1. Introduction
Hox genes play an important role in the development of most animals and plants. Some Hox genes form clusters which are crucial for the Embryogenesis of Metazoa. The importance of this clustering was first noticed by E.B. Lewis who studied the genetics of Drosophila [
1]. In 1978, Lewis observed that some genes of the genome (later coined Hox genes) were located in order along the telomeric to centromeric direction as (Hox1, Hox2, Hox3,…). Lewis noticed that in the Hox gene clusters the genes are sequentially ordered and they are expressed in the same order along the antero-posterior axis of the Drosophila embryo [
1]. This is an astonishing event since this correlation occurs between extremely distant locations - the genetic sequence in the cell nucleus in one hand and the Drosophila embryo in the other. These two local domains are about 4 orders of magnitude apart from each other. Biomolecular interaction alone cannot create such correlations [
2]. This surprising phenomenon was named Spatial Collinearity (SC). Some years later, another collinearity was observed particularly in the Vertebrates: temporal collinearity (TC). According to TC, the first Hox gene (Hox1) of the Hox cluster started being expressed. Later, Hox2 was expressed and even later Hox3 followed until all Hox genes were expressed following the sequence Hox1, Hox2, Hox3,…[
3].
In order to explain these phenomena, a biophysical model (BM) was proposed in 2001 according to which, pulling physical forces can justify the data [
2,
4]. Several experimental findings were successfully compared to the BM predictions [
5,
6].
A simple heuristic expression for these pulling forces F was proposed [
7].
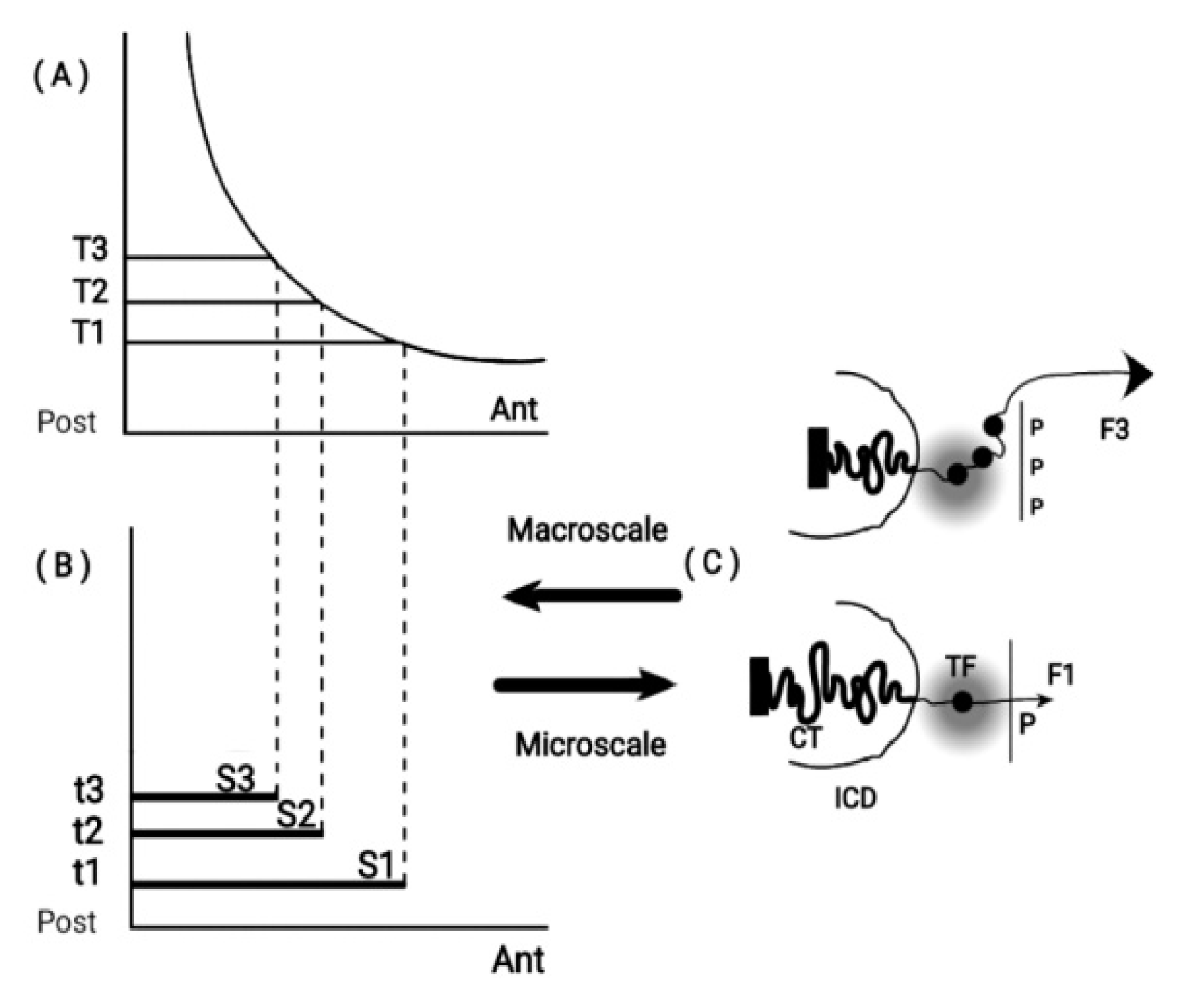
In the above equation, the pulling physical forces F are located opposite the telomeric end of the Hox cluster (
Figure 1). In Eq. (1), F stands for a quasi - Coulomb force where, for simplicity, the relative distance between the electric charges is omitted [
8]. It turns out that this omission reflects a deep connection to the fundamental phenomenon of Symmetry (see the Appendices). Ν and P are standing for the negative and positive charges acting on a Hox cluster. In the above heuristic formulation, the Hox cluster consists of a deployed finite sequence of Hox genes along the telomeric to centromeric ends of the cluster (Hox1, Hox2, Hox3,…). The numbers assign the gene order in the cluster. These numbers determine the order membership to the evolutionary Paralogy Group (PG). (Here is followed Duboule’s definitions of PG [
9]).
The contemporary cephalochordate Amphioxus is a descendant of the Amphioxus considered the ancestor of both Drosophila and vertebrates [
9]. Amphioxus lived even before the Cambrian period of evolutionary explosion 500 million years ago (Mya). Vertebrates and Drosophila appeared a few Mya later. Amphioxus has 14 Hox genes whereas vertebrates and Drosophila have 13 (Hox14 is missing).
As mentioned above, N represents the microscopic contribution to F and it is a real entity – the negative electric charge of the DNA sequence. P represents a positively charged molecular structure located opposite the telomeric end of the Hox cluster (
Figure 1). Contrary to N, P is a fictitious entity as yet, standing for the embryonic-macroscopic contribution to F. The known morphogens of the present time like Sonic Hedgehog, Fiber growth factors, Retinoic acid and the plethora of other morphogenetic factors were fictitious fifty years ago. The existence of P does not contradict any first principle so it is legitimate to anticipate its existence as advocated in [
4]. F pulls the Hox genes sequentially out of the cluster (
Figure 1). Eq.(1) is a heuristic expression that was successfully tested in several experiments [5 - 8].
Hox genes control the normal development of animals (wild type). Spontaneous mutation of these genes cause severe malformations (Homeosis), consisting of parts of the animal growing in the wrong location of the body. In Homeosis PG ordering is violated.
About twenty years ago, an important advancement was achieved concerning the transfer of specific molecules from outside the cell into the inner domain of the cell nucleus [10 -13]. For example, it was noticed that significant amounts of Activin are gathered outside the cell nucleus. Controlled amounts of this activin were transduced inside the nucleus causing specific modifications on the genome). It is assumed that BM combined with the action of transduction technology can affect Hox gene expression. This possibility is incorporated in the present hypothesis.
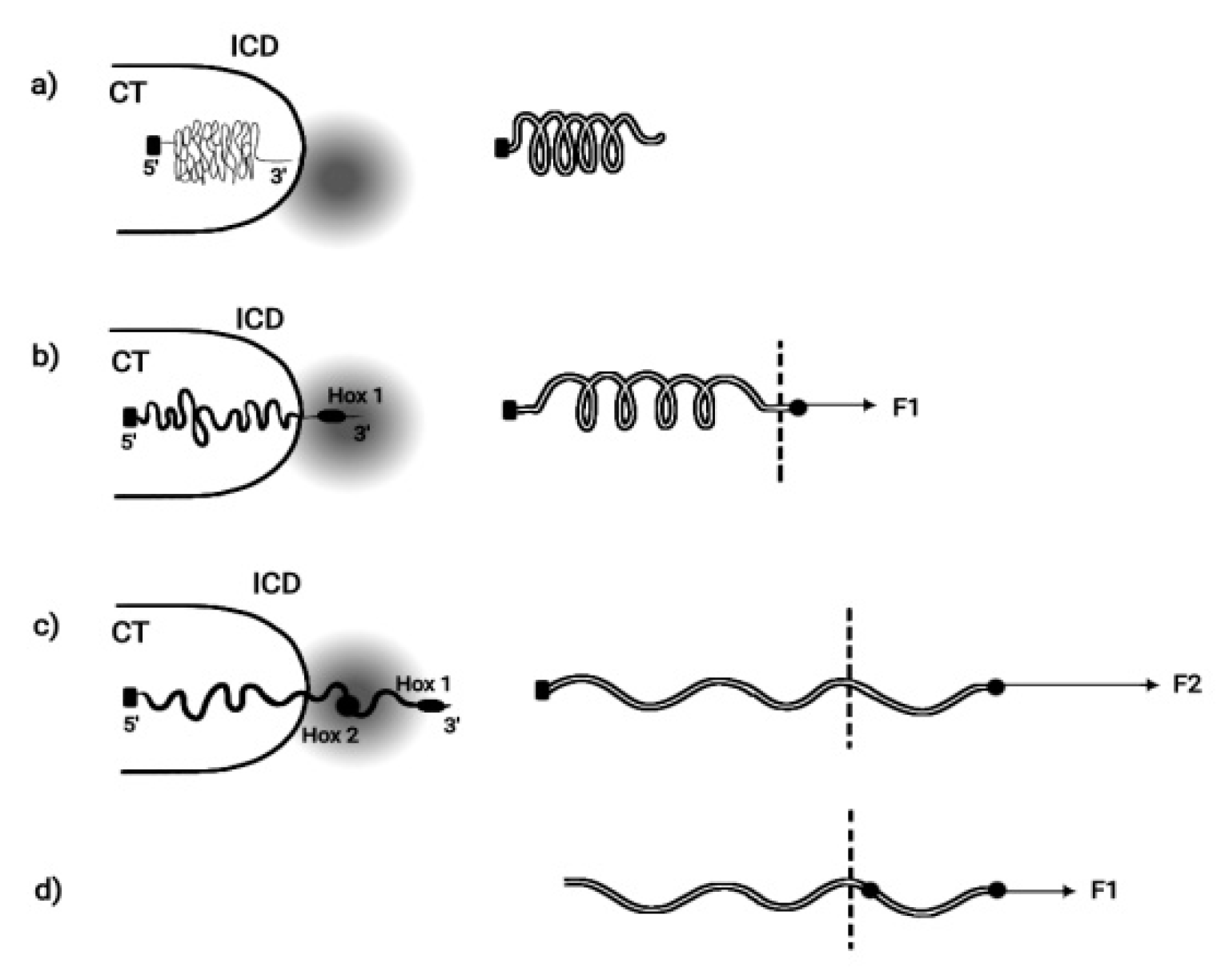
BM is based on the hypothesis that pulling forces are applied at the telomeric end of the Hox cluster. This hypothesis was elaborated in detail and it was concluded that the cluster is elongated along the direction of the force [
14]. This BM prediction was later confirmed [15-17]. In some cases the measured elongation of the activated Hox cluster was five times longer than the length of the inactive Hox cluster [
16]. When Hox cluster activation is initiated, a weak force (F1) pulls the first gene of the cluster (Hox1) out of its niche toward the interchromosome domain (ICD) (
Figure 2).
Particularly Hox1 is directed towards the transcription factory domain (TFD) where Hox gene activation (expression) is possible [
18], [
19]. When the pulling force increases to F2, Hox2 is extruded from its niche. This process continues until all Hox genes are transferred in the TF. For the efficient function of an elongated elastic spring, besides the pulling force at one of its ends, a proper fastening should be applied at its other end. Accordingly, the Hox cluster should be fastened at the centromeric end (
Figure 2).
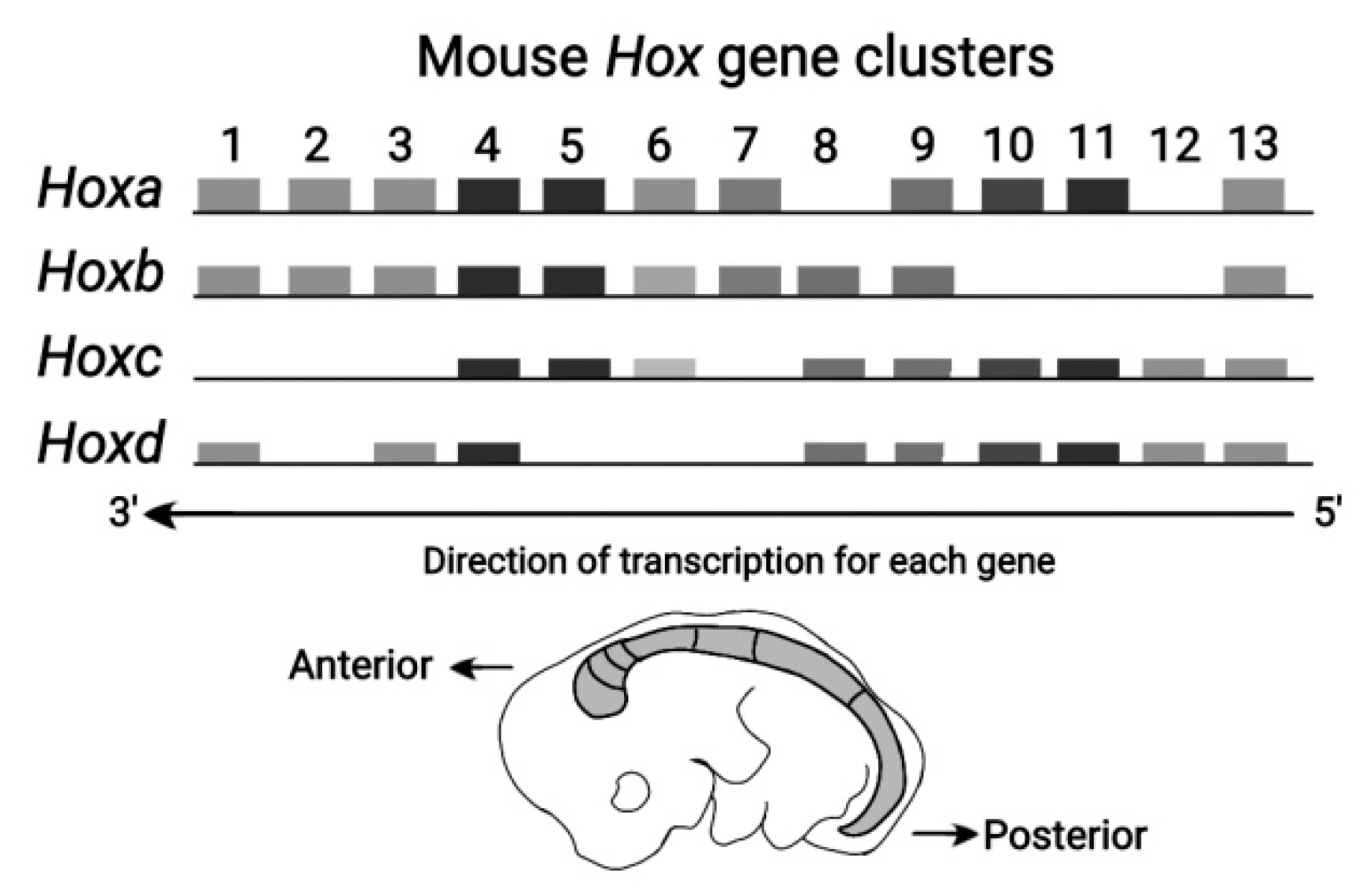
The Vertebrate Hox clusters comprise four homologue clusters
(HoxA, HoxB, HoxC , HoxD) as shown in
Figure 3 [
11]. Each homologue cluster is included in a separate chromosome. In these homologue clusters the PG identity is conserved. However, in the course of Evolution, modifications of the mouse genes are possible up to the point of gene deletion.This ordered mouse Hox clusters remind of a ratchet allowing motion in one direction only [
8]. Note that some ‘teeths’ of the ratchet may be missing.
2. Symmetries entangled with gene ordering in Hox gene clusters
Symmetry is an important concept in Science as stressed in [20 - 21] (see also the Appentices below).
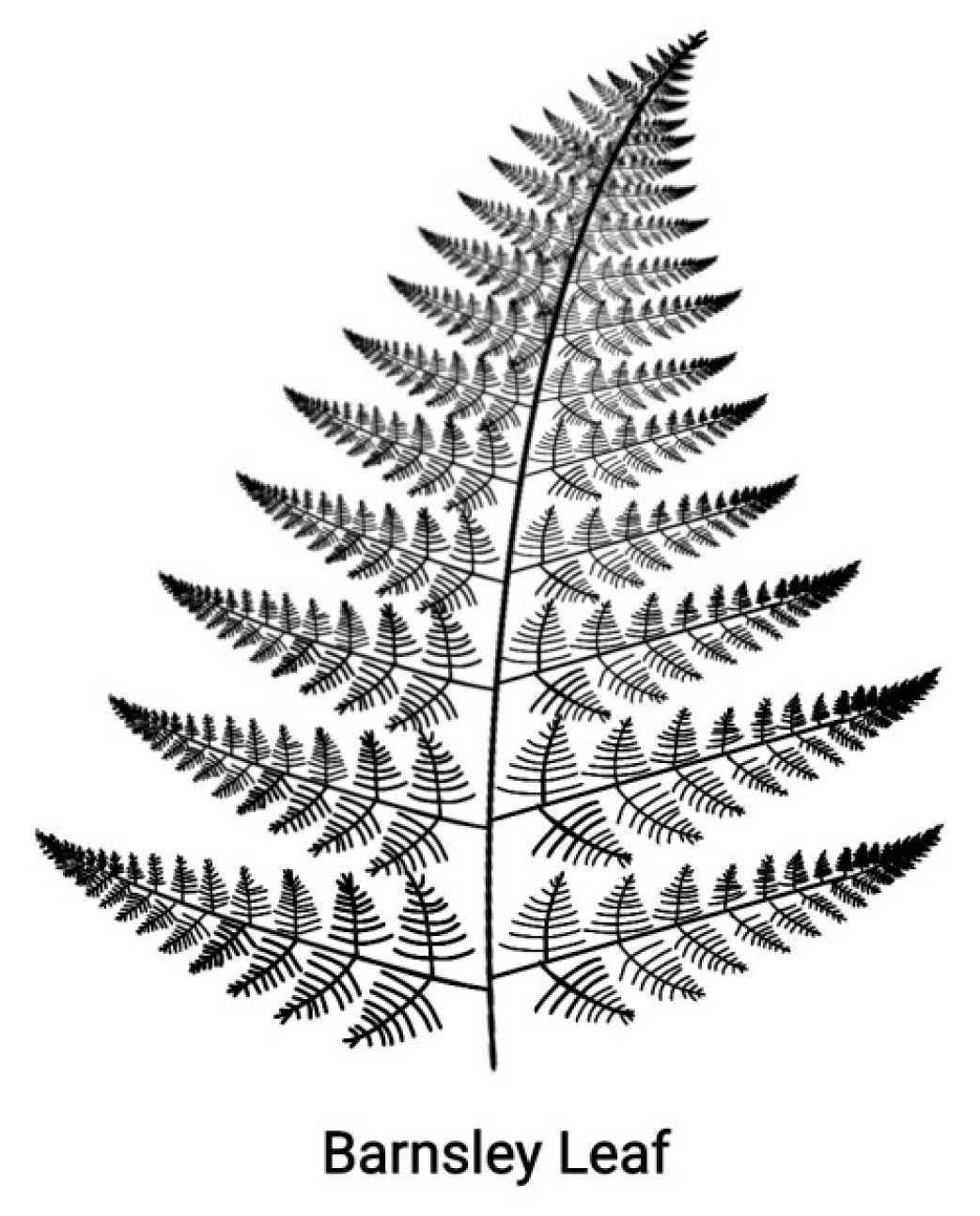
Self-similarity is the particular symmetry of objects which, although different, they look the same if depicted under a suitable scale unit. Such objects are the
fractals where the part looks like the whole with a typical example being the Barnsley fern shown in
Figure 4 [
22]. Self-similarity is a continuous symmetry extending to all geometric scales. In contrast, the linear ordering (Hox1, Hox2,…Hox13) of the Hox genes of a Hox cluster and the ontogenetic units of the embryo along the AP-axis refer to only two geometric scales. In this spirit, the two entities can be considered as defective self similar [8, 21]. In this defective self-similarity, the PG ordering is conserved where some Hox genes of the cluster may fade out up to extinction during the Whole Genome Duplication D of the evolutionary process [20-21].
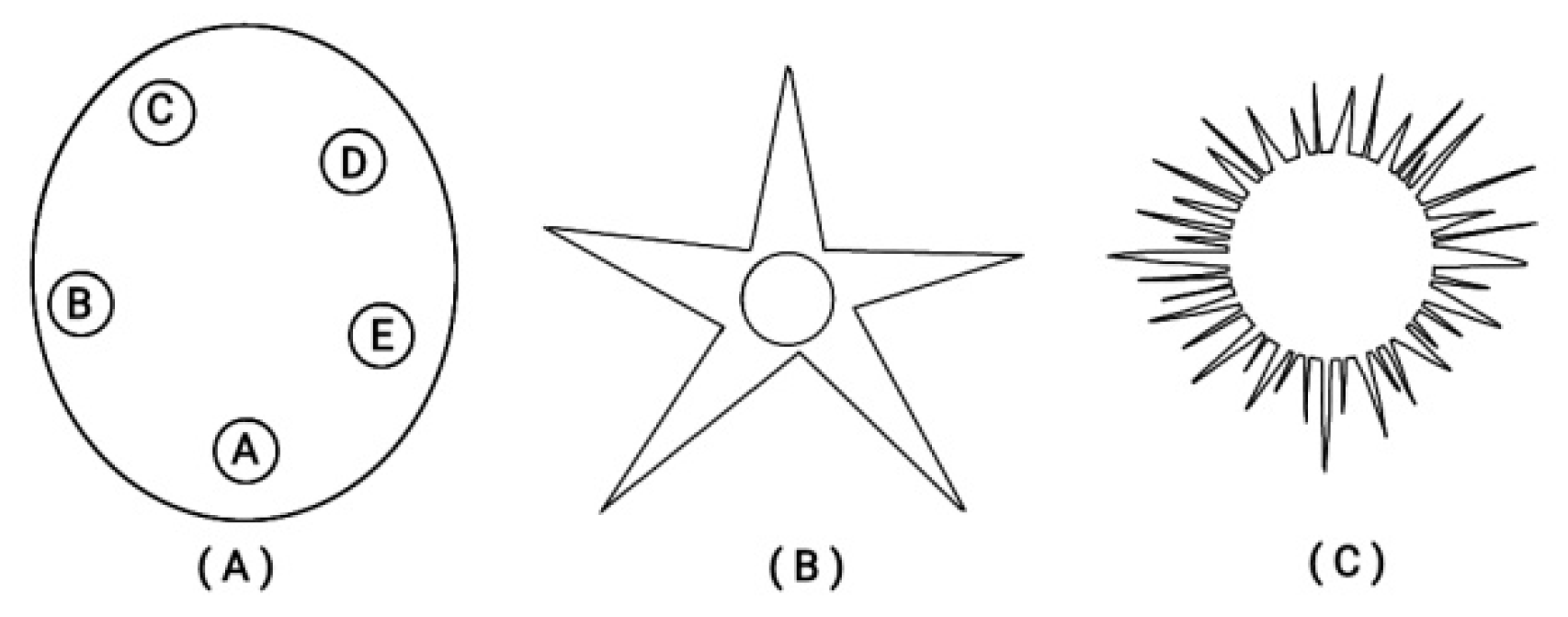
Besides this ordering of Hox genes on a finite straight line, in many early larva embryos (e.g. the echinoderms) a circular ordering is superimposed as shown in
Figure 5 [
23].
Surprisingly, a self-similarity rule applies so that a circular ordering of the Hox cluster corresponds to the embryonic circular ordering as shown in
Figure 6. (See details in [
8] and [
23]).
In Figure 6B the two ends of the cluster are attached to the 3’ and 5’ ends of the flanking chromosome. If the 3’ end of the flanking chromosome is attached to Hox1(and 5’ to Hox13) no novelty is created and
A.planci Hox gene order gene is reproduced. In contrast, if the 5’ end of the flanking chromosome is attached to Hox1, Hox2. Hox3 (shown in
Figure 6C) a novelty is created. A second breaking follows leading to a new Hox gene order which corresponds to the Hox gene order of the
sea urchin [
23]
.
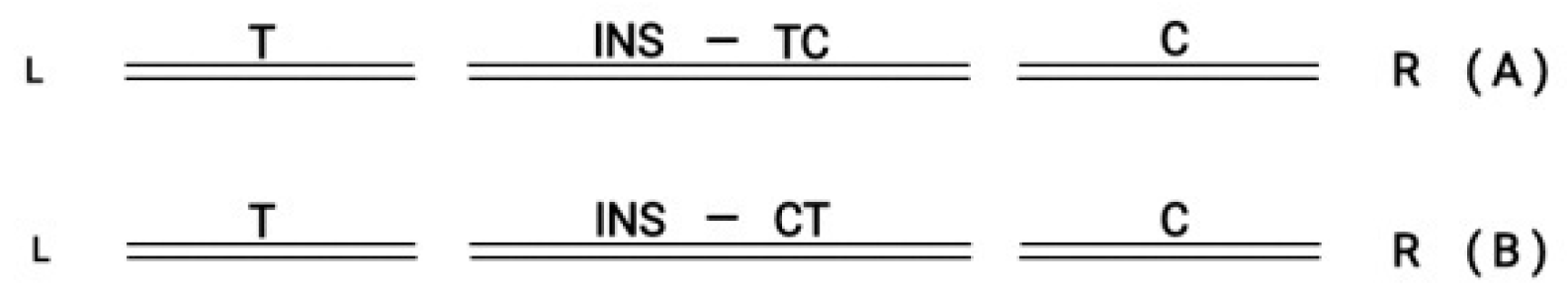
The circular Hox gene clusters can be incorporated in the flanking DNA sequence of the genome. A recent review by T. Hanscom refers to the well known technique of double strand break (DSB) [
24]. In the above review, besides the usual medical applications of the DSB methodology, it is extensively emphasized the novel trends of research to explore how DSB can leverage genome evolution. It is here schematically depicted the incorporation of a Hox cluster in the flanking genome (
Figure 7).
In (A) the inserted graft in the middle follows the orientation T→ C In (B) the inserted graft in the middle follows the reverse orientation (T ← C).
3. Complete vs split Hox clusters
Hox Gene Collinearity (both Spatial and Temporal) has been unequivocally confirmed in the Vertebrates. However, in recent years it was found that in many other animal species this is not true, particularly in invertebrates. For instance, it was observed that Hox Colinearity is violated in the lophotrochozoa and this violation was associated with the brachiopods whose Hox cluster is broken [
25]. In brachiopods both spatial and temporal collinearities are violated,while lophotrochozoan morphological novelties result from Hox Collinearity violation [
25]. It was argued above that for the insertion of a circularly organized Hox cluster in the flanking genome, a break (split) of the cluster is necessary as shown in
Figure 6C. It is clear that Hox cluster splitting is a necessary step for evolutionary novelties.
It has been emphasized that tight Hox clustering is lost during Evolution [26-29]. More specifically D. Ferrier and P. Holland assumed that Hox clusters are necessarily constrained in their gene order by TC. Moreover, when TC is no more needed, ‘Hox gene clusters may fall apart’ [26, 27]. Similar arguments were put forward before and after the above assumption [
28,
29].
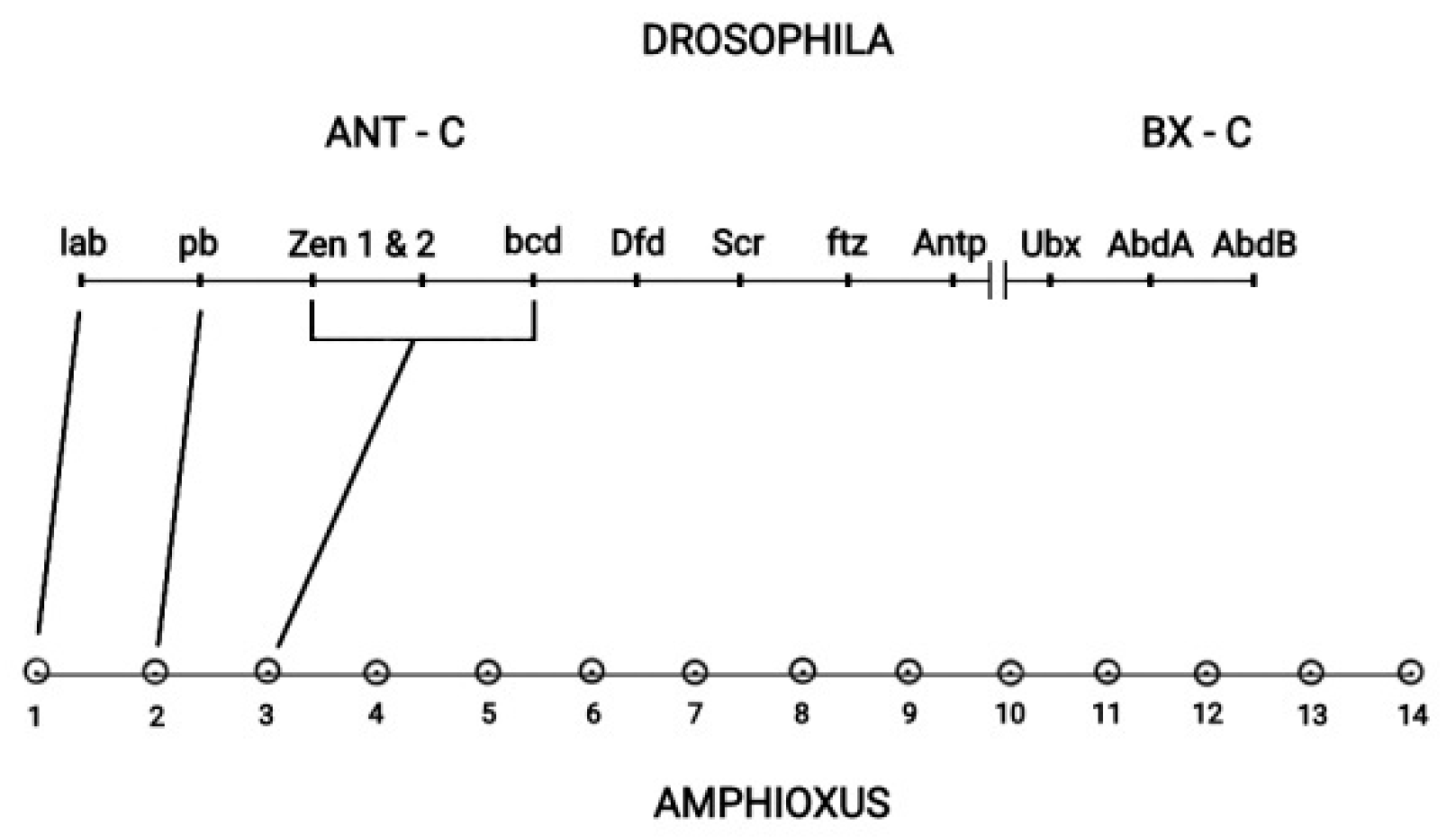
The Drosophila Hox cluster has 13 genes contained in the two subclusters ANT-C and BX-C depicted in
Figure 8 [
27]. Drosophila has a typically split Hox cluster. It was shown that this cluster consists of two subclusters Ant-C and BX-C. Drosophila together with the vertebrate Hox cluster originate from a large ancestral Hox cluster [
27]. Cloning had later identified Amphioxus as the common ancestor of insects and vertebrates [
28], and a one-to-one correspondence between the Amphioxus Hox genes and the Drosophila Hox genes was confirmed [
27]. However in this correspondence some Drosophila Hox genes of the ANT-C subcluster developed novel evolutionary non-Hox functions. For instance, the Drosophila complex of Hox genes (zen1, zen2, bcd) corresponds to the ancestral Amphioxus Hox3 gene. Some Central genes have evolved from tandem evolutionary duplications [
27]. (see in [
27]
Figure 1). The BX-C subcluster consists of the 3 last genes (Ubx, Abd-A, Abd-B) of the Drosophila Hox cluster. The summarizing conclusion from the above analysis is that TC is responsible for a
complete Hox cluster. If this is not possible (or not needed) the Hox cluster is split. In any case SC is a necessity for a Hox cluster [
26].
An Empirical Rule (ER) formulated by D. Duboule is a useful guide [
28]: ‘A complete cluster controlling development in time along the Anterior-Posterior axis is non-split, whereas animals developing according to a time-independent mechanism to produce their main body axis are licensed to split their clusters…’ In the framework of BM, a ‘prediction in retrospect’ was proposed according to which TC really disappears and not as if it were real [
30]. This disappearance occurs if an extended upstream DNA domain of the Hox cluster is cut-off [
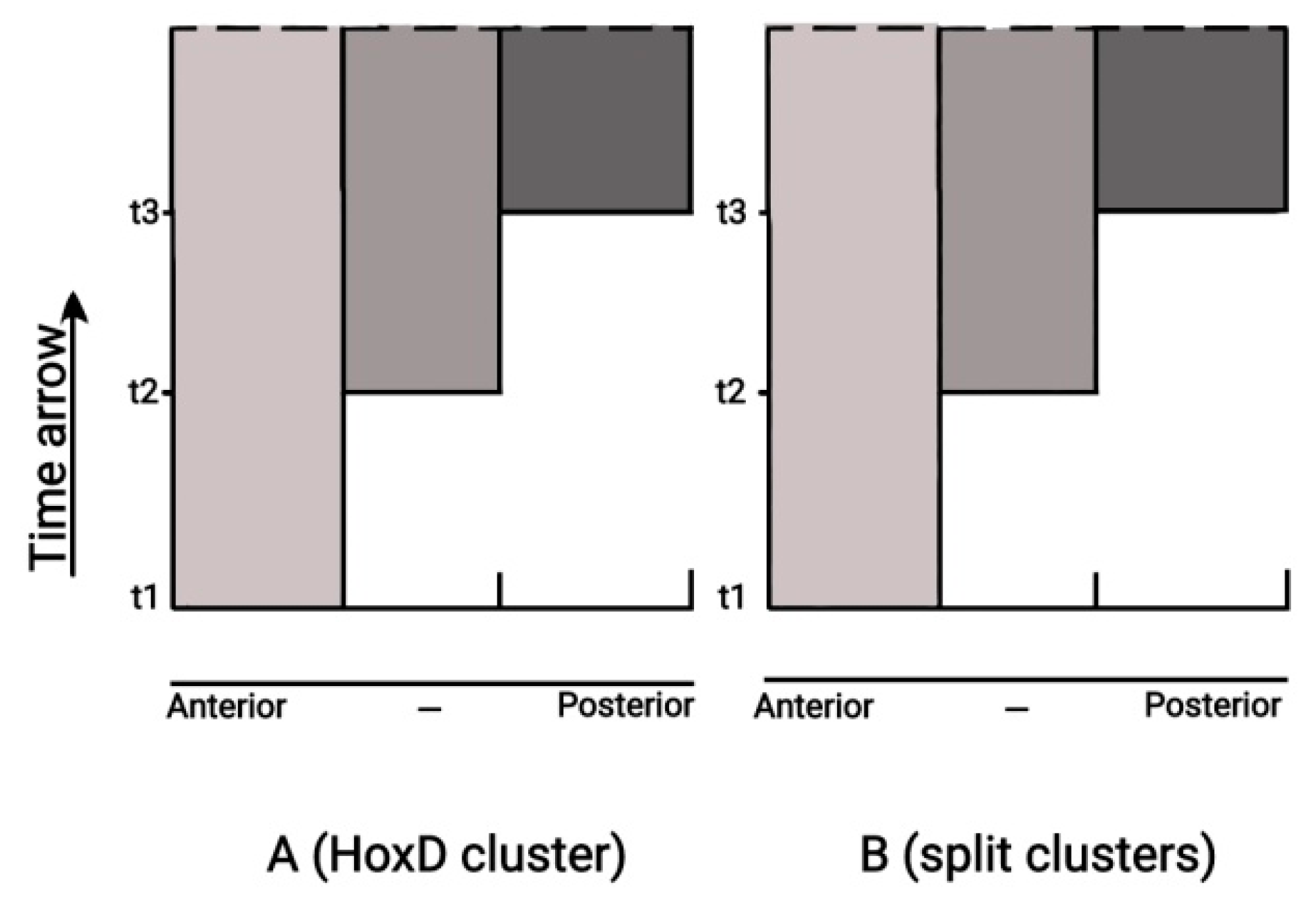
31]. In this spirit it is shown in
Figure 9 how a split Hox cluster approaches sequentially in time (t1, t2, t3) the state of completeness where TC is no more needed. In this interpretation, the pulling forces of BM provide a strong theoretical support of the Empirical Rule.
In
Figure 2D, an alternative situation is shown: if the Hox cluster is not fastened at all, a minimal pulling force can slide the cluster in the transcription factory domain. In the evolutionary course, a spontaneous mutation may occur which dismantles the fastening of the Hox cluster with dramatic repercussions on both evolution and genetic structure and function of the cluster:
the disappearance of TC. Therefore, the loss of TC in Drosophila could serve as a further confirmation of the ER.
5. Conclusion
New technological advances (e.g. STORM- the stochastic optical reconstruction microscopy) made possible the measurement of quantities and properties that were inaccessible before. Physical tension in Hox clusters is such a case and more specifically the tension of DNA topological domains which are important for Hox gene activation. A.R. Amàndio
et al. have recently measured mouse HoxD clusters under physical tension [
33]. The origin of this tension is elusive. This team has even considered the possibility of physical forces of BM to be responsible for this phenomenon. In this case they argue that ‘the forces would be generated by the local chromatin interactions themselves, rather than through an asymmetrically localized point of attachment to the nuclear environment’[
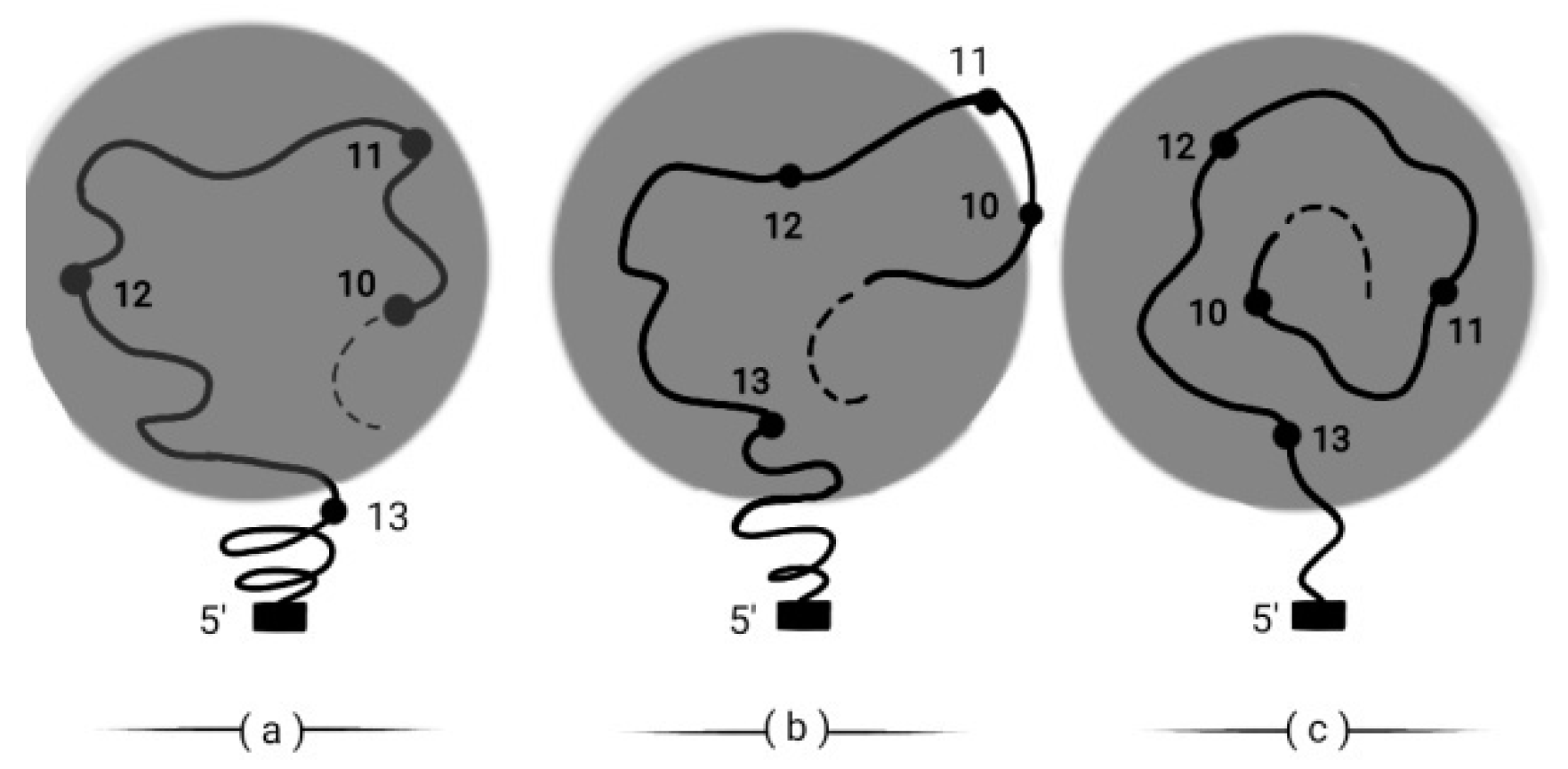
33]. Indeed this is most probable. Moreover, in the framework of BM, it is expected that in the global Hox cluster activation, there appear specific genes of the cluster (e.g. a small DNA strip containing Hox10 and Hox11) which remain inactive for a variable time interval. The Hox cluster is activated in the shadowed circle. The strip is pushed out of this Hox activation domain shown in (b) of
Figure 10 [
34].
In conclusion in
Figure 10 are shown: (a) Hox10,…Hox12 are activated. (b) (Hox10, Hox11) are pushed out of the activation domain. (c) At a later time, (Hox10, Hox11) reenter in the activation domain, according to the BM increased pulling force.
It is interesting that the theoretical prediction of ‘Biophysics
preceds Biochemistry’[
34] was experimentally confirmed soon after ‘…structural organization of HoxD cluster may
predate transcriptional activation…’ [
16].
Natural Sciences are connected to the other branches of intellectual activity. A short epistemological overview is included in [
40].
Figure 1.
Macro-scale and Micro-scale Hox gene clustering (adapted from S. Papageorgiou, Biology 2017: 6,32). (A) Concentration (T1, T2. T3) along the Ant. - Post. Axis. (B) Time sequence (t1, t2. T3) combine with (T1, T2,T3) for expression domains of Hox1, Hox2, Hox3. (C) (bottom) a small force F1 pulls Hox1 out of the chromatin territory CT toward the Interchromosome Domain (ICD) and the Transcription Factory (TF) regime (grey domain). Polar Molecule P is opposite the telomeric end. At a later stage (top), a stronger force F3 pulls Hox1, Hox2, Hox3 out of CT in TF.
Figure 1.
Macro-scale and Micro-scale Hox gene clustering (adapted from S. Papageorgiou, Biology 2017: 6,32). (A) Concentration (T1, T2. T3) along the Ant. - Post. Axis. (B) Time sequence (t1, t2. T3) combine with (T1, T2,T3) for expression domains of Hox1, Hox2, Hox3. (C) (bottom) a small force F1 pulls Hox1 out of the chromatin territory CT toward the Interchromosome Domain (ICD) and the Transcription Factory (TF) regime (grey domain). Polar Molecule P is opposite the telomeric end. At a later stage (top), a stronger force F3 pulls Hox1, Hox2, Hox3 out of CT in TF.
Figure 2.
Mechanical analogue of Hox cluster decondensation (adapted from S. Papageorgiou, Current Genomics 2012, 13:3). a) (left) Before activation Hox cluster is condensed inside (CT). (right) Mechanical analogue: uncharged elastic spring fixed at its left end. b) (left) BM pulling force decondenses the cluster and Hox1 is extruded in (ICD) in the transcription factory (TF) domain (shadow disc) The cluster is fastened posteriorily. (right) A small force F1 slightly expands the spring and black spot moves beyond the dashed line. Spring fixed posteriorily. c) (left) Hox cluster is further decondensed and Hox1, Hox2, Hox3 move in (ICD). (right) F2> F1 and the spring is further expanded. d) (left) The fastening of the cluster is removed and, with a smaller force F1, the cluster can slide beyond the dashed line. (right) the loose spring slides freely beyond the dashed line.
Figure 2.
Mechanical analogue of Hox cluster decondensation (adapted from S. Papageorgiou, Current Genomics 2012, 13:3). a) (left) Before activation Hox cluster is condensed inside (CT). (right) Mechanical analogue: uncharged elastic spring fixed at its left end. b) (left) BM pulling force decondenses the cluster and Hox1 is extruded in (ICD) in the transcription factory (TF) domain (shadow disc) The cluster is fastened posteriorily. (right) A small force F1 slightly expands the spring and black spot moves beyond the dashed line. Spring fixed posteriorily. c) (left) Hox cluster is further decondensed and Hox1, Hox2, Hox3 move in (ICD). (right) F2> F1 and the spring is further expanded. d) (left) The fastening of the cluster is removed and, with a smaller force F1, the cluster can slide beyond the dashed line. (right) the loose spring slides freely beyond the dashed line.
Figure 3.
Mouse Hox Gene Clusters (adapted from Z. Afzal and R. Krumlauf, J Dev Biol 2022) HoxA, HoxB, HoxC and HoxD are depicted in the direction of transcription for each gene. The mouse embryo is also shown in the Anterior – Posterior direction.
Figure 3.
Mouse Hox Gene Clusters (adapted from Z. Afzal and R. Krumlauf, J Dev Biol 2022) HoxA, HoxB, HoxC and HoxD are depicted in the direction of transcription for each gene. The mouse embryo is also shown in the Anterior – Posterior direction.
Figure 4.
A self-similar design. The Barnsley fern reproduced from Wikipedia - the free Encyclopedia.
Figure 4.
A self-similar design. The Barnsley fern reproduced from Wikipedia - the free Encyclopedia.
Figure 5.
Circular organization of echinoderms at different developmental stages. (A) Strongylocentratus purpuratus larva (with five podia). (B) An adult starfish (with five podia). (C) A juvenile sea urchin.
Figure 5.
Circular organization of echinoderms at different developmental stages. (A) Strongylocentratus purpuratus larva (with five podia). (B) An adult starfish (with five podia). (C) A juvenile sea urchin.
Figure 6.
Circular ordering of Hox gene clusters (adapted from S. Papageorgiou 2016, Current Genomics).(A) Bending of the 13 genes in a circle. (B) The ends of the cluster (Hox1 and Hox13) are attached to the 3’ and 5’ ends of the flanking chromosome. (C) A different attachment of Hox1 and Hox13 to the flanking chromosome. (see the text below).
Figure 6.
Circular ordering of Hox gene clusters (adapted from S. Papageorgiou 2016, Current Genomics).(A) Bending of the 13 genes in a circle. (B) The ends of the cluster (Hox1 and Hox13) are attached to the 3’ and 5’ ends of the flanking chromosome. (C) A different attachment of Hox1 and Hox13 to the flanking chromosome. (see the text below).
Figure 7.
(A) L Double Strand Break Left (T) and Right (C) in the middle ( INS) follows orientation (T→C) R. (B) L Double Strand Break Left (T) and Right (C) in the middle (INS) follows orientation (T←C) R .
Figure 7.
(A) L Double Strand Break Left (T) and Right (C) in the middle ( INS) follows orientation (T→C) R. (B) L Double Strand Break Left (T) and Right (C) in the middle (INS) follows orientation (T←C) R .
Figure 8.
The ANT-C and BX-C subclusters of Drosophila and Amphioxus (adapted from D.E.K. Ferrier in Hox Gene Expression editor S. Papageorgiou. Editions Springer and Landes Bioscience 2007).
Figure 8.
The ANT-C and BX-C subclusters of Drosophila and Amphioxus (adapted from D.E.K. Ferrier in Hox Gene Expression editor S. Papageorgiou. Editions Springer and Landes Bioscience 2007).
Figure 9.
Quantitative Collinearity (left) and Split clusters (right). The split clusters of section 3 and Quatitative collinearity of section 4.3 are described by identical mechanisms.
Figure 9.
Quantitative Collinearity (left) and Split clusters (right). The split clusters of section 3 and Quatitative collinearity of section 4.3 are described by identical mechanisms.
Figure 10.
A small fragmental strip of the HoxD cluster is pushed out of the cluster expression domain in (b).
Figure 10.
A small fragmental strip of the HoxD cluster is pushed out of the cluster expression domain in (b).