1. Introduction
Fluorine (F) is a nonmetal element in nature, which has been recognized a strong oxidant because of its unique chemical structure [
1]. Fluoride is a salted compound of fluorine, which maintains physiological homeostasis and is an essential component for bone mineralization and enamel formation [
2,
3]. It has been reported that appropriate intake of fluoride helps with skeletal formation, prevention of dental caries, promotion of the neuron conduction, and anti-aging [
4]. Supplement of fluoride in drinking waters has been used extensively worldwide for disinfection and prevention of dental diseases [
5,
6,
7]. However, the concentration of fluoride in drinking water for prevention of dental caries should not exceed 0.7 mg/L [
8]. Fluoride is a double-edged sword, given a low dose of fluoride ingestion in drinking water helps to prevent dental caries, but ingestion of fluoride in drinking water in excess of the doses described above can cause fluorosis and threaten human life health. Fluorosis leads to skeletal disorders and arthrosis, which result in deformity. Chronic fluorosis has been reported to cause encephalopathy, leading to impaired central nervous system function [
9]. It has been known that fluorosis is an irreversible and a major public health threat in China [
4]. There are three potential resources to get exposed with fluoride, including drinking water, coal-burning, and drinking tea [
10]. Fluorosis becomes an endemic disease in China because of the habits of drinking tea and uncontrolled coal-burning [
11]. Recently, the studies in regard to the molecular mechanism of action of fluorosis and explore a traditional Chinese medication to intervene fluorosis has become popular [
12].
It has been recognized recently that Nrf2/ARE pathway plays a key role to mitigate oxidative stress during inflammation [
13]. Liver has been known an important organ to regulate or initiate Nrf2/ARE pathway [
14,
15]. Furthermore, it has been shown that Nrf2 pathway is activated during fluoride induced hepatopathy to alleviate oxidative stress [
16]. Therefore, one of the aims of this study is to explore the mechanism of action and the role of Nrf 2 pathway during fluoride-induced hepatopathy.
Over the past few decades, several studies have demonstrated the benefits of using natural products to counter oxidative stress via the Nrf2/ARE pathway[
13]. Tetramethylpyrazine (TMP) is an active ingredient extracted from Ligusticum chuanxiong Hort (
Ligusticum wallichii) [
17], which has been proven that to be an effective component with anti-inflammatory and antioxidant properties [
18]. It has been reported that the antioxidant effect of TMP is mainly by activating Nrf2/HO-1 pathway in liver, which scavenges free radicals, inhibits NF-κB, enhances the expressions of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and other associated antioxidants, and promotes the antioxidant capacity of hepatocytes [
19,
20]. Use of traditional Chinese herb extractions, such as Longyanshen polysaccharide, Astragalus membranaceus, stilbene glycoside, gastrodin, resveratrol, and so on, to intervene fluorosis has been studied extensively [
21]. Therefore, the second purpose of this study is to clarify the clinical effects and the mechanism of actions of TPM in treating fluoride-induced hepatopathy.
2. Materials and Methods
2.1. Chemicals
Sodium fluoride (NaF), sodium pentobarbital (C11H17N2NaO3), TMP, alanine aminotransferase (ALT, C009-2-1), aspartate aminotransferase (AST, C010-2-1), malondialdehyde (MDA, A003-1-2), reduced glutathione (GSH, A006-2-1), total superoxide dismutase (T-SOD, A001-1-2), and total antioxidant Capacity (T-AOC, A015-3-1) were purchased from Jiancheng Bioengineering Institute, Nanjing, China. (Kit name: PrimeScriptTM RT Reagent KitRR086A). SYBR R Premix Ex TaqTM II (cat no. RR920A) and RNAiso Plus (cat no. 9178) were provided by Takara Biotech-nology Co., Ltd. (Dalian, Liaoning, China).
2.2. Experimental Animals and Methods
The use of animals was approved by Animal Protection and Use Committee of Sichuan Agricultural University (Approval No.: dyy - 20203012). Sixty four-week-old male ICR mice with body weight from 18 to 22g were used in this study (Pegatron Biotech Co., Ltd., Chengdu, China; Animal License No.:SCXK [chuang] 2020-030). Mice were housed in a standard SPF facility with controlled room temperature at 25±2℃ under a 12-hour light-dark cycle, and fed with standard pellet diet and water
ad libitum. Subjects were randomly divided into 5 groups (n = 12 in each group): (1) control group (CON); (2) model group (F), (3) low dose TMP group (LT): 40mg/kg of TMP; (4) medium dose TMP group (MT): 80mg/kg of TMP; (5) high dose TMP group (HT): 160mg/kg of TMP. TMP was dissolved in distilled water and administered to mice by gastric tube for 14 days in three TMP groups. Mice in the control and model groups were administered the same volume of distilled water for 14 days. After the last administration, mice in model group and TMP group were given NaF dissolved in distilled water (35 mg/kg body weight)[
22] after the last treatment to induce acute hepatopathy; the same volume of distilled water was administered intragastrically to the control group. After 24 hours of Fluoride challenge, the mice were euthanized by administration of 80 mg/kg 2% pentobarbital sodium intraperitoneally, followed by cervical dislocation. Blood samples were collected from the Orbital venous plexus of the mice. Serum alanine aminotransferase (ALT), aspartate am-inotransferase (AST) and biomarkers of oxidative stress were measured. Livers were harvested, weighed, and stored for further histopathological and biochemical analysis, including histopathological changes, ultrastructural changes, biomarkers of oxidative stress, and gene expressions.
2.3. Body Weight, Organ Index and liver Index
Body weights were measured and recorded weekly. At the end of the experiment, brain, heart, liver, kidney, lungs, and spleen of each subject were collected, weighed and photographed. Organ index is calculated by the following formula[
23]:
2.4. Detection of Serum Levels of ALT and AST
Blood samples were collected from the mice and stored at 4℃ for 2 hours, then centrifuged at 3000 rpm for 10 min. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured according to the user’s manual from the manufacture (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Detection of Serum Levels of Oxidative Stress Biomarkers
Blood samples were collected from the mice and left to stand at 4°C for 2 hours, followed by centrifuged at 3000 rpm for 10min to obtain serum. Total superoxide dismutase (T-SOD) activity, total antioxidant capacity (T-AOC), glutathione (GSH) and malondialdehyde (MDA) content were measured by a biochemical reagent kit provided by China Jiancheng Bioengineering Institute (Nanjing, China).
2.6. Detection of Hepatic Oxidative Stress Biomarkers
Liver tissues were rinsed in isotonic saline solution at 4℃, weighed and homogenized in ice-cold isotonic saline solution at a ratio of 1:9 (w/v), followed by centrifugation (3500 rpm, 10 min at 4℃). The suspension was collected for measurement of the concentration of total protein by Bradford protein assay. The hepatic levels of T-SOD, T-AOC, GSH and MDA were detected by a biochemical reagent kit provided by China Jiancheng Bioengineering Institute (Nanjing, China).
2.7. Hepatic Histopathological Changes
The remaining liver tissues were prepared for histopathological examination. The liver tissue was repaired and rinsed with water for 4 h, Alcohol gradient dehydration was performed, and the dissolved paraffin was infiltrated into the tissue blocks, which became hard wax blocks after cooling. The wax blocks were placed on a slicer (Leica, Heidelberg, Germany), cut into 5-μm slices, and transferred to slides. The wax blocks were placed in a temperature box at 60 °C for 2 h and dried, Paraffin sections were immersed in hematoxylin for 5 min, followed by eosin for 2 min, and slides were dehydrated and sealed with neutral resin. Leica DM1000 microscope (Leica, Heidelberg, Germany) was used to observe histopathological changes [
24].
2.8. Ultrastructural Changes of Hepatocytes
Samples of liver preserved in glutaraldehyde were taken and ultrathin sections were made according to standard protocols, and for ultrastructural changes examination by a JEM-1400-FLASH Transmission Electron Microscope (Jeol, Japan).
2.9. RNA Extraction and qRT-PCR
Liver samples were stored at -80℃ for RNA extraction. Total liver RNA was extracted by RNAiso Plus (9108, Takara, Otsu, Japan) followed the manufacturer ‘s instructions. cDNA was synthesized simultaneously using 1 ug of RNA with the Prim-Script. TM. RT Reagent Kit (9108, Takara, Japan) and the SYBR Premix Ex Taq. TM. II Kit (9108A, Takara, Japan). The expressions of Nrf2, HO-1, CAT, GSH-Px, and SOD-1 were detected by Real-Time quantitative PCR (Bio-Rad, USA). β-actin was applied as a housekeeping gene for normalization. RNA expression levels were calculated by 2
-△△CT method [
25]. Primers used in this study were designed by PREMIER 5 (PREMIER Biosoft International, Palo Alto, Calif.) and shown in
Table 1.
2.10. Data Statistics and Analysis
All data were analyzed using SPSS 27.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) was used to determine overall differences among groups. All parameters were expressed as mean ± standard deviation. Significance was recognized when P < 0.05.
4. Discussion
Fluorine is a nature element in the environment and fluoride has been used extensively in daily life, such as sewage treatment. However, excessive exposure to fluoride lead to fluorosis, resulting in severe hepatopathy. Fluorosis-induced hepatopathy has been reported associate with reactive oxygen species (ROS) activate oxidative stress. Excess ROS cause cellular damage due to oxidative stress reactions [
26]. TMP is an active ingredient of a traditional Chinese medicine, extracted from chuanxiong, which has been reported to be an effective antioxidant and anti-inflammatory agent. TMP has been used to treat liver issues, such as ischemia-reperfusion injury, hepatic fibrosis, septic liver injury, and so on [
19,
20,
27].
The results of the current study revealed that overdose of fluoride induced liver damages. The serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are two important intracellular biomarkers to assess liver function [
25]. The serum levels of ALT and AST increase during hepatocytes injuries due to increased permeability of cell membrane [
28,
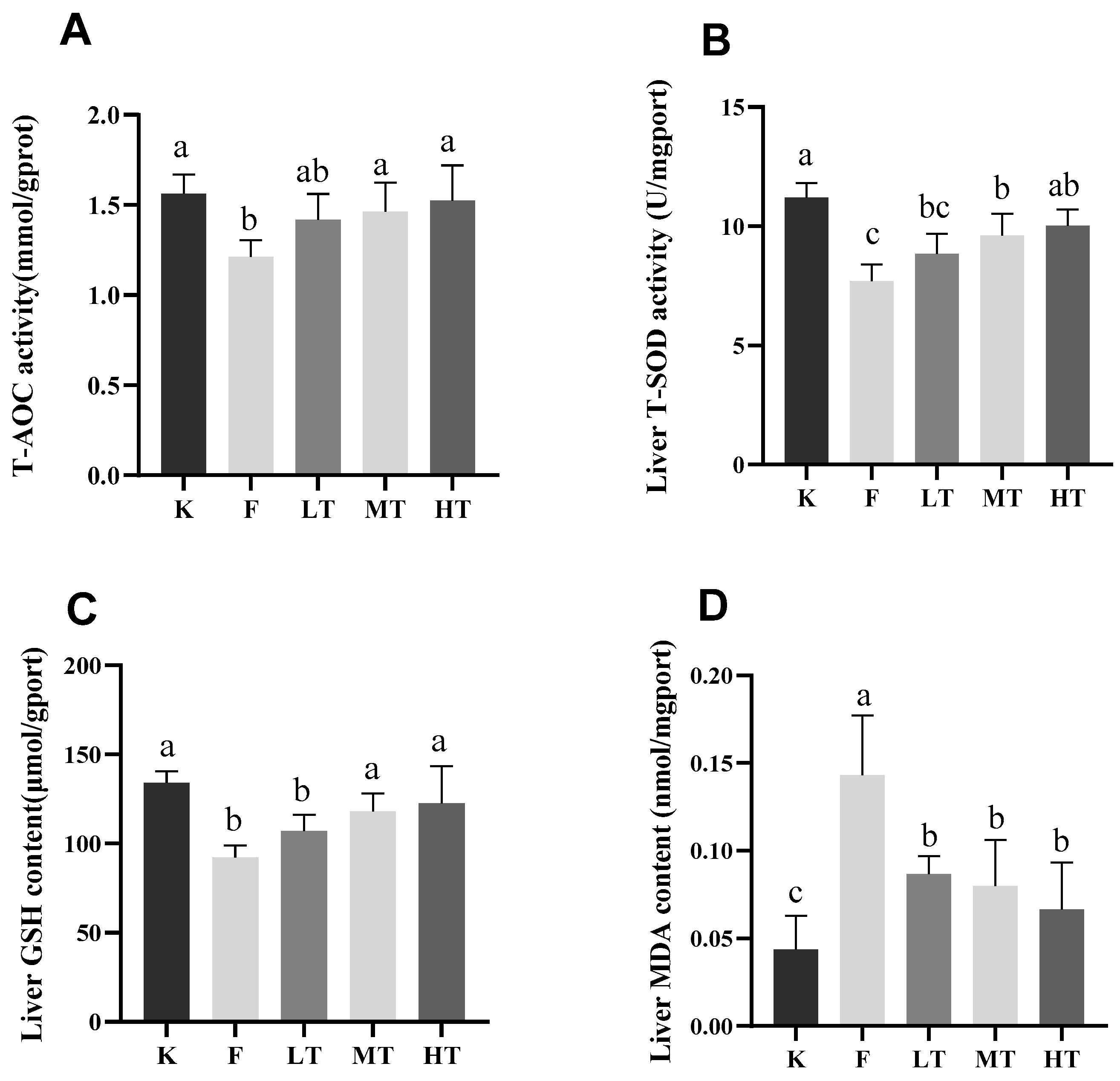
29]. T-SOD is an antioxidant which effectively scavenges free radicals and alleviates cellular oxidative damages. GSH is another antioxidant synthesized in low-molecular-weight cells which inhibits the production of free radicals. Nonetheless, MDA is an indicator of lipid peroxidation and indirectly reflects the increase in contents of free radicals during oxidative stress. Furthermore, T-AOC represents the total antioxidant capacity of the body [
30,
31]. When oxidative stress is in progress, reactive oxygen species (ROS) activate the chain reactions of antioxidants, leading to elevation of the amount T-SOD and GSA, and reduction of the level of T-AOC. Meanwhile, the serum content of MDA increased. The results of this study are in line with the ROS-triggered antioxidant chain reactions after fluoride-challenged hepatopathy. After fluoride challenged, the serum levels of ALT, AST, and MDA increased significantly; however, the serum levels of T-SOD, GSH, and T-AOC decreased significantly, which in accordance with previous studies [
14,
30,
32]. Among treatment groups, especially in groups of MT and HT, the levels of T-SOD, GSH, and T-AOC increased; whereas the content of MDA decreased, which suggested that the intervention of TMP alleviated the fluoride-induced oxidative stress and hepatic injury.
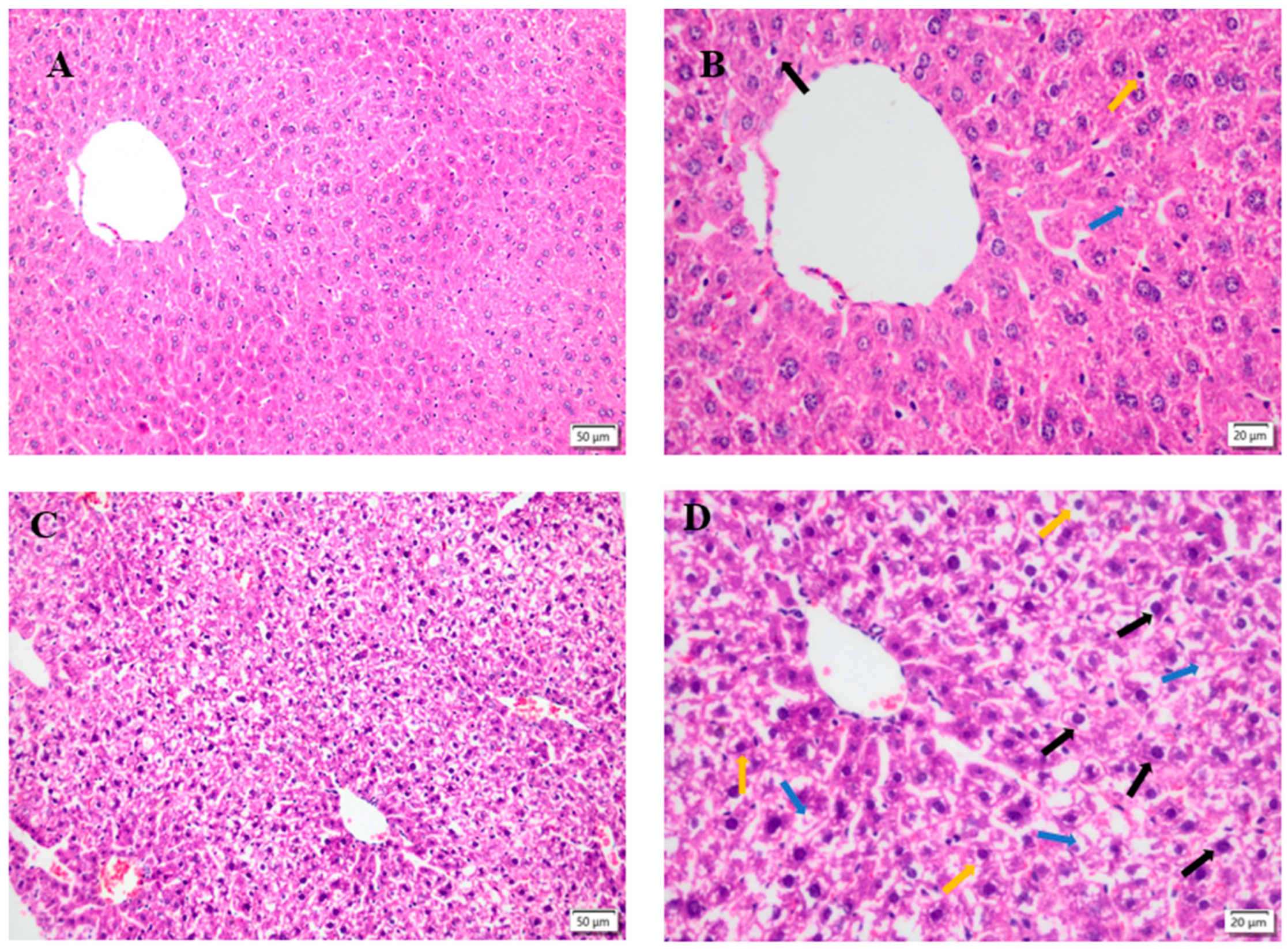
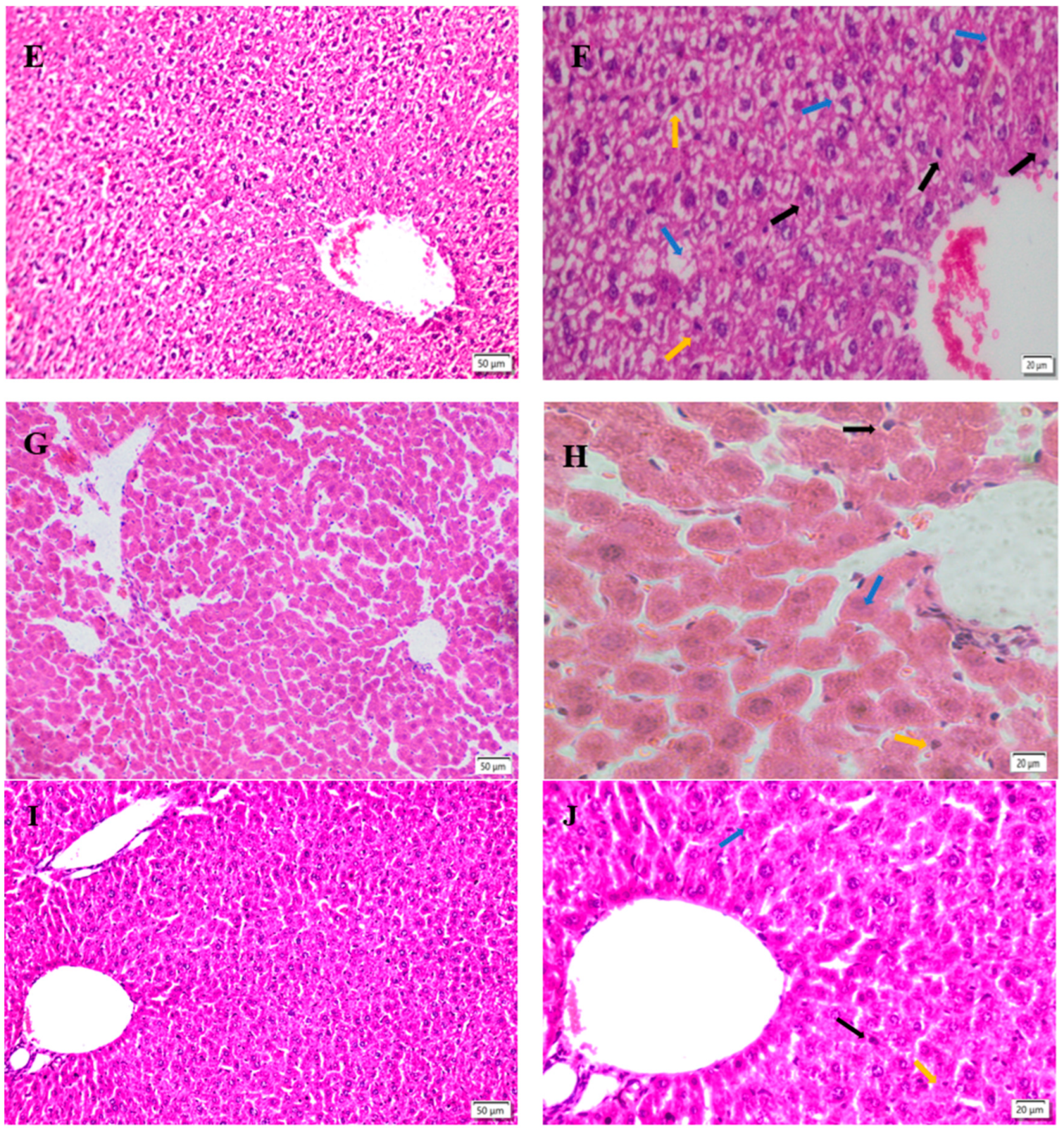
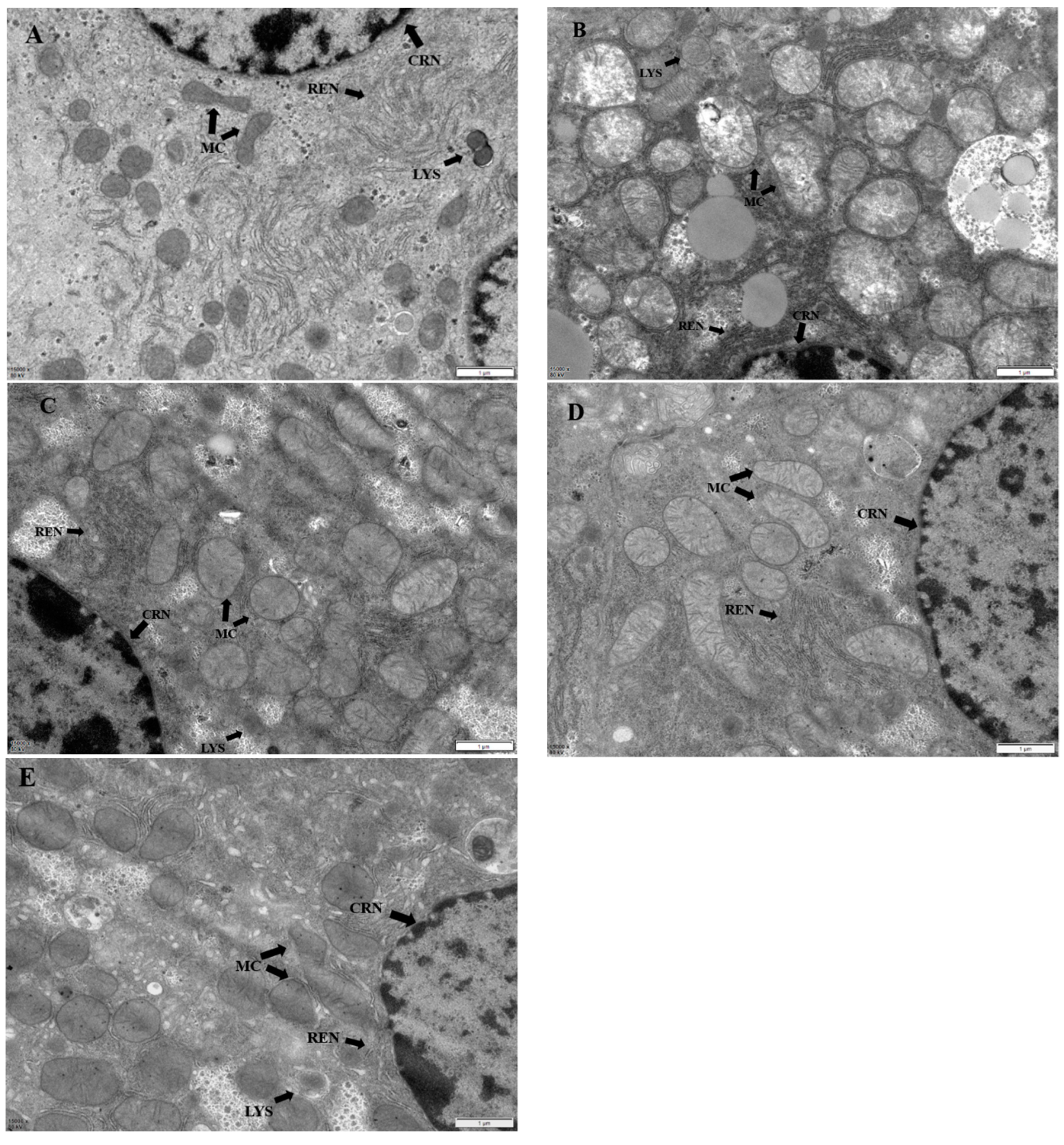
Histopathological changes directly reflect the cellular damages, which is helpful to evaluate the severity of organ damages. The severity of hepatocytes damages predisposes the progress of ROS associated damages. The results of this study showed that fluoride induced hepatopathy because of significant observation of hepatocyte vacuolation and swollen mitochondria, indicating severe ongoing oxidative stress after fluoride challenges. The findings in this study are consistent with previous reports [
23,
33,
34]. The interventions of different dosages of TMP alleviated the severity of hepatocytes damages in the current study, especially in groups of MT and HT. The morphologies of hepatocytes, including mitochondria, in these groups were relatively normal compared to group F, indicating the potential protection of TPM on fluoride-induced hepatopathy.
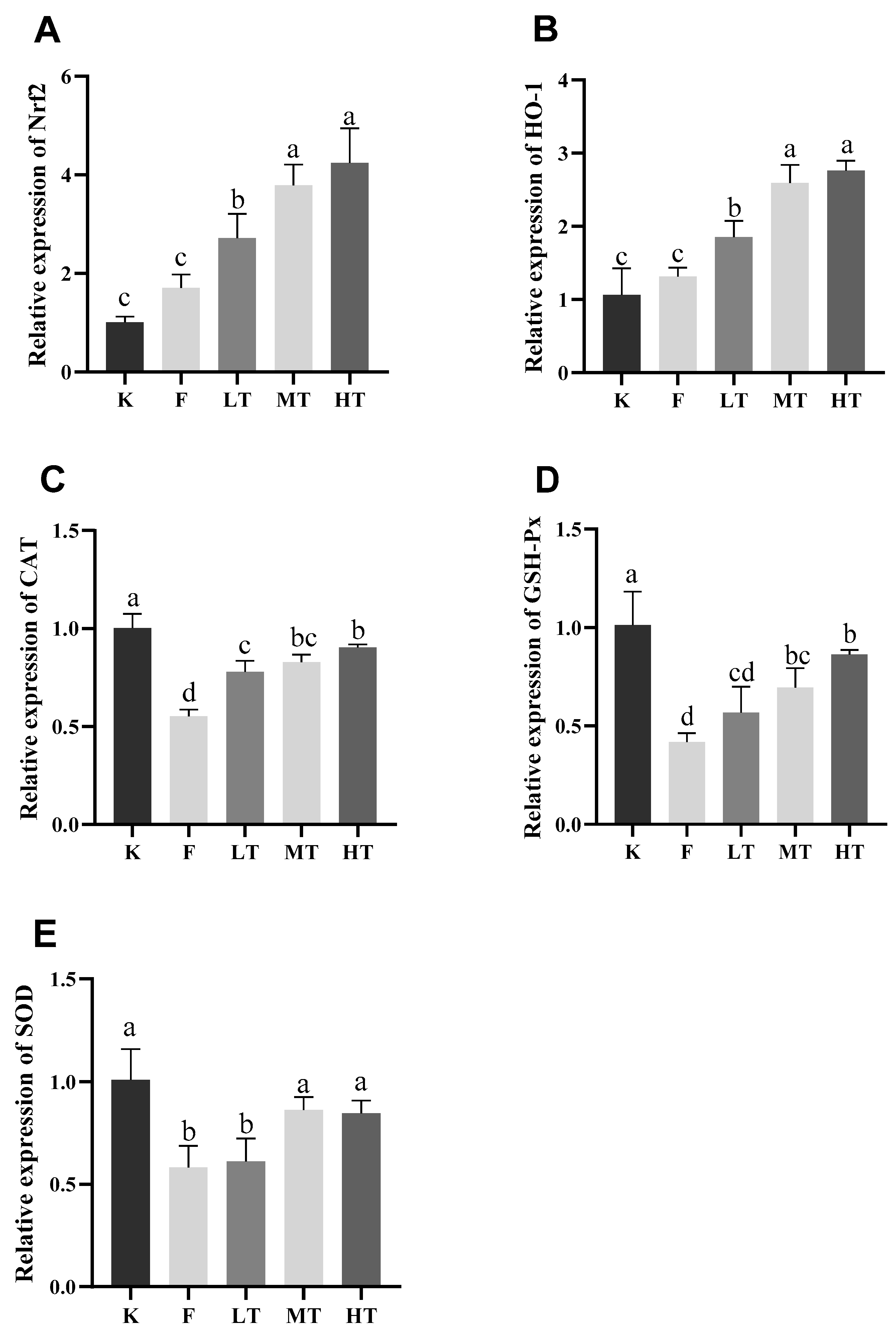
Nuclear factor E2-related factor 2 (Nrf2) is ubiquitous in mammalian cells, which combines to antioxidant counter-response factors and triggers the transcription and expression of antioxidant enzymes such as SOD, GSH-Px, etc.; however, which is inhibited during oxidative stress. Nrf2 has been reported involvement in regulating oxidation balance and protecting antioxidant and phase II detoxification reactions in organisms [
35]. The expressions of Heme oxygenase-1 (HO-1) is regulated by Nrf2, which promotes heme degradation. Physiologically, the expression of Nrf2 is regulated Keap1 protein in cytoplasm; however, Nrf2 is uncoupled from Keap1 and rapidly translocated to nucleus when oxidative stress occurs, in terms of Nrf2-dependent cellular defense mechanism which activates the transcriptions of HO-1, SOD, CAT, and GSH-Px in order to enhance cellular antioxidant capacities. Nrf2/HO-1 signaling pathway is critical in improving the capability of organisms to defense oxidative stress induced cellular damages, which has been recognized a most important endogenous antioxidant signaling pathway in mammalians [
36]. The results of the current study showed that the hepatic mRNA expressions of Nrf2 and HO-1 increased concurrently with decreased expressions of SOD and CAT decreased in group F, suggesting that acute excessive fluoride intake led to oxidative stress and compromise the ability to scavenge free radicals. Previous studies have shown that fluorosis activates Keap1-Nrf2 signaling pathway to prevent oxidative stress induced hepatic or renal injuries in chronic fluorosis rats [
1,
14]; Nevertheless, fluoride-induced oxidative stress promotes the expressions of intracellular ROS, inducing peroxiredox-dependent unfolded protein response and triggering the autophosphorylation of Nrf2 [
37], which reduces the expression of downstream genes of Nrf2 pathway and exacerbates oxidative stress induced hepatopathy. According to the results of this study, the expressions of genes associated with Nrf2 pathway in the liver are enhanced by the TMP, suggesting that TMP is effective to promotes the expressions of the Nrf2 pathway associated genes by activating the Nrf2/HO-1 signal pathway and facilitate elimination of excessive ROS in fluoride-induced hepatopathy mice. The finding is consistent with previous studies [
19,
20,
38,
39]. Furthermore, it has been reported that TMP improves the expressions of Nrf2 pathway associated genes in Nrf2 gene knockout mice [
40].
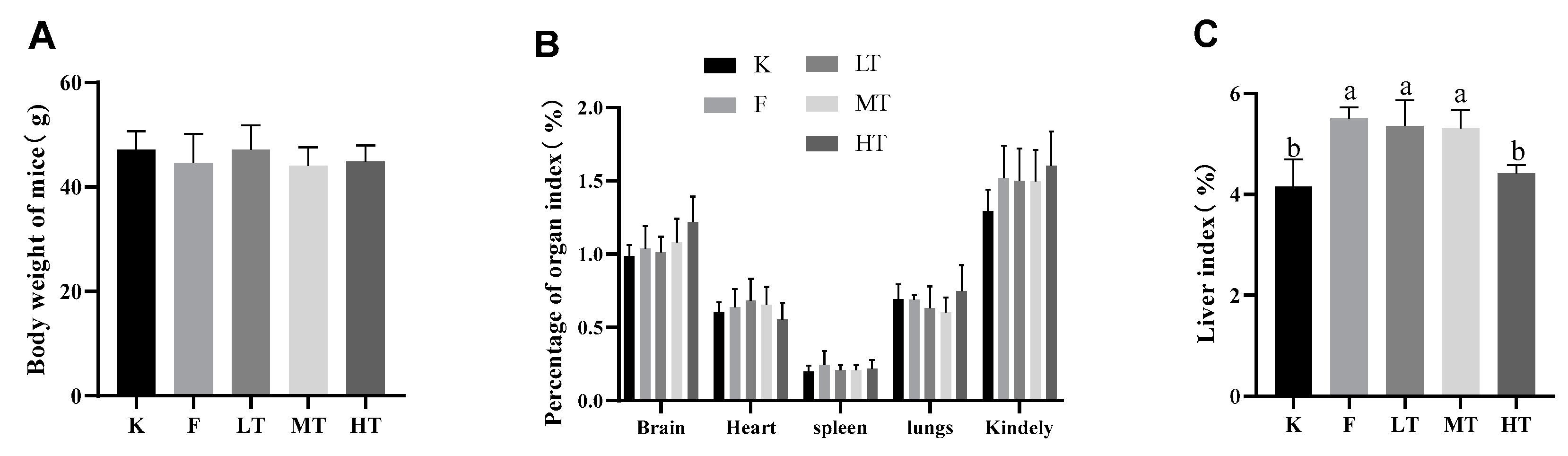
Figure 1.
Body weight and organ indices. (A) Body weight of mice; (B) Organ indices; (C) Organ index of liver. Data are presented as mean ± SD. Different alphabets indicate statistically significant differences among groups (P < 0.05). K: control group; F: model group; LT, low-dose TMP group; MT, medium-dose TMP group; HT, high dose TMP group.
Figure 1.
Body weight and organ indices. (A) Body weight of mice; (B) Organ indices; (C) Organ index of liver. Data are presented as mean ± SD. Different alphabets indicate statistically significant differences among groups (P < 0.05). K: control group; F: model group; LT, low-dose TMP group; MT, medium-dose TMP group; HT, high dose TMP group.
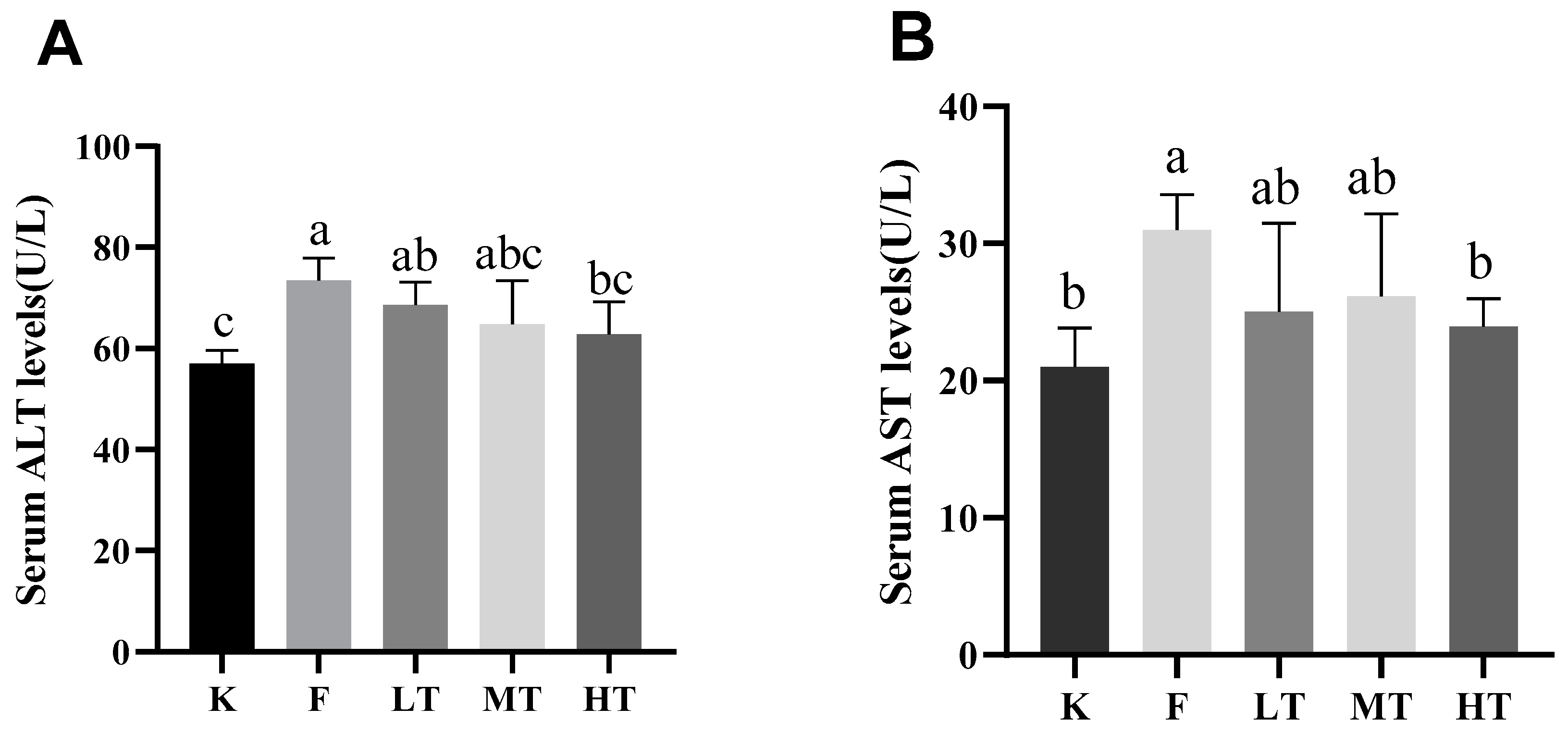
Figure 2.
| Serum levels of ALT and AST. (A) serum level of ALT; (B) serum level of AST. Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 2.
| Serum levels of ALT and AST. (A) serum level of ALT; (B) serum level of AST. Different alphabets indicate statistically significant differences among groups (P < 0.05).
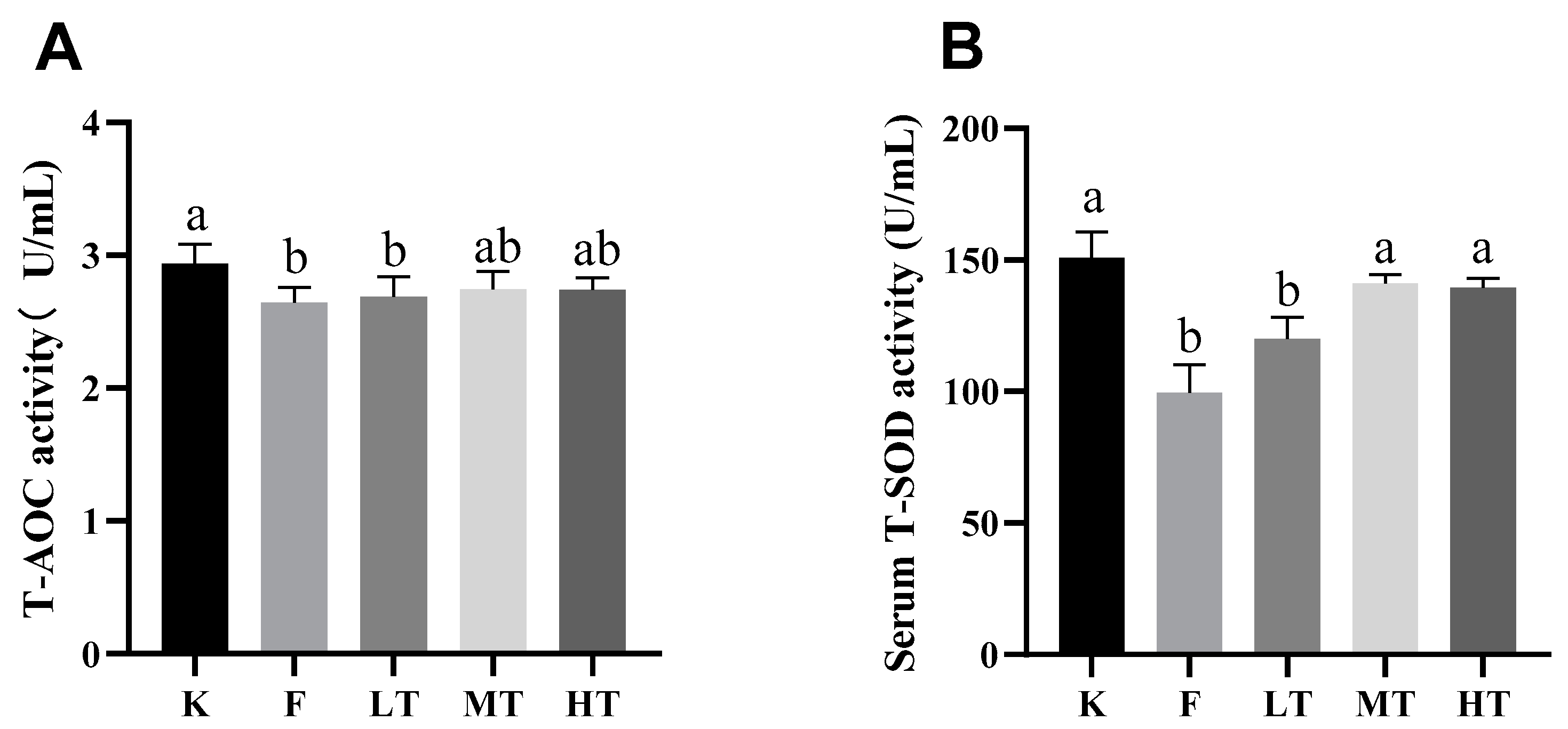
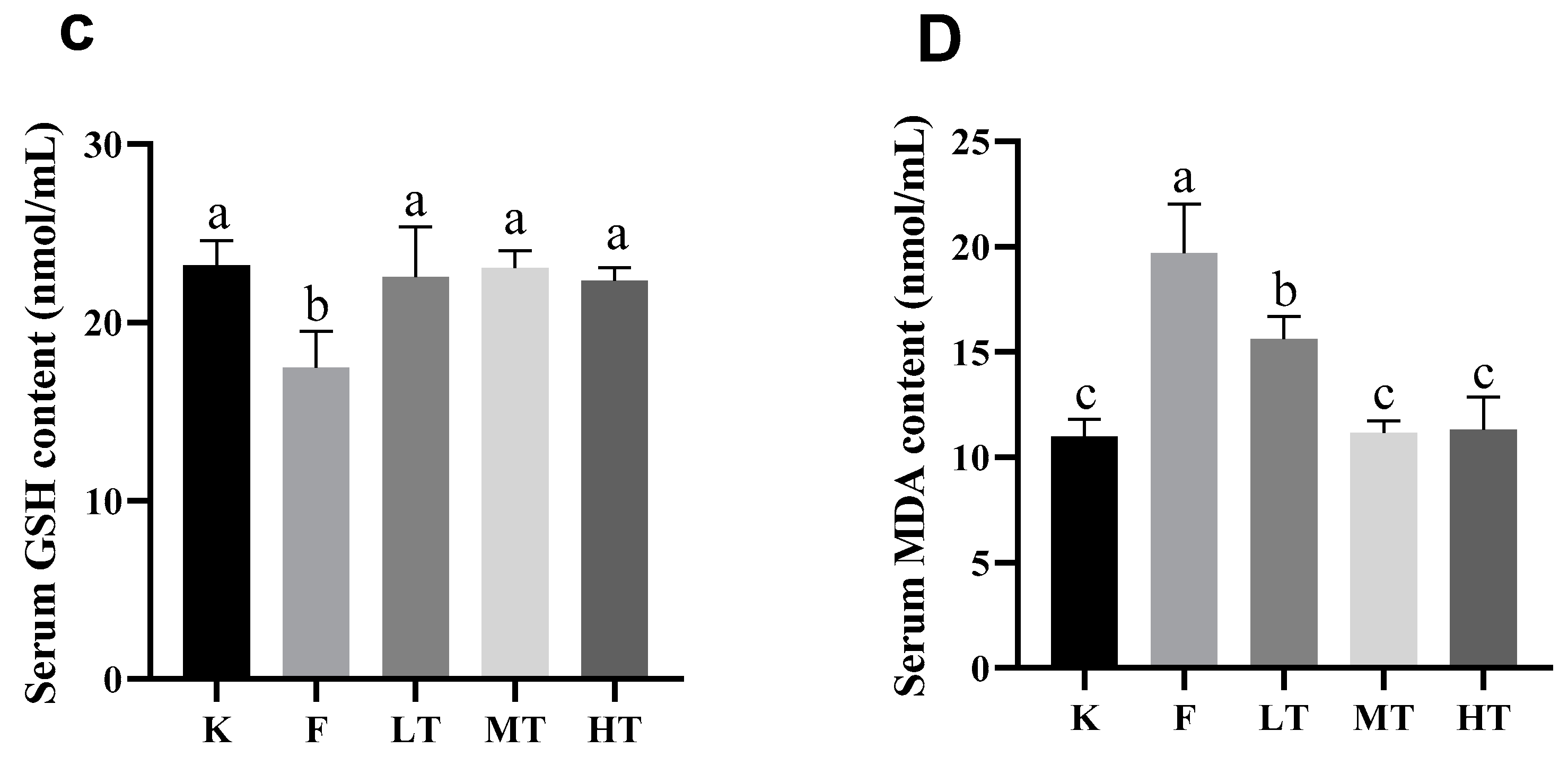
Figure 3.
Serum antioxidant enzyme activity and oxidative concentration. (A) total antioxidant capacity T-AOC; (B) superoxide dismutase T-SOD; (C) Glutathione (GSH); (D) Malondialdehyde (MDA). Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 3.
Serum antioxidant enzyme activity and oxidative concentration. (A) total antioxidant capacity T-AOC; (B) superoxide dismutase T-SOD; (C) Glutathione (GSH); (D) Malondialdehyde (MDA). Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 4.
Liver antioxidant index results. (A) total antioxidant capacity T-AOC; (B) superoxide dismutase T-SOD; (C) glutathione GSH; (D) MDA. Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 4.
Liver antioxidant index results. (A) total antioxidant capacity T-AOC; (B) superoxide dismutase T-SOD; (C) glutathione GSH; (D) MDA. Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 5.
Liver morphology, left magnification: 50 µ m, 200×;Right magnification: 20 µ m, 400 ×. (A, B) is the liver degeneration of the control group. (C, D) shows the changes in the liver of the model group. (E, F) indicates the changes of liver in low dose TMP group. (G, H) represents the changes of liver in the middle dose TMP group. (I, J) indicates the liver changes in high-dose TMP group. Black arrow: nuclear pyknosis; Blue snip: nuclear lysis; Yellow arrow: indicates neutrophil infiltration.
Figure 5.
Liver morphology, left magnification: 50 µ m, 200×;Right magnification: 20 µ m, 400 ×. (A, B) is the liver degeneration of the control group. (C, D) shows the changes in the liver of the model group. (E, F) indicates the changes of liver in low dose TMP group. (G, H) represents the changes of liver in the middle dose TMP group. (I, J) indicates the liver changes in high-dose TMP group. Black arrow: nuclear pyknosis; Blue snip: nuclear lysis; Yellow arrow: indicates neutrophil infiltration.
Figure 6.
Ultrastructural image of liver, magnification: 1 µm, 15000X. MC: mitochondria; CRN: nucleus; REN: rough endoplasmic reticulum; LYS: Lysosomes. (A) is that structure of hepatocyte in the control group. (B) It shows the structure of hepatocytes in model group. (C) It is the structure of liver cells in low dose TMP group. (D) It represents the structure of liver cells in middle dose TMP group. (E) The structure of liver cells in high dose TMP group.
Figure 6.
Ultrastructural image of liver, magnification: 1 µm, 15000X. MC: mitochondria; CRN: nucleus; REN: rough endoplasmic reticulum; LYS: Lysosomes. (A) is that structure of hepatocyte in the control group. (B) It shows the structure of hepatocytes in model group. (C) It is the structure of liver cells in low dose TMP group. (D) It represents the structure of liver cells in middle dose TMP group. (E) The structure of liver cells in high dose TMP group.
Figure 7.
The relative expression of Nrf2, HO-1, CAT, GSH-Px, SOD mRNA in the liver was measured. (A) Nrf2, (B) HO-1, (C) CAT, (D) GSH-Px, (E) SOD. Different alphabets indicate statistically significant differences among groups (P < 0.05).
Figure 7.
The relative expression of Nrf2, HO-1, CAT, GSH-Px, SOD mRNA in the liver was measured. (A) Nrf2, (B) HO-1, (C) CAT, (D) GSH-Px, (E) SOD. Different alphabets indicate statistically significant differences among groups (P < 0.05).
Table 1.
qRT-PCR Primer Sequence Listing.
Table 1.
qRT-PCR Primer Sequence Listing.
| Gene |
Accession number |
Primer sequence (5′-3′) |
Product length(bp) |
| Nrf2 |
NM-010902.4 |
F: AGCGGTAGTATCAGCCAR: GCCCAGTCCCTCAATAGC |
150 |
| HO-1 |
NM-010442.2 |
F: TGTTGCGCTCTATCTCCR: GTACACATCCAAGCCGAG |
136 |
| CAT |
NM-009804.2 |
F: TTCCTGAGCAAGCCTTCR: CACATGAATGGCTATGGATCACA |
138 |
| GSH-Px |
NM-008160.6 |
F: TCTCTTCATTCTTGCCATR: GACTACACCGAGATGAACGA |
112 |
| SOD1 |
NM-011434.2 |
F: CACCTTTGCCCAAGTCAR: TCCATTGAAGATCGTGT |
97 |
| β-Actin |
NM-007393 |
F: CGCTCGTTGCCAATAGTGR: GCTGTGCTATGTTGCTCTAG |
117 |