Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
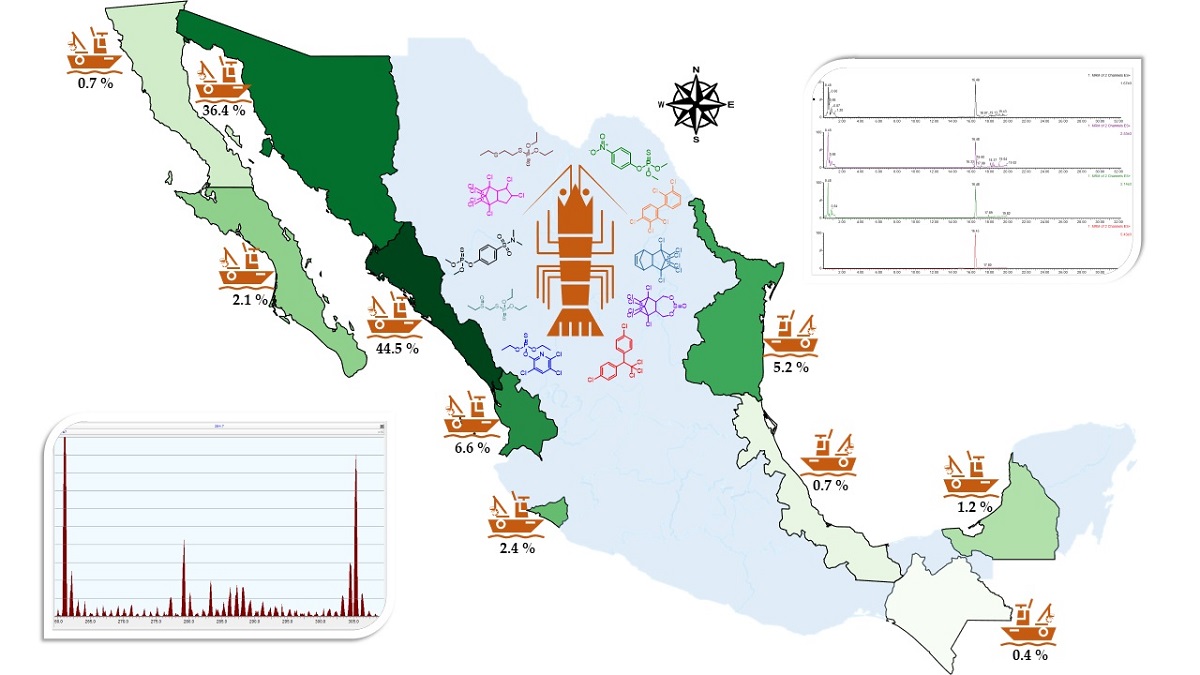
Keywords:
1. Introduction
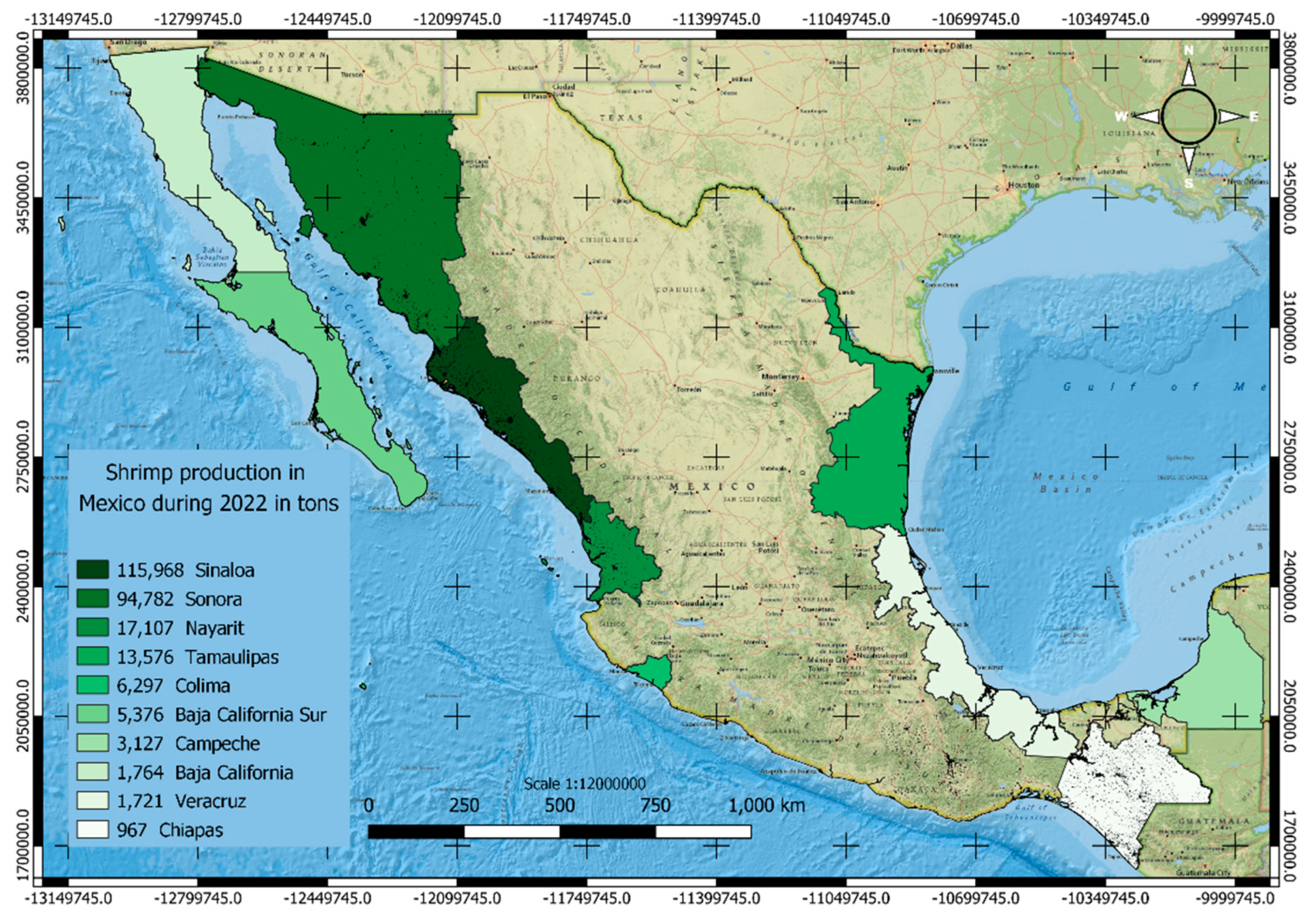
1.1. Shrimp farming in Mexico an Overview
2. Pesticides
3. Pesticide Studies in Shrimp in Mexico
3.2. Pesticides in the Gulf of Mexico
4. Methods of Extraction and Identification of Pesticides in Shrimp in Studies Reported in Mexico
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAO Yearbook of Fishery and Aquaculture Statistics 2007; FAO, 2009. Available online: https://www.fao.org/documents/card/es/c/974997d3-409e-556b-808d-9f0c9f1d3022/ (accessed on 22 January 2023).
- OECD Review of Fisheries. OECD publishing. 2017. Available online: https://one.oecd.org/document/TAD/FI.
- Vázquez-Vera, L.; Chávez-Carreño, P. Diagnóstico de la acuacultura en México; Fondo Mexicano para la Conservación de la Naturaleza, A.C: México, 2022. Available online: https://www.researchgate.net/profile/Leonardo-Vazquez-Vera/publication/361250469_Diagnostico_de_la_acuacultura_en_Mexico/links/62a63e6f55273755ebe7acfd/Diagnostico-de-la-acuacultura-en-Mexico.pdf.
- Comisión Nacional de Acuacultura y Pesca (CONAPESCA). Anuario Estadístico de Acuacultura y Pesca 2020. 2020. Available online: https://nube.conapesca.gob.mx/sites/cona/dgppe/2020/ANUARIO_ESTADISTICO_DE_ACUACULTURA_Y_PESCA_2020.pdf (accessed on 22 January 2023).
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Panorama Agroalimentario 2022. 2022. Available online: https://www.gob.mx/siap/acciones-y-programas/panorama-agroalimentario-258035 (accessed on 15 January 2023).
- Instituto Nacional de Pesca. Acuacultura Camarón blanco del Pacífico. 2018. Available online: https://www.gob.mx/inapesca/acciones-y-programas/acuacultura-camaron-blanco-del-pacifico. (accessed on 28 April 2023).
- Chávez Sánchez, M.C.; Higuera Ciapara, I. Manual de buenas prácticas de producción acuícola de camarón para la inocuidad alimentaria. Centro de Investigación en Alimentación y Desarrollo, A.C: Unidad Mazatlán en Acuicultura y Manejo Ambiental y el Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria, 2003. https://cesasin.mx/wp-content/uploads/2017/12/Cam-Manual-de-buenas-practicas-de-producción-acuicola-de-camarón-para-la-inocuidad-alimentaria.pdf.
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. En Natural Remedies for Pest, Disease and Weed Control; Elsevier, 2020; pp 29–42. [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Q&A on Pests and Pesticide Management. Food and Agriculture Organization of the United Nations, 2021. Available online: https://www.fao.org/newsroom/detail/Q-A-on-Pests-and-Pesticide-Management/en (accessed on 15 January 2023).
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18(3), 1112. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6(2), 48–60. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Pesticides use, pesticides trade and pesticides indicators; FAO, 2022. [CrossRef]
- Hayes, T.B.; Hansen, M.; Kapuscinski, A.R.; Locke, K.A.; Barnosky, A. From silent spring to silent night: Agrochemicals and the anthropocene. Elem Sci Anth. 2017, 5. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I. H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of endocrine disruptor pesticides: a review. Int. J. Environ. Res. Public Health. 2011, 8(6), 2265–2303. [Google Scholar] [CrossRef] [PubMed]
- Bernal-González, K.G. , Covantes-Rosales, C.E., Camacho-Pérez, M.R., Mercado-Salgado, U., Barajas-Carrillo, V.W., Girón-Pérez, D.A.,... & Girón-Pérez, M.I. Organophosphate-Pesticide-Mediated Immune Response Modulation in Invertebrates and Vertebrates. Int. J. Mol. Sci.. 2023, 24(6), 5360. [CrossRef]
- Botello, A.V.; Rueda-Quintana, L.; Díaz-González, G.; Toledo, A. Persistent Organochlorine Pesticides (POPs) in Coastal Lagoons of the Subtropical Mexican Pacific. Bull. Environ. Contam. Toxicol. 2000, 64(3), 390–397. [Google Scholar] [CrossRef]
- Galindo-Reyes, J.G.; Fossato, V.U.; Villagrana-Lizarraga, C.; Dolci, F. Pesticides in Water, Sediments, and Shrimp from a Coastal Lagoon off the Gulf of California. Mar. Pollut. Bull. 38(9), 837–841. [CrossRef]
- Rueda, L.; Botello, A.V.; Diaz, G. Presencia de plaguicidas organoclorados en dos sistemas lagunares del estado de Chiapas, México. Rev. Int. de Contam. Ambient. 1997, 13, 55–61. [Google Scholar]
- Bamber, S.; Rundberget, J.T.; Kringstad, A.; Bechmann, R.K. Effects of simulated environmental discharges of the salmon lice pesticides deltamethrin and azamethiphos on the swimming behaviour and survival of adult Northern shrimp (Pandalus borealis). Aquatic Toxicology 2021, 240, 105966. [Google Scholar] [CrossRef]
- Lewchenko, V. , Hariharan, P. J.. Quantum chemical calculations for understanding and predicting toxicity. II. The phosphorylation step in the inhibition of ache by organophosphorus anticholinesterases. Int J Quantum Chem. 1982, 22(S9), 275–280. [Google Scholar] [CrossRef]
- Mena, F.; González-Ortegón, E.; Solano, K.; Araújo, C.V. The effect of the insecticide diazinon on the osmoregulation and the avoidance response of the white leg shrimp (Penaeus vannamei) is salinity dependent. Ecotoxicol. Environ. Saf 2020, 206, 111364. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.P.; Sanaye, S.V.; Shyama, S.; Sreepada, R.A.; Dake, A.S. Effects of salinity and temperature on the acute toxicity of the pesticides, dimethoate and chlorpyrifos in post-larvae and juveniles of the whiteleg shrimp. Aquaculture Reports 2020, 16, 100240. [Google Scholar] [CrossRef]
- Mostafalou, S.; Mohammad, A. The link of organophosphorus pesticides with neurodegenerative and neurodevelopmental diseases based on evidence and mechanisms. Toxicology 409. 2018, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.T. L.; Escalona, R.L. Organochlorine residues in organisms of two different lagoons of Northwest Mexico. Bull. Environ. Contam. Toxicol. 1983, 30(1), 456–463. [Google Scholar] [CrossRef] [PubMed]
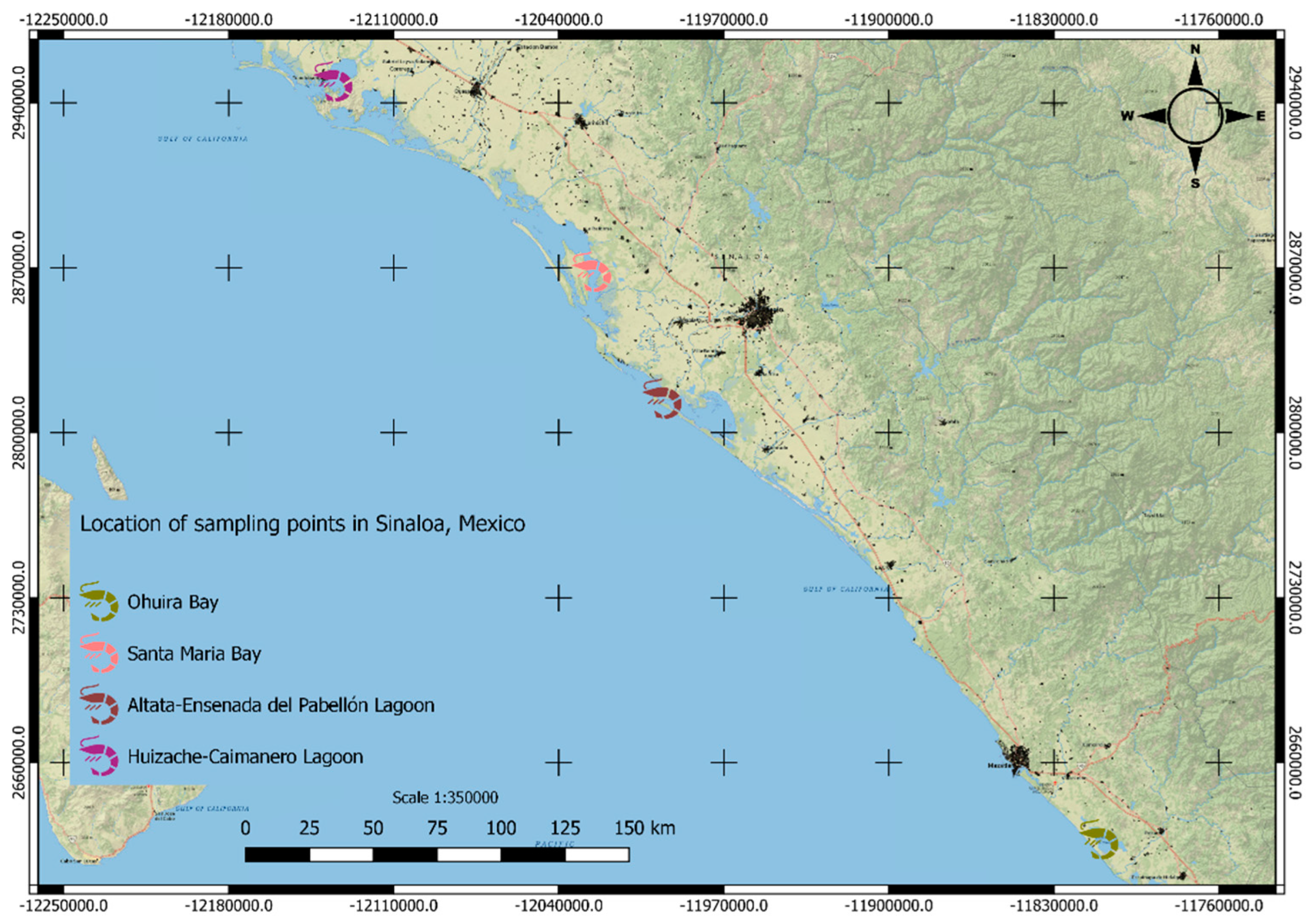
- Galindo-Reyes, J.G.; Guerrero Ibarra, M.A.; Villagrana Lizárraga, C.; Quezada Urenda, L.G. Contaminación por plaguicidas en agua, sedimentos, camarón y almeja, de dos ecosistemas costeros de Sinaloa, México. Trop. Ecol. 1992, 33, 172–180. [Google Scholar]
- Galindo-Reyes, J.G.; Villagrana L., C.; Alvarez, G.L. Environmental Conditions and Pesticide Pollution of Two Coastal Ecosystems in the Gulf of California, Mexico. Ecotoxicol. Environ. Saf. 1999, 44(3), 280–286. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Gonzalez-Farias, F.; Villeneuve, J.P.; Cattini, C.; Hernandez-Garza, M.; Mee, L.D.; Fowler, S.W. Distribution, Fate and Effects of Pesticide Residues in Tropical Coastal Lagoons of Northwestern Mexico. Environ Technol. 2002, 23(11), 1257–1270. [Google Scholar] [CrossRef]
- Osuna-Flores, I.; Riva, M.C. Organochlorine Pesticide Residue Concentrations in Shrimps, Sediments, and Surface Water from Bay of Ohuira, Topolobampo, Sinaloa, Mexico. Bull. Environ. Contam. Toxicol. 2002, 68 (4), 532–539. [CrossRef]
- Osuna-Flores, I.; Riva, M.C. Plaguicidas organofosforados en camarones, sedimento y agua superficial de la Bahía de Ohuira, Topolobampo, Sinaloa, México. Afinidad 2004, 61, 387–392. [Google Scholar]
- Burgos-Hernández, A.; Zapién, M.G. L.; Madrid, M.L. A.; Sifuentes, C.O. G.; Gil, C.I. M.; Burgos, E.C. R.; Olivas, R.R. Presence of insecticides in shrimp farms adjacent to the sea of cortes: detection, quantification, and toxicity testing. Eur. Food Res. Technol. 2006, 222 (3-4), 380–384. [CrossRef]
- Robledo-Marenco, M.L.; Botello, A.V.; Romero-Banuelos, C.A.; Diaz-Gonzalez, G. Presence of persistent organochlorine pesticides in estuaries of the subtropical Mexican Pacific. Int. J. Environ. Pollut. 2006, 26 (1/2/3), 284. [CrossRef]
- Bozada, R.L. M.; Berajano, G.F. Los contaminantes orgánicos persistentes en el istmo mexicano 2006, Mexico: International POPs Elimination Network (IPEN). https://www.rapam.org/wp-content/uploads/2015/12/Los-Contaminantes-Istmo-Opti.pdf.
- Gold-Bouchot, G.; Silva-Herrera, T.; Zapata-Pérez, O. Chlorinated pesticides in the Rio Palizada, Campeche, Mexico. Mar. Pollut. Bull. 1993, 26(11), 648–650. [Google Scholar] [CrossRef]
- Vidal-Martínez, V.M.; Aguirre-Macedo, M.L.; Del Rio-Rodríguez, R.; Gold-Bouchot, G.; Rendón-von Osten, J.; Miranda-Rosas, G.A. The pink shrimp Farfantepenaeus duorarum, its symbionts and helminths as bioindicators of chemical pollution in Campeche Sound, Mexico. J. Helminthol. 2006, 80(2), 159–174. [Google Scholar] [CrossRef]
- Maia, M.L.; Delerue-Matos, C.; Calhau, C.; Domingues, V.F. Validation and Evaluation of Selected Organic Pollutants in Shrimp and Seawater Samples from the NW Portuguese Coast. Molecules 2021, 26(19), 5774. [Google Scholar] [CrossRef] [PubMed]

| Pesticide | Concentration (ng g-1) | Shrimp | Location | Reference |
| HCH | 1 | P. stylirostris (without head and shell) | Huizache-Caimanero Lagoon, Sinaloa (Jan/ 1981) | [25] |
| heptachlor epoxide | 0.34 | |||
| dieldrin | 1.06 | |||
| DDT | 0.55 | |||
| HCH | 0.6 | P. vannamei (without head and shell) | Huizache-Caimanero Lagoon, Sinaloa (Oct/ 1980) | |
| aldrin | 0.68 | |||
| heptachlor epoxide | 1.1 | |||
| dieldrin | 1.88 | |||
| endrin | 0.21 | |||
| DDT | 0.33 | |||
| HCB | 9 | P. vannamei | Altata-Ensenada del Pabellón Lagoon, Sinaloa (1989) | [28] |
| p,p´-DDE | 1.1 | |||
| dieldrin | 1.1 | |||
| endosulfan sulfate | 3 | |||
| aroclor-1254 | 2.7 | |||
| HCB | 0.1 | Altata-Ensenada del Pabellón Lagoon, Sinaloa (1991) | ||
| β-HCH | 1.3 | |||
| γ-HCH | 0.08 | |||
| α- endosulfan | 0.7 | |||
| p,p´-DDE | 6 | |||
| dieldrin | 1 | |||
| p,p´-DDT | 0.5 | |||
| aroclor-1254 | 1.3 | |||
| DDMU | 0.1 | |||
| heptachlor | 19.6 | P. vannamei | Altata-Ensenada del Pabellón Lagoon, Sinaloa (Dec/ 1997) | [18] |
| endosulfan sulfate | 127.5 | |||
| DDE | 20.5 | |||
| aldrin | 2.6 | P. vannamei | Altata-Ensenada del Pabellón Lagoon, Sinaloa (Sep/ 1998) | |
| α endosulfan | 1.07 | |||
| endosulfan sulfate | 1.9 | |||
| heptachlor | 70.6 | P. vannamei | Santa Maria Bay, Sinaloa (Dec/ 1997) | |
| dieldrin | 15.6 | |||
| endrin | 45.6 | |||
| endosulfan sulfate | 106.8 | |||
| DDE | 2.1 | P. vannamei | Santa Maria Bay, Sinaloa (Sep/ 1998) | |
| endosulfan | 11.67 | |||
| γ-HCH | BDL-132.0 | Penaeus sp | Ohuira Bay, Sinaloa | [29] |
| δ-HCH | 48.8-127.0 | |||
| heptachlor | 18.0-127.0 | |||
| heptachlor epoxide | BDL-58 | |||
| α- endosulfan | 47.0-2000.5 | |||
| DDE | 19.0-29.0 | |||
| α- endosulfan | 210.01 | P. vannamei | Ohuira Bay, Sinaloa. Station 2(Sep/ 1996) | [27] |
| DDE | 29.03 | |||
| heptachlor | 126.04 | P. vannamei | Ohuira Bay, Sinaloa. Station 4 (Sep/ 1996) | |
| α- endosulfan | 200.44 | |||
| DDE | 19 | |||
| γ-HCH | 125.03 | P. vannamei | Ohuira Bay, Sinaloa. Station 1 (Nov/ 1996) | |
| heptachlor | 121.04 | P. vannamei | Ohuira Bay, Sinaloa. Station 2 (Nov/ 1996) | |
| α- endosulfan | 57.93 | |||
| γ-HCH | 48.02 | P. vannamei | Ohuira Bay, Sinaloa. Station 5 (Nov/ 1996) | |
| heptachlor | 107.03 | |||
| α- endosulfan | 194.14 | |||
| heptachlor | 17.05 | P. vannamei | Ohuira Bay, Sinaloa. Station 1 (Jan/ 1997) | |
| chlordane | 112.51 | P. vannamei | Ohuira Bay, Sinaloa. Station 3 (Jan/ 1997) | |
| γ-HCH | 132.05 | P. vannamei | Ohuira Bay, Sinaloa. Station 3 (Jan/ 1997) | |
| heptachlor | 95.06 | |||
| α- endosulfan | 59.73 | |||
| HCH | 0.23 | P. stylirostris (without head and shell) | Moroncarit Lagoon, Sonora (Jul/ 1980) | [25] |
| heptachlor epoxide | 0.67 | |||
| dieldrin | 3.87 | |||
| DDT | 2 | |||
| HCH | 1.71 | P. vannamei (without head and shell) | Moroncarit Lagoon, Sonora (Jul/ 1981) | |
| aldrin | 0.68 | |||
| heptachlor epoxide | 1.1 | |||
| dieldrin | 1.88 | |||
| endrin | 0.21 | |||
| DDT | 1.33 | |||
| HCH | 0.11 | P. stylirostris (without head and shell) | Yavaros Lagoon, Sonora (Oct/ 1980) | |
| heptachlor epoxide | 0.21 | |||
| dieldrin | 3.87 | |||
| DDT | 0.85 | |||
| HCH | 0.46 | P. stylirostris (without head and shell) | Yavaros Lagoon, Sonora (Oct/ 1981) | |
| heptachlor epoxide | 0.89 | |||
| dieldrin | 0.62 | |||
| endrin | 0.4 | |||
| DDT | 0.8 | |||
| HCH | 0.78 | T. s. pacificus (without head and shell) | Yavaros Lagoon, Sonora (Oct/ 1981) | |
| heptachlor epoxide | 1.24 | |||
| dieldrin | 1.06 | |||
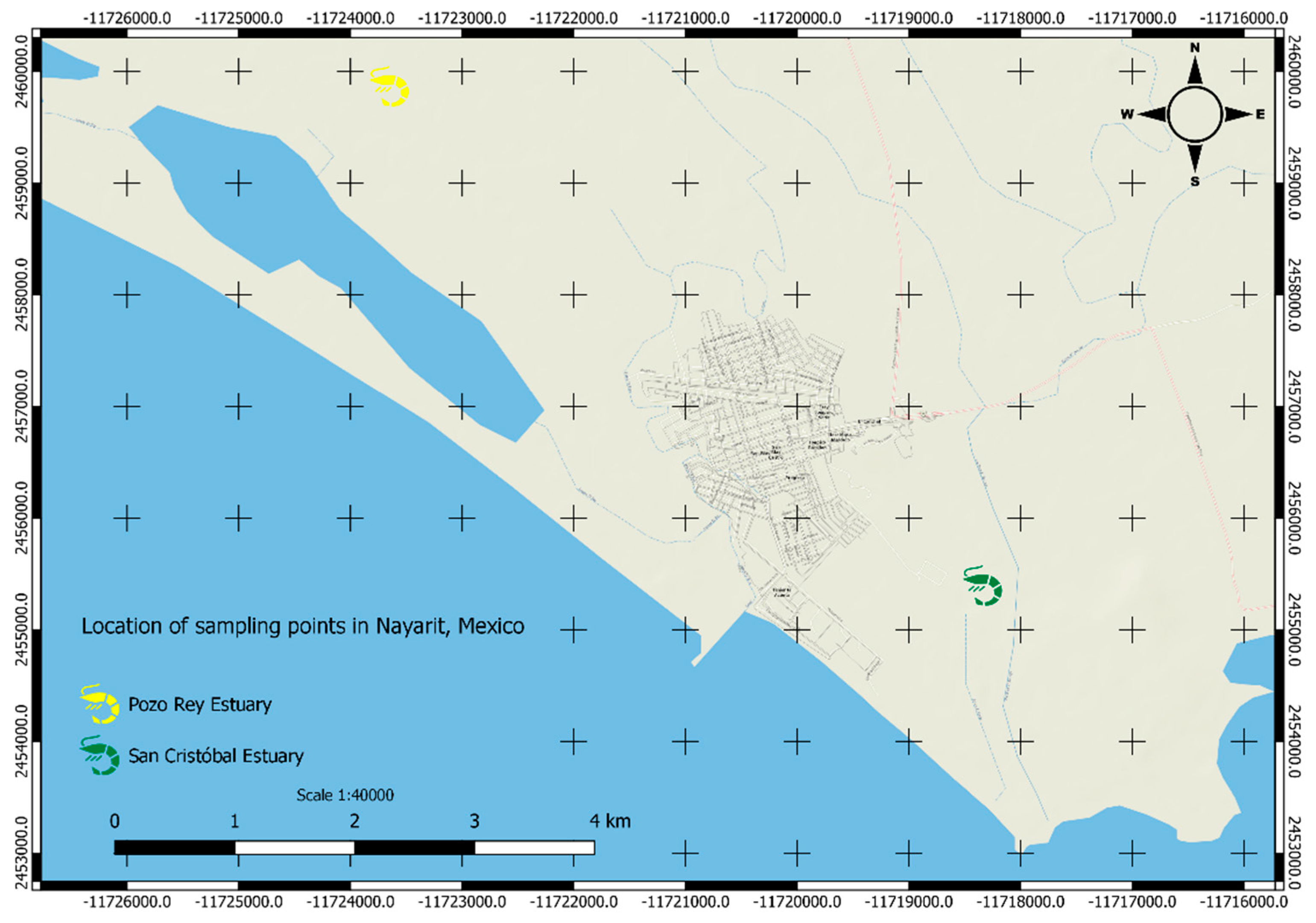
| α - HCH | 1 | Penaeus sp | Pozo Rey Estuary, Nayarit ( 1996-1997) | [32] |
| β-HCH | 2.9 -7.9 | |||
| δ-HCH | 15.37 | |||
| aldrin | 0.9 | |||
| heptachlor epoxide | 18.89 | |||
| α- endosulfan | 9.54-13.33 | |||
| p,p´-DDE | 0.98 | |||
| dieldrin | 1.11 | |||
| endrin | 9.24-12.25 | |||
| β- endosulfan | 8.14 | |||
| p,p´-DDD | 0.98- 25.45 | |||
| endrin aldehyde | 2.45 | |||
| endosulfan sulfate | 1.70- 8.99 | |||
| p,p´-DDT | 3.02 | |||
| α - HCH | 0.14 | Penaeus sp | San Cristobal Estuary, Nayarit ( 1996-1997) | |
| β -HCH | 9.0-33.49 | |||
| δ-HCH | 0.7-13.49 | |||
| aldrin | 4.05 | |||
| α- endosulfan | 3 | |||
| p,p´-DDD | 0.84-22.76 | |||
| endrin aldehyde | 0.57 | |||
| DDT | 1.53 | |||
| γ-HCH | 0.2 | P. vannamei | Sta. Rosa 1. Oaxaca | [33] |
| α- HCH | 0.57 | Sta. Rosa 2. Oaxaca | ||
| γ-HCH | 1.54 | |||
| δ-HCH | 0.61 | |||
| heptachlor | 0.2 | |||
| endosulfan sulfate | 1.8 | |||
| α- HCH | 1.5 | Jaltepec 1. Oaxaca | ||
| γ-HCH | 6.71 | |||
| δ-HCH | 2.77 | |||
| dieldrin | 0.49 | |||
| endosulfan sulfate | 2.27 | |||
| α - HCH | 0.89 | Jaltepec 2 A. Oaxaca | ||
| γ-HCH | 2.11 | |||
| δ-HCH | 1.98 | |||
| heptachlor | 1.83 | |||
| α- HCH | 1.44 | Jaltepec 2 B. Oaxaca | ||
| γ-HCH | 4.64 | |||
| heptachlor | 5 | P. vannamei (tissue) | Carreteras–Pereira Lagoon, Chiapas | [17] |
| p,p´-DDE | 2 | |||
| HCH | 2 | P. vannamei (exoskeleton) | ||
| p,p´-DDE | 0.5 | |||
| δ-HCH | 2.18 | P. vannamei (exoskeleton) | Chantuto-Panzacola Lagoon, Chiapas (Feb/ 1995) | [19] |
| heptachlor | 3.3 | |||
| p,p´-DDE | 15.94 | |||
| heptachlor | 4.66 | P. vannamei (tissue) | Laguna Carretas- Pereyra Lagoon, Chiapas (Apr/ 1994) | |
| p,p´-DDE | 1.97 | |||
| δ-HCH | 1.54 | P. vannamei (exoskeleton) | ||
| p,p´-DDE | 0.55 |
| Pesticide | Concentration (ng g-1) | Shrimp | Location | Reference |
| methyl parathion | 98.62 | P. vannamei | Ohuira Bay, Sinaloa (Sep/ 1996) | [27] |
| methyl parathion | 113.81 | P. vannamei | Ohuira Bay, Sinaloa (Jan/ 1997) | |
| methyl parathion | 103 | P. vannamei | Ohuira Bay, Sinaloa (Oct/ 1997) | |
| chlorpyrifos | 100-400 | Penaeus sp | Ohuira Bay, Sinaloa | [30] |
| disulfoton | BDL-900 | |||
| phorate sulfoxide | BDL-400 | |||
| famfur | BDL-300 | |||
| chlorpyrifos | 13 | Penaeus sp | Atanasia-Santo Domingo Estuarie, Sonora | [31] |
| Sample | Analyte | Area (location) | Extraction | Detection | Reference | ||||
| Extraction type | Solvent | Clean-up | Type of chromatography | Column | Detector | ||||
| Shrimp (Penaeus vannamei and Penaeus stylirostris) whole (without head and exoskeleton) | OC | Lagoon systems in northwestern Mexico | Soxhlet | Hexane | 1st deactivated alumina > Hexane 2nd Deactivated silica > Hexane + 10% diethyl ether |
GC | Chromosorb W/HP 80/100, 8% DC-200 y 6% QF-I | ECD 63Ni | [25] |
| OC | Teacapan Estuary Sinaloa (south) | Dry 60-65 °C Anhydrous sodium sulfate milling Soxhlet |
Hexane | 1st alumina, 2nd silica-gel and metallic copper | GC | 4% OV-101 phase packed glass columns (2 mm x 2 m) | ECD | [26] | |
| Shrimp (Penaeus vannamei) | OC and OP | Santa María Bay and Ensenada de Pabellon, | Dry 40-45 °C Soxhlet |
Hexane | Silica gel/aluminum oxide/fluorisil/anhydrous sulfate column | GC | Restek-5x (fused silica, 30 m x 0.32 mm) | FID> OP ECD> OC |
[18] |
| Shrimp (Penaeus vannamei) | Pesticides | Ohuira Bay, California gulf, Sinaloa, (northwest) | Freeze-drying Soxhlet |
Hexane | Silica gel/aluminum oxide/florisil column | [27] | |||
| Shrimp (Peneaus spp) | OC | Ohuira Bay | Dry Soxhlet |
Hexane | Alumina column/ silica gel deactivated/ sodium sulfate | GC | Restek-5x (fused silica, 30 m x 0.32 mm) | ECD | [29] |
| Shrimp (peneaus vannamei) | OC and OP | Altata-Ensenada de Pabellon Sinaloa Lagoons, México | Soxhlet | Hexane | Inactivated Florisil | GC | Capillary column | ECD> OC FPD, NPD > OP Confirmation> MS |
[28] |
| Shrimp (Peneaus spp) | OP | Ohuira Bay | Dry (60-65 °C) Mortar grinding Soxhlet |
Hexane | Alumina column, silica/sodium sulfate/silica gel | GC | Restek RTX-5. 5 % diphenyl-95% dimethylpolysiloxane (30 m x 0.25 mm) | FID | [30] |
| Aquaculture shrimps | OC | Laguna La Atanasia-Santo Domingo, Cajeme, Sonora, Mexico | Dry (60-65 °C) Soxhlet |
Hexane | Silica gel/aluminum oxide/fluorisyl/anhydrous sodium sulfate column | GC | Durabon 608 (30 mm × 0.32 mm) | ECD | [31] |
| OC | Estuarine system (San Cristóbal and Pozo-Rey) San Blas coast, Nayarit, Mexico | Dry (60 °C) Mortar grinding Soxhlet |
Hexane | Florisil column and anhydrous sodium sulfate | GC | Capillary column SPB-5 (30 m × 0.25 mm x 0.25 μm) | ECD 63Ni | [32] | |
| Shrimp (Penaeus setiferus). | OC | Palizada River, Campeche, Mexico (Gulf of Mexico) |
Soxhlet (8h) Kuderna-Danish concentrator Freeze-drying |
Hexane | Florisil column | GC | Capillary column SE-54 (30 m×200 μm) | ECD | [34] |
| Shrimp (Farfantepenaeus duorarum) | OC | Campeche, Mexico (Gulf of Mexico) | Soxhlet (8h) Kuderna-Danish concentrator |
Hexane/Dichloromethane | Column chromatography with silica gel and alumina. Size exclusion column. | GC | ND | ECD | [35] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





