Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
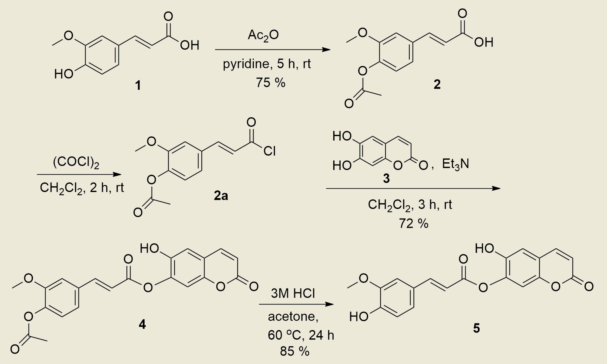
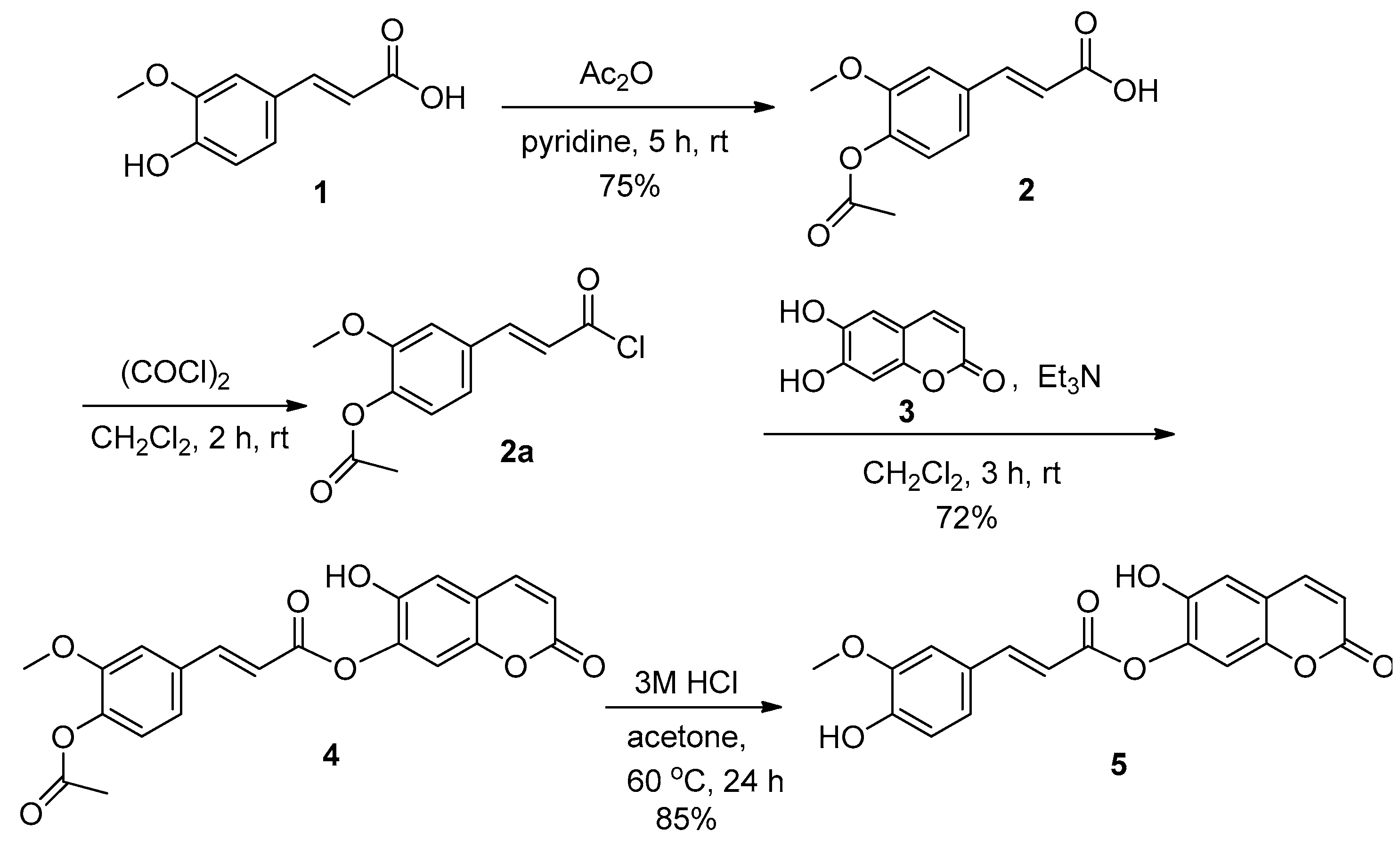
3.2. Synthesis of (E)-6-Hydroxy-2-Oxo-2H-Chromen-7-yl 3-(4-Acetoxy-3-Methoxyphenyl)Acrylate (4)
3.3. Synthesis of (E)-6-Hydroxy-2-Oxo-2H-Chromen-7-yl 3-(4-Hydroxy-3-Methoxyphenyl)Acrylate (5)
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Morales, V.; Villasana-Ruíz, A.P.; Garza-Veloz, I.; González-Delgado, S.; Martinez-Fierro, M.L. Therapeutic effects of coumarins with different substitution patterns. Molecules 2023, 28, 2413. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, M.; Gašo-Sokač, D.; Jokič, S.; Molnar, M. Recent advances in the synthesis of coumarin derivatives from different starting materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer potentials of coumarin and its derivatives. Mini Rev. Med. Chem. 2021, 21, 2996–3029. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Budagumpi, S.; Balappa Somappa, S. Synthetic and natural coumarins as potent anticonvulsant agents: A review with structure-activity relationship. J. Clin. Pharm. Ther. 2022, 47, 915–931. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patel, H.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; Pasupuleti, V.R.; Deshmukh, P.K. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef]
- Li, Z.; Kong, D.; Liu, Y.; Li, M. Pharmacological perspectives and molecular mechanisms of coumarin derivatives against virus disease. Genes Dis. 2021, 9, 80–94. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, T.Z.; Zhao, Y.L.; Fan, D.W.; Xia, Y. J.; Zhang, P. Synthesis, crystal structure, photo- and electro-luminescence of 3-(4-(anthracen-10-yl)phenyl)-7-(N,N’-diethylamino)coumarin. Synth. Met. 2010, 160, 1642–1647. [Google Scholar] [CrossRef]
- Voutsadaki, S.; Tsikalas, G.K.; Klontzas, E.; Froudakis, G.E.; Katerinopoulos, H.E. A ‘turn-on” coumarin-based fluorescent sensor with high selectivity for mercury ions in aqueous media. Chem. Commun. 2010, 46, 3292–3294. [Google Scholar] [CrossRef]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer. 2016, 15, 64. [Google Scholar] [CrossRef]
- Wang, G.; Lu, M.; Yao, Y.; Wang, J.; Li, J. Esculetin exerts antitumor effect on human gastric cancer cells through IGF-1/PI3K/Akt signaling pathway. Eur. J. Pharmacol. 2017, 814, 207–215. [Google Scholar] [CrossRef]
- Kim, S.H.; Kang, K.A.; Zhang, R.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Chae, S.W.; Kang, S.S.; Lee, K.H.; Kang, H.K.; et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Huang, C.J.; Yen, T.L.; Hsia, C.W.; Sheu, J.R.; Bhavan, P.S.; Huang, W.C.; Hsieh, C.Y.; Hsia, C.H. Activation of Nrf2 by esculetin mitigates inflammatory responses through suppression of NF-κB signaling cascade in RAW 264.7 Cells. Molecules 2022, 27, 5143. [Google Scholar] [CrossRef] [PubMed]
- Rzodkiewicz, P.; Gasińska, E.; Gajewski, M.; Bujalska-Zadrożny, M.; Szukiewicz, D.; Maśliński, S. Esculetin reduces leukotriene B4 level in plasma of rats with adjuvant induced arthritis. Reumatologia 2016, 54, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.Y.; Ham, J.R.; Lee, M.K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem. Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clini. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Singh Tuli, H.; Kumar, A.; Ramniwas, S.; Coudhary, R.; Aggarwal, D.; Kumar, M.; Sharma, U.; Chaturvedi Parashar, N.; Haque, S.; Sak, K. Ferulic Acid: A Natural Phenol That Inhibits Neoplastic Events through Modulation of Oncogenic Signaling. Molecules 2022, 27, 7653. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent advances in the neuroprotective properties of ferulic acid in Alzheimer’s disease: A narrative review. Nutrients 2022, 14, 3709. [Google Scholar] [CrossRef]
- Li, Y.; Sair, A.T.; Zhao, W.; Li, T.; Liu, R.H. Ferulic acid mediates metabolic syndrome via the regulation of hepatic glucose and lipid metabolisms and the insulin/IGF-1 receptor/PI3K/AKT pathway in palmitiate-treated HepG2 cells. Agric. Food Chem. 2022, 70, 14706–14717. [Google Scholar] [CrossRef]
- Shirai, A.; Kajiura, M.; Omasa, T. Synergistic photobactericidal activity based on ultraviolet-A irradiation and ferulic acid derivatives. Photochem. Photobiol. 2015, 91, 1422–1428. [Google Scholar] [CrossRef]
- Um, J.N.; Mim, J.W.; Joo, K.S.; Kang, H.C. Antioxidant, anti-wrinkle activity and whitening effect of fermented mixture extracts of angelica gigas, paeonia lactiflora, rehmannia chinensis and cnidium officinale. Korean J. Medicinal Crop Sci. 2017, 25, 152–159. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations of by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




