1. Introduction
Surgical treatment for obesity began in the 1950s. Sleeve gastrectomy, which was developed in the 2000s, has become popular worldwide with the progress of laparoscopic surgery, and is currently the most performed bariatric surgical procedure [
1]
. In this surgery, the weight loss effect and the metabolic improvement effect are remarkably observed. Therefore, this surgery has been re-recognized as a metabolic surgery, and then the number of surgical cases is increasing [
2,
3]
. The low invasiveness, weight loss effect, and metabolic improvement effect of this procedure have been highly evaluated, and research on the factors that influence the effect has progressed [
4,
5,
6]. On the other hand, with the spread of this technique, it is important to perform it safely for short-term and long-term results. A management system is in place for this purpose. In the U.S., ACS (American College of Surgeons) and ASMBS (American Society for Metabolic and Bariatric Surgery) have their own education and certification systems to ensure safety [
7,
8]
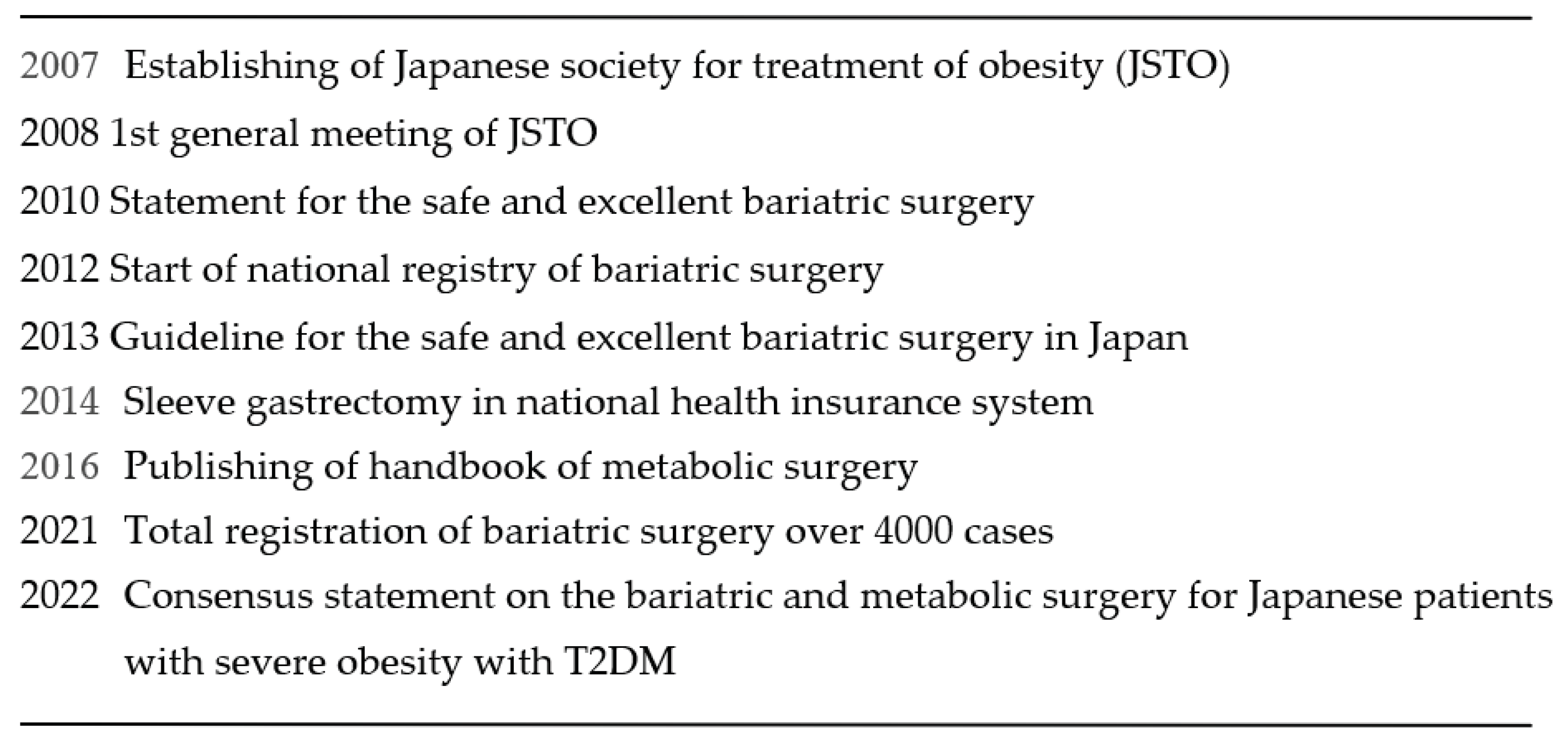
. In Japan, bariatric surgical treatment was started in 1982. The first case was done by open method. After that, the Japanese Society for Treatment of Obesity(JSTO) was established in 2007 in the background of an increase in diabetes and an increase in the trend toward obesity among adult men
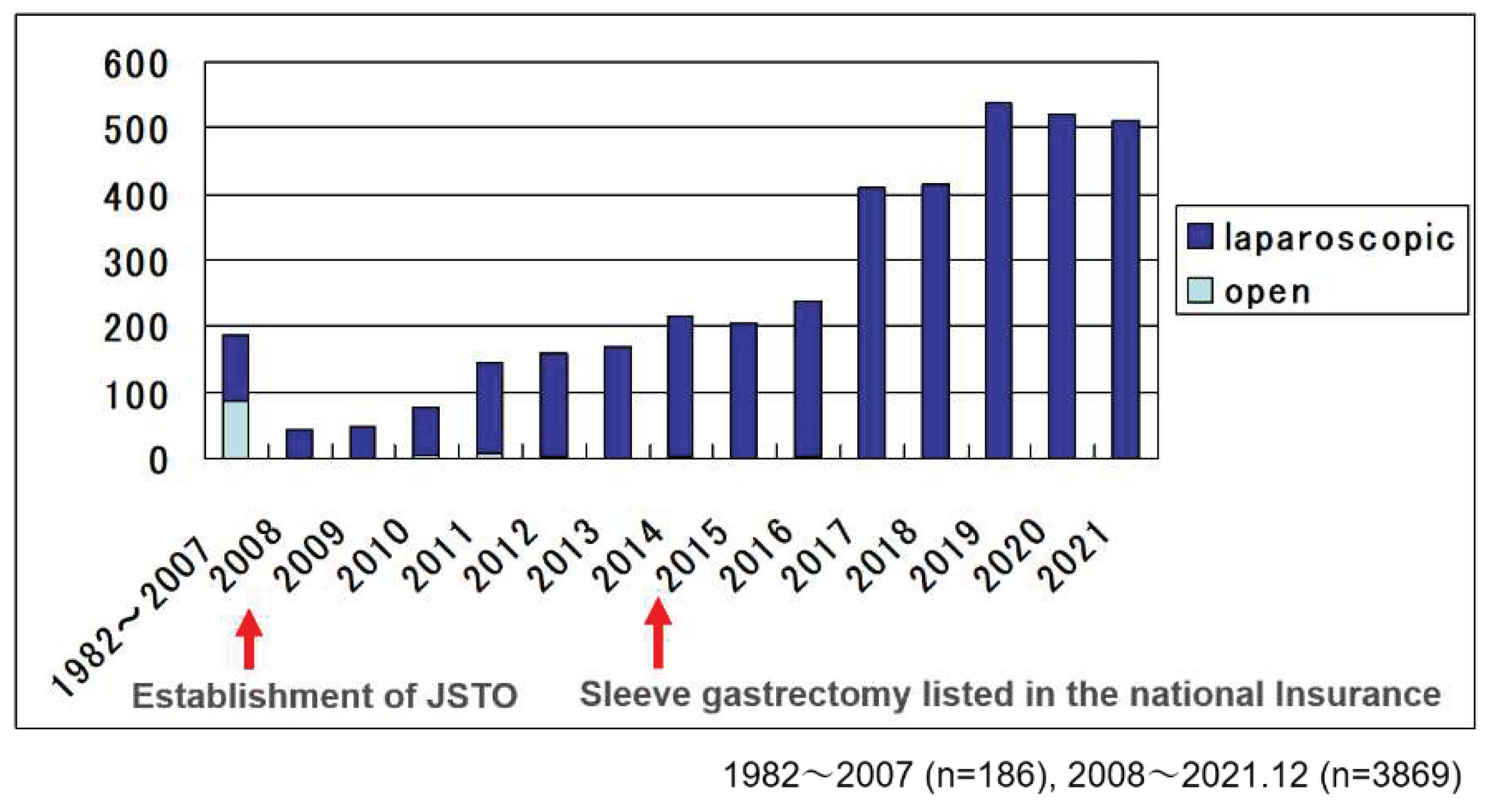
, and then JSTO started national registry of bariatric surgery cases and multidisciplinary educational program(Table 1). Before the establishment of JSTO, total cases were 186, and half of them were done by open method. After the establishment of JSTO, the numbers were increasing and reached 4055 in 2021 totally
(Figure 1).
In this article, we verified the safety and efficacy of sleeve gastrectomy in Japan based on national registration by JSTO.
2. Subjects and Methods
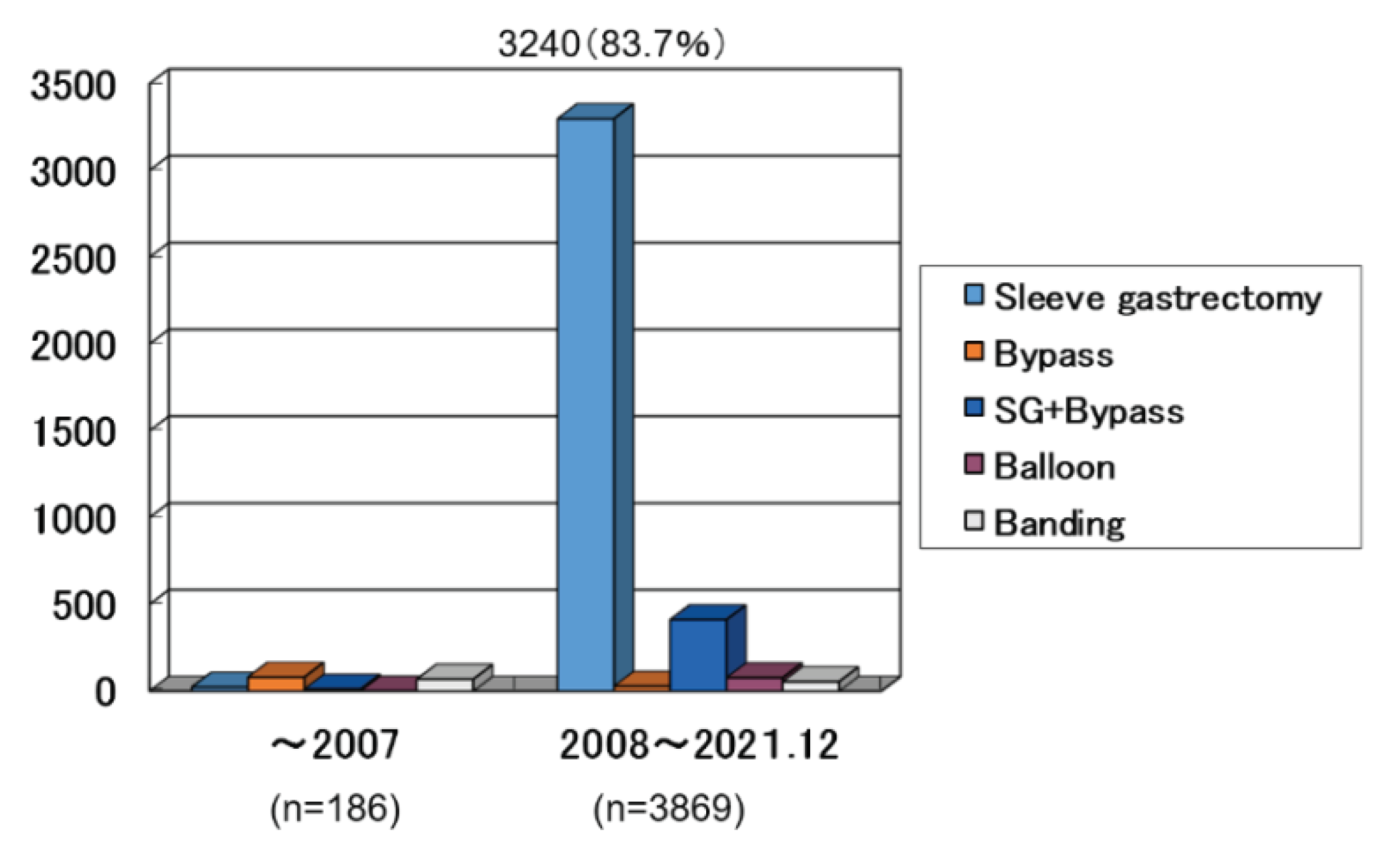
44 facilities registered 4055 bariatric surgical cases until 2021. In Japan, before 2008, the half were Gastric Bypass and the half were Banding method and sleeve gastrectomy. After 2008, sleeve gastrectomy was mostly done (
Figure 2), and the next was sleeve bypass method [
9]. In this study, the purpose is to clarify the indication, the safety and the effectiveness of the sleeve gastrectomy using national registry data base compiled by JSTO. The number of the subjects were 3240. The period of the surgery was from 2008 to 2021. The evaluated factors were preoperative BMI, coexisting comorbidities, intraoperative incidents, postoperative complications, reoperation, morbidity, mortality, hospital stay and the effect on the body weight and the metabolic comorbidities.
After 2008, sleeve gastrectomy was mostly done (
Figure 2) in 3240 cases (83.7%).
3. Results
3.1. Indications of surgery
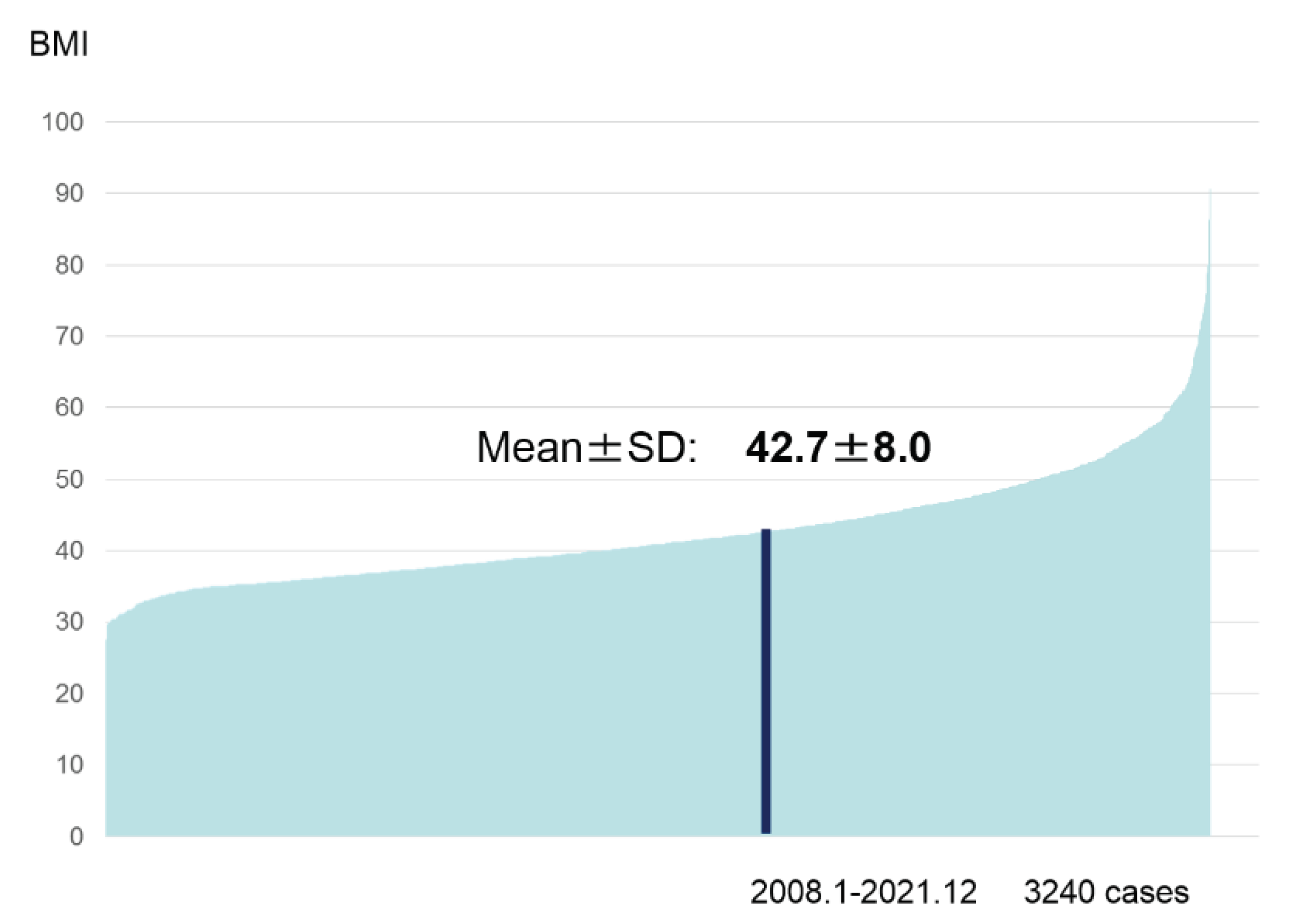
The evaluated factors were preoperative BMI and the coexisting morbidities. Preoperative BMI was 27.6 to 90.7, and the mean value was 42.7(
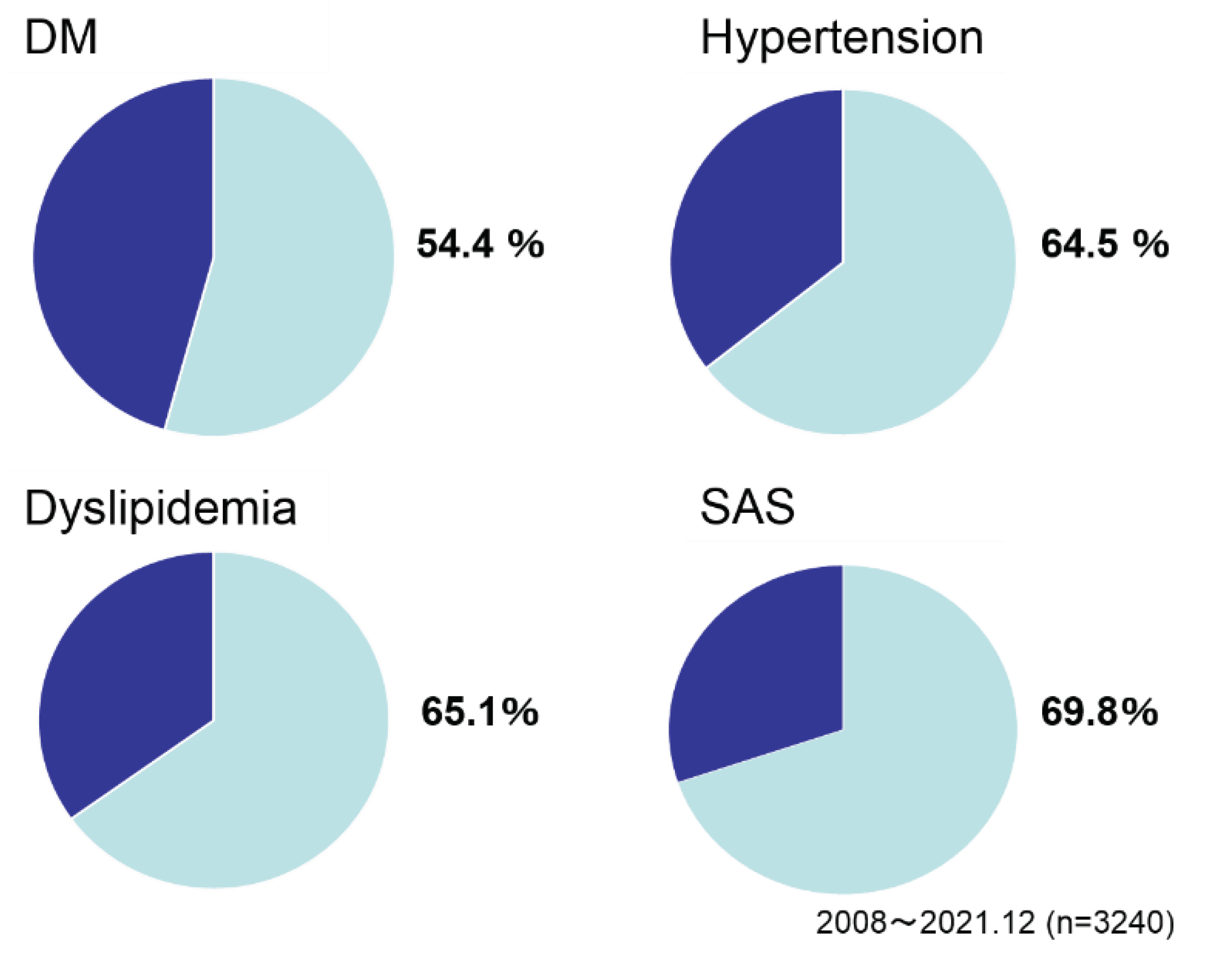
Figure 3). As gender, men/women was 1/1.3. Age was 42.2 as mean. As preoperative comorbidities, the ratio of DM was 54.4%, hypertension 64.5%, dyslipidemia 65.1 and sleep apnea syndrome (SAS) 69.8% (
Figure 4).
The value data ranged from 27.6 to 90.7 and mean value was 42.7.
3.2. Evaluation of the safety of the surgery
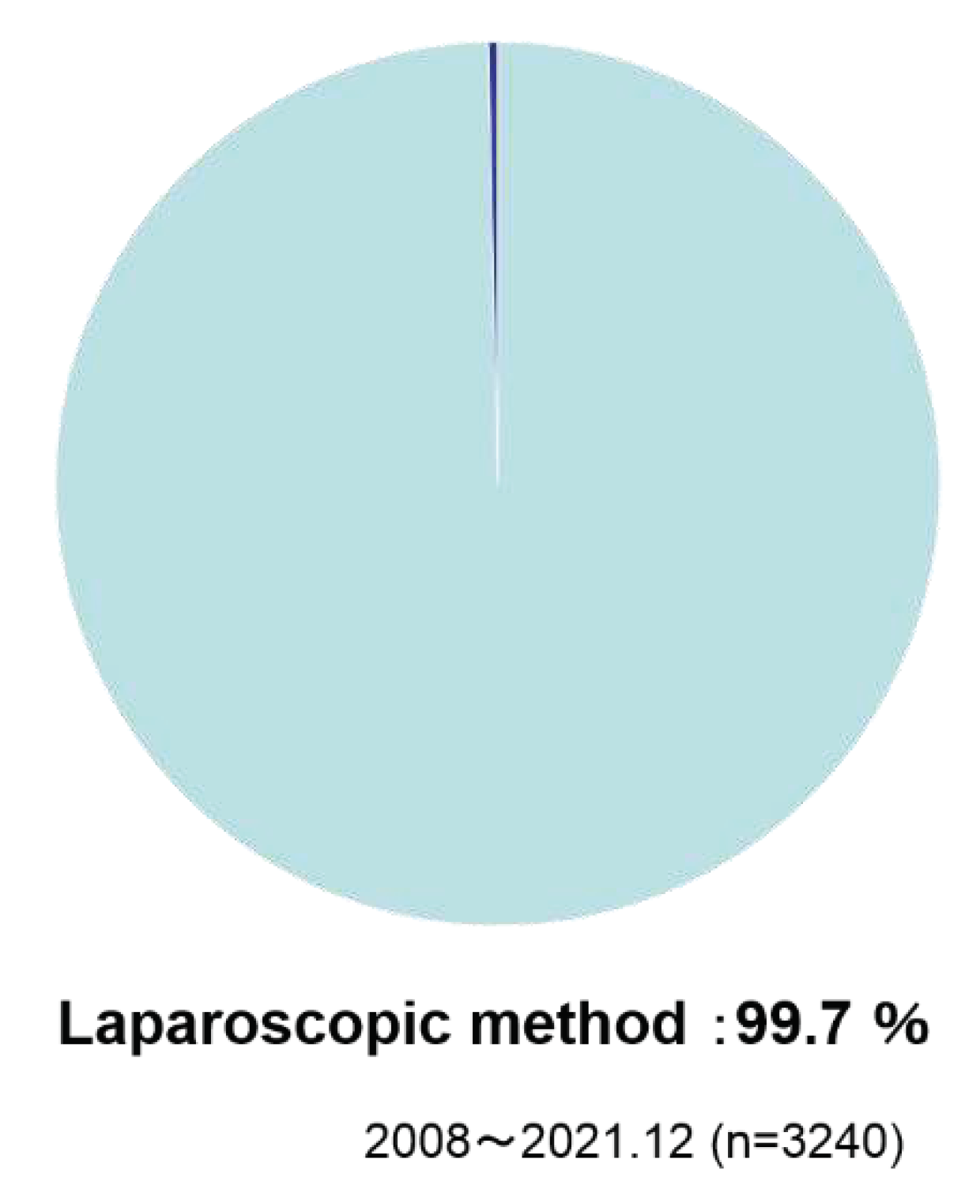
As operation method, laparoscopic methods were taken in 99.7% of the cases (
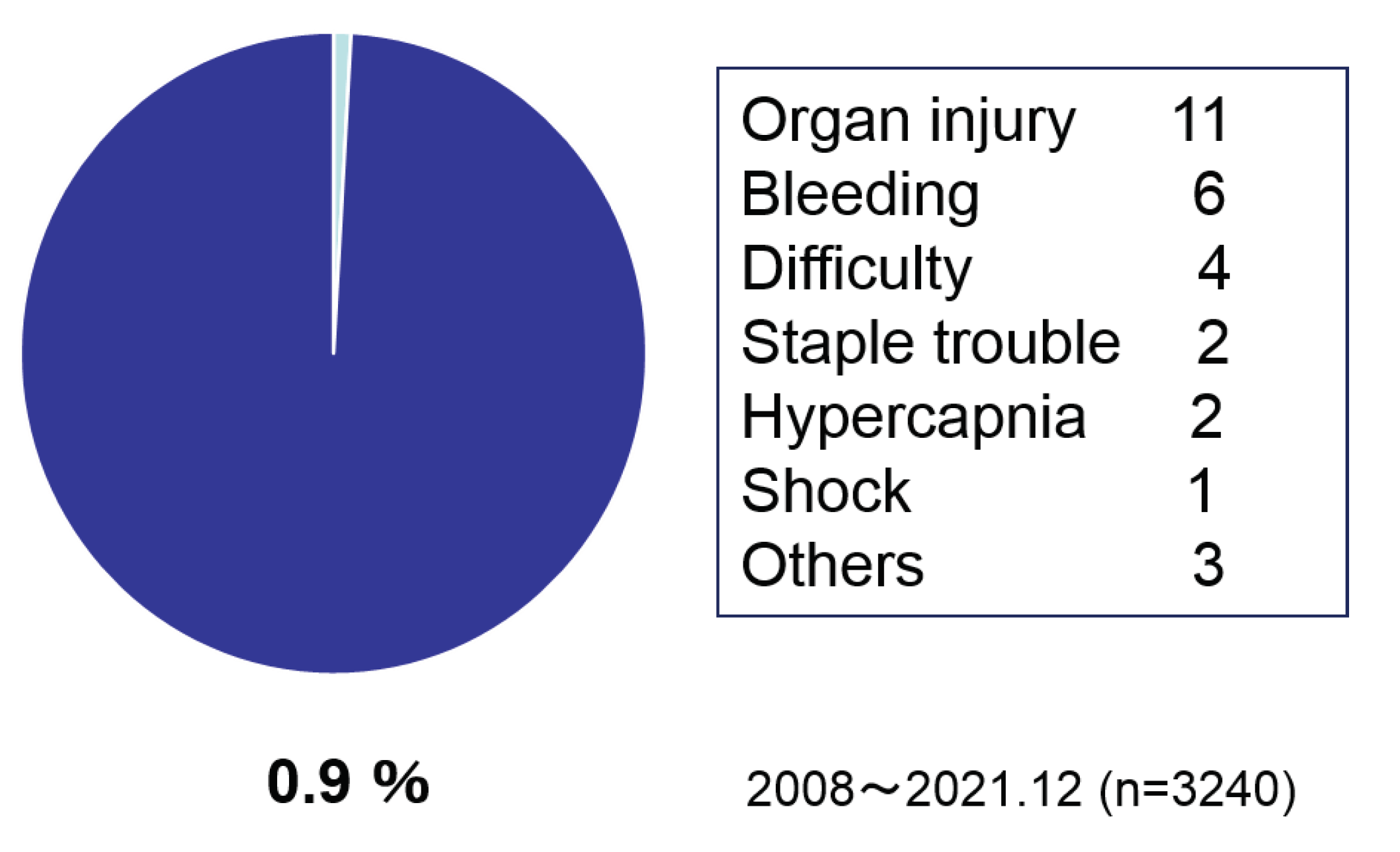
Figure 5). The intra operative incidents rate was 1.3 % which included injury, bleeding, staple trouble, method change for difficulties and drug induced shock (
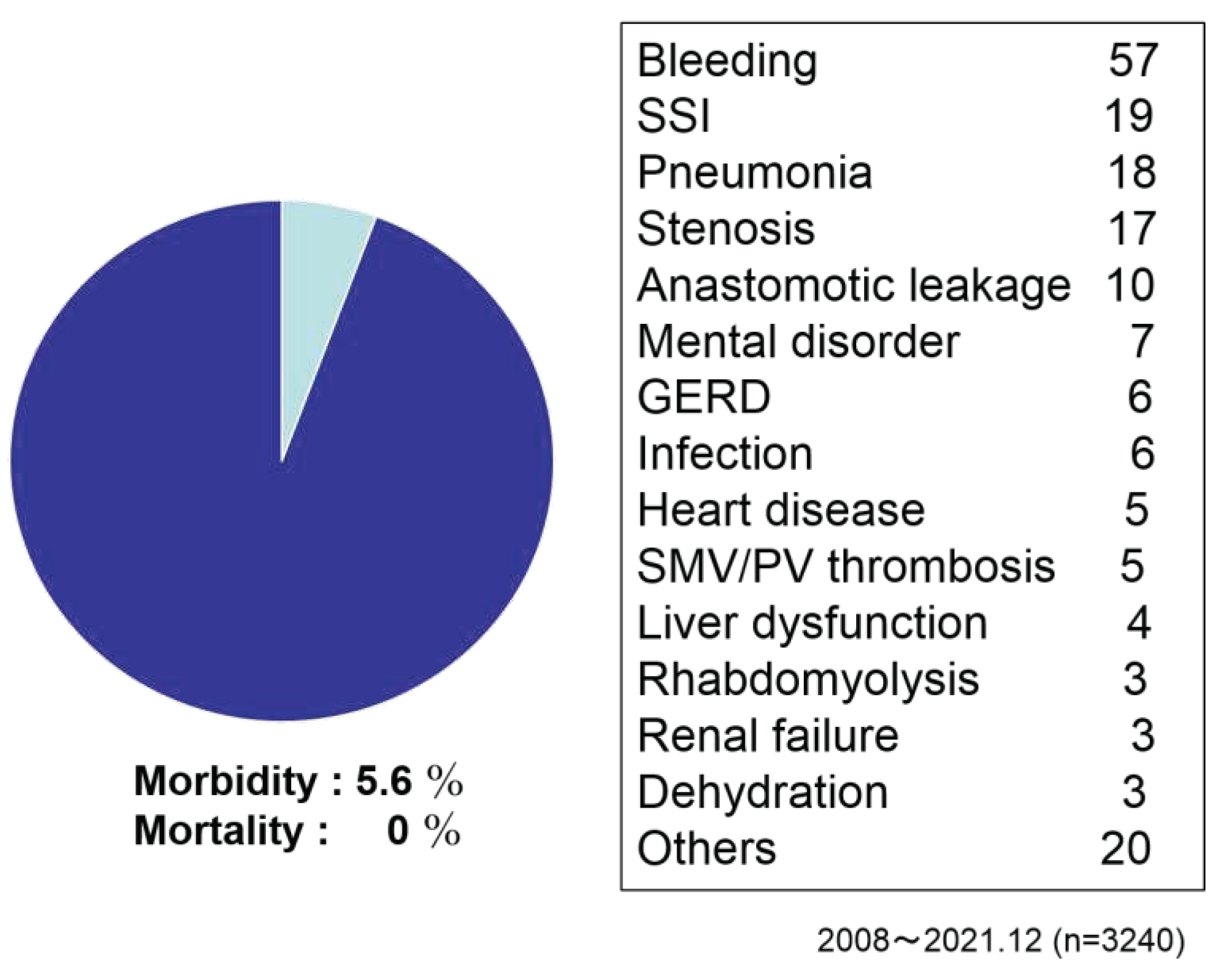
Figure 6). Conversion rate to open from laparoscopic methods was 1.1%. As postoperative complications, bleeding, SSI, pneumonia, stenosis, anastomotic leakage, mental disorder, GERD, thrombosis and heart disease were reported. Morbidity ratio was 5.6% and mortality was 0 % (
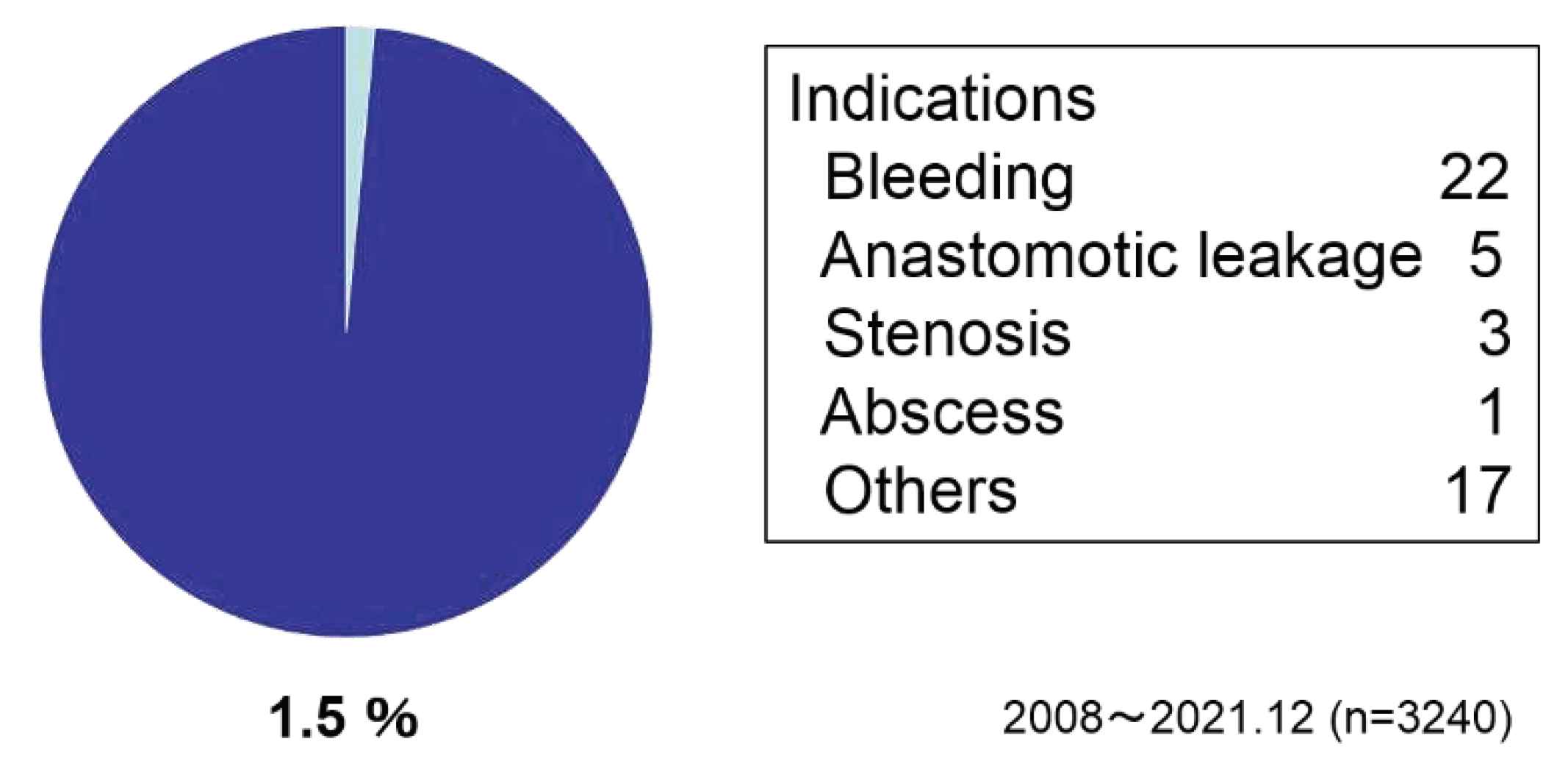
Figure 7). Reoperations were performed in 1.5 % of the cases due to bleeding, anastomotic leakage, stenosis, and others (
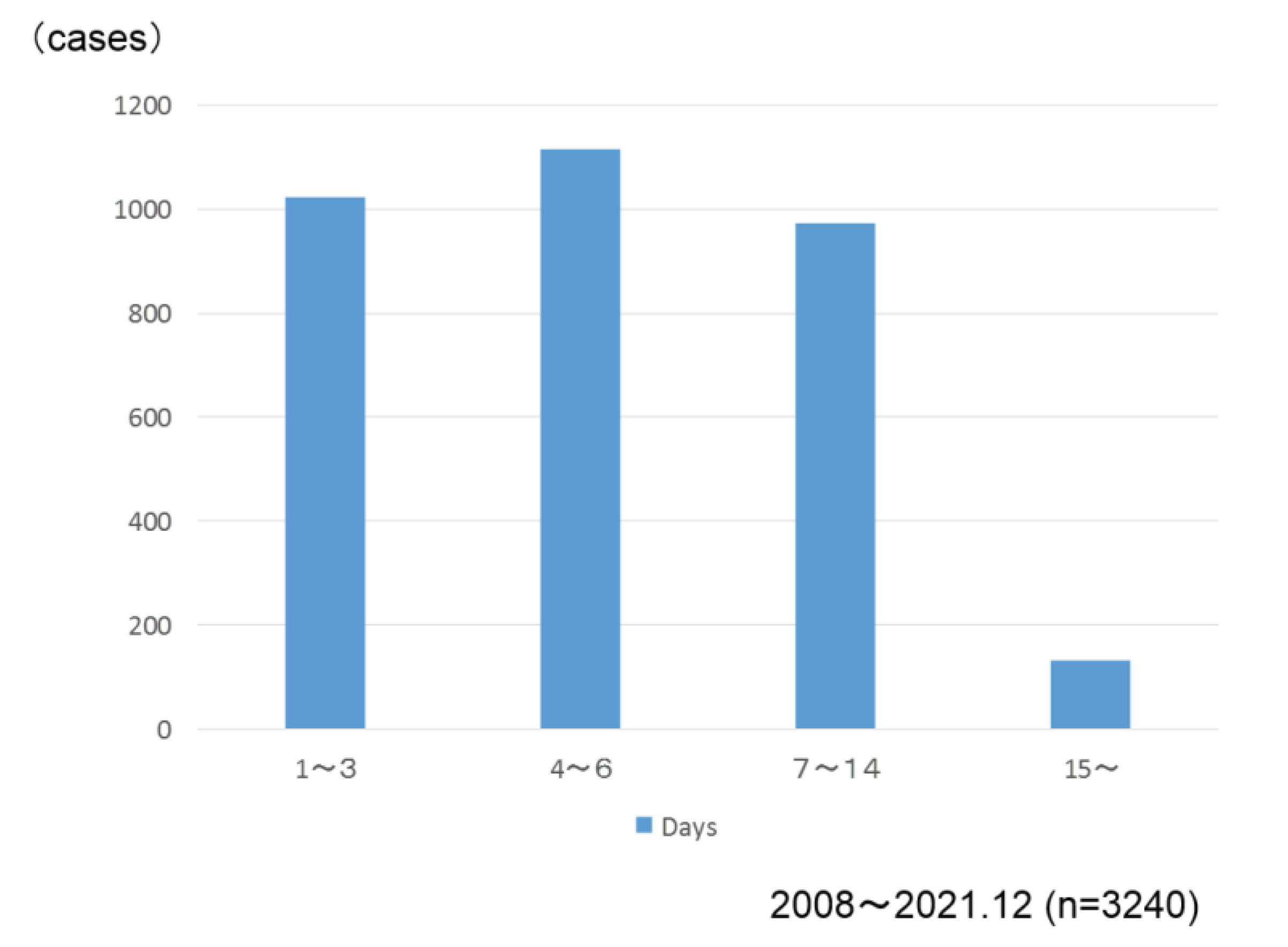
Figure 8). Postoperative hospital stay was 5 days in the median value (
Figure 9).
Laparoscopic methods were taken in 99.7% of the cases.
Occurrence rate was 0.9%.
The total morbidity ratio was 5.6% and mortality was 0 %.
Reoperations were performed in 1.5 % of the cases due to bleeding or others.
The median was 5 days.
3.3. Evaluation of the outcome of metabolic effects
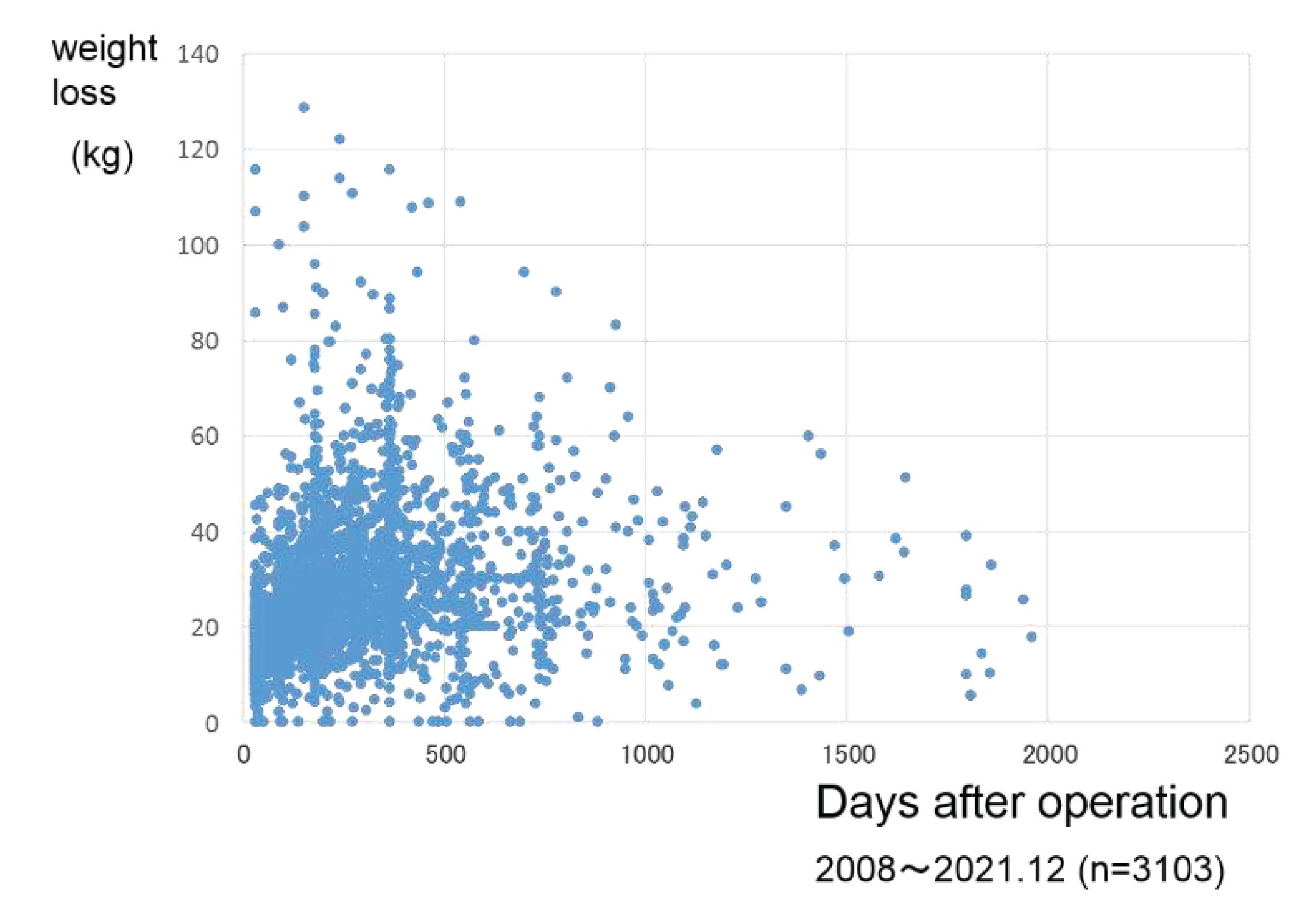
Body weight loss was 27.6 kg in the follow up days of 279±245 after surgery (
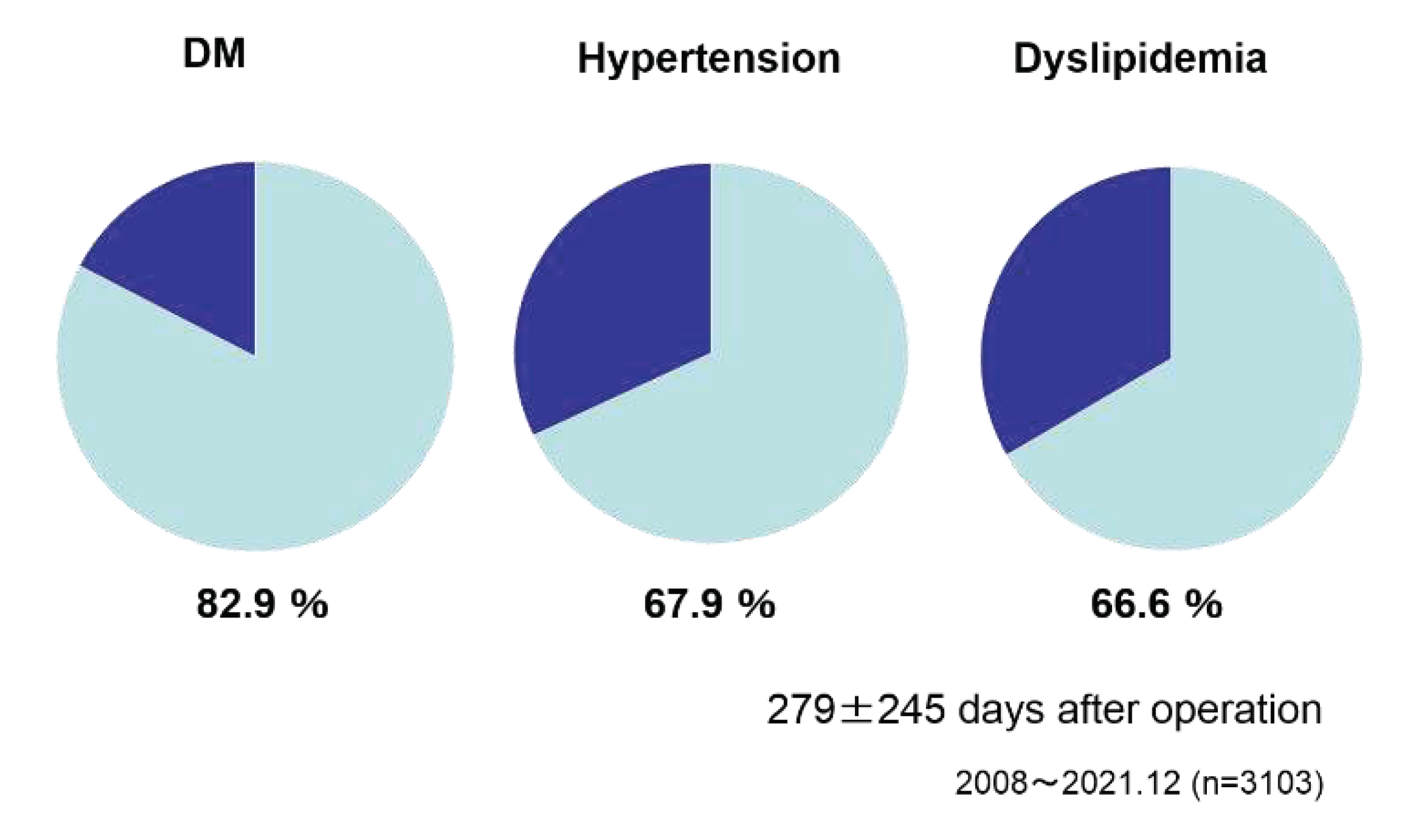
Figure 10). As the effect on the preoperative metabolic comorbidities, DM improved in 82.9 % of the cases, hypertension 67.9 % and dyslipidemia 66.6% after 279±245 days after surgery (
Figure 11).
Weight loss was 27.6 ±15.5 (kg) in the cases of 279±245 days after operation.
4. Discussion
The obese population (BMI>30) in Japan is about 3%, which is one of the lowest in the world [
10]. On the other hand, the diabetic population is on the rise in Japan [
11], and the relationship between obesity and diabetes has also been reported [
12]. In particular, Asians are said to be underweight and prone to diabetes compared to Westerners [
13]. In the 2000s, the metabolic improvement effect of bariatric surgery was recognized, and it came to be called ‘metabolic surgery’ [
14]. In particular, it is highly effective in improving diabetes, and its indications have been determined by academic societies worldwide [
15,
16,
17]. A lower BMI indication has been shown for Asians [
18].
In Japan, JSTO was established in 2008 with the aim of safe increasing of surgery while feeling the need to expand bariatric surgical treatment. Under the leadership of the society, we have promoted the guideline making, the surgical case registration, the multi-professional education program, and the facility certification business. As of 2008, there were less than 200 surgical cases, which rapidly increased thereafter, reaching over 4,000 cases in 2021 after being covered by health insurance in 2014. At JSTO, we have reported on safety and efficacy at each annual meeting, and both have shown good results and are improving year by year. In the present analysis, the intraoperative incident rate was 1.3% (
Figure 6) and the postoperative complication rate was 7.0%. The reoperation rate was 1.5% and there were no deaths (
Figure 7,8). The median postoperative hospital stay was 5 days (
Figure 9), and it was considered that the surgical treatment was performed safely. Looking at the improvement up to 1 year after surgery, the weight loss effect was an average of 27.2% reduction[fig.10], and the improvement of comorbidities was observed in the majority of patients, with diabetes mellitus 86.9%, hypertension 68.7%, and dyslipidemia 67.9%. prominent in diabetes (
Figure 11). Efficacy in long-term outcomes has also been reported by multiple institutions[
19,
20,
21]
As described above, the usefulness of ‘sleeve gastrectomy’ has been shown and verified that it is safely administered in Japan, which is located in East Asia and is prone to diabetes. The laparoscopy rate was 99.5%, and the conversion to open rate was 0.98% (
Figure 5,6). Minimally invasive procedures are thought to contribute to safety. On the other hand, the characteristic postoperative complications of sleeve gastrectomy, such as a stump of suture failure, twisting, stenosis, and reflux esophagitis, have also been registered. It is considered necessary to devise ways to avoid these problems and to further consider treatments to be taken at the time of onset. In addition, multi-disciplinary education [
22], facility certification, surgical case registration, and verification of safety effectiveness are considered important, and we would like to continue them in the future.
5. Conclusions
The safety and efficacy of sleeve gastrectomy in Japan were verified using national registry data base compiled by JSTO.
Funding
This research received no external funding.
Review Board Statement by Japanese Society of Treatment of Obesity (JSTO)
Statements regarding ethics and consent were obtained from the Ethics Committee of JSTO (IRB #2022-03). All procedures involving human participants were performed in accordance with the ethical standards of the ethical committee of JSTO and the 1964 Helsinki Declaration and its later amendments of comparable ethical standards.
Informed Consent Statement
Informed consent was obtained from all participants included in the study.
Data Availability Statement
Our data used in this study is available on request form the corresponding author.
Acknowledgments
We would like to thank the office of JSTO for several managements.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes Surg. 2015, 25, 1822–32. [Google Scholar] [CrossRef]
- Rubino, F.; Shukla, A.; Pomp, A.; Moreira, M.; Ahn, S.M.; Dakin, G. Bariatric, metabolic, and diabetes surgery: what's in a name? Ann Surg. 2014, 59, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Brown WA, Kow L, Shikora S, Liem R, Welbourn R, Dixon J. 6th IFSO global registry report 2021.
- Chambers, A.P.; Kirchner, H.; Wilson-Perez, H.E.; Willency, J.A.; Hale, J.E.; Gaylinn, B.D. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 2013, 144, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Myronovych, A.; Kirby, M.; Ryan, K.K.; Zhang, W.; Jha, P.; Setchell, K.D.; Dexheimer, P.J.; Aronow, B.; Seeley, R.J.; Kohli, R. Vertical sleeve gastrectomy reduces hepatic steatosis while increasing serum bile acids in a weight-loss-independent manner. Obesity 2014, 22, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Tovar, J.; Zubiaga, L.; Llavero, C.; Diez, M.; Arroyo, A.; Calpena, R. Serum cholesterol by morbidly obese patients after laparoscopic sleeve gastrectomy in additional physical activity. Obes Surg 2014, 24, 385–389. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Root, J.; Zainabadi, K.; Sabio, A.; Chalifoux, S.; Stevens, C.M.; Mavandadi, S.; Longoria, M.; Wilson, S.E. Accelerated growth of bariatric surgery with the introduction of minimally invasive surgery. Arch Surg. 2005, 140, 1198–202. [Google Scholar] [CrossRef]
- Demaria, E.J.; Winegar, D.A.; Pate, V.W.; Hutcher, N.E.; Ponce, J.; Pories, W.J. Early postoperative outcomes of metabolic surgery to treat diabetes from sites participating in the ASMBS bariatric surgery center of excellence program as reported in the Bariatric Outcomes Longitudinal Database. Surg. 2010, 252, 59–66. [Google Scholar] [CrossRef]
- Kasama K, Tagaya N, Kanehira E, Oshiro T, Seki Y, Kinouchi M, Umezawa A, Negishi Y, Kurokawa Y. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg. 2009, 9, 1341–1345. [Google Scholar]
- Yoshiike, N.; Miyoshi, M. Epidemiological aspects of overweight and obesity in Japan-international comparisons. Nihon Rinsho. 2013, 71, 207–16. [Google Scholar]
- Goto, A.; Goto, M.; Noda, M.; Tsugane, S. Incidence of type 2 diabetes in Japan: a systematic review and meta-analysis. PLoS One. 2013, 6, e74699. [Google Scholar]
- Malone, J.I.; Hansen, B.C. Does obesity cause type 2 diabetes mellitus (T2DM)? Or is it the opposite? .Pediatr Diabetes. 2019, 20, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.B.; Oza-Frank, R.; Staimez, L.R.; Ali, M.K.; Narayan, K.M. Type 2 diabetes in Asians: prevalence, risk factors, and effectiveness of behavioral intervention at individual and population levels. Annu Rev Nutr. 2012, 32, 417–439. [Google Scholar] [CrossRef]
- Rubino, F.; R'bibo, S.L.; del Genio, F.; Mazumdar, M.; McGraw, T.E. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrionol. 2010, 6, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Kaplan, L.M.; Schauer, P.R.; Cummings, D.E. The diabetes Surgery Summit consensus conference recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010, 251, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.B.; Zimmet, P.; Alberti, K.G.; Rubino, F. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011, 28, 628–642. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes 2015. Diabetes Care. 2015, 38, 46–48. [Google Scholar]
- Kasama, K.; Mui, W.; Lee, W.J.; Lakdawala, M.; Naitoh, T.; Seki, Y.; Sasaki, A.; Wakabayashi, G.; Sasaki, I.; Kawamura, I.; Kow, L.; Frydenberg, H.; Chen, A.; Narwaria, M.; Chowbey, P. IFSO-APC Consensus Statement 2011. Obes Surg. 2012, 22, 677–684. [Google Scholar] [CrossRef]
- Seki, Y.; Kasama, K.; Hashimoto, K. Long-term outcomes of laparoscopic sleeve gastrectomy in morbidly obese Japanese patients. Obes Surg 2016, 26, 138–145. [Google Scholar] [CrossRef]
- Haruta, H.; Kasama, K.; Ohta, M.; Sasaki, A.; Yamamoto, H.; Miyazaki, Y.; Oshiro, T.; Naitoh, T.; Hosoya, Y.; Togawa, T.; Seki, Y.; Lefor, A.K.; Tani, T. Long-term outcomes of bariatric and metabolic surgery in Japan: Results of multi-institutional survey. Obes Surg 2017, 27, 754–762. [Google Scholar] [CrossRef]
- Saiki, A.; Yamaguchi, T.; Tanaka, S.; Sasaki, A.; Naitoh, T.; Seto, Y.; Matsubara, H.; Yokote, K.; Okazumi, S. Background characteristics and postoperative outcomes of insufficient weight loss after laparoscopic sleeve gastrectomy in Japanese patients. Ann Gastroenterol Surg 2019, 26, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-term weight loss maintenance for Obesity: a multidisciplinary approach. Diabetes Metab Syndr Obes. 2016, 26, 37–46. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).