Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
Introduction
2. Methods
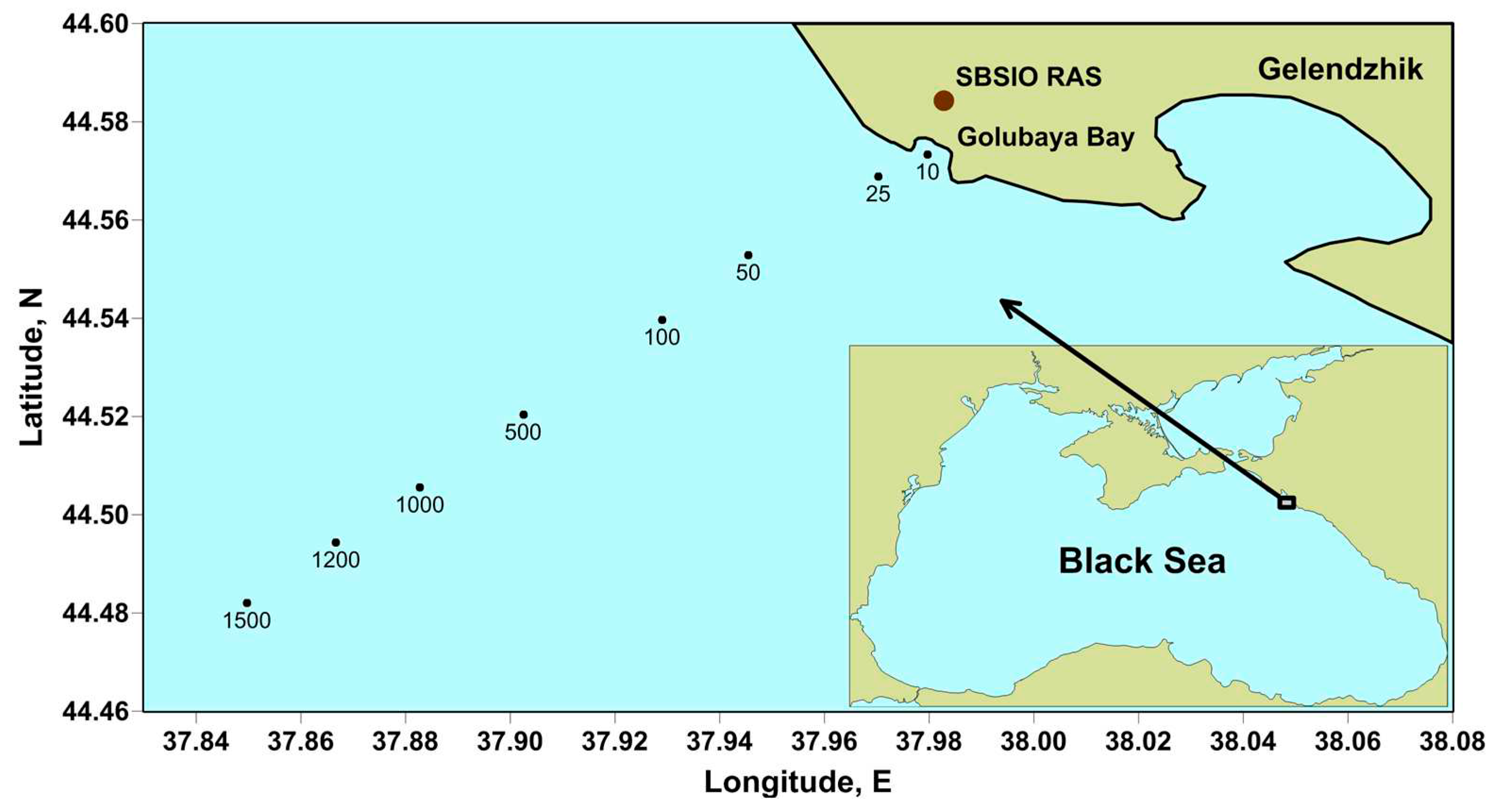
2.1. Field Sampling
2.2. Field Sample Analyses
2.3. Experimental studies
3. Results
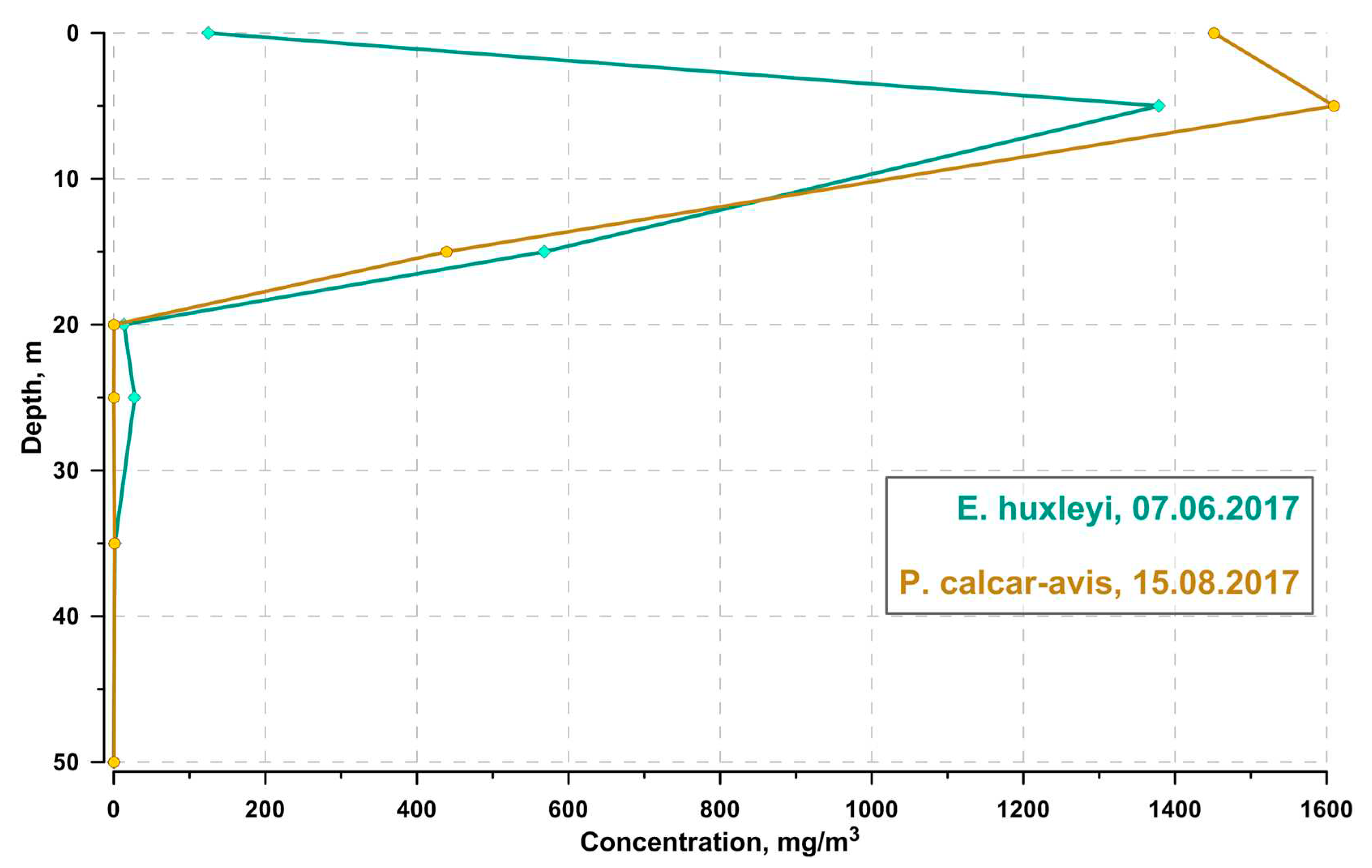
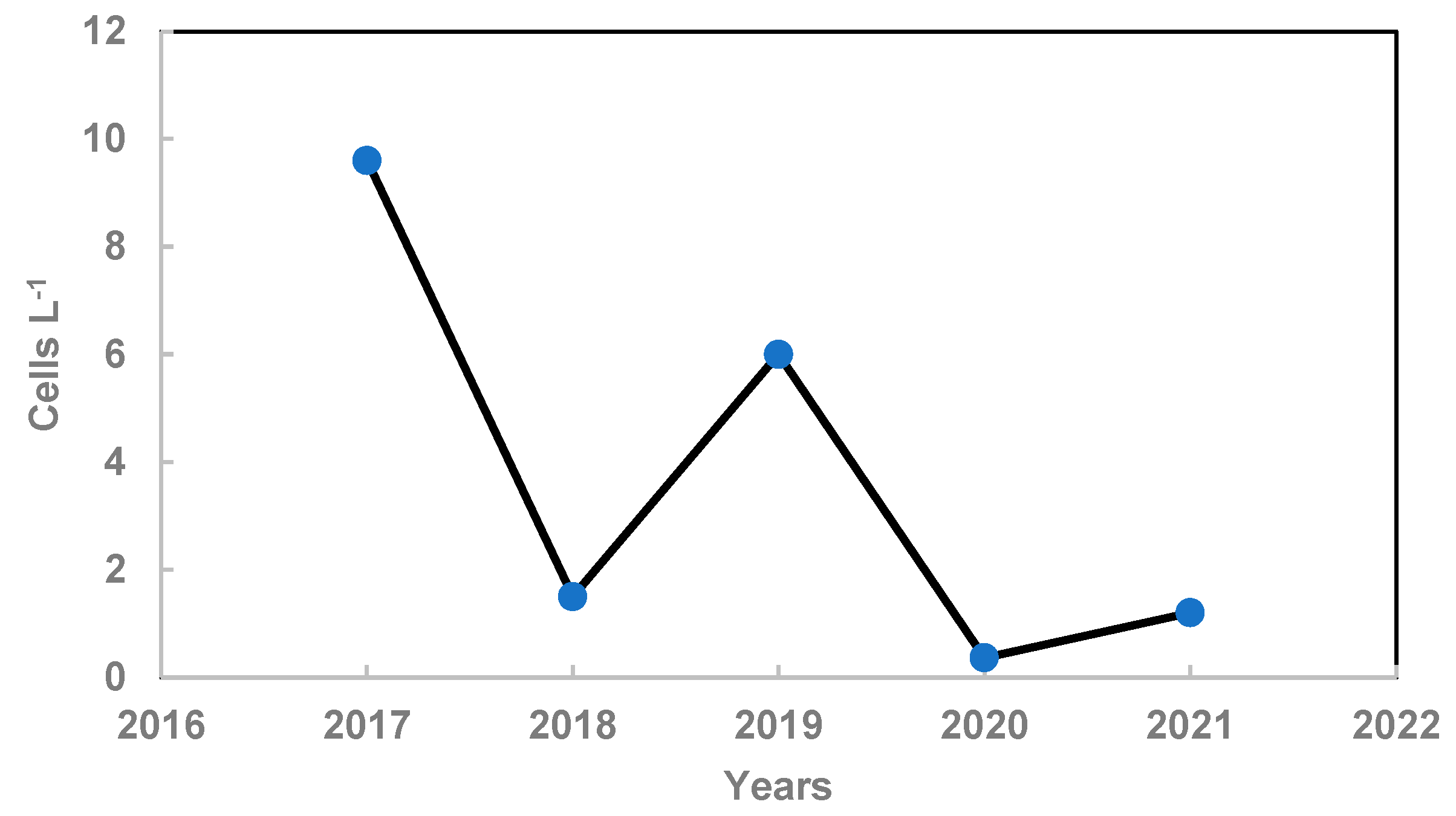
3.1. Dominance of coccolithophores and large diatoms in the Black Sea
3.1.1. Dominance of coccolithophores
3.1.2. The dominance of large diatoms.
3.2. Physics of the water column.
| Parameter | E. huxleyi | P. calcar-avis | p, t-test |
| Temperature | 20,89 | 25.93 | 5.1·10-26 |
| Salinity | 17.74 | 17.97 | 0.0004 |
| Depth of seasonal thermocline | 6.87 | 17.96 | 1·10-7 |
| Depth of layer 10 °C | 27.47 | 42.67 | 5.1·10-7 |
| The thickness of seasonal thermocline | 20.60 | 24.41 | 0.031 |
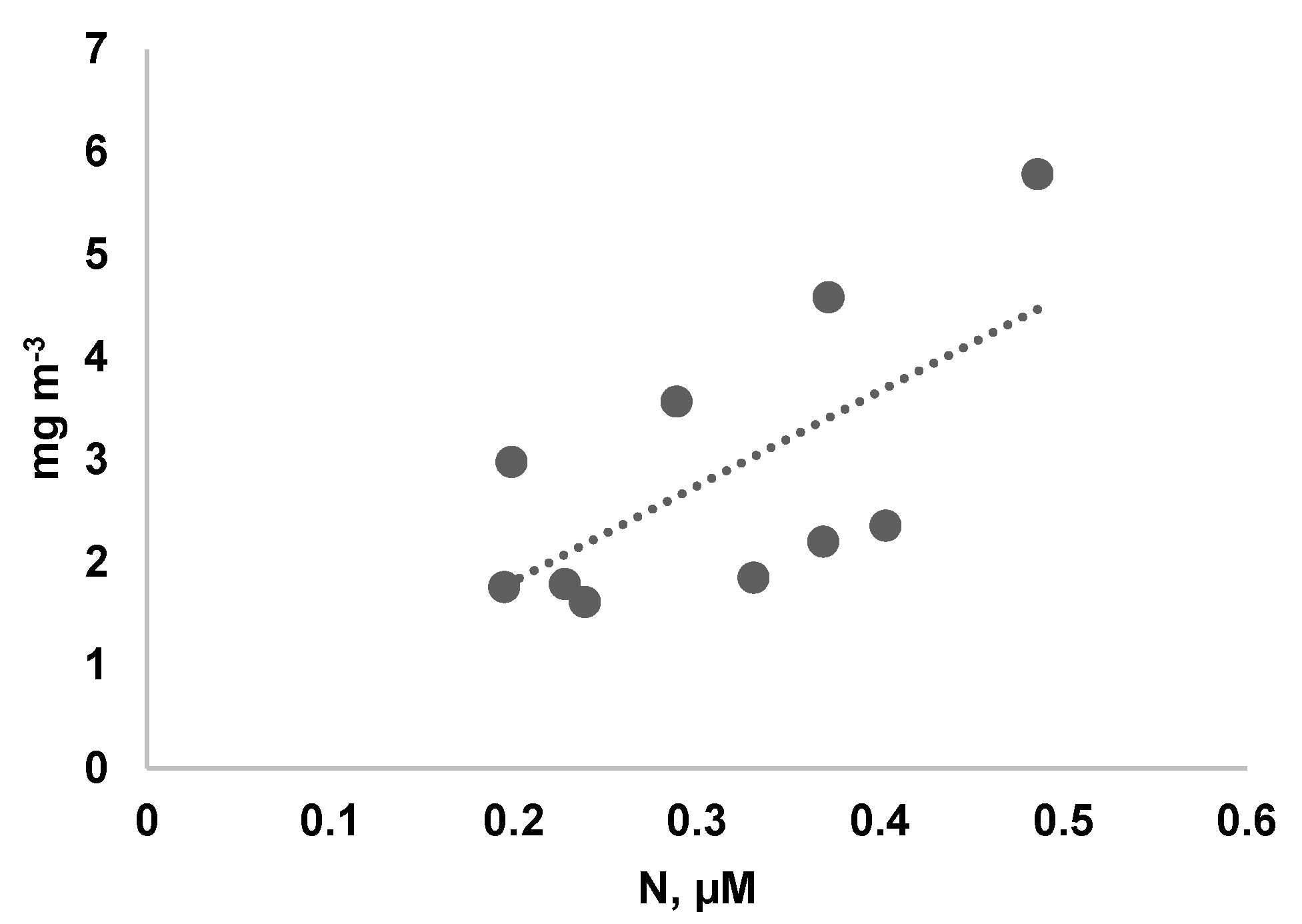
3.3. UML Chemistry
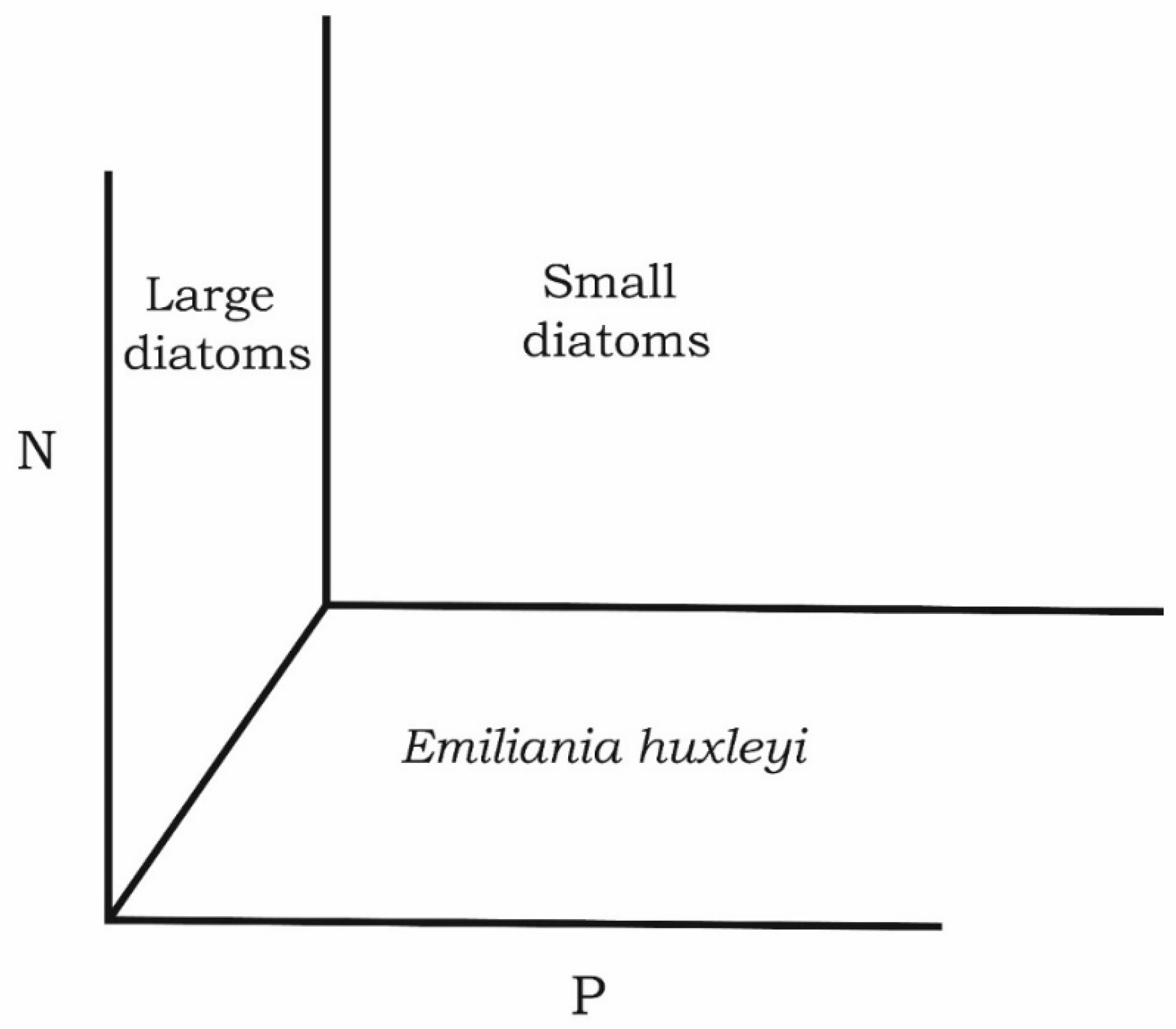
3.4. The effect of nitrogen and phosphorus additives on the dynamics of phytoplankton.
Discussion
Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Inte-grating terrestrial and oceanic components. Science 1998, 281(5374), 237–240. [Google Scholar] [CrossRef] [PubMed]
- Volk, T.; Hoffert, M.I. Ocean carbon pumps: Analysis of relative strengths and efficiencies in ocean-driven atmospheric CO2 changes. In The Carbon Cycle and Atmospheric CO2 Natural Variations Archean to Present. In Geophysical Monograph Series, Sundquist, E.T., Broecker, W.S., Eds. American Geophysical Union, Washington, DC: 1985; 32, 99-110.
- Sarmiento, J.L.; Gruber, N. Ocean Biogeochemical Dynamics. Princeton University Press, Princeton, NJ: 2006; p. 526.
- Henson, S.A.; Sanders, R.; Madsen, E.; Morris, P.J.; Le Moigne, F.; Quartly, G.D. A reduced estimate of the strength of the ocean's biological carbon pump. Geophys. Res. Lett. 2011, 38. p. L04606. [CrossRef]
- Siegel, D.A.; Buesseler, K.O.; Doney, S.C.; Sailley, S.F.; Behrenfeld, M.J.; Boyd, P.W. Global assessment of ocean carbon export by combining satellite observations and food-web models. Global Biogeochemical Cycles. 2014, 28, 181–196. [Google Scholar] [CrossRef]
- Legendre, L.; Rivkin, R.B.; Weinbauer, M.G.; Guidi, L.; Uitz, J. The microbial carbon pump concept: Potential biogeochemical significance in the globally changing ocean. Prog. Oceanogr. 2015, 134, 432–450. [Google Scholar] [CrossRef]
- Cermeno, P.S.; Dutkiewicz, R.P.; Harris, M.; Follows, O.; Schofield and Falkowski, P.G. The role of nutricline depth in regulating the ocean carbon cycle. Proc. Natl. Acad. Sci. USA 2008, 105, 20344–20349. [Google Scholar] [CrossRef] [PubMed]
- Tréguer, P.; Bowler, C.; Moriceau, B.; et al. Influence of diatom diversity on the ocean biological carbon pump. Nat Geosci 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Milliman, J.D. Production and accumulation of calcium carbonate in the ocean: budget of a non-steady state. Global Biogeochemical Cycles 1993, 7, 927–957. [Google Scholar] [CrossRef]
- Poulton, A.J.; Adey, T.R.; Balch, W.M.; Holligan, P.M. Relating coccolithophore calcification rates to phytoplankton community dynamics: Regional differences and implications for carbon export. Deep Sea Research Part II. 2007, 54, 538–557. [Google Scholar] [CrossRef]
- Tanioka T., Catherine, A.G.; Alyse A. L.; Nathan, S.G.; Adam, J.; Fagan, 1. & Adam, C.; Martiny. Global patterns and predictors of C:N:P in marine ecosystems. Communications Earth & Environment 2022, 3, 271. [CrossRef]
- Dutkiewicz, S.; et al. Capturing optically important constituents and properties in a marine biogeochemical and ecosystem model. Biogeosciences 2015, 12, 4447–4481. [Google Scholar] [CrossRef]
- DeVries, T.; Deutsch, C. Large-scale variations in the stoichiometry of marine organic matter respiration. Nat. Geosci. 2014, 7, 890–894. [Google Scholar] [CrossRef]
- Moreno, A.R.; Martiny, A.C. Ecological Stoichiometry of Ocean Plankton. Annual Review of Marine Science. 2018, 10, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Lomas, M.W.; Steven, E.; Baer, C.M.; Kristina, X.T.; Debra, A.L.; Mark, A.A.; Adam, C.M. Varying influence of phytoplankton biodiversity and stoichiometric plasticity on bulk particulate stoichiometry across ocean basins. Commun. Earth Environ. 2021, 2, 143. [Google Scholar] [CrossRef]
- Inomura, K.; Deutsch, C.; Jahn, O.; Stephanie, D.; Michael, J.F. Global patterns in marine organic matter stoichiometry driven by phytoplankton ecophysiology. Nat. Geosci. 2022, 15, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M. and Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecology Letters. 2000, 3, 540–550. [CrossRef]
- Sterner, R.W. and Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton: Princeton University Press, 2003. [CrossRef]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; Hood, J. and Sterner, R.W. Growth rate–stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [CrossRef]
- Ågren, G.I. The C:N:P stoichiometry of autotrophs – theory and observations. Ecol. Lett. 2004, 7, 185–91. [Google Scholar] [CrossRef]
- Hessen, D.O.; Elser, J.J.; Sterner, R.W.; Urabe, J. Ecological stoichiometry: an elemental approach using basic principles. Limnol. Oceanogr. 2013, 58, 2219–2236. [Google Scholar] [CrossRef]
- Moody, E.K.; Rugenski, A.T.; Sabo, J.L.; Turner, B.L. and Elser, J.J. Does the Growth Rate Hypothesis Apply across Temperatures? Variation in the Growth Rate and Body Phosphorus of Neotropical Benthic Grazers. Front. Environ. Sci. 2017, 5, 14. [CrossRef]
- Hessen, D.O.; Hafslund, O.T.; Andersen, T.; Broch, C.; Shala, N.K. and Wojewodzic, M.W. Changes in Stoichiometry, Cellular RNA, and Alkaline Phosphatase Activity of Chlamydomonas in Response to Temperature and Nutrients. Front. Microbiol. 2017, 8, p 18. [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.V. and Raven, J.A., Phytoplankton in a changing world: cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [CrossRef]
- Finkel, Z.V.; Follows, M.J.; Liefer, J.D.; Brown, C.M.; Benner, I.; Irwin, A.J. (2016) Phylogenetic Diversity in the Macromolecular Composition of Microalgae. PLoS ONE 2016, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; Elser, J.; Gruber, N.; Hibbard, K.; Hogberg, P.; Linder, S.; Mackenzie, F.T.; Moore, B.; Pedersen, T.; Rosenthal, Y.; Seitzinger, S.; Smetacek, V.; Steffen, W. The global carbon cycle: a test of our knowledge of earth as a system. Science 2000, 290(5490), 291–296. [Google Scholar] [CrossRef] [PubMed]
- Geider, R. and La Roche, J. Redfield revisited: Variability of C:N:P in marine microalgae and its biochemical basis. European Journal of Phycology 2002, 37, 1–17. [CrossRef]
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004, 429, 171–74. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, H.; Steinert, G.; Boersma, M.; Malzahn, A.; Meunier, C.; Plum, L.C.; Ptacnik, R. Goldman revisited: Faster-growing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol. Oceanogr. 2013, 58(6), 2076–2088. [Google Scholar] [CrossRef]
- Flynn, K.J.; Raven, J.A.; Rees, T.A.; Finkel, Z.; Quigg, A.; Beardall, J. Is the growth rate hypothesis applicable to microalgae? J. Phycol. 2010, 46, 1–12. [Google Scholar] [CrossRef]
- Cembella, A.D.; Antia, N.J.; Harrison, P.J. and Rhee, G-Y. The Utilization of Inorganic and Organic Phosphorous Compounds as Nutrients by Eukaryotic Microalgae: A Multidisciplinary Perspective: Part 2, Critical Reviews in Microbiology 1984, 11:1, 13-81. [CrossRef]
- Droop, M.R. Vitamin B12 and marine ecology. The kinetics of uptake, growth and inhibition of Monochrysis lutheri. J. Mar. Biol. Assoc. UK. 1968, 48, 689–733. [Google Scholar] [CrossRef]
- Droop, M.R. The nutrient status of algal cells in continuous culture. J. Mar. Biol. Assoc UK. 1974, 54, 825–855. [Google Scholar] [CrossRef]
- Smith, S.L.; Pahlow, M.; Merico, A. and Wirtz, K.W. Optimality-based mo deling of planktonic organisms. Limnol. Oceanogr. 2011, 56, 2080–2094. [CrossRef]
- Bonachela, J.A.; Allison, S.D.; Martiny, A.C.; Levin, S.A. A model for variable phytoplankton stoichiometry based on cell protein regulation. Biogeosciences Discuss. 2013, 10, 3241–3279. [Google Scholar] [CrossRef]
- Pahlow, M. and Oschlies, A. Optimal allocation backs droop s cell-quota model. Mar. Ecol. Prog. Ser. 2013, 473, 1–5. [Google Scholar] [CrossRef]
- Meunier, C.L.; Boersma, M.; El-Sabaawi, R.; Halvorson, H.M.; Herstoff, E.M.; Van de Waal, D.B.; Vogt, R.J. and Litchman, E. From Elements to Function: Toward Unifying Ecological Stoichiometry and Trait-Based Ecology. Front. Environ. Sci. 2017, 5, p 18. [CrossRef]
- Litchman, E. and Klausmeier, C.A. Trait-based community ecology of phytoplankton. Ann. Rev. Ecol. Evol. Syst. 2008, 39, 615–639. [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M. et al. The role of functional traits and trade-offs in structuring phytoplankton com munities: scaling from cellular to ecosystem level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E.; Klausmeier, C.A.; Yoshiyama, K. Contrasting size evolution in marine and freshwater dia-toms. Proc. Nat. Acad. Sci. USA 2009, 106, 2665–2670 http://dx. [Google Scholar] [CrossRef] [PubMed]
- Verdy, A.; Follows, M.J. and Flierl, G. Optimal phytoplankton cell size in an allometric model. Mar. Ecol. Prog. Ser. 2009, 379, 1–12. [CrossRef]
- Mei, Z.-P.; Finkel, Z.V.; Irwin, A.J. Phytoplankton growth allometry and size dependent C:N stoichiometry revealed by a variable quota model. Mar. Ecol. Prog. Ser. 2011, 434, 29–43. [Google Scholar] [CrossRef]
- Kremer, C.T.; Williams, A.K.; Finiguerra, M.; Fong, A.A.; Kellerman, A.; Paver, S.F.; Tolar, B.B.; Toscano, B.J. Realizing the potential of trait-based aquatic ecology: new tools and collaborative approaches. Limnol. Oceanogr. 216, 62, 253-271. [CrossRef]
- Falkowski, P.G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 1997, 387, 272–275. [Google Scholar] [CrossRef]
- Redfield, A.C. The biological control of chemical factors in the environment. Am. Sci. 1958, 46, 205–221. [Google Scholar]
- Tilman, D. Resource competition between planktonic algae: an experimental and theoretical approach. Ecology 1977, 58, 338–348. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure. 1982. Princeton University Press 1982, p. 296.
- Klausmeier, C.A.; Litchman, E.; Daufresne, T.; Levin, S.A. Phytoplankton stoichiometry. Ecol. Res. 2008, 23, 479–485. [Google Scholar] [CrossRef]
- Sorokin, Yu.I. The Black Sea ecology and oceanography. Backhuys Publishers, Leiden, The Netherlands 2002, p. 875.
- Kostianoy, A.G.; Kosarev, A.N. The Black Sea Environment, Springer-Verlag Berlin Heidelberg, Hdb Env Chem 2008, 5, Part Q: XIV, p. 457.
- Konovalov, S.K. Konovalov, S.K. and Murray, J.W. Variations in the Chemistry of the Black Sea on a Time Scale of Decades (1960-1995). Journal of Marine Systems 2001, 31, 217–243. [Google Scholar] [CrossRef]
- Oguz, T.; Fach, B.; Salihoglu, B. Invasion dynamics of the alien ctenophore Mnemiopsis leidyi and its impact on anchovy collapse in the Black Sea. J. Plankton Res. 2008, 30, 1385–1397. [Google Scholar] [CrossRef]
- Oguz, T.; Velikova, V. Abrupt transition of the northwestern Black Sea shelf ecosystem from a eutrophic to an alternative pristine state. Mar. Ecol. Prog. Ser. 2010, 405, 231–242. [Google Scholar] [CrossRef]
- Pakhomova, S.; Vinogradova, E.; Yakushev, E.; Zatsepin, A.; Shtereva, G.; Chasovnikov, V. and Podymov, O. Interannual variability of the Black Sea Proper oxygen and nutrients regime: the role of climatic and anthropogenic forcing. Estuar. Coast. Shelf Sci. 2014, 140, 134–145. [Google Scholar] [CrossRef]
- Bodeanu, N.; Andrei, C.; Boicenco, L.; Popa, L.; Sburlea, A. A new trend of the phytoplankton structure and dynamics in the Romanian marine waters. Cercetari Marine 2004, 35, 77–86. [Google Scholar]
- Moncheva, S.; Petrova-Karadjova, V.; Palasov, A. Harmful algal blooms along the Bulgarian Black Sea coast and possible patterns of fish and zoobenthic mortalities. Harmful Marine Algal Blooms, Lavoisier Publ. Incorp, Paris 1995, 193-198.
- Moncheva, S.; Gotsis-Skretas, O.; Pagou, K.; Krastev, A. Phytoplankton Blooms in Black Sea and Medi-terranean Coastal Ecosystems Subjected to Anthropogenic Eutrophication: Similarities and Differences. Estuar. Coast. Shelf 2001, 53, 281–295. [Google Scholar] [CrossRef]
- Velikova, V.; Moncheva, S.; Petrova, D. Phytoplankton dynamics and Red Tides (1987–1997) in the Bulgarian Black Sea. Water Sci. Technol. 1999, 39(8), 27–36. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Stanichny, S.V.; Zatsepin, A.G. Interannual variability of Danube waters propagation in summer period of 1992–2015 and its influence on the Black Sea ecosystem. Journal of Marine Systems 2018, 179, 10–30. [Google Scholar] [CrossRef]
- Zatsepin, A.G.; Baranov, V.I.; Kondrashov, A.A.; Korzh, A.O.; Kremenetskiy, V.V.; Ostrovskii, A.G.; Soloviev, D.M. Submesoscale Eddies at the Caucasus Black Sea Shelf and the Mechanisms of Their Gen-eration. Oceanology 2011, 51(4), 554–567. [Google Scholar] [CrossRef]
- Kubryakov, A.I.; Korotaev, G.K.; Dorofeev, V.L.; Ratner, Y.B.; Palazov, A.; Valchev, N.; Malciu, V.; Matescu, R. and Oguz, T. Black Sea coastal forecasting system. Ocean. Sci. 2012, 8, 183–196 http://dx. [Google Scholar] [CrossRef]
- Konovalov, S.K.; Murray, J.W.; Murray, Luther G.W. Basic Processes of Black Sea Biogeochemistry. Oceanography 2005, 18(2), 24–35. [CrossRef]
- Kubryakova, E.A.; Kubryakov, A.A.; Stanichny, S.V. Impact of Winter Cooling on Water Vertical Entrainment and Intensity of Phytoplankton Bloom in the Black Sea. Physical Oceanography 2018, 25(3), 191–206. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Belokopytov, V.N.; Zatsepin, A.G.; Stanichny, S.V. and Piotukh, V.B. The Black Sea Mixed Layer Depth Variability and Its Relation to the Basin Dynamics and Atmospheric Forcing. Physical Oceanography 2019, 26(5), 397–413. [CrossRef]
- Pautova, L.A.; Mikaelyan, A.S.; Silkin, V.A. The structure of plankton community in shelf waters of north-eastern part of the Black Sea in the period of mass bloom of Emiliania huxleyi in 2002-2005. Oceanology 2007, 47, 408–417. [Google Scholar] [CrossRef]
- Mikaelyan, A.S.; Silkin, V.A.; Pautova, L.A. Coccolithophorids in the Black Sea: interannual and long-term changes. Oceanology 2011, 51, 45–53. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Pakhomova, S.V.; Lifanchuk, A.V.; Yakushev, E.V. and Chasovnikov, V.K. Environmental control on phytoplankton community structure in the NE Black Sea. J. Exp. Mar. Biol. Ecol. 2014, 461, 267–274. [Google Scholar] [CrossRef]
- Silkin, V.A.; Pautova, L.A.; Giordano, M.; Chasovnikov, V.K.; Vostokov, S.V.; Podymov, O.I.; Pakhomova, S.V.; Moskalenko, L.V. Drivers of phytoplankton blooms in the northeastern Black Sea. Mar. Poll. Bull. 2019, 138, 274–284. [Google Scholar] [CrossRef]
- Cokacar, T.; Kubilay, N.; Oguz, T. Structure of Emiliania huxleyi blooms in the Black Sea surface waters as detected by SeaWIFS imagery. Geophysical Research Letters 2001, 28, 4607–4610. [Google Scholar] [CrossRef]
- Kopelevich, O.; Burenkov, V.; Sheberstov, S.; Vazyulya, S.; Kravchishina, M.; Pautova, L.; Silkin, V.; Ar-temiev, V.; Grigoriev, A. Satellite monitoring of coccolithophore blooms in the Black Sea from ocean col-or data. Remote Sensing of Environment 2014, 146, 113–123. [Google Scholar] [CrossRef]
- Bordovskiy, O.K.; Chernyakova, A.M. Modern Methods of the Ocean Hydrochemical Investigations; P.P. Shirshov Institute of Oceanology 1992, 200 p. (In Russian).
- Grashoff, K.; Kremling, K.; Ehrhard, M. Methods of Seawater Analysis; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore; Toronto, ON, Canada 1999; 420 p.
- Tomas, C.R. (Ed.). Identifying Marine Phytoplankton; Academic Press: San Diego, CA, USA 1997, 858 p. ISBN 0-12-693018-X.
- Throndsen, J.; Hasle, G.R.; Tangen, K. Norsk Kystplanktonflora; Almater: Oslo, Norway 2003, 341 p.
- Hillebrand, H.; Durselen, C.; Kirschtel, D.; Pollingher, U.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationship for dinoflagellates, diatom, and other protist plahkton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Maksimov, V.N. Mnogofactornyi experiment v boilogii (Multyfactorial experiment in biology). Moscow, Nauka 1980, 164-190. (In Russian).
- Barton, A.D.; Lozier, M.S.; Williams, R.G. 11. Physical controls of variability in North Atlantic phyto-plankton communities. Limnol. Oceanogr. 2014, 60, 181–197. [Google Scholar] [CrossRef]
- Lindemann, C.; John, M.A.St. A seasonal diary of phytoplankton in the North Atlantic. Front. Mar. Sci. 1, A37. [CrossRef]
- Krivosheya, V.G.; Moskalenko, L.V.; Melnikov, V.A.; Skirta, A.Yu. Effects of the Wind and Thermal Conditions Variability on the Structure and Dynamics of the Seawater in the Northeastern Black Sea. Oceanology 2012, 52, 453–466. [Google Scholar] [CrossRef]
- Arkhipkin, V.S.; Gippius, F.N.; Koltermann, K.P.; Surkova, G.V. Wind waves in the Black Sea: results of a hind cast study. Nat. Hazards Earth Syst. Sci. 2014, 14, 2883–2897. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Stanichny, S.V.; Zatsepin, A.G.; Kremenetskiy, V.V. Long-Term Variations of the Black Sea Dynamics and Their Impact on the Marine Ecosystem. Journal of Marine Systems, 2016, 163, 80–94. [Google Scholar] [CrossRef]
- Bach, L.T.; Ulf, R.; Magdalena, A.G.; Luisa, F.; Schulz, K.G. A unifying concept of coccolithophore sensitivity to changing carbonate chemistry embedded in an ecological framework. Progress in Oceanography 2015, 135, 125–138. [Google Scholar] [CrossRef]
- Iglesias-Rodrigez, M.D.; Brown, C.W.; Doney, S.C.; Kleypas, J.; Kolber, D.; Kolber, Z.; Hayes, P.K. and Falkowski, P.G. Representing key phytoplankton functional groups in ocean cycle models: Coccolithophorids. Global. Biogem. Cy. 2002, 16, 1–20. [Google Scholar] [CrossRef]
- Fielding, S.R. Emiliania huxleyi specific growth rate dependence on temperature. Limnol. Oceanogr. 2013, 58(2), 663–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Klapper, R.; Lohbeck, K.T.; Bach, L.T.; Schulz, K.G.; Thorsten, B.H. Reusch and Ulf, R. Between- and within-population variations in thermal reaction norms of the coccolithophore Emiliania huxleyi. Limnol. Oceanogr. 2014, 59(5), 1570-1580. [CrossRef]
- Mikaelyan, A.S.; Pautova, L.A.; Chasovnikov, V.K.; Mosharov, S.A.; Silkin, V.A. Alternation of diatoms and coccolithophores in the northeastern Black Sea: a response to nutrient changes. Hydrobiologia 2015, 755, 89–105. [Google Scholar] [CrossRef]
- Kubryakov, A.A.; Mikaelyan, A.S.; Stanichny, S.V. Extremely strong coccolithophore blooms in the Black Sea: the decisive role of winter vertical entrainment of deep water. Deep Sea Research Part I Oceanographic Research Papers 2021, 173(4), 103554. [Google Scholar] [CrossRef]
- Iglesias-Rodrigez, M.D.; Brown, C.W.; Doney, S.C.; Kleypas, J.; Kolber, D.; Kolber, Z.; Hayes, P.K. and Falkowski, P.G. Representing key phytoplankton functional groups in ocean cycle models: Coccolithophorids. Global. Biogem. Cy. 2002, 16, 1–20. [Google Scholar] [CrossRef]
- Paasche, E. A review of the coccolithophorid Emiliania huxleyi (Prymnesiophyceae), with particular reference to growth, coccolith formation, and calcification-photosynthesis interactions. Phycologia 2002, 40, 503–529. [Google Scholar] [CrossRef]
- Eppley, R.W.; Rogers, J.N.; McCarthy, J.J. Half saturation constants for uptake of nitrate and ammonium by marine phytoplankton. Limnology and Oceanography 1968, 14, 912–920. [Google Scholar] [CrossRef]
- Sunda, W.G. and Hardison, R.D. Ammonium uptake and growth limitation in marine phytoplankton. Limnol. Oceanogr. 2007, 52(6), 2496–2506. [CrossRef]
- Silkin, V.A. and Khailov, K.M. Bioecological Mechanisms of Aquaculture Management. Leningrad, Nauka, 1988, p. 230. (In Russian).
- Lessard, E.J.; Merico, A.; Tyrell, T. Nitrate:phosphate ratios and Emiliania huxleyi blooms. Limnol. Oceanogr. 2005, 50, 1020–1024. [Google Scholar] [CrossRef]
- Loebl, M.; Cockshutt, A.M.; Campbell, D.A. and Finkel Z.V. Physiological basis for high resistance to photoinhibition under nitrogen depletion in Emiliania huxleyi. Limnol. Oceanogr. 2010, 55(5), 2150–2160. [CrossRef]
- Paasche, E. Roles of nitrogen and phosphorus in coccolith formation in Emiliania huxleyi (Prymnesiophyceae). Eur. J. Phycol. 1998, 33, 33–42. [Google Scholar] [CrossRef]
- Shiraiwa, Y. Physiological regulation of carbon fixation in the photosynthesis and calcification of coccolithophorids. Comparative Biochemistry and Physiology 2003, 136, 775–783. [Google Scholar] [CrossRef]
- Oviedo, A.M.; Langer, G.; Ziver, P. Effect of phosphorus limitation on coccolith morphology and element ratios in Mediterranean strains of the coccolithophore Emiliania huxleyi. Journal of Experimental Marine Biology and Ecology 2014, 459, 105–113. [Google Scholar] [CrossRef]
- Smetacek, V. Diatoms and the ocean carbon cycle. Protist 1999, 150, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; Colomban de Vargas; Bittner, L.; Zingone, A. and Bowler, S. Insights into global diatom distribution and diversity in the world’s ocean. PNAS 2016, 113(11), E1516-E1525. [CrossRef]
- Rhee, G.-Y. Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol. Oceanogr. 1978, 23, 10–25. [Google Scholar] [CrossRef]
- Goldman, J.C.; Mccarthy, J.J.; Peavey, D.G. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 1979, 279, 210–215. [Google Scholar] [CrossRef]
- Raven, J.A. The role of vacuoles. New Phytol. 1987, 106, 357–422. [Google Scholar] [CrossRef]
- Margalef, R. Life forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1978, 1, 493–509. [Google Scholar]
- Villamaña, M.; Marañón, E.; Cermeño, P.; Estrada, M.; Fernández-Castro, B.; Figueiras, F.G.; Latasa, M.; Luis Otero-Ferrer, J.; Reguera, B. and Mouriño-Carballido, B. The role of mixing in controlling resource availability and phytoplankton community composition. Progress in Oceanography 2019, 178, 102181 https:// doiorg/101016/jpocean2019102181.
- Smayda, T.J. Ecological features of harmful algal blooms in coastal upwelling ecosystems. South African Journal of Marine Science 2000, 22(1), 219–253. [Google Scholar] [CrossRef]
- Smayda, T.J.; Reynolds, C.S. Community assembly in marine phytoplankton: Application of recent models to harmful dinoflagellate blooms. J. Plankton Res. 2001, 23, 447–461. [Google Scholar] [CrossRef]
- Glibert, P.M. Margalef revisited: A new phytoplankton mandala incorporating twelve dimensions, including nutritional physiology. Harmful Algae 2016, 55, 25–30. [Google Scholar] [CrossRef]
- Silkin, V.; Fedorov, A.; Flynn, K.J.; Paramonov, L.; Pautova, L. Protoplasmic streaming of chloroplasts enables rapid photoacclimation in large diatoms. J. Plankton Res. 2021, 43, 831–845. [Google Scholar] [CrossRef]
- Siegel, D.A.; Doney, S.C.; Yoder, J.A. The North Atlantic spring phytoplankton bloom and Sverdrup’s critical depth hypothesis. Science 2002, 296, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Adrian, R.; De Senerpont, D.L.; Elser, J.J.; Gaedke, U.; Ibelings, B.; Jeppesen, E.; Lürling, M.; Molinero, C.J.; Mooij, W.M.; van Donk, E.; Winder, M. The Plankton Ecology Group (PEG) Model: mechanisms driving plankton succession. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 429–448. [Google Scholar] [CrossRef]
- Sverdrup, H.U. On conditions for the vernal blooming of phytoplankton. J. Cons. Int. Explor. Mer. 1953, 18, 287–295. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.S. Resurrecting the Ecological Underpinnings of Ocean Plankton Blooms. Annu. Rev. Marine. Sci. 2014, 6, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Mikaelyan, A.S.; Chasovnikov, V.K.; Kubryakov, A.A.; Stanichny, S.V. Phenology and drivers of the win-ter-spring phytoplankton bloom in the open Black Sea: the application of Sverdrup's hypothesis and its re-finements. Prog. Oceanogr. 2017, 151, 163–176. [Google Scholar] [CrossRef]
- yrrell, T.; Merico, M. Emiliania huxleyi: bloom observations and the conditions that induce them. In Coccolithophores from Molecular Processes to Global Impact, Thierstein, H.R., Young, J.R., Eds. Springer, Berlin, Heidelberg 2004, 75-97.
- Raven, J.; Crawfurd, K. Environmental controls on coccolithophore calcification. Mar. Ecol. Prog. Ser. 2012, 470, 137–166. [Google Scholar] [CrossRef]
- Goldman, J.C. Potential role of large oceanic diatoms in new primary production. Deep-Sea Res. 1993, 40, 159–168. [Google Scholar] [CrossRef]
- Kemp, A.; Pearce, R.B.; Grigorov, I.; Rance, J.; Lange, C.B.; Quilty, P. and Salteet, I. Production of giant marine diatoms and their export at oceanic frontal zones: Implications for Si and C flux from stratified oceans. Global Biogeochem Cycles 2006, 20, 1–3. [Google Scholar] [CrossRef]
- Villareal, T.A.; Brown, C.G.; Brzezinski, M.A.; Krause, J.W. and Wilson, C. Summer diatom blooms in the north Pacific subtropical gyre: 2008–2009. PLoS ONE 2012, 7, e33109. [CrossRef]
- Pautova, L.; Silkin, V.; Kravchishina, M.; Klyuvitkin, A.; Kudryavsteva, E.; Glukhovets, D.; Chultsova, A.; Politova, N. Phytoplankton of The High-Latitude Arctic: Intensive Growth Large Diatoms Porosira Glacialis in The Nansen Basin. J. Mar. Sci. Eng. 2022, 10, x. [Google Scholar] [CrossRef]
- Edwards, K.F.; Thomas, M.K.; Christopher, A.; Klausmeier, A.C. and Litchman, E. Allometric scaling and taxonomic variation in nutrient utilization traits and maximum growth rate of phytoplankton. Limnol. Oceanogr. 2012, 57, 554–566. [Google Scholar] [CrossRef]
| Date | Max biomass |
% of total biomass |
Dominant species |
| 07.2017 | 1609.75 | 98.9 | Pseudosolenia calcar-avis |
| 07.2018 | 1121.0 | 91.0 | Pseudosolenia calcar-avis |
| 07.2019 | 214.0 | 67.3 | Pseudosolenia calcar-avis |
| 07.2020 | 4300,9 | 98.5 | Pseudosolenia calcar-avis+ Proboscia alata |
| 08.2021 | 3914.0 | 95.3 | Pseudosolenia calcar-avis + Proboscia alata |
| Species | 17.08.2017 | 22.07.2020 | 31.07.2020 | 16.08.2020 | 26.08.2020 | 17.08.2021 |
| Proboscia alata | 0 | 112.9 | 902.6 | 557.6 | 5.4 | 1298.5 |
| Pseudosolenia calcar-avis | 1430.4 | 3496.2 | 556.7 | 124.0 | 356.8 | 2058.4 |
| Dactyliosolen fragilissimus | 0 | 0 | 0 | 0 | 0 | 500 |
| Pseudo-nitzschia delicatissima | 13.6 | 0 | 0 | 0 | 0 | 0 |
| N | P | Si. | N:P | Si:N | Si:P | ||||||
| E. h. | P. c-a | E. h. | P. c-a | E. h. | P. c-a | E. h. | P. c-a | E. h. | P. c-a | E. h. | P. c-a |
| 44 | 86 | 44 | 86 | 44 | 86 | 44 | 86 | 44 | 86 | 44 | 86 |
| 0,77 | 1.04 | 0.10 | 0.04 | 5.54 | 2.19 | 12.63 | 60.29 | 8,74 | 4,35 | 99.4 | 87.0 |
| p = 0.04 | p = 1.7·10-8 | p = 6.25·10-13 | p = 0.0003 | p = 0.003 | p = 0.51 | ||||||
| Date (day, month) |
Species | Regression equations Mg m-3 |
Confi-dence interval |
| 2019 | |||
| 21.05. | Emiliania huxleyi | 7111.9+5083.5 N +5291.8 P +5166.1 NP | 551.0 |
| 21.05. | Pseudo-nitzschia delicatissima | 2205.0+1991.2 N +1382.4 P +1340.7 NP | 419.4 |
| 21.05. | Dactyliosolen fragilissimus | 1613.7+310.4 N -294.3 P -133.6 NP | 479.8 |
| 2020 | |||
| 11.06. | Emiliania huxleyi | 494.6-49.3 N +327.6 P -51.1 NP | 78.6 |
| 11.06. | Cerataulina pelagica | 3378.5+2353.5 N +1797.1 P +1055.0 NP | 1783.2 |
| 11.06. | Leptocylindrus danicus | 5599.2+4267.9 N +4081.3 P +3779.9 NP | 903.7 |
| 2021 | |||
| 11.06. | Proboscia alata | 2854.0+780.3 N - 480.9 P -732.2 NP | 788.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





