Preprint
Article
Mushroom-Legume-Based Minced Meat: Physico-chemical and Sensorial Properties
Altmetrics
Downloads
281
Views
65
Comments
0
A peer-reviewed article of this preprint also exists.
† These authors contributed equally to this work.
This version is not peer-reviewed
Submitted:
06 May 2023
Posted:
08 May 2023
You are already at the latest version
Alerts
Abstract
A growing number of health conscious consumers are looking for animal protein alternatives with similar texture, appearance, and flavor. There has been a lot of interest in meat analogs as potential meat substitutes. However, research and development still needs to find any alternative non-meat materials. The objective of the current research was to develop fungi minced meat alternative (FMMA) from edible mushrooms. Pleurotus Sajor-caju (SC) was used as the starting material. SC mushroom was selected for starting materials based on high protein content (41.99%.) and sensory attributes. Chickpea flour was used to improve the textural properties by mixing with SC mushroom at a ratio of 0:50, 12.5:37.5, 25:25, 37.5:12.5, and 50:0 (w/w). Textural and sensory attributes suggest that SC mushroom to chickpea at a ratio of 37.5:12.5, shows higher acceptability of FMMA with the protein content up to 47%. Beetroot extract 0.2% (w/w), and 5% (w/w) canola oil showed the most acceptable color parameters and consumer acceptability. This research suggested that SC mushroom with 12.5% chickpea flour, 0.2% beetroot extract and 5% canola oil could be suitable ingredients for the mushroom-based FMMA.
Keywords:
Subject: Biology and Life Sciences - Food Science and Technology
1. Introduction
Meat consumption has played a crucial role in the development of prehistoric Homo sapiens and has become an important part of the human diet in many parts of the world with unforeseen consequences [2]. Beef, pork, mutton, and chicken are the products with the highest demand, and the countries with the highest yearly meat consumption per capita are the United States and Australia [3]. The demand for meat has increased globally by 58% over the past two decades due to an increase in the global population, and rapid economic expansion [4]. Nowadays, there are major concerns about the harmful consequences of meat consumption on human health and the inefficiency of meat production when compared to crop cultivation [5]. In addition, because of the pandemics of COVID-19 and the African swine flu, scientists and researchers have started to reevaluate the safety of low-temperature meat supply chains [15]. A recent analysis of 90 dietary recommendations from around the world found that 37% suggested substituting meat protein for vegetable protein [16], which shows the importance of establishing a sustainable source of human protein nutrition. To address these issues, food scientists and the food industry are exploring ways to offer meat alternatives of plant and fungi origin with the aim of mimicking original animal tissue in terms of texture, aroma, taste, and appearance [6,7]. According to predictions by Union de Banques Suisses (UBS) [8], the market for non-animal meat either from plants and/or edible fungus or insects is expected to grow from $4.6 billion in 2018 to $85.0 billion in 2030 and, as a notable milestone, reaching $30.9 billion by the year 2026 [9]. This new market appears to be well-positioned for the development and invention of new meat alternatives.
The food research community is now studying three main meat substitutes: plant-based meat (developed from plant proteins mainly using mechanical structural techniques) [12], fungi-based using mainly ascomycetes or basidiomycetes, and cultured meat based on animal tissue engineering [13]. Recent years have seen an increase in interest in meat analogs developed from more environmentally friendly non-animal proteins [14]. However, as of now, mainly plant-based (PB) alternatives are on the market, whereas only a few fungi-based meat alternatives have been released (for example Quorn products). Most frequently, soy and wheat gluten are used as potential sources of protein for the processing of meat analogs [23]. Nevertheless, sources of protein from fungi like mycoprotein and mushrooms as well as legumes like peas, faba beans, kidney beans, and others have also been used for the development of meat substitutes [24]. Mushrooms are attractive and highly valued due to their unique flavors, textures and their high nutritional value with 4g/100 g protein (depending on species), and high dietary fiber content, which is mainly composed of β-glucan [25]. Nowadays, edible mushrooms are used for the processing of meat analogs in the human diet because they are good sources of macro-nutrients (i.e., protein, fiber), and micro-nutrients (i.e., vitamins, and minerals). Edible mushrooms contain low amounts of fat, sodium, and possess low energy content [26,27,28,29]. Mushrooms also contain a few bioactive compounds, such as proteoglycans, phenolic compounds, terpenes, and lectins [25].
Mushrooms have yet to be introduced as a raw material for the production of minced meat alternatives. Some earlier studies by Yuan et al. [30] developed a fibrous meat analog utilizing thermo-extrusion and developed different formulas for manufacturing sausage analogs. However, extrusion requires high temperature and shear conditions and might not be readily available everywhere. Thus, there is a need to develop meat alternatives based on mushrooms that do not require the use of extrusion techniques. Moreover, mushrooms aid in the development of primary organoleptic properties such as the appearance, flavor, and texture, which might be negatively influenced by extrusion. Consequently, the goal of this project is to develop fungi-based minced meat substitutes (FMMA) that may be claimed as a clean-label product and to produce value-added meat substitutes that could satisfy customer demand. The objectives of this study are to (i) develop a FMMA by using a common mushroom, (ii) optimize the formulation using clean-label ingredients, and (iii) reveal the textural as well as the sensory properties of the developed formulations.
2. Materials and Methods
2.1. Materials
Raw edible mushroom Pleurotus Sajor-caju was purchased from the local fresh market (Bandu, Chiang Rai, Thailand). Canola oil was purchased from the departmental store (Big C, Chiang Rai, Thailand). Chickpea flour (moisture 11.85±0.01, protein 22.18±0.09, fat 5.52±0.07, ash 2.61±0.01, and carbohydrate content 69.70±0.05, wt%, d.b.) was purchased from Huglamool farm (Amnat Charoen, Thailand). Beetroot extract was purchased from Narah herb company (Chiang Mai, Thailand). Vital wheat gluten (moisture 8.84±0.01, and protein content 87.94±0.39, wt%, d.b.), soy protein isolate (moisture 8.93±0.02, and protein content 90.17±0.17, wt%, d.b.) was purchased from UNION SCIENCE. CO., Ltd (Chiang Mai, Thailand). Yeast extract was purchased from Thai Food and Chemical Co. Ltd. (Bangkok, Thailand).
2.2. Mushrooms preparation
At first, SC raw mushrooms were washed with potable water several times to remove foreign materials. The cleaned mushrooms were blanched at 100°C for 5 min to ensure their storability before mincing them in a meat mincer (SIR1-TC12E, SEVENFIVE DISTRIBUTOR CO., LTD., Nonthaburi, Thailand) followed by drying at 60°C in the cabinet tray dryer (Model BP-80, KluayNamThai, Bangkok, Thailand Bangkok, Thailand). The final moisture content in mushrooms was 65%. Dried mushrooms were packed in vacuum bags, and stored at -20 °C until further use.
2.3. Mushroom-based FMMA preparation
2.3.1. Base formulation
Frozen mushrooms were thawed in the refrigerator overnight at 4°C. Dried mushrooms (25%, w/w) were mixed with chickpea flour (25%, w/w) vital wheat gluten (4.8%, w/w), distilled water (28% w/w), soy protein isolate (10%, w/w), canola oil (5%, w/w), beetroot extract (0.2%, w/w), and yeast extract (2%, w/w). These ingredients were selected to accurately mimic the taste and appearance of minced meat. All ingredients were blended in a mixing bowl until homogeneously mixed. The mixture was placed into a meat mincer (SIR1-TC12E, SEVENFIVE DISTRIBUTOR CO., LTD., Nonthaburi, Thailand) to form the typical minced meat shape, and transferred in a baking oven at 150°C for 30 min [31]. Each sample was individually packed in a vacuum bag for further analysis. Figure 1 shows the flow diagram for processing mushroom-based FMMA.
2.3.2. Formulation optimization
In order to further optimize the FMMA, three treatments were designed: i) effect of different concentrations (0, 12.5, 25, 37.5, 50% w/w) of chickpea flour on the textural and sensory attributes of FMMA, ii) effect of different concentrations of beetroot extract (0.2, 0.4, 0.6, 0.8 and 1.0% w/w) on the color parameter and sensory quality of FMMA, and iii) effect of canola oil concentrations (1, 2, 3, 4, and 5% w/w) on sensory attributes of the mushroom-based FMMA (See supplemental materials for formulations; Table S1-3).
2.4. Chemical analysis of mushroom-based FMMA
2.4.1. Proximate composition analysis
Proximate composition, i.e., moisture, ash, protein, and fat content, were determined using 2019 AOAC guidelines: moisture content was determined by transferring 3 to 5 g of sample into a convection oven at 105°C for at least 16 h [32]. Ash content was measured by muffle furnace combustion at 525°C for 6 h [33]. Protein content was measured using Kjeldahl method utilizing a nitrogen-to-protein conversion factor of 5.99 [34]. Fat content was measured using Soxhlet extraction method [35]. The amount of total carbohydrates was calculated in accordance with FAO guidelines as the remainder [36].
2.4.2. Determination of total dietary fiber (TDF)
TDF content was determined by using an enzymatic-gravimetric method [37]. Duplicate portions of defatted and dried samples were gelatinized and partially digested by α-amylase before being enzymatically digested with protease and amyloglucosidase to remove the protein and starch from the sample. Acetate buffer (5 ml, 0.1 M, pH 5.0) containing 100 µl thermostable α-amylase was mixed with about 300 g of FMMA before being incubated at 96°C for 60 min in a tightly sealed tube. The sample was then incubated at 60oC for 4 h after 400 µl of amyloglucosidase and proteases were added. Subsequently, 80% aqueous ethanol was added to precipitate soluble fibers. The samples were centrifuged at 2,000 rpm for 20 min to collect the total fiber. The residue was then washed with ethanol and acetone, followed by drying and weighing. One part of the residue was tested for protein, and the other part for ash. TDF is computed as a percentage of the initial sample weight by subtracting the residue's weight from that of the protein and ash.
2.4.3. Amino acid analysis
The sample for analysis of amino acids was prepared according to the method described by Borokini et al. [38]. Each fresh mushroom sample (20 g) was weighed accurately and pulverized using 100 ml of phosphate buffer with 2% SDS (sodium dodecyl sulfate) by a blender. The homogenate was filtered using double-layered cheesecloth. The filtrate was precipitated by using an ammonium sulphate salt precipitation technique at 65% saturation. The proteins are pelleted by centrifugation, concentrated by dialysis, and freeze-dried for amino acid analysis. Four (4) g protein isolate was hydrolyzed and evaporated using a rotary evaporator. The amino acid composition of the fresh SC and LS mushroom was analyzed in the Central Laboratory, Chiang Rai, Thailand using in-house method TE-CH-372 based on the Official Journal of the European Journal of Communities, L 257/16 by amino acid analysis and the result was reported as, g/100 g sample.
2.4.4. SDS polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was used to see the protein patterns of the developed sample. The samples were mixed with sample buffer (0.5 M Tris-HCl, pH 6.8 containing 4% SDS, 20% glycerol, 0.03% bromophenol blue with/without 10% DTT) at 1:1 ratio and boiled for 3 min. The protein-dyes were loaded into the Roti-PAGE Gradient (4-20%) precast gels and run in an electrophoresis tank filled with buffer solution at a constant current of 60 mA using a Biostep® GmbH power supply (Burkhardtsdorf, Germany). The gels were stained in a staining solution (Coomassie Blue R-250 methanol-acetic acid) and gentle shaking at 50 rpm was continued for overnight. The gel was de-stained with de-staining solution I and II (methanol-acetic acid-water) until the background was clear and then dried.
2.5. Physical analysis of FMMA
2.5.1. Textural profile analysis (TPA)
TPA of FMMA was determined using Tasnim et al. [39] methods with slight modifications. For the sample preparation, FMMA was formed into a patty using a petri dish to transform them into a round-shaped structure (1.5 cm x 5.0 cm) (height x length). TPA was measured by using a TA.XT plus Texture Analyzer (Surrey, UK). A cylindrical probe (SMSP/36R, cylinder diameter = 36 mm) was used for TPA. The testing conditions were as follows: pre-test speed = 1 mm/s; test speed = 5 mm/s; post-test speed = 5 mm/s; strain = 50%; trigger force = 10 g; the time interval between the two compressions: 5 s. The results were calculated by EXPONENT CONNECT®software (Stable Microsystems, Surrey, UK) as hardness (g), chewiness (g.s), springiness (%), cohesiveness, and gumminess.
2.5.2. Cooking loss
The cooking loss was determined using five different FMMA samples and calculating the ratio of weight before and after cooking. FMMA was soaked to distilled water in the ratio 1:1 (w/v) for 1 h (soaked/uncooked) and cooked in a pan without oil for 15 min and cooling at room temperature. The cooking loss was determined using the following equation 1 [40]:
2.5.3. Color determination
The color of the FMMA was measured using a colorimeter (Hunter Lab/colorQuest XE, Reston, Color Global, Bangkok, Thailand) employing a 10° standard observer and illuminant D65. The device was calibrated with a standard white plate. The CIE L*, a*, b* values of the samples were recorded using ten randomly chosen samples. The L* represented the lightness of the sample, ranging from black (L* = 0) to white (L* = 100). The a* is the red spectrum (positive a*) and green (negative a*) and b* indicate yellow (positive b*) and blue (negative b*) of the samples. These attributes were also used to determine ΔE and whiteness, according to Lee, et al. [41]. A ΔE>2.0 is often considered to be a color difference.
2.6. Sensory evaluation
Sensory evaluation of FMMA was studied by using a 9-point hedonic scale (1= dislike extremely, and 9 = like extremely) [42]. Sensory analysis was carried out by the Food Sensory Lab (S4) (Mae Fah Luang University, Chiang Rai, Thailand) with ethical approval (Protocol No.: EC22177-14) of consumer testing. Evaluation of the products was permitted by Mae Fah Luang University, Chiang Rai, Thailand (CoE158/2022). Samples were evaluated by at least 46, 46, 34, 35, and 120 - untrained panelists for selecting mushroom types, optimum concentration of CF and SC mushroom, optimum concentration of beetroot extract, optimum concentration of canola oil, and for the final product, respectively. Each study of mushroom-based FMMA was evaluated for sensory attributes, including appearance, texture, odor, flavor, and overall acceptability. Samples were given to panelists once at a time in an order to avoid the effect of sample order presentation. Panelists were asked to drink water to rinse their palate among samples. A sensory session of FMMA was performed at 25 °C in isolated rooms (individual cabins) under controlled environmental conditions with white light (300 lx) and relative humidity of 54%. Moreover, to avoid the impact of shocks, all panelists were informed in advance that new products were designed to replace conventional animal minced meat.
2.7. Statistical analysis
Each data was based on triplicate measurements and expressed as mean ± standard deviation. All the data was analyzed using one-way analysis of variance (ANOVA) by the Statistical Tool for Agricultural Research (STAR) software tool (International Rice Research Institute, Manila, The Philippines). The 95% significance level (p≤0.05) was considered to be statistically significant among different samples.
3. Results and Discussion
3.1. Properties of Pleurotus Sajor-caju mushroom
Before the FMMA was prepared, the SC was analyzed for its morphological attributes and composition. According to the literature search, it is shown that SC has among the highest essential amino acids (except for lysine) when compared to two other mushrooms (Shiitake, Lentinus squarrosulus; data not show). Moreover, overall sensory acceptance was highest for SC in FMMA formulations (see supplemental material). For this reason, SC was chosen to prepare and optimize FMMA formulations. Before preparation, the content of ash, protein, fat, dietary fiber, and essential amino acids in SC was analyzed (Table 1).
The major component of SC mushrooms is dietary fiber, followed by proteins and other carbohydrates. This dietary fiber is mainly composed of β-glucan, which was present in SC at 25.72 g/100 g DW. β-glucan stimulates the host immune system to protect the host against bacterial, viral, fungal, or parasitic infections [43]. By attaching to the receptor (dectin-1) of the host cells, β-glucan stimulates macrophages, neutrophils, and natural killer cells [44,45]. On a final note, SC containes considerable amounts of indispensable amino acids with many of them being present at concentrations higher than those recommended by the FAO for age groups of adults. However, actual bioavailability data and PDCAAS/DIAAS values are currently missing for this mushroom to draw a final conclusion on the protein quality.
3.2. Properties of base formulation
After the main components of SC mushroom were identified, a FMMA was prepared using SC as the main ingredient (50%, w/w). The results showed that the moisture content of SC-based FMMA was 28.39±0.17%. The protein content of the SC formulation was (41.99±0.55%), which was considerably higher than the initial protein content of the mushrooms, and regular minced lean meat. This can be attributed to the formulation that contained chickpea flour, wheat gluten, and soy protein. The sensory attributes provide information about the overall acceptability, appearance, aroma, color, taste, and texture of FMMA formulated with SC (Table 2). The results showed that the overall acceptability of the FMMA base formulation is in the range of “Like Slightly”. This is not surprising since this is a new type of food and many consumers reject foods when they try it for the first time [47,48]. Nonetheless, the acceptability was already high using the base formulation, but especially taste and smell, were ranked rather low. This might be due to aroma compounds that are typically found in mushrooms, such as 1-octen-3-ol, hexadecanoic acid and octadecenoic acid [49-51] ). These compounds are not commonly found in real meat products and therefore might have caused an adverse rating in such a product that is designed to replace real animal food. However, as the base FMMA formulation was overall positively evaluated by the panelists, further formulation improvements were investigated which will be discussed in the following sections.
3.3. Effect of concentrations of chickpea flour (CF)
CF is a commonly used food ingredient and is also regularly utilized as a binding and texturizing agent in meat alternatives [52,53]. Typically, it is observed that the hardness of food matrices is increased when CF is added [54]. For this reason, the aim of these experiments was to elucidate the effect of increasing chickpea flour concentration on the textural and sensory properties of FMMA. For this, SC was replaced with CF at concentrations from 0-50 % (see supplemental material for formulations).
The moisture and protein content (on a weight dry basis, g/100g) of SC mushroom–based FMMA are shown in Table 3. In particular, moisture content was significantly (p<0.05) increased while increasing the SC mushroom in the FMMA. This might be due to the residual moisture content of SC mushrooms (MC = 65%) after drying. The moisture content of SC mushroom-based FMMA was the highest in SC to CF ratio (50:0) and significantly different from other samples (p<0.05). However, the moisture content of mushroom-based FMMA was much lower than minced beef and pork, which are 61 and 53%, respectively [55,56]. The highest protein content was observed for FMMA with SC to CF ratio of 37.5: 12.5 and 50: 0 and the values were 47.03, and 47.59%, respectively. There was no significant difference (p>0.05) between FMMA with SC to CF ratio 37.5: 12.5 and 50: 0 in terms of protein content. Table 3 suggests that increasing the mushroom content increased the protein content of the FMMA. These results show that SC-based FMMA can contribute considerably to the protein supply in the human diet and future protein quality assessments should be carried out to analyze the bioavailability of the amino acids [57]. The result suggested that the protein content of SC mushroom-based FMMA was higher than minced beef (25.53%), and minced pork (25.7%) [55,56].
In the next step, the change in the texture of FMMA with increasing CF was analyzed. TPA measurements showed that the addition of CF to FMMA has a considerable influence on its textural attributes. Hardness values ranging from 1,983.35 N (SC: CF = 50:0) to 9,441.01 N (SC: CF = 0:50), springiness from 0.65 mm (SC: CF = 0:50) to 0.90 mm (SC: CF = 50:0), and cohesiveness from 0.35 (SC: CF = 0:50) to 0.63 (SC: CF = 50:0) was detected. Hardness and chewiness show similar patterns among treatments, with 50% chickpea flour showing the highest values for both hardness and chewiness. The treatments with the highest SC concentration 37.5% and 50% from mushrooms exhibited low hardness values (2610.23 and 1983.355 N, respectively) (Table 3). The result indicated that this treatment significantly reduced the force required to compress the sample, which can have important consequences for the mouth feel of a product. The reason for this is most likely the higher porosity induced by the higher concentration of SC and the lower crosslinking by water-soluble proteins that is expected to increase the hardness in samples containing higher amounts of CF. FMMA with 50% CF showed the highest hardness due to an increase in bulk density, decreased porosity, and decreased water-holding capacity [58].
The findings revealed that FMMA made with increasing CF concentration decreased the springiness of the sample and that FMMA with pure SC mushrooms had the highest potential to regain its original dimension following compression. This shows a high degree of protein texturization that allows for energy conservation and thus elastic deformation, presumably in the form of disulfide bond cross-links [58]. The 0% CF (50% SC mushroom) FMMA had a sponge-like springiness following hydration, which, however, was not a meat-like texture. All treatments with additional CF had significantly less springiness, which indicated a strong influence of the addition of starch to the formulation that contributed to changing the textural properties of the FMMA matrix [58]. A low springiness value, on the other hand, suggests that the material was plastically deformed [59]. Moreover, the maximum chewiness was observed in formulations with 50% CF (0% SC mushroom). The result corresponds with the hardness value. While chewiness is a computed measure that is partially derived from hardness and springiness, hardness predominates due to its higher values when compared to the other treatments. Table 3 suggested that chewiness was dramatically reduced by more than 50% with the addition of 12.5 to 37.5% SC mushroom.
For meat analog products to be as widely accepted by consumers as possible, textural characteristics should, however, closely resemble those found in meat products. The TPA measurements revealed that controlling the protein-to-starch ratio by optimizing the CF and SC content can be a crucial factor in determining these desirable qualities attributes. Due to the negative effects of decreased chewiness, a higher value of springiness without sufficient hardness, as in the case of 50% SC mushroom treatment, may reduce consumer acceptability. In light of the aforementioned discussion, it can be anticipated that a FMMA product made with 12.5% CF and 37.5% SC mushroom has the highest level of customer acceptance (Table 3). To elucidate this question, a sensory test was carried out.
3.4. Sensory properties of Sajor-caju mushroom-based minced meat alternative
The composition analysis suggested that FMMA with SC to CF ratio 37.5: 12.5 and 50:0 had the highest protein concentration, but FMMA with 50% SC-mushroom is most likely less suited for food applications because of the adverse textural attributes revealed in the TPA measurements. As a result, consumer preference was conducted by sensory evaluation of 46 untrained panelists. As already projected in the TPA measurements, the sensory analysis suggested that 37.5% SC mushroom and 12.5% CF exhibit the highest overall acceptability, followed by other FMMAs. The appearance ratings of FMMA also suggested that 37.5% SC mushroom and 12.5% CF based FMMA is rated the best among all ratios. Similarly, 37.5% SC mushroom and 12.5% CF FMMA show better texture acceptability by the panelists following other samples. FMMA containing 50% CF shows the least acceptability by the consumer due to hard texture and a high chewiness. The consumer acceptability of meat substitutes depends on the taste, color, and flavor of the products [47]. Consumers who eat meat have the tendency to compare meat substitutes with traditional beef, mutton, or pork. Customers have been advised to reduce their meat intake for health and environmental reasons. A possible solution is to replace meat with plant protein and fungi-based substitutes; however, consumer acceptance of these products is still low, possibly due to taste and flavor [48]. As a result, it is important to identify sensorial attributes that must be optimized to improve palatability [47]. In our study, more than two-thirds of consumers were classified as omnivores, implying that meat played a significant role in the average diet. However, the purchase and/or like of plant/fungi-based meat alternatives varied from country to country. As for example, i) those who are particularly connected to meat in the United States are relatively unlikely to buy or liking PB meat alternatives. Appeal, excitement, and low disgust were all attitudinal predictors of purchase intent. ii) In China, women are more likely than men to purchase PB meat alternatives. Meat eaters are substantially more likely to purchase PB meat alternatives than vegetarians and vegans, and higher meat attachment predicts higher purchase likelihood. Higher familiarity and lesser food neophobia are predictive of purchase intent. iii) Omnivores and individuals who consume more meat are more likely to consume PB meat alternatives in India. Higher income groups as well as more educated and politically liberal consumers exhibited a greater interest in PB meat alternatives. Food neophobia indicated lower purchase intent, but familiarity with the products predicted higher buy intent. Perceived sustainability, excitement, necessity, and goodness were predictors of PB meat alternative purchasing intent in India [60]. One-third (or less) of consumers identified themselves as vegetarians, vegans, or pescetarians. An increased emphasis on environmental and health-related reasons might aid in the adoption of PB meat substitutes. Despite a few obstacles, there is undeniably a large market potential for PB meat substitutes, especially FMMA, which is projected to grow in the future as environmental and health awareness grows [48]. Table 3 indicated that 37.5% SC mushroom, and 12.5% CF based FMMA showed the highest textural and sensory acceptability. As a result, FMMA formulation containing 37.5% SC mushroom, and 12.5% CF was selected for the following experiments.
3.5. Effect of canola oil on sensory characteristics
The base formulation contained 5% of canola oil, which may adversely affect purchasing decisions of consumers who are looking for a low-fat product. For this reason, the effect of decreasing the oil content was investigated. For this part of the study, 37.5% w/w SC mushroom and 12.5% w/w CF were utilized as these have been determined to be the optimum concentration in the previous section. From there, the canola oil concentration was changed to the range of 1-5% and a sensory analysis was carried out. The results showed that the formulation containing 5% w/w canola oil significantly (p<0.05) exhibited the highest consumer acceptability. Although 1, 2, and 3% w/w canola oil had similarities in terms of appearance, texture, juiciness, and overall acceptability, the sensory acceptance of these formulations was lower than for matrices containing 5% of canola oil (Table 4). In general, 5% w/w canola oil exhibited the highest score for appearance, texture, and juiciness, from 35 untrained panelists. This is in line with other studies, such as the results reported by Wi et al. [61] who used 15-35% canola oil for the processing of meat analogs and found that the addition of canola oil reduces the cooking loss, increased the water holding capacity and sensorial properties. In addition, Selani et al. [62] discovered that using canola oil as a fat substitute in a beef burger improved its cohesion and springiness in its sensory attributes. Typically, animal fats are replaced with vegetable oils such as olive oil [63,64], palm oil [65], canola oil, and coconut oil [66,67] to lower the saturated fatty acid and cholesterol levels of certain meat alternatives. Depending on the raw materials, different quantities of oil are used to provide a more meat-like texture and to improve the flavor, juicy quality, tenderness, and sensorial attributes of meat substitutes [68]. Canola oil is often regarded as healthy oil due to its low saturated fat content (7%), which further supports the utilization of canola oil in FMMA formulations, since canola oil includes omega-6 and omega-3 fatty acids in a 2:1 ratio, which is thought to lower low-density lipoprotein (LDL) and total cholesterol levels [69].
3.6. Effect of beetroot extract on color and sensory characteristics
The visual appearance of food products considerably influences consumer acceptability [70]. After establishing the optimum texture, attention should be given to the color or changes in color during product processing and cooking. Beetroot extract is commonly used as a natural coloring agent to mimic the red-pink appearance of uncooked meat [71]. For this reason, beetroot extract was chosen as a coloring agent to enhance the appearance of the FMMA, which appear brownish without a colorant. Moreover, beetroot extracts undergo color changes as a result of thermal deterioration and thereby mimic the natural color change that occurs when cooking meat [72]. For these experiments, 37.5% w/w SC mushroom, 12.5% w/w CF, and 5% w/w canola oil was used and the beetroot extract concentration varied from 0.2% w/w to 1.0% w/w. shows the results of the color measurements before and after cooking in a pan at low flame heat for 8 to 10 min until browned as well as for the sensory trials for these formulations.
Low concentrations of beetroot extract in both fresh and cooked samples had significantly highest (p<0.05) lightness (L*)-value, positive a*(redness), and positive b*-value (yellowness). Moreover, the a*-value increased with the addition of beetroot extract, which was expected due to the high coloring power of the betanin found in this ingredient [72]. The a*-value then decreased upon cooking due to the thermal degradation of betanin [72]. This was in line with the increase in L* values of cooked FMMA at each concentration when compared to the fresh samples (Table 5). It is often difficult to mimic the color change that occurs during cooking meat. Therefore, it is necessary to replace or mix a heat-stable natural or artificial food grade color or combination of colors that enable a color change similar to animal meat during cooking, grilling, or frying. For example, myoglobin denaturation results in a color change in beef muscle tissue at around 75°C [72,73]. To mimic this color pattern in meat analogs, beetroot extract, and betanin are suggested to be added as food additives to mimic raw meat color [74-76] and exhibit color changes as a result of thermal degradation [77]. Beetroot extract is also added as a food colorant for the burger formulation of Beyond MeatTM [78,79].
The effect of beetroot extract and cooking on the physical appearance of FMMA is illustrated in Figure 2 and the results of the sensory analysis are presented in Table 5. In general, the inclusion of beetroot extract enhanced the overall acceptance scores of FMMA. The overall acceptability, appearance, fresh and cooked color acceptance of each sample were significantly different (p<0.05) from each other. The analysis suggested that increasing the beetroot extract content in FMMA decreases the consumer preference possibly because the overall redness is too intense and may be perceived as artificial. In fact, a higher concentration of beetroot extract resulted in a dark pink color (Figure 2) and two-way ANOVA suggested that 0.2% w/w beetroot extract exhibited significantly higher appearance acceptability.
The addition of different concentrations of beetroot extract did not show any significant effect (p>0.05) on the texture properties reported by the panel. The aroma scores of cooked FMMA scores were significantly (p<0.05) higher than the aroma of fresh FMMA, which implies that cooking did not result in the generation of off-flavors and may even contribute to the flavor development. This is in line with Sucu and Turp [80] who discovered that adding beetroot powder (0.12, 0.24, and 0.35%) to cooked fermented sausages resulted in a statistically significant (p<0.05) increase in inner color scores. Moreover, other studies with fresh pork sausage that included additional beetroot extract (1 ml/kg) had a higher consumer acceptance score than control (no colorant) sausages [81]. Overall, these findings indicated that adding beetroot extract as a natural colorant to FMMA improves its sensory qualities and that 0.2% w/w beetroot extract is enough to achieve high sensory acceptance.
3.7. Analysis of the optimized Sajor-caju mushroom-based minced meat alternative
The previous results revealed that SC FMMA is based on 37.5% SC mushroom, 12.5% w/w chickpea flour, 0.2% w/w beet root extract, and 5% w/w canola oil shows high consumer acceptance in terms of color, texture, and sensory properties. The aim of this last section was to thoroughly evaluate the properties of this optimized formulation.
3.7.1. Appearance and textural properties
The appearance of the optimized FMMA recipe in terms of fresh (Figure 3a) and cooked (Figure 3b) is shown in Figure 3. It looks similar to commercial PB minced meat in terms of coarse particle size.
The proximate composition including moisture, protein, fat, ash, carbohydrate content, and the dietary fiber on a dry weight basis (g/100 g) are shown in Table 6. The developed FMMA had considerable amounts of protein (47.90%), fat (12.51%), ash (4.32%), carbohydrate (23.21%), and dietary fiber (9.63%). Especially dietary protein is necessary for functional needs like enhancing health, building muscle, and promoting growth [82]. Consumption of SC mushroom-based FMMA may substantially contribute towards the recommended dietary intake (RDA) for protein and dietary fiber, with a recommended intake of 0.8 grams of protein per kilogram of body weight and 14 g of dietary fiber per 1,000 calories of food [83].
The proximate composition of SC mushroom-based FMMA is affected by cooking, as shown in Table 6. Raw FMMA displayed higher moisture content (p≤0.05) than cooked samples, which may limit its shelf-life. However, 18% moisture reduction was achieved upon cooking. This significant moisture reduction may prevent degradation and spoilage of the cooked product, but more storage studies are needed to establish the actual shelf-life. In addition, Table 6 shows that more than 14% of fat was expelled due to the cooking process. This is quite high compared to other researchers who reported a lower fat loss during cooking such as Olagunju and Nwachukwu [84] who found that cooked beef products lost 2.74-2.90% of fat. However, only a slight reduction in protein content was observed in the present study. The decrease in protein content might be due to the denaturation of protein that occurs at high temperatures, which can also foster the fat expulsion from the food matrix. Further studies should investigate how the fat retention can be optimized during cooking to ensure a optimum quality.
The appearance and texture measurements also revealed a considerable influence of cooking on the appearance and textural properties of the FMMA. As already discussed in the previous section, cooking induced an increase in lightness and a decrease in redness and yellowness values due to the breakdown of betanin from the beetroot extract [72]. Moreover, the textural attributes of the FMMA changed upon cooking, with a significant increase in hardness, chewiness, and cohesiveness (Table 6). The increase in hardness during cooking is related to a number of factors. The unfolding and aggregation of more proteins during cooking promotes more protein-protein interactions and the formation of a gel-like network. Moreover, the leaking of water and fat most likely resulted in a denser structure that is further enhanced by residual starch gelation, which both together result in a change in textural attributes [85].
3.7.2. Protein patterns of different minced meat alternatives
The protein pattern was analyzed by SDS-PAGE to observe the profile of SC mushroom-based FMMA, and compared it with pork and commercial PB minced meat substitutes. The SDS-PAGE profile of SC mushroom-based FMMA (a), commercial product (b), and pork minced (c) are shown in Figure 4. For every sample, three major bands were observed at ~65, 100, and ~130 kDa likely corresponding patterns for protein composition of the three samples.
Blanching and cabinet drying of mushrooms can cause protein denaturation and alter the molecular weight profile of the protein [23]. Albumins, globulins, glutelin-like materials, glutelins, prolamins, and prolamin-like materials were the major protein fractions in mushrooms. The majority of soy protein is made up of the two common legume proteins, 7S β-conglycinin (approximately 40% of total protein) and 11S glycinin (about 30% of total protein) [86]. About 85% of the proteins in wheat gluten are made up of gliadin and glutenin, which particularly have distinctive properties that set them apart from other plant proteins [86]. These two proteins combine with water to form the viscoelastic matrix typical of bread dough and help form the disulfide bonds that give textured plant proteins their fibrous structure [87]. Chickpea is an additional protein source included in the FMMA in addition to soy protein and wheat gluten. Protein pattern of legumin, which makes up 32% of the protein in chickpeas, has a protein pattern with molecular weights of 75 and 70 kDa. Vicilin was higher soy protein than legumin, which is larger in size and contains more sulfide groups. Despite having a lesser concentration, it is an essential component of the protein's texturization due to the sulfide groups it contributes [88].
3.7.3. Sensorial properties
In this last part of the study, the sensory acceptance (for cooked FMMA) was studied for the optimized FMMA formulation. The sensory analysis revealed that SC mushroom-based MMS containing 37.5% SC mushrooms, 12.5% chickpea flour, 0.2% beetroot extract, and 5% canola oil has an overall high acceptance as reported by 120 untrained panelists. Figure 5 illustrates the appearance, color, aroma, taste, texture, and overall acceptability of the developed and optimized FMMA. The overall acceptability of FMMA is higher than 8.0, however, texture, taste, color, and appearance were just below 8.0 scores. Also, the aroma score is less than 8.0, indicating that some volatile compounds may have negatively influenced such as one derived from legume ingredients that are often reported to induce off-flavors and thereby decrease the aroma acceptance [89]. Nonetheless, overall the product was highly accepted and it satisfies the sensory qualities of meat products. These high ratings may help to introduce such new FMMA formulations to the market as flavor and texture are key drivers in consumer decisions [90]. The findings are consistent with those of Sirimuangmoon et al. [91], who discovered that 50 or 80% of the meat substituted with mushrooms increased overall sensory acceptance. The use of mushrooms in the manufacturing of meat analogs, on the other hand, revealed that the organoleptic criterion for FMMA highly depends on the overall formulation, which was also reported in the present study. For example, Nivetha et al. [92] showed that a FMMA with a high sensory score can be obtained by the addition of wheat gluten whereas the addition of paneer was less accepted. Overall, FMMA formulations containing SC mushroom 37.5% w/w, 12.5% w/w CF, vital wheat gluten (4.8%, w/w), distilled water (28% w/w), soy protein isolate (10%, w/w), canola oil (5%, w/w), beetroot extract (0.2%, w/w), and yeast extract (2%, w/w) show promising texture and flavor profiles, which may lead to a higher consumer adoption of meat alternatives.
4. Conclusions
This study described a novel method for producing a mushroom-based minced meat alternative using Sajor-caju as a main ingredient along with chickpea flour, soy protein isolated, and vital wheat gluten as a protein source. Results suggested that SC mushroom shows the best sensory acceptance for the processing of FMMA. Overall, an optimum formulation containing 37.5% w/w SC mushroom, 12.5% w/w chickpea flour, 5% w/w canola oil, and 0.2% w/w beetroot extract was selected for the production of FMMA based on nutritional, textural, and sensory characteristics. These results show that it is possible to formulate a nutritious meat analog with a high consumer acceptance based on mushrooms. The development of FMMA is expected to expand the applications of mushrooms, expand the meat alternative portfolio and respond to consumers' demands and sustainability of future food supply. Further research could also focus on determining the storage stability, the bioavailability, and in vivo analysis of FMMA and its impact on allergenicity.
Author Contributions
Conceptualization, S.R., Methodology, M.A.R., S.S., P.P., S.K., S.R.; Formal Analysis, M.A.R., S.S., P.P.; Resources, S.K., S.R.; Data curation, M.A.R., S.S., P.P S.K.; Writing—original draft preparation, M.A.R., S.S., P.P., S.K.; Writing—review and editing, M.A.R., S.K., M.C, L.G, S.R.; Supervision, S.K., S.R.; Project administration, S.K., S.R.; Funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from Mae Fah Luang University via the Reinventing University Program Fund (652A04045/2565-2566) and Post-Doctoral fellowship by Mae Fah Luang University, Chiang Rai, Thailand (Grant No.: 09/2023). .
Data Availability Statement
Not applicable.
Acknowledgments
The authors also warmly thank the staff/scientist of Food Science and Technology and Scientific & Technological Instruments Center (STIC), Mae Fah Luang University for facilities support and technical support of using any advanced instrument.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the result.
References
- Stanford, C.B.; Bunn, H.T. Meat-Eating and Human Evolution; Oxford University Press: New York, USA, 2001. [Google Scholar]
- Williams, A.C.; Hill, L.J. Meat and nicotinamide: A causal role in human evolution, history, and demographics. Int. J. Tryptophan Res. 2017, 10, 1178646917704661. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Rosado, P.; Roser, M. Meat and Dairy Production; OurWorldInData.org, 2017 https://ourworldindata.org/meat-production (accessed on 29 January, 2023).
- Whitnall, T.; Pitts, N. ; Global trends in meat consumption. Agric. Commodit. 2019, 9, 96–99. [Google Scholar]
- Marinova, D.; Bogueva, D. Planetary health and reduction in meat consumption. Sustain. Earth. 2019, 2, 3. [Google Scholar] [CrossRef]
- Kumar, P.; Chatli, M.K.; Mehta, N.; Singh, P.; Malav, O.P.; Verma, A.K. Meat analogues: health promising sustainable meat substitutes. Crit. Rev. Food Sci. Nutr. 2017, 57, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Malav, O.P.; Talukder, S.; Gokulakrishnana, P.; Chand, S. Meat analog: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1241–1245. [Google Scholar] [CrossRef]
- UBS. Market News; 2019. https://www.ubs.com/global/en/wealthmanagement/marketnews/home/article.1441202.html/ (accessed on 29 January, 2023).
- Watson, J. Plant-based Meat Market to Reach USD 30.92 Billion by 2026. Reports and Data, 2019. https://www.globenewswire.com/news-release/2019/10/14/ 1929284/0/en/Plant-based-Meat-Market-To-Reach-USD-30-92-Billion-By-2026- Reports-And-Data.html/ (accessed on 29 January, 2023).
- Yaffe-Bellany, D. The New Makers of Plant-based Meat? The New York Times, 2019. https://www.nytimes.com/2019/10/14/business/the-new-makers-ofplant-based-meat-big-meat-companies.html/ (accessed on 29 January, 2023).
- Lucas, A. Impossible Foods is Launching Meatless Pork and Sausage as it Prepares for a Global Push. 2020. https://www.cnbc.com/2020/01/06/impossible-foods-is-launching-meatless-pork-and-sausage-as-it-prepares-for-a-globalpush.html/ (accessed on 29 January, 2023).
- Bhat, Z. F.; Fayaz, H. Prospectus of cultured meat—advancing meat alternatives. J. Food Sci. Technol. 2011, 48, 125–140. [Google Scholar] [CrossRef]
- Joshi, V.K.; Kumar, S. Meat analogues: plant based alternatives to meat products-A review. Int. J. Food Ferment. Tech. 2015, 5, 107–119. [Google Scholar] [CrossRef]
- United Nations, UN. Global Population Growth and Sustainable Development. UN DESA. United Nations Department of Economic and Social Affairs, 2019. https://desapublications.un.org/file/649/download (accessed on 30 January, 2023).
- De Angelis, D.; Kaleda, A.; Pasqualone, A.; Vaikma, H.; Tamm, M.; Tammik, M.-L.; Squeo, G.; Summo, C. Physicochemical and sensorial evaluation of meat analogues produced from dry-fractionated pea and oat proteins. Foods 2020, 9, 1754. [Google Scholar] [CrossRef]
- He, J.; Evans, N. M.; Liu, H.; Shao, S. A Review of research on plant-based meat aternatives: driving forces, history, manufacturing, and consumer attitudes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2639–2656. [Google Scholar] [CrossRef]
- Tso, R.; Lim, A. J.; Forde, C.G. A Critical appraisal of the evidence supporting consumer motivations for alternative proteins. Foods, 2020, 10, 24. [Google Scholar] [CrossRef]
- Curtain, F.; Grafenauer, S. Plant-based meat substitutes in the flexitarian age: An audit of products on supermarket shelves. Nutrients, 2020, 11, 2603. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F. Plant-based meat analogues: from niche to mainstream. European Food Res. Tech. 2020, 247, 297–308. [Google Scholar] [CrossRef]
- Agriculture, Agri-Food Canada. Market Access Secretariat. Global analysis report. Health and wellness series, Vegetarian and vegan Food in Germany. 2017, Htt ps://docplayer.net/45339983-Vegetarian-and-vegan-food-in-germany.html (accessed on 01 February, 2023). /.
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat analog as future food: A Review. J. Animal Sci. Tech. 2020, 62, 111. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Keppler, J. K.; Goot, V. D. A. J. Functionality of ingredients and additives in plant-based meat analogues. Foods, 2021, 10, 600. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Dekkers, B.; van der Goot, A.J. Chapter 6 - Plant-based meat analogues. In Sustainable Meat Production and Processing, Galanakis, C.M., Ed.; Academic Press: 2019; 103-126.
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel protein sources for applications in meat-alternative products—insight and challenges. Foods, 2022, 11, 957. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Asgar, M.A.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A.A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Croan, S.C. Conversion of conifer wastes into edible and medicinal mushrooms. For. Prod. J. 2004, 54, 68–76. [Google Scholar]
- Synytsya, A.; Míčková, K.; Jablonský, I.; Sluková, M.; Čopíková, J. Mushrooms of genus Pleurotus as a source of dietary fibres and glucans for food supplements. Czech J. Food Sci. 2009, 26, 441–446. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdullah, N.; Nuruddin, N.N. Yield and nutritional composition of oyster mushrooms: An alternative nutritional source for rural people. Sains Malays. 2016, 45, 1609–1615. [Google Scholar]
- Yuan, X.; Jiang, W.; Zhang, D.; Liu, H.; Sun, B. Textural, Sensory and Volatile Compounds Analyses in Formulations of Sausages Analogue Elaborated with Edible Mushrooms and Soy Protein Isolate as Meat Substitute. Foods 2022, 11, 52. [Google Scholar] [CrossRef]
- Egbert, R.; Borders, C. Achieving success with meat analogs. Food Tech. 2006, 60, 28–34. [Google Scholar]
- AOAC International, Official Method 950.46. Moisture in Meat and Meat Products; 21st ed., 2019, Gaithersburg, MD.
- AOAC International, Official Method 920.153. Ash in Meat and Meat Products; 21st ed., 2019, Gaithersburg, MD.
- AOAC International, Official Method 981.10. Crude Protein in Meat and Meat Products; 21st ed., 2019. Gaithersburg, MD.
- AOAC International, Official Method 922.06. Fat in Grain and Flou;, 21st ed., 2019, Gaithersburg, MD.
- Food and Agriculture Organization, Food Energy-Methods of Analysis and Conversion Factors; 2003, http://www.fao.org/uploads/medi a/FAO_2003_Food_Energy_02.pdf (accessed on 05 February, 2023).
- AOAC International, Official Method 985.29. Total Dietary Fiber in Foods; 21st ed., 2019, Gaithersburg, MD.
- Borokini, F.; Lajide, L.; Olaleye, T.; Boligon, A.; Athayde, M.; Adesina, I. Chemical profile and antimicrobial activites of two edible mushrooms (Termitomyces robustus and Lentinus squarrosulus). J. Microbiol. Biotech. Food Sci. 2016, 5, 416–423. [Google Scholar] [CrossRef]
- Tasnim, T.; Das, P.C.; Begum, A.A.; Nupur, A.H.; Mazumder, M.A.R. Nutritional, textural and sensory quality of plain cake enriched with rice rinsed water treated banana blossom flour. J. Agric. Food Res. 2020, 2, 100071. [Google Scholar] [CrossRef]
- Botella-Martínez, C.; Viuda-Martos, M.; Fernández-López, J. A.; Pérez-Alvarez, J. A.; Fernández-López, J. Development of plant-based burgers using gelled emulsions as fat source and beetroot juice as colorant: effects on chemical, physicochemical, appearance and sensory characteristics. LWT, 2022, 114193. 1141.
- Lee, J.-S.; Oh, H.; Choi, I.; Yoon, C.S.; Han, J. Physico-chemical characteristics of rice protein-based novel textured vegetable proteins as meat analogues produced by low-moisture extrusion cooking technology. LWT 2022, 157, 113056. [Google Scholar] [CrossRef]
- Noordraven, L.E.C.; Buvé, C.; Grauwet, T.; Van Loey, A.M. Effect of experimental flour preparation and thermal treatment on the volatile properties of aqueous chickpea flour suspensions. J. Integ. Agric. 2022, 21, 2445–2455. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity. 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Hong, F.; Yan, J.; Baran, J.T. Allendorf, D.J.; Hansen, R.D.; Ostroff, G.R.; Xing, P.X.; Cheung, N-K.V.; Ross, G.D. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J. Immunol. 2004, 173, 797–806. [Google Scholar] [CrossRef]
- Lee, D.; Ji, I.; Chang, H.; Kim, C. High-level TNF-α secretion and macrophage activity with soluble β-glucans from Saccharomyces cerevisiae. Biosci. Biotech. Biochem. 2002, 66, 233–238. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Protein quality evaluation. Report of joint expert consultation, Food and Agricultural Organization of the United Nations: Rome, Italy, 1991; pp. 51.
- Cordelle, S.; Redl, A.; Schlich, P. Sensory acceptability of new plant protein meat substitutes. Food Qual. Prefer. 2022, 98, 104508. [Google Scholar] [CrossRef]
- Szenderák, J.; Fróna, D.; Rákos, M. Consumer Acceptance of Plant-Based Meat Substitutes: A Narrative Review. Foods 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Gogavekar, S.S.; Rokade, S.A.; Ranveer, R.C.; Ghosh, J.S.; Kalyani, D.C.; Sahoo, A.K. Important nutritional constituents, flavour components, antioxidant and antibacterial properties of Pleurotus sajor-caju. J. Food Sci. Technol. 2014, 51, 1483–91. [Google Scholar] [CrossRef] [PubMed]
- Misharina, T.A.; Muhutdinova, S.M.; Zharikova, G.G.; Terenina, M.B.; Krikunova, N.I. The composition of volatile components of Cepe (Boletus edulis) and oyster mushrooms (Pleurotus ostreatus). Appl. Biochem. Microbiol. 2009, 45, 187–193. [Google Scholar] [CrossRef]
- Caglarırmak, N. The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds. Food Chem. 2007, 105, 1188–1194. [Google Scholar] [CrossRef]
- Jukantil, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. British J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Sharima-Abdullah, N.; Hassan, C.Z.; Arifin, N.; Huda-Faujan, N. Physicochemical properties and consumer preference of imitation chicken nuggets produced from chickpea flour and textured vegetable protein. Int. Food Res. J. 2018, 25, 1016–1025. [Google Scholar]
- Sanjeewa, W.G.T.; Wanasundara, J.P.D.; Pietrasik, Z.; Shand, P.J. Characterization of chickpea (Cicer arietinum L.) flours and application in low-fat pork bologna as a model system. Food Res. Int. 2010, 43, 617–626. [Google Scholar] [CrossRef]
- USDA. Beef, Ground, 80% Lean meat/ 20% Fat, Raw; Agricultural Bulletin, U.S. Department of Agriculture: Washington, DC, 2018. [Google Scholar]
- USDA. Pork, Fresh, Ground, Raw; Agricultural Bulletin, U.S. Department of Agriculture: Washington, DC, 2018. [Google Scholar]
- The Surprising Protein Composition of Mushrooms. https://blog.designsforhealth.com/node/1101#:~:text=Both%20cooked%20and%20uncooked%20mushrooms,superiority%20to%20other%20protein%20sources (accessed on 28 March, 2023).
- Jongrak Attarat, J.; Phermthai, T. Bioactive Compounds in Three Edible Lentinus Mushrooms. Walailak J. Sci. Tech. 2015, 12(6), 491–504. [Google Scholar]
- Toontom, N.; Namyota, C.; Nilkamheang, T.; Wongprachum, K.; Bourneow, C. ; Tudpor,K. Nutraceutical stability in Lentinus squarrosulus after drying and frying for snack production. Int. J. Health Sci. 2022, 6, 8762–8774. [Google Scholar]
- Bryant, C.; Szejda, K.; Parekh, N.; Deshpande, V.; Tse, B. A survey of consumer perceptions of plant-based and clean meat in the USA, India, and China. Front. Sustain. Food Syst. 2019, 3, 11. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the Physicochemical and Structural Properties and the Sensory Characteristics of Meat Analogues Prepared with Various Non-Animal Based Liquid Additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef]
- Selani, M.M.; Shirado, G.A.N.; Margiotta, G.B.; Saldana, E.; Spada, F.P.; Piedade, S.M.S.; Contreras-Castillo, C.J.; Canniatti-Brazaca, S.G. Effects of pineapple byproduct and canola oil as fat replacers on physicochemical and sensory qualities of low-fat beef burger. Meat Sci. 2016, 112, 69–76. [Google Scholar] [CrossRef]
- Bayram, M.; Bozkurt, H. The use of bulgur as a meat replacement: bulgur-sucuk (a vegetarian dry-fermented sausage). J. Sci. Food Agric. 2007, 87, 411–419. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Improved functional properties of meat analogs by laccase catalyzed protein and pectin crosslinks. Sci. Rep. 2021, 11, 16631. [Google Scholar] [CrossRef]
- Mazlan, M.M.; Talib, R.A.; Chin, N.L.; Shukri, R.; Taip, F.S.; Nor, M.Z.M.; Abdullah, N. Physical and microstructure properties of oyster mushroom-soy protein meat analog via single-screw extrusion. Foods 2020, 9, 1023. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Sabikun, N.; Hwang, Y.-H.; Joo, S.-T. A Novel approach for tuning the physicochemical, textural, and sensory characteristics of plant-based meat analogs with different levels of methylcellulose concentration. Foods 2021, 10, 560. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Evaluation of rheological and sensory characteristics of plant-based meat analog with comparison to beef and pork. Food Sci. Anim. Resour. 2021, 41, 983–996. [Google Scholar] [CrossRef]
- Mazumder, M.A.R.; Sujintonniti, N.; Chaum, P.; Ketnawa, S.; Rawdkuen, S. Developments of plant-based emulsion-type sausage by using grey oyster mushrooms and chickpeas. Foods 2023, 12, 1564. [Google Scholar] [CrossRef]
- Saedi, S.; Noroozi, M.; Khosrotabar, N.; Mazandarani, S.; Ghadrdoost, B. ; How canola and sunflower oils affect lipid profile and anthropometric parameters of participants with dyslipidemia. Med J. Islam Repub Iran. 2017, 2017 31, 5. [Google Scholar] [CrossRef]
- Hutchings, J.B. The important of visual apperance of foods to food processor and the consumer. J. Food Qual. 1977, 1(3), 267–278. [Google Scholar] [CrossRef]
- Domínguez, R.; Munekata, P.E.S.; Pateiro, M.; Maggiolino, A.; Bohrer, B.; Lorenzo, J.M. Red Beetroot. A Potential Source of Natural Additives for the Meat Industry. Appl. Sci. 2020, 10, 8340. [Google Scholar] [CrossRef]
- Pakula, C.; Stamminger, R. Measuring changes in internal meat colour, colour lightness and colour opacity as predictors of cooking Time. Meat Sci. 2012, 90, 721–727. [Google Scholar] [CrossRef]
- Hollenbeck, J.J.; Apple, J.K.; Yancey, J.W.S.; Johnson, T.M.; Kerns, K.N.; Young, A.N. Cooked color of precooked ground beef patties manufactured with mature bull trimmings. Meat Sci. 2019, 148, 41–49. [Google Scholar] [CrossRef]
- Rolan, T.; Mueller, I.; Mertle, T.J.; Swenson, K.; Conley, C.; Orcutt, M.W.; Mease, L. Ground Meat and Meat Analog Compositions Having Improved Nutritional Properties; U.S. Patent 11/963,375, 30 October 2008. [Google Scholar]
- Hamilton, M.N.; Ewing, C.E. Food Coloring Composition; 2000, https://patents.google.com/patent/CA2314727C/en (accessed on 10 February 2023).
- Kyed, M.-H.; Rusconi, P. Protein Composition for Meat Products or Mmeat Aanalog Products; U.S. Patent 12/389,148, 20 August 2009. [Google Scholar]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2006, 69, C491–C498.
- Vrljic, M.; Solomatin, S.; Fraser, R.; O’reilly Brown, P.; Karr, J.; Holz-Schietinger, C.; Eisen, M.; Varadan, R. Methods and Compositions forConsumables; Patent. WO2013010042A1, 12 July 2012. [Google Scholar]
- Fraser, R.; Davis, S.C.; Brown, P.O. Secretion of heme-containing polypeptides; U.S. Patent 20170342131A1, 9 August 2017. [Google Scholar]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–66. [Google Scholar] [CrossRef]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncales, P. Comparative effect of red yeast rice (Monascus purpureus), red beet root (Beta vulgaris) and betanin (E-162) on colour and consumer acceptability of fresh pork sausages packaged in a modified atmosphere. J. Sci. Food Agric. 2006, 86, 500–8. [Google Scholar] [CrossRef]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Adam, T.; Westerterp-Plantenga, M. Dietary protein and energy balance in relation to obesity and co-morbidities, Front. Endocrinol. 2018, 9, 1–5. [Google Scholar]
- USDA, Composition of Foods Raw, Processed, Prepared - USDA National Nutrient Database for Standard Reference, Release 28; Agricultural Bulletin, U.S. Department of Agriculture: Washington, DC, 2015.
- Olagunju, A.I.; Nwachukwu, I.D. The differential effects of cooking methods on the nutritional properties and quality attributes of meat from various animal sources. Croat. J. Food Sci. Technol. 2020, 12, 37–47. [Google Scholar] [CrossRef]
- Vu, G.; Zhou, H.; McClements, D.J. Impact of cooking method on properties of beef and plant-based burgers: appearance, texture, thermal properties, and shrinkage. J. Agric. Food Res. 2022, 9, 100355. [Google Scholar] [CrossRef]
- Webb, D.; Li, Y.; Alavi, S. Chemical and physicochemical features of common plant proteins and their extrudates for use in plant-based meat. Trends in Food Science & Technology 2023, 131, 129–138. [Google Scholar]
- Samard, S.; Ryu, G.-H. Physicochemical and functional characteristics of plant protein-based meat analogs. J. Food Proc. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Rolan, T.; Mueller, I.; Mertle, T.J.; Swenson, K.; Conley, C.; Orcutt, M.W.; Mease, L. Ground Meat and Meat Analog Compositions Having Improved Nutritional Properties; U.S. Patent 11/963,375, 30 October 2008. [Google Scholar]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile Compounds in Pulses: A Review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef]
- Andreani, G.; Sogari, G.; Marti, A.; Froldi, F.; Dagevos, H.; Martini, D. Plant-Based Meat Alternatives: Technological, Nutritional, Environmental, Market, and Social Challenges and Opportunities. Nutrients 2023, 15, 452. [Google Scholar] [CrossRef]
- Sirimuangmoon, C.; Lee, S.M.; Guinard, J.X.; Miller, A.M. A Study of using mushrooms as a plant-based alternative for a popular meat-based dish. KKU Res. J. 2016, 21, 156–167. [Google Scholar]
- Nivetha, B.R.; Sudha, K.; Narayanan, R.; Vimalarani, M. Development and sensory evaluation of meat analog. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 1283–1288. [Google Scholar] [CrossRef]
Figure 1.
Processing of mushroom-based minced meat alternative.
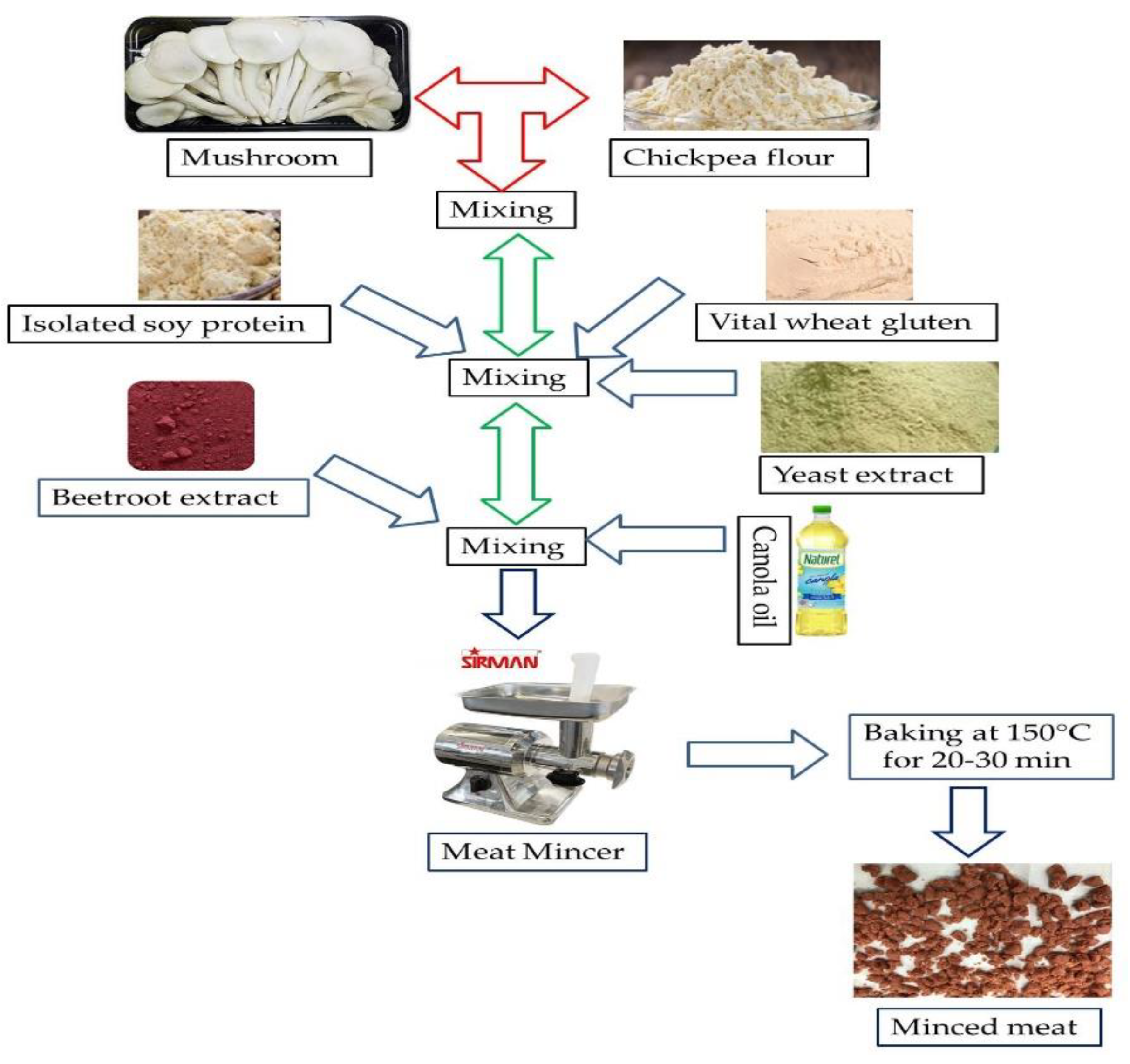
Figure 2.
Effect of different concentrations of beetroot extracts on the appearance of fresh (raw) and cooked Sajor-caju mushroom-based minced meat alternative.
Figure 2.
Effect of different concentrations of beetroot extracts on the appearance of fresh (raw) and cooked Sajor-caju mushroom-based minced meat alternative.

Figure 3.
Appearance of the Sajor-caju mushroom-based minced meat alternative (fresh and cooked produced with the optimized recipe.
Figure 3.
Appearance of the Sajor-caju mushroom-based minced meat alternative (fresh and cooked produced with the optimized recipe.

Figure 4.
Electrophoresis patterns of protein profile of different minced meat alternatives, (a) Mushroom-based minced meat alternative; (b) Pork minced meat; (c) Commercial plant-based minced meat alternative; (d) Standard marker.
Figure 4.
Electrophoresis patterns of protein profile of different minced meat alternatives, (a) Mushroom-based minced meat alternative; (b) Pork minced meat; (c) Commercial plant-based minced meat alternative; (d) Standard marker.

Figure 5.
Sensory evaluation of the Sajor-caju mushroom-based minced meat alternative by using 9-point hedonic scale.
Figure 5.
Sensory evaluation of the Sajor-caju mushroom-based minced meat alternative by using 9-point hedonic scale.
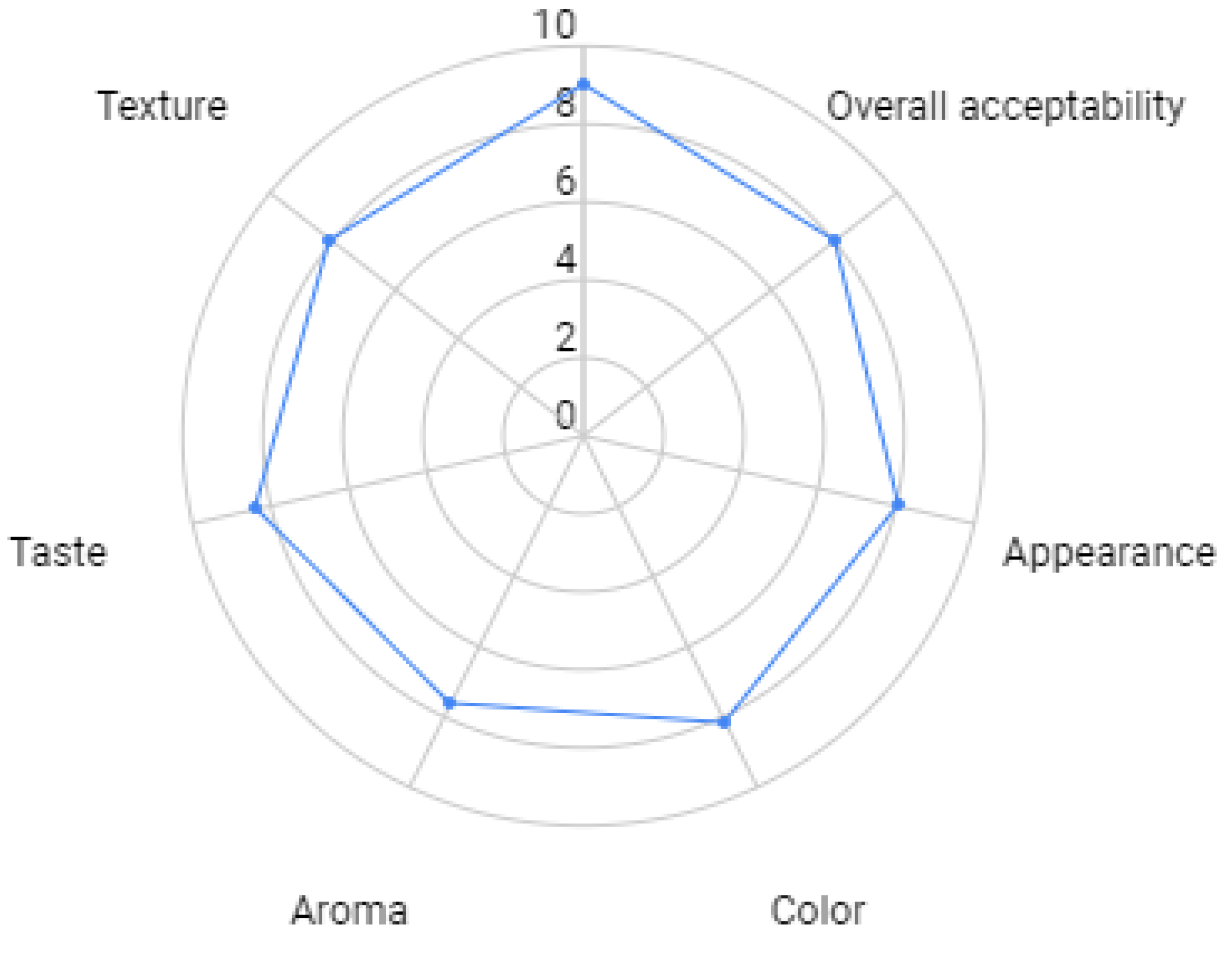
Table 1.
Morphological characteristics and nutritional properties of Sajor-caju mushroom.
| Properties | Sajor-caju | % RP |
|---|---|---|
| Morphology | ||
| Size | Stalk length: 2.8 cm; Stalk diameter: 1.1 cm; Diameter of cap = 6 cm | - |
| Shape | Cap is fleshy shell or spatula shaped (pileus), stipe (stalk) is lateral (short or long) or central stalk; gills (lamellae) is long ridges and furrows underneath pileus | - |
| Weight/Age | 28 to 35 g /25 to 30 days | - |
| Nutritional properties (% Dry weight) | ||
| Ash | 7.85±0.09 | - |
| Protein | 24.79±0.9 | - |
| Fat | 1.15±0.08 | - |
| Dietary fiber | 43.75±3.50 | - |
| Essential amino acids (g/100g sample) | ||
| Histidine | 2.20 | 1.9 |
| Lysine | 4.94 | 5.08 |
| Isoleucine | 4.61 | 2.8 |
| Leucine | 7.17 | 6.6 |
| Tryptophan | 1.13 | - |
| Phenylalanine | 6.05 | 6.3a |
| Threonine | 4.74 | 3.4 |
| Methionine | 1.59 | 2.5b |
| Valine | 5.07 | 3.5 |
All values are means ± SD of three replicates; % RP = Requirement pattern in protein (%) for adults [46], a = Phenylalanine with tyrosine, b = Cysteine with methionine.
Table 2.
Moisture, protein content and sensory attributes of Sajor-caju mushroom minced meat alternative base formulation.
Table 2.
Moisture, protein content and sensory attributes of Sajor-caju mushroom minced meat alternative base formulation.
| Properties | SC FMMA | |
|---|---|---|
| Moisture (%) | 28.39±0.17 | |
| Protein (% db) | 41.99±0.55 | |
| Sensory attributes | Overall acceptability | 6.43±1.80 |
| Appearance | 6.80±1.47 | |
| Color | 6.78±1.74 | |
| Aroma | 5.93±1.68 | |
| Taste | 5.91±1.81 | |
| Texture | 6.43±1.82 | |
All values are means ± SD of three replicates.
Table 3.
Effect of different concentrations of chickpea flour on the properties of Sajor-caju mushroom-based minced meat alternative.
Table 3.
Effect of different concentrations of chickpea flour on the properties of Sajor-caju mushroom-based minced meat alternative.
| Properties | SC Mushroom: Chickpea flour (by weight) | |||||
|---|---|---|---|---|---|---|
| 0: 50 | 12.5: 37.5 | 25: 25 | 37.5: 12.5 | 50: 0 | ||
| Moisture (%) | 12.30± | 12.99± | 13.74± | 14.86± | 16.16± | |
| Protein (%db) | 34.29± | 37.74± | 39.69± | 47.03±0.28a | 47.59± | |
| Textural properties | Hardness (N) | 9441.01 ± | 3668.28 ± | 2721.81 ± | 2610.23 ± | 1983.35 ± |
| Chewiness (N) | 3422.55 ± | 1347.78± | 1220.32 ± | 1171.32 ± | 789.84± | |
| Springiness (mm) | 0.65 ± | 0.76 ± | 0.86 ± | 0.88 ± | 0.90 ± | |
| Gumminess | 826.99±91.31a | 791.45±90.29a | 660.54±456.18b | 673.47±88.52ab | 775.54±80.97a | |
| Cohesiveness | 0.35 ± | 0.50 ± | 0.57 ± | 0.45 ± | 0.63 ± | |
| Sensory attributes | Overall acceptability | 4.44± | 5.09± | 5.47± | 7.24± | 6.24± |
| Appearance | 5.24± | 5.18± | 5.53± | 7.21± | 6.00± | |
| Aroma | 5.53± | 6.06± | 6.77± | 7.91± | 7.04± | |
| Texture | 3.03± | 4.26± | 5.41± | 7.65± | 6.24± | |
All values are means ± SD of three replicates; Mean values with different letters in each row are significantly (p<0.05) different.
Table 4.
Effects of canola oil concentrations on sensory properties of Sajor-caju mushroom-based minced meat alternative.
Table 4.
Effects of canola oil concentrations on sensory properties of Sajor-caju mushroom-based minced meat alternative.
| Canola Oil (%, w/w) | Overall acceptability | Appearance | Juiciness | Aroma | Texture | |
| 1 | 6.37± | 6.60± | 5.46± | 5.60± | 6.26± | |
| 2 | 6.46± | 6.60± | 5.57± | 6.05± | 6.26± | |
| 3 | 6.46± | 6.60± | 5.54± | 6.70± | 6.14± | |
| 4 | 6.80± | 6.51± | 5.06± | 6.81± | 6.69± | |
| 5 | 6.97± | 6.89± | 6.60± | 7.21± | 6.74± |
All values are means ± SD of triplicates; Mean value with different superscripts in each column is significantly different (p≤0.05).
Table 5.
Color attributes and sensorial properties of Sajor-caju mushroom-based minced meat alternative by using different levels of beetroot extract.
Table 5.
Color attributes and sensorial properties of Sajor-caju mushroom-based minced meat alternative by using different levels of beetroot extract.
| Properties | Concentration of beetroot extract (%, w/w) | |||||
|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | 1.0 | ||
| Fresh FMMA color | ||||||
| Whiteness | 34.55±0.55 | 30.85±1.55 | 29.45±0.90 | 29.291.11 | 27.79±2.01 | |
| ΔE | 25.76±1.03 | 23.03±1.25 | 22.20±1.35 | 22.10±0.95 | 20.70±0.75 | |
| L* | 38.89± | 34.79± | 33.55± | 33.40± | 31.34± | |
| a* | 7.83± | 7.74± | 8.01± | 8.27± | 8.39± | |
| b* | 6.87± | 4.40± | 3.45± | 2.94± | 0.41± | |
| Cooked FMMA color | ||||||
| Whiteness | 75.25±1.03 | 72.47±0.98 | 71.72±0.85 | 71.25±2.03 | 70.12±2.20 | |
| ΔE | 56.90±1.15 | 54.40±1.95 | 53.85±1.22 | 53.35±1.75 | 53.25±1.55 | |
| L* | 85.32± | 82.12± | 81.27± | 80.76± | 80.38± | |
| a* | 4.37± | 4.45± | 4.93± | 4.43± | 4.76± | |
| b* | 4.65± | 4.43± | 3.13± | 2.38± | 2.13± | |
| Sensory attributes | Overall acceptability | 6.85± | 4.62± | 6.56± | 5.50± | 5.50± |
| Appearance | 7.15± | 5.21± | 6.62± | 5.62± | 5.59± | |
| Fresh texture | 7.93± | 7.85± | 7.78± | 7.61± | 7.55± | |
| Cooked texture | 7.75± | 7.70± | 7.55± | 7.41± | 7.35± | |
| Fresh aroma | 7.22± | 6.89± | 6.72± | 6.59± | 6.41± | |
| Cooked aroma | 7.64± | 7.05± | 6.82± | 6.64± | 6.58± | |
| Fresh color | 6.82± | 3.79± | 5.62± | 5.09± | 4.41± | |
| Cooked color | 6.94± | 4.59± | 7.12± | 5.74± | 5.18± | |
All values are means ± SD of triplicates; Mean value with different superscripts in each row is significantly different (p≤0.05).
Table 6.
Nutritional, physico-chemical and textural properties of the Sajor-caju mushroom-based minced meat alternative.
Table 6.
Nutritional, physico-chemical and textural properties of the Sajor-caju mushroom-based minced meat alternative.
| Properties | Fresh fungi minced meat alternative | Cooked fungi minced meat alternative |
|---|---|---|
| Nutritional composition | ||
| Moisture (%) | 12.06 ± | 9.78± |
| Protein (%) | 47.90 ± | 45.06± |
| Fat (%) | 12.51 ± | 10.76± |
| Ash (%) | 4.32 ± | 3.97± |
| Carbohydrate (%) | 23.21±0.95b | 30.43±3.53a |
| Dietary fiber (%) | 9.63 ± | 8.65± |
| Cooking loss (%) | 44.76 | |
| Color parameters | ||
| Whiteness | 32.25±1.55 | 70.02±1.35 |
| ΔE | 23.76±1.75 | 53.25±1.55 |
| L* | 36.11± | 79.51± |
| a* | 7.88 ± | 4.21± |
| b* | 6.81± | 4.14± |
| Textural properties | ||
| Hardness (N) | 2109.34 ± | 2457.85± |
| Chewiness (N) | 1477.95 ± | 1747.58± |
| Springiness (mm) | 0.93 ± | 0.98 ± |
| Cohesiveness | 0.52 ± | 0.77 ± |
All values are means ± SD of three replicates. Mean values with different letters in each row are significantly different (p<0.05).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






