Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
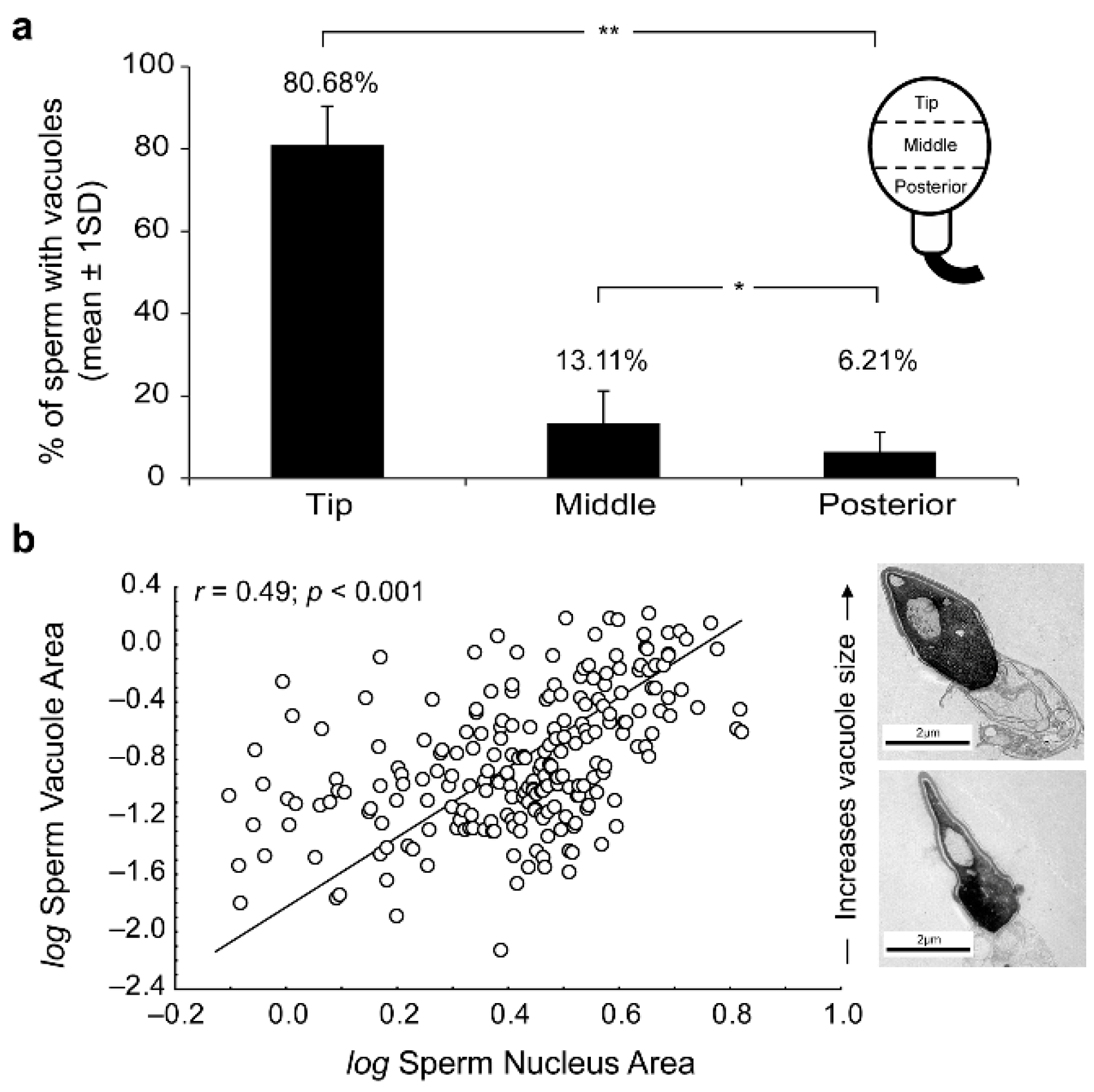
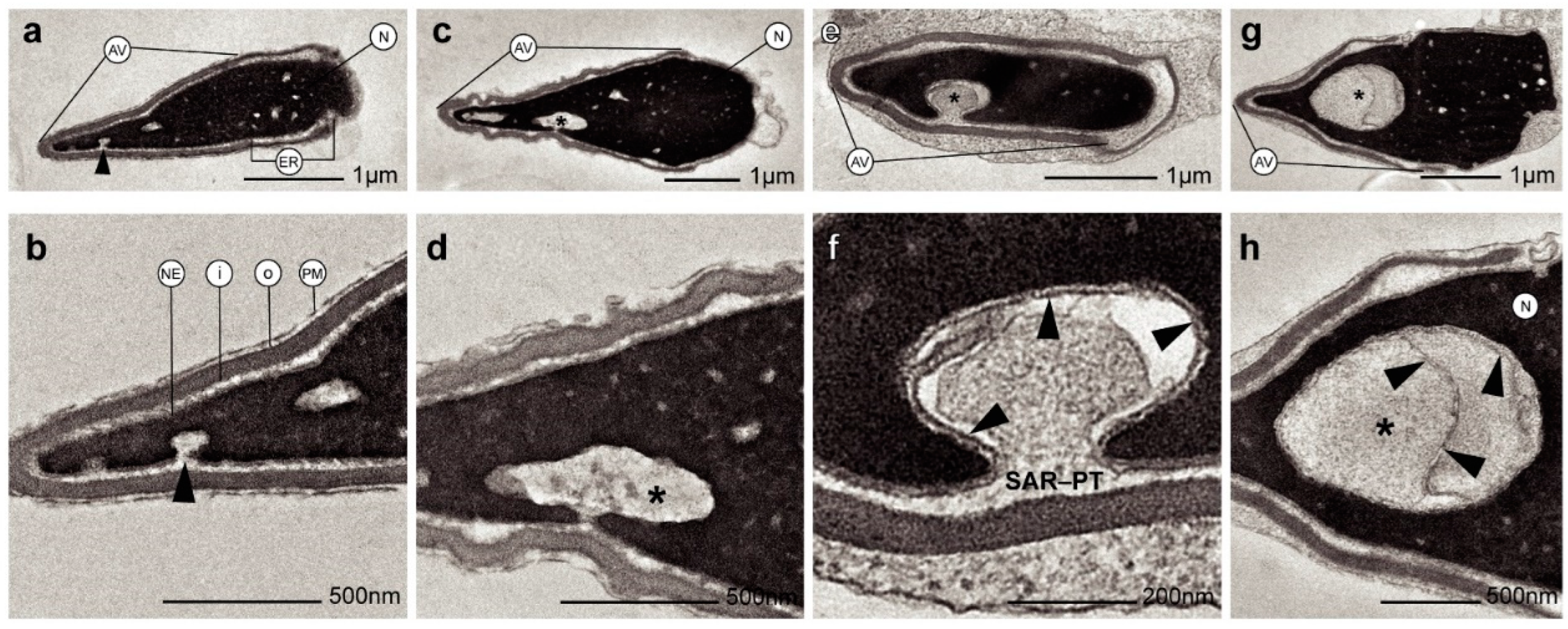
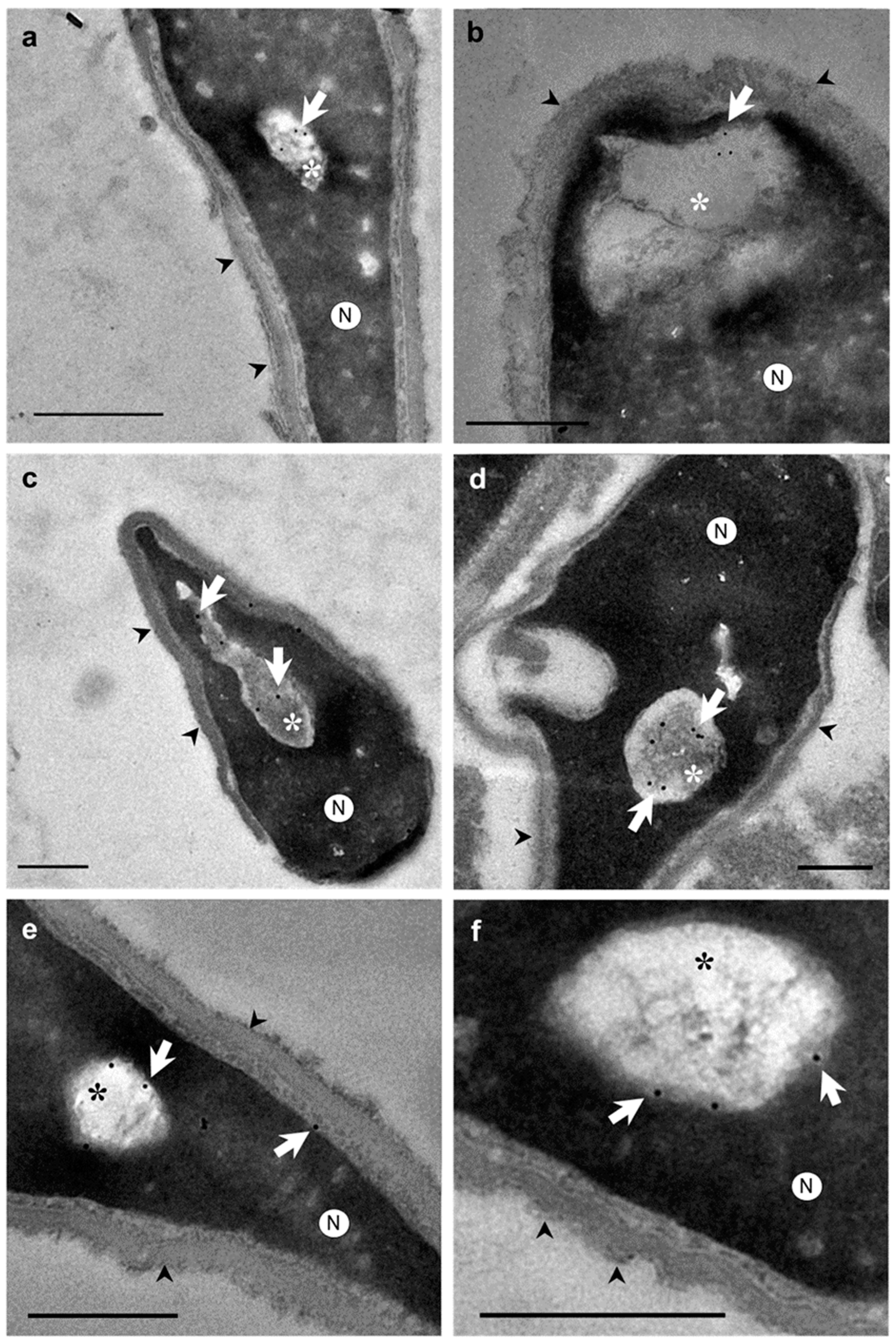
2. Results
| Sample | Sperm cells (n) |
Vacuolated sperm (%) | Vacuoles/cell (n) |
Relative vacuole area (RVA)a |
|---|---|---|---|---|
| 1 | 101 | 42.574 | 1.615 | 9.659 |
| 2 | 96 | 64.583 | 1.455 | 9.204 |
| 3 | 124 | 43.548 | 1.238 | 10.081 |
| 4 | 98 | 51.020 | 1.391 | 7.365 |
| 5 | 170 | 24.118 | 1.167 | 1.144 |
| 6 | 106 | 58.491 | 1.455 | 9.310 |
| 7 | 83 | 48.193 | 1.391 | 7.826 |
| 8 | 75 | 30.667 | 1.294 | 10.185 |
| 9 | 167 | 16.766 | 1.409 | 11.379 |
| 10 | 134 | 38.060 | 1.150 | 8.893 |
| 11 | 100 | 65.000 | 1.235 | 9.160 |
| 12 | 106 | 48.113 | 1.500 | 6.540 |
| 13 | 109 | 34.862 | 1.100 | 11.039 |
| 14 | 106 | 45.283 | 1.118 | 10.119 |
| 15 | 106 | 70.755 | 1.200 | 11.370 |
| 16 | 104 | 62.500 | 1.667 | 11.550 |
| 17 | 123 | 66.667 | 1.391 | 6.359 |
| Mean | 47.718 | 1.340 | 9.658 | |
| SD | 15.678 | 0.170 | 1.978 |
3. Discussion
4. Materials and Methods
4.1. Semen Samples Preparation
4.2. Electron Microscopy
4.3. Immunocytochemical Study
4.4. Sperm Head Vacuole Count and Morphological Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol 2008, 636, 1–15. [Google Scholar]
- Oko, R.; Morales, C.R. Molecular and cellular biology of novel cytoskeletal proteins in spermatozoa. In Cellular and Molecular Regulation of Testicular Cells; Desjardins, C., Ed.; Springer: New York, NY, USA, 1996; pp. 135–165. [Google Scholar]
- Oko, R. Occurrence and formation of cytoskeletal proteins in mammalian spermatozoa. Androl 1998, 30, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Toshimori, K.; Eddy, E.M. The spermatozoon. In Knobil and Neill's Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–148. [Google Scholar]
- Ito, C.; Akutsu, H.; Yao, R.; Kyono, K.; Suzuki-Toyota, F.; Toyama, Y.; Maekawa, M.; Noda, T.; Toshimori, K. Oocyte activation ability correlates with head flatness and presence of perinuclear theca substance in human and mouse sperm. Hum Reprod 2009, 24, 2588–2595. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Kyono, K.; Yao, R.; Noda, T.; Toshimori, K. Appearance of an oocyte activation-related substance during spermatogenesis in mice and humans. Hum Reprod 2013, 25, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Oko, R.; Sutovsky, P. Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J Reprod Immunol 2009, 83, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Protopapas, N.; Hamilton, L.E.; Warkentin, R.; Xu, W.; Sutovsky, P.; Oko, R. The perforatorium and postacrosomal sheath of rat spermatozoa share common developmental origins and protein constituents. Biol Reprod 2019, 100, 1461–1472. [Google Scholar] [CrossRef]
- Breitbart, H.; Cohen, G.; Rubinstein, S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reprod 2005, 129, 263–268. [Google Scholar] [CrossRef]
- Dvoráková, K.; Moore, H.D.; Sebková, N.; Palecek, J. Cytoskeleton localization in the sperm head prior to fertilization. Reprod 2005, 130, 61–69. [Google Scholar] [CrossRef]
- Lécuyer, C.; Dacheux, J.L.; Hermand, E.; Mazeman, E.; Rousseaux, J.; Rousseaux-Prévost, R. Actin-binding properties and colocalization with actin during spermiogenesis of mammalian sperm calicin. Biol Reprod 2000, 63, 1801–1810. [Google Scholar] [CrossRef]
- Chyra-Jach, D.; Kaletka, Z.; Dobrakowski, M.; Machoń-Grecka, A.; Kasperczyk, S.; Birkner, E.; Kasperczyk, A. The Associations between Infertility and Antioxidants, Proinflammatory Cytokines, and Chemokines. Oxid Med Cell Longev 2018, 16, 8354747. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the Actin Cytoskeleton During Mammalian Sperm Capacitation and Acrosome Reaction. Biol of Reprod 2003, 68, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, T.; Noda, Y.; Narimoto, K.; Shiotani, M.; Mori, T.; Matsuda, T.; Yoshida, O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum Reprod 1992, 7, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, E.; Zhang, C.; Zhang, H. Study of the Changes of Acrosomal Enzyme, Nitric Oxide Synthase, and Superoxide Dismutase of Infertile Patients with Positive Antisperm Antibody in Seminal Plasma. Cell Biochem Biophys 2015, 73, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Boitrelle, F.; Ferfouri, F.; Petit, J.-M.; Segretain, D.; Tourain, C.; Bergere, M.; Bailly, M.; Vialard, F.; Albert, M.; Selva, J. Large human sperm vacuoles observed in motile spermatozoa under high magnification: nuclear thumbprints linked to failure of chromatin condensation. Hum Reprod 2011, 26, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Fortini, D.; Menegazzo, M.; De Toni, L.; Nicoletti, V.; Moretti, A.; Selice, R.; Engl, B.; Foresta, C. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online 2008, 17, 610–616. [Google Scholar] [CrossRef]
- Franco, J.G., Jr.; Baruffi, R.L.; Mauri, A.L.; Petersen, C.G.; Oliveira, J.B.; Vagnini, L. Significance of large nuclear vacuoles in human spermatozoa: implications for ICSI. Reprod Biomed Online 2008, 17, 42–45. [Google Scholar] [CrossRef]
- Perdrix, A.; Travers, A.; Chelli, M.H.; Escalier, D.; Do Rego, J.L.; Milazzo, J.P.; Mousset Siméon, N.; Macé, B.; Rives, N. Assesment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum Reprod 2011, 26, 47–58. [Google Scholar] [CrossRef]
- Perdrix, A.; Rives, N. Motile sperm organelle morphology examination (MSOME) and sperm head vacuoles: state of the art in 2013. Hum Reprod Update 2013, 19, 527–541. [Google Scholar] [CrossRef]
- Boitrelle, F.; Albert, M.; Petit, J.M.; Ferfouri, F.; Wainer, R.; Bergere, M.; Bailly, M.; Vialard, F.; Selva, J. Small human sperm vacuoles observed under magnification are pocket like-nuclear concavities linked to chromatin condensation failure. Reprod Biomed Online 2013, 27, 201–211. [Google Scholar] [CrossRef]
- Gatimel, N.; Léandri, R.D.; Marino, L.; Esquerre-Lamare, C.; Parinaud, J. Sperm vacuoles cannot help to differentiate fertile men from infertile men with normal sperm parameter values. Hum Reprod 2014, 1–9. [Google Scholar] [CrossRef]
- Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R.D. Sperm morphology: assessment, pathophysiology, clinical relevance, and state of the art in 2017. Androl 2017, 5, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, D.W. The structure of the mammalian spermatozoon. Intern Rev Cytology 1958, 7, 195–234. [Google Scholar]
- Schnall, M.D. Electron microscopic study of human spermatozoa. Fertil Steril 1952, 3, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Larsen, J. The morphology of the human sperm; electron microscopic investigations of the ultrastructure. Acta Path Microbiol Scand Suppl 1958, 44, 1–121. [Google Scholar]
- Perdrix, A.; Saïdi, R.; Ménard, J.F.; Gruel, E.; Milazzo, J.P.; Macé, B.; Rives, N. Relationship between conventional sperm parameters and motile sperm organelle morphology examination (MSOME). Int J Androl 2012, 35, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nagayoshi, M.; Tanaka, I.; Kusunoki, H. Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil Steril 2012, 98, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Tanaka, A.; Fujii, S.; Mizunuma, H.; Fukui, A.; Fukuhara, R.; Nakamura, R.; Yamada, K.; Tanaka, I.; Awata, S.; Nagayoshi, M. An investigation of the potential effect of vacuoles in human sperm on DNA damage using a chromosome assay and the TUNEL assay. Hum Reprod 2011, 26, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Silva, L.F.I.; Felipe, V.; Cavagna, M.; Pontes, A.; Baruffi, R.L.R.; Oliveira, J.B.A.; Vagnini, L.D. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int J Androl 2012, 35, 46–51. [Google Scholar] [CrossRef]
- Fekonja, N.; Strus, J.; Tusek Znidaric, T.; Knez, K.; Bokal, V.; Verdenid, I.; Virant-Klun, I. Clinical and structural features of sperm head vacuoles in men included in the in vitro fertilization programme. Biomed Res Int 2014, 927841. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tomita, K.; Hashimoto, S.; Matsumoto, H.; Satoh, M.; Kato, H.; Hosoi, Y.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J Reprod 2016, 62, 599–606. [Google Scholar] [CrossRef]
- Kacem, O.; Sifer, C.; Barraud-Lange, V.; Ducot, B.; De Ziegler, D.; Poirot, C.; Wolf, J. Sperm nuclear vacuoles, as assessed by motile sperm organellar morphological examination, are mostly of acrosomal origin. Reprod Biomed Online 2010, 20, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Montjean, D.; Belloc, S.; Benkhalifa, M.; Dalleac, A.; Menezo, Y. Sperm vacuoles are linked to capacitation and acrosomal status. Hum Reprod 2012, 27, 2927–2932. [Google Scholar] [CrossRef] [PubMed]
- Balaban, B.; Yakin, K.; Alatas, C.; Oktem, O.; Isiklar, A.; Urman, B. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: a prospective randomized study. Reprod Biomed Online 2011, 22, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Bartoov, B.; Berkovitz, A.; Eltes, F.; Kogosowski, A.; Menezo, Y.; Barak, Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J Androl 2002, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, A.; Eltes, F.; Yaari, S.; Katz, N.; Barr, I.; Fishman, A.; Bartoov, B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum Reprod 2005, 20, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Berkovitz, A.; Eltes, F.; Ellenbogen, A.; Peer, S.; Feldberg, D.; Bartoov, B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum Reprod 2006, 21, 1787–1790. [Google Scholar] [CrossRef]
- Vanderzwalmen, P.; Hiemer, A.; Rubner, P.; Bach, M.; Neyer, A.; Stecher, A.; Uher, P.; Zintz, M.; Lejeune, B.; Vanderzwalmen, S.; Cassuto, G.; Zech, N.H. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod Biomed Online 2008, 17, 617–627. [Google Scholar] [CrossRef]
- Utsuno, H.; Miyamoto, T.; Oka, K.; Shiozawa, T. Morphological alterations in protamine-deficient spermatozoa. Hum Reprod 2014, 29, 2374–2381. [Google Scholar] [CrossRef]
- Skowronek, F.; Casanova, G.; Alciaturi, J.; Capurro, A.; Cantu, L.; Montes, J.M.; Sapiro, R. DNA sperm damage correlates with nuclear ultrastructural sperm defects in teratozoospermic men. Androl 2012, 44, 56–65. [Google Scholar] [CrossRef]
- Fortunato, A.; Boni, R.; Leo, R.; Nacchia, G.; Liguori, F.; Casale, S.; Bonassisa, P.; Tosti, E. Vacuoles in sperm head are not associated with head morphology, DNA damage and reproductive success. Reprod Biomed Online 2015, 32, 154–161. [Google Scholar] [CrossRef]
- Monqaut, A.L.; Zavaleta, C.; López, G.; Lafuente, R.; Brassesco, M. Use of high-magnification microscopy for the assessment of sperm recovered after two different sperm processing methods. Fertil Steril 2011, 95, 277–280. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction; WHO: Concern, Singapore, 1980. [Google Scholar]
- World Health Organization. WHO laboratory manual for the examination of Human Semen and Semen-Cervical mucus interaction; WHO: Cambridge, UK, 1987. [Google Scholar]
- World Health Organization. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction; WHO: Cambridge, UK, 1992. [Google Scholar]
- World Health Organization. WHO laboratory manual for the examination of Human Semen and Semen-Cervical mucus interaction; WHO: Cambridge, UK, 1999. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneve, Switzerland, 2010. [Google Scholar]
- Bartoov, B.; Berkovitz, A.; Eltes, F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N Engl J Med 2001, 345, 1067–1068. [Google Scholar] [CrossRef] [PubMed]
- Danis, R.B.; Samplaski, M.K. Sperm Morphology: History, Challenges, and Impact on Natural and Assisted Fertility. Curr Urol Rep 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Kovac, J.R.; Smith, R.P.; Cajipe, M.; Lamb, D.J.; Lipshultz, L.I. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J Androl 2017, 19, 39–42. [Google Scholar] [PubMed]
- Garolla, A.; Fortini, D.; Menegazzo, M.; De Toni, L.; Nicoletti, V.; Moretti, A.; Selice, R.; Engl, B.; Foresta, C. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod Biomed Online 2008, 17, 610–616. [Google Scholar] [CrossRef]
- Komiya, A.; Watanabe, A.; Kawauchi, Y.; Fuse, H. Sperm with large nuclear vacuoles and semen quality in the evaluation of male infertility. Syst Biol Reprod Med 2013, 59, 13–20. [Google Scholar] [CrossRef]
- Joshi, N.V.; Medina, H.; Colasante, C.; Osuna, A. Ultrastructural investigation of human sperm using atomic force microscopy. Arch Androl 2000, 44, 51–57. [Google Scholar]
- Moretti, E.; Sutera, G.; Collodel, G. The importance of transmission electron microscopy analysis of spermatozoa: Diagnostic applications and basic research. Syst Biol Reprod Med 2016, 62, 171–183. [Google Scholar] [CrossRef]
- Visco, V.; Raffa, S.; Elia, J.; Delfino, M.; Imbrogno, N.; Torrisi, M.R.; Mazzilli, F. Morphological sperm defects analysed by light microscopy and transmission electron microscopy and their correlation with sperm motility. Int J Urol 2010, 17, 259–266. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Petersen, C.G.; Massaro, F.C.; Baruffi, R.L.; Mauri, A.L.; Silva, L.F.; Ricci, J.; Franco, J.G. Jr. Motile sperm organelle morphology examination (MSOME): intervariation study of normal sperm and sperm with large nuclear vacuoles. Reprod Biol Endocrinol 2010, 8, 56. [Google Scholar] [CrossRef]
- Bedford, J.M. Observations on the fine structure of spermatozoa of the bush baby (Galago senegalensis), the African green monkey (Cercopithecus aethiops) and man. Am J Anat 1967, 121, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, L.; Zemjanis, R.; Stefanini, M. The fine structure of monkey and human spermatozoa. Anat Rec 1971, 169, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Movilla, M.; Avilés, M.; Gómez-Torres, M.J.; Fernández-Colom, P.J.; Castells, M.T.; de Juan, J.; Romeu, A.; Ballesta, J. Carbohydrate analysis of the zona pellucida and cortical granules of human oocytes by means of ultrastructural cytochemistry. Hum Reprod 2004, 19, 1842–1855. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, S.P.; Winfrey, V.P.; Olson, G.E. Localization of actin in human, bull, rabbit, and hamster sperm by immunoelectron microscopy. Anat Rec 1988, 221, 599–610. [Google Scholar] [CrossRef]
- Senn, A.; Germond, M.; De Grandi, P. Immunofluorescence study of actin, acrosin, dynein, tubulin and hyaluronidase and their impact on in-vitro fertilization. Hum Reprod 1992, 7, 841–849. [Google Scholar] [CrossRef]
- Paranko, J.; Salonen, I. Calicin in human sperm fertilizing zona-free hamster eggs in vitro. Reprod Fertil Dev 1995, 7, 97–105. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





