3. The Many Places and the Many Faces
The term peritoneal carcinomatosis (PC) refers to a spread of malignant epithelial cells as tumour deposits to the peritoneum {9}.
PC represents a very complex condition at imaging, more than any other metastatic site.
The appearance of the peritoneal deposits is determined not only by the histological characteristics of the primary tumour, the time-point of the tumour-evolution and the treatment [
10] but also, and more interestingly, by the peritoneal space where they occur. Complications secondary to deposits will also vary according to the location, depending on the organs involved.
Some anatomic knowledge will be required in the quest for deposits. The aim of this review is not to cover a comprehensive description of peritoneal anatomy but sufficiently to be sure that all locations will be thoroughly investigated.
The peritoneal cavity is the virtual space that exists between the parietal and visceral peritoneum [
11] and in normal conditions, it contains a small amount of plasma-like fluid.
The parietal peritoneum delineates the periphery of the peritoneal cavity:
The peritoneum invaginates to fully cover most of the abdominal viscera, anterior and posteriorly, becoming the visceral peritoneum. It is organized into a folded disposition as ligaments, folds, and mesenteries to nurture, innervate and support the intraperitoneal organs, connecting them to the posterior parietal peritoneum.
As a common rule, abnormal thickening and pathological enhancement of surfaces covered by the peritoneum may be the only initial imaging finding in PC.
It may be difficult, in the absence of ascites, to differentiate parietal peritoneal deposits from their visceral counterparts on locations where the two leaves are adjacent (e.g.: a deposit within the parietal peritoneum covering the lateral abdominal wall or within the visceral peritoneum covering the liver). For the sake of simplicity, some examples of parietal peritoneal deposits will be shown now, the rest will be included within their peritoneal space (
Figure 4).
If the deposit lies within a fat containing peritoneal space, the spectrum of presentation ranges from nodular focal fat stranding to irregular haziness, evolving towards solid lesions. These solid lesions may be initially millimetric, appearing as either solitary to multiple soft tissue nodules that will eventually grow and merge to form plaques or sheets and then masses. Most commonly, PC appears as a combination of all these findings.
Peritoneal spaces are classified as supra or inframesocolic [
12,
13]. The anatomic landmark that enables this division is the
mesentery of the transverse colon (transverse colon mesocolon). Unfortunately, it is also an easy route for carcinomatosis spread as it communicates on both sides with other ligaments and centrally with the small bowel mesentery.
Deposits within the transverse mesocolon will appear either on its mesentery (
Figure 5) or /and on the serosa covering the transverse colon [
14], and differentiation between them may not always be feasible. Deposits may cause different degrees of luminal stenosis with or without signs of bowel obstruction.
Imaging features of serosal bowel deposits include nodular lesions, segmental parietal thickening, and diffuse infiltration. Their detection may be an arduous task, more so if bowel is not sufficiently distended. In addition, it is important to bear in mind that layered implants will blend in with the bowel contour, whereas nodular deposits will alter it and thus are easier to detect (
Figure 6).
This description of serosal bowel deposits has the same validity as for the rest of the gastrointestinal tract.
A cranial-to-caudal and lateral-to-medial approach will be used to review the peritoneal spaces.
3.1. Supramesocolic Spaces
Supramesocolic cavity comprehends the subphrenic, perihepatic and perisplenic spaces, periportal space, lesser omentum, lesser sac and right subhepatic space. These locations need to be carefully assessed using multiplanar reconstructions as deposits within them may require a complex surgery or in some cases may be considered unresectable.
3.1.1. Subphrenic Spaces
Imaging features include thickening and pathological enhancement of the diaphragm, nodules, and masses (
Figure 7 and
Figure 8).
3.1.2. Perihepatic and Perisplenic Spaces
Deposits can be identified as a continuum from abnormal peritoneal enhancement to subtle nodularity and well-defined nodules, often showing a biconvex morphology.
Perihepatic deposits may occur on the superficial visceral peritoneum that surrounds most of the liver surface (except for the liver bare area, the porta hepatis and the attachment site of the gallbladder to the liver) and/or underneath Glisson capsule, within the subcapsular space. Glisson´s capsule is a thick fibrous membrane that lies deep to the visceral peritoneum. It is assumed that deposits infiltrate the liver capsule upon deposition on the visceral peritoneum. Liver capsule covers the entire hepatic surface, including the periportal space, and is in communication with the lesser omentum, thus becoming another route for deposits to reach the subcapsular space. Both the periportal space and the lesser omentum will be reviewed next.
In the event of subcapsular deposits, secondary parenchymal invasion may occur, resulting in a characteristic scalloping of the underlying parenchyma (
Figure 9). Despite this sign, sometimes it may be difficult to distinguish between solely subcapsular deposits and those with parenchymal invasion. A sign that has been found to be highly sensitive to rule out secondary hepatic invasion is the presence of a well-defined interface between the lesion and the liver and/or a clear plane either fatty or from ascites [
15] (
Figure 10).
3.1.3. Periportal Space
Periportal deposits need to be well differentiated from parenchymal metastases. Deposits within the periportal space will appear as nodular or plaque-like lesions, predominantly at the porta hepatis and along the left branch of the portal vein; they are usually ill defined and not circumferentially surrounded by hepatic parenchyma, unlike their intraparenchymal counterparts (Figure11).
A proven useful tip is the distention of the periportal space over time due to the presence of deposits. As with any radiological examination, comparison with prior images in the setting of peritoneal carcinomatosis is mandatory (
Figure 12).
Deposits within this location may cause biliary obstruction, which usually presents at a late stage. Dilatation of the intrahepatic biliary ducts (usually segmentary) with no identifiable cause should raise the possibility of deposits within the periportal spaces. Another secondary finding may be a progressive compressive effect on the portal vein over time (Figure13).
3.1.4. Lesser Omentum
This portion of the peritoneum suspends the liver and the lesser curvature of the stomach and separates the first two centimetres of the duodenum from the liver. It is formed by the gastrohepatic and hepatoduodenal ligaments.
Deposits within this space show features common to any fat-containing space. Furthermore, since the porta hepatis runs in the hepatoduodenal ligament, biliary obstruction or portal vein compression may be found as indirect signs of PC (
Figure 14).
As previously seen, once the disease is in the lesser omentum it can easily spread to the periportal space, thanks to the surrounding connective tissue of the Glisson sheath, which makes them continuous. This is a particularly important spread route for pancreatic and gastrointestinal tumours.
3.1.5. Lesser Sac
Potential space between the pancreas and the stomach. Its distention, either by a solid lesion or by ascites, as will be reviewed later, is a sign of its involvement by PC (
Figure 15). It communicates with the rest of the peritoneal cavity (greater sac) through an opening, immediately posterior to the lesser omentum, the foramen of Winslow.
3.1.6. Right Subhepatic Space
Pouch inferior to segment VI of the liver. It communicates with the right subphrenic space, the right paracolic gutter and the lesser sac. Deposits within this space tend to be more subtle, partly due to small size of this space, and range from an ill-defined outer hepatic contour to focal fat stranding and nodules (
Figure 16).
3.2. Inframesocolic Spaces
Inframesocolic spaces will also be described through the same cranial-to-caudal and lateral-to-medial approach: greater omentum, paracolic gutters, small bowel mesentery, sigmoid mesocolon and pelvis recesses.
3.2.1. Greater Omentum
The greater omentum is the main peritoneal fold. It connects the stomach to the anterior surface of the transverse colon and then extends caudally into the pelvis covering the small bowel loops. It lies mainly inferior to the transverse colon mesocolon though its smaller cranial portion (the gastrocolic ligament) is within the supramesocolic space.
The uniqueness of the imaging features of deposits in this location is the omental cake, which occurs when nodular deposits collide (
Figure 17) and blend through one another boosting a fibrotic response and replacing the omental fat (
Figure 18).
A helpful diagnostic sign, as the infiltration progresses, is the subsequent displacement of the bowel loops. Enlargement of the fatty content and mass effect due to omental seeding may be more apparent than the actual deposits (
Figure 19).
Omental deposits are more easily detected on MR. However, in the early stages and especially in thin patients, CT appears to perform better, even though the scarce fatty content in thin patients may negatively contribute to identifying peritoneal deposits (
Figure 20).
3.2.2. Small Bowel Mesentery
The small bowel mesentery is a fan-shaped peritoneal double layer that surrounds the small bowel and then extends diagonally from the ligament of Treitz in the left upper quadrant to the ileocecal junction, anchoring at these two locations and at the posterior parietal peritoneum.
Mesenteric deposits show a rather unique and more complex appearance when compared to deposits elsewhere in the abdominal cavity. Deposits may be found:
within the mesenteric fat: As in the rest of the fat containing peritoneal spaces, they may range from focal nodular fat stranding to irregular haziness to nodules and masses (
Figure 21).
within the SB and caecal serosa: Deposits may also lie within the serosa covering the small bowel and the caecum (
Figure 22).
Figure 22.
Axial T2WI (A), axial DWI (B), CE portal phase FST1WI (C). PC from duodenal adenocarcinoma: Deposit within the distal ileum serosa. Axial CE-CT (D). PC from breast carcinoma: Deposits within the caecal serosa.
Figure 22.
Axial T2WI (A), axial DWI (B), CE portal phase FST1WI (C). PC from duodenal adenocarcinoma: Deposit within the distal ileum serosa. Axial CE-CT (D). PC from breast carcinoma: Deposits within the caecal serosa.
involving both the mesentery and the serosa: As in the transverse mesocolon, deposits may appear both within the mesentery and the serosa covering the small bowel loops and the caecum (
Figure 23).
Figure 23.
Axial CE-CT. PC from ovarian carcinoma: mesenteric seeding. Mesenteric involvement may happen as a combination of deposits involving both the mesentery and the bowel serosa, as in this case. Observe the clustered SB loops appearance. The calcified content of some of the deposits enhances their presence (arrow). Omental deposits (*).
Figure 23.
Axial CE-CT. PC from ovarian carcinoma: mesenteric seeding. Mesenteric involvement may happen as a combination of deposits involving both the mesentery and the bowel serosa, as in this case. Observe the clustered SB loops appearance. The calcified content of some of the deposits enhances their presence (arrow). Omental deposits (*).
within the mesenteric leaves: Deposits within the mesenteric leaves may go unperceived. The nodular thickening and enhancement of the mesenteric leaves is usually more conspicuous on MR but can be also spotted on CT and it becomes more noticeable when accompanied by ascites (
Figure 24 and
Figure 25).
Figure 24.
Axial CE-CT (A), axial T2WI (B), axial CE portal phase FS T1WI (C). PC from endometrial carcinoma: Deposit seeding within the mesenteric leaves.
Figure 24.
Axial CE-CT (A), axial T2WI (B), axial CE portal phase FS T1WI (C). PC from endometrial carcinoma: Deposit seeding within the mesenteric leaves.
Figure 25.
Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.
Figure 25.
Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.
Stellate mesentery: Diffuse mesenteric infiltration leads to a stellate appearance, which is commonly associated with breast (especially lobular carcinoma) [
16], gastric, pancreatic, and ovarian tumours [
17]. This deposition pattern follows the distribution of the mesenteric vessels, causing thickening and rigidity of perivascular bundles (
Figure 26).
Figure 26.
Axial CE-CT (A). PC from stomach adenocarcinoma: Stellate mesentery. Axial CE portal phase FST1WI (B). PC from lobular breast adenocarcinoma: Stellate mesentery, notice the perivascular distribution. Axial CE-CT (C), axial T2WI (D). PC from stomach adenocarcinoma: Isolated perivascular deposit within the mesentery, as a soft tissue mass surrounding a branch of the SMV.
Figure 26.
Axial CE-CT (A). PC from stomach adenocarcinoma: Stellate mesentery. Axial CE portal phase FST1WI (B). PC from lobular breast adenocarcinoma: Stellate mesentery, notice the perivascular distribution. Axial CE-CT (C), axial T2WI (D). PC from stomach adenocarcinoma: Isolated perivascular deposit within the mesentery, as a soft tissue mass surrounding a branch of the SMV.
As a result of tumour infiltration, the mesentery becomes rigid and loses its usual free wavering. SB loops will appear thickened, with restricted distensibility, looking initially separated and angulated and finally, clustered (
Figure 27). With time, the retraction caused by the deposits will cause a decrease in size of the mesenteric fat (
Figure 28). These effects on the SB loops and the mesenteric fat may happen to be more conspicuous than actual deposits.
The main complication of deposits lying within this space is bowel obstruction, which usually occurs at a late stage and its diagnosis is not challenging. However, it may also be the presenting sign. It typically involves more than one small bowel loop, and the degree of occlusion may vary. Another not so frequent complication is bowel ischaemia due to perivascular infiltration (
Figure 29).
3.2.3. Paracolic Gutters
Peritoneal recesses between the colon, partially covered by the posterior parietal peritoneum (on its anterior, medial, and lateral walls) and the lateral parietal peritoneum on each side. They constitute the attachments of the ascending and descending colon to the posterior parietal peritoneum.
Both are in continuity with the peritoneal spaces in the pelvis. Superiorly, the right paracolic gutter communicates with the right subphrenic and right subhepatic spaces, whereas the left paracolic gutter, much smaller, is partially separated from the left subphrenic space, by the phrenicocolic ligament (
Figure 1b).
The infiltrated peritoneum will typically appear nodulary thickened showing pathological enhancement (
Figure 30).
The oblique orientation of the small bowel mesentery described earlier divides the inframesocolic compartment into two compartments: right and left, the latter being larger. The only structure standing on the way of the free communication between the left inframesocolic space and the pelvis is the sigmoid mesocolon, thus the reason why it constitutes a common site of deposits, as it is an area of arrested flow. Deposits within the sigmoid mesocolon will lie on its fat and/or on the serosa and differentiation between them may not always be feasible. Sigmoid luminal stenosis with/without signs of obstruction are a frequent consequence of the seeding (
Figure 31).
The mesentery of the appendix is anchored to the lower end of the small bowel mesentery, close to the ileocecal junction and to the tip of the appendix. Despite its small size, it may be an important site in appendiceal neoplasms: in case of rupture, deposits will likely appear there first.
3.2.4. Peritoneal Recesses of the Pelvis-Ovarian Metastases
Deposits within the pelvis may be quite tricky to find as the anatomy is rather complex and there are several structures that fit in a small cavity, therefore a careful exploration using multiplanar reconstructions is recommended.
The parietal peritoneum that covers the abdominal wall goes down to the pelvis, where it does not reach the pelvis floor, as it reflects on the pelvis organs (peritoneal reflexion). The peritoneal reflexion covers the dome of the urinary bladder, then descends along its posterior wall and laterally forms the paravesical spaces: a fold over the ureters, and in men, it also covers the deferent ducts and the seminal vesicles. It continues towards the rectum and then ascends partially cloaking the upper and middle rectum (thus subperitoneal, the rest is extraperitoneal) and the lateral pelvic walls (
Figure 32). In women, it also coats the uterine fundus and body and the posterior part of the vagina and extends laterally (broad ligament) wrapping up the tubes and suspending the ovaries.
Two blind-ends pouches are therefore found in women: the uterovesical, anteriorly and the rectouterine, posteriorly, while there is only one in men, the rectovesical [
4].
Pelvic organs, except for the tubes, which are intraperitoneal, are only covered by the peritoneum superiorly and laterally. Thus, deposits will be identified as enhancing nodular peritoneal thickenings, either on the pelvic walls (
Figure 33) or within the peritoneal reflexion, either surrounded by fat or on the partially peritonealised organ surfaces (
Figure 34).
As ureters are in close contact with the peritoneum at the paravesical spaces, pelvis deposits may be the cause of ureterohydronephrosis and this is frequently overlooked, especially as the only sign of PC. Any deposit along the posterior parietal peritoneum in the proximity of the course of the ureter may also be the cause of a urinary obstruction, but the pelvis is a very frequent location (
Figure 35).
As a result of pelvis deposits within the peritonealised portion of the rectum, occlusion may occur.
Another common site of PC in the pelvis is the ovaries. As seen previously, they are extraperitoneal organs but considered intraperitoneal as they communicate with the peritoneal cavity. This is the reason why ovaries are included amongst the locations of PC.
Compared to primary ovarian tumours, ovarian metastases seem to be smaller, more frequently bilateral, showing more uniform cysts and more moderate enhancement of the solid portions [
18]. Although a solid appearance may also be found or even characteristics resembling the primary tumour (
Figure 36).
The term Krukenberg tumour is sometimes misused in the setting of ovarian metastases from a gastrointestinal tumour, as its use should be limited to ovarian metastasis from a poorly differentiated adenocarcinoma with signet ring cell features [
19]. Krukenberg tumour should be considered in the differential diagnosis when solid bilateral ovarian masses containing intratumoral cystic component are detected, even in the absence of a primary malignancy [
20].
4. Peritoneal Fluid Circulation-Ascites
Now that the peritoneal anatomy has been revised, the focus will be placed on the fluid contained in the peritoneal cavity, as it will play a determinant role in the seeding of the deposits.
Peritoneal fluid circulates following the path determined by ligaments and mesenteries, under the influence of the abdominal pressure fluctuations caused by respiration and intestinal peristalsis.
In case of tumour spread to the peritoneum, the quantity of peritoneal liquid will rise due to two conditions: the obstruction of the lymphatics in charge of the resorption and the over-production of fluid caused by the vascular permeability factor secreted by the tumour cells [
21,
22].
Fluid in the inframesocolic space naturally goes down on the right of the small bowel mesentery [
23], through the mesenteric leaves, and on the left, through the medial mesosigmoid, thus an area of arrested flow. It then reaches the pelvic recesses, the most gravity dependent spaces. After filling the pelvis, it goes up the paracolic gutters: preferently on the right, as the left gutter is shallower, and the flow is limited cranially by the phrenicocolic ligament [
24] (
Figure 1b). On the right, it reaches the subhepatic space and finally, the right subphrenic space, where most of the peritoneal lymphatic clearance will take place, along with the omentum [
25]. Therefore, places that normally constitute a free route or barrier for the flow or where most of the resorption takes place, need to be particularly scrutinized.
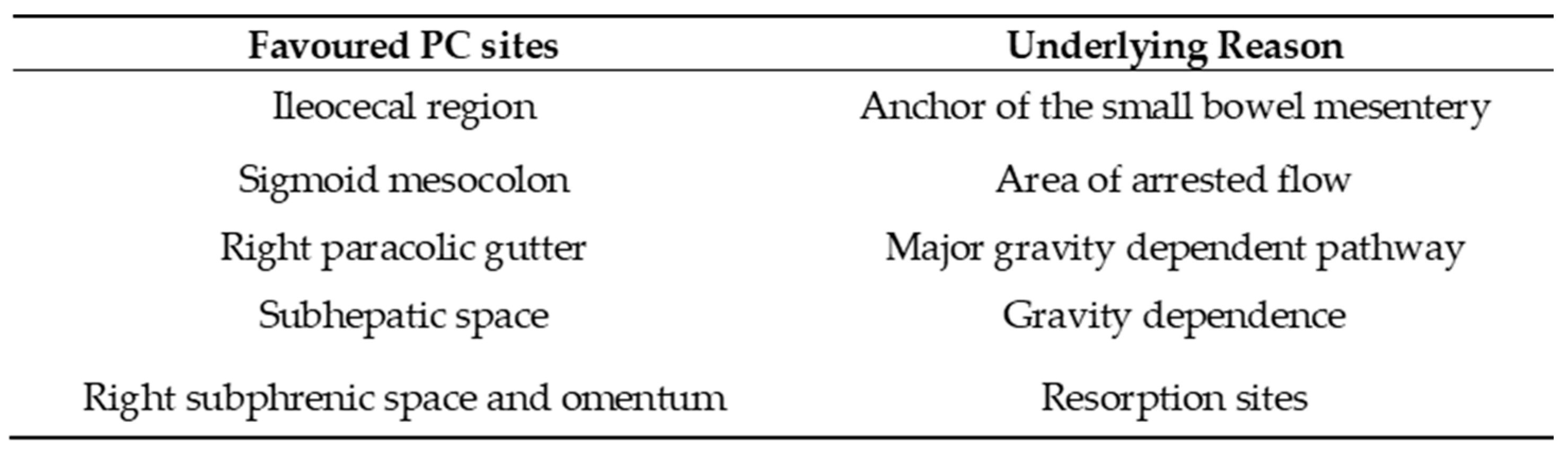
Table 1 sums up the favoured locations for the peritoneal seeding and the underlying reasons.
Ascites may be one of the first signs of PC. Its appearance correlates closely with its volume: if minimal will only be found in the pelvic recesses, surrounding the liver and the spleen or between the small bowel loops, in a triangle-shaped fashion (
Figure 37). If quantity increases, it will fill up the gutters, the omentum, and the mesentery leaves.
Suspicious signs may be ascites with rounded or bulging contours or when present concomitantly in the greater and lesser sacs (
Figure 38).
Another important clue is how SB loops behave to peritoneal liquid: in non-neoplastic ascites or in early stages of PC, they will float freely, with an anterior location, whereas in advanced PC, as the mesentery becomes fibrotic and rigid, SB loops will be pulled back centrally and posteriorly (the tethered-bowel sign) {26}. Characteristically, there will be little quantity of fluid between these rigid infiltrated mesenteric leaves, while predominant elsewhere in the peritoneal cavity (
Figure 39).
In the setting of portal hypertension and non-malignant ascites, though, collateral vessels within the parietal peritoneum should be born in mind cautiously so as not to misdiagnose them as deposits (
Figure 40).
5. Deposit Behaviour on Cross Sectional Images
Signal wise, the deposit does not fall far from the primary tumour, that is, deposits will usually show a density/signal intensity and enhancement pattern resembling the primary tumour, as extra-peritoneal metastases do.
Thus, one should always bear in mind the underlying histology and imaging features of the primary tumour and contemplate the possibility of a second primary tumour if a discrepancy is found.
In addition, in the event of no known primary tumour, which happens in about 3 to 5% of cases of PC [
2], the behaviour on the different MR sequences and, on a lesser degree, on CT, may be helpful hints to suggest the origin, despite the non-specificity of the imaging findings.
Indeed, knowledge of the underlying deposit content responsible for the appearance of high signal intensity on T1 and T2 weighted images of peritoneal deposits is an important diagnostic criterion that can contribute to the diagnosis of the primary tumour.
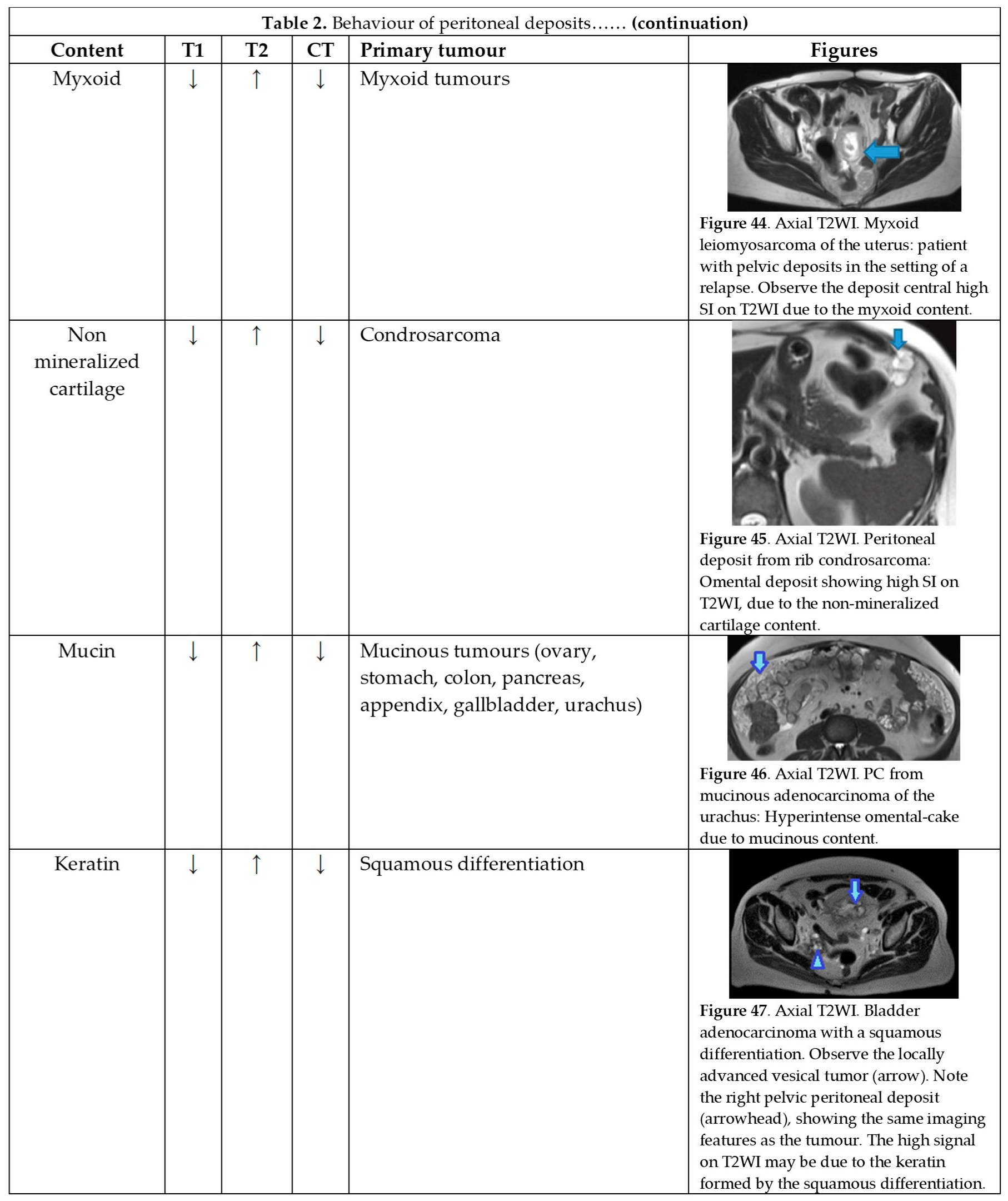
Table 2 summarizes the behaviour of the peritoneal deposits according to their appearance on MR and CT, their content, and the corresponding primary tumour regardless of the cell line.
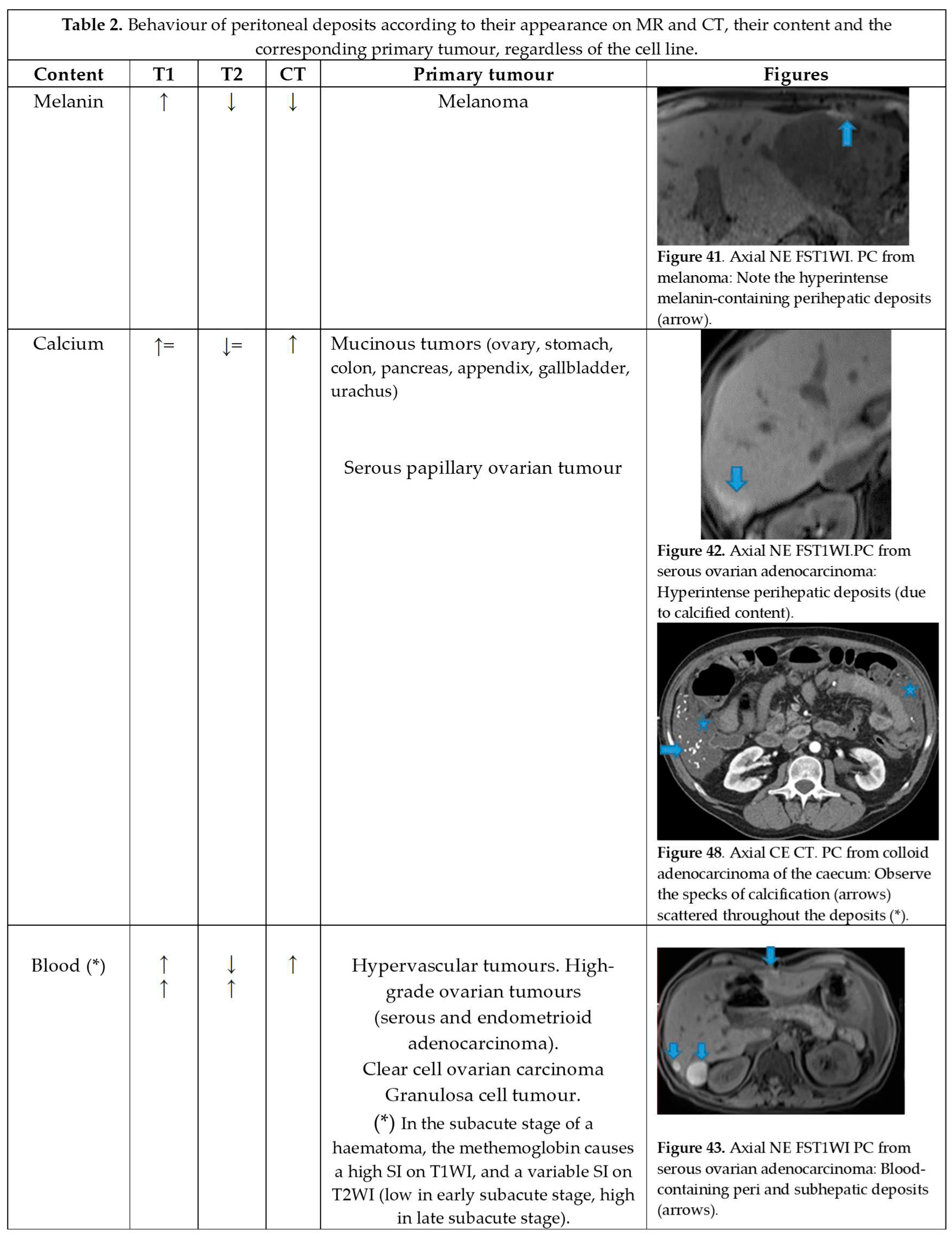
T1 hyperintensity may be observed within a deposit due to three contents: melanin, blood, and calcium.
Melanin-containing deposits: from melanoma (Figure 48 of Table 2).
Calcium-containing deposits: Mucinous tumours of different origins (ovary, stomach, colon, pancreas, appendix, gallbladder, urachus) may calcify (Figure 48 of Table 2).
Blood-containing deposits: Blood content is frequently found in peritoneal deposits from hypervascular tumours of different origins (for instance, clear cell and granulosa ovarian tumours) (Figure 48 of Table 2).
T2 hyperintensity can reflect different contents within deposits: myxoid, non-mineralized cartilage or mucin. It may also be due to keratin in the setting of a squamous differentiation.
Myxoid-containing deposits: as in myxoid liposarcoma (Figure 48 of Table 2).
Non mineralized cartilage-containing deposits: from chondrosarcoma (Figure 48 of Table 2).
Mucin-containing deposits: from mucinous tumours arising on different organs, namely ovary, stomach, colon, pancreas, appendix, gallbladder and the urachus (Figure 48 of Table 2).
Keratin-containing deposits: from tumours showing a squamous differentiation (Figure 48 of Table 2).
CT hyperdensity can be due to calcium (Figure 48 of Table 2) or blood content. Calcification may occur secondary to treatment.
The vascular pattern of deposits also correlates with the characteristics of the primary tumour and may have an impact on the differential diagnosis (
Table 3) depending on whether deposits are hyper (Figure 49 of
Table 3) or hypovascular (Figure 50 of
Table 3).
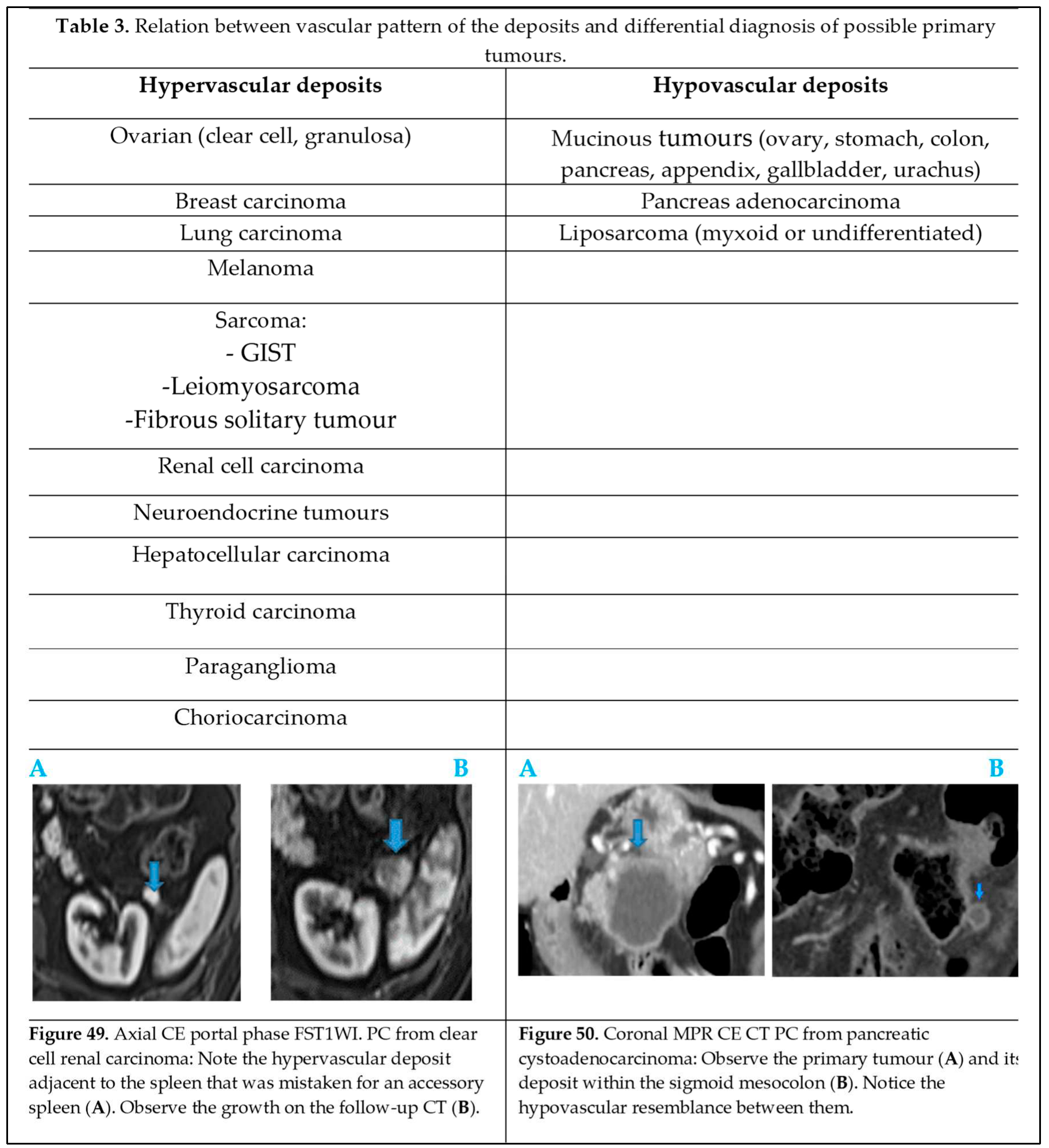
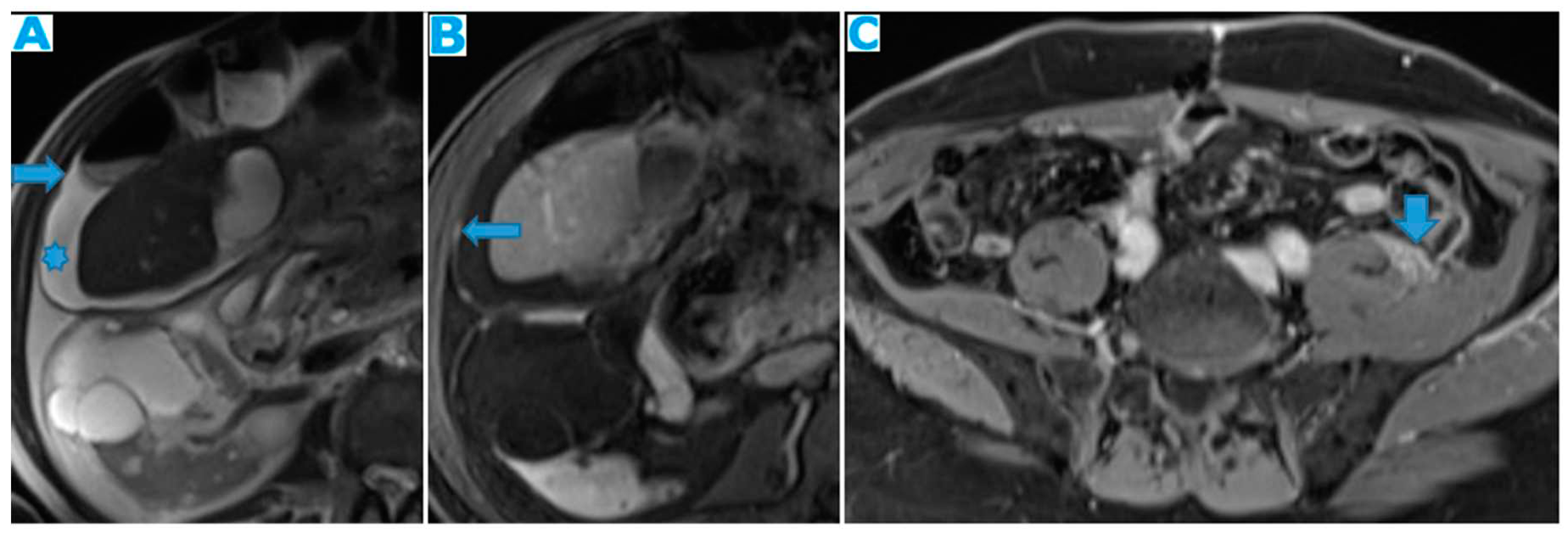
Figure 1.
Axial CE-CT (A) and coronal MPR (B). Massive ascites, helpful to differentiate the peritoneal spaces. Observe on A how the posterior margin of the diaphragm (arrow) is not covered by the parietal peritoneum as it is directly in contact with the retroperitoneal fat. The attachment sites of ligaments on the diaphragmatic surface are not covered by peritoneum; Identify on A the falciform and on B, the phrenicocolic ligaments (arrowhead). Notice on B how the phrenicocolic ligament partially separates the left parietocolic gutter from the left subphrenic space (*), whereas the right parietocolic gutter fully communicates with the right subphrenic space (curved arrow). Identify the bare area of the liver on B (arrow), an area directly attached to the diaphragm by connective tissue, thus not covered by peritoneum as neither is its diaphragmatic attachment site.
Figure 1.
Axial CE-CT (A) and coronal MPR (B). Massive ascites, helpful to differentiate the peritoneal spaces. Observe on A how the posterior margin of the diaphragm (arrow) is not covered by the parietal peritoneum as it is directly in contact with the retroperitoneal fat. The attachment sites of ligaments on the diaphragmatic surface are not covered by peritoneum; Identify on A the falciform and on B, the phrenicocolic ligaments (arrowhead). Notice on B how the phrenicocolic ligament partially separates the left parietocolic gutter from the left subphrenic space (*), whereas the right parietocolic gutter fully communicates with the right subphrenic space (curved arrow). Identify the bare area of the liver on B (arrow), an area directly attached to the diaphragm by connective tissue, thus not covered by peritoneum as neither is its diaphragmatic attachment site.
Figure 2.
Axial T2WI.PC from neuroendocrine tumour: Deposit within anterior parietal peritoneum.
Figure 2.
Axial T2WI.PC from neuroendocrine tumour: Deposit within anterior parietal peritoneum.
Figure 3.
Axial T2WI. PC from neuroendocrine tumour: Deposits within the posterior parietal peritoneum (arrows) (which is immediately anterior to the anterior pararenal fascia).
Figure 3.
Axial T2WI. PC from neuroendocrine tumour: Deposits within the posterior parietal peritoneum (arrows) (which is immediately anterior to the anterior pararenal fascia).
Figure 4.
Axial T2WI (A), axial CE portal phase FST1WI (B), same patient. PC from colon adenocarcinoma: Notice the nodular thickening of the parietal peritoneum due to deposits. Notice how easy it is to distinguish the parietal peritoneum from the visceral perihepatic peritoneum thanks to the ascites (*). Axial CE portal phase FST1WI (C). PC from neuroendocrine tumour: Deposit within the posterior parietal peritoneum. The retroperitoneal space is very thin at this location and so the posterior parietal peritoneum is adjacent to the abdominal wall.
Figure 4.
Axial T2WI (A), axial CE portal phase FST1WI (B), same patient. PC from colon adenocarcinoma: Notice the nodular thickening of the parietal peritoneum due to deposits. Notice how easy it is to distinguish the parietal peritoneum from the visceral perihepatic peritoneum thanks to the ascites (*). Axial CE portal phase FST1WI (C). PC from neuroendocrine tumour: Deposit within the posterior parietal peritoneum. The retroperitoneal space is very thin at this location and so the posterior parietal peritoneum is adjacent to the abdominal wall.
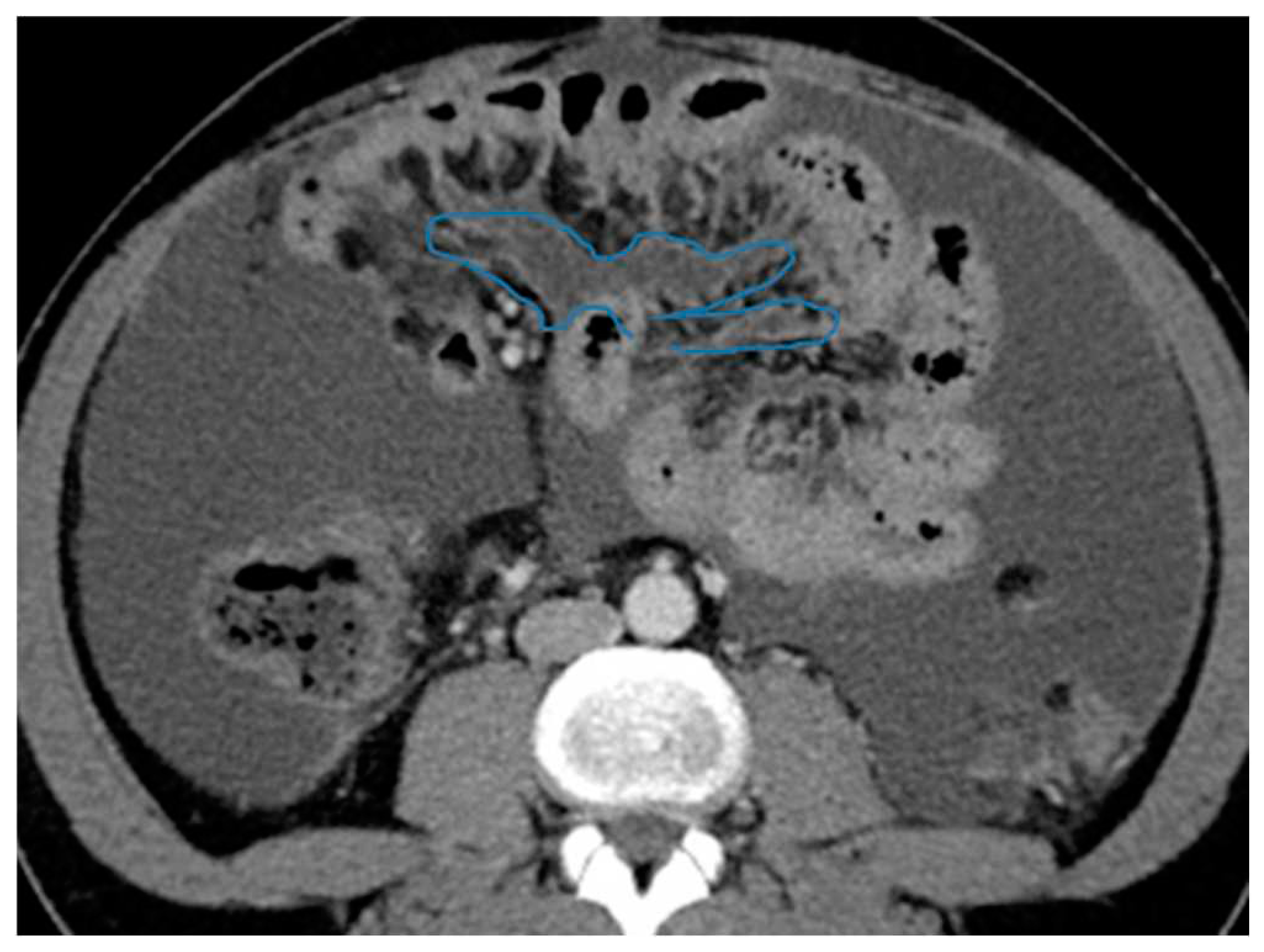
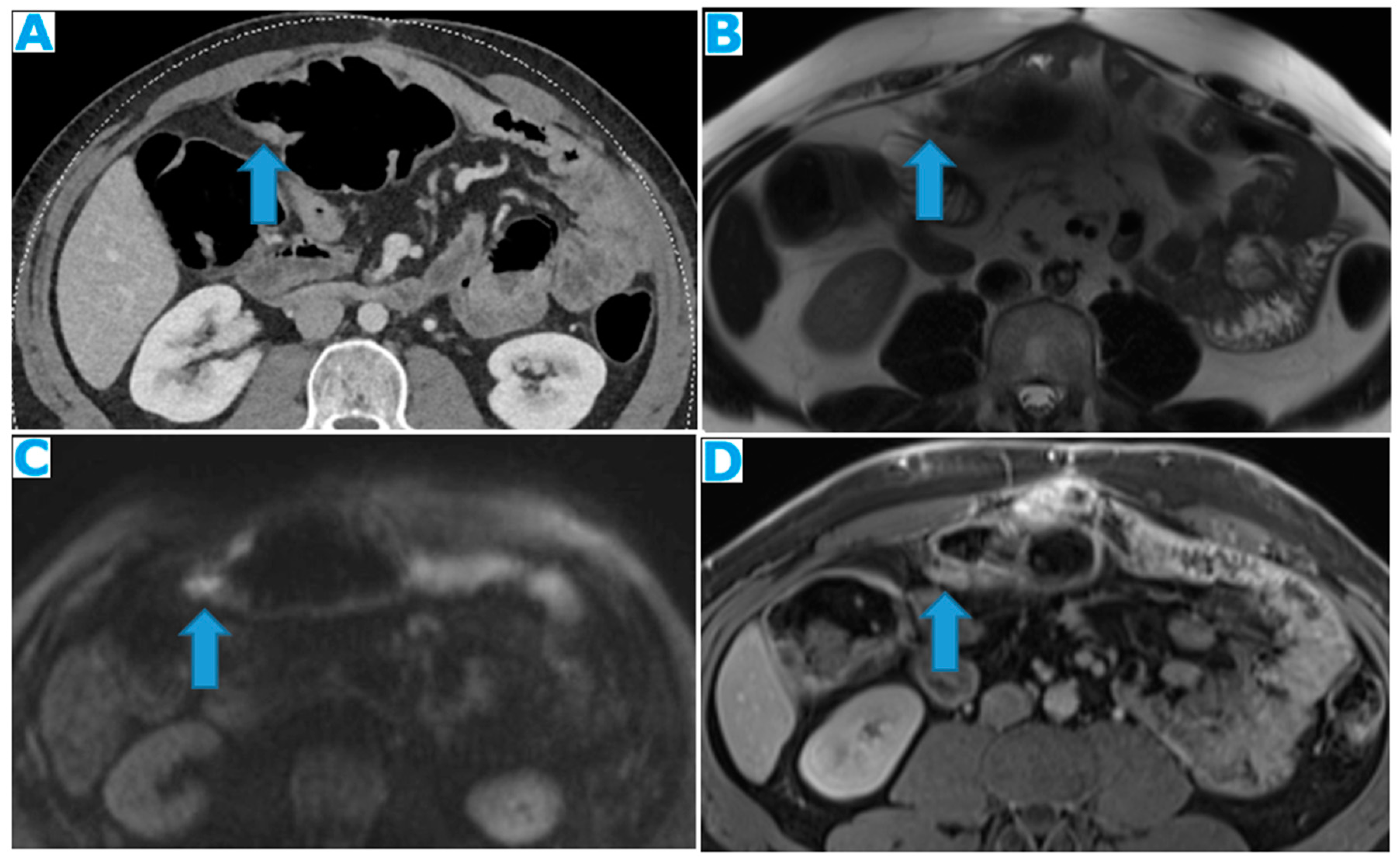
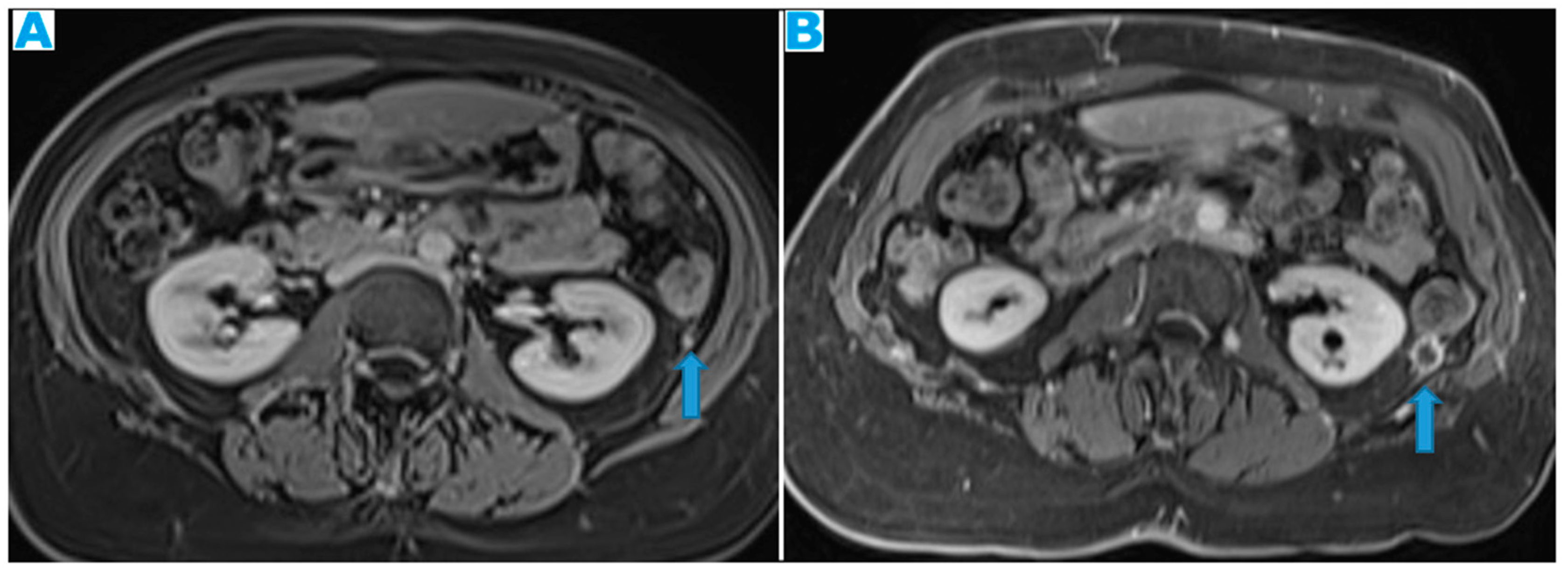
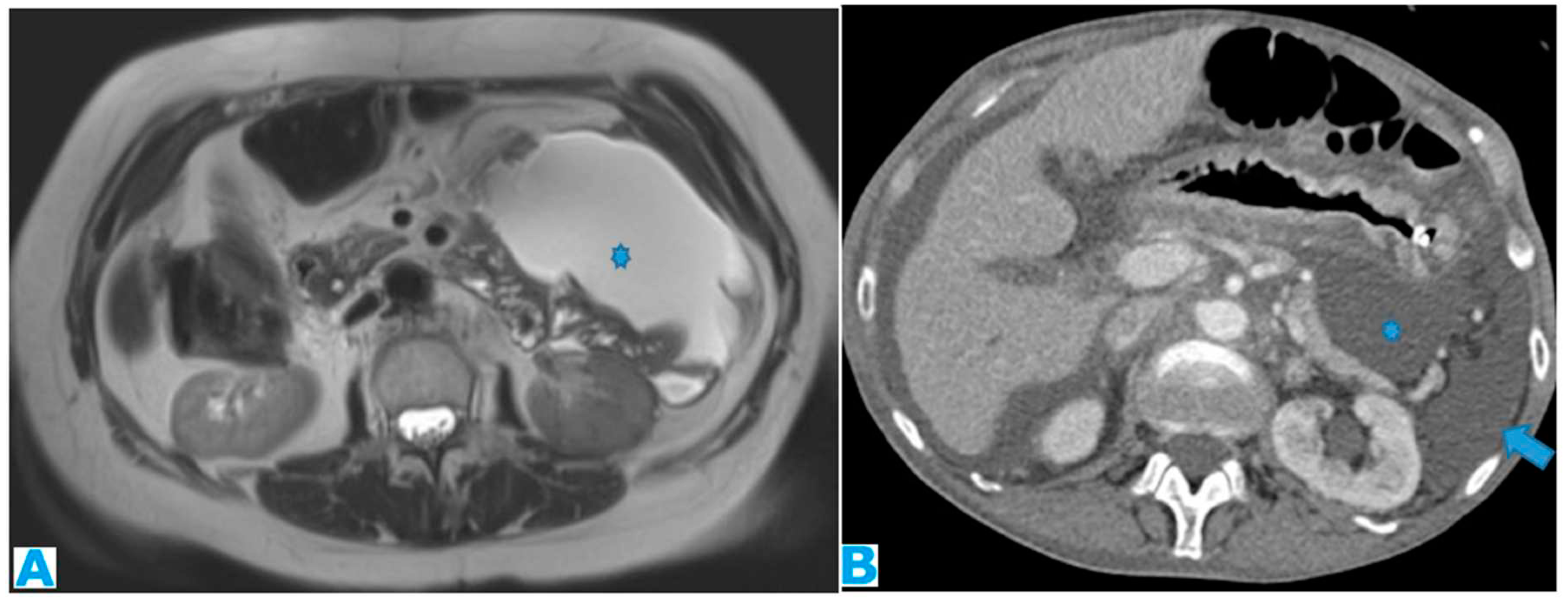
Figure 5.
Axial CE-CT (A), axial T2WI (B), DWI (C), axial CE portal phase FST1WI (D). PC from gastric adenocarcinoma: Subtle deposit within the transverse mesocolon on CT, more conspicuous on MR.
Figure 5.
Axial CE-CT (A), axial T2WI (B), DWI (C), axial CE portal phase FST1WI (D). PC from gastric adenocarcinoma: Subtle deposit within the transverse mesocolon on CT, more conspicuous on MR.
Figure 6.
Axial CE-CT (A). PC from breast carcinoma: Deposits within the transverse colon serosa (arrows), causing luminal stenosis. Axial CE-CT (B). PC from breast carcinoma: Layered deposits within the transverse colon serosa (arrow), notice the concentric pattern and the luminal stenosis. Ascites (*). Coronal CE-CT MPR (C-D). C: PC from signet ring cell gastric carcinoma. Diffuse serosal infiltration of the transverse colon. D: PC from breast carcinoma: Segmental parietal thickening of the transverse colon due to nodular deposits within the serosa (arrow). Observe how layered deposits on C blend in with the bowel contour and may be more difficult to detect than nodular deposits on D.
Figure 6.
Axial CE-CT (A). PC from breast carcinoma: Deposits within the transverse colon serosa (arrows), causing luminal stenosis. Axial CE-CT (B). PC from breast carcinoma: Layered deposits within the transverse colon serosa (arrow), notice the concentric pattern and the luminal stenosis. Ascites (*). Coronal CE-CT MPR (C-D). C: PC from signet ring cell gastric carcinoma. Diffuse serosal infiltration of the transverse colon. D: PC from breast carcinoma: Segmental parietal thickening of the transverse colon due to nodular deposits within the serosa (arrow). Observe how layered deposits on C blend in with the bowel contour and may be more difficult to detect than nodular deposits on D.
Figure 7.
CE-CT coronal MPR. PC from ovarian carcinoma: Multiple bilateral diaphragmatic nodular deposits (arrows). Notice how useful ascites (*) is to distinguish peritoneal deposits within the parietal peritoneum from deposits within the visceral (hepatic) peritoneum (arrowhead). Hepatic metastases (thin arrow).
Figure 7.
CE-CT coronal MPR. PC from ovarian carcinoma: Multiple bilateral diaphragmatic nodular deposits (arrows). Notice how useful ascites (*) is to distinguish peritoneal deposits within the parietal peritoneum from deposits within the visceral (hepatic) peritoneum (arrowhead). Hepatic metastases (thin arrow).
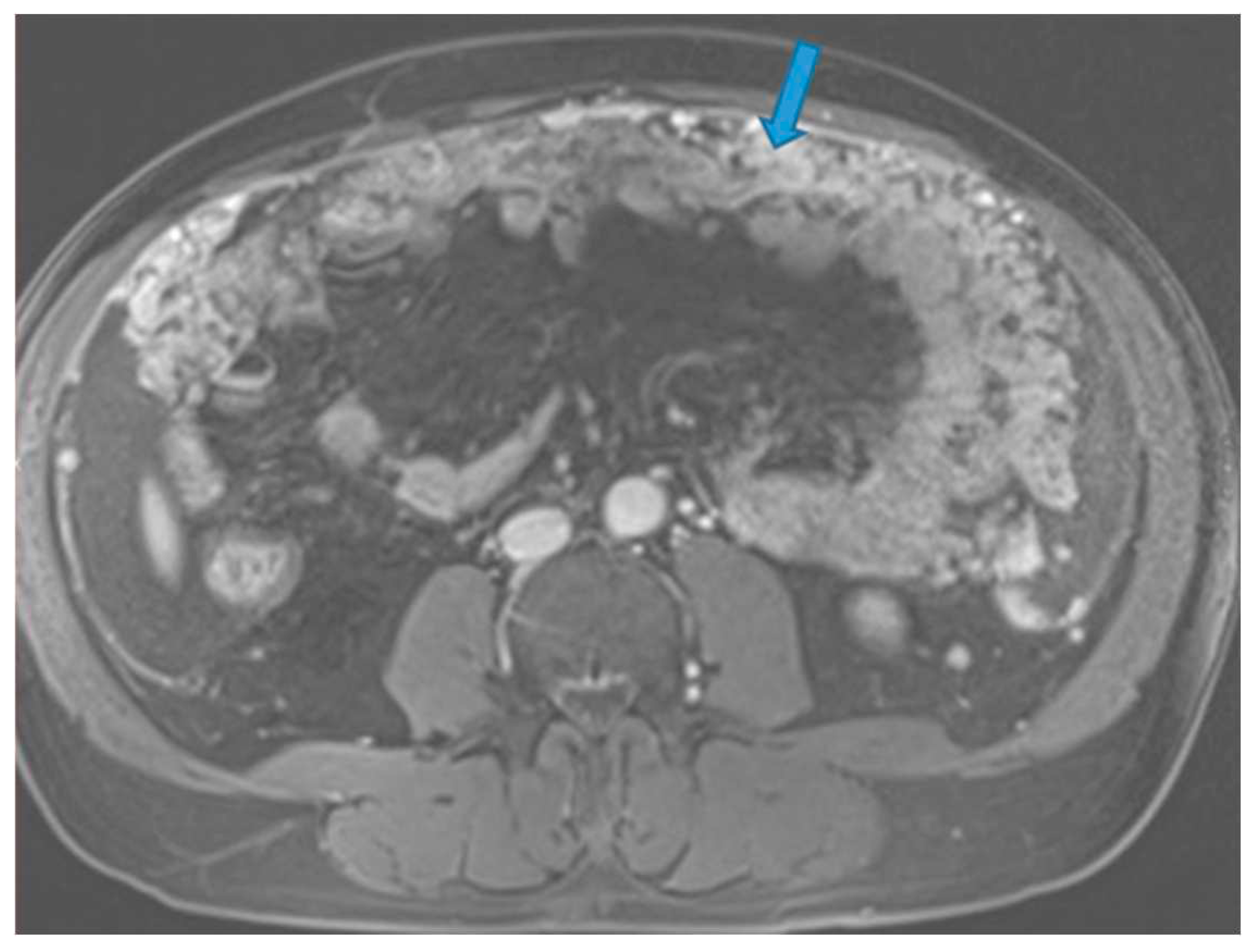
Figure 8.
Coronal T2WI (A, from five years prior) and CE-CT coronal MPR (B, current follow up). PC from ovarian granulosa cell tumour: Notice on B a deposit within the right subphrenic space, bulging into the hepatic capsule. See how subtle it originally was on A, easily overlooked on axial imaging.
Figure 8.
Coronal T2WI (A, from five years prior) and CE-CT coronal MPR (B, current follow up). PC from ovarian granulosa cell tumour: Notice on B a deposit within the right subphrenic space, bulging into the hepatic capsule. See how subtle it originally was on A, easily overlooked on axial imaging.
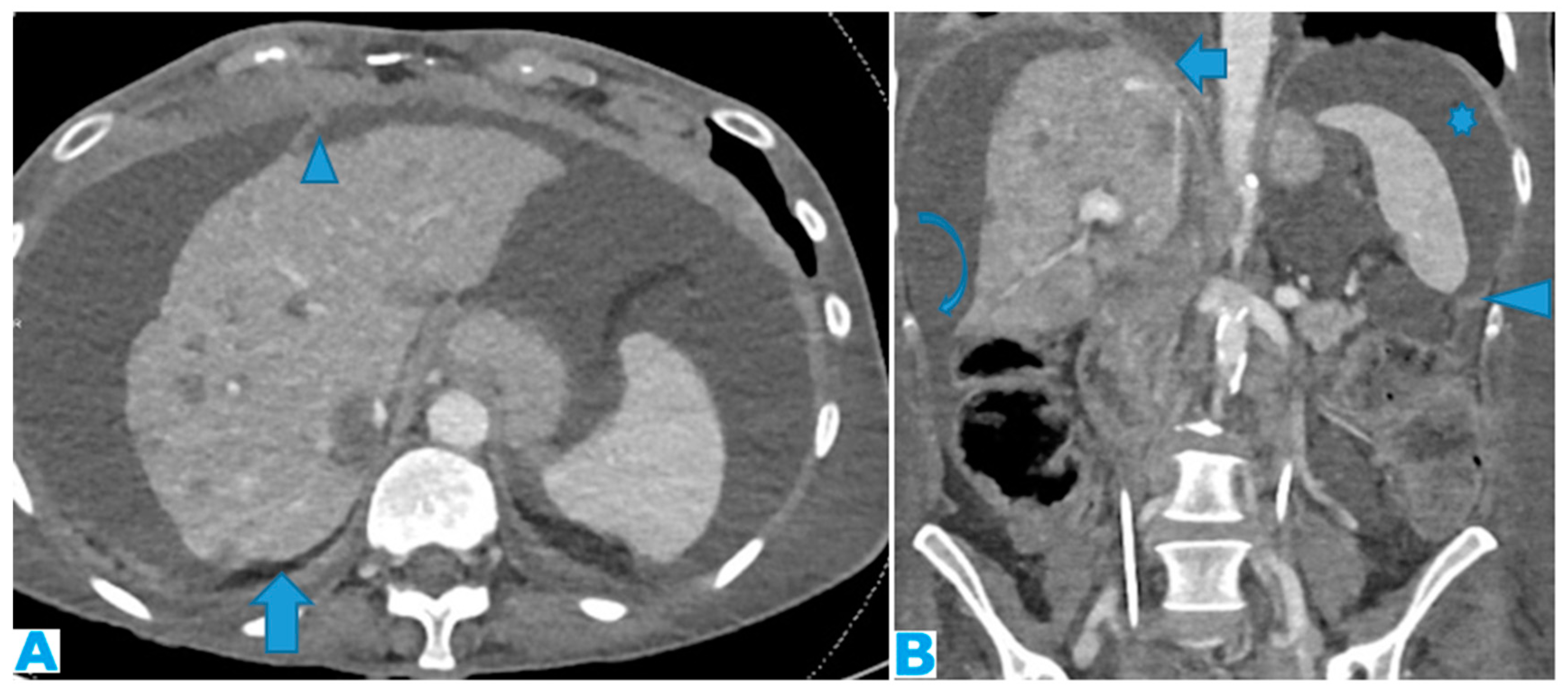
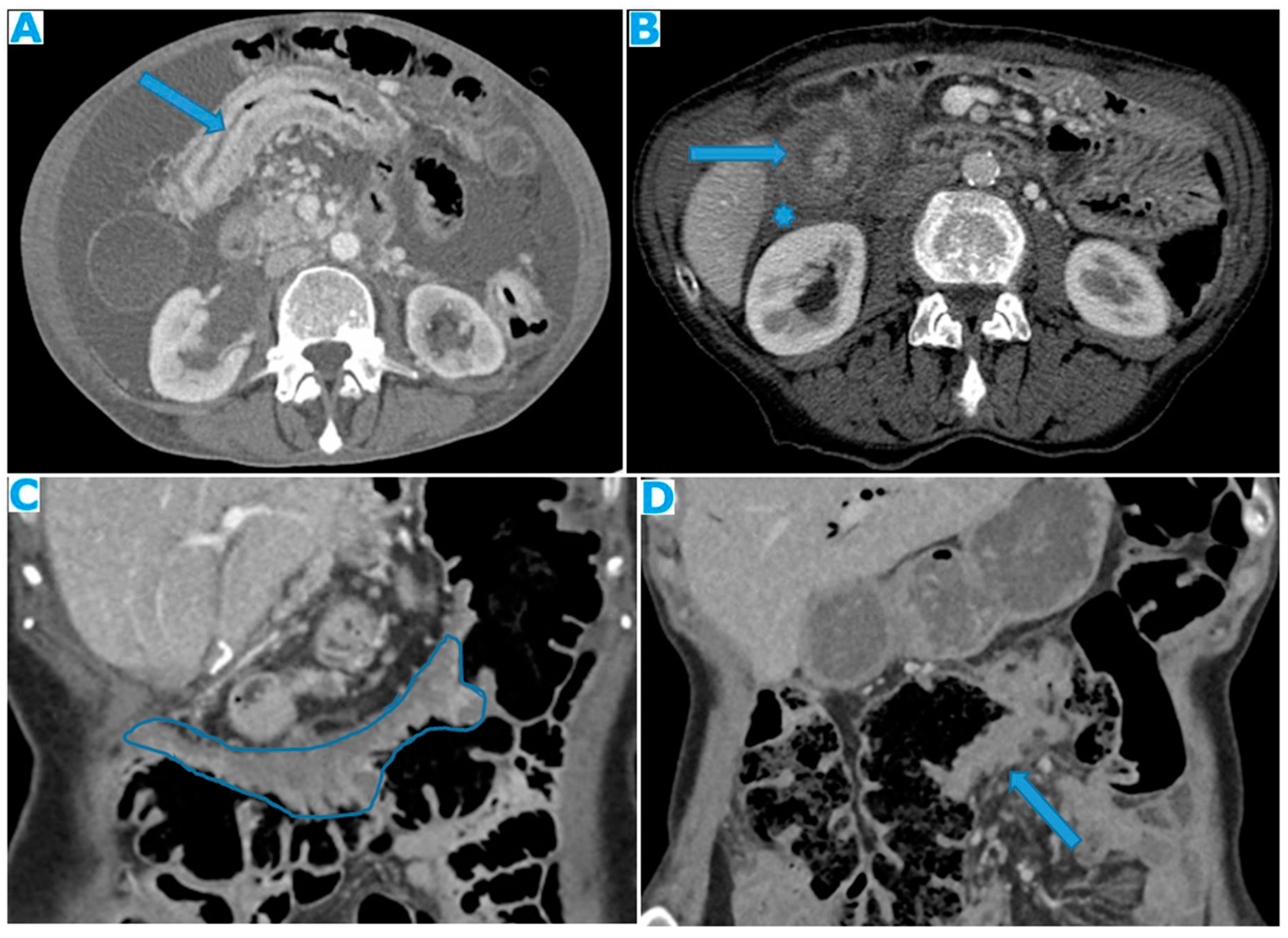
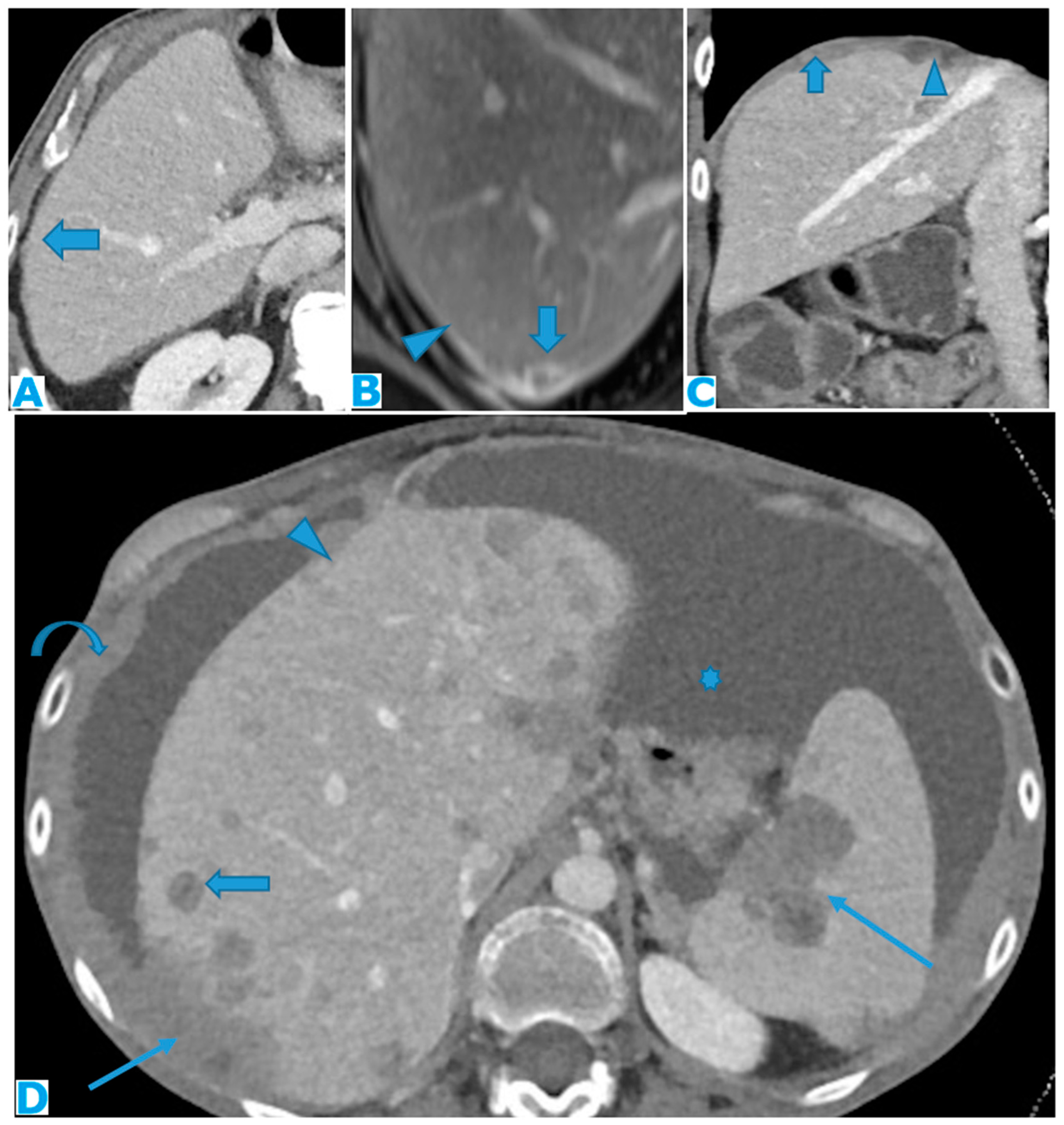
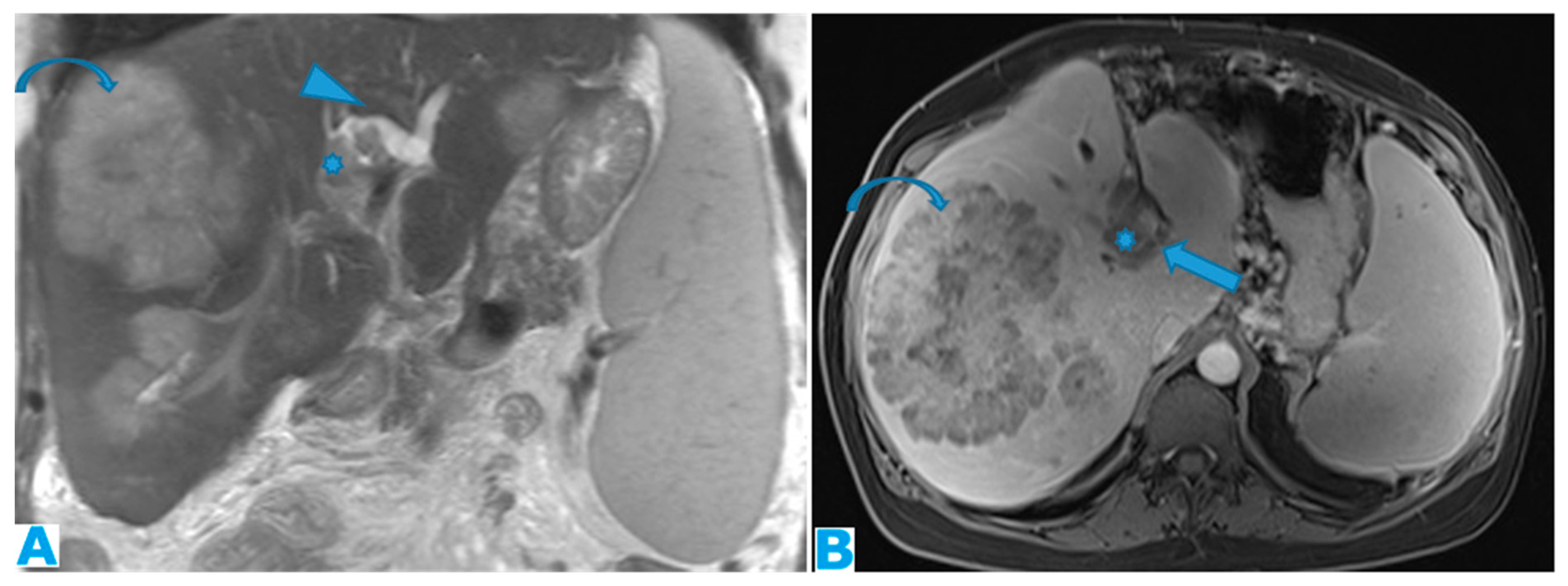
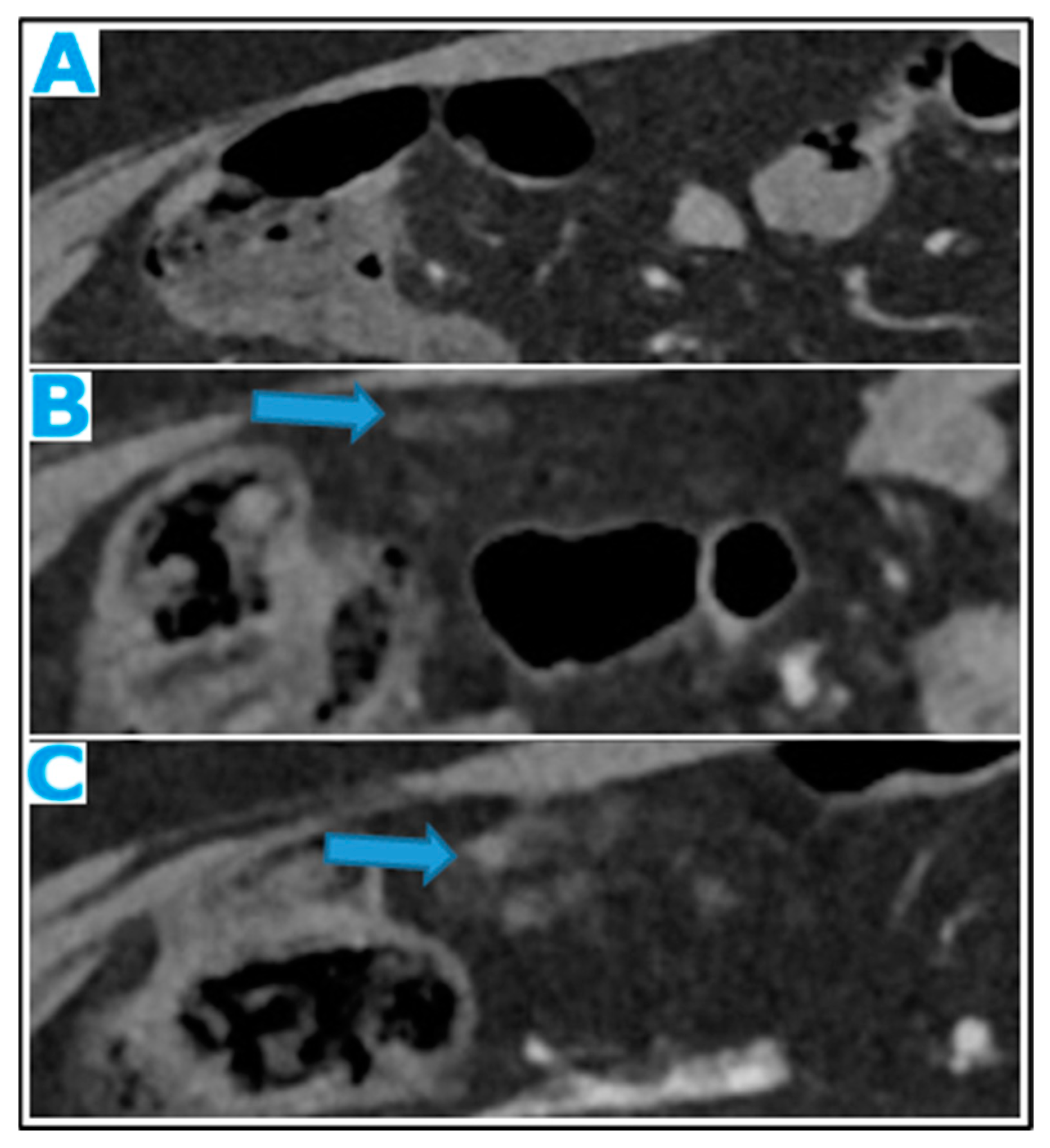
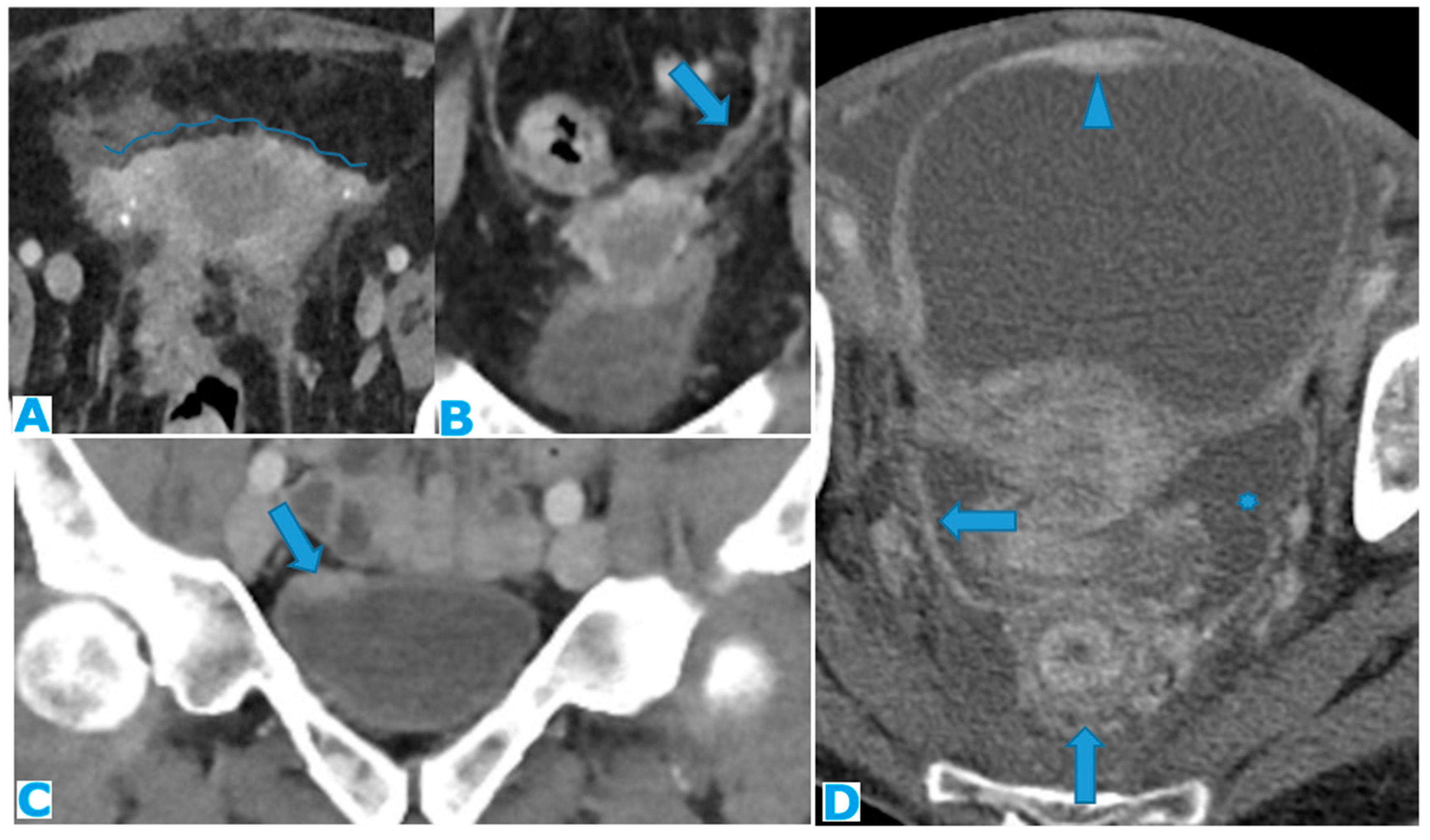
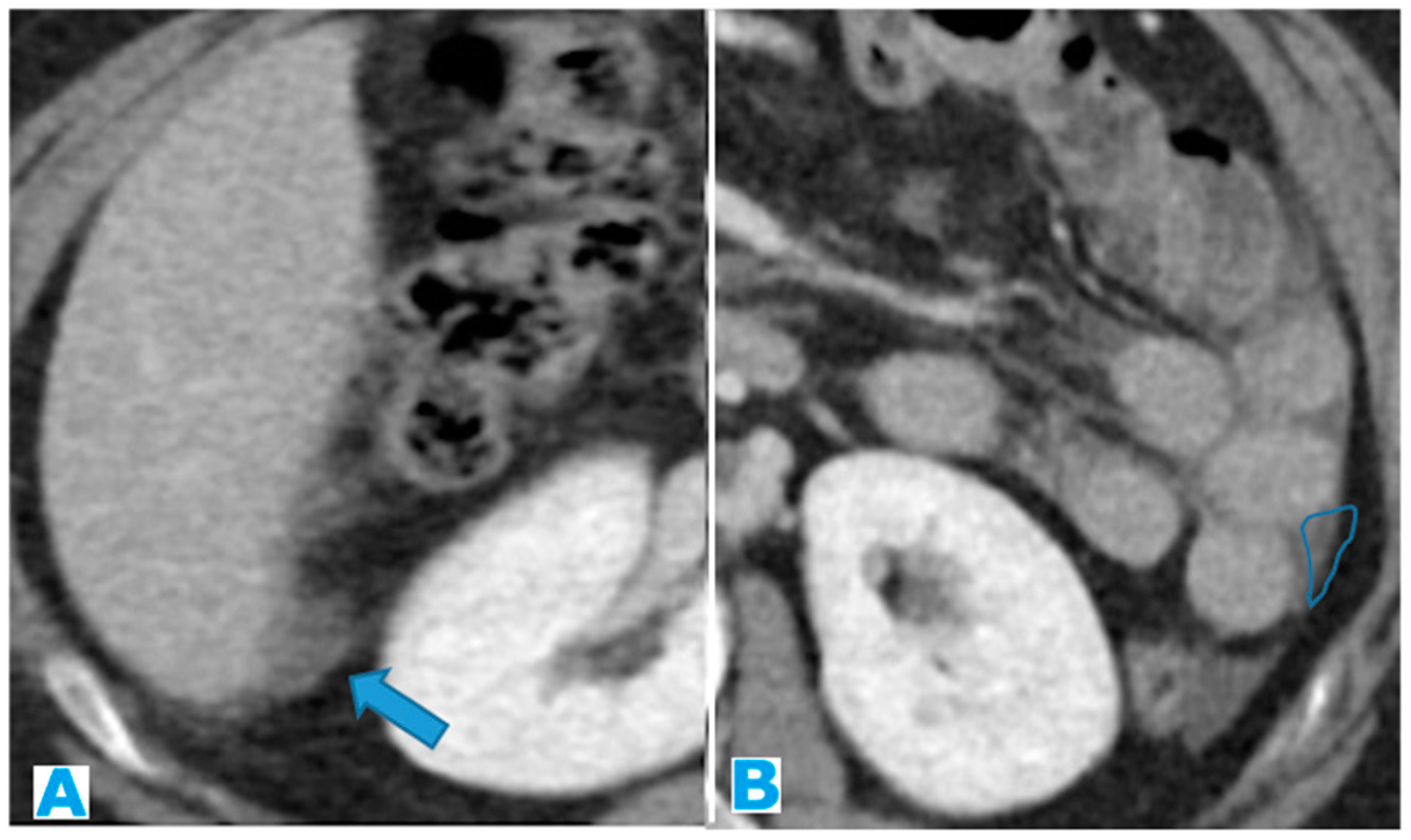
Figure 9.
Axial CE-CT (A): PC from colon carcinoma: Note the subtle irregularities of the liver contour caused by the deposit seeding within the peritoneum covering the hepatic surface. CE portal phase FST1WI (B): PC from cervical carcinoma: Notice the difference between the linear subcapsular deposits (arrowhead) and the biconvex subcapsular deposit with parenchymal invasion (arrow): observe the scalloped appearance of the underlying parenchyma. CE-CT coronal MPR (C): PC from colon carcinoma: Thickening of the right diaphragm caused by deposit seeding (arrow). Notice the difference with the subcapsular deposits that scallop the liver contour (arrowhead). Axial CE-CT (D): PC from ovarian carcinoma: Subcapsular deposits with parenchymal invasion (thin arrows), both hepatic and splenic. Observe how they differ from hepatic metastases, well defined and completely surrounded by parenchyma (arrow). Note also perihepatic (arrowhead) and right subphrenic deposits (curved arrow). Ascites (*).
Figure 9.
Axial CE-CT (A): PC from colon carcinoma: Note the subtle irregularities of the liver contour caused by the deposit seeding within the peritoneum covering the hepatic surface. CE portal phase FST1WI (B): PC from cervical carcinoma: Notice the difference between the linear subcapsular deposits (arrowhead) and the biconvex subcapsular deposit with parenchymal invasion (arrow): observe the scalloped appearance of the underlying parenchyma. CE-CT coronal MPR (C): PC from colon carcinoma: Thickening of the right diaphragm caused by deposit seeding (arrow). Notice the difference with the subcapsular deposits that scallop the liver contour (arrowhead). Axial CE-CT (D): PC from ovarian carcinoma: Subcapsular deposits with parenchymal invasion (thin arrows), both hepatic and splenic. Observe how they differ from hepatic metastases, well defined and completely surrounded by parenchyma (arrow). Note also perihepatic (arrowhead) and right subphrenic deposits (curved arrow). Ascites (*).
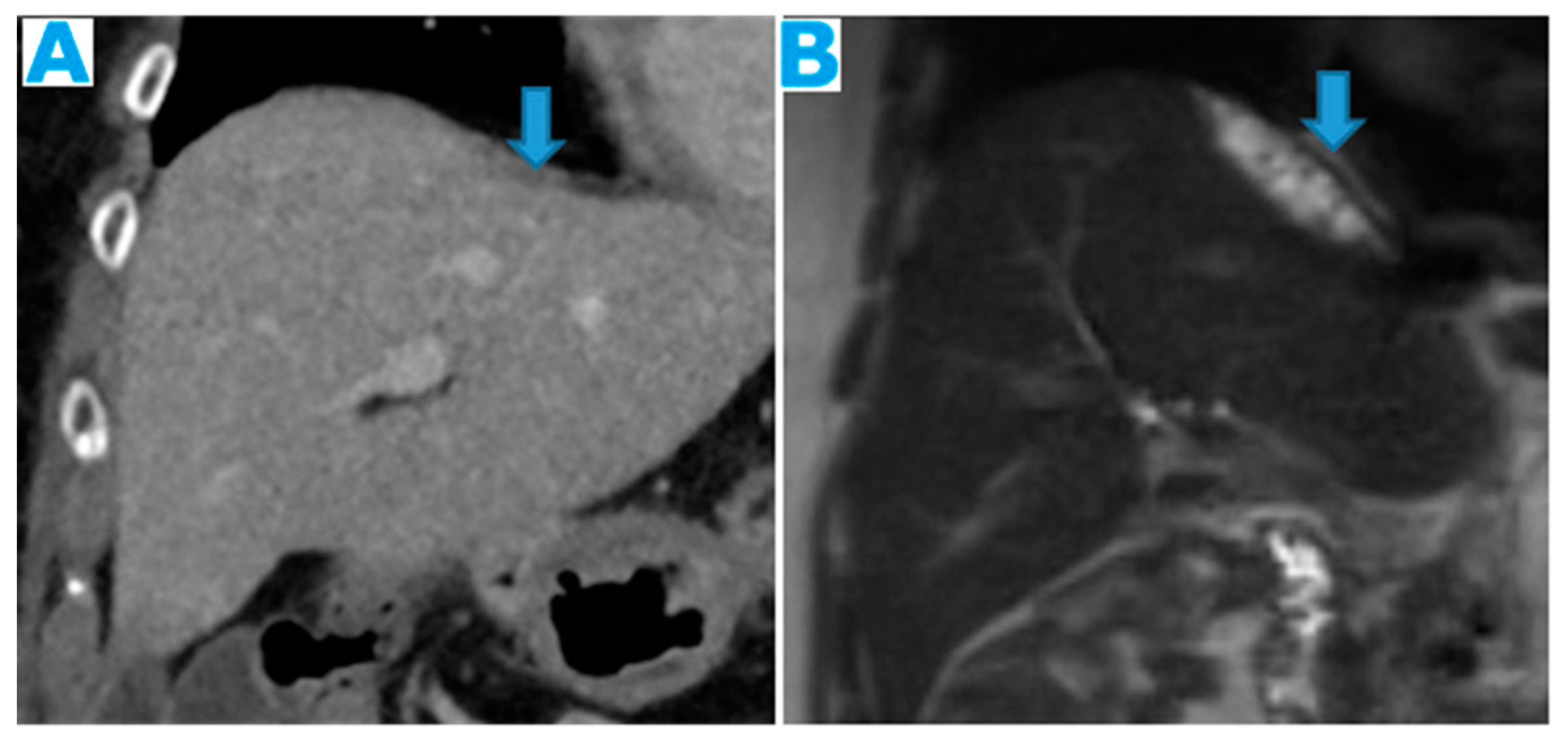
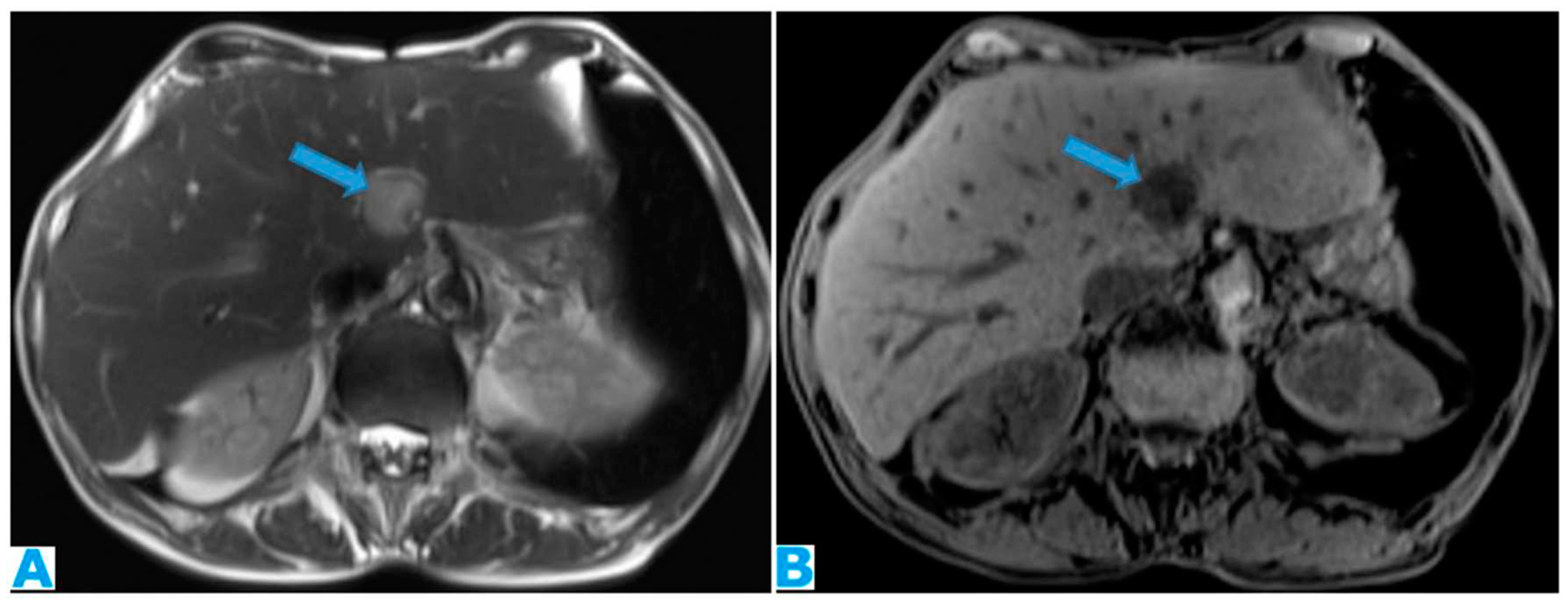
Figure 10.
Axial T2WI (A), axial NE FST1WI (B). PC from ovarian serous carcinoma: Subcapsular hepatic deposit, presenting a fatty plane around (arrow) that excludes secondary hepatic invasion.
Figure 10.
Axial T2WI (A), axial NE FST1WI (B). PC from ovarian serous carcinoma: Subcapsular hepatic deposit, presenting a fatty plane around (arrow) that excludes secondary hepatic invasion.
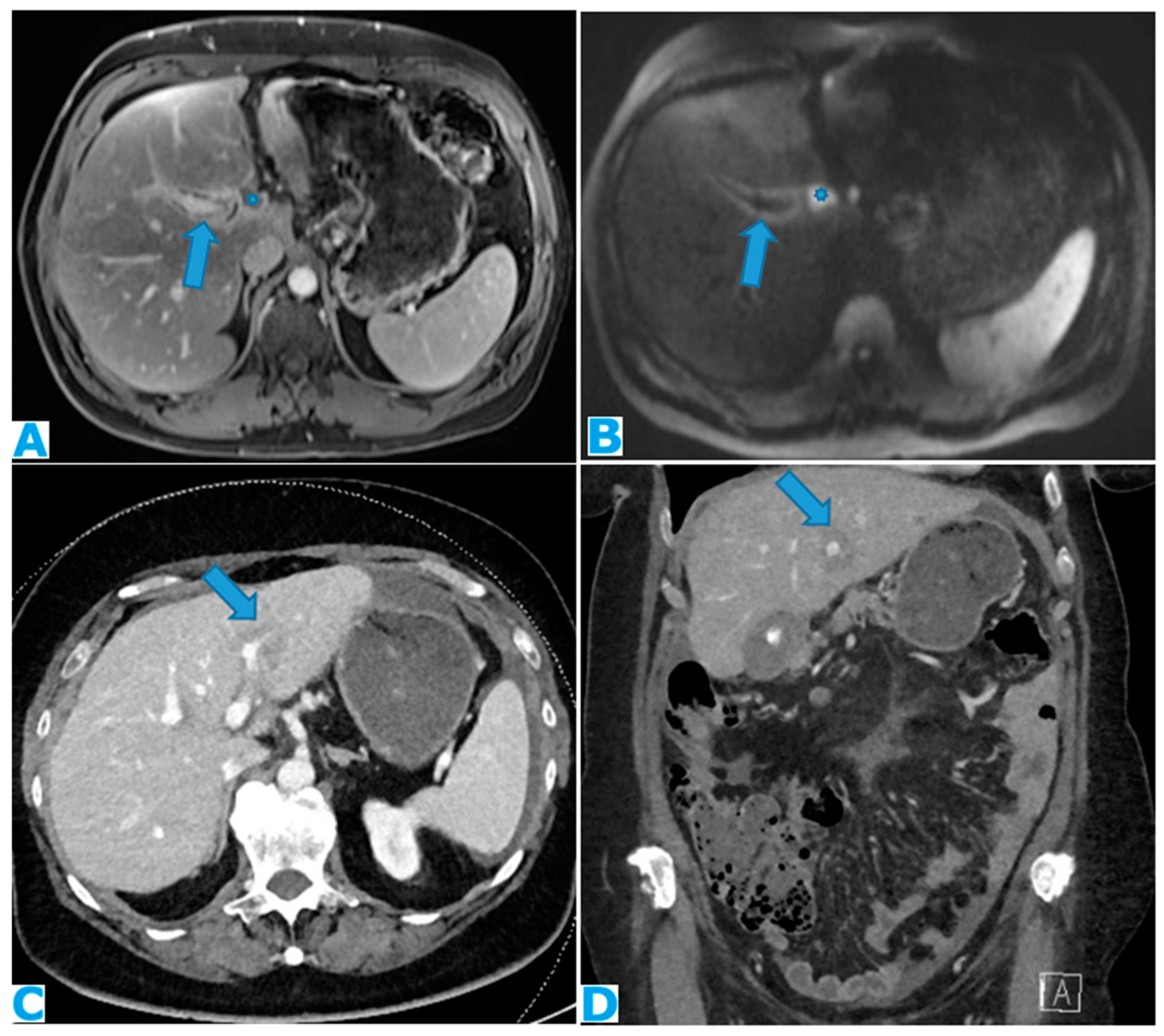
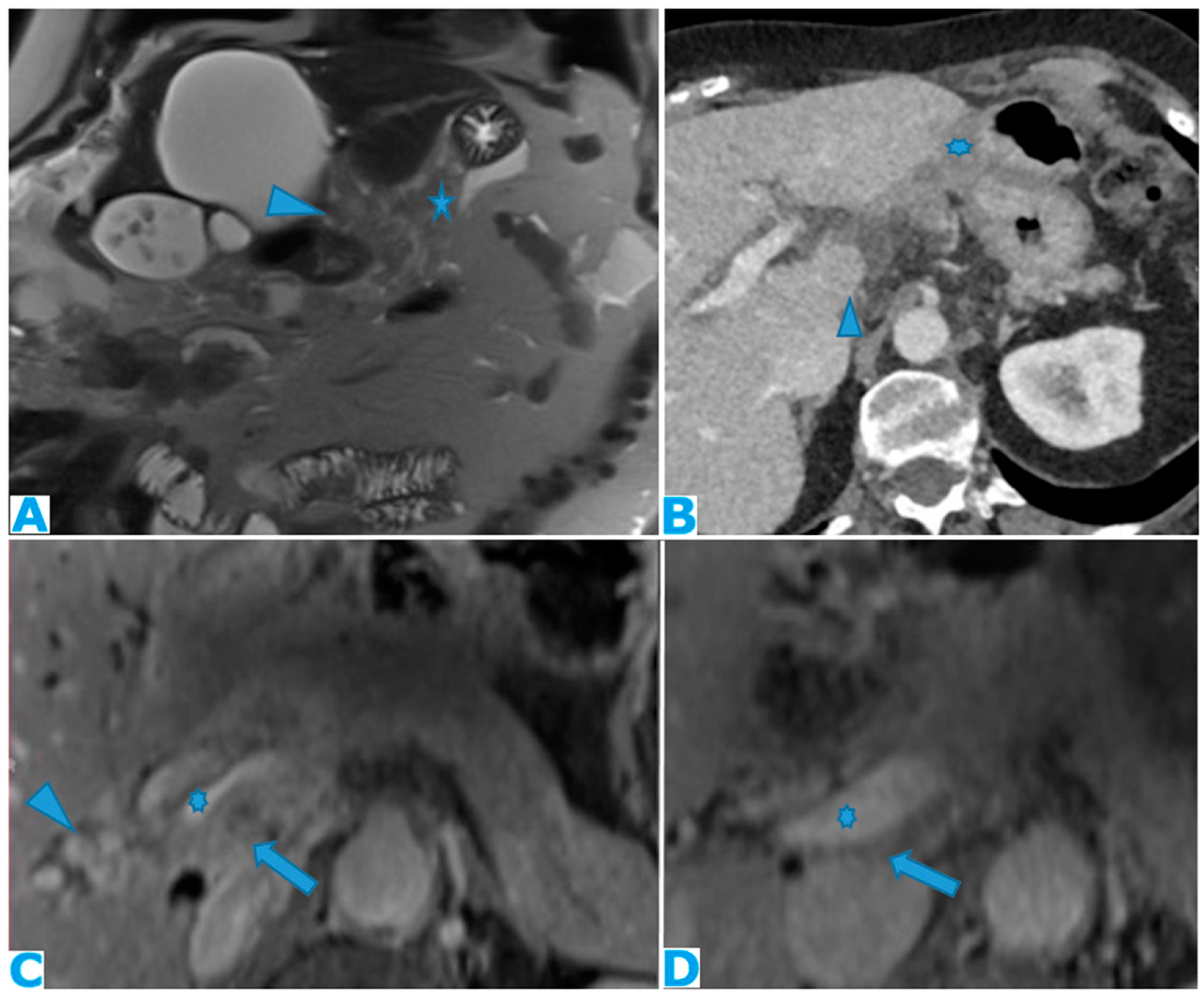
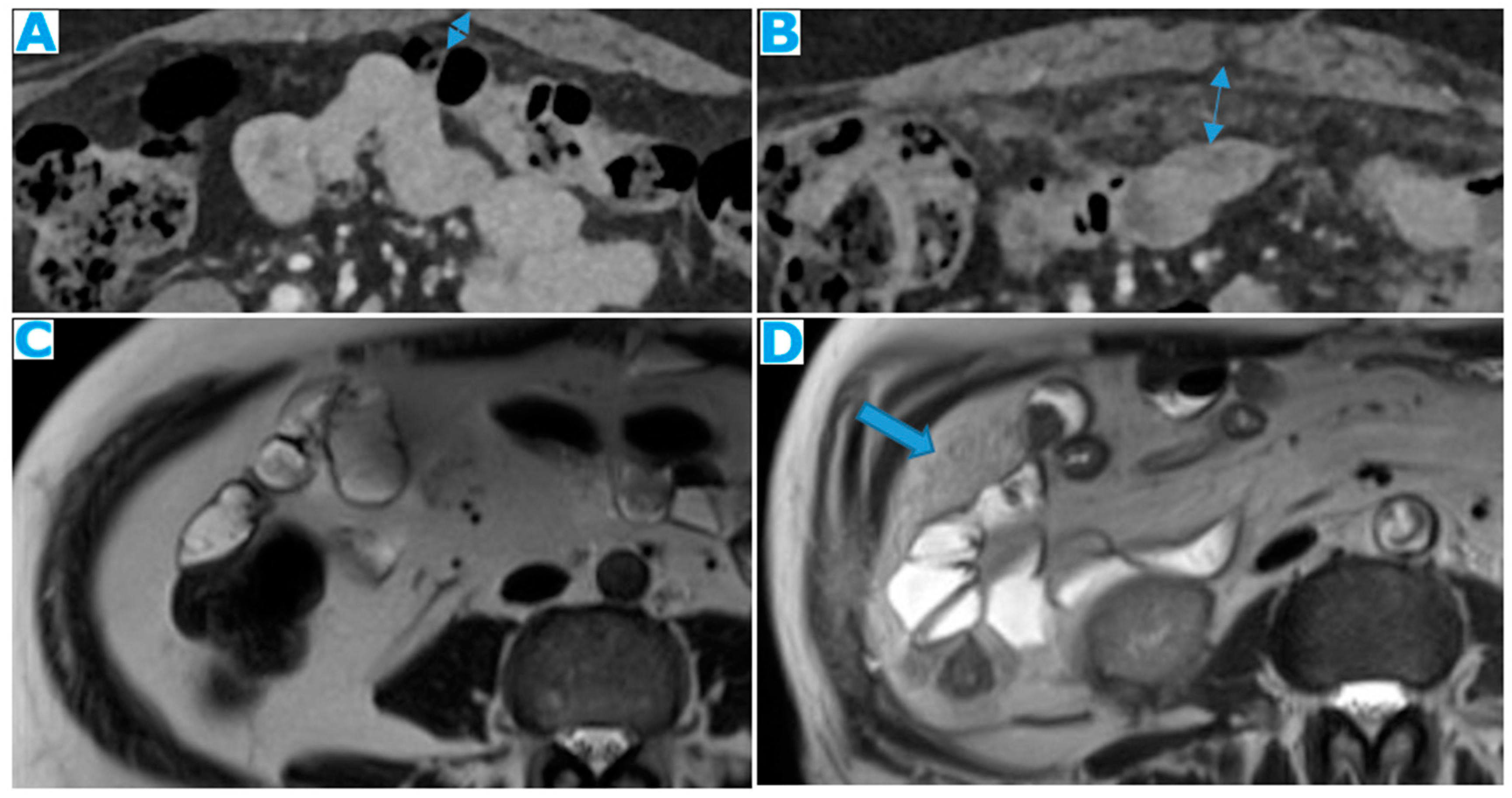
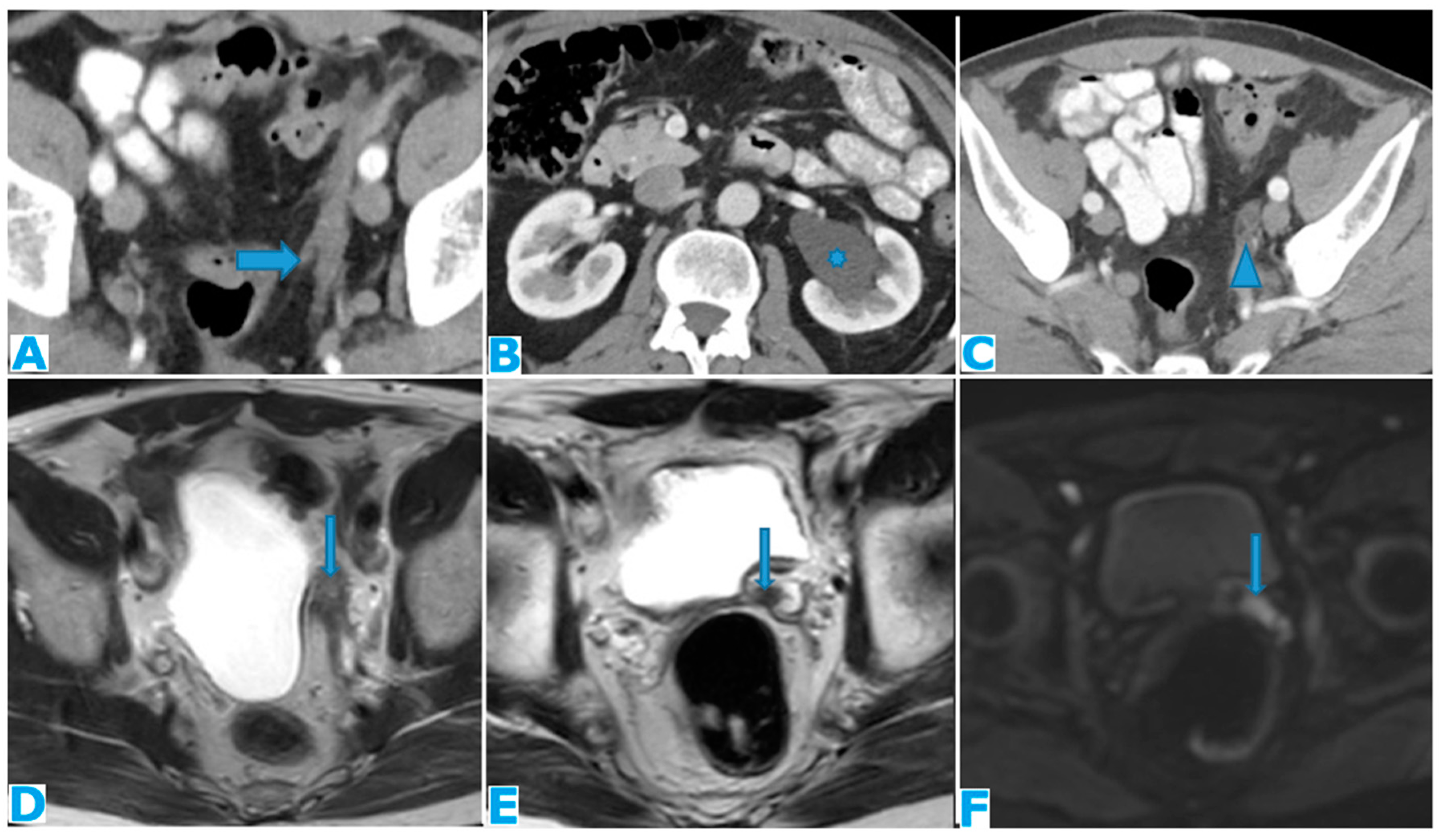
Figure 11.
DWI (A), axial CE portal phase FST1WI (B). PC from colon carcinoma: deposits within the periportal space. Observe the diffusion restriction and enhancement around the periportal space (arrows); also note the nodular deposit (*). Axial CE -CT(C) and coronal MPR (D). PC from ovarian carcinoma: Note the periportal deposit as a soft tissue mass around the left portal branch, more conspicuous on the MPR.
Figure 11.
DWI (A), axial CE portal phase FST1WI (B). PC from colon carcinoma: deposits within the periportal space. Observe the diffusion restriction and enhancement around the periportal space (arrows); also note the nodular deposit (*). Axial CE -CT(C) and coronal MPR (D). PC from ovarian carcinoma: Note the periportal deposit as a soft tissue mass around the left portal branch, more conspicuous on the MPR.
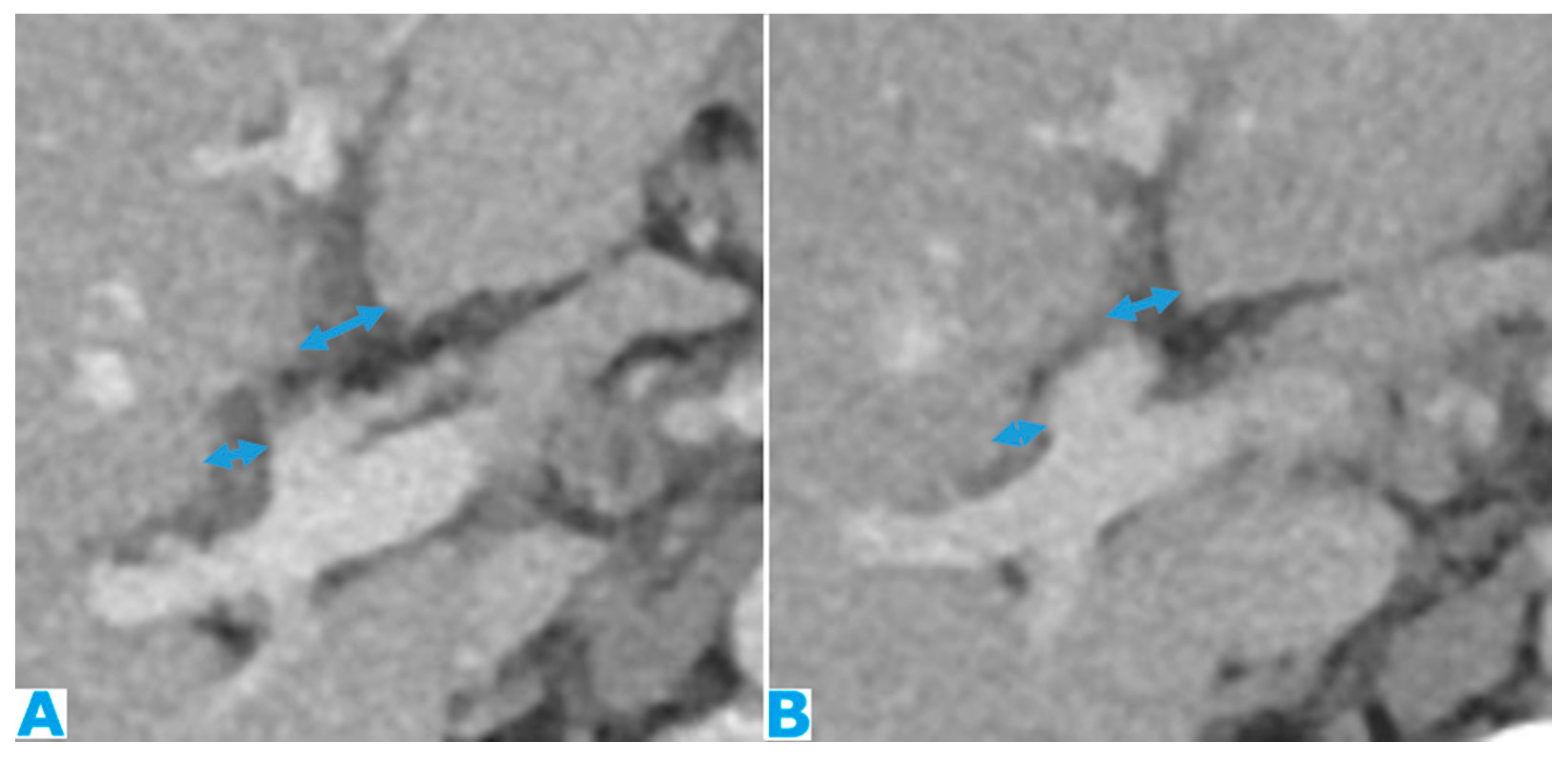
Figure 12.
Axial CE-CT (A, current study, and B, from one year prior). PC from gastric adenocarcinoma: Subtle soft tissue mass occupying the periportal space. Observe the periportal space enlargement, more conspicuous if compared to the previous CT.
Figure 12.
Axial CE-CT (A, current study, and B, from one year prior). PC from gastric adenocarcinoma: Subtle soft tissue mass occupying the periportal space. Observe the periportal space enlargement, more conspicuous if compared to the previous CT.
Figure 13.
Coronal T2WI (A), axial CE portal phase FST1WI (B). PC from colon adenocarcinoma: periportal deposit (*) that causes segmental intrahepatic biliary dilatation (arrowhead) and portal vein compression (arrow). The patient had a known portal hypertension due to massive hepatic metastases (curved arrow) and splenomegaly.
Figure 13.
Coronal T2WI (A), axial CE portal phase FST1WI (B). PC from colon adenocarcinoma: periportal deposit (*) that causes segmental intrahepatic biliary dilatation (arrowhead) and portal vein compression (arrow). The patient had a known portal hypertension due to massive hepatic metastases (curved arrow) and splenomegaly.
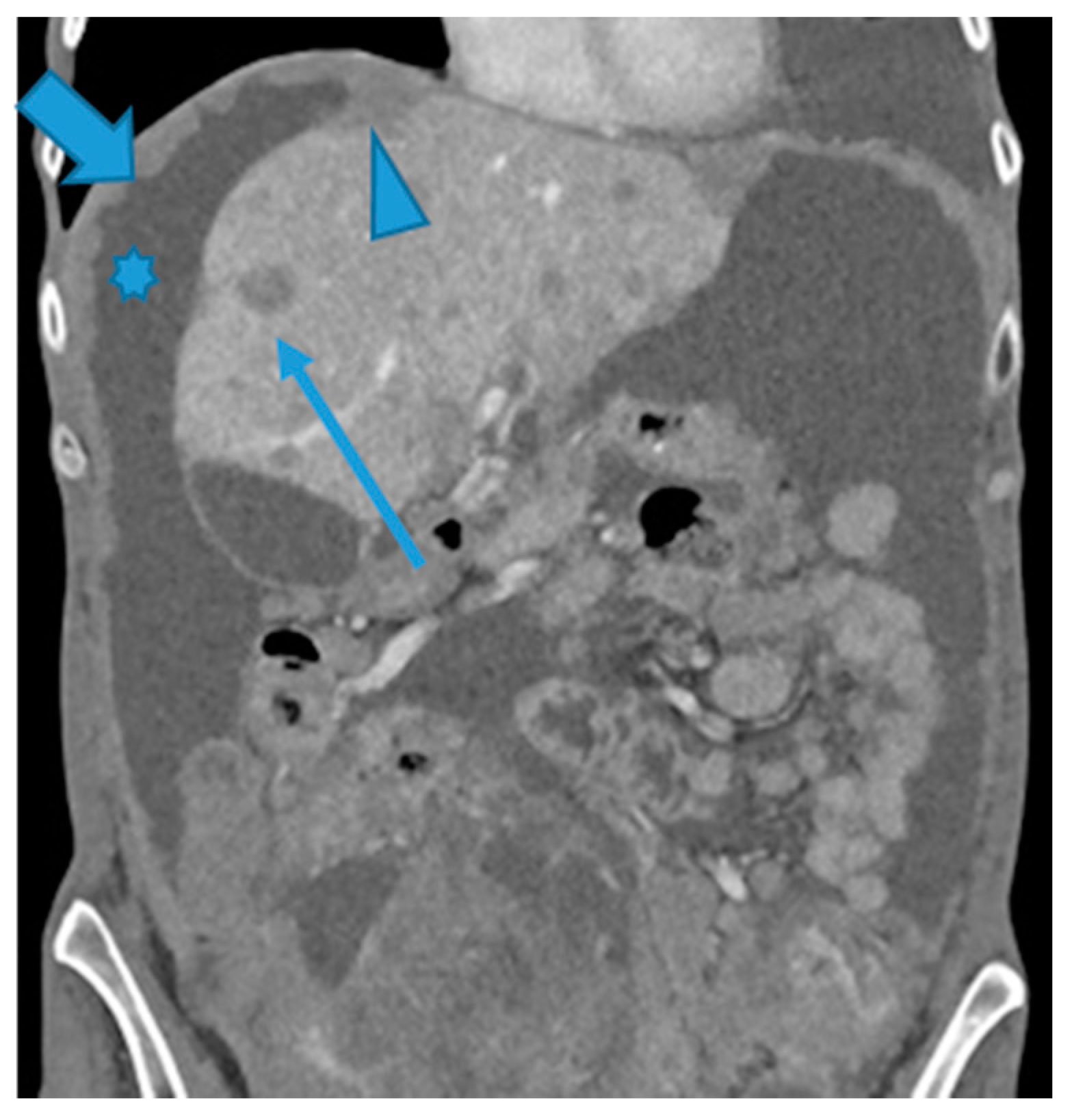
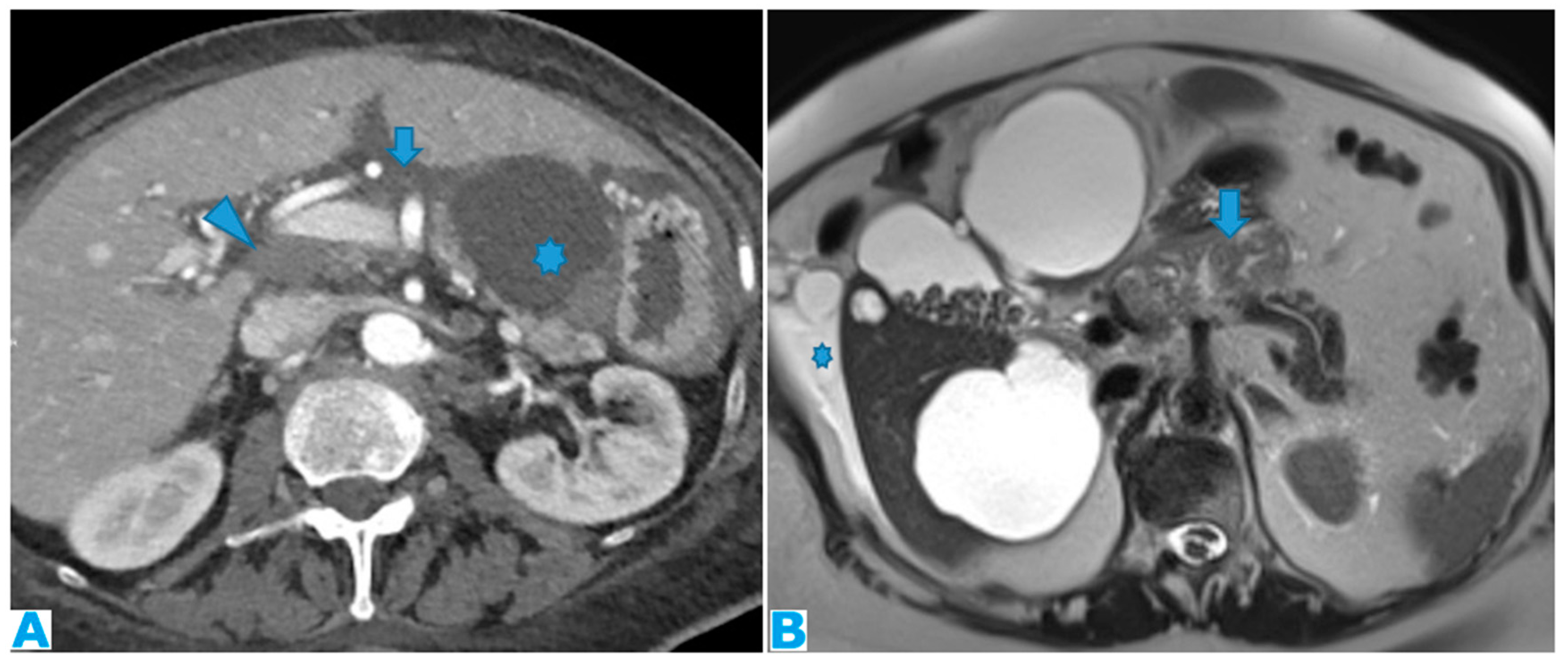
Figure 14.
Axial CE-CT (A). PC from ovarian carcinoma: deposits within the gastrohepatic (*) and the hepatoduodenal ligaments (arrowheads). Coronal T2WI (B). PC from ovarian carcinoma: deposits within the lesser omentum: Identify its two components, the gastrohepatic (*) and the hepatoduodenal (arrowhead) ligaments. Axial CE portal phase FST1W1 (C-D), the current study (C) and from one year prior (D), for comparison. PC from colon adenocarcinoma: Deposits within the gastrohepatic ligament (lesser omentum) (arrows). Note the compression of the portal vein (*) on C and the development of collateral vessels (arrowheads).
Figure 14.
Axial CE-CT (A). PC from ovarian carcinoma: deposits within the gastrohepatic (*) and the hepatoduodenal ligaments (arrowheads). Coronal T2WI (B). PC from ovarian carcinoma: deposits within the lesser omentum: Identify its two components, the gastrohepatic (*) and the hepatoduodenal (arrowhead) ligaments. Axial CE portal phase FST1W1 (C-D), the current study (C) and from one year prior (D), for comparison. PC from colon adenocarcinoma: Deposits within the gastrohepatic ligament (lesser omentum) (arrows). Note the compression of the portal vein (*) on C and the development of collateral vessels (arrowheads).
Figure 15.
Axial CE-CT (A). PC from ovarian carcinoma: mass-like deposit within the lesser sac (*). Also notice the seeding within the lesser omentum (arrow). Portacaval lymph node (arrowhead). Axial T2WI (B). PC from ovarian carcinoma: mass-like deposit within the lesser sac (arrow). Ascites (*).
Figure 15.
Axial CE-CT (A). PC from ovarian carcinoma: mass-like deposit within the lesser sac (*). Also notice the seeding within the lesser omentum (arrow). Portacaval lymph node (arrowhead). Axial T2WI (B). PC from ovarian carcinoma: mass-like deposit within the lesser sac (arrow). Ascites (*).
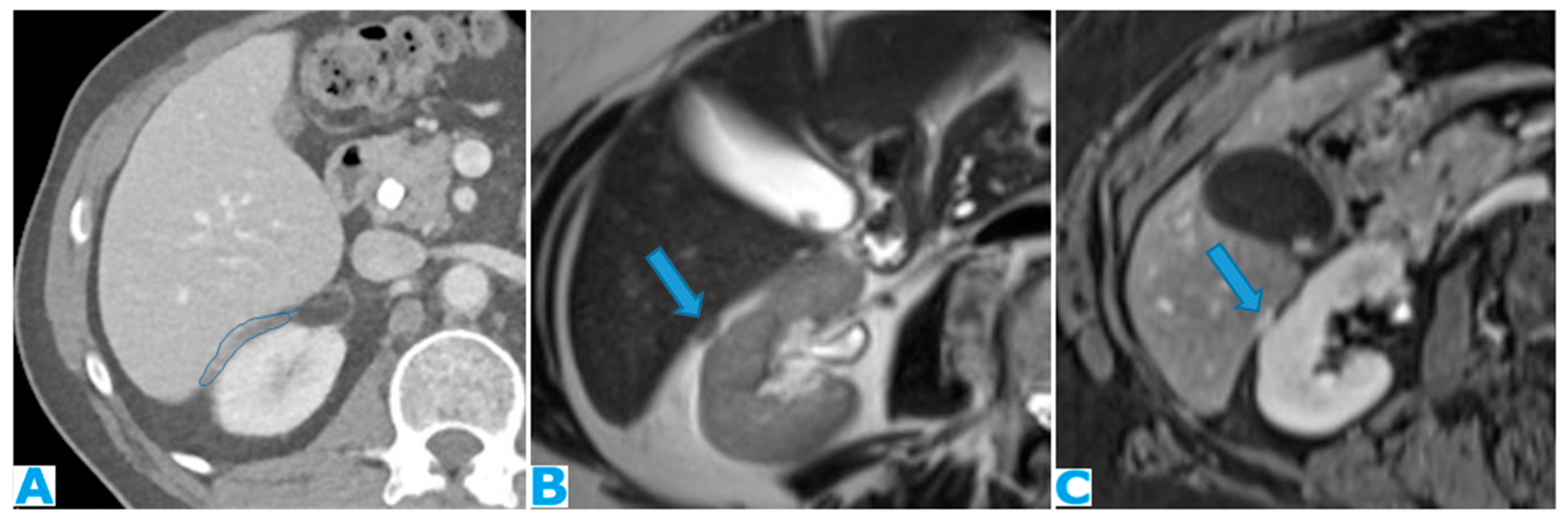
Figure 16.
Axial CE-CT (A): PC from breast carcinoma: deposits within the subhepatic space as reticulation of its fatty content. Axial T2WI CE-CT (B), CE portal phase FST1WI (C): PC from colon carcinoma: nodular deposit within the subhepatic space (arrow).
Figure 16.
Axial CE-CT (A): PC from breast carcinoma: deposits within the subhepatic space as reticulation of its fatty content. Axial T2WI CE-CT (B), CE portal phase FST1WI (C): PC from colon carcinoma: nodular deposit within the subhepatic space (arrow).
Figure 17.
Axial CE-CT (A): from one year prior (disease-free peritoneum), B from four months prior, C current follow up. Note the early stages and evolution of omental deposits (arrows).
Figure 17.
Axial CE-CT (A): from one year prior (disease-free peritoneum), B from four months prior, C current follow up. Note the early stages and evolution of omental deposits (arrows).
Figure 18.
Axial CE portal phase FST1W. PC from melanoma: Omental cake (arrow) replacing the omental fat.
Figure 18.
Axial CE portal phase FST1W. PC from melanoma: Omental cake (arrow) replacing the omental fat.
Figure 19.
Axial CE-CT (A from one-year prior, B current follow up). PC from breast carcinoma: Note the omental infiltration on B associated with a posterior displacement of the small bowel loops. Axial T2WI (C peritoneal-disease-free study from two years prior, D current follow up). PC from renal cell carcinoma: Enlargement of the fatty content and mass effect due to omental seeding (arrow) that may be more conspicuous than the actual deposits.
Figure 19.
Axial CE-CT (A from one-year prior, B current follow up). PC from breast carcinoma: Note the omental infiltration on B associated with a posterior displacement of the small bowel loops. Axial T2WI (C peritoneal-disease-free study from two years prior, D current follow up). PC from renal cell carcinoma: Enlargement of the fatty content and mass effect due to omental seeding (arrow) that may be more conspicuous than the actual deposits.
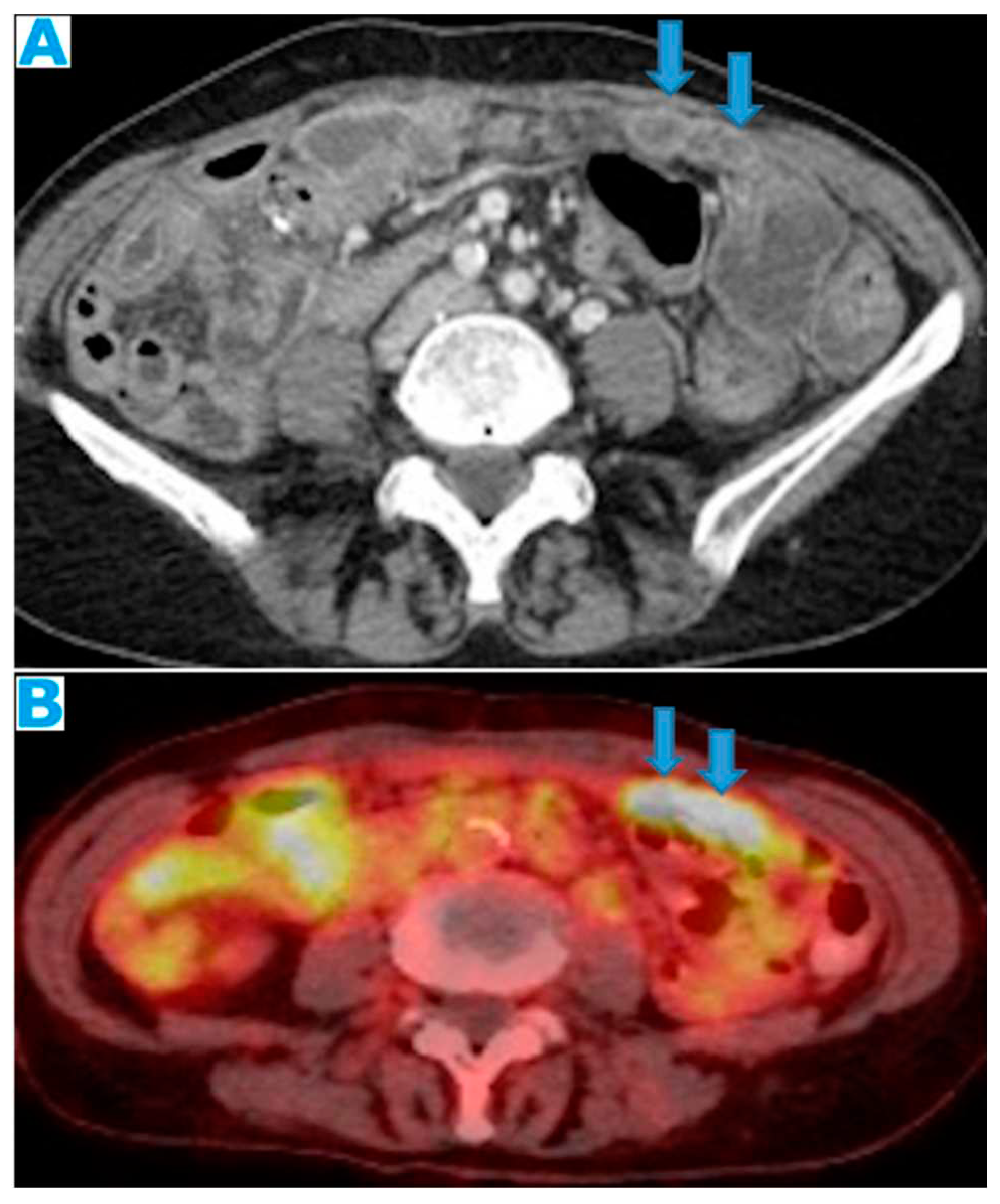
Figure 20.
Axial CE CT (A), FDG PET CT (B). PC from colon adenocarcinoma: Nodular deposits within the omentum that were originally mistaken for SB loops on CT, due to scarce intrabdominal fat.
Figure 20.
Axial CE CT (A), FDG PET CT (B). PC from colon adenocarcinoma: Nodular deposits within the omentum that were originally mistaken for SB loops on CT, due to scarce intrabdominal fat.
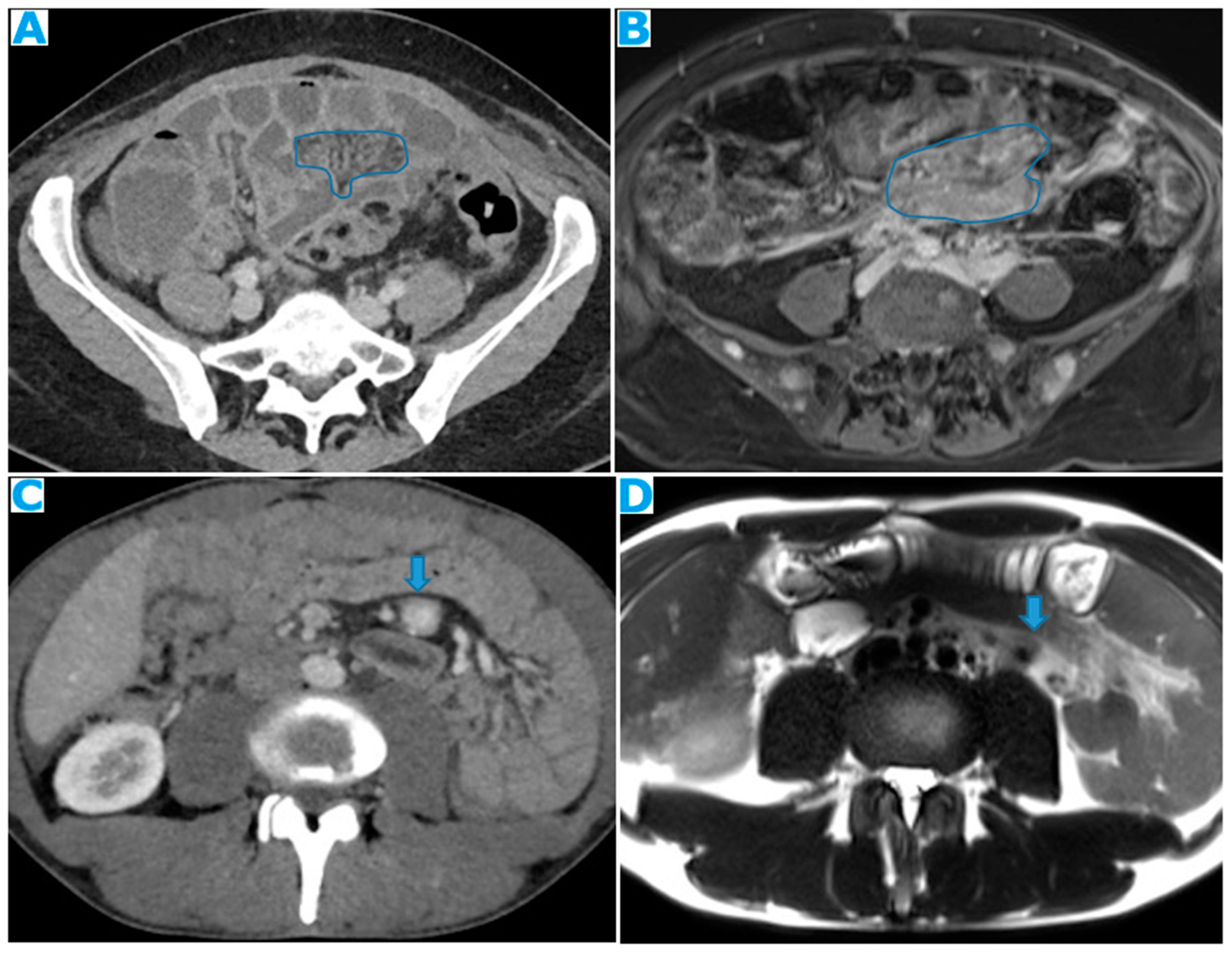
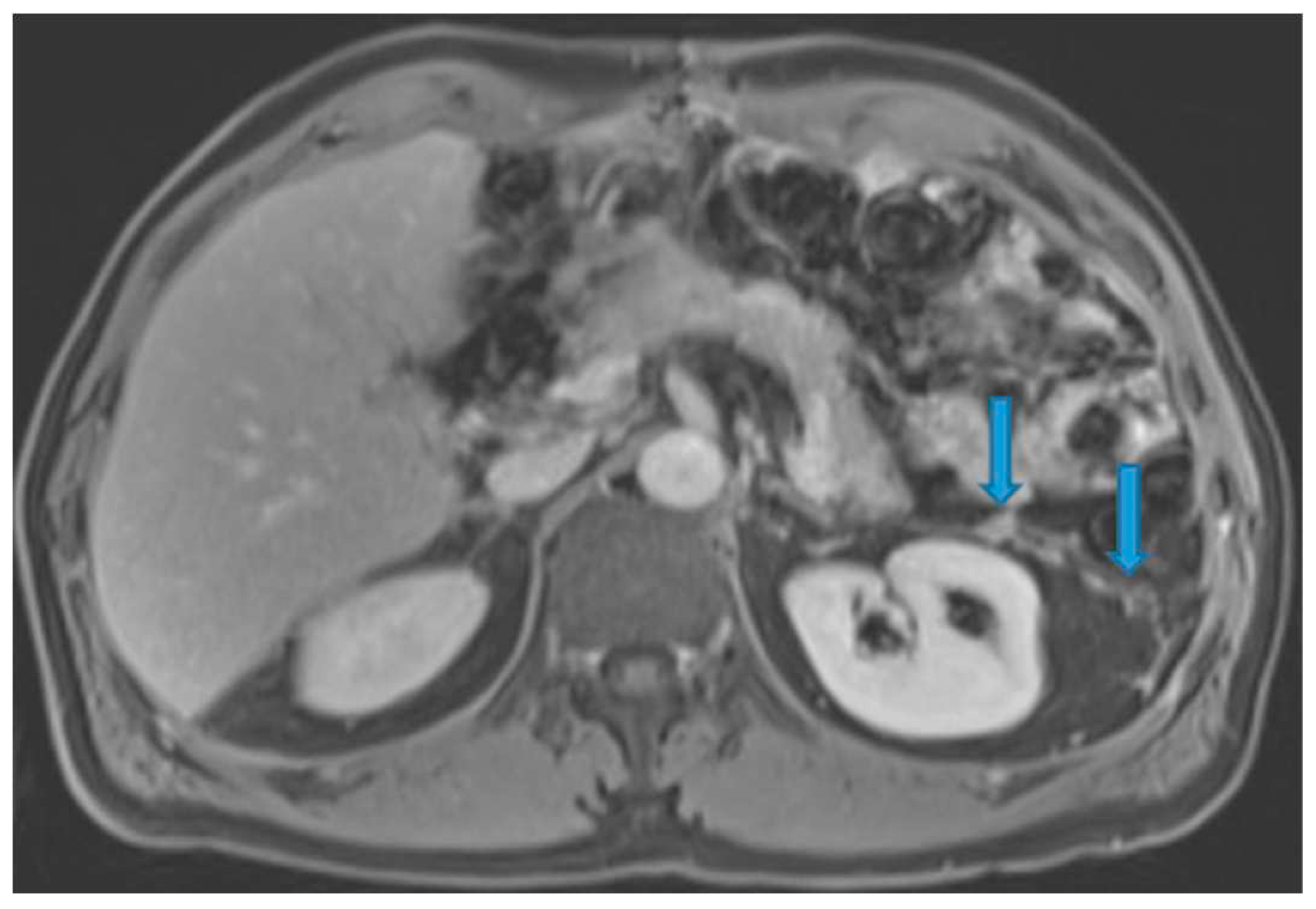
Figure 21.
Axial CE-CT. PC from colon adenocarcinoma: Nodular deposit within the mesentery.
Figure 21.
Axial CE-CT. PC from colon adenocarcinoma: Nodular deposit within the mesentery.
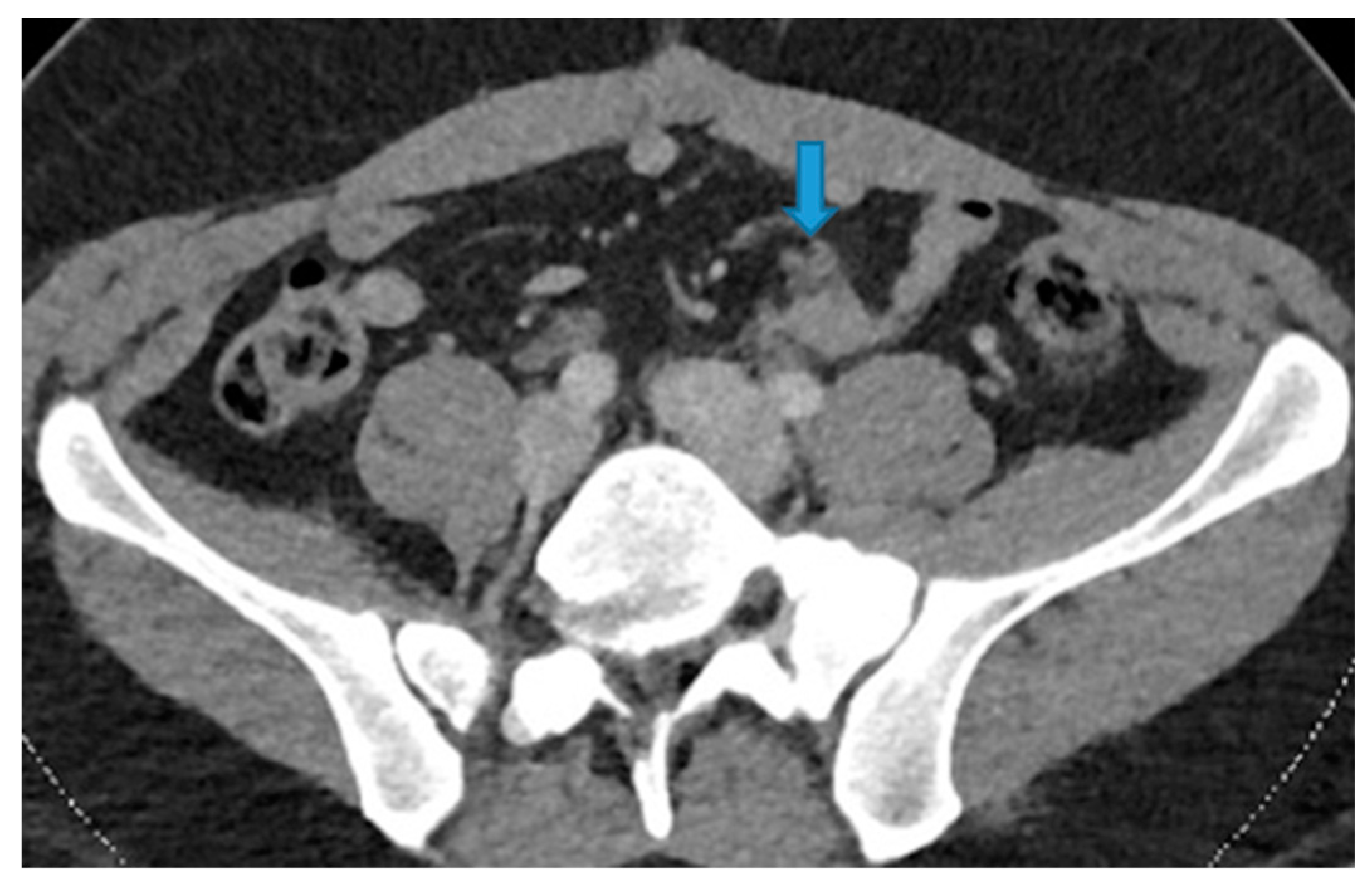
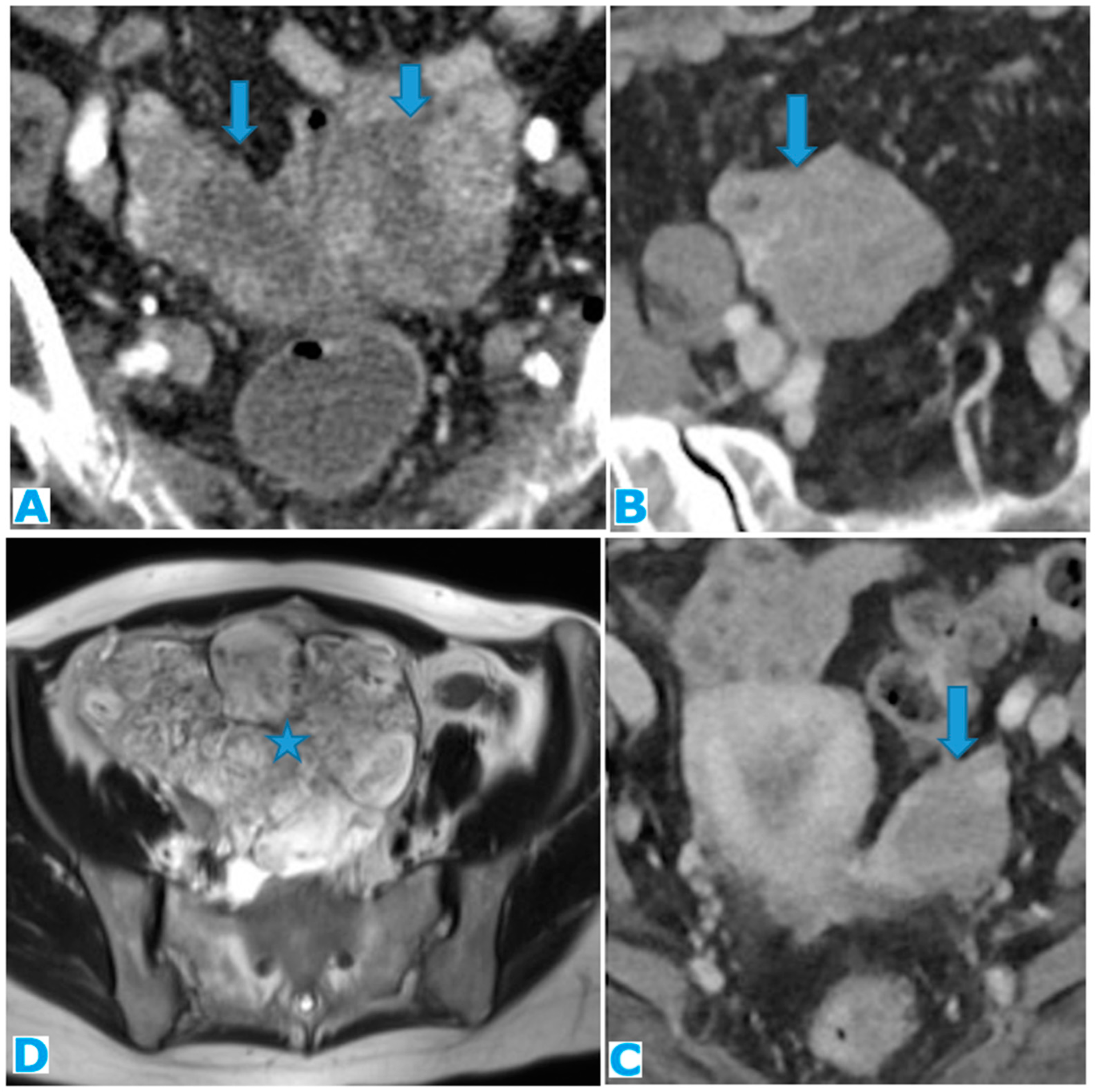
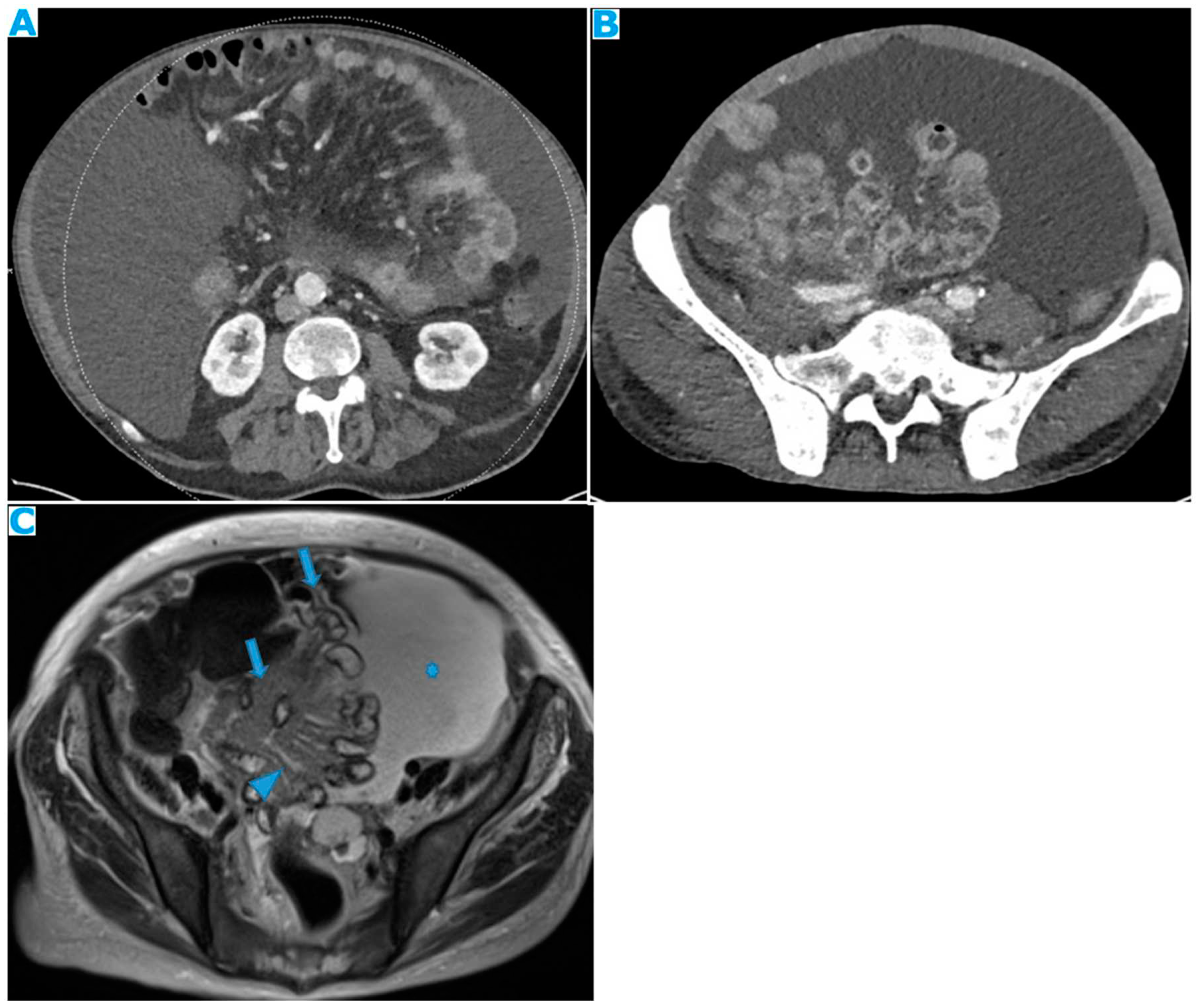
Figure 27.
Axial T2WI (A). PC from mucinous adenocarcinoma of the uracus: Notice the hyperintense mesenteric deposits (*) (signal due to mucin content) and how the SB loops appear separated and angulated (arrow). Axial T2WI (B), axial CE portal phase FST1WI (C). PC from breast carcinoma: Mesenteric deposits (arrowheads on B) make SB loops appear thickened and separated. Notice how deposits are more conspicuous on T2WI due to its high tissue contrast. Axial CE-CT (D). PC from ovarian carcinoma: Clustered small bowel loops as the end point of mesenteric seeding.
Figure 27.
Axial T2WI (A). PC from mucinous adenocarcinoma of the uracus: Notice the hyperintense mesenteric deposits (*) (signal due to mucin content) and how the SB loops appear separated and angulated (arrow). Axial T2WI (B), axial CE portal phase FST1WI (C). PC from breast carcinoma: Mesenteric deposits (arrowheads on B) make SB loops appear thickened and separated. Notice how deposits are more conspicuous on T2WI due to its high tissue contrast. Axial CE-CT (D). PC from ovarian carcinoma: Clustered small bowel loops as the end point of mesenteric seeding.
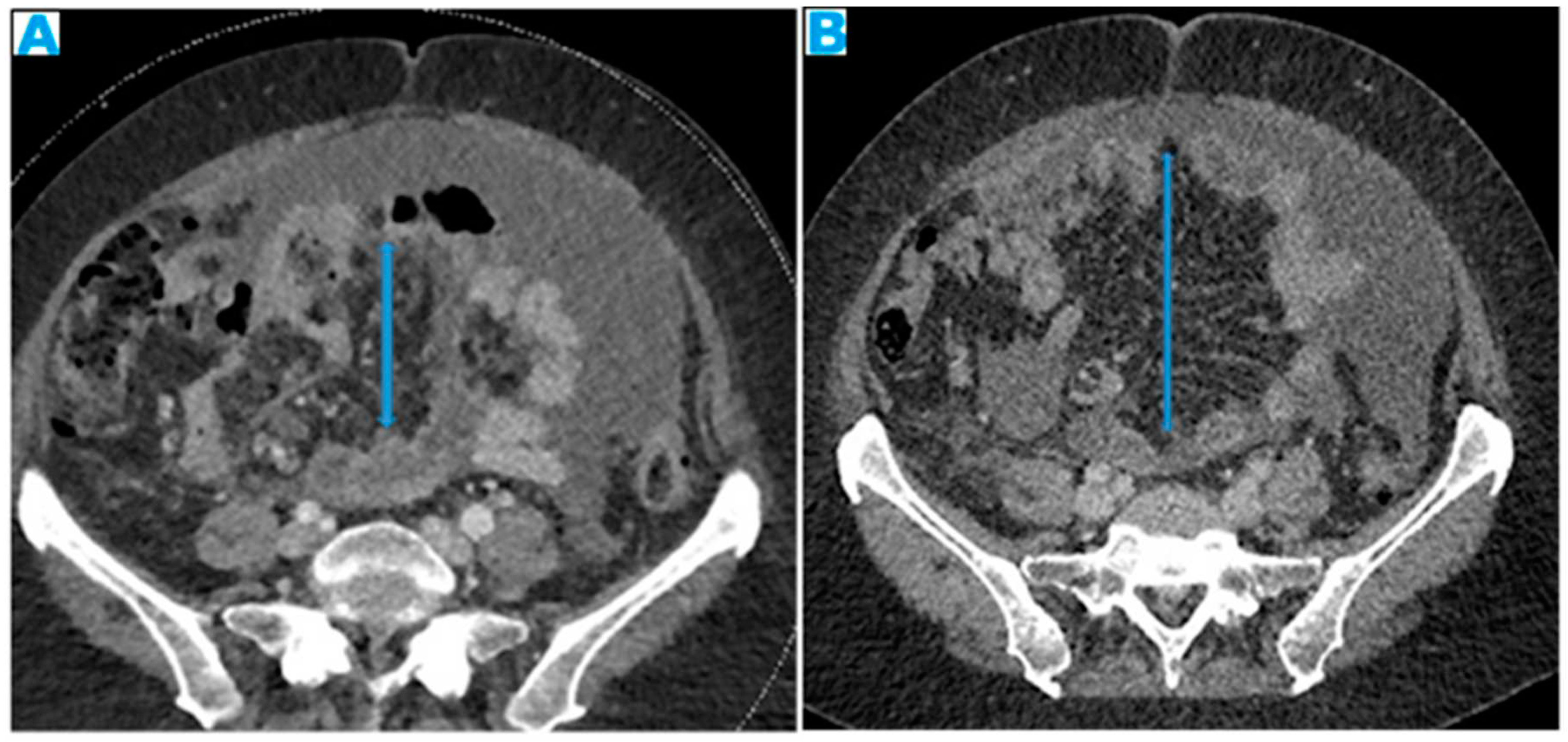
Figure 28.
Axial CE-CT, current study (A) and from six months prior (B). PC from colon carcinoma: The mesentery becomes fibrotic as the seeding evolves and, secondary to the retraction effect, a decrease in size of the mesenteric fat will occur (arrows). This effect may be more conspicuous than the actual deposits.
Figure 28.
Axial CE-CT, current study (A) and from six months prior (B). PC from colon carcinoma: The mesentery becomes fibrotic as the seeding evolves and, secondary to the retraction effect, a decrease in size of the mesenteric fat will occur (arrows). This effect may be more conspicuous than the actual deposits.
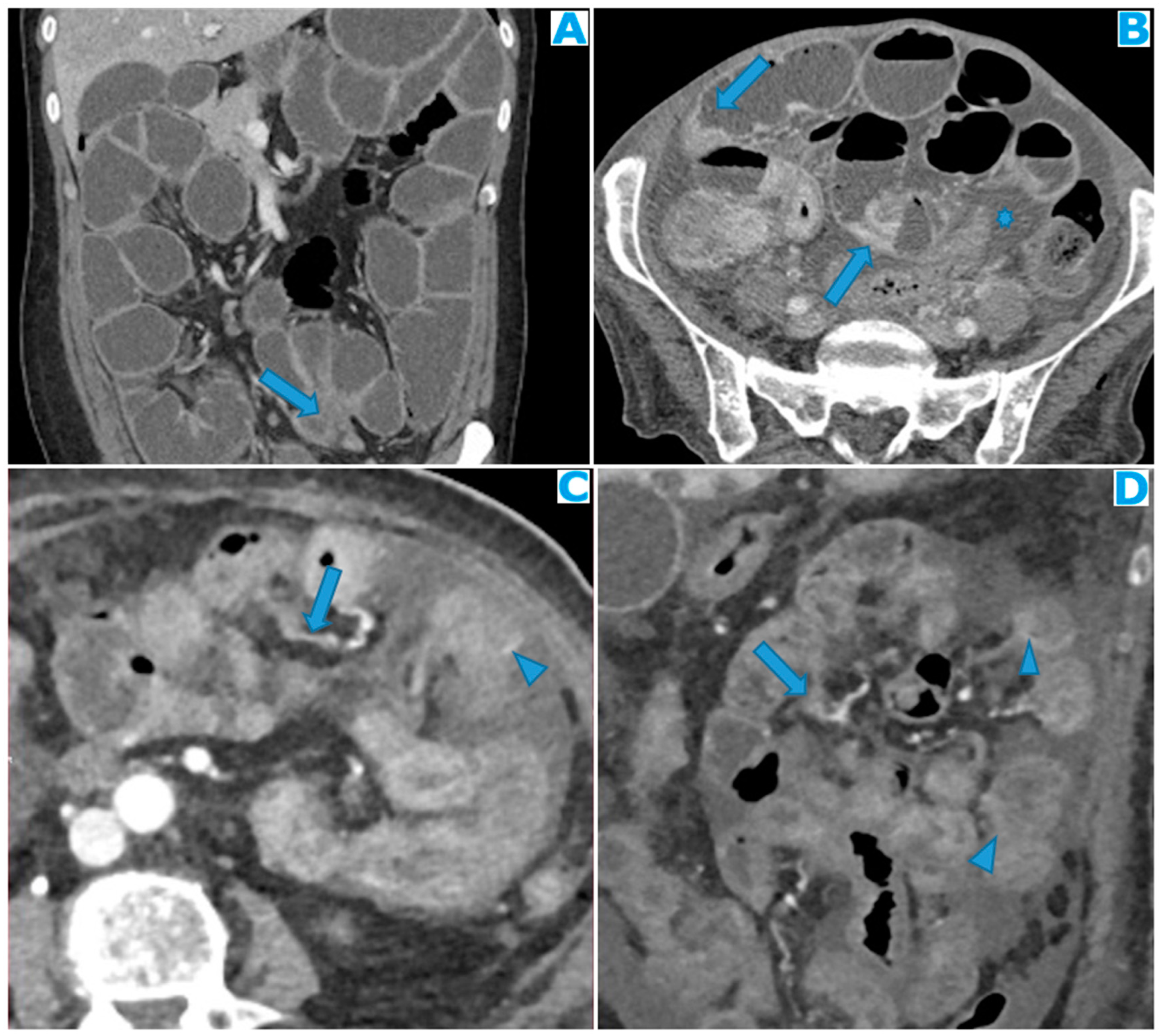
Figure 29.
Coronal MPR (A). PC from mucinous colon adenocarcinoma: Solitary mesenteric deposit (arrow) as the cause of a SB obstruction, involving several loops. Axial CE-CT (B). PC from breast carcinoma: SB multifocal obstruction, due to several deposits within the SB serosa (arrows). Ascites (*). Axial CE-CT (C) and coronal MPR (D). PC from adenocarcinoma of the urachus: Severe mesenteric infiltration causing SB ischaemia as distal SMA and SMV branches are compressed and infiltrated by deposits (arrows). Observe the SB loops thickening and the heterogenous and patchy bowel wall enhancement (arrowheads) due to the ischaemia.
Figure 29.
Coronal MPR (A). PC from mucinous colon adenocarcinoma: Solitary mesenteric deposit (arrow) as the cause of a SB obstruction, involving several loops. Axial CE-CT (B). PC from breast carcinoma: SB multifocal obstruction, due to several deposits within the SB serosa (arrows). Ascites (*). Axial CE-CT (C) and coronal MPR (D). PC from adenocarcinoma of the urachus: Severe mesenteric infiltration causing SB ischaemia as distal SMA and SMV branches are compressed and infiltrated by deposits (arrows). Observe the SB loops thickening and the heterogenous and patchy bowel wall enhancement (arrowheads) due to the ischaemia.
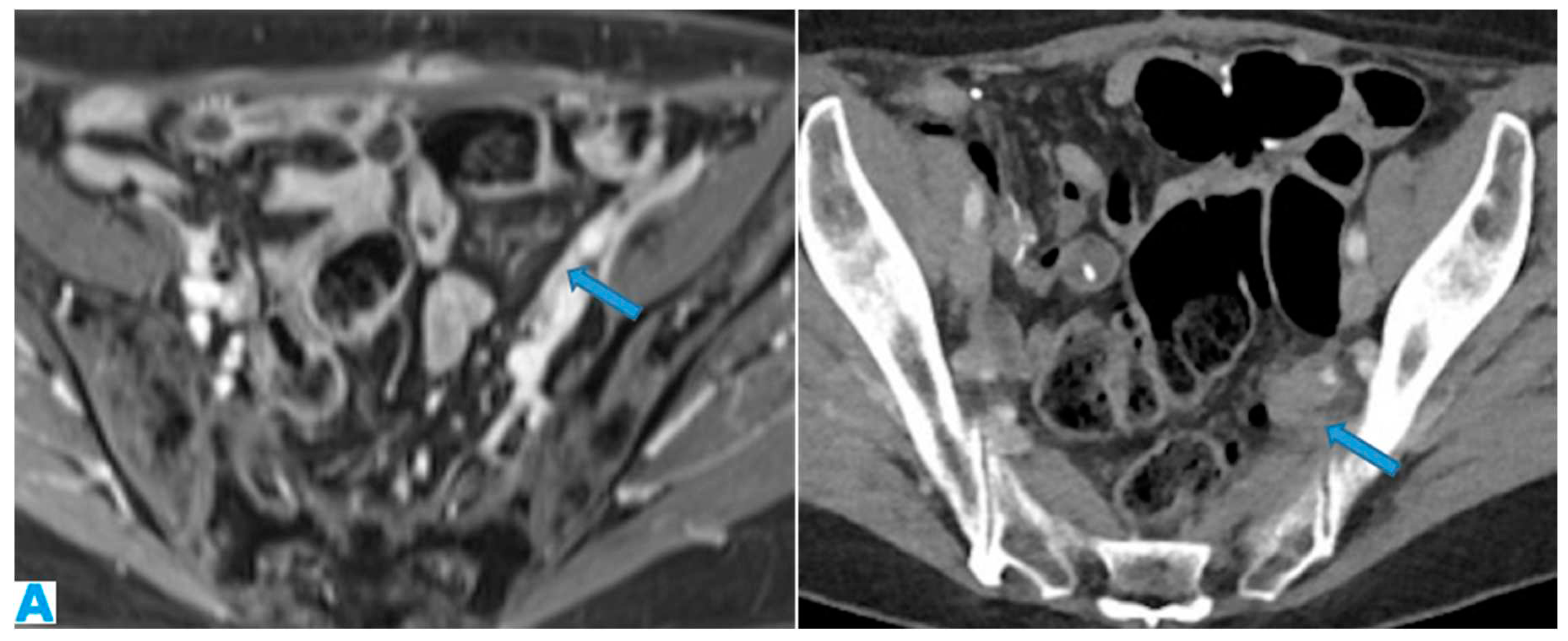
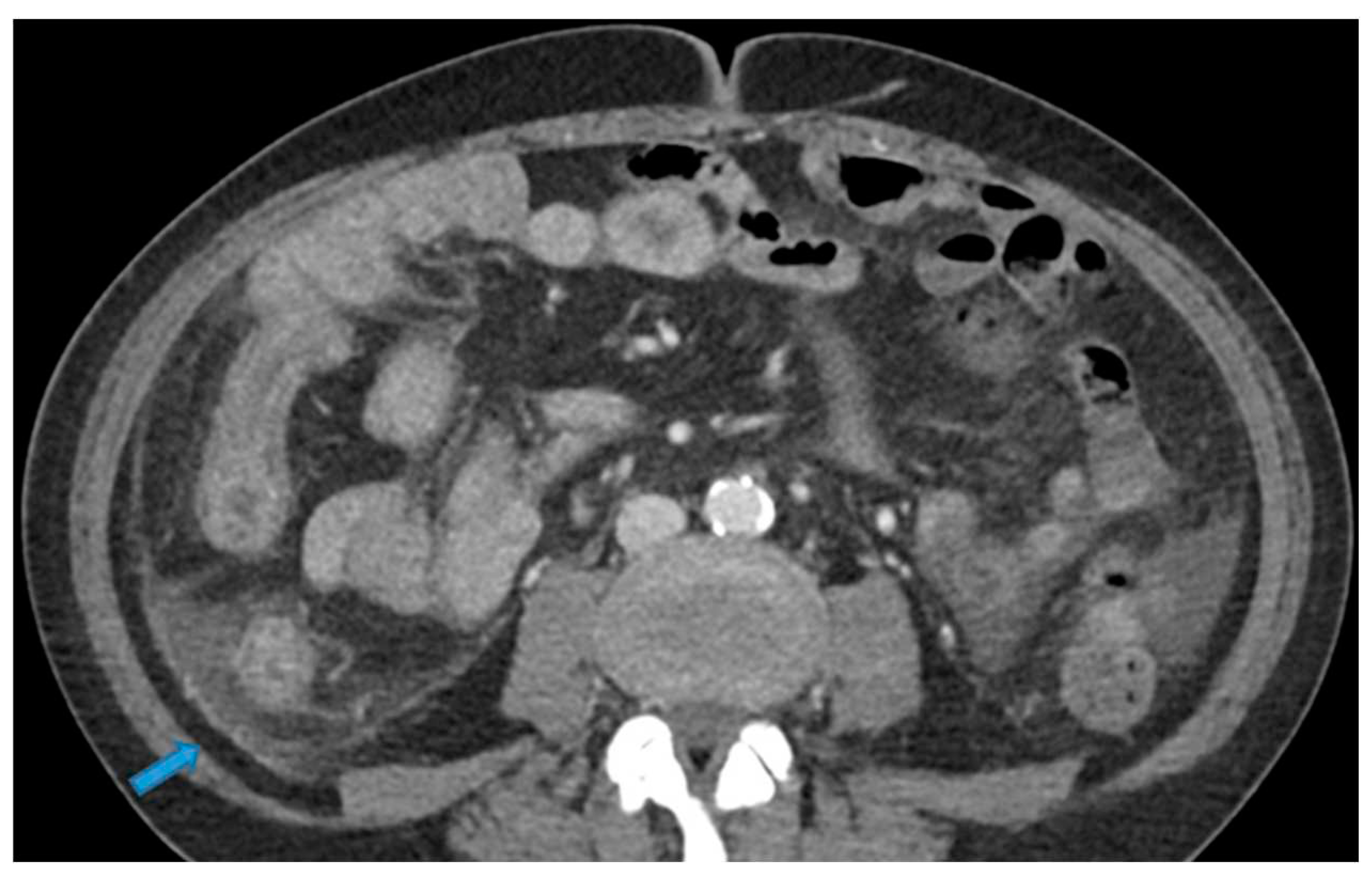
Figure 30.
CE portal phase FST1WI (A, from four months prior, B, current study). PC from colon adenocarcinoma: Nodular deposit within the left paracolic gutter that was undercalled. See the growth on B.
Figure 30.
CE portal phase FST1WI (A, from four months prior, B, current study). PC from colon adenocarcinoma: Nodular deposit within the left paracolic gutter that was undercalled. See the growth on B.
Figure 31.
Axial T2WI (A). PC from mucinous adenocarcinoma of the terminal ileum: Hyperintense nodular deposits (due to mucin-content) within the sigmoid mesocolon. Axial CE-CT (B). PC from undifferentiated carcinoma of unknown origin: Deposits within both the sigmoid mesocolon (arrows), outlining the epiploic appendices, and within the sigmoid serosa, making the sigmoid colon appear thickened (arrowheads). Axial CE-CT (C, D). PC from colon adenocarcinoma: A. Observe on C the seeding involving the sigmoid mesocolon (arrowhead) and the serous layer of the bowel (arrow), partially obstructing the sigmoid lumen. On B the two components cannot be differentiated but a luminal stenosis is clearly identified (arrow).
Figure 31.
Axial T2WI (A). PC from mucinous adenocarcinoma of the terminal ileum: Hyperintense nodular deposits (due to mucin-content) within the sigmoid mesocolon. Axial CE-CT (B). PC from undifferentiated carcinoma of unknown origin: Deposits within both the sigmoid mesocolon (arrows), outlining the epiploic appendices, and within the sigmoid serosa, making the sigmoid colon appear thickened (arrowheads). Axial CE-CT (C, D). PC from colon adenocarcinoma: A. Observe on C the seeding involving the sigmoid mesocolon (arrowhead) and the serous layer of the bowel (arrow), partially obstructing the sigmoid lumen. On B the two components cannot be differentiated but a luminal stenosis is clearly identified (arrow).
Figure 32.
CE-CT Coronal MPR (A). PC from colon carcinoma: Observe how the parietal peritoneum does not reach the pelvis floor. Ascites (*). Deposits within the pelvic reflexion (arrow). Sagittal MPR (B), axial CE-CT (C). PC from adenocarcinoma of the appendix: Irregularly thickened peritoneal reflexion due to seeding. Observe on the sagittal MPR how the peritoneal reflexion covers the dome of the urinary bladder and then descends, following its posterior wall.
Figure 32.
CE-CT Coronal MPR (A). PC from colon carcinoma: Observe how the parietal peritoneum does not reach the pelvis floor. Ascites (*). Deposits within the pelvic reflexion (arrow). Sagittal MPR (B), axial CE-CT (C). PC from adenocarcinoma of the appendix: Irregularly thickened peritoneal reflexion due to seeding. Observe on the sagittal MPR how the peritoneal reflexion covers the dome of the urinary bladder and then descends, following its posterior wall.
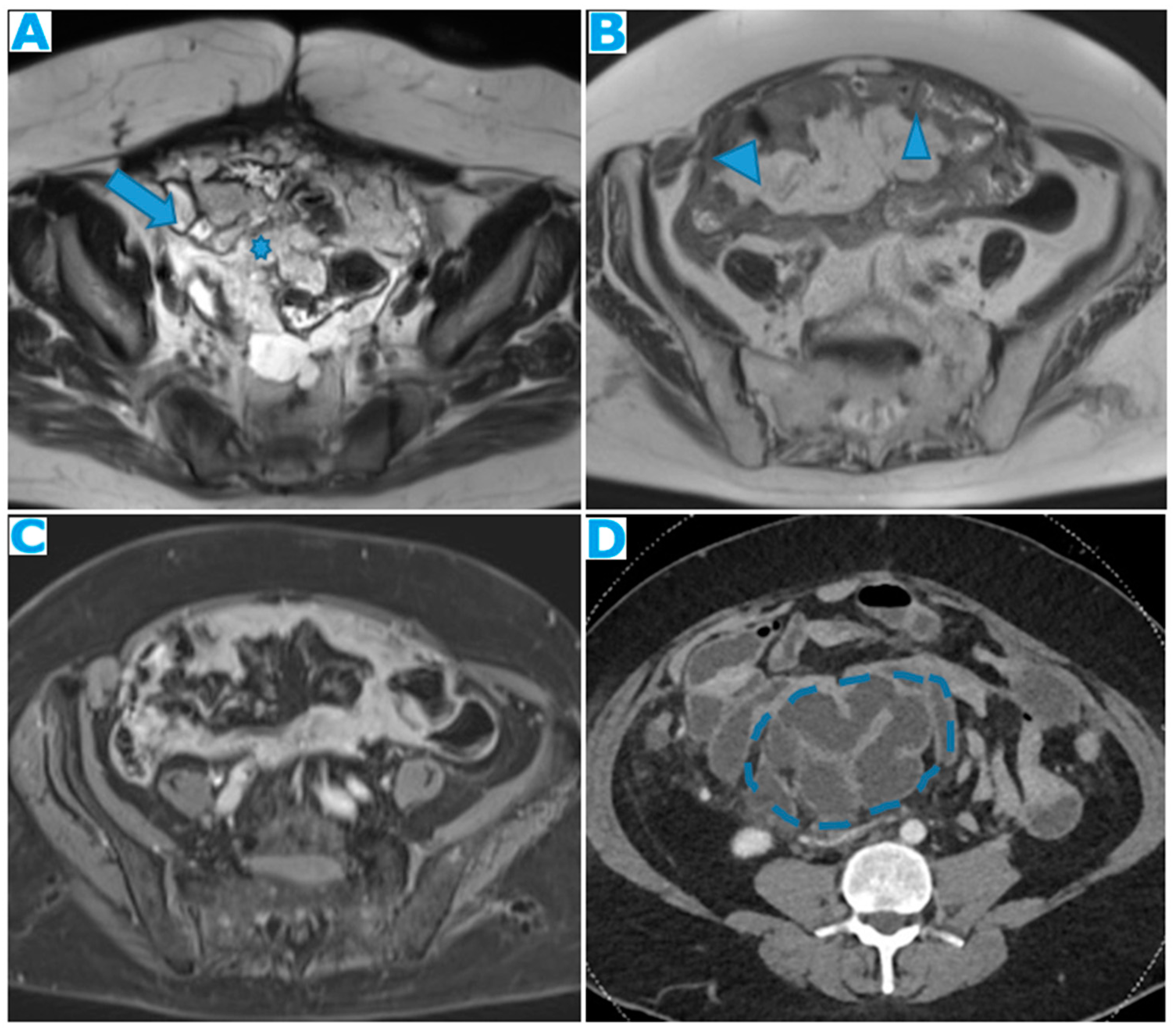
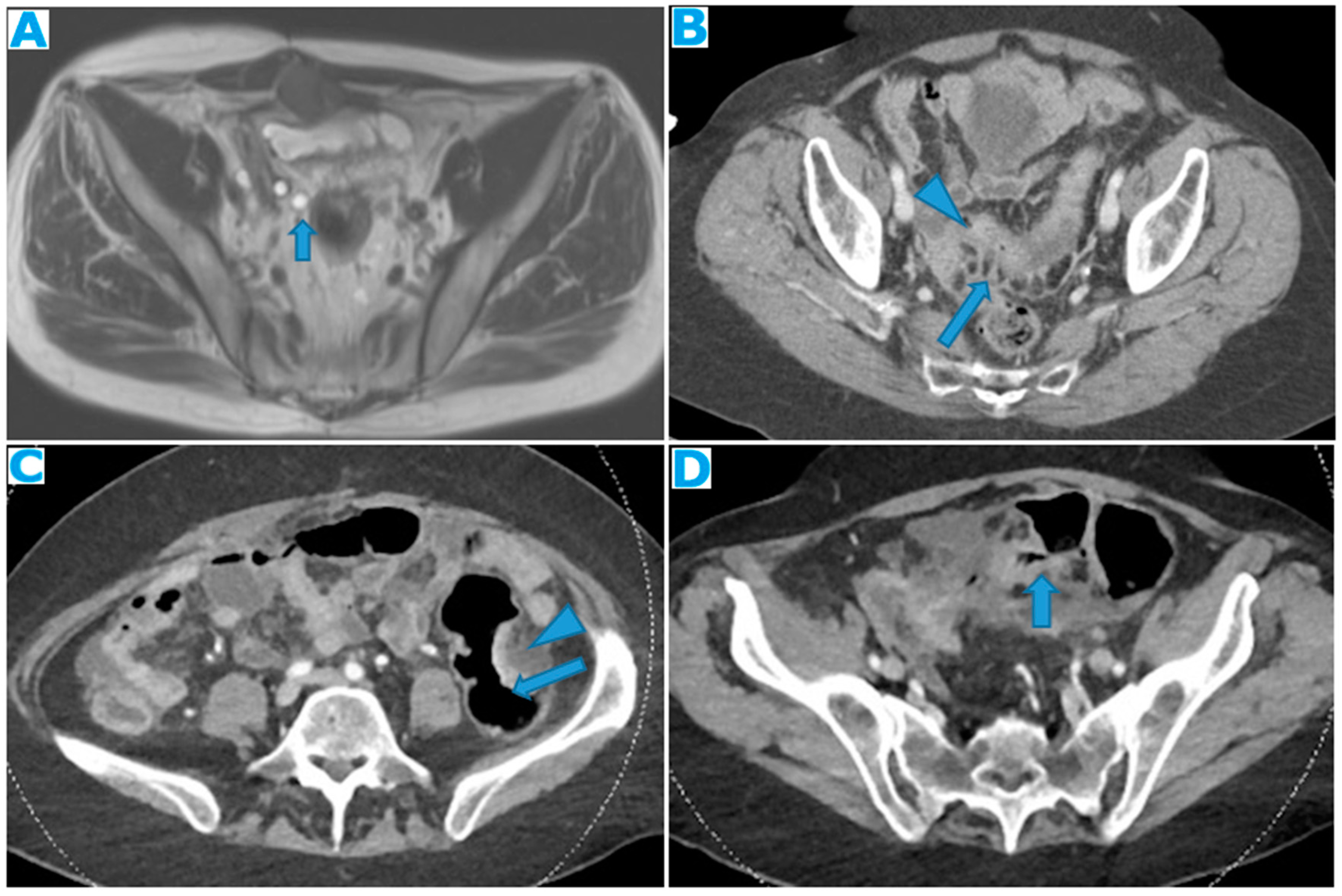
Figure 33.
Axial CE portal phase FST1WI (A). PC from duodenal adenocarcinoma: Deposit seeding within the peritoneum that covers the left pelvic wall, which looks diffusely thickened (arrow). Axial CE-CT (B). PC from undifferentiated caecal adenocarcinoma: Nodular deposit within the peritoneum that covers the left pelvic wall (arrow).
Figure 33.
Axial CE portal phase FST1WI (A). PC from duodenal adenocarcinoma: Deposit seeding within the peritoneum that covers the left pelvic wall, which looks diffusely thickened (arrow). Axial CE-CT (B). PC from undifferentiated caecal adenocarcinoma: Nodular deposit within the peritoneum that covers the left pelvic wall (arrow).
Figure 34.
Axial CE-CT (A), CE-CT coronal MPR (B). PC from breast carcinoma: The peritoneal reflexion covers the uterine fundus and body and the posterior part of the vagina and extends laterally (broad ligament, arrow on B). Note the nodular appearance of the peritonealised uterine surface and the broad ligaments (arrow on B) due to deposit seeding. CE-CT coronal MPR (C). PC from cardia adenocarcinoma: Deposit within the peritoneal reflexion covering the vesical dome (arrow). Axial CE-CT (D). PC from breast carcinoma: Notice the peritoneal seeding on the peritonealised surfaces of the bladder (arrowhead), and rectum (vertical arrow). Also, within the peritoneum that covers the pelvic walls (horizontal arrow). Ascites (*).
Figure 34.
Axial CE-CT (A), CE-CT coronal MPR (B). PC from breast carcinoma: The peritoneal reflexion covers the uterine fundus and body and the posterior part of the vagina and extends laterally (broad ligament, arrow on B). Note the nodular appearance of the peritonealised uterine surface and the broad ligaments (arrow on B) due to deposit seeding. CE-CT coronal MPR (C). PC from cardia adenocarcinoma: Deposit within the peritoneal reflexion covering the vesical dome (arrow). Axial CE-CT (D). PC from breast carcinoma: Notice the peritoneal seeding on the peritonealised surfaces of the bladder (arrowhead), and rectum (vertical arrow). Also, within the peritoneum that covers the pelvic walls (horizontal arrow). Ascites (*).
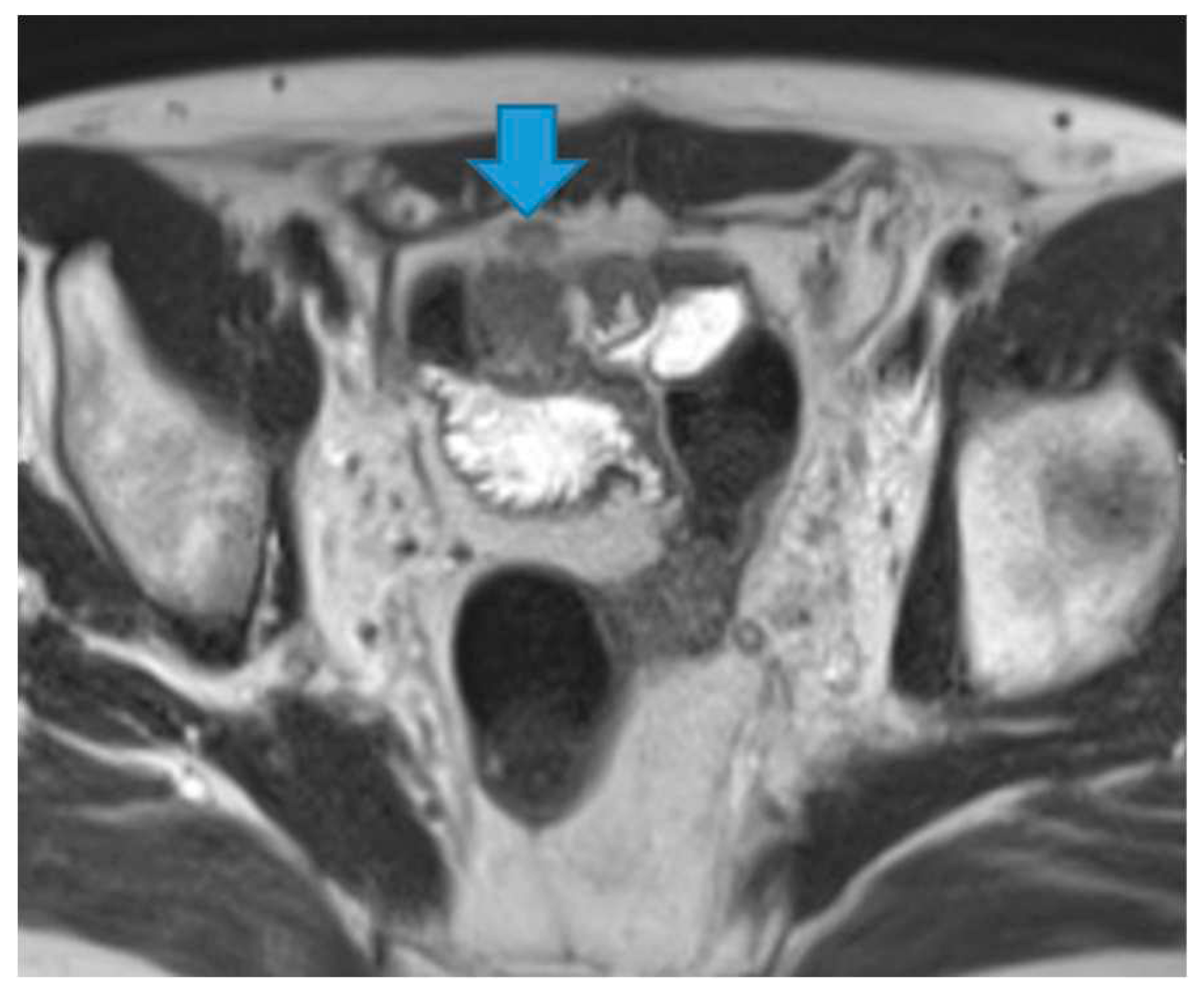
Figure 35.
Axial CE-CT (A, B, C). Axial T2WI (D and E), DWI (F). PC from mucinous adenocarcinoma of the appendix: Notice the peritoneal deposit within the left lateral pelvis (arrow on A) as an elongated soft tissue mass. The patient presented with a left uretero-hydronephrosis (* on B) due to the pelvic deposit which obstructed the ureter (arrowhead on C). Paravesical spaces are peritoneal recesses that cover on each side the distal ureter, the seminal vesicle, and the deferent duct. Note the deposit within the left paravesical space and how it obstructs the left ureter (arrow on C). The deposit also follows the course of the left deferent duct (arrow on E and F).
Figure 35.
Axial CE-CT (A, B, C). Axial T2WI (D and E), DWI (F). PC from mucinous adenocarcinoma of the appendix: Notice the peritoneal deposit within the left lateral pelvis (arrow on A) as an elongated soft tissue mass. The patient presented with a left uretero-hydronephrosis (* on B) due to the pelvic deposit which obstructed the ureter (arrowhead on C). Paravesical spaces are peritoneal recesses that cover on each side the distal ureter, the seminal vesicle, and the deferent duct. Note the deposit within the left paravesical space and how it obstructs the left ureter (arrow on C). The deposit also follows the course of the left deferent duct (arrow on E and F).
Figure 36.
Axial CE-CT (A). PC from sigmoid adenocarcinoma: Bilateral ovarian metastasis as complex cystic masses with solid poles. Axial CE-CT (B, D). PC from colon adenocarcinoma: Bilateral ovarian metastasis as solid masses. Axial T2WI (C). PC from mucinous tumour of the appendix: Left ovarian metastases (*) presenting as a predominantly hyperintense mass due to the mucin content.
Figure 36.
Axial CE-CT (A). PC from sigmoid adenocarcinoma: Bilateral ovarian metastasis as complex cystic masses with solid poles. Axial CE-CT (B, D). PC from colon adenocarcinoma: Bilateral ovarian metastasis as solid masses. Axial T2WI (C). PC from mucinous tumour of the appendix: Left ovarian metastases (*) presenting as a predominantly hyperintense mass due to the mucin content.
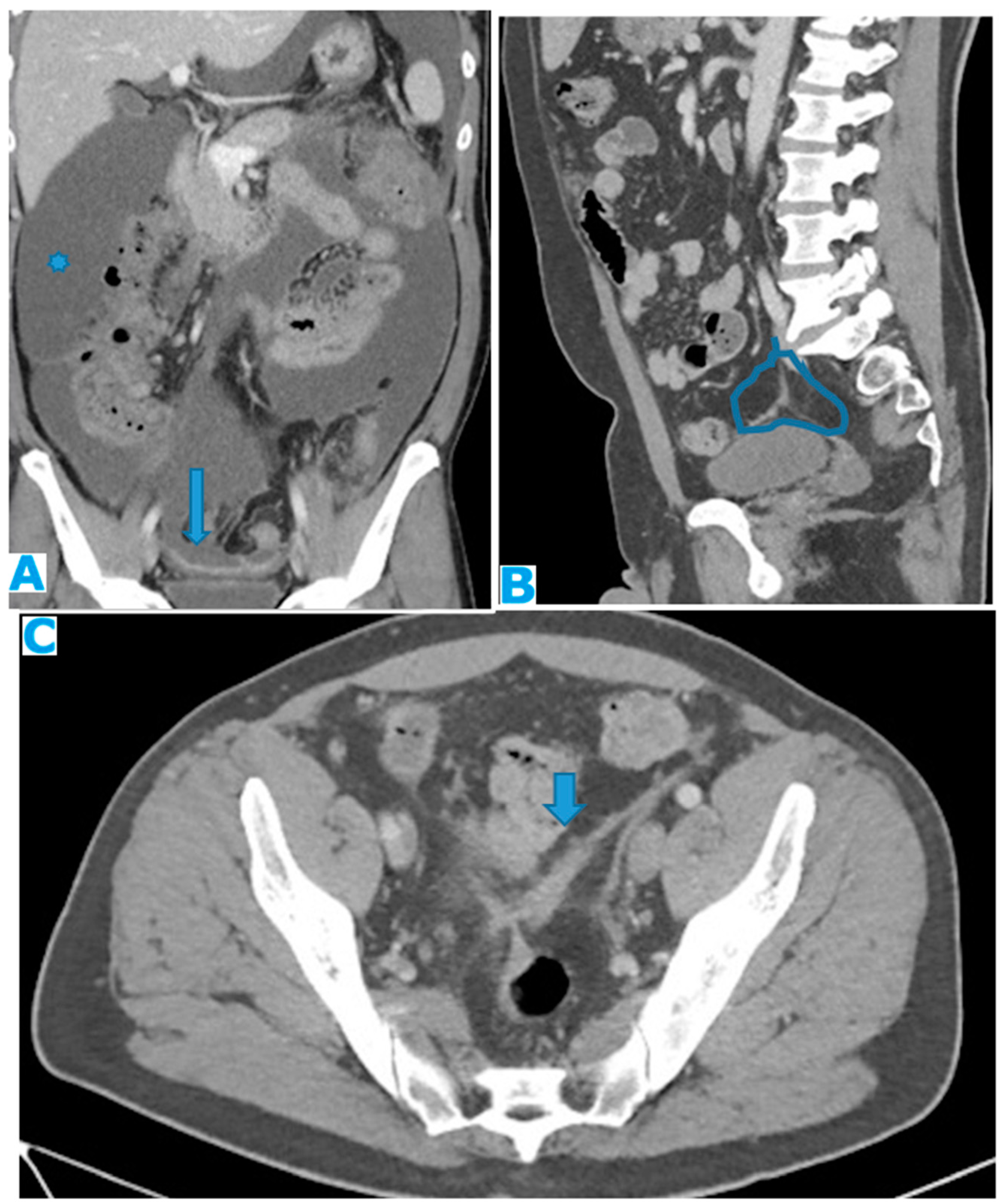
Figure 37.
Axial CE-CT (A, B). PC from ovarian carcinoma: Minimal ascites within the subhepatic space (arrow on A) and between the SB loops, triangle shaped (B).
Figure 37.
Axial CE-CT (A, B). PC from ovarian carcinoma: Minimal ascites within the subhepatic space (arrow on A) and between the SB loops, triangle shaped (B).
Figure 38.
Axial T2WI (A). PC from ovarian adenocarcinoma : Loculated ascites within the mesentery (*). Axial CE-CT (B). PC from breast carcinoma: Concomitant ascites within greater (arrow) and lesser (*) sacs.
Figure 38.
Axial T2WI (A). PC from ovarian adenocarcinoma : Loculated ascites within the mesentery (*). Axial CE-CT (B). PC from breast carcinoma: Concomitant ascites within greater (arrow) and lesser (*) sacs.
Figure 39.
Axial CE-CT (A, B). A : non-malignant ascites, B : malignant ascites from colon adenocarcinoma. Observe how SB loops float freely on A, with an anterior disposition. On a malignant ascites (B), SB loops are drawn posteriorly and lose contact with the anterior abdominal wall (tethered-bowel sign) due to the rigid infiltrated mesenteric leaves. Axial T2WI (C). PC from endometrial carcinoma: Diffuse mesenteric deposit seeding (arrow) which causes SB retraction (tethered-bowel sign). Notice that despite massive ascites (*), there is scarce quantity of liquid between the rigid infiltrated mesenteric leaves (arrowhead).
Figure 39.
Axial CE-CT (A, B). A : non-malignant ascites, B : malignant ascites from colon adenocarcinoma. Observe how SB loops float freely on A, with an anterior disposition. On a malignant ascites (B), SB loops are drawn posteriorly and lose contact with the anterior abdominal wall (tethered-bowel sign) due to the rigid infiltrated mesenteric leaves. Axial T2WI (C). PC from endometrial carcinoma: Diffuse mesenteric deposit seeding (arrow) which causes SB retraction (tethered-bowel sign). Notice that despite massive ascites (*), there is scarce quantity of liquid between the rigid infiltrated mesenteric leaves (arrowhead).
Figure 40.
Axial CE-CT. Observe the enhancing pseudonodular parietal peritoneum (arrows) which corresponds to collaterals vessels, not to be mistaken for peritoneal deposits.
Figure 40.
Axial CE-CT. Observe the enhancing pseudonodular parietal peritoneum (arrows) which corresponds to collaterals vessels, not to be mistaken for peritoneal deposits.
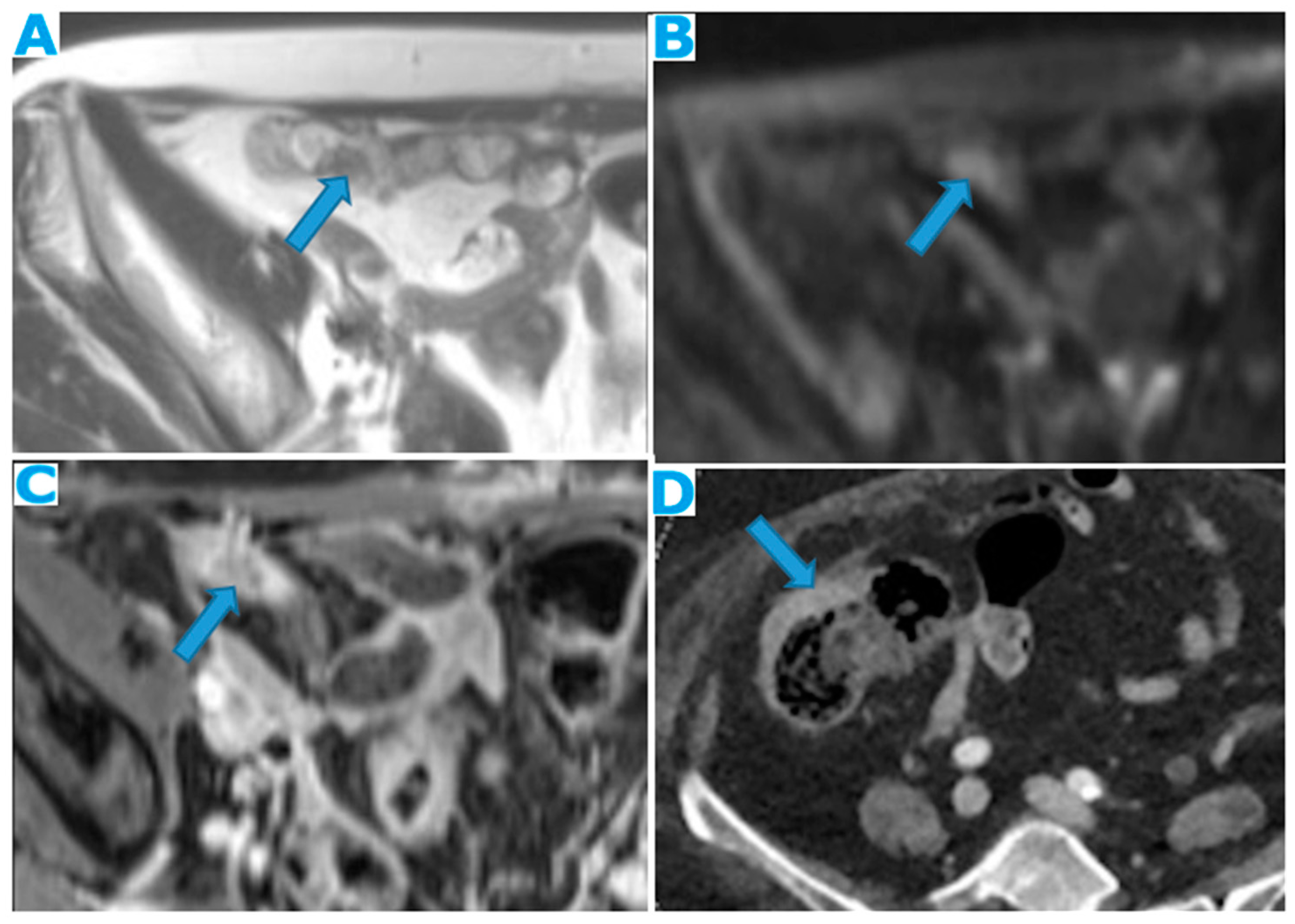
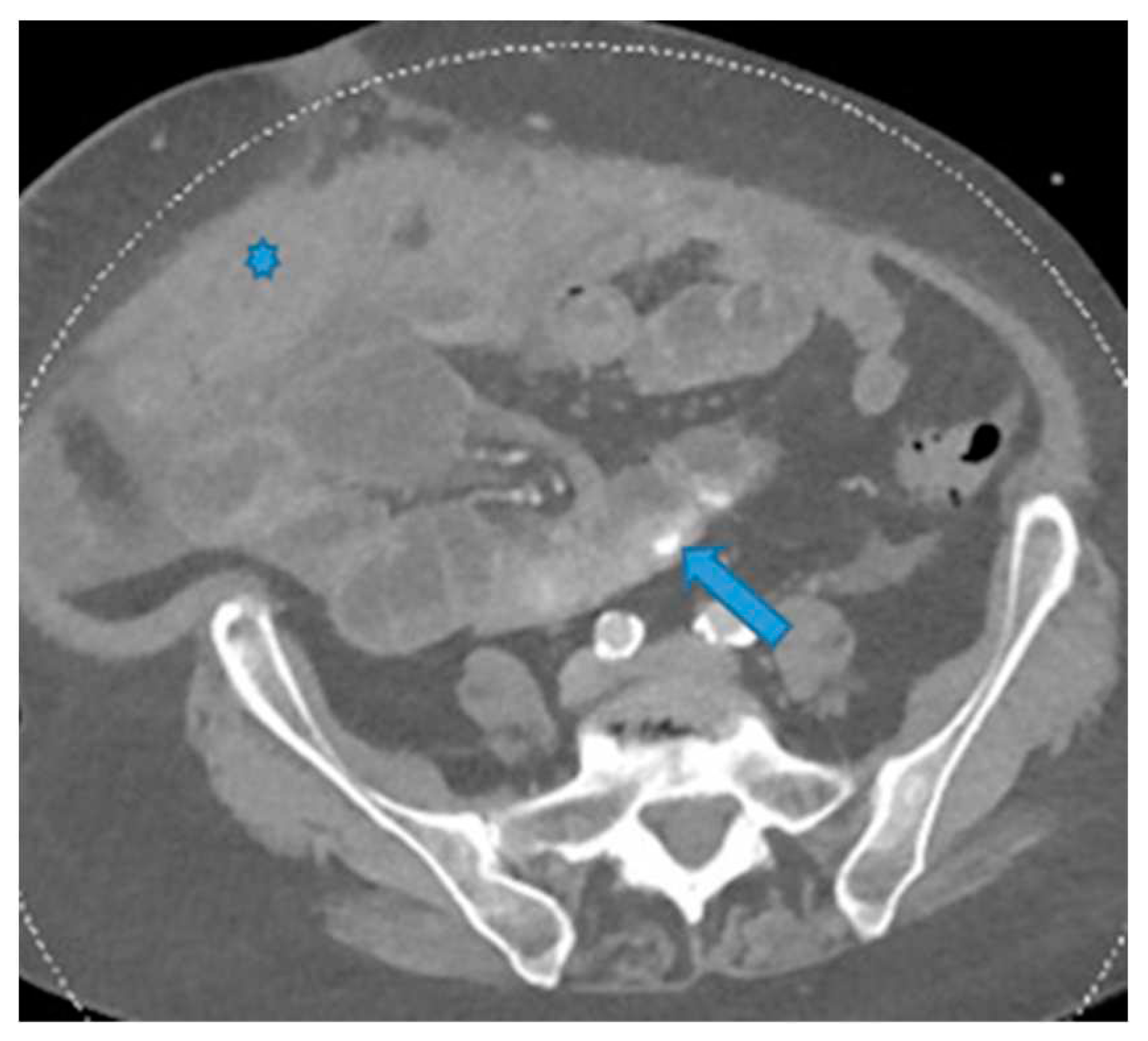
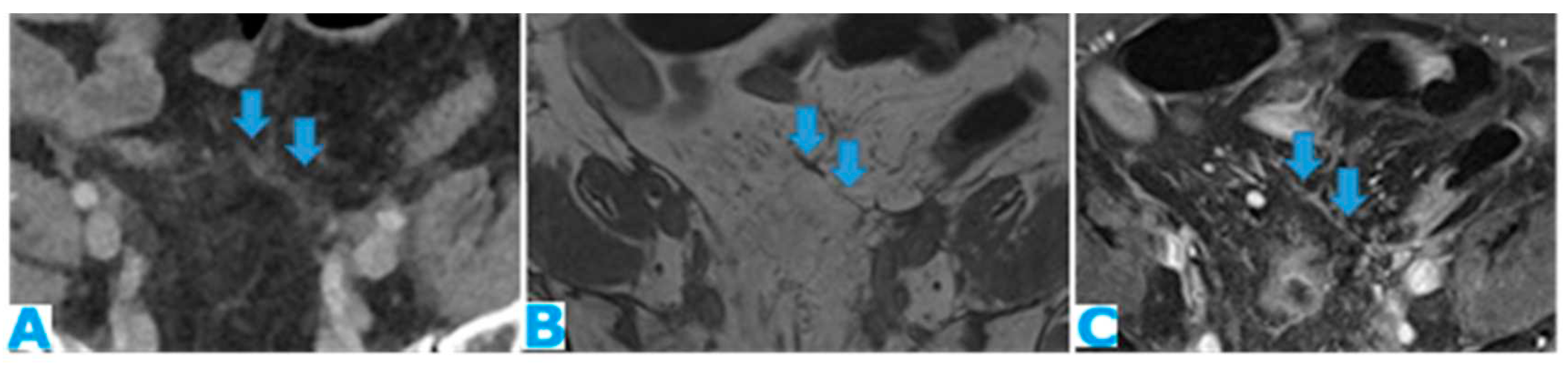 Figure 25. Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.Figure 25. Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.
Figure 25. Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.Figure 25. Axial CE-CT. PC from colon adenocarcinoma: Involvement of the mesenteric leaves (note the nodular thickening and enhancement) that becomes more apparent with ascites.