Submitted:
03 May 2023
Posted:
08 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Selection and description of subjects
2.2. CT scan protocol
2.3. Data recording and image analysis
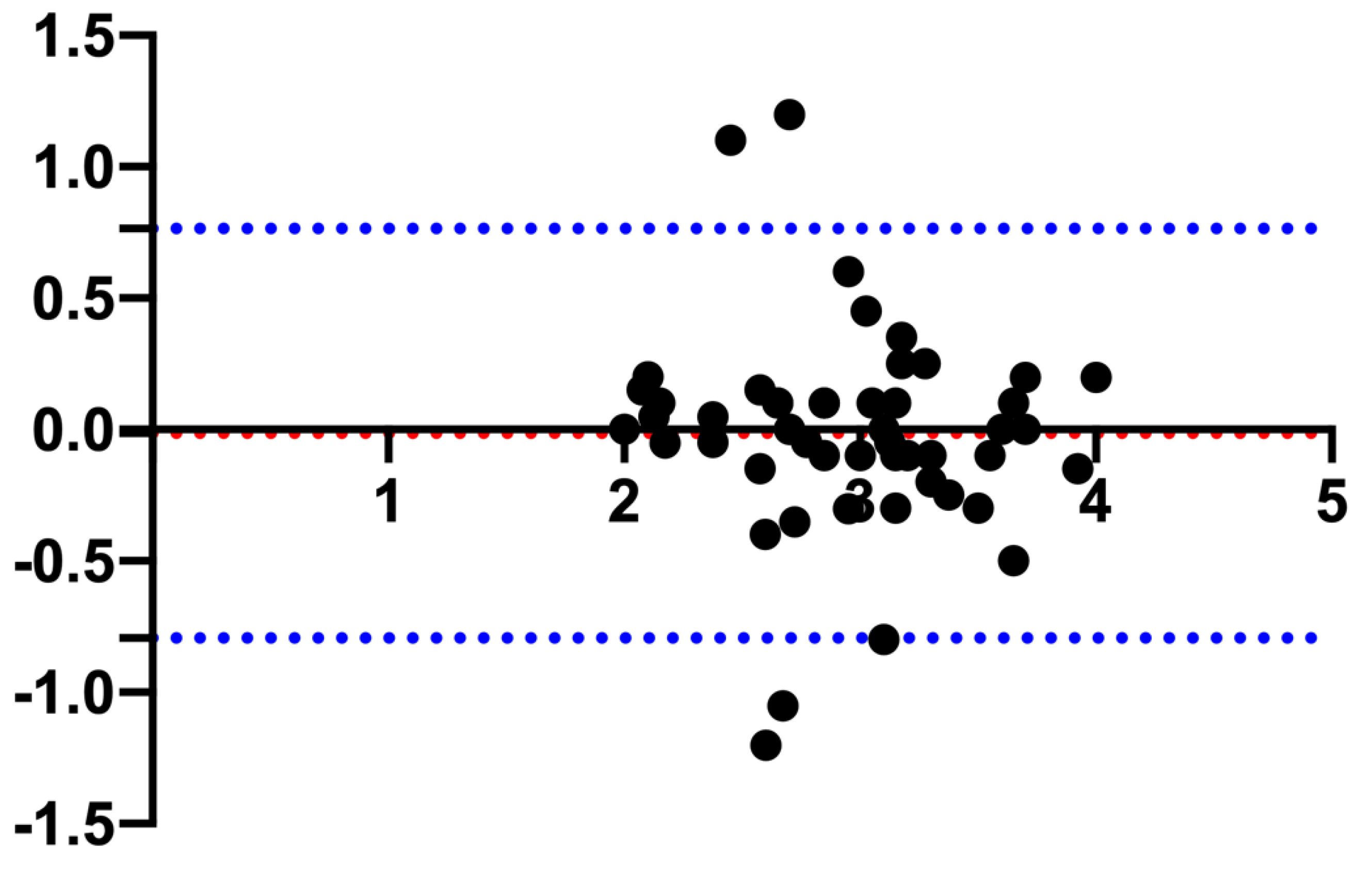
2.4. Statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, S.W.; Ellsworth, B.S.; Peréz Millan, M.I.; Gergics, P.; Schade, V.; Foyouzi, N.; Brinkmeier, M.L.; Mortensen, A.H.; Camper, S.A. Pituitary gland development and disease: from stem cell to hormone production. Curr Top Dev Biol 2013, 106, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Rosol, T.J.; Meuten, D.J. Tumors of the Endocrine Glands. In Tumors in Domestic Animals; 2016; pp. 766-833. [CrossRef]
- Polledo, L.; Grinwis, G.C.M.; Graham, P.; Dunning, M.; Baiker, K. Pathological Findings in the Pituitary Glands of Dogs and Cats. Vet Pathol 2018, 55, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Lunn, K.F.; Boston, S.E. 26 - Tumors of the Endocrine System. In Withrow and MacEwen's Small Animal Clinical Oncology (Sixth Edition), Vail, D.M., Thamm, D.H., Liptak, J.M., Eds.; W.B. Saunders: St. Louis (MO), 2020; pp. 565–596. [Google Scholar]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathologica 2017, 134, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.; Galac, S.; Meij, B.P. Pituitary tumour types in dogs and cats. Vet J 2021, 270, 105623. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.P. Imaging the Feline Neurologic System. In Feline Diagnostic Imaging; 2020; pp. 77-111.
- Owen, T.J.; Martin, L.G.; Chen, A.V. Transsphenoidal Surgery for Pituitary Tumors and Other Sellar Masses. Veterinary Clinics of North America: Small Animal Practice 2018, 48, 129–151. [Google Scholar] [CrossRef]
- Hecht, S.; Schwarz, T. Pituitary Gland. In Veterinary Computed Tomography; 2011; pp. 197-203. [CrossRef]
- Gunn-Moore, D. Feline endocrinopathies. Veterinary Clinics of North America: Small Animal Practice 2005, 35, 171–210. [Google Scholar] [CrossRef]
- Reusch, C.E. Chapter 2 - Disorders of Growth Hormone. In Canine and Feline Endocrinology (Fourth Edition), Feldman, E.C., Nelson, R.W., Reusch, C.E., Scott-Moncrieff, J.C.R., Eds.; W.B. Saunders: St. Louis, 2015; pp. 37–76. [Google Scholar]
- Niessen, S.J.M.; Forcada, Y.; Mantis, P.; Lamb, C.R.; Harrington, N.; Fowkes, R.; Korbonits, M.; Smith, K.; Church, D.B. Studying Cat (Felis catus) Diabetes: Beware of the Acromegalic Imposter. PLOS ONE 2015, 10, e0127794. [Google Scholar] [CrossRef]
- Tyson, R.; Graham, J.P.; Bermingham, E.; Randall, S.; Berry, C.R. Dynamic computed tomography of the normal feline hypophysis cerebri (Glandula pituitaria). Vet Radiol Ultrasound 2005, 46, 33–38. [Google Scholar] [CrossRef]
- Wallack, S.T.; Wisner, E.R.; Feldman, E.C. Mensuration of the pituitary gland from magnetic resonance images in 17 cats. Vet Radiol Ultrasound 2003, 44, 278–282. [Google Scholar] [CrossRef]
- Nadimi, S.; Molazem, M.; Jarolmasjed, S.; Esmaili Nejad, M.R. Volumetric evaluation of pituitary gland in dog and cat using computed tomography. Vet Res Forum 2018, 9, 337–341. [Google Scholar] [CrossRef]
- Häußler, T.C.; von Pückler, K.H.; Thiel, C.; Enderlein, S.; Failing, K.; Ondreka, N.; Kramer, M.; Schmidt, M.J. Measurement of the normal feline pituitary gland in brachycephalic and mesocephalic cats. J Feline Med Surg 2018, 20, 578–586. [Google Scholar] [CrossRef]
- Kooistra, H.S.; Voorhout, G.; Mol, J.A.; Rijnberk, A. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J Endocrinol 1997, 152, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Costanza, D.; Coluccia, P.; Castiello, E.; Greco, A.; Meomartino, L. Description of a low-cost picture archiving and communication system based on network-attached storage. Vet Radiol Ultrasound 2022, 63, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Reed, A.H.; Henry, R.J.; Mason, W.B. Influence of statistical method used on the resulting estimate of normal range. Clin Chem 1971, 17, 275–284. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline - Third Edition. CLSI document EP28-A3c 2008.
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J Chiropr Med 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Geffré, A.; Concordet, D.; Braun, J.-P.; Trumel, C. Reference Value Advisor: a new freeware set of macroinstructions to calculate reference intervals with Microsoft Excel. Veterinary Clinical Pathology 2011, 40, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.; Adams, W.H. MRI of brain disease in veterinary patients part 1: Basic principles and congenital brain disorders. Vet Clin North Am Small Anim Pract 2010, 40, 21–38. [Google Scholar] [CrossRef]
- Verdun, F.R.; Racine, D.; Ott, J.G.; Tapiovaara, M.J.; Toroi, P.; Bochud, F.O.; Veldkamp, W.J.H.; Schegerer, A.; Bouwman, R.W.; Giron, I.H.; et al. Image quality in CT: From physical measurements to model observers. Physica Medica 2015, 31, 823–843. [Google Scholar] [CrossRef]
- Faby, S.; Flohr, T. Multidetector-Row CT Basics, Technological Evolution, and Current Technology. In Body MDCT in Small Animals: Basic Principles, Technology, and Clinical Applications, Bertolini, G., Ed.; Springer International Publishing: Cham, 2017; pp. 3–33. [Google Scholar] [CrossRef]
- Bertolini, G. Basic Principles of MDCT Angiography. In Body MDCT in Small Animals: Basic Principles, Technology, and Clinical Applications, Bertolini, G., Ed.; Springer International Publishing: Cham, 2017; pp. 37–51. [Google Scholar] [CrossRef]
- Schwarz, T.; O'Brien, R. CT Acquisition Principles. In Veterinary Computed Tomography; 2011; pp. 9-27. [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Argyropoulou, M.; Perignon, F.; Brunelle, F.; Brauner, R.; Rappaport, R. Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatric Radiology 1991, 21, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Sari, E.; Akgun, V.; Ozcan, E.; Ince, S.; Saldir, M.; Babacan, O.; Acikel, C.; Basbozkurt, G.; Ozenc, S.; et al. Measures of pituitary gland and stalk: from neonate to adolescence. Journal of Pediatric Endocrinology and Metabolism 2014, 27, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.N.; Doraiswamy, P.M.; Husain, M.M.; Boyko, O.B.; Ellinwood, E.H., Jr.; Figiel, G.S.; Krishnan, K.R. In vivo assessment of pituitary gland volume with magnetic resonance imaging: the effect of age. J Clin Endocrinol Metab 1990, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- van der Vlugt-Meijer, R.H.; Meij, B.P.; Voorhout, G. Intraobserver and interobserver agreement, reproducibility, and accuracy of computed tomographic measurements of pituitary gland dimensions in healthy dogs. Am J Vet Res 2006, 67, 1750–1755. [Google Scholar] [CrossRef]
- Van Hoe, L.; Haven, F.; Bellon, E.; Baert, A.L.; Bosmans, H.; Feron, M.; Suetens, P.; Marchal, G. Factors influencing the accuracy of volume measurements in spiral CT: a phantom study. J Comput Assist Tomogr 1997, 21, 332–338. [Google Scholar] [CrossRef]
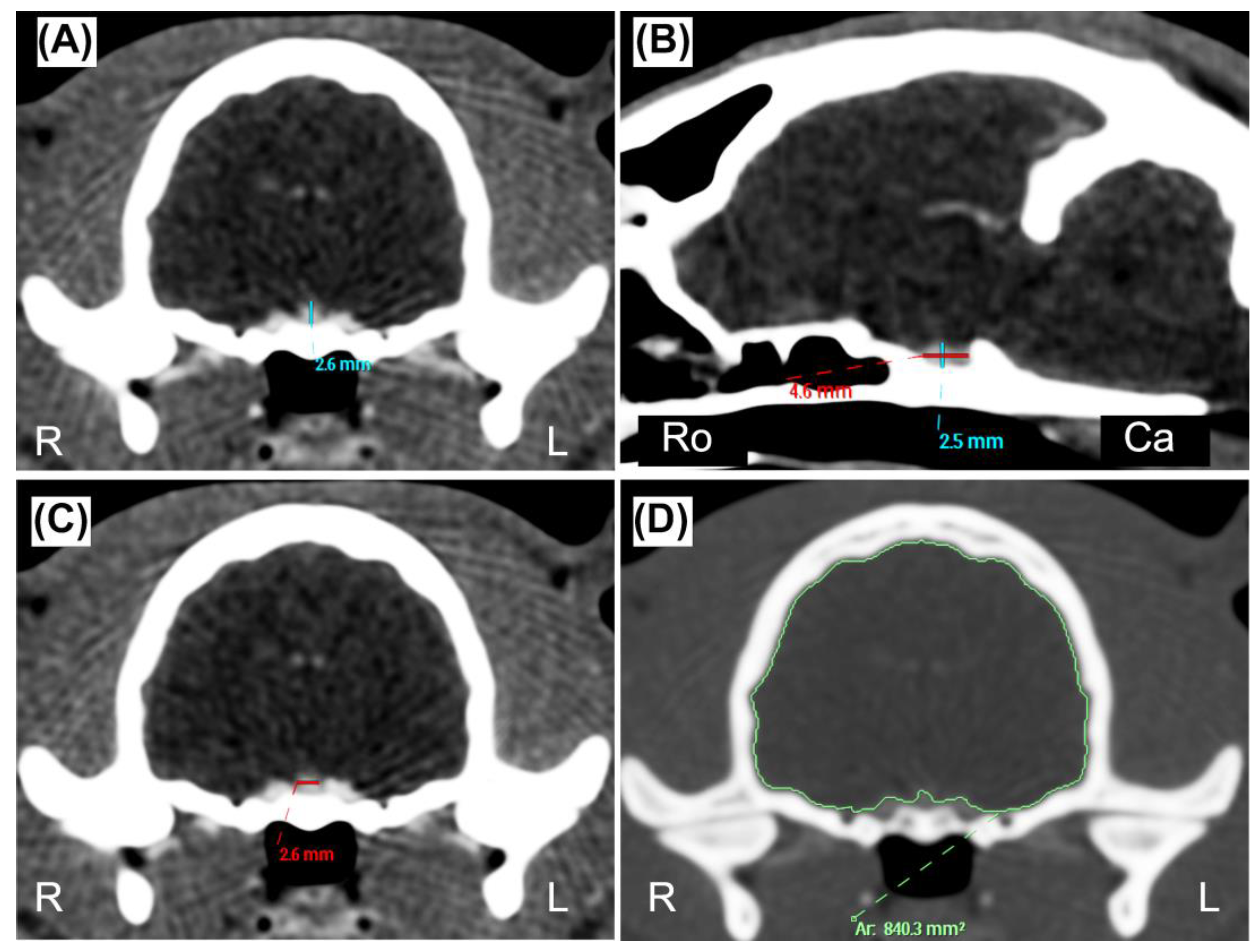
| Measurement | Mean (±SD) | Range (Min–Max) |
Lower Limit (90% CI) |
Upper Limit (90% CI |
|---|---|---|---|---|
| PHT (mm) | 2.94 (± 0.52) | 2.0 – 4.1 | 1.88 (1.68 – 2.10) |
4.01 (3.81 – 4.20) |
| PHS (mm) | 2.95 (± 0.55) | 1.9 – 4.0 | 1.84 (1.63 – 2.08) |
4.10 (3.89 – 4.27) |
| PL (mm) | 3.17 (± 0.52) | 2.0 – 4.4 | 2.13 (1.92 – 2.37) |
4.23 (4.03 – 4.42) |
| PW (mm) | 3.24 (± 0.61) | 2.1 – 4.8 | 1.92 (1.66 – 2.15) |
4.41 (4.12 – 4.71) |
| BA (mm2) | 789.02 (± 61.85) | 647.87 – 962.85 | 659.51 (634.51 – 687.61) |
910.96 (883 – 938.01) |
| P:B ratio | 0.37 (± 0.06) | 0.25 – 0.48 | 0.25 (0.23 – 0.28) |
0.49 (0.47 – 0.51) |
| Measurement | Body weight | p-value | Age | p-value |
|---|---|---|---|---|
| PHT (mm) | r = 0.39 | 0.004 | rs = 0.22 | 0.12 |
| PHS (mm) | r = 0.30 | 0.032 | rs = 0.35 | 0.012 |
| PL (mm) | r = 0.28 | 0.044 | rs = 0.36 | 0.009 |
| PW (mm) | r = 0.35 | 0.011 | rs = 0.12 | 0.39 |
| BA (mm2) | r = 0.29 | 0.036 | rs = 0.17 | 0.24 |
| P:B ratio | r = 0.29 | 0.038 | rs = 0.14 | 0.33 |
| Measurement | ICC | 95% CI | p-value |
|---|---|---|---|
| PHT | 0.69 | 0.38 – 0.84 | < 0.0001 |
| PHS | 0.58 | 0.37 – 0.74 | < 0.0001 |
| PL | 0.58 | 0.36 – 0.73 | < 0.0001 |
| PW | 0.66 | 0.46 – 0.79 | < 0.0001 |
| BA | 0.81 | 0.50 – 0.91 | < 0.0001 |
| Measurement | Operator 1 | Operator 2 | ||||
|---|---|---|---|---|---|---|
| ICC | 95% CI | p-value | ICC | 95% CI | p-value | |
| PHT | 0.81 | 0.63 – 0.91 | < 0.0001 | 0.66 | 0.17 – 0.86 | <0.0001 |
| PHS | 0.87 | 0.71 – 0.94 | < 0.0001 | 0.60 | -0.04 – 0.85 | <0.0001 |
| PL | 0.51 | 0.19 – 0.73 | 0.001 | 0.70 | 0.10 – 0.89 | <0.0001 |
| PW | 0.78 | 0.59 – 0.89 | < 0.0001 | 0.60 | 0.08 – 0.83 | <0.0001 |
| BA | 0.82 | 0.66 – 0.91 | < 0.0001 | 0.92 | 0.84 – 0.96 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





