5.2% risk reduction in CVD death. The PRODIGY trial, which investigated prolonged DAPT after coronary stenting, showed consistent benefits in the PAD subgroup [
38,
39,
40,
41,
42].
Anticoagulant Therapy: The Warfarin Antiplatelet Vascular Evaluation trial (WAVE) compared combination antiplatelet and oral anticoagulant therapy with antiplatelet drugs alone in PAD patients. Warfarin (an anticoagulant) didn't decrease CVD death, but there was a greater than threefold increase in life-threatening bleeding. However, using new oral anticoagulants (ruvaroxaban) for patients who underwent revascularization was found to be beneficial, and decreased limb ischemia, amputation rate, incidence of MI, stroke, and death from CVD causes [
43].
Diet: The Prevención con Dieta Mediterránea (PREDIMED), a multicenter primary prevention clinical trial that examined he effect of diet on patients with T2DM. The study found that Mediterranean diet decreased PAD significantly [
44]. Diet is part of the PAD therapy, mainly foods rich in anti-inflammatory and antioxidants [
45]. Other diets showed that that fish and shellfish could be beneficial for diabetic patients with PAD [
46].
Walking: Supervised exercise programs improved functional measures in patients with PAD. A Cochrane review found that supervised exercise was more beneficial and clinically effective than non-supervised exercise. Supervised exercise training is also recommended for patients with PAD by the American heart association [
47].
Exercise therapy for patients with PAD is known since 1966 when 6 months of unsupervised intermittent walking improved walking time (PWT). Clinical trials and meta-analyses support the management of patients with PAD by exercise, which improves functioning and quality of life. Supervised treadmill exercise therapy is the most popular form of training for patients with PAD and claudication. Recent evidence shows that arm ergometry, leg cycling, and resistance training, all of them also improve walking time and functioning of patients with PAD [
48].
Weight reduction: Perivascular adipose tissue (PVAT) is an adipose tissue surrounding blood vessels. It regulates the vascular tone, the intravascular thermoregulation, and vascular smooth muscle cell (VSMC) proliferation. Physical activity improves PVAT function by reducing oxidative stress and the inflammatory state caused by obesity. Diet, which is another non-invasive strategy in weight reduction may reduce weight and reduce inflammation and oxidative stress [
49].
Frailty: Frailty is associated with worse prognosis in patients with PAD undergoing revascularization. Amputation is an important factor leading to high risk Frailty. The risk of amputation and the prognosis after revascularization may be modified by medications, life style modification, diet rich in proteins and antioxidants, and physical activity [
50].
Medications
Anti inflammatory:
Many PAD patients suffer from CAD. High levels of markers of inflammation may suggest that these patients suffer from CAD, and a CVD risk stratifications recommended in these patients [
51].
Anti-inflammatory medications (Canakinumab [anti interleukin 1]) and CAD:
High-sensitivity C-reactive protein (CRP) was decreased in patients who got anti Interleukin 1 antibodies (canakinumab) [
51]. Colchicine reduced CVD events in patients post MI [
52].
IL-6 receptor inhibition (tolicizumab): Interleukin-6 (IL-6) plays a key role in inflammation. A monoclonal antibody against IL-6 receptor (Tolicizumab) improved endothelial function and arterial stiffness, but increased total, HDL, and LDL cholesterol and triglyceride levels [
53].
Statins in PAD: Statins reduced major adverse cardiac events (MACE) and decreased mortality in patients with PAD. In the Incremental Decrease in End Points Through Aggressive Lipid Lowering Study Group (IDEAL trial) high dose atorvastatin was compared with low-moderate-dose intensity (LMI) simvastatin in post MI patients and found that high dose atorvastastin decreased PAD compared with LMI simvastatin. In patients with PAD amputation and mortality rates decreased with statins [
53]. High Intensity (HI) statins improved survival (p=0.004) and decreased MACE (p=0.02) [
54,
55].
Antioxidant therapy: The prevention of progression of arterial disease and diabetes (POPADAD) trial examined the effect of aspirin and antioxidants on PAD progression in diabetic patients with PAD. There was no benefit from each one of them (or combined) on CVD [
56]. A systematic review on antioxidants and PAD found 18 studies that fulfilled the inclusion criteria. 16.6% of the studies used natural antioxidants, and 83.3% used synthetic antioxidants. Natural antioxidants were beneficial in treating PAD, while the synthetic antioxidants were less effective [
57].
The angiotensin-converting enzyme inhibitors (ACE-I): ACEi increase NO production and secretion from endothelial cells. Ramipril improved maximum walking distance by and symptoms of intermittent claudication [
58].
Phosphodiesterase 5 inhibitor (PDE5i):
Viagra that was given to Swedish men post MI or revascularization decreased mortality recurrent MI, heart failure and hospitalizations with heart failure, CVD mortality, and revascularization rate. Male patients with stable CAD who were treated with Viagra decreased overall mortality rate, occurrence of MI, heart failure, and revascularization [
59]. Sildenafil increased blood flow and angiogenesis in ischemic tissues [
60].
Vascular Intervention
Peripheral Artery Surgery: Surgical revascularization improves symptoms in patients with disabling claudication and is indicated in patients who suffer rest pain with critical limb ischemia. A systematic review of 29 studies from 1970 to 2007 that compared 5738 patients who underwent aortobifemoral bypass surgery found an operative mortality rate of 4%, although high-volume centers in the United States report lower mortality rates. Five-year patency rates for aortobifemoral bypass grafts exceed 80% [
61].
Femoral-Popliteal Endovascular Intervention - Atherosclerotic occlusive disease is 3 to 5 times more common in the femoral-popliteal artery than in the iliac artery. The femoral-popliteal arteries are 3 times more likely to be occluded than to be stenotic, a distribution that is reversed in the aortoiliac system. A meta-analysis attempted to compare the 4 different approaches to this disease, i. e., surgical revascularization, vs. conservative / exercise and found that any type of revascularization as well as SET were superior to OMT with respect to walking distance , pain, and claudication [
62,
63].
Drug-Coated Balloons - The 3-year data from ADMIRAL INPACT confirmed the long-term efficacy of DCB therapy. The 3-year patency of DCB versus PTA was 69.5% vs. 45.1% (p = 0.001) and CD-TLR was 84.5% with DCB vs. 70.4% in PTA (p =0.001). At 5 years, DCB treatment had much more favorable results compared to PTA alone [
64,
65].
Grafts (Covered Stents) – The studies VlABAHN Endoprosthesis with Propaten Bioactive Surface versus Bare Nitinol Stent in the Treatment of long lesions in Superficial Femoral Artery Occlusive Disease (VIASTAR) and VlABAHN Endoprosthesis versus Bare Nitinol Stent in the Treatment of Long Lesion [~8 cm] Superficial Femoral Artery Occlusive Disease (VlBRANT) have shown a clinical benfit for covered stents compared to self-expanding BMS in the femoral-popliteal territory [
66]. The covered stent's 1-year patency was 78%, which was superior to BMS (p = 0.009). [
67].
Drug-Eluting Stents - The Zilver pactlotaxel (PTX) drug eluting stent (DES) had better clinical results compared to BMS at 5-years follow with a >40% relative risk reduction for restenosis and revascularization compared to BMS [
68].
Drug-Eluting Stents for below the knee PAD - For patients with lesions smaller than 120 mm, the 1-year restenosis rate for DES 41. 9% compared with 22% for BMS (p = 0.019). Several meta-analyses showed that infrapopliteal DES had better clinical results compared to PTA or BMS with better patency, less interventions, much less amputations, and improved event-free survival [
69,
70].
The Gap in the Therapeutic Landscape
Right now there is no curable treatment, and even the endovascular approach seems to be more like a palliative approach, that is definitely helpful, but time limited.
Advanced Technologies - Stem Cell Therapy
Progenitor cells generated from monocytes or non-monocytes cells have been shown to be beneficial for transplantation and regeneration of damaged blood vessels.
Mesenchymal stem cells are CD105
+ CD90
+ cells capable of differentiation including osteogenic, chondrogenic and adipogenic lineages. The unique characteristics include low immunogenicity, which is an advantage for stem cells' transplantation [
69]. Intramyocardial injections of Exosomes and later on MSC into hearts after myocardial infarction increased cardiac ejection function, decreased infarct size and enhanced neovascularization [
70]. A systemic review found that autologous stem cell transplantation for PAD is safe and effective in patients with chronic leg ischemia. Still, high quality, larger clinical studies should be done to support clinical application of stem cell transplantation for PAD [
71,
72,
73].
Limitations of cell therapy:
High cost because of the special sterility ("clean rooms") needed for human cells transplantation.
Special high quality personnel.
There is always the threat of infections and mutations, so the preparation of cells for transplantation has to be performed in special centers who are dedicated to this procedure and treatment.
Still, more clinical randomized trials are needed in order to prove this approach.
A Novel approach to treat PAD using an endovascular device
The endovascular approach has been shown to be the most advanced and successful one for patients who suffer from end stage CLI, mainly patients with diabetes mellitus who already have ischemic leg ulcers and/or gangrene, planned for amputation.
The main approach today is to invade a narrowed artery and to implant a stent that is dilating the narrowed vessel and regains blood flow the ischemic zone. The main challenge is that in many patients with CLI caused by BKA PAD the arteries are tortuous and the lesions are longer than 6 cm, fibrotic and calcified, and the intervention has high rate of dissections and failure. The long outcome is also not promising for patients with these lesions that tend to block the vessels that were successfully invaded.
We know from a pathophysiologic point of view, that stent insertion causes tear and break of the integrity of the endothelium and endothelial cells are damaged altogether with the middle layer composed mainly of smooth muscle cells.
Our novel idea is to have a different approach, the curative "do no harm" approach, by introducing a device close to the narrowed diseases vessels, but not to invade inside the vessel but to affect it from a near-by location. It will be deployed proximal to the lesion, proximal to the narrowed arteries and proximal to the ischemic zone, where the arterial wall is intact and no harm will be caused to the endothelial layer. The deployment will be gentle and without generating extreme force on the arterial wall, so that no harm will be caused to the endothelial layer and it will stay safe and intact. The endovascular device (a graft, a stent with small spaces between the shafts) will contain mesenchymal stem cells (MSCs) that will be donated by a healthy live young donor from the bone marrow. These cells can be drawn, like in the blood bank, on demand, or can be drawn and stored in a tissue bank (frozen in optimal conditions) and will be used as needed and will be prepared in advance.
MSCs will be stored already within the device, frozen, and will be used as required, delivered to the catheterization/operating room as planned.
The endovascular device will be implanted intra-arterially and will be deployed in a healthy segment of the femoral artery, proximal to the damaged narrowed artery, above the knee. From this location the MSCs will be delivered to the local region and to the narrowed blocked arteries, with a possibility of top-on of MSCs on a monthly basis through a one-way valve that will be echo-lucent.
Keeping a "tissue bank" of MSCs for an immediate delivery MSCs will be drawn from healthy young donors and will be kept frozen at -2700C, and will be prepared in small vials (frozen) [after undergoing all the safety issues of stem cells for human use] and will be kept in a special deep freeze container – as a tissue bank –for later on immediate use as will be needed in the catheterization laboratory or in the operating theater.
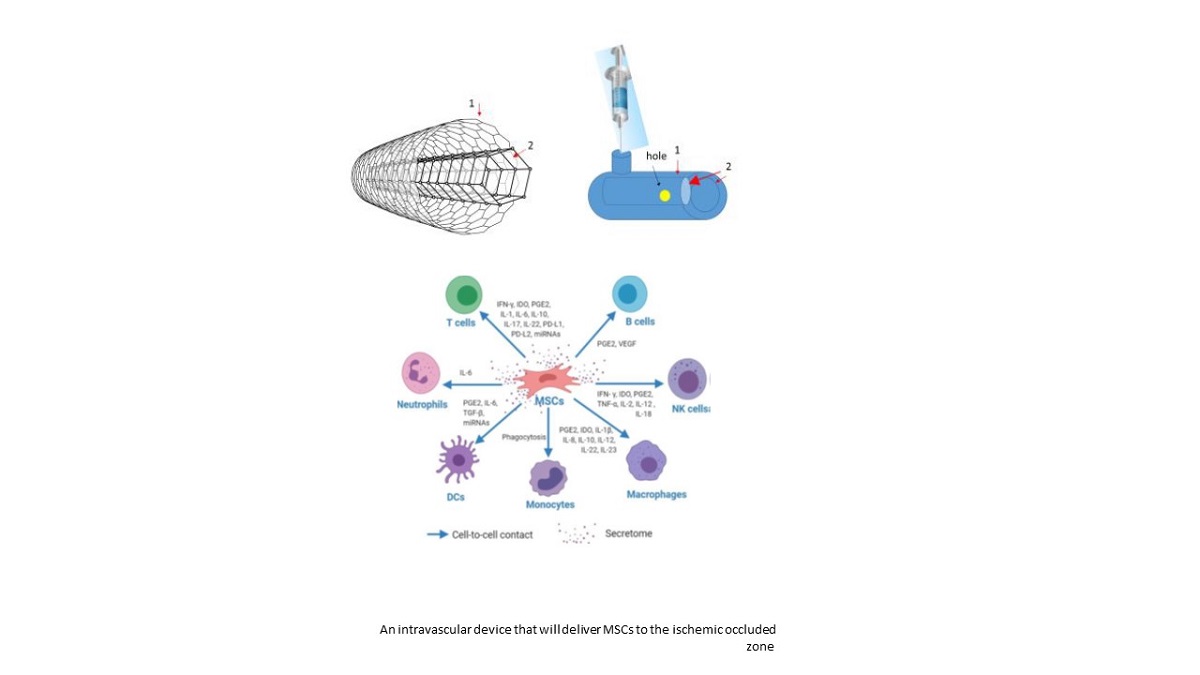
The endovascular device:
The endovascular device will be composed of 2 layers:
An outer layer/membrane (1), with a larger diameter (about 4 mm diameter).
An inner layer (2) that will be in a smaller diameter (about 3 mm diameter) and will be designed like a conus, with the smaller diameter in the proximal part of the conus.
The shaft of both membranes will be composed as a net (shown in the sketch above) with holes that will be smaller than 10 µm, so that mesenchymal stem cells (MSCs) will not be able to run away from the device, and red and white blood cells will not be able to enter it, as well as platelets. The net construction is for the delivery of oxygen to the inner vessel wall by diffusion, without leaking of cells through these holes that will compose the net/shaft of the device (both layers).
The space between the outer membrane/stent and the inner membrane/stent will be able to contain around 50 µL of solution, with 250,000 cells (MSCs).
The cells will be released through the hole (in yellow) in the inner layer (in the inner side). The hole will be in the size that will permit the secretion of 1 cell per second. That will be a slow release mechanism that will allow the secretion of all the cells (250,000) within the container to be released within 3 days.
Another bolus of 250,000 MSCs will be supplied 30 days after the first delivery, and they will be transferred through a syringe that will deliver the cells through the one-way valve, guided by an ultra sound that will demonstrate the orifice of the valve that will be located in the outer surface of the outer membrane (1).
Future directions
PAD is an unsolved enigma in medicine. Millions of people are suffering from this disease on a daily basis at different levels of severity, and many more have PAD in the subclinical stage, that will be developed in the upcoming years. PAD is more common among the elderly population, most of them have diabetes mellitus, most of the patients have other co-morbidities like heart failure, hypertension, renal failure, many also belong to the lower socio economic classes.
The solutions offered today are limited, and offer a life style modifications, that is important but hardly work for the long run, and interventional endovascular solutions, that are more helpful for medium sized arteries of the leg (above the knee), but with a very low yield and success rate for below the knee arterial disease, mainly because the pathophysiology of the below the knee arteries is different – longer than 6 cm tortuous very narrow arteries, most of them are fibrotic and calcified, and the ability to intervene and to open these long chronic fibrotic-calcified lesion is very small and dangerous. More than half of the interventions there are complicated with dissections, and many vascular surgeons choose not to intervene in many of these patients because of the complexity and the danger of complications following any endovascular intervention there.
A local delivery endovascular device will secrete mesenchymal stem cells to the area of interest (occluded or damaged blood vessels), and since the delivery will be local and in proximity to the diseased area, it will have a much more potent and significant effect.
This device has a one-way valve that can be used for top-on mesenchymal stem cells after a few weeks that can be done by a direct injection under US guidance in the out-patient clinic. I suggest to repeat this top-on method every few months in order to promote the regenerative effect, lower inflammation and oxidative stress by inducing endogenous nitric oxide production, and to regenerate the damaged blood vessels and to induce growth of de novo and existing collateral blood vessels.
Our hypothesis is that local endovascular delivery device and repeated periodic top-up of stem cells will cure the local ischemic ulcer and will also fight systemic atherosclerosis. That way, patients at different stages of atherosclerosis will benefit from this procedure, and the mortality rate will decrease (below the 30% death rate in 3 years after a successful intervention in the larger arteries).
We believe this novel approach will be used not just to treat patients at a critical CLI stage, but also as a preventive means for patients with milder forms of CLI and PAD, and will be used as a general management/approach to treat atherosclerosis.
Authors' contributions
I am the sole author.
Funding
I was funded by Abbott Ireland.
Acknowledgments
I would like to thank Mr. Alan Hughes (MSc.) and Mr. Diarmuid Wall (M.Sc.) – both are engineers that work for Abbott, who helped me to build my novel idea and to design the endovascular device.
Ethical Approval
This is a review paper with a novel idea in the end of the review. No ethical approval was needed.
Consent to publish
I agree to publish my MN in the European Journal of Medical Research.
Competing interests
No competing interests.
List of abbreviations
CLI (chronic leg ischemia), PAD (peripheral artery disease), CAD (coronary artery disease), CVD (cardiovascular disease).
References
- Faxon DP, Fuster V, Libby P, et al. Atherosclerotic Vascular Disease Conference: Writing Group III: pathophysiology. Circulation 2004; 109:2617. [CrossRef]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473:317. [CrossRef]
- Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol 2009; 53:323. [CrossRef]
- Anderson TJ, Meredith IT, Charbonneau F, et al. Endothelium-dependent coronary vasomotion relates to the susceptibility of LDL to oxidation in humans. Circulation 1996; 93:1647. [CrossRef]
- Harrison DG, Armstrong ML, Freiman PC, Heistad DD. Restoration of endothelium-dependent relaxation by dietary treatment of atherosclerosis. J Clin Invest 1987; 80:1808. [CrossRef]
- John S, Schlaich M, Langenfeld M, et al. Increased bioavailability of nitric oxide after lipid-lowering therapy in hypercholesterolemic patients: a randomized, placebo-controlled, double-blind study. Circulation 1998; 98:211. [CrossRef]
- Mancini GB, Henry GC, Macaya C, et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study. Circulation 1996; 94:258. [CrossRef]
- Levine GN, Frei B, Koulouris SN, et al. Ascorbic acid reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation 1996; 93:1107. [CrossRef]
- Stein JH, Keevil JG, Wiebe DA, et al. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation 1999; 100:1050. [CrossRef]
- Park YM, R Kashyap S, A Major J, Silverstein RL. Insulin promotes macrophage foam cell formation: potential implications in diabetes-related atherosclerosis. Lab Invest 2012; 92:1171. [CrossRef]
- Newman JD , Rockman CB, Kosiborod M, et al. Diabetes mellitus is a coronary heart disease risk equivalent for peripheral vascular disease. Am Heart J 2017 Feb; 184:114-120. [CrossRef]
- Saely CH, Rein P, Vonbank A, et al. Type 2 diabetes and the progression of visualized atherosclerosis to clinical cardiovascular events. Int J Cardiol 2013; 167:776. [CrossRef]
- Blum A, Socea D, Sirchan R: Vascular responsiveness in type 2 diabetes mellitus. QJM 2016; 109(12): 791-796. [CrossRef]
- Blum A, Pastukh N, Socea D, Jabaly H. Levels of adhesion molecules in peripheral blood correlate with stages of diabetic retinopathy and may serev as bio-markers for microvascular complications. Cytoline 2018; 106: 76-79. [CrossRef]
- Blum A, Socea D, Pastukh N, Jabaly H. Colony forming units – endothelial progenitor cells (CFU-EPCs): a surrogate marker for diabetic retinopathy and high cardiovascular mortality rate. International Journal of Pharma Research & Review 2016; 5(5): 57-62.
- Blum A, Meerson A, Rohana H, Jabaly H, Nahul N, celesh D, Romanenko O, Tamir S. micro-RNA 423 may regulate diabetic vasculopathy. Clin Exp Med 2019; 19(4): 469-477. [CrossRef]
- Criqui MH, Aboyans V: Epidemiology of peripheral artery disease. Circ Res 2015; 116: pp. 1509-1526. [CrossRef]
- Fowkes FG, Rudan D, Rudan I, et. al.: Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet 2013; 382: pp. 1329-1340. [CrossRef]
- Wang JC, Bennett M. Aging and Atherosclerosis. Circulation Research 2012; 111: 245-259. [CrossRef]
- Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral artery disease. Circulation 2005; 112: 2703-2707. [CrossRef]
- Abola MT, Bhatt DL, Duval S, et. al.: REACH Investigators. Fate of individuals with ischemic amputations in the REACH Registry: three-year cardiovascular and limb-related outcomes. Atherosclerosis 2012; 221: pp. 527-535. [CrossRef]
- McDermott MM, Guralnik JM, Criqui MH, et. al.: Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014; 130: pp. 61-68. [CrossRef]
- Howard DP, Banerjee A, Fairhead JF, et. al.: Oxford Vascular Study: population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation 2015; 132: pp. 1805-1815. [CrossRef]
- Mihatov N, Secemsky EA. Peripheral Matters. Peripheral and coronary artery disease: two sides of the same coin. Amerucan College of Cardiology; Cardiology Magazine Sep 20, 2019.
- Fowkes FGR, Low LP, Tuta S, et al. Ankle brachial index and extent of atherothrombosis in 8891 patients with or at risk of vascular disease: results of the international AGATHA study. Eur Heart J 2006; 27(15): 1861-1867. [CrossRef]
- Pande RL, Perlstein TS, Beckman JA, Creager MA: Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011; 124: pp. 17-23. [CrossRef]
- Conen D, Everett BM, Kurth T, et. al.: Smoking, smoking cessation, [corrected] and risk for symptomatic peripheral artery disease in women: a cohort study. Ann Intern Med 2011; 154: pp. 719-726. [CrossRef]
- Alvarez LR, Balibrea JM, Surinach JM, et. al.: FRENA Investigators. Smoking cessation and outcome in stable outpatients with coronary, cerebrovascular, or peripheral artery disease. Eur J Prev Cardiol 2013; 20: pp. 486-495. [CrossRef]
- Inzucchi SE, Bergenstal RM, Buse JB, et. al.: Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: pp. 1364-1379. [CrossRef]
- Bianchi C, Del Prato S: Metabolic memory and individual treatment aims in type 2 diabetes: outcome-lessons learned from large clinical trials. Rev Diabet Stud 2011; 8: pp. 432-440. [CrossRef]
- Zinman B, Wanner C, Lachin JM, et. al.: Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. EMPA-REG OUTCOME Investigators. N Engl J Med 2015; 373: pp. 2117-2128. [CrossRef]
- US Food and Drug Administration: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate. FDA Drug Safety Communication. http://www.fda.gov/Drugs/DrugSafety/ucm500965.htm.
- Marso SP, Daniels GH, Brown-Frandsen K, et. al.: Liraglutide and cardiovascular outcomes in type 2 diabetes. LEADER Steering Committee. N Engl J Med 2016; 375: pp. 311-322. [CrossRef]
- Marso SP, Bain SC, Consoli A, et. al.: SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016; 375: pp. 1834-1844. [CrossRef]
- Bavry AA, Anderson RD, Gong Y, et. al.: Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension 2010; 55: pp. 48-53. [CrossRef]
- Wright JT, Williamson JD, Whelton PK, et. al.: A randomized trial of intensive versus standard blood-pressure control. SPRINT Research Group. N Engl J Med 2015; 373: pp. 2103-2116. [CrossRef]
- Paravastu SC, Mendonca DA, Da Silva A: Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev 2013; CD005508. [CrossRef]
- Baigent C, Blackwell L, Collins R, et. al.: Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Antithrombotic Trialists' (ATT) Collaboration. Lancet 2009; 373: pp. 1849-1860. [CrossRef]
- Fowkes FG, Price JF, Stewart MC, et. al.: Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. Aspirin for Asymptomatic Atherosclerosis Trialists. JAMA 2010; 303: pp. 841-848. [CrossRef]
- Mora S, Ames JM, Manson JE: Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA 2016; 316: pp. 709-710. [CrossRef]
- Hiatt WR, Fowkes FG, Heizer G, et. al.: Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. EUCLID Trial Steering Committee. N Engl J Med 2017; 376: pp. 32-40. [CrossRef]
- Jones WS, Baumgartner I, Hiatt WR, et. al.: Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. International Steering Committee and Investigators of the EUCLID Trial (Examining Use of tiCagreLor In paD). Circulation 2017; 135: pp. 241-250. [CrossRef]
- Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020; 382(21): 1994-2004. [CrossRef]
- Ruiz-Cnela M, Estruch R, Corella D, et al. Association of Mediterranean Diet with Peripheral Artery Disease: the PREDIMED randomized trial. JAMA 2014; 311(4): 415-417. [CrossRef]
- Nosova EV, Conte MS, Grenon SM. Advancing beyond the "Heart Healthy Diet" for peripheral artery disease. J Vasc Surg 2015; 61(1): 265-274. [CrossRef]
- Lilja E, Bergwall S, Sonestedt E, Gottsäter A, Acosta S. The association between dietary intake, lifestyle and incident symptomatic peripheral arterial disease among individuals with diabetes mellitus: insights from the Malmö Diet and Cancer study. Ther Adv Endocrinol Metab. 2019; 10: 2042018819890532. [CrossRef]
- Hamburg NM, Balady GJ. Exercise Rehabilitation in Peripheral Artery Disease: Functional Impact and Mechanisms of Benefits. Circulation. 2011 Jan 4; 123(1): 87–97. [CrossRef]
- Treat-Jacobson D, M. McDermott MM, Bronas UG, et al. Optimal Exercise Programs for Patients With Peripheral Artery Disease A Scientific Statement From the American Heart Association. Circulation. 2019; 139:e10–e33. [CrossRef]
- Stanek A, Brożyna-Tkaczyk K , Myśliński W. The Role of Obesity-Induced Perivascular Adipose Tissue (PVAT) Dysfunction in Vascular Homeostasis. Nutrients 2021 Oct 28; 13(11):3843. [CrossRef]
- Jakubiak GK , Pawlas N, Cieślar G , and Stanek A. Chronic Lower Extremity Ischemia and Its Association with the Frailty Syndrome in Patients with Diabetes. Int J Environ Res Public Health 2020 Dec 14; 17(24):9339. [CrossRef]
- Brevetti G, Giugliano G, Brevetti L, Hiatt WR. Inflammation in peripheral artery disease. Circulation 2010 Nov 2; 122(18):1862-75. [CrossRef]
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017; 377:1119-1131. [CrossRef]
- Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med 2020; 383:1838-1847. [CrossRef]
- Antonopoulos AS, Papanikolaou E, Vogiatzi G, Oikonomou E, Tousoulis D Anti-inflammatory agents in peripheral arterial disease. Curr Opin Pharmacol 2018; 39:1-8. [CrossRef]
- Arya S, Khakharia A, Binney ZO, et al. Association of Statin Dose with Amputation and Survival in Patients with Peripheral Artery Disease. Circulation 2018 3; 137(14):1435-1446. [CrossRef]
- Foley TR, Singh GD, Kokkinidis DG, et al. High-Intensity Statin Therapy Is Associated With Improved Survival in Patients With Peripheral Artery Disease. J Am Heart Assoc 2017 Jul 15; 6(7):e005699. [CrossRef]
- Keramat S , Sharebiani H , Patel M, et al. The Potential Role of Antioxidants in the Treatment of Peripheral Arterial Disease: A Systematic Review. Antioxidants (Basel) 2022 Oct 28; 11(11):2126. [CrossRef]
- Falconer D, Papageorgiou N, Salem K., et al. Nitric oxide donors for peripheral artery disease. Curr Opin Pharmacol 2018; 39: 77-85. [CrossRef]
- Andersson DP, Landucci L, Lagerros YT, et al. Association of Phosphodiesterase-5 Inhibitors Versus Alprostadil With Survival in Men With Coronary Artery Disease. J Am Coll Cardiol 2021 Mar 30;77(12):1535-1550. [CrossRef]
- Nguyen H, Amanullah AM. Therapeutic potentials of phosphodiesterase-5 inhibitors in cardiovascular disease. Rev Cardiovasc Med 2014;15(2):158-67. [CrossRef]
- Burke CR, Henke PK, Hernandez R, et. al.: A contemporary comparison of aortofemoral bypass and aortoiliac stenting in the treatment of aortoiliac occlusive disease. Ann Vasc Surg 2010; 24: pp. 4-13. [CrossRef]
- NordanstigJ, Taft C, Hensater M, et al. Improved quality of life after one year with an invasive versus a non-invasive treatment strategy in claudicants: one yea r results of the IRONIC trial. Circulation 2014;130(12):939-947. [CrossRef]
- Malgor RD, Alahdab F, Elraiyah TA, et al. A systematic review of treatment of intermittent claudication in the lower extremities. J Vase Surg. 2015;61:545-735. [CrossRef]
- Jones WS, Schmit KM , Vemulapalli S, et al. Treatment Strategies for Patients with Peripheral Artery Disease. Comparative Effectiveness Review No. 118. (Prepa red by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I.) AHRQ Publication No. 13-EHC090-EF; 2013. Available at www.effectivehealthcare.
- Tepe G, Schnorr B, Albrecht T, et al. Angioplasty of femoralpopliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial.jACC Cardiovasc lnterv. 2015;8: 102-108. [CrossRef]
- Katsanos K, Spiliopou los S, Kitrou P, et al. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a stematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e0 11245. [CrossRef]
- Lammer J, Zeller T, Hausegger KA , et al. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease).] Am Coll Cardiol. 2013;62:1320-1327. [CrossRef]
- Uhl C, Dadras A, Reichmann F, et al. Long-term results of the heparin-bonded Viabahn stent graft in femoropopliteal TASC C and D lesions with a covered stent length of minimum 25 cm. Vascular 2019;27(5):553-559. [CrossRef]
- Siablis D, Kitrou P, Spiliopoulos S, Katsanos K, Karnabatidis D. Paclitaxel-coated balloon angioplasty versus dug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the !DEAS randomized controll ed trial. JACC Cardiovase lnte 2014; 7: 1048-1056. [CrossRef]
- Katsanos K, Spiliopou los S, Diamantopoulos A, et al. Systematic review of infrapopliteal drug-eluting stents: a meta-analysis of randomized controlled trials. Cardiovase ln tervent Radio. 2013; 36: 645-658. [CrossRef]
- Blum A, Balkan W, Hare JM. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis 2012; 223(2): 269-77. [CrossRef]
- Huang P , Wang L , Li Q. , et al. Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Res Ther. 2019 Oct 10; 10(1):300. [CrossRef]
- Baocheng Xie , Houlong Luo , Yusheng Zhang, et al. Autologous Stem Cell Therapy in Critical Limb Ischemia: A Meta-Analysis of Randomized Controlled Trials. Stem Cells Int. 2018 May 24; 2018:7528464. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).