1. Introduction
Acute manifestations of atherosclerosis remain the most common cause of premature death, yet the mechanisms shaping this process are still not fully understood [
1]. Even though the inflammatory component of atherogenesis has gained the attention of researchers over recent years, multiple hypotheses still exist regarding the molecular interplay, which drives arterial inflammation and accelerates plaque creation [
2,
3]. An array of recent data suggests that anti-inflammatory therapy may be of added benefit in the treatment of coronary artery disease, though efficacy in the treatment of peripheral disease is uncertain [
4,
5].
The inner layer of arteries is composed of the endothelium, which plays the role of an interface between molecules circulating in blood and cells residing within the vessel wall. Impairment of its proper function is assumed to be the origin point for atherosclerotic processes and is incited by a pro-inflammatory milieu [
6].
Leukotrienes (LT) are lipid molecules, which exert multiple physiologic functions within the vasculature and organ systems, also being crucial mediators in the initial stages of inflammation. Throughout the process of atherogenesis, the abundance of pro-inflammatory signals also contributes to the development of unstable plaques.
Recent data from Sweden suggests that asthmatic patients treated with leukotriene receptor antagonists are less vulnerable to cardiovascular disease [
7]. Studies in acute coronary syndrome report a reduction in LT levels by 12 weeks, with significant differences observed for the incidence of atherosclerotic plaques and plaque volume [
8]. The link between LTs and atherosclerosis has been shown to have a genetic component [
9].
The clinical utility of arachidonic acid metabolites as biomarkers of peripheral vascular disease remains not fully explored. In patients with intermittent claudication, the occurrence of restenosis after endovascular treatment was related to urinary leukotriene E4 (LTE4) concentration, while perioperative thromboxane B2 levels were tied to the rate of major adverse cardiovascular events [
10,
11]. A comprehensive literature review was recently conducted to examine the association between elevated LT and the presence of atherosclerotic disease, showing the existing dependence, but not answering the question of its mechanism [
12].
While the role of LT as mediators has been established in the process of atherogenesis, the relationship with endothelial function and vascular measures is not fully known. To our knowledge, the relationship between LT concentrations and alterations in endothelial function have not been studied prospectively in the context of peripheral arterial disease.
2. Materials and Methods
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
2.1. Study Design
Middle-aged (45 to 75 years), consecutive patients with intermittent claudication (Rutherford 3) and without signs of inflammation, qualified for lower limb percutaneous transluminal angioplasty (PTA) were enrolled. The number of patients included was based on the power and sample size calculations. Detailed clinical characteristics including co-morbidity burden and cardiovascular treatment were gathered at baseline, prior to endovascular procedures. Individuals were followed up at pre-set intervals of 1, 3, 6 and 12 months after discharge. Patient clinical assessment, vascular imaging, endothelial parameters assessment and collection of urine samples were performed at each of those time points.
The primary outcome of the study was to assess the relationship between urinary LTE4 and LTB4 levels and non-invasive measures of endothelial parameters and its functions described below.
2.1.1. Flow-Mediated Dilatation (FMD)
The brachial artery was localized with the use of an ultrasound transducer 2-5 cm above the cubital fossa. The cuff was placed on the forearm and was pumped 50 mm Hg above systolic blood pressure for 5 min to restrict artery inflow. From 60 s to 90 s after deflation, all vasodilation measurements with vessel diameter changes to reactive hyperemia were made [
13]. FMD was measured with the Hitachi Arietta 850 automatic software.
2.1.2. Shear Rate (SR)
The calculation of blood shear force defined as the blood velocity divided by vessel internal diameter. It was assessed during FMD measurement.
2.1.3. Corrected Augmentation Index (AI75)
Represents a backward traveling pressure waves, defined as the ratio of difference in pressure between the early and late systolic shoulders to pulse pressure, reflecting the arterial wall stiffness. In this study we used the standardized index to a heart rate of 75 bpm as it has been proved to be more reliable.
2.1.4. Intima-Media Thickness (IMT)
The measurement of the IMT was conducted in the common carotid artery 1cm proximal to the bulb, on the 10 mm-long part of the far and near wall and calculated by the automatic software provided in Hitachi Arietta 850. The assessment was performed on both sides and the higher result was included in the calculations.
The secondary goal was the evaluation of LTE4 and LTB4 levels and potential associations with clinical characteristics and achievement of a composite endpoint of treated limb-artery restenosis or death at one-year follow-up was performed.
Urine samples were collected at all study visits, centrifuged and stored in a freezer at -80°C until testing. LTE4 and LTB4 were assayed with a competitive Abbexa ELISA kit. The results were recalculated by the concentration of creatinine in urine.
2.2. Ethical Aspects
The study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans and the proper consent for this study has been obtained from the constituted committee for human subjects or animal research on Jagiellonian University Medical College (1072.6120.365.2020). All of the participants signed the informed consent.
2.3. Statistical Analysis
Continuous variables are summarized as mean (standard deviation) or median (interquartile range). Variable distribution was assessed using QQ plots and the Shapiro-Wilk test. Simple linear regression models were fit for comparison of the relationship between the discussed variables. Skewed variables were transformed to approximate the normal distribution. Right-skewed variables were log-transformed. The decision to include clinically significant covariates in multivariable models was limited due to sample size and the investigators decided, before conducting analyses, that the understanding of the impact of gender and coronary artery disease is theoretically justified.
3. Results
3.1. Characteristics of the Study Population
Out of 126 patients screened, the inclusion criteria were met by 50, who were all enrolled into the study. The participants' mean age of the sample was 65.76 years with a male predominance (66%). The cardiovascular burden of the population was high – 74% had hypertension, 56% previously diagnosed dyslipidemia, 44% documented coronary artery disease, and 44.0% were active smokers. (Supplementary Material,
Table S1) During the hospitalization, all patients were treated with acetylsalicylic acid, clopidogrel, proton pump inhibitors and statins. If necessary, hypotensive drugs were introduced.
3.2. Urinary Leukotriene Levels and Their Relationship with Parameters Reflecting Vascular Function
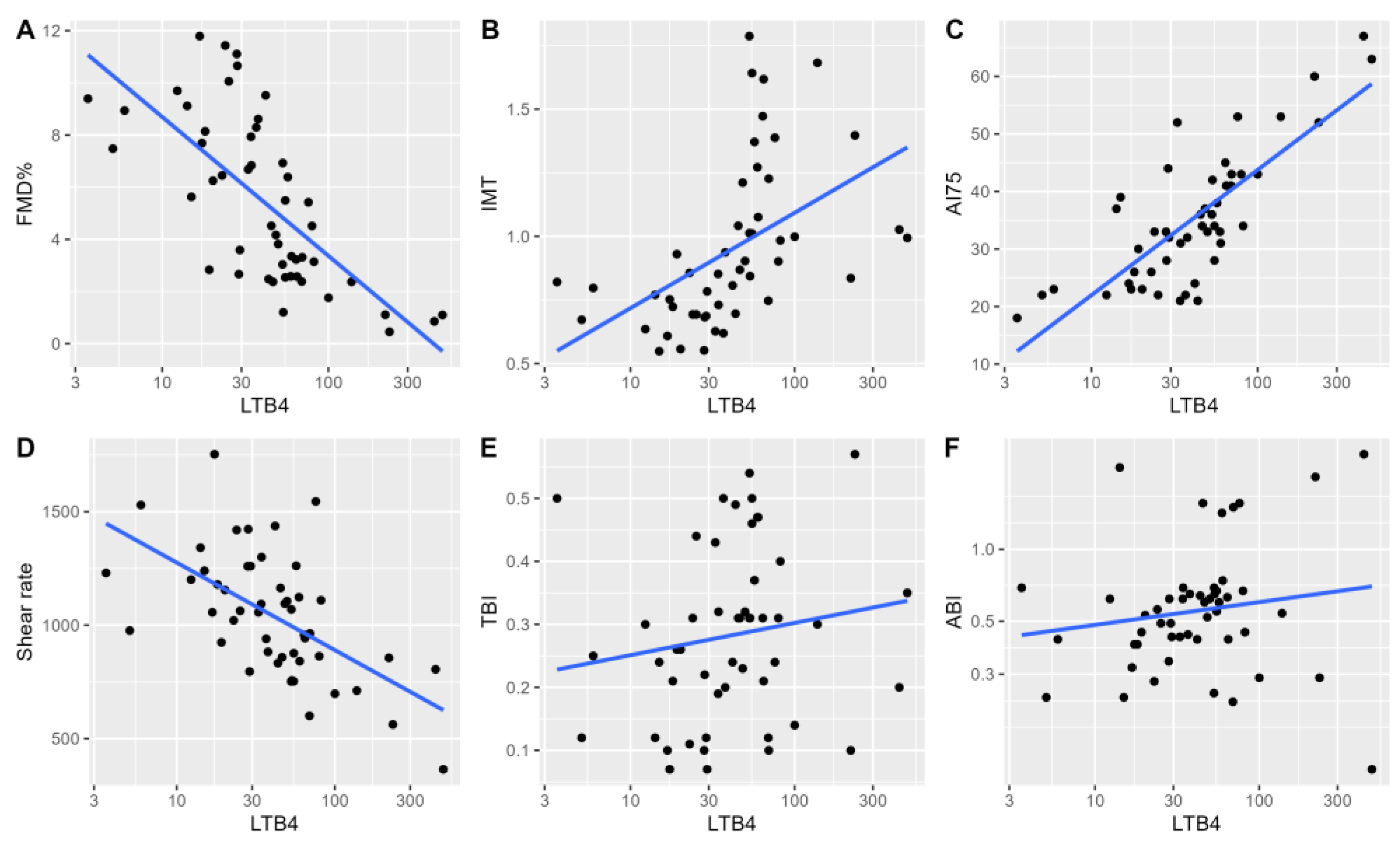
In univariable analyses, we observed a significant relationship between log-transformed LTB4 concentration and ultrasound measures reflecting vascular functions and stiffness (
Figure 1). A significant univariable relationship between log-transformed LTB4 levels and flow-mediated dilatation was noted (adjusted R2 = 0.47, P<0.001), and was maintained in a multivariable model adjusting for patients’ age and sex (adjusted R2 = 0.45, P<0.001). Similar observations were noted in univariable baseline analyses for mean IMT (adjusted R2=0.23, P<0.001), AI75 (adjusted R2=0.61, P<0.001) and SR (adjusted R2=0.33, P<0.001). In multivariable models adjusting for the presence of documented coronary artery disease (CAD) and patient sex, we observed LTs to be significant independent predictors of flow-mediated dilatation, arterial augmentation index and vessel wall shear rate. (
Supplementary Material, Table S2)
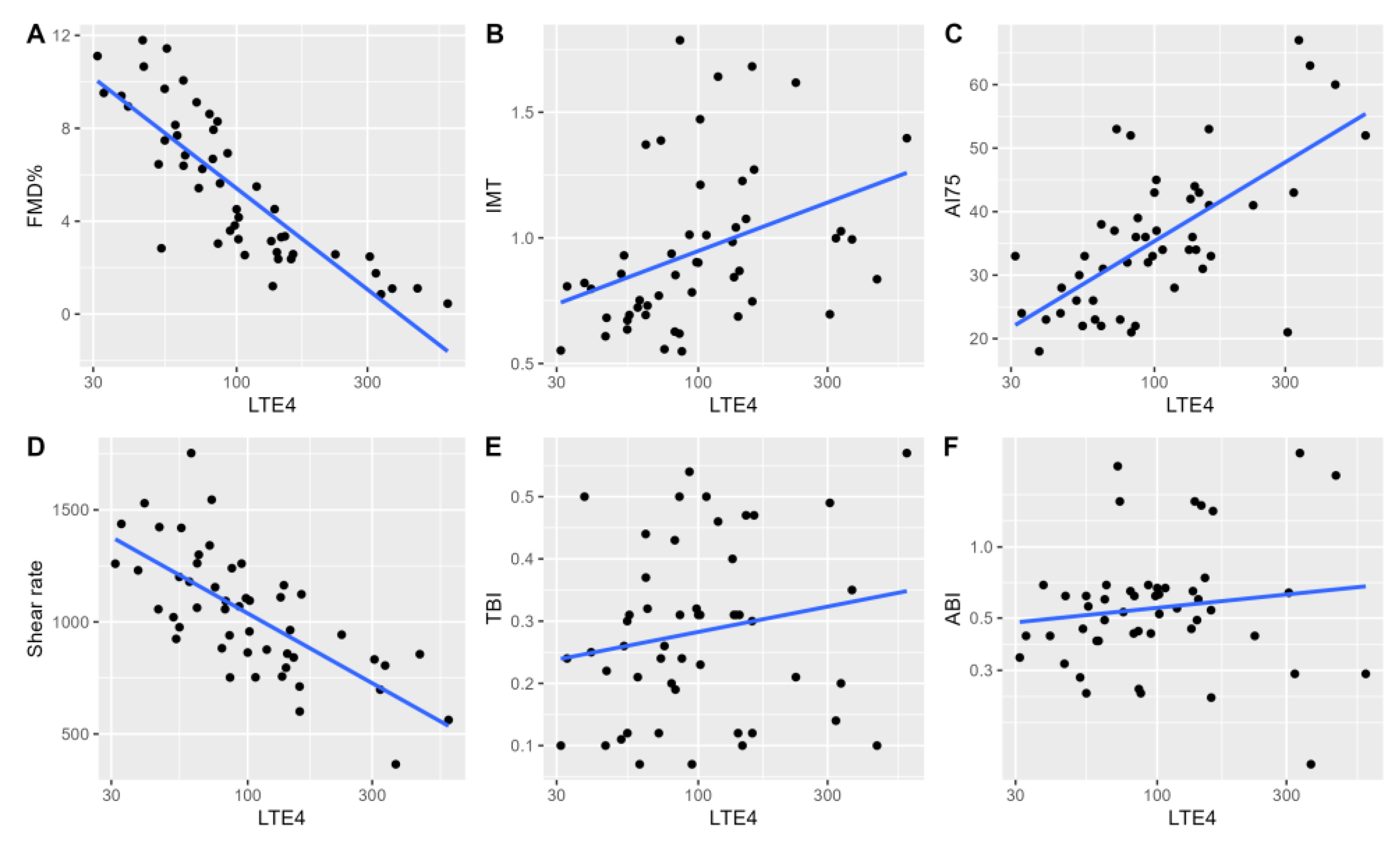
In univariable analyses for LTE4, we observed similar relationships between indirect measures of vascular function and stiffness. A significant univariable relationship between log-transformed LTE4 levels and flow-mediated dilatation was noted (adjusted R2 = 0.69, P<0.001. Similar baseline observations were noted in univariable analyses for mean IMT (adjusted R2=0.12, P<0.01), AI75 (adjusted R2=0.43, P<0.001), SR (adjusted R2=0.48, P<0.001). Similar to LTB4, no relationship with log-transformed ABI and TBI was noted (P=0.36, P=0.21). (
Figure 2)
3.3. Changes in Urinary Leukotriene Levels and Their Relationship with Alterations in Arterial Function Measures
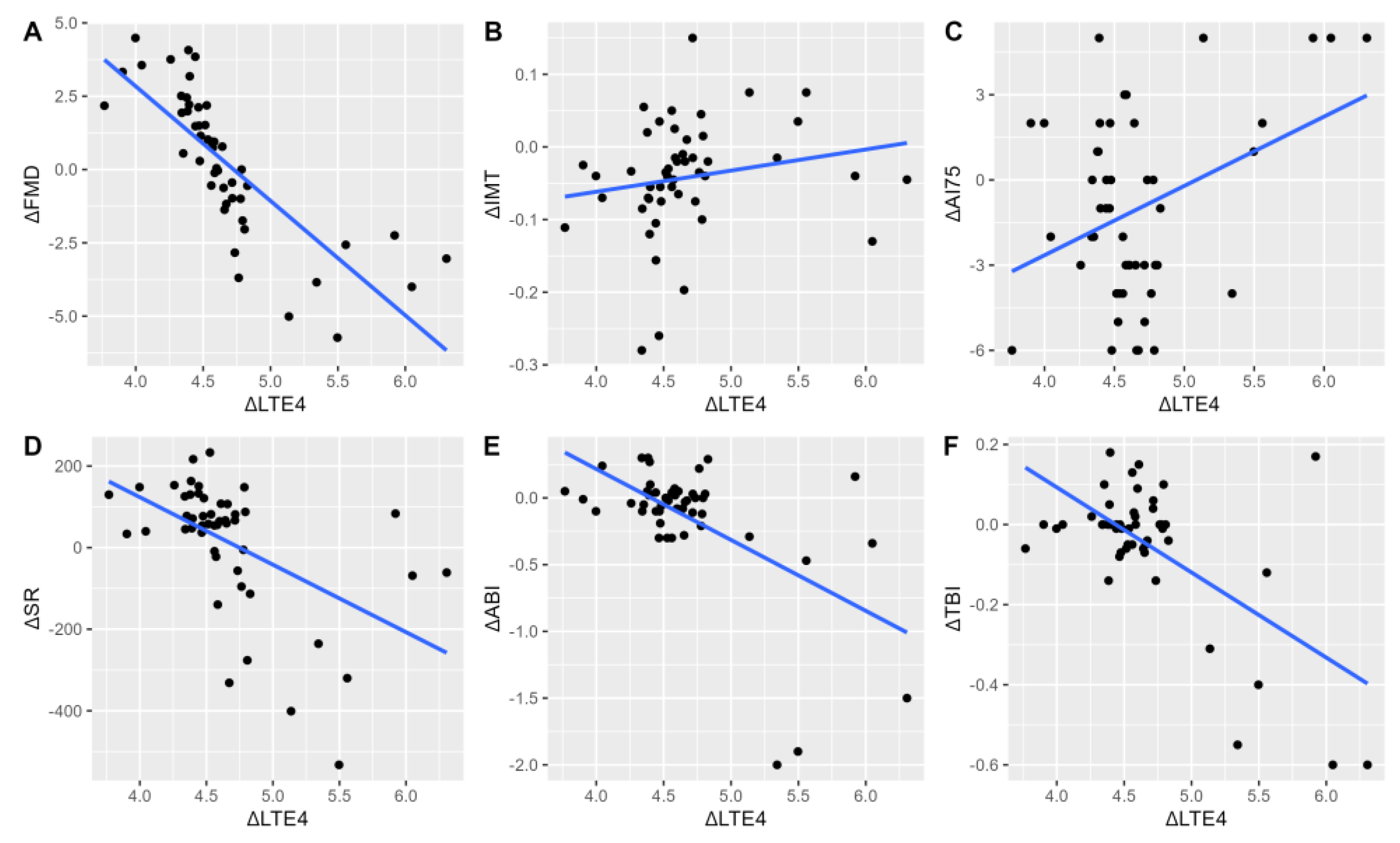
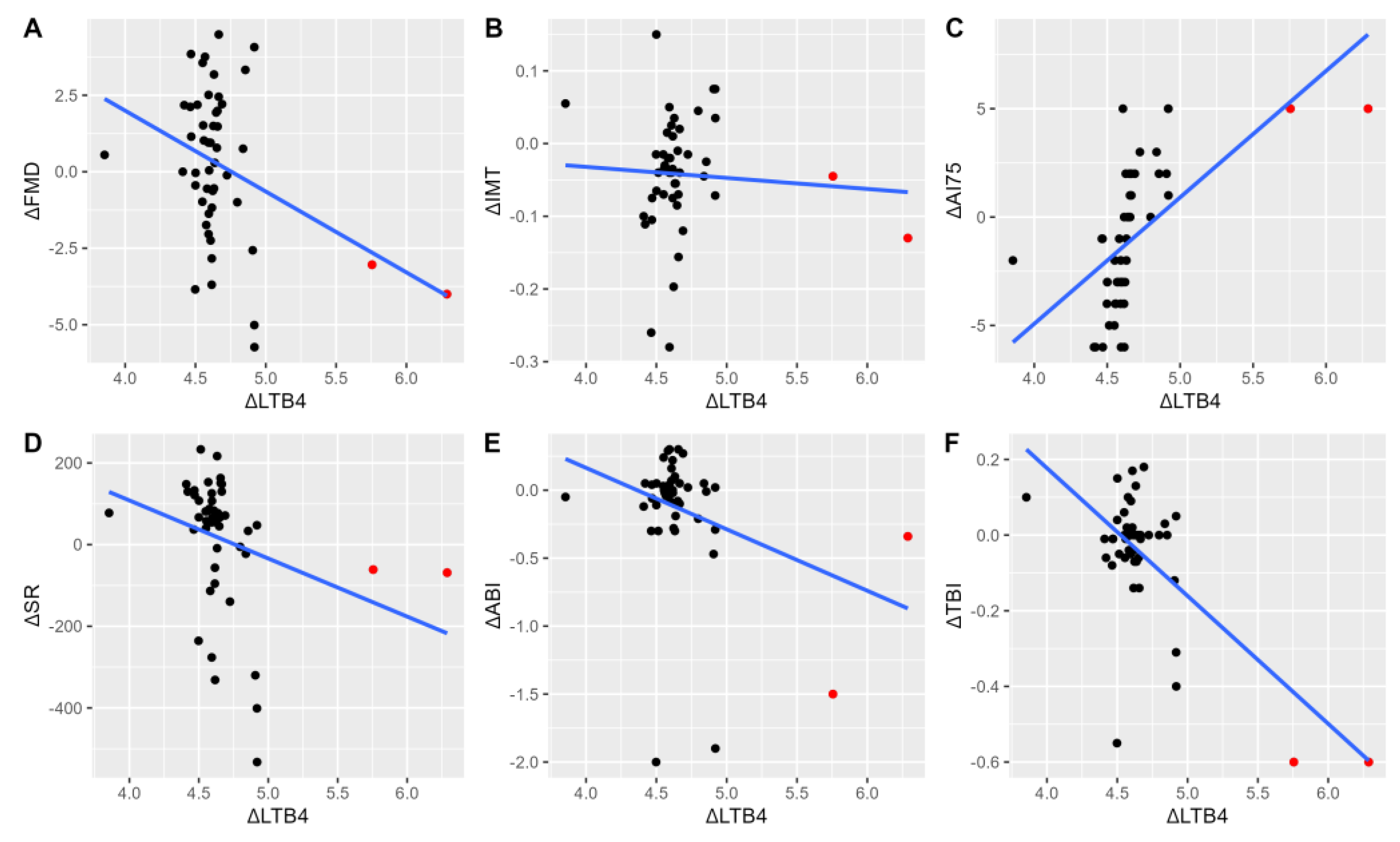
In univariable analyses, significant relationships were observed between the change in LTE4 and LTB4 levels defined as the change respective to the 1-month as an earliest post-procedural stable assay by 6 months (LT at six months/LT at one month; ΔLT). This time point was chosen as it was the earliest time at which restenosis was reported, which would suggest that sufficient pathological changes within the vascular bed have occurred to be of clinical significance. Analyses were conducted to evaluate whether alterations in LT levels are associated with the progression of arterial function impairment and would aid in the detection of clinical progression with a reference set at earliest pre-procedural assay (ΔFMD, ΔIMT, ΔAI75, ΔSR). ΔLTE4 (adjusted R2=0.63, P<0.001) and ΔLTB4 (adjusted R2=0.06, P=0.047) were significant predictors of ΔFMD values. Similarly, ΔLTE4 (adjusted R2=0.42, P<0.001) and ΔLTB4 (adjusted R2=0.29, P<0.001) were significantly related to ΔSR. ΔLTE4 was not associated with ΔAI75 (P=0.33), in contrast to ΔLTB4 (adjusted R2=0.40, P<0.001). A significant relationship between ΔIMT and ΔLTE4 (adjusted R2=0.008, P=0.02), but not ΔLTB4 was noted (P=0.13). (
Figure 3 and
Figure 4)
4. Discussion
4.1. The Significance of Leukotrienes in Atherosclerosis
We have previously shown that serial measurements of LTE4 are a useful prognostic factor to aid in the prediction of angioplasty failure [
11]. Across studies, urinary LTE4 levels have been treated as a proxy measure of LT generation, and are significantly higher in patients with myocardial infarction, as compared with stable coronary artery disease cases [
14]. Aside from LTE4, LTB4 is another candidate biomarker of emerging significance. It is characterized as a potent chemoattractant, which is produced on-site within vascular lesions and activates immune cell subsets [
15]. LTB4 levels were further tied to luminal carotid diameter in subjects with low arterial oxygen saturation [
16]. Taken together, these studies identify an association between processes shaping cardiovascular disease and LTE4 / LTB4 generation. The inhibition of leukotrienes pathways is believed to represent a new opportunity in the pharmacological treatment of atherosclerosis [
17,
18].
A salient finding of the present study is that both LTB4 and LTE4 levels share a significant relationship with various indices of vascular function and vessel remodeling. Even though there is clear dependence between those two types of LTs (
Supplementary Material, Figure S1) their impact on the individual vascular parameters differs, which may reflect varying involvement in pathobiological processes.
4.2. The Impact of LTs on Vascular Endothelium Parameters
According to the latest suggestions, the thickness of the carotid intima-media layer should not be treated as the marker of subclinical atherosclerosis [
19], though it still remains one of the most recognizable cardiovascular disease risk factors [
20]. Our results showed that there is a relationship between LTs concentrations and IMT at the baseline, supporting the concept of leukotriene-injured-based endothelium dysfunction theory. The correlation between the change of carotid IMT and LTs level dynamics was not pronounced, which is likely due to the short study timeframe. Furthermore, all patients were treated according to best practices, which include intensive statin therapy, which has been documented to lower the risk of IMT expansion [
21].
The relation between arterial wall stiffness and LTs has been the subject of only one in vivo human study so far, and LTB4 was assayed during only a single time point [
22]. Although a causal effect cannot be established by our study due to the presence of potential confounding factors, elevations in LTE4 and LTB4 are related to measures of arterial stiffness. (
Figure 3C,
Figure 4C). As increased arterial stiffness causes the progression of atherosclerosis, we can assume that a sufficient increase in the concentration of leukotrienes may lead to a higher rate of recurrence of lower limb ischemia.
There are multiple factors influencing vascular wall dilatation properties, with a prominent role in inflammation [
23]. Our results show that the vascular milieu, represented by increasing values of LTB4 and LTE4 is accompanied by impaired vasodilatation. This observation is consistent both prior to and after endovascular treatment, where patients with increased LTs have lower rates of FMD. Moreover, increasing concentrations of LTs were reflected by the further reduction of FMD. (
Figure 3A,
Figure 4A). Whether a direct pathophysiologic relationship between the reduction of FMD and the development of peripheral artery disease patients exists remains unclear [
24].
4.3. Study Limitations
Firstly, the small sample and restenosis count warrant careful consideration of the conclusions drawn at present. While our results are in line with theoretical assumptions that are derived from literature, these findings should be treated as identifying a trend worthy of further evaluation. Although the study sample is moderately heterogeneous in terms of cardiometabolic burden, the recruitment criteria were designed to limit the influence of significant confounders, such as the exclusion of significant renal impairment, which is known to affect urinary LT levels. Last, but not least, the study was conducted only on patients with intermittent claudication, but not with chronic limb-threatening ischemia. This is due the fact that in patients with ischemic wounds concentration of LT will be disturbed by the lower limb inflammation. As the consequence, the conclusions of this study may not be universally adapted for all groups of lower limb ischemia patients.
4.5. Clinical Implications of the Study
To our best knowledge, this is the first prospective study to evaluate the utility of urinary LTB4 and LTE4 as surrogate measures of endothelial dysfunction and vascular impairment in patients with moderate-grade peripheral artery disease undergoing endovascular treatment. Over subsequent follow-up time points, LT levels do not change significantly, which may reflect an individual propensity for “steady state” production. This inherent level of LT generation could reflect the extent and severity of vascular disease, as patients with greater baseline LT levels are at greater risk for restenosis.
This, as well as the fact that both examined LT may significantly induce the recreation of atherosclerotic plaque, supports the existing idea of therapy based on anti-leukotriene drugs [
17,
25] and significantly contribute to a new pathway of pharmacological treatment development.
5. Conclusions
The findings of the present study show the significant impact of proinflammatory biomarkers represented by LT on the vascular endothelium and its functioning: the vascular inflammation enlarges the thickness of the intima-media complex, increases the vascular wall stiffness and impairs its vasodilatation properties. This is the next step for a hypothetical framework for the utility of leukotrienes as biomarkers of incident restenosis and/or vascular disease progression, but also significantly contributes to a new pathway of pharmacological treatment development for peripheral arterial disease patients.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Scatterplot with line of linear fit illustrating the relationship between urinary LTE4 and LTB4; Table S1: Summary of cardiometabolic co-morbidities of patients at baseline stratified by endpoint status at 12 months. All patients were receiving dual platelet therapy, IPP, and statin at recommended/tolerated dose. No differences in pharmacotherapy were observed apart from more common diuretic use in incident cases (72.7% vs. 35.9%, P=0.042); Table S2: Multivariable regression model (N=50) for baseline FMD, AI75 and shear rate including leukotriene B4 or E4 levels and co-variates of patient gender and presence of coronary artery disease.
Author Contributions
Conceptualization, Agnieszka Wachsmann-Maga, Piotr Terlecki and Paweł Maga; Data curation, Mikołaj Maga; Formal analysis, Krzysztof Batko; Funding acquisition, Agnieszka Wachsmann-Maga; Investigation, Agnieszka Wachsmann-Maga, Aleksandra Włodarczyk, Mikołaj Maga and Katarzyna Bogucka; Methodology, Agnieszka Wachsmann-Maga, Mikołaj Maga and Paweł Maga; Project administration, Agnieszka Wachsmann-Maga and Mikołaj Maga; Resources, Maria Kapusta; Software, Agnieszka Wachsmann-Maga, Aleksandra Włodarczyk and Mikołaj Maga; Supervision, Piotr Terlecki and Paweł Maga; Validation, Piotr Terlecki and Paweł Maga; Visualization, Agnieszka Wachsmann-Maga and Krzysztof Batko; Writing – original draft, Agnieszka Wachsmann-Maga, Aleksandra Włodarczyk, Mikołaj Maga, Krzysztof Batko, Katarzyna Bogucka and Maria Kapusta; Writing – review & editing, Mikołaj Maga, Piotr Terlecki and Paweł Maga. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Polish National Science Centre with PRELUDIUM, grant number 2017/27/N/NZ5/02191. The APC was funded by Jagiellonian University Medical College.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Jagiellonian University Medical College Bioethical Committee (1072.6120.365.2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We would like to thank the Jagiellonian Centre for Experimental Therapeutics JCET for providing us with measurement equipment.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Libby, P. The Changing Landscape of Atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W. Inflammation Revisited: Atherosclerosis in the Post-CANTOS Era. Eur. Cardiol. Rev. 2017, 12, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; MacNamara, K.C. Atherosclerosis Is a Major Human Killer and Non-Resolving Inflammation Is a Prime Suspect. Cardiovasc. Res. 2021, 117, 2563–2574. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.S.; Yates, D.P.; Kramer, C.M.; Feller, A.; Mahling, P.; Colin, L.; Clough, T.; Wang, T.; LaPerna, L.; Patel, A.; et al. A Randomized, Placebo-Controlled Trial of Canakinumab in Patients with Peripheral Artery Disease. Vasc. Med. (United Kingdom) 2019, 24, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, E.; Yin, L.; Bäck, M. Nationwide Cohort Study of the Leukotriene Receptor Antagonist Montelukast and Incident or Recurrent Cardiovascular Disease. J. Allergy Clin. Immunol. 2012, 129. [Google Scholar] [CrossRef] [PubMed]
- Gaztanaga, J.; Farkouh, M.; Rudd, J.H.F.; Brotz, T.M.; Rosenbaum, D.; Mani, V.; Kerwin, T.C.; Taub, R.; Tardif, J.-C.; Tawakol, A.; et al. A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study of the Effect of VIA-2291, a 5-Lipoxygenase Inhibitor, on Vascular Inflammation in Patients after an Acute Coronary Syndrome. Atherosclerosis 2015, 240, 53–60. [Google Scholar] [CrossRef]
- Freiberg, J.J.; Tybjaerg-Hansen, A.; Nordestgaard, B.G. Novel Mutations in Leukotriene C4 Synthase and Risk of Cardiovascular Disease Based on Genotypes from 50000 Individuals. J. Thromb. Haemost. 2010, 8, 1694–1701. [Google Scholar] [CrossRef]
- Maga, P.; Sanak, M.; Jawien, J.; Rewerska, B.; Maga, M.; Wachsmann, A.; Koziej, M.; Gregorczyk-Maga, I.; Nizankowski, R. 11-Dehydro Thromboxane B2 Levels after Percutaneous Transluminal Angioplasty in Patients with Peripheral Arterial Occlusive Disease during a One Year Follow-up Period. J. Physiol. Pharmacol. 2016, 67, 377–383. [Google Scholar]
- Maga, P.; Sanak, M.; Rewerska, B.; Maga, M.; Jawien, J.; Wachsmann, A.; Rewerski, P.; Szczeklik, W. Urinary Cysteinyl Leukotrienes in One-Year Follow-up of Percutaneous Transluminal Angioplasty for Peripheral Arterial Occlusive Disease. Atherosclerosis 2016, 249, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Wachsmann-Maga, A.; Kaszuba, M.; Maga, M.; Włodarczyk, A.; Krężel, J.; Kaczmarczyk, P.; Bogucka, K.; Maga, P. Leukotrienes in the Atherosclerotic Cardiovascular Diseases – a Systematic Review. Acta Angiol. 2022, AA4. [Google Scholar] [CrossRef]
- Mitchell, A.; Newby, D.E.; Mills, N.L.; Fujisawa, T.; Cruden, N.L.M. Reproducibility of Radial Artery Flow-Mediated Dilatation and Feasibility as a Model of Mechanical Vascular Injury in Man. Atherosclerosis 2015, 241, e49. [Google Scholar] [CrossRef]
- Stodólkiewicz, E.; Rewerska, B.; Rzeszutko, M.; Tomala, M.; Chrustowicz, A.; Zmudka, K.; Sanak, M.; Szczeklik, W. Leukotriene Biosynthesis in Coronary Artery Disease. Polish Arch. Intern. Med. 2018, 128, 934–942. [Google Scholar]
- Riccioni, G.; Bäck, M. Leukotrienes as Modifiers of Preclinical Atherosclerosis? Sci. World J. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Pépin, J.L.; Baguet, J.P.; Tamisier, R.; Roustit, M.; Riedweg, K.; Bessard, G.; Lëvy, P.; Stanke-Labesque, F. Leukotriene B4: Early Mediator of Atherosclerosis in Obstructive Sleep Apnoea? Eur. Respir. J. 2008, 32, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Alomair, B.M.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Al-Hamash, S.M.; De Waard, M.; Sabatier, J.-M.; Saad, H.M.; El-Saber Batiha, G. Montelukast and Acute Coronary Syndrome: The Endowed Drug. Pharmaceuticals 2022, 15, 1147. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and Its Resolution in Atherosclerosis: Mediators and Therapeutic Opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef]
- Raggi, P.; Stein, J.H. Carotid Intima-Media Thickness Should Not Be Referred to as Subclinical Atherosclerosis: A Recommended Update to the Editorial Policy at Atherosclerosis. Atherosclerosis 2020, 312, 119–120. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Lind, L. Effect of New Statin Treatment on Carotid Artery Intima-Media Thickness: A Real-Life Observational Study over 10 Years. Atherosclerosis 2020, 306, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Labat, C.; Temmar, M.; Nagy, E.; Bean, K.; Brink, C.; Benetos, A.; Bäck, M. Inflammatory Mediators in Saliva Associated with Arterial Stiffness and Subclinical Atherosclerosis. J. Hypertens. 2013, 31, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Honda, A.; Tahara, N.; Nitta, Y.; Tahara, A.; Igata, S.; Bekki, M.; Nakamura, T.; Sugiyama, Y.; Kaida, H.; Kurata, S.; et al. Vascular Inflammation Evaluated by [ 18 F]-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography Is Associated With Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, P.; Maga, P.; Niżankowski, R.; Januszek, R.; Frołow, M.; Maga, M.; Kościelniak, J.; Belowski, A. The Relationship between Pulse Waveform Analysis Indices, Endothelial Function and Clinical Outcomes in Patients with Peripheral Artery Disease Treated Using Percutaneous Transluminal Angioplasty during a One-Year Follow-up Period. Cardiol. J. 2020, 27, 142–151. [Google Scholar] [CrossRef]
- Gautier-Veyret, E.; Bäck, M.; Arnaud, C.; Belaïdi, E.; Tamisier, R.; Lévy, P.; Arnol, N.; Perrin, M.; Pépin, J.-L.; Stanke-Labesque, F. Cysteinyl-Leukotriene Pathway as a New Therapeutic Target for the Treatment of Atherosclerosis Related to Obstructive Sleep Apnea Syndrome. Pharmacol. Res. 2018, 134, 311–319. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).