Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Citizen Science for the monitoring of pufferfish species
2.1.1. Organization of the monitoring activities
2.1.2. Project dissemination
2.2. Pufferfish specimens’ collection and identification
2.2.1. Specimens’ collection
2.2.2. Specimens’ identification
2.3. Collection of records of S. pachygaster in the Mediterranean and Italian waters
2.4. TTXs detection
3. Results and Discussion
3.1. Citizen science for monitoring the presence of pufferfish species in the Strait of the Sicily (Lampedusa Island)
3.2. Occurrence of S. pachygaster in the Mediterranean and Italian waters: data from our specimens’ collection and literature.
3.2.1. Overall specimens’ number and date of catch
3.2.2. Specimens’ total length and body weight
3.2.3. Additional information on catch areas and fishing method
3.3. TTXs detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tonon, L. A. C.; de Azevedo, G. P.; Monteiro, A. F.; Bernardi, D. I.; Gubiani, J. R., Ioca, L. P.; Mattsson, H.K.; Moreira, A. P. B.; Gomes, A. F.; Pires, O. R. jr.; Pedrosa, C. S. G.; Souza, L. R.Q.; Rehen. S. K.; Thompson, C. C.; Thompson, F. L.; Berlinck, R. G. New tetrodotoxin analogs in Brazilian pufferfishes tissues and microbiome. Chemosphere 2020, 242, 125211. [CrossRef]
- Guardone, L.; Gasperetti, L.; Maneschi, A.; Ricci, E.; Susini, F.; Guidi, A.; Armani, A. Toxic invasive pufferfish (Tetraodontidae family) along Italian coasts: Assessment of an emerging public health risk. Food Control 2018,91, 330-338. [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A. R.; Campàs, M.; Ozogul, F. An updated review of tetrodotoxin and its peculiarities. Mar. drugs 2022, 20(1), 47. [CrossRef]
- Fernández-Ortega, J. F.; Morales-de los Santos, J. M.; Herrera-Gutiérrez, M. E.; Fernández-Sánchez, V.; Loureo, P. R.; Rancaño, A. A.; Téllez-Andrade, A. Seafood intoxication by tetrodotoxin: First case in Europe. J Emerg. Med. 2010; 39(5), 612-617. [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin–distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. drugs 2008, 6(2), 220-242. [CrossRef]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6(2), 693-755. [CrossRef]
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916. [CrossRef]
- Gao, W.; Kanahara, Y.; Yamada, M.; Tatsuno, R.; Yoshikawa, H., Doi, H. et al. Contrasting toxin selectivity between the marine pufferfish Takifugu pardalis and the freshwater pufferfish Pao suvattii. Toxins 2019, 11(8), 470. [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on the risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues inmarine bivalves and gastropods. EFSA J. 2017, 15, 4752.
- Cembella, A.D.; Durán-Riveroll, L.M. Chapter One—Marine guanidinium neurotoxins: Biogenic origins and interactions, biosynthesis and pharmacology. In Advances in Neurotoxicology; Novelli, A., Fernández-Sánchez, M.T., Aschner, M., Costa, L.G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–47. [CrossRef]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A global retrospective study on human cases of tetrodotoxin (TTX) poisoning after seafood consumption. Food Rev. Int. 2020, 36(7), 645-667. [CrossRef]
- Gao, Y.; Yu, R. C.; Murray, S. A.; Chen, J. H.; Kang, Z. J.; Zhang, Q. C.; Kong, F.Z.; Zhou, M. J. High specificity of a quantitative PCR assay targeting a saxitoxin gene for monitoring toxic algae associated with paralytic shellfish toxins in the Yellow Sea. Appl. Environ. Microbiol. 2015, 81(20), 6973-6981. [CrossRef]
- Cusick, K.D.; Sayler, G.S. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [CrossRef]
- Raposo, M. I.; Gomes, M. T. S.; Botelho, M. J.; Rudnitskaya, A. Paralytic shellfish toxins (PST)-transforming enzymes: A review. Toxins 2020, 12(5), 344. [CrossRef]
- Denac, H.; Mevissen, M.; Scholtysik, G. Structure, Function and Pharmacology of Voltage-gated Sodium Channels. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362(6), 453–479. [CrossRef]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.E.; Etheridge, S.M.; Deeds, J.R.; Van Dolah, F.M.; Leighfield, T.A.; Zou, Y.; Beaudry, C.G.; Benner, R.A.; Rogers, P.L.; Scott, P.S.; Kawabata, K.; Wolny, J.L.; Steidinger, K.A. Saxitoxin puffer fish poisoning in the United States, with the first report of Pyrodinium bahamense as the putative toxin source. Environ. Health Perspect 2006 114, 1502–1507. [CrossRef]
- Abbott, J. P.; Flewelling, L. J.; Landsberg, J. H. Saxitoxin monitoring in three species of Florida puffer fish.Harmful Algae 2009, 8(2), 343-348. [CrossRef]
- udi, M.; Abdelmouleh, A., Jamoussi, K., Kammoun, A.; El Feki, A. Hematological toxicity associated with tissue extract from poisonous fish Lagocephalus lagocephalus—Influence on erythrocyte function in wistar rats. J Food Sci. 2008, 73(7), H155-H159. [CrossRef]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi; A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54(1), 50-55. [CrossRef]
- Reverté, L.; De La Iglesia, P.; Del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847. [CrossRef]
- Bane, V.; Brosnan, B., Barnes, P.; Lehane, M.; Furey, A. High-resolution mass spectrometry analysis of tetrodotoxin (TTX) and its analogues in puffer fish and shellfish. Food Addit. Contam. Part A 2016, 33(9), 1468-1489. [CrossRef]
- Kırımer, N.; Göger, F.; Kürkçüoğlu, M.; Özbek, E. Ö.; Coban, B.; Balcıoğlu, E. B.; Öztürk, B.; Güven, K. C.. Tetrodotoxin and fatty acids contents of Lagocephalus sceleratus (Gmelin, 1789) collected in Antalya, Turkey, by MS/MS and GC/MS analyses. J. Black Sea/Medit. Environ 2016, 22(3), 278-288.
- Kosker, A. R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J. M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food chem. 2016, 210, 332-337. [CrossRef]
- Acar, C.; Ishizaki, S.; Nagashima, Y. Toxicity of the Lessepsian pufferfish Lagocephalus sceleratus from eastern Mediterranean coasts of Turkey and species identification by rapid PCR amplification. Eur. Food Res.Technol. 2017, 243, 49-57. [CrossRef]
- Alabssawy, A. N. Antimicrobial activity of Tetrodotoxin extracted from liver, skin and muscles of puffer fish, Lagocephalus sceleratus inhabiting Mediterranean Sea, Egypt. Int. J. Cancer Biomed. Res. 2017, 1(1), 2-10. [CrossRef]
- Rambla-Alegre, M.; Reverté, L.; Del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Elliott, C. T.; Maillaude, C.; Boundy, M. J.; Harwoodf, D. T.; et al. Evaluation of Tetrodotoxins in Puffer Fish Caught along the Mediterranean Coast of Spain. Toxin Profile of Lagocephalus Sceleratus. Environ. Res. 2017, 158, 1–6. [CrossRef]
- Kosker, A. R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Šimat, V.; Özogul, Y. First report on TTX levels of the yellow spotted pufferfish (Torquigener flavimaculosus) in the Mediterranean Sea. Toxicon 2018, 148, 101-106. [CrossRef]
- Leonardo, S.; Kiparissis, S.; Rambla-Alegre, M.; Almarza, S.; Roque, A., Andree, K. B.; Christidis, A.; Flores, C.; Caixach, J., Campbell, K. et al. Detection of tetrodotoxins in juvenile pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the North Aegean Sea (Greece) by an electrochemical magnetic bead-based immunosensing tool. Food chemistry 2019, 290, 255-262. [CrossRef]
- Kosker, A. R.; Özogul, F., Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J. M.; Özogul, Y. Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean sea. Chemosphere 2019, 219, 95-99. [CrossRef]
- Akbora, H.D.; Kunter, ˙I.; Erçetin, T.; Elagöz, A.M.; Çiçek, B.A. Determination of tetrodotoxin (TTX) levels in various tissues of the silver cheeked puffer fish (Lagocephalus sceleratus (Gmelin, 1789)) in Northern Cyprus Sea (Eastern Mediterranean). Toxicon 2020, 175, 1–6. [CrossRef]
- Ujević, I.; Roje-Busatto, R.; Dragičević, B.; Dulčić, J. Tetrodotoxin in invasive silver-cheeked toadfish lagocephalus sceleratus (Gmelin, 1789) in the Adriatic Sea. The Montenegrin Adriatic Coast: Mar. Chem. Poll. 2021, 141-149. [CrossRef]
- Christidis, G.; Mandalakis, M.; Anastasiou, T. I.; Tserpes, G.; Peristeraki, P.; Somarakis, S. Keeping Lagocephalus sceleratus off the Table: Sources of Variation in the Quantity of TTX, TTX Analogues, and Risk of Tetrodotoxication. Toxins 2021, 13(12), 896. [CrossRef]
- Farrag, M. M. Toxicity pattern of pufferfish Lagocephalus sceleratus (Gmelin, 1789), Mediterranean Sea, Egypt: Awareness and food safety. Aqua. Aquar. Conserv. Legis. 2022, 15(2), 941-962.
- Hassoun, A. E. R.; Ujević, I.; Jemaa, S.; Roje-Busatto, R.; Mahfouz, C.; Fakhri, M.; Nazlić, N. Concentrations of tetrodotoxin (TTX) and its analogue 4, 9-anhydro TTX in different tissues of the silver-cheeked pufferfish (Lagocephalus sceleratus, Gmelin, 1789) caught in the South-Eastern Mediterranean Sea, Lebanon. Toxins 2022, 14(2), 123. [CrossRef]
- Ulman, A.; Tekin, H.; Aysal, A. I.; Akbora. H.D.; Çiçek, B. A. Contribution to puffer toxicity: TTX analysis of three pufferfish species in the eastern mediterranean. In Abstract and Proceeding book of 2nd International symposium on Pufferfish/Lionfish. Turan, C. (Eds), Published by Natural and Engineering Sciences, Iskenderun, Turkey, 20-22 May 2022, pp 11.
- Zenetos A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; Garcia Raso, J.; Cinar, M.; Almogi-Labin, A.; Ates, A.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13(2), 328–352. [CrossRef]
- Ragonese, S.; Morara, U. Scientific note evidence of short-term discard mortality of trawled Sphoeroides pachygaster (Osteichthyes, Tetraodontidae) off the southern coast of Sicily (Central Mediterranean Sea). Pan-Am. J. Aquat. Sci. 2012, 7, 73-76.
- Antonelli, P.; Salerno, B.; Bordin, P.; Peruzzo, A.; Orsini, M.; Arcangeli, G.; Barco, L.; Losasso, C. Tetrodotoxin in live bivalve mollusks from Europe: Is it to be considered an emerging concern for food safety? Compr. Rev. Food Sci. and Food Saf., 2022 21(1), 719-737. [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First detection of tetrodotoxin and high levels of paralytic shellfish poisoning toxins in shellfish from Sicily (Italy) by three different analytical methods. Chemosphere 2019, 215, 881–895. [CrossRef]
- Poursanidis, D.; Zenetos, A. The role played by citizen scientists in monitoring marine alien species in Greece. Cah. Biol. Mar. 2013, 54, 419-426.
- Vohland, K.; Land-Zandstra, A.; Ceccaroni, L.; Lemmens, R.; Perelló, J.; Ponti, M.; Samson, R.; Wagenknecht, K. The Science of Citizen science. Gewerbestrasse: Springer 2021. [CrossRef]
- Directorate-General for Maritime Affairs and Fisheries, 2022. Dealing with a wicked guest, with some EU help. https://oceans-and-fisheries.ec.europa.eu/news/dealing-wicked-guest-some-eu-help-2022-06-02_enISPRA, 2016. Attenzione al pesce palla maculato è tossico e non va mangiato! https://www.isprambiente.gov.it/files/comunicati-stampa/2015/Locandina_pesce_palla.pdf.
- Guidi, A.; Armani, A.; Guardone, L.; Guarducci, M.; Gasperetti, L.; Susini, F.; Longo, M.; Maneschi, A., Dadovich, N. Climate change and food safety: Citizen science for monitoring the presence of toxic alien fish species in Italian waters. J. Bio. Res. 2016, (Italy), 89(1S), 13.
- Amante, C. and B. W. Eakins, ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis. NOAA Technical Memorandum NESDIS NGDC-24, 19 pp, March 2009.
- Boyer, Tim P.; García, Hernán E.; Locarnini, Ricardo A.; Zweng, Melissa M.; Mishonov, Alexey V.; Reagan, James R.; Weathers, Katharine A.; Baranova, Olga K.; Paver, Christopher R.; Seidov, Dan; Smolyar, Igor V. (2018). World Ocean Atlas 2018. [indicate subset used]. NOAA National Centers for Environmental Information. Dataset. https://www.ncei.noaa.gov/archive/accession/NCEI-WOA18. Accessed [5 may 2023].
- Malloggi, C.; Tinacci, L.; Giusti, A.; Galli F. Dall'Ara, S.; Marconi, P.; Gasperetti, L.; Armani, A. Accidental discovery of a Tetraodontidae (Sphoeroides marmoratus) within a cuttlefish (Sepia officinalis) bought in a fish shop in Italy: Risk assessment associated with the presence of Tetrodotoxin- Ital. J. Food. Saf. 2023, Accepted in press.
- Cammilleri, G.; Graci, S.; Buscemi, M. D.; Collura, R.; Costa, A.; Dico, G. M. L., Cumbo, V. New report of Anikasis larvae from Blunthead Puffer, Sphoeroides pachygaster caught off Strait of Sicily. Biodivers. J. 2019, 10(4), 475-478. [CrossRef]
- Carlucci, R.; Mentino, D.; Semeraro, D.; Ricci, P.; Sion, L.; Scillitani, G. Comparative histochemical analysis of intestinal glycoconjugates in the blunthead pufferfish Sphoeroides pachygaster and grey triggerfish Balistes capriscus (Teleostei: Tetraodontiformes). J. Fish Biol. 2019, 94(1), 122-131. [CrossRef]
- Ragheb, E.; Rizkalla, S. I; Analyses of the non-target catch from the Egyptian Mediterranean trawlers, off Port Said. Egypt. J. Aquat. Res. 2019, 45(3), 239-246. [CrossRef]
- Vella, A.; Vella, N.; Karakulak, F. S.; Oray, I. DNA barcoding of Tetraodontidae species from the Mediterranean Sea: Filling knowledge gaps for improved taxonomic accuracy. Genet. Aquat. Org. 2017, 1(2), 61-69. [CrossRef]
- Gerovasileiou, V., Akel, E. S. H. K., Akyol, O. K. A. N.; Alongi, G.; Azevedo, F.; Babali, N et al. New New Mediterranean Biodiversity Records (July 2017). Mediterr. Mar. Sci. 2017, 18/2, 355-384. [CrossRef]
- Hussein, K. B.; Bensahla-Talet, L.; Chakouri, A. A review on the occurrence of the Blunthead puffer, Sphoeroides pachygaster (Müller & Troschel, 1848) in the Mediterranean with a new occurrence from Oran Bay (Western Algeria). Aquat. Scie. Eng. 2021,36(2), 89-94. [CrossRef]
- Akbora, H. D.; Snape, R.; Deniz, A. Y. A. S.; Çiçek, B. A. The first substantiated record of blunthead puffer Sphoeroides pachygaster (Müller and Troschel, 1848), from the coast of northern Cyprus (eastern Mediterranean). Mar. Sci. Tech. Bull. 2021, 10(1): 1-7. [CrossRef]
- Kizilkaya, İ. T.; Akyol, O. Additional Record of Sphoeroides pachygaster (Tetraodontidae) in the Aegean Sea (Fethiye, Turkey). COMU-JMSF 2020, 3(2), 136-139. [CrossRef]
- Gazzetta del Sud online- Messina. Available online: https://messina.gazzettadelsud.it/foto/cronaca/2021/08/20/pesce-palla-velenoso-ritrovato-gia-morto-nelle-acque-dello-stretto-foto-cc52a465-ea70-456c-bade-f04180224512/2/ (Accessed 5 may 2023).
- Karakuş, M.; İsmail Dal, Coşkun M. A.The first record of age findings of sphoeroides pachygaster (muller and troschel, 1848) in the eastern mediterranean sea. In Abstract and Proceeding book of 2nd International symposium on Pufferfish/Lionfish. Turan, C. (Eds), Published by Natural and Engineering Sciences, Iskenderun, Turkey, 20-22 May2022., pp 12.
- Pinto, E. P.; Rodrigues, S. M.; Gouveia, N.; Timóteo, V.; Costa, P. R. Tetrodotoxin and saxitoxin in two native species of puffer fish, Sphoeroides marmoratus and Lagocephalus lagocephalus, from NE Atlantic Ocean (Madeira Island, Portugal). Mar. Environ. Res. 2019, 151, 104780. [CrossRef]
- Boundy, M. J.; Selwood, A. I.; Harwood, D. T.; McNabb, P. S.; Turner, A. D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1-12. [CrossRef]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the Pufferfish Toxin. Tetrodotoxin in European Bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [CrossRef]
- Eitzel, M.V.; Cappadonna, J.L.; Santos-Lang, C.; Duerr, R.E.; Virapongse, A.;West, S.E.; Kyba CC, M.; Bowser, A.; Cooper, C.B.;Sforzi, A.; et al. Citizen Science Terminology Matters: Exploring Key Terms. Citiz. Sci. Theory Pract. 2017, 2, 1–20. [CrossRef]
- European Commission. An Inventory of Citizen Science Activities for Environmental Policies. 2018 Brussels: European Commission.
- Science Europe (2018). Science Europe Briefing Paper on Citizen Science: D/2018/13.324/2. Brussels: Science Europe.
- Theobald, E. J.; Ettinger, A. K., Burgess, H. K.; DeBey, L. B., Schmidt, N. R.; Froehlich, H. E.; Wagner, C.; HilleRisLambers, J.; Tewksbury, J.; Harsch, M.A.; Parrish, J. K. Global change and local solutions: Tapping the unrealized potential of citizen science for biodiversity research. Biol. Conserv. 2015,181, 236-244. [CrossRef]
- Kousteni, V.; Tsiamis, K.; Gervasini, E.; Zenetos, A.; Karachle, P. K.; Cardoso, A. C. Citizen scientists contributing to alien species detection: The case of fishes and mollusks in European marine waters. Ecosphere,2022, 13(1), e03875. [CrossRef]
- Clements, K. R.; Karp, P.; Harris, H. E.; Ali, F.; Candelmo, A.; Rodríguez, S. J.; Balcàzar- Escalera, C.; Fogg, A. Q.; Green, S. J.; Solomon, J. N. The Role of Citizen Science in the Research and Management of Invasive Lionfish across the Western Atlantic. Diversity, 2021, 13(12), 673. [CrossRef]
- Encarnação, J.; Teodósio, M.A.; Morais, P. Citizen Science and Biological Invasions: A Review. Front. Environ. Sci. 2021, 8, 602980. [CrossRef]
- Nader, M. R., Indary, S., & Boustany, L. The puffer fish Lagocephalus sceleratus (Gmelin, 1789) in the Eastern Mediterranean. EastMed Technical Documents (FAO) 2012, GCP/INT/041/EC–GRE–ITA; FAO: Rome, Italy.
- Ragonese, S.; Jereb, P.; Morara, U. Morphometric relationships of Sphoeroides pachygaster (Pisces - Tetraodontidae) of the Strait of Sicily (Mediterranean Sea). Cah. de Bio. Mar. 1997, 38, 283e289.
- Katsanevakis, S.; Bogucarskis, K.; Gatto, F.; Vandekerkhove, J.; Deriu, I.; Cardoso, A. C. Building the European Alien Species Information Network (EASIN): A novel approach for the exploration of distributed alien species data. BioInvasions Records 2012, 1, 235–245. [CrossRef]
- Consoli, P.; Esposito, V.; Battaglia, P.; Altobelli, C.; Perzia, P.; Romeo, T. et al. Fish distribution and habitat complexity on banks of the Strait of Sicily (Central Mediterranean Sea) from remotely-operated vehicle (ROV) explorations. PLoS ONE 2016, 11(12), e0167809. [CrossRef]
- Ragonese, S.; Rivas, G.; Jereb, P. Spreading of the puffer Sphoeroides pachygaster Günther, 1870) (Pisces: Tetraodontidae) in the Sicilian channel. Is it an “exploding” population? Rapp. Comm. Int. Mer. 1992, 33, 308.
- Matsuura, K.; Tyler, J. C. Tetraodontiform fishes, mostly from deep waters, of New Caledonia. p.173-208. In Séret B. (ed.) Paris, France, 1997. Résultats des Campagnes MUSORSTOM, vol. 17. no.9. Mem. Mus. Natl. Hist. Nat. MNHN (Mémoires du Muséum National d’Histoire Naturelle. Zoologie), 174, 215 p 174.
- Golani, D.; Orsi- Relini, L.; Massutí, E.; Quignard, J. CIESM Atlas of Exotic Species in the Mediterranean. Vol. 1. Fishes. D 2003Sep.3067(3):377-8. Available at: https://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/489. 8Accessed 5 may 2023).
- Jarboui, O.; Ceriola, L.; Fiorentino, F. Current fisheries management in the Strait of Sicily and progress towards an ecosystem approach. Fisheries and Aquaculture Technical Paper (FAO) 2022, 681,147-162. FAO: Rome, Italy.
- Zenetos, A.; Çinar, M. E.; Pancucci-Papadopoulou, M. A.; Harmelin, J. G.; Furnari, G.; Andaloro, F.; Zibrowius, H. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Med. mar. sci. 2005, 6(2), 63-118. [CrossRef]
- Regulation (EC) No 853/2004. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. OJ L 139, 30.4.2004, 55–205.
- Turner, A. D.; Hatfield, R. G.; Maskrey, B. H.; Algoet, M.; & Lawrence, J. F. Evaluation of the new European Union reference method for paralytic shellfish toxins in shellfish: A review of twelve years regulatory monitoring using pre-column oxidation LC-FLD. TrAC 2019, 113, 124-139. [CrossRef]
- Masuda, H.; K. Amaoka, C.; Araga, T.; Uyeno T.; Yoshino, T. The fishes of the Japanese Archipelago. Tokai University Press, Tokyo, Japan, 1984, 1, 437.
- Tani, T. Nihonsan fugu no chudokugakutekikenkyu (Toxicological studies on Japanese puffer). Tokyo, Teikokutosho, 1945, 15-27.
- Jeong, D. Y.; Kim, D. S.; Lee, M. J.; Kim, S. R.; Byun, D. S.; Kim, H.D.; Park, Y. H. Toxicity of several puffers collected at a fish market of Pusan, Korea. Korean J. Fish. Aquat. Sci. 1994, 27(6): 682-689.
- Amano, M.; Takatani, T.; Sakayauchi, F.; Oi, R.; Sakakura, Y. The brain of the wild toxic marine pufferfishes accumulates tetrodotoxin. Toxicon 2022, 218, 1-7. [CrossRef]
- Hashiguchi, Y., Lee, J.M., Shiraishi, M., Komatsu, S., Miki, S., Shimasaki, Y.,Mochioka, N., Kusakabe, T., Oshima, Y. Characterization and evolutionary analysis of tributyltin-binding protein and pufferfish saxitoxin and tetrodotoxin-binding protein genes in toxic and nontoxic pufferfishes. J. Evol. Biol. 2015, 28, 1103e1118. [CrossRef]
- Nagashima, Y.; Ohta, A.; Yin, X.; Ishizaki, S.; Matsumoto, T.; Doi, H.; Ishibashi, T. Difference in Uptake of Tetrodotoxin and Saxitoxins into Liver Tissue Slices among Pufferfish, Boxfish and Porcupinefish. Mar. Drugs 2018 Jan 8;16(1):17. [CrossRef]
- Ochoa, J. L.; Sánchez-Paz, A.; Cruz-Villacorta, A.; Nunez-Vázquez, E.; Sierra-Beltrán, A. Toxic Events in the Northwest Pacific Coastline of Mexico during 1992–1995: Origin and Impact. In Asia-Pacific Conference on Science and Management of Coastal Environment; Springer: Dordrecht, 1997; 195–200. [CrossRef]
- Shiu, Y. C.; Lu, Y. H.; Tsai, Y. H.; Chen, S. K.; Hwang, D. F. Occurrence of Tetrodotoxin in the Causative Gastropod Polinices Didyma and Another Gastropod Natica Lineata Collected from Western Taiwan. J. Food Drug Anal. 2003, 11(2), 159–163. [CrossRef]
- Quilliam, M.; Wechsler, D.; Marcus, S.; Ruck, B.; Wekell, M.; Hawryluk, T. Detection and identification of paralytic shellfish poisoning toxins in Florida pufferfish responsible for incidents of neurologic illness. Harmful algae 2002, 116-118.
- Deeds, J. R.; White, K. D.; Etheridge, S. M.; Landsberg, J. H. Concentrations of Saxitoxin and Tetrodotoxin in Three Species of Puffers from the Indian River Lagoon, Florida, the Location for Multiple Cases of Saxitoxin Puffer Poisoning from 2002 to 2004. Trans. Am. Fish. Soc. 2008, 137(5), 1317–1326. [CrossRef]
- Katikou, P. Public health risks associated with tetrodotoxin and its analogues in European waters: Recent advances after the EFSA scientific opinion. Toxins 2019, 11, 240. [CrossRef]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236.
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS Potentially Linked to the Presence of the Dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [CrossRef]
- Nakamura, M.; Oshima, Y.; Yasumoto, T. Occurrence of saxitoxin in puffer fish. Toxicon 22 1984, 381–385. [CrossRef]
- Kaufmann, M.J.; Santos, F.; Maranhão, M.; Checklist of nanno- and microphytoplankton off Madeira Island (Northeast Atlantic) with some historical notes. Nova Hedwig. 2015, 101, 205–232. [CrossRef]
- Bacchiocchi, S.; Campacci, D.; Siracusa, M.; Dubbini, A.; Accoroni, S.; Romagnoli, T. et al. A Hotspot of TTX Contamination in the Adriatic Sea: Study on the Origin and Causative Factors. Mar. Drugs 2023, 21(1), 8. [CrossRef]
- Finch, S. C.; Boundy, M. J.; Harwood, D. T. The acute toxicity of tetrodotoxin and tetrodotoxin–saxitoxin mixtures to mice by various routes of administration. Toxins 2018, 10(11), 423. [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First Occurrence of Tetrodotoxins in Bivalve Mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [CrossRef]
- Leão, J. M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary results on the evaluation of the occurrence of tetrodotoxin associated to marine Vibrio spp. in bivalves from the Galician Rias (Northwest of Spain). Marine Drugs 2018, 16(3), 81.
- Turner, J.W., Good, B., Cole, D., & Lipp, E. K. (2009). Plankton composition and environmental factors contribute to Vibrio seasonality. ISME Journal 2009, 3(9), 1082–1092. [CrossRef]
- Boundy, M. J.; & Harwood, D. T. Review of literature to help identify risks associated with Tetrodotoxin in seafood, including bivalve molluscs. Prepared for MPI. Cawthron Institute Report 2020, NO 2986A, 46.
- Jen, H. C.; Nguyen, A.-T.; Wu, Y J.; Hoang, T.; Arakawa,O.; Lin,W. F.; Hwang,D. F. Tetrodotoxin and paralytic shellfish poisons in gastropod species from Vietnam analyzed by high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2014, 22(2), 178–188. [CrossRef]
| Specie | N° of specimens | Catch area | TTX results | Reference |
|---|---|---|---|---|
| L. lagocephalus | 10 | WMED-Tunisia, Gabes region | positive | Saoudi et al. [19] |
| L. sceleratus | 6 | EMED-Aegean Sea | positive | Katikou et al. [20] |
| S.pachygaster | 1 | WMED-Spain | negative | Revertè et al. [21] |
| L.sceleratus | 1 | EMED-Greece | positive | |
| L sceleratus | 1 | EMED-Aegean Sea | positive | Bane et al. [22] |
| L. sceleratus | 5 | EMED- Turkey | positive | Kırımer et al. [23] |
| L. sceleratus | 16 | EMED-Mersin Bay | positive | Kosker et al. [24] |
| L. sceleratus | 20 | EMED- Turkey | positive | Acar et al. [25] |
| L. sceleratus | 20 | EMED-Egypt | positive | Alabssawy [26] |
| L. lagocephalus | 14 | WMED-Spain | negative | Rambla-Alegre et al. [27] |
| L. sceleratus | 1 | positive | ||
| S. pachygaster | 5 | negative | ||
| Torquigener flavimaculosus | 16 | EMED-Mersin Bay | positive | Kosker et al. [28] |
| L. sceleratus | 2 | EMED-Aegean Sea | positive | Leonardo et al. [29] |
| L. sceleratus | 40 | EMED-Mersin Bay | positive | Kosker et al. [30] |
| Lagocephalus spadiceus | 40 | EMED-Mersin Bay | negative | |
| Lagocephalus suezensis | 40 | EMED-Mersin Bay | positive | |
| L. sceleratus | 16 | EMED-Cyprus | positive | Akbora et al. [31] |
| L. sceleratus | 1 | ADRIA | positive | Ujević et al. [32] |
| L. sceleratus | 83 | EMED- Crete | positive | Christidis et al. [33] |
| L. sceleratus | 112 | EMED- Egypt | positive | Farrag et al. [34] |
| L. sceleratus | 3 | EMED-Lebanon | positive | Hassoun et al. [35] |
| Lagocephalus guentheri | 1 | EMED | negative | Ulman et al. [36] |
| L. suezensis | 1 | Positive | ||
| S. pachygaster | 1 | Negative |
| Date of catch | Catch area | N of specimens | Reference | Type of record | Fishing method | Total Lenght (mm) |
Body Weight (g) |
Depth (m) |
Type of seabed |
|---|---|---|---|---|---|---|---|---|---|
| 2012-2015 | CMED- Strait of Sicily |
7 | Cammilleri et al. [49] | Caught | Trawl | - | 130-1750 | - | - |
| 2014-2016 | WMED- Spain | 5 | Rambla- Alegre et al. [27] | Caught | - | - | - | - | - |
| 2015-2016 | CMED- Ionian Sea | 5 | Carlucci et al. [50] | Caught | Trawl | - | - | - | - |
| 2016- 2017 | EMED - Egypt | 4 | Ragheb et al. [51] | Caught | Trawl | - | - | - | - |
| NR | CMED - Malta | 9 | Vella et al. [52] | Caught | - | 266-465 | 385-2275 | - | - |
| April 2017 | EMED - Lebanon | 3 | Gerovasileiou et al. [53] | Caught | Long line | 344-417 | - | - | Soft |
| 2020 | WMED - Algerian coast | 2 | Hussein et al. [54] | Caught | Fisherman Trawl |
360.6 409.5 |
2250 2420 |
10 120 |
- |
| March 2020 |
EMED - Northen Cyprus | 1 | Akbora et al. [55] | Caught | Trammel- net | 520 | 1200 | 250 | - |
| May 2020 | EMED - Turkey | 1 | Erguden et al. [reprted in 56] | Caught | - | 490 | - | 400 | - |
| July 2020 | EMED - Turkey | 1 | Kizilkaya and Akyol [56] | Caught | Trammel-net | 257 | - | 125 | - |
| August 2021 | CMED - Strait of Messina | 1 | [57] | stranded | - | - | - | - | - |
| March 2022 | EMED - Turkey | 5 | Karakuş et al. [58] | caught | Trawl | 190-410 | 10-1405 | 140-240 | - |
| NR | EMED - Turkey | 1 | Ulman et al. [36] | caught | - | - | - | - | - |
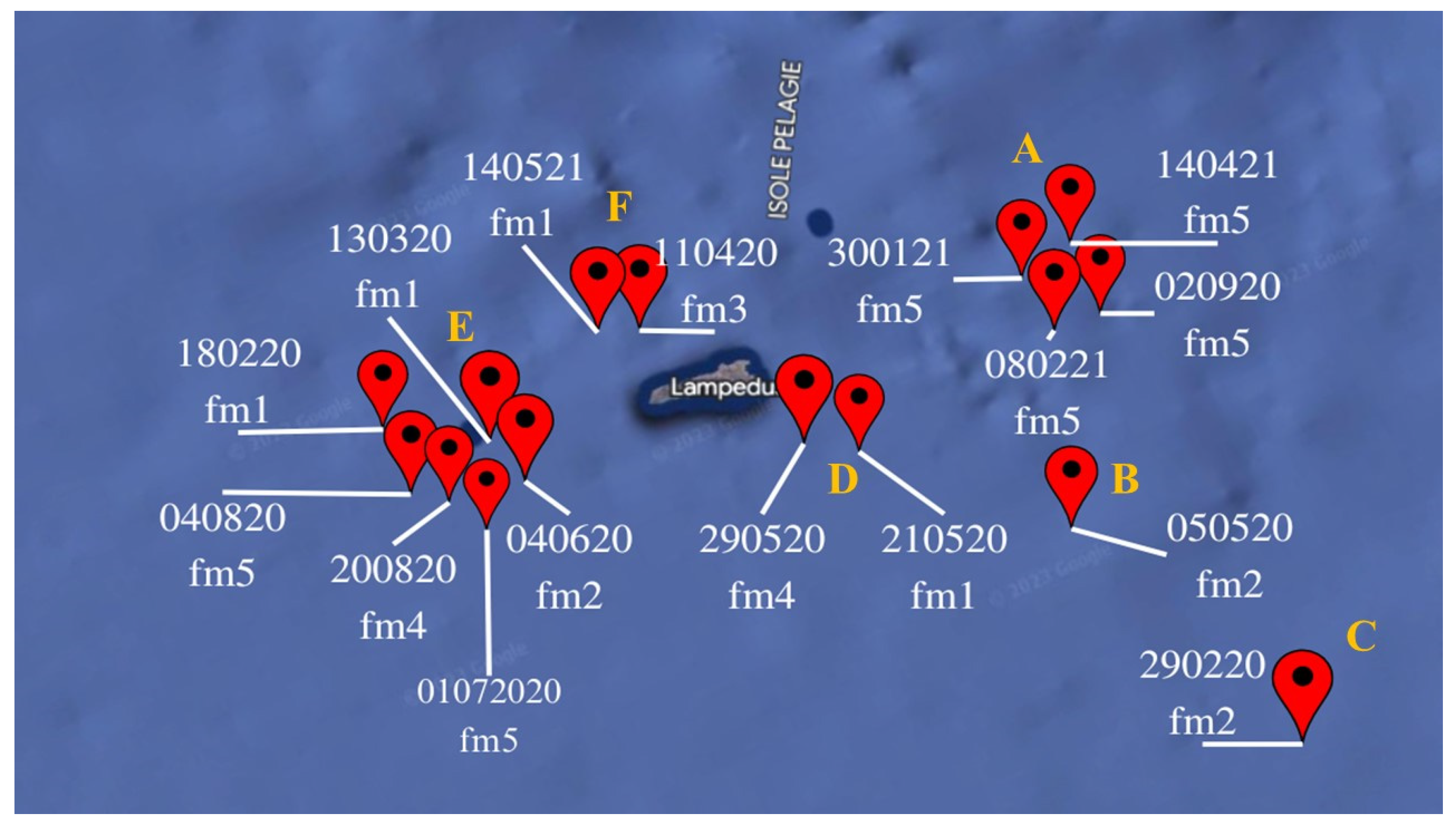
| Catch date (Fishermam) |
N of specimens | Specimen code |
Body Weight (gr) | Total Lenght (cm) | Size | Toxin Analysis |
|---|---|---|---|---|---|---|
| 180220 (Fm1) |
1 | P01 | 196 | 175 | S | TTXs |
| 290220 (Fm2) |
2 | P02(A) | 199 | 220 | S | TTXs STXs |
| P02(B) | 215 | 190 | S | - | ||
| 130320 (Fm1) |
3 | P03(A) | 670 | 270 | S | TTXs |
| P03(B) | 122 | 190 | S | TTXs STXs |
||
| P03(C) | 100 | 160 | S | - | ||
| 110420 (Fm3) |
3 | P04(A) | 687 | 260 | S | TTXs STXs |
| P04(B) | 170 | 117 | S | - | ||
| P04(C) | 160 | 131 | S | - | ||
| 50520 (Fm2) |
2 | P05(A) | 743 | 260 | S | TTXs |
| P05(B) | 264 | 220 | S | TTXs STXs |
||
| 210520 (Fm1) |
3 | P06(A) | 2570 | 420 | L | TTXs |
| P06(B) | 136 | 190 | S | - | ||
| P06(C) | 202 | 210 | S | - | ||
| 210520 (Fm4) |
1 | P07(A) | 841 | 360 | S | TTXs STXs |
| 290520 (Fm2) |
2 | P08(A) | 1726 | 430 | L | TTXs |
| P08(B) | 394 | 250 | S | TTXs STXs |
||
| 40620 (Fm5) |
3 | P09(A) | 207 | 210 | S | TTXs |
| P09(B) | 179 | 190 | S | - | ||
| P09(C) | 169 | 200 | S | - | ||
| 10720 (Fm5) |
12 | P10(A) | 349 | 220 | S | TTXs STXs |
| P10(B) | 199 | 210 | S | - | ||
| P10(C) | 163 | 190 | S | - | ||
| P10(D) | 296 | 235 | S | - | ||
| P10(E) | 202 | 200 | S | - | ||
| P10(F) | 378 | 250 | S | - | ||
| P10(G) | 574 | 260 | S | - | ||
| P10(H) | 290 | 220 | S | - | ||
| P10(I) | 205 | 210 | S | - | ||
| P10(J) | 296 | 220 | S | - | ||
| P10(K) | 523 | 270 | S | - | ||
| P10(L) | 496 | 270 | S | - | ||
| 40820 (Fm4) |
3 | P11(A) | 1655 | 360 | L | TTXs |
| P11(B) | 523 | 270 | S | TTXs STXs |
||
| P11(C) | 496 | 270 | S | - | ||
| 200820 (Fm5) |
3 | P12(A) | 2145 | 445 | L | TTXs STXs |
| P12(B) | 271 | 220 | S | TTXs STXs |
||
| P12(C) | 203 | 200 | S | - | ||
| 20920 (Fm4) |
4 | P13(A) | 264 | 230 | S | - |
| P13(B) | 2280 | 450 | L | TTXs STXs |
||
| P13(C) | 1850 | 450 | L | TTXs STXs |
||
| P13(D) | 1640 | 420 | L | TTXs STXs |
||
| 300121 (Fm5) |
5 | P14(A) | ND | ND | - | - |
| P14(B) | ND | ND | - | - | ||
| P14(C) | ND | ND | - | - | ||
| P14(D) | ND | ND | - | - | ||
| P14(E) | ND | ND | - | - | ||
| 080221 (Fm5) |
5 | P15(A) | 860 | 360 | S | - |
| P15(B) | ND | ND | - | - | ||
| P15(C) | ND | ND | - | - | ||
| P15(D) | ND | ND | - | - | ||
| P15(E) | ND | ND | - | - | ||
| 140421 (Fm5) |
2 | P16(A) | ND | ND | - | - |
| P16(B) | ND | ND | - | - | ||
| 140521 (Fm1) |
2 | P17(A) | 1148 | 410 | L | - |
| P17(B) | 1407 | 430 | L | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





