1. Introduction
The wiring diagram of jaw-muscle activation during chewing has been studied extensively, yet the in vivo control mechanism of jaw muscles of conscious subjects has remained an enigma [
1]. The causative relationship of dental occlusion and tooth contacts eliciting jaw movement activity has been much debated and disputed. In the early years of 20th century jaw-closing and the concomitant reciprocal inhibition of jaw-opening muscles were found to be elicited by tooth contact stimuli [
2] whereas today some authors completely deny the importance of occlusal contacts for eliciting dysfunctional jaw muscle activity [
3]. Nevertheless, we still don’t understand why tooth wear and cracks appear in some people more easily.
The present-day consensus suggests that masticatory movements are caused by fast-conducting bilateral, corticobulbar outputs from the pre-central gyruses of the parietal lobes, the masticatory motor cortex [
4]. Special emphasis has been advocated for the dorsal part of the brain stem, adjacent to the trigeminal nuclei, where astrocytes are postulated to contribute calcium-dependent rhythmogenic outputs resulting in repetitive open-close jaw-movement sequences of mastication [
5,
6]. Most dentistry textbooks vaguely explain mammalian mastication as “rhythmic, stereotyped activity of the jaw-closing and -opening muscles guided by the masticatory central pattern generator (CPG)”. Since the pace and force rate of rhythmic masticatory movements are subject to variability according to food texture, it has been explained that the innate consistency of different food items “modulates the pace and force of mastication by peripheral sensory inputs”.
However, the plausible mechanisms of this specific “peripheral sensory feedback modulation of the CPG output” remain to be explained satisfactorily. To provoke rhythmic, “fictive mastication”, the test animals must be subjected to the carefully adjusted flow of repeated electrical or transcranial magnetic stimuli. Half a century ago, in their classic study Dellow and Lund [
7], stimulated the putamen and the corticobulbar pathways of rabbits with trains of repeated 1 millisecond (ms) electrical pulses. The frequency of these pulsations was adjusted at 40 Hz,
i.e., the pulsation train provoked an electrical stimulus every 25 ms. They reported that if the train of pulsations was set at 20 Hz (50 ms interval between pulses) the rhythmic jaw muscle activity phenomenon was not always predictably attained. Furthermore, in their “Results” section Dellow and Lund specifically stated that
“Jaw opening was always the first movement observed following a latent period from the beginning of stimulation”. My philosophical question is, why should food-crushing start with jaw opening? The rhythmic jaw movements of the test animal were found to be synchronous with depolarisation pulses of the hypoglossal nucleus. Perhaps, the artificially induced “fictive mastication”, that looks and appears as if it was “true” masticatory movement might actually be a compound of dysfunctional, repetitive patterns of interrupted attempts for swallowing reflexes, distorted by the artificially hasty trains of repeated electric or magnetic stimuli subjected to the descending pathways of the sensory-motor cortex of a test animal.
The innate vigilance of our neural system does a fairly good job of preventing tooth-fracture emergencies during mastication. Predetermined and stereotyped patterns of jaw movements may be good for drinking and swallowing, but to chew the soft and hard parts of fruits-and-nuts-cake, instantaneous increase/decrease -adjusting of jaw muscle force is required. The hard parts of composite foodstuffs constantly cause unexpected tilts and unilateral jolts for the jaw-lever with possibly catastrophic consequences, were it not for rapid-responding feedback from peripheral afferent innervation.
Natural empty mouth jaw-closing without any tooth-food contacts is executed by bilateral muscle activity. The jaw-opening part of the chewing cycle is also executed by a bilaterally equal amount of activity of jaw muscles. In the absence of tooth contacts (when speaking, yawning, jumping, or walking et.c.) the mere stretching of jaw muscle spindles, without tooth contacts, does not provoke nor inhibit the activity of the masseter and temporalis muscles.
It is not quite clear why the original, bilaterally executed motor efferent feed for the rhythmic open-close activity of jaw muscles abruptly turns into powerful unilateral reflex actions. The switching over from centrally driven, bilateral jaw muscle activity to the unilateral mode of activity of food-crushing is extremely rapid and apparently monosynaptic. The excitatory reflex response appears to be exclusive to the working side jaw-closing muscles; and occurs within 12 ms after a hard particle is placed between molars of a laboratory animal undergoing fictive mastication movements [
8]. For natural mastication, an unilaterally located piece of food causes the activation of jaw-closing muscles from the working side only, ipsilaterally to the piece of food [
9].
In humans, the activity of jaw-closing muscles can be temporarily inhibited by tapping an incisor tooth. The “silent period” follows after a latency of about 10 ms. The inhibition of jaw-closing muscles lasts some dozens of ms, after which the temporal and masseter muscles resume their activity [
1,
10]. Previously, I conducted a clinical study to demonstrate the qualitative difference of the kinematics of natural jaw-closures starting from an anterior tooth (ANT) contact, as compared to the kinematics of jaw-closings starting from a first contact in the back-tooth (BAT) -area. Bites starting from an ANT-contact stalled the jaw-closure movement for some dozens of ms, but bites starting from BAT-contacts were accelerated more immediately [
11]. The reciprocity of neural output between ANT and BAT contacts can also be demonstrated by placing a tongue blade between the ANT so that the BAT contacts are prevented. As a result, the test subjects’ ability for forceful jaw clenching is reduced [
12]. Instant stalling of the masseter and temporalis muscles is also induced during horizontal, gliding jaw movements [
13,
14]. Apparently, this is because of the “canine guidance phenomenon”. Human ANT-contacts cause minute disclusion of the lower BAT-contacts against the upper BAT during horizontal food-grinding movements [
11]. Interestingly, in addition to animal studies that demonstrate the rapid excitatory quality of BAT-contacts [
8], evidence of the dichotomous reciprocity (“ANT is not BAT”) of the human masticatory wiring diagram is also available. Two experiments, analogous in design, have demonstrated that a tap on a human incisor causes a short latency inhibitory silent period of the masseteric EMG activity, whereas a tap-stimulus on a molar tooth rather causes rapid-onset excitatory activity of working side masseter muscle [
15,
16].
Real-time monitoring of unexpected tooth-contacts and muscular strains of food-crushing by primary afferent neurons (PAN) is essential for the control and reflex responses to the instantaneously changing and unpredictable kinematics of the jaw-lever during mastication. Spatially precise and rapidly responding reflex surveillance of oral peripheral sensory inputs has been a necessity for the coherence of food-crushing starting from the earliest jawed vertebrates. This article prospects and examines the existing research data on the evolution of the vertebrate jaw and its’ neural control. Coincidental with the evolution of the jaw, the conduction velocity of PANs and their respective peripheral sensor organs also improved together with an innovative neural unit in the brain stem, the trigeminal mesencephalic ganglion (Vmes). I propose a hypothesis explaining the neural mechanism that controls bite force during food crushing.
2. Background
2.1. The evolution of the triangular jaw
The jawless vertebrates with their sharp and caudally inclined teeth were inadequately prepared for the protective-thickness-amassing chitin-exoskeleton arms race of the Devonian seas. With their relatively inefficient peristaltic pulsations of their circular mouth the jawless vertebrates were able to grasp, pierce, and swallow only the soft-skinniest of prey animals. They were probably slow to respond to the unexpected soft-hard variabilities in the consistency of food, not to mention of controlling the “unwillingness-to-become-eaten-up-resistance” and the escape-potential of living prey. The cartilaginous rims of their circular mouth did not have the mechanical rigidity, nor neural feedback mechanism fast enough to target the available muscle force for cracking the weak spots of the body armour of e.g., trilobites. The lower jaw (a.k.a inferognathal, when talking about early jawed vertebrates), evolving originally for placoderms in the Silurian marine ecosystems some 430 million years ago, was a novel type of a “feeding limb”, a mechanic lever for crushing hard chitin shells.
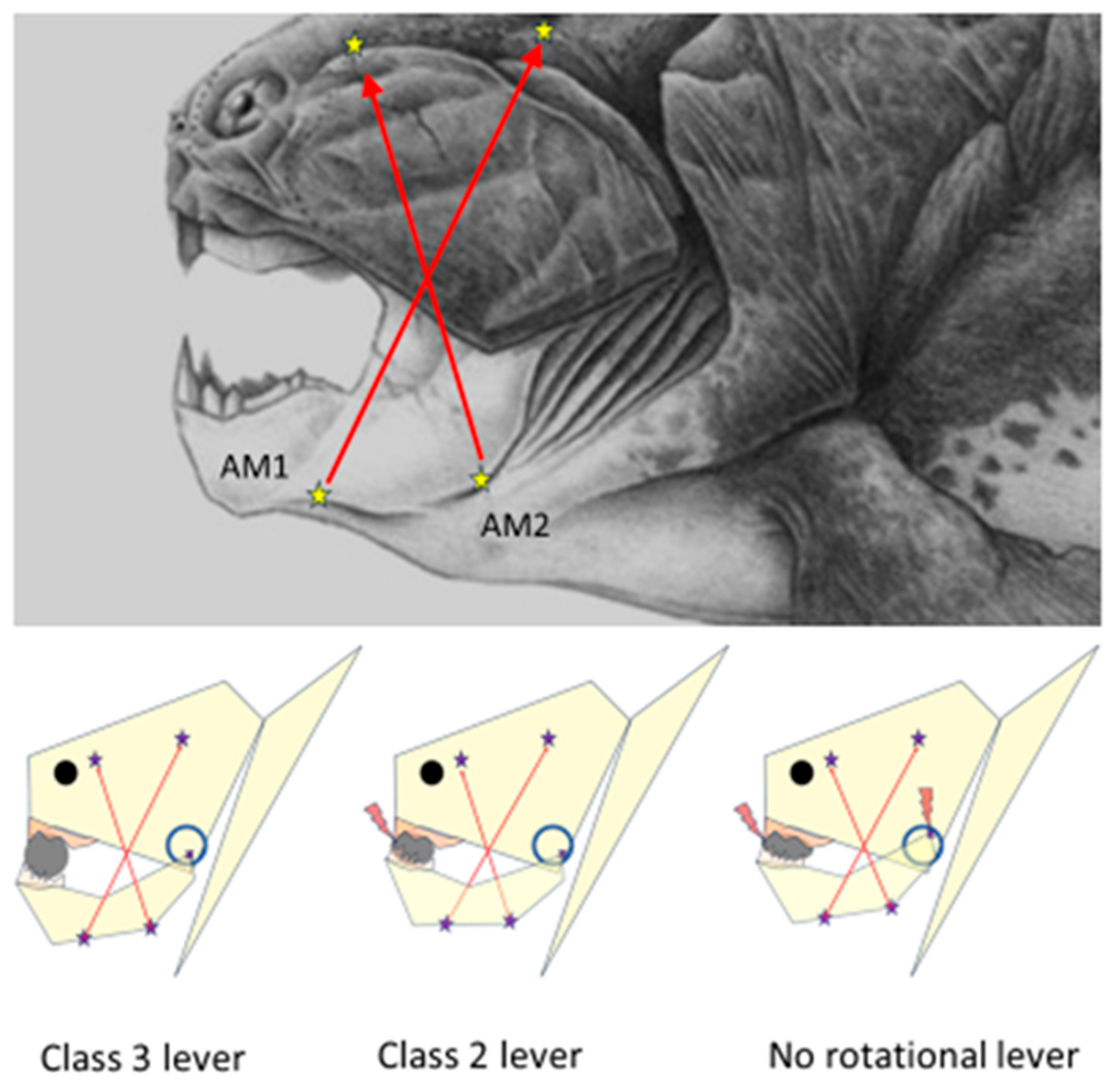
Overall, the lower jaw of placoderms may be considered a rigid, muscle-wrapped triangular pendulum suspended to the skull by the articular endings of the jaw arms. The rostral parts of the symmetrical jaw-arms were connected with a more or less rigid symphysis. The upward movements of the symphyseal part of the jaw triangle were limited by the unyielding contact against the upper jaw. Caudally, the rigid arms of the lower jaw were joined to the corresponding quadrate bone by bilateral articulations. The resiliency of the synovial spaces of the casings of the articular fossae of the quadrate bone, the quadratomandibular joint, probably also allowed for a certain amount of freedom of movement (translation and distraction) of condyles. Studies on the fossil remains of the Devonian apex predator
Dunkleosteus terrelli have allowed the speculation that the translatory and rotational movements of the mandibular condyles and the distraction of the quadratomandibular articulation during jaw closure necessitated at least two differently oriented configurations of mandible adductor muscles, the AM1 and AM2 [
17], with insertion points along the ramal base of the inferognathal, and projecting to the attachment sites on the visceral side of skull roof and extending just above the upper margin of the rigid suborbital plate of
Dunkleosteus which was fused to the palatoquadrate plate. The attachment locations and force orientation vectors of AM1 and AM2 muscles are depicted in
Figure 1. It is plausible that the bulging of AM1 and AM2 muscles should have caused lateral expansion of the lower rim of the orbital plates and the palatoquadrate – and caused an upward shift in the location of the quadratomandibular joint (the circle in
Figure 1 depicts the hypothetical range of movement of the condyle head in relation to the skull). For a
Dunkleosteus engulfing a trilobite, the jaw opening (with the AM1 and AM2 relaxed) possibly lowered the location of the condylar fulcrum in relation to the skull, thus also enabling a larger gape.
Biomechanical models of food crushing should consider the instantaneous changes in the mode of force leverage that occur during food crushing. The condyle-ends of the jaw are often considered as fixed rotational fulcrums for class 3 type of force leverage by the jaw arms. However, as soon as the resistance of the compacting food bolus exceeds that required for the downward distraction of the articular head from its synovial counterpart, the class 3 mode of force leverage ceases to exist. In the case of the unfortunate trilobite undergoing compressive strains between the jaws of Dunkleosteus terrelli this is more than likely. Suddenly the food-crushing lever system becomes transcended into a class 1 mode of force lever, where the fulcrum is the hard piece of food – the trilobite. Bite force increases, and the trilobite starts to lose its integrity until the extremely hard, interlocking, and unyielding anterior “teeth”, the dental plates of the upper and lower jaw make contact. Another transcendence of force leverage system takes place. Now, the anterior dental plates act as the fulcrum of a class 2 force lever, and the previous, downward distracting strain on the synovial ends and the suborbital plate of the jaw lever system is relieved. Class 2 type of force leverage continues squeezing the trilobite and the synovia of the articulations until their resistance equals that of the anterior dental plates.
2.2. The food-crushing class 1 lever
The jaw is a versatile mechanical tool capable of instantaneous transformations of its mode of force leverage. The class 1 lever mode may be the most efficient one for levering force inputs since all input force is transmitted entirely to the piece of food itself – nothing is wasted as reaction energy. In the class 2 mode of force leverage a part of the input forces are lost as reaction force which is transmitted to compress the ANT fulcrum. Also, in the class 3 mode of food-crushing a part of the input force is lost as reaction force to distract the synovia of the articulations.
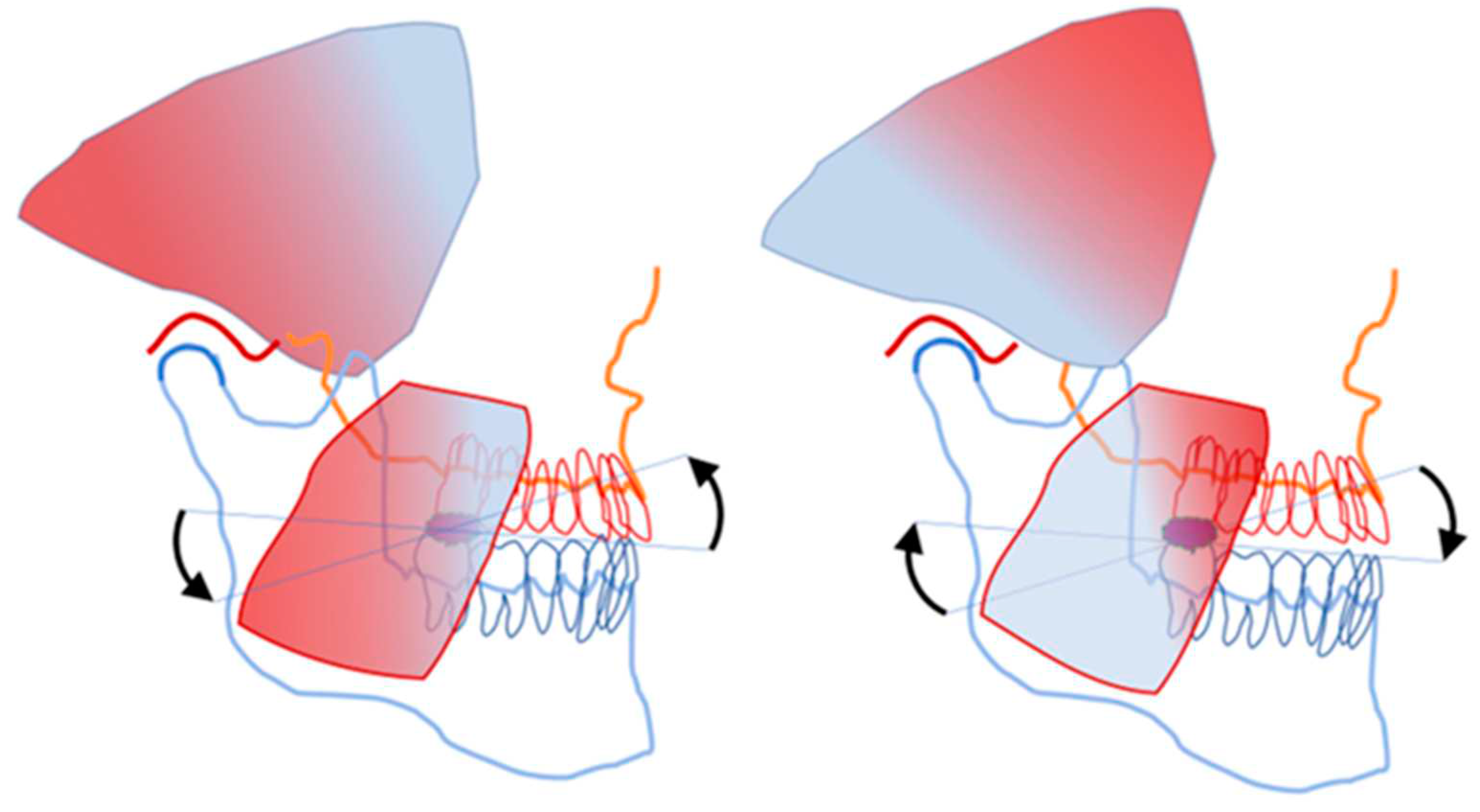
Vertebrate mandibles are equipped with many flanges and distant extensions to extend the length of force-leverage arms. The coronoid process of the human mandible is the site of insertion of the fan-shaped temporalis muscle. The motor units of the anterior part of the human temporalis muscle are directed almost perpendicular to the motor units of the posterior part of the same muscle. The masseter muscle comprises of two slightly differently oriented parts, the superficial and deep bellies. The overall directional configurations of the motor units of the masseter and temporalis muscles are very differently oriented, yet they seem to operate harmoniously during food crushing. Separate innervation for differently oriented motor units of jaw closing muscles may be assumed. The superficial and deep bellies of the masseter muscle get active/turn slack according to the rapidly changing functional needs of food crushing. The almost perpendicularly oriented parts of the temporalis muscle must also have a neural control mechanism that prevents simultaneous activation of contrasting force vectors. The cooperation between muscle stretch-sensing spindles and the bite force controlling mechanism of the central nervous system is essential to ensure differentially oriented functional stretching of the fan-shaped jaw muscles to instantly target specifically the activity of the stretched motor units only [
19].
Figure 2 illustrates the heterogeneity of the stretching of the temporalis and masseter muscles during food crushing.
To control the jolts of the unstable mandible during food crushing, counter-directional excitatory stretch reflexes of the differentially oriented motor unit of jaw-closing muscles must alternate between the caudal and rostral parts of the jaw-lever. Food crushing within the class 1 lever mode may be regarded as a see-saw that perpetuates reciprocal reflex movements of the mandible until the food-bolus fulcrum is disintegrated to the occlusal plane level.
2.3. The caveats of powerful force-leverage
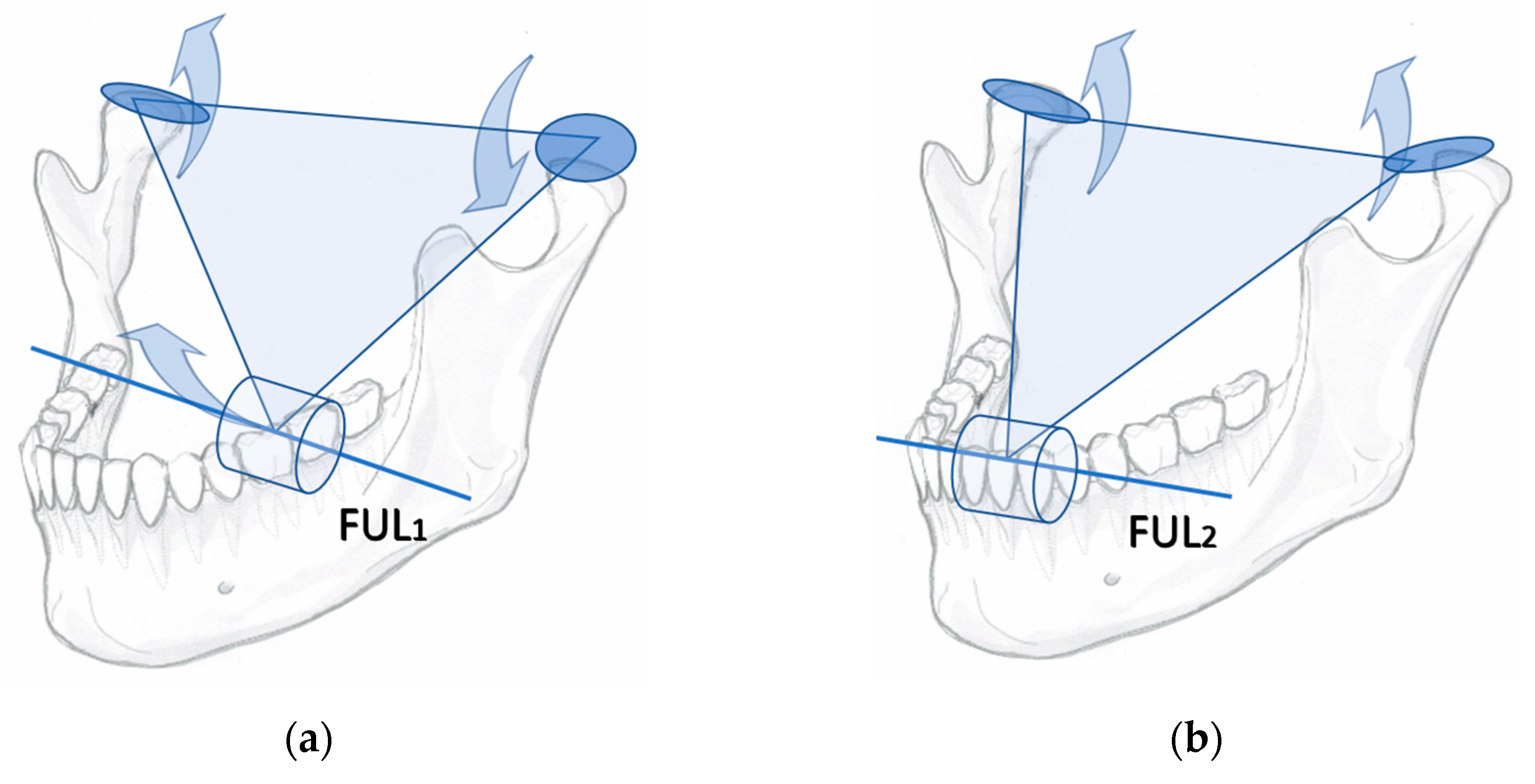
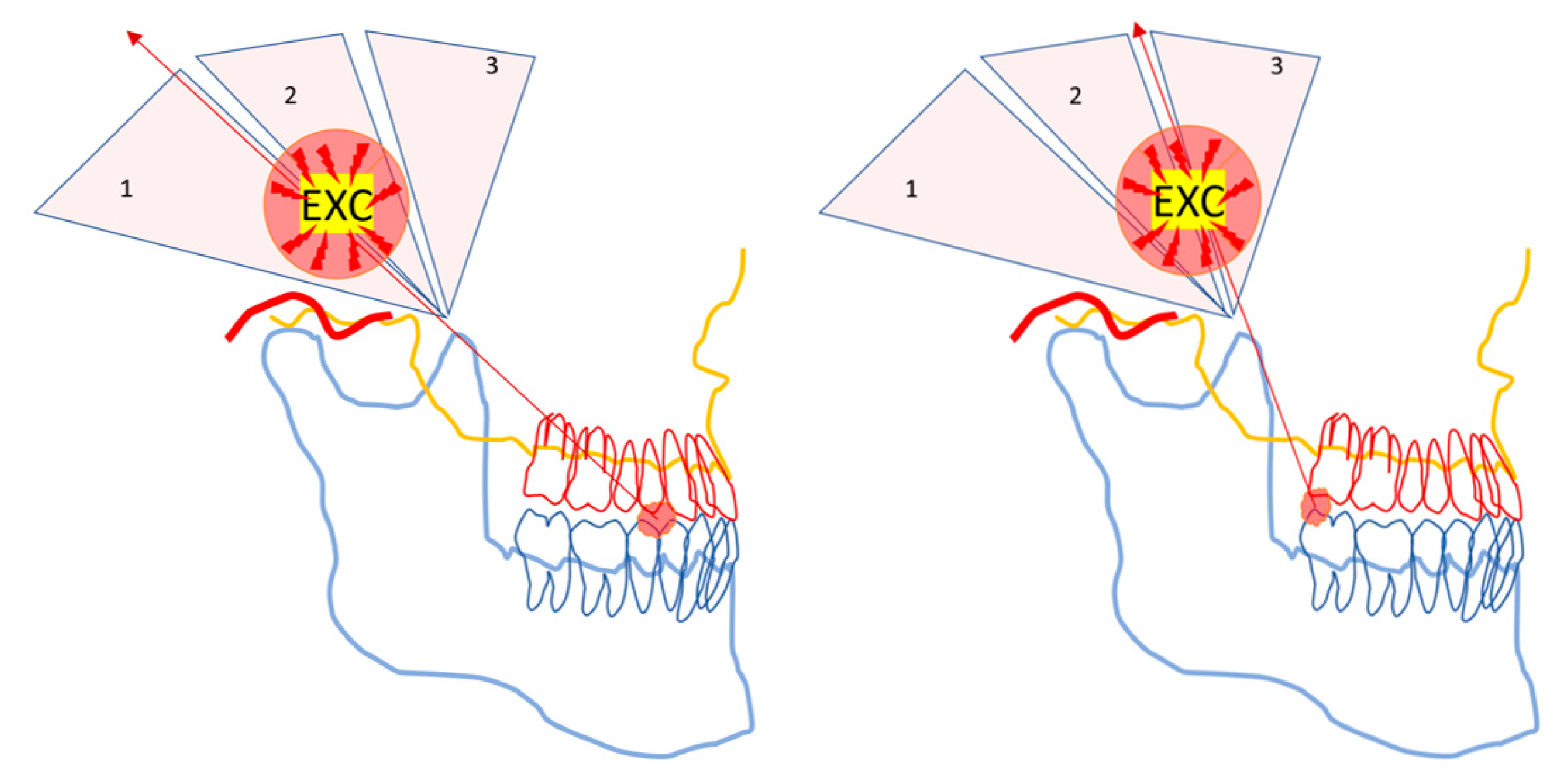
Any kind of powerful mechanical tool must be handled with caution. There are caveats to using the jaw-lever to multiply muscle force. Food crushing with the jaw-lever may cause rapidly occurring, unexpected, and potentially harmful strains for the jaw articulation and the ANT (
Figure 3).
Distraction and tearing of the articulation occur ipsilateral to the hard piece of food, while the contralateral jaw-joint might be damaged by the sudden leverage of an unfavourably placed food-particle fulcrum in the BAT area. Therefore, the three ends of the triangularly arranged jaw-lever must be protected by inhibitory, short latency withdrawal reflexes.
2.4. The two parallel neural infrastructures for vertebrate jaw operations
The excitation and inhibition for circumoral, mouth-constricting muscles of early jawless vertebrates were most likely conveyed by peripheral afferent inputs. Lampreys and hagfishes are the few jawless fish species remaining extant today. To extrapolate our understanding of the neural systems of lampreys to that of extinct jawless vertebrates it might be speculated that the PANs projecting sensory feedback from tactile receptors of the jawless mouth synapsed and crossed over to the midbrain to be further processed in the primitive forebrain pallium. In turn, reflex-like sets of sequenced synaptic spikes would have been produced in the pallium to be executed to the midbrain motor nuclei for repetitive and stereotyped rostro-caudally propagating pulsatory food-intake movements of oral muscles [
21]. The oromotor neural systems of hagfishes and lampreys are also distinct from jawed vertebrates by the absence of Vmes and myelin [
22]. Without myelin, the conduction velocity of the reflex pathways of the early jawless vertebrates was inadequate and slow to respond coherently to the instantaneous changes of the location, hardness, and the number of shattered food particles across and about the circular mouth.
The mammalian jaw appears to have two parallel and alternating modes of neural control. The trigeminal, Vmes-mediated control pathway is reserved for strictly unilateral jaw movement reflexes, whereas the controlling of digastric and tongue muscle reflexes are organized bilaterally [
23]. The latter, hemisphere-crossing pathway might be regarded as the original one that existed for jawless vertebrates. However, for jawed vertebrates, all of which are in possession of Vmes, the bilateral, masticatory pallium-driven feed for jaw movement patterns appears to be temporarily overrun by independent and autonomous unilateral jaw-muscle reflexes.
2.5. The Vmes is a “shortcut pathway” for controlling unexpected jaw movements
For the jawed vertebrates, unilateral and independent control of sensorimotor reflexes was required to monitor and control the proprioceptive tooth contacts and the heterogenous stretching of motor units of the right and left side muscles. To accommodate the rapidly alternating excitatory and inhibitory needs of the jaw-levering muscles, a novel type of sensory ganglion was necessary for the autonomy of right- and left-side reflexes, analogous to the spinal dorsal root ganglia (DRG) monitoring the independent reflex responses of bilateral fins and limbs. An “add-on proprioceptive supplement”, the Vmes, was installed to the original vertebrate neural bauplan. The PAN-mediated feedback from the right- and left-side teeth and jaw muscles were independently collected by the ipsilateral Vmes, the shortcut switch in the rostral part of the midbrain. The Vmes evolved as the universal hallmark of jawed vertebrates to handle the rapidly changing and unexpected kinematics of the triangularly arranged jaw-lever, the symphysis-joined pair of bilateral feeding limbs.
As result, jawed vertebrates have two distinct modes for the control of jaw movement, the fast, monosynaptic Vmes connection is there to rapidly surpass the slow, polysynaptic pallium connection. The more rapid Vmes pathway delivers ipsilaterally executed proprioception, while the slow- responding, polysynaptic mode of jaw movement control, mediated by masticatory pallium, with many synaptic connections, is slow to respond to afferent sensory stimuli. The polysynaptic neural connection between mouth and brain is reserved for the more-or-less stereotyped cascades of low-force movement tasks, such as swallowing. Another example of the slow, bilaterally executed polysynaptic mode of control is the jaw opening. As there are no tooth-food contacts, opening an empty mouth does not require sensory monitoring of peripheral inputs. Swallowing, yawning, and the repeated initiations of the opening phases of the masticatory cycle, as well as the closing phase of the jaw of an empty mouth, are also examples of the bilateral, polysynaptic, and centrally driven control mode of jaw movement.
Should the upper and lower jaws happen to have something hard between teeth, the fast Vmes mode, the food-crushing reflex would instantly be unleashed to overrun and replace the slow polysynaptic mode of control of jaw muscle activity. First come, first served.
2.6. The simplified, dichotomous perspective on mammalian dental formulas
For most mammaliform species, four different types of teeth (incisors, canines premolars, and molars) are positioned in the rostral and caudal parts of dentition, respectively. The rationale of the four different types of teeth of mammalian dental formulas could probably be simplified as a dichotomy according to the functional properties of their PAN connections to the Vmes. Simply, there are two kinds of teeth, inhibition-causing ANT and excitatory BAT.
The reciprocity of the neural wiring diagram, the “ANT is not BAT”-principle is probably the guiding determinant in embryonal tooth development. All teeth originate from cranial neural crest cells [
24]. The ANT of human upper jaw dentition consists of embryonal prosencephalon-derived premaxillary incisors and canines, the ANT. whereas premolars and molars, the upper BAT. are derived from the two sides of maxilla proper originating from the first branchial arch. The lower jaw incisor primordia develops from symphyseal mesenchymal condensations. Mandibular incisor precursor cells (i.e., lower jaw ANT) do not express the Barx1 homeobox gene, whereas the expression of Barx1-positivity characterizes the precursor cells of BAT. the molars [
25,
26,
27] and premolars of most mammals [
28]. The homeobox gene Barx1 may be the general characteristic to determine which type of PAN precursor cell axons become targeted for BAT during embryogenesis. It would be plausible that the “inhibitory type PAN precursor cells” in turn, would have a predilection for axon growth towards the ANT periodontal ligament. Conversely, the PAN precursor cells equipped with excitatory neurotransmitter production abilities would be targeted for BAT. The mechanisms of migration and axonal growth of neural cells towards the target sensory organs is not completely understood [
29], and even less is known of axonal growth cones of embryonal teeth. Perhaps, the Barx1-negative gene-expression phenotypes of the primordia of ANT are enticing for the growth of the axons of the inhibitory-phenotype PANs, to which this text refers as “PAN-pmr-ANT” while the mesenchymal primordial buds of Barx1-positive BAT would provide specific axonal growth cone cues for attracting excitatory type of PAN (“PAN-pmr-BAT”).
2.7. Improved velocity of reflexes
Coinciding with the first jawed vertebrates, myelin-producing Schwann cells also appeared for the first jawed vertebrates to improve the conduction velocity between sensory receptors and motor efferent nerves of jaws and fins. Indeed, no evidence of myelin sheath can be demonstrated for fossils of the sister clade of the same era, the jawless
Osteostraci [
30]. Rapid proprioceptive sensors acting within milliseconds of a proprioceptive stimulus certainly would have been useful for the neural infrastructure operating the unpredictable kinematics of food-crushing by jaw-lever. However plausible and fascinating the existence of such neural mechanism would be, there is no fossil evidence of the existence of any kind of muscle stretch sensors of early jawed vertebrates. The stretching of muscles was perhaps sensed by the Piezo1 or Piezo2 type of calcium channel units [
31], or early exemplars of the muscle spindle evolution line were already available [
32]. No doubt, the enhanced rapidity of muscle reflexes provided by myelinated neurons certainly was of great importance for locomotion, especially for large-sized animal species, such as
Dunkleosteus terrelli, but the improved velocity of their reflex actions must have been indispensable to control the unexpected, sudden kinematic tilts of their trilobite-cracking jaw-lever.
2.8. The sensory ganglia controlling jaw movements.
The mechanoreceptors of the periodontal ligament (pmr) of mammalian teeth are important proprioceptive sensors providing essential information to control jaw operations. Interestingly, these “tooth-contact-sensors” have two different pathways to the motor efferent neurons of the jaw muscles. For the vertebrate jaw, two main sensory ganglia and two phenotypes of PAN are operational. The Vmes houses the perikarya of PAN-Mes-phenotype of neurons, and the trigeminal ganglion (TG) houses the cell bodies of PAN-TG-phenotype of neurons. The TG and Vmes together are the exclusive and the only ganglia to collect the primary afferent neural inputs from the pmr of mammalian teeth. This kind of dual-pathway sensory arrangement of tooth-contact information (either PAN-TG-pmr, or PAN-Mes-pmr) may seem curious. However, the second important source of afferent inputs involved in the control of bite force, the muscle spindles, is not a “dual pathway”. The sensory information from the spindles of masticatory muscles is exclusively mediated by the pseudounipolar PAN-Mes-sp that have their perikarya within the Vmes only [
33,
34,
35]. The Vmes-housed PANs are involved with the muscle spindles, and the pmrs, whereas the TG-housed PANs are projecting only to the tooth mechanoreceptors and not at all to the jaw muscle spindles.
Another important difference between TG- and V-Mes-housed PAN cells can be identified by their axonal vesicle contents. Both ganglia house an heterogenous array of many different cell-types. The neurochemical properties of TG and its cells are distinct from the cells of the Vmes ganglia. The PAN-TG can express a wide array of peptide neurotransmitters, such as calcitonin-gene-related peptide, Substance P. et.c., with tissue morphogenetic functions. In addition to the PAN-TG neurons, TG has many different types of cells, satellite glial cells, fibroblasts and macrophage-like cells [
36,
37]. In distinction to the PAN-TG. the PAN-Mes neurons don’t express any peptide neurotransmitters [
38].
The outgoing axons of PAN-TG-pmr-phenotype submit neural information from tooth contacts via synapses in the midbrain sensory nuclei crossing over to the contralateral nuclei of brain stem, thalamus and the sensory cortex. The PAN-TG-pmr neural pathway appears to be the original mode of vertebrate proprioceptive information conveyed to the “masticatory-pallium”. In contrast, the Vmes-connection has ipsilateral, monosynaptic connections with motor efferent neurons of the jaw muscles. For the present text, I chose to consider the PAN-Mes neurons “functionally more oriented” for executing rapid, unilateral reflexes, as compared to the PAN-TG neurons. I postulate the PAN-Mes-mediated primary afferent neural output for the motor efferent neurons of jaw muscles is of more relevant importance for the control of the unpredictable and rapidly developing kinematic events of the vertebrate jaw.
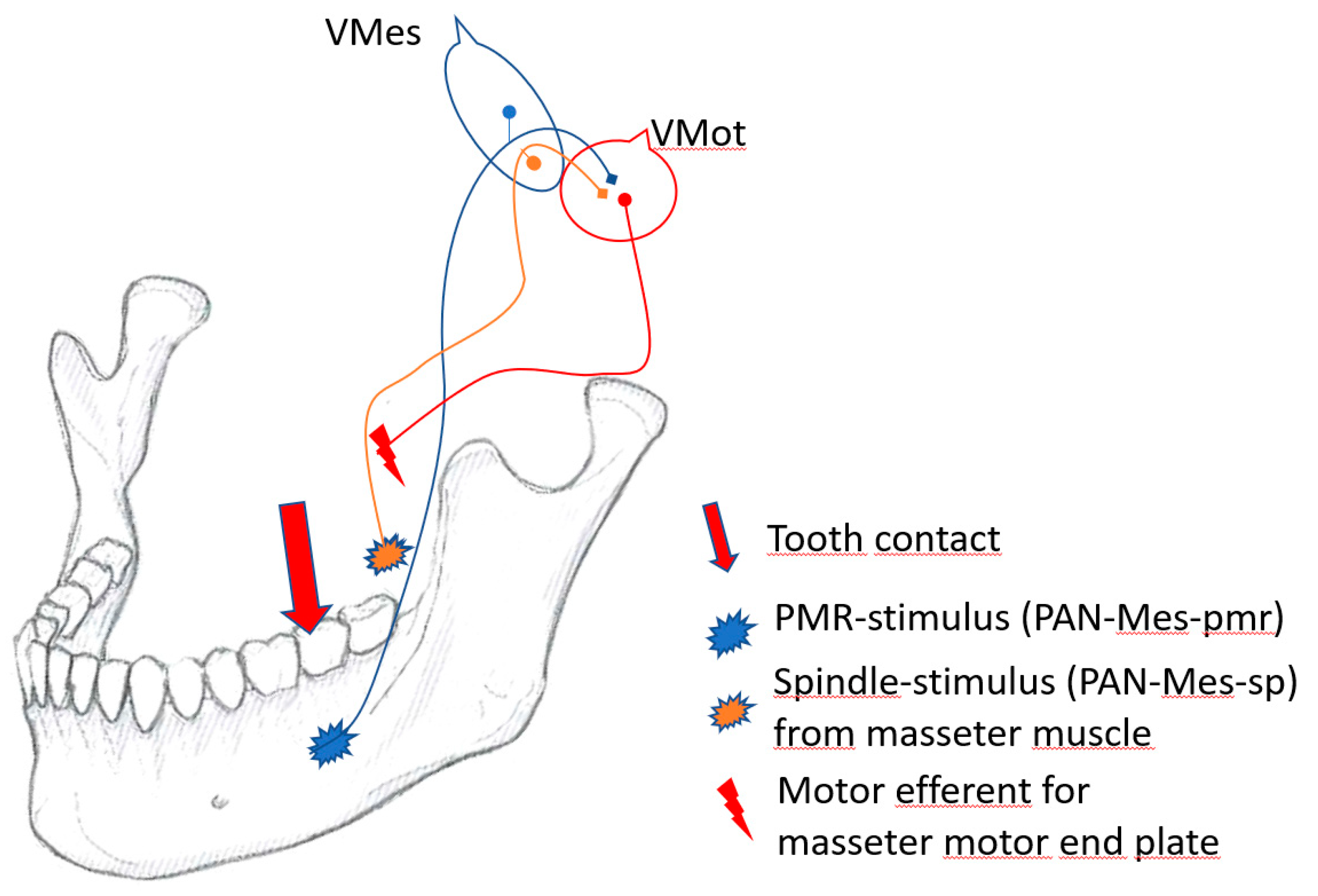
2.9. The coupling of spindle and pmr-inputs in Vmes
In the Vmes, two different kinds of PAN-Mes sensory neurons communicate. The PAN-Mes-sp and PAN-Mes-pmr convey information from two important sources. The two sources of proprioceptive information are from:
Muscle stretch sensing spindles, conveyed by PAN-Mes-sp neurons;
Tooth-contact-sensing periodontal mechanoreceptors, conveyed by PAN-Mes-pmr neurons.
Figure 4 illustrates the “presynaptic coupling” of the cell bodies and axons of the pseudounipolar, peripheral afferent neurons from two different sources, that are brought in close proximity with each other in the Vmes.
At the instant of tooth contact with solid food, neural inputs from both sources, the spindles and the pmrs, are delivered almost simultaneously to Vmes. In the Vmes, the axons and cell bodies of PAN-Mes-sp and PAN-Mes-pmr neurons are brought in physical proximity to each other. In my opinion, this is in order for the PAN-Mes-sp and PAN-Mes-pmr to communicate with each other. The within Vmes interplay between these two types of neurons does not necessarily need to be inter-axonal synaptic transmission [
39], but is probably conveyed by direct (and rapid), electrical gap-junction connections [
40,
41].
Vmes can be regarded as the control panel of the on-off switches for each of the differentially directed force vectors of the fan-shaped jaw-closing muscles. Jaw-muscle motor efferent neurons are not fully functional unless for simultaneous inputs from both muscle-stretching, and mechanoreceptor sources. The simultaneous feedforward from both sources may be essential for generating proper masticatory force. In the absence of proprioceptive neural inputs from the pmr the masticatory performance is reduced, as shown for dental patients with all their natural teeth missing and replaced by titanium implants [
42].
2.10. The pin-point targeting of muscle force
The PAN-Mes-sp are needed to answer the question: “Which motor units should be active to crack the hardest part of food?” Only the taut motor units can send monosynaptic firing for the homonymous motor efferent neurons via Vmes. The piece of food is the unstable fulcrum of the jaw-lever. See-saw type reciprocally alternating tilts of the jaw around the food-fulcrum cause alternating stretching and relaxing of the opposite halves of the fanned jaw muscles, as demonstrated for the human temporalis muscle (
Figure 2 and
Figure 5).
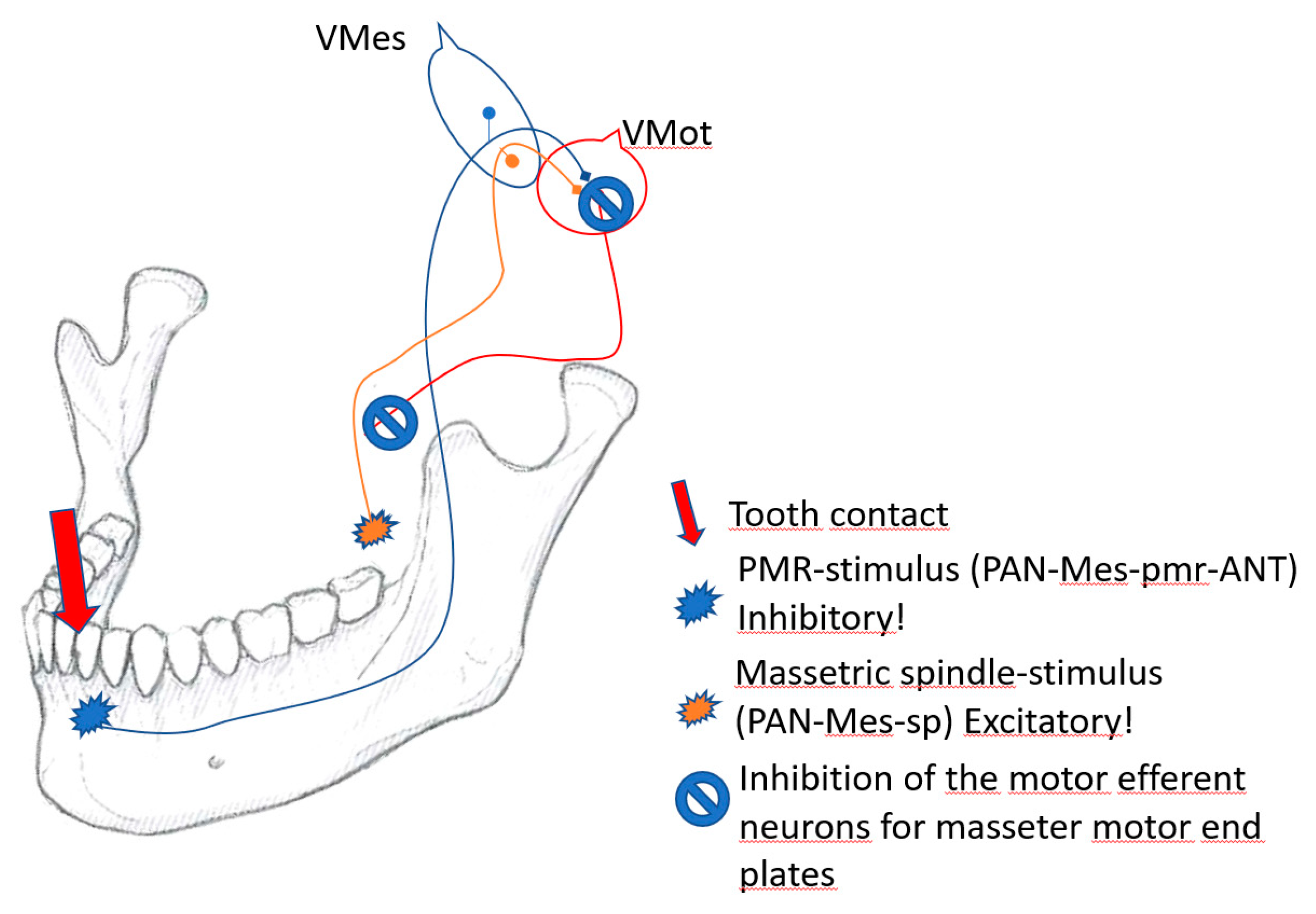
2.11. The Vmes-switch for the inhibitory withdrawal of muscle force
Vmes is the control panel of jaw muscle activity, where the PAN-Mes-sp and PAN-Mes-pmr neurons communicate with each other. Before executing their simple, monosynaptic commands both PAN-Mes-sp and the PAN-Mes-pmr neuron must be in mutual agreement: “Is it OK to direct jaw-muscle force for this specific tooth-contact-location at this very instant?” If the force leverage for this location might have noxious consequences for either the ANT or the jaw articulations, a “power-off switch” is there to protect the rostral tip of the jaw, and the jaw joints from harmful, excessive force multiplication by jaw-lever.
Passive jaw muscle stretching happens all the time (during speaking, yawning, sudden head movements et. c.), but, the reflex activation of jaw muscles seems to occur only if the tooth pmrs are also firing simultaneously. Those specific motor units that are stretched by the piece of food are activated only if they are “permitted” by the specific tooth, upon which the corresponding food-fulcrum is located. This feedback is monosynaptic, and conveyed by PAN-Mes-pmrs to the motor efferent neurons of jaw-closing muscles. Should the spatial location of the food-fulcrum happen to be located on the BAT. the perikarya, and the axonal part of the PAN-Mes-sp, would receive the presynaptic excitatory “go active” permission, by connection from the adjacent, excitatory PAN-Mes-pmr-BAT phenotype neurons. Both sources of the spindle and pmr inputs are in agreement.
However, the neural outputs of PAN-Mes-pmr-ANT should be considered inhibitory [
1,
10]. A masticatory contact from ANT- area causes a silent period by the inhibitory output within a latency of about 10 ms. For several dozens of ms, the excitatory motor efferent neurons of jaw-closing muscles can’t execute their mission. Furthermore, direct electrical inhibition of the axons and perikarya of the excitatory PAN-Mes-sp by gap-junction connections probably also takes place within Vmes (
Figure 6).
2.12. Evolutionary implications
Normally, in natural, empty mouth closures of humans, the anterior part of the dentition is most likely to subdue the impact of the first tooth contact [
11,
43]. The ANT and the rostral part of the jaw-lever are also subject to potentially noxious strains by forceful class 2 lever operations (
Figure 3). It makes sense for the ANT to be equipped with a protective sensory infrastructure for inhibitory withdrawal reflexes, while excitatory PAN-pmr-BAT neurons are needed to boost jaw muscle activity for BAT-area food-crushing. The ANT of the mammalian lower jaw normally occlude matching harmoniously with the upper jaw ANT only. However, there are distinctive species-specific exceptions to this rule. The shape of dental arches and dental formulas reflect the feeding habits and masticatory kinematics of any given species. The “C/P
3 honing complex” of most primate species, or the lack thereof in humans, demonstrates the extensive evolutionary implications of the “ANT is not BAT” -principle in the species-specific dental formulas. The large canines of apes prevent direct upper and lower jaw teeth contacts in the BAT-area during the horizontal phase of food grinding. For horizontal food grinding, non-hominin primates must protrude their jaw slightly to disconnect the interlocking large canines and then use their voluminous incisors for the lateral, food-grinding strokes. Hominins instead, have relatively the smallest canine teeth of the primate clade and effectively grind their food by broad horizontal strokes, guided by their canine teeth, and using their entire set of BAT. the premolars and molars, probably because the continuous flow of excitatory BAT-contacts boosts the grinding efficiency. For the non-hominin primates, the excitatory lower jaw first premolars (P
3, equipped with excitatory Pan-pmr-BAT) are arranged to contact with large and overlapping upper jaw canines (that are equipped with inhibitory PAN-pmr-ANT). This is probably why the bite force of the vertically oriented jaw closures of non-hominin primates is not properly inhibited during chewing activities. Constantly repeating, uninhibited shearing of the enamel of the non-hominin upper canine/P
3-contact characterizes the “simean honing complex”. Weapon-like attributes have been associated with the sharp-honed canines of e.g.,
Gorilla, but mostly, their honing complex serves as practical tableware for cutting bamboo shoots into more amenable chunks for chewing. Because of the “hominin, entire BAT-area extended horizontal honing complex” valuable nutrients of starchy underground yams tubers and from the tiny seeds of figs are more easily triturated. Grinding down the tendinous and cartilaginous parts of meats with horizontal strokes by the human BAT-area is more efficient than the vertical-only-directed BAT-area crushing strokes of non-hominin primates.
The superior efficiency of the human “BAT-area extended honing complex” comes with a cost. Progressive wear of human teeth is probably causally associated with this unique, hominin ability for horizontal food grinding in the BAT-area. Tooth wear is unavoidable and results in continuous age-dependent loss of vertical dimension of occlusion, which is causally concomitant with another common problem acknowledged by dentists, the “loss of canine guidance”, which in turn ultimately leads to accelerated wear of teeth and jaw joints and a wide array of dental and temporomandibular calamities [
44,
45], that are almost exclusive for the human lineage, and practically non-existent to other primates.
2.13. Coda
The first branchial arch of pre-gnathostomes stiffened into more rigid jaw-arms to enforce water flow for suction feeding [
46]. The early jaw was also useful for cracking the hard parts of food, an evolutionary determinant to evolve jaw muscles. Improved force-leverage and complicated kinematics of the symphysis-connected bilateral jaw-arms necessitated autonomous control of the neural afferent-efferent feed for the left and right sides of the jaw. Vmes is analogous to the DRG in executing rapid reflex responses to unilateral proprioceptive stimuli. However, the inhibitory withdrawal reflexes of DRG are disynaptic. No inhibitory synaptic transmitters are found for the first-order PANs of the DRG reflex arc, but inhibitory synaptic messenger molecules are characteristic for the PANs of Vmes [
38], Perhaps, the “need for monosynaptic, inhibitory PANs”, that are readily available in the hox-negative cranial domain, explains why the Vmes is the only primary afferent ganglion located within the central nervous system [
47]. As a result, the original, peristaltic food-intake mechanisms of the vertebrate mouth remained, but were supplemented with the evolutionary novelty, the food-crushing, neuronal plug-in gadget. The jaw itself is the plug-in hardware, the motor cortex is the main board of the computer, and the Vmes is the add-on microchip monitoring jaw movements. The Vmes and the triangular jaw-lever have been around for some 430 million years to monitor the consistency of food, and to switch on the jaw-muscle force to be dispensed whenever anything harder than water is caught between teeth. The unilateral, excitatory, and inhibitory food-crushing reflexes (the UFCR) is the underlying characteristic that manifests in the exorbitantly varied anatomical diversities of the extinct and extant vertebrate jaws demonstrating the natural history of feeding habits of the jawed vertebrate clade. Understanding the evolution of vertebrate jaw, and its neural control should open prospects for the systematic assessment of the causal conditions for “bite-issue-emergencies”. In the future, perhaps, a fractured tooth cusp will be classified as a preventable disease entity.