1. Introduction
The human parvovirus B19 (B19) is a linear, non-enveloped DNA virus, classified in the Parvoviridae family, genus Erythroparvovirus [
1] with three distinct genotypes: 1, 2 and 3 [
2]. It is a highly infectious virus, disseminated by respiratory secretions, transfusion of infected blood products, and vertically from mother to child [
3,
4]. It has a selective tropism for cells in the erythroid lineage in the bone marrow. Virus binding to the P antigen can induce severe hemolytic reactions such as chronic anemia, aplastic crisis and hydrops fetalis [
5]. Most cases of B19 infection tend to be asymptomatic, are more frequent in childhood and have a variable seroprevalence from 40% to 87% [
6,
7,
8].
B19 is associated with a wide range of clinical manifestations, from asymptomatic forms to severe neurological disorders [
8,
9,
10]. Encephalitis and encephalopathy are the most common consequences of parvovirus B19 infection in the central nervous system (CNS) and accounts for 38.8% of the total B19- associated neurological sequelae [
11].
B19 is clinically indistinguishable from several other viruses; they co-circulate with measles virus, rubella virus, and arboviruses. In remote areas of Brazil several emerging and re-emerging viruses are present in the population and differential diagnostic tests are scarce. Due to the similarity of B19 clinical symptoms with other viruses, the true B19 clinical and epidemiological impact on public health remains unknown. The percentage of cases due to B19 infection are often mistakenly reported [
12,
13]. In Brazil, neurological consequences of a parvovirus B19 infection are considered rare, but laboratory tests are not always available to rule out their occurrence[
14].
Metagenomic next-generation sequencing (mNGS) technology is a high-throughput approach that can simultaneously investigate all genetic material in a biologic sample. It has been utilized as a very useful exploratory method for the identification of viral infections of undetermined etiology when specific agents are not detected by standard diagnostic tests [
15,
16,
17,
18].
Although B19 has previously been reported to cause a variety of clinical symptoms in patients with different immune disorders, B19 infection in patients younger than 1 year of age is not common. Here, we report the use of mNGS for the diagnosis of an unusual case of acute viral meningitis in a one-month-old newborn, admitted to the Hospital das Clinicas da Faculdade de Medicina, Sao Paulo University São Paulo, Brazil, with fever, Haemophilus Influenzae meningitis, and hydrocephalus secondary to the infection. Using metagenomics as an alternative method to aid in the diagnosis of meningitis, we detected and characterized the entire genome of B19 from the cerebrospinal fluid (CSF) of this newborn. Metagenomics analysis of CSF in a patient with a severe neurological disorder may be an effective tool for investigating the presence of sequence diversity in a biological sample and represents a novel protocol that may yield a more precise insight into viral evolution dynamics.
2. Setting and case report
The present case report evolved from an analysis of CSF samples collected from patients with suspected CNS infection enrolled in a hospital-based surveillance study conducted in Sao Paulo, Brazil, from March 2018 to November 2019. A total of 600 CSF samples were collected at the Hospital das Clinicas da Faculdade de Medicina da Universidade de Sao Paulo, a public hospital (300 samples) and from other different private hospitals in the same city (300 samples), from individuals (both genders and any age group) with clinical suspicion of acute infectious encephalitis or meningitis.
After collection, as per the study protocol, all CSF samples were tested for a range of pathogens using the diagnostic work-up performed in the clinical study which initially included the latex agglutination test, India ink staining, and bacterial, fungi and tuberculosis cultures. The metagenomic approach was performed as a complementary exploratory analysis to determine the etiology of cases of meningitis, encephalitis, or acute meningoencephalitis in which no cause was identified after this previous screening. A specific infectious etiology was defined in 300 cases but remained unknown in the remaining 300 cases.
The remaining volume of the CSF samples were stored at −80°C for further testing in the Virology Laboratory at the Tropical Medicine Institute at Sao Paulo University Medical School where a molecular screening by real time PCR for Human Enterovirus, Parvovirus B19 , Parechovirus [
19,
20] and Herpesvirus was performed in a viral panel as with some modifications [
21] [
22]. Among all 600 samples tested, only one sample (0.17%) was positive for Parvovirus B19 by real time PCR.
2.1. Case report
A 38-day old girl was admitted to our hospital with a diagnosis of bacterial meningitis, seizures, and acute hydrocephalus on May 28th, 2019. She had been delivered by cesarean section without complications and achieved normal growth until the 30th day of life, when she presented with fever and vomiting. She was immediately referred for additional analysis. In the hospital unit, meningitis due to Haemophilus Influenzae was diagnosed by a Polymerase Chain Reaction (PCR) assay and antibiotic therapy was initiated. Her symptoms worsened to persistent seizures requiring orotracheal intubation (OTI) and sedation. Computer tomography (CT) performed 2 days after symptom onset revealed supratentorial hydrocephalus, in addition to diffuse cerebral edema. The baby was then referred to our hospital for hydrocephalus management, where she was admitted to the neonatal intensive care unit on May 28, 2019. Blood, urine, and CSF samples were collected to confirm the diagnosis of bacterial meningitis and to rule out other co-morbidities.
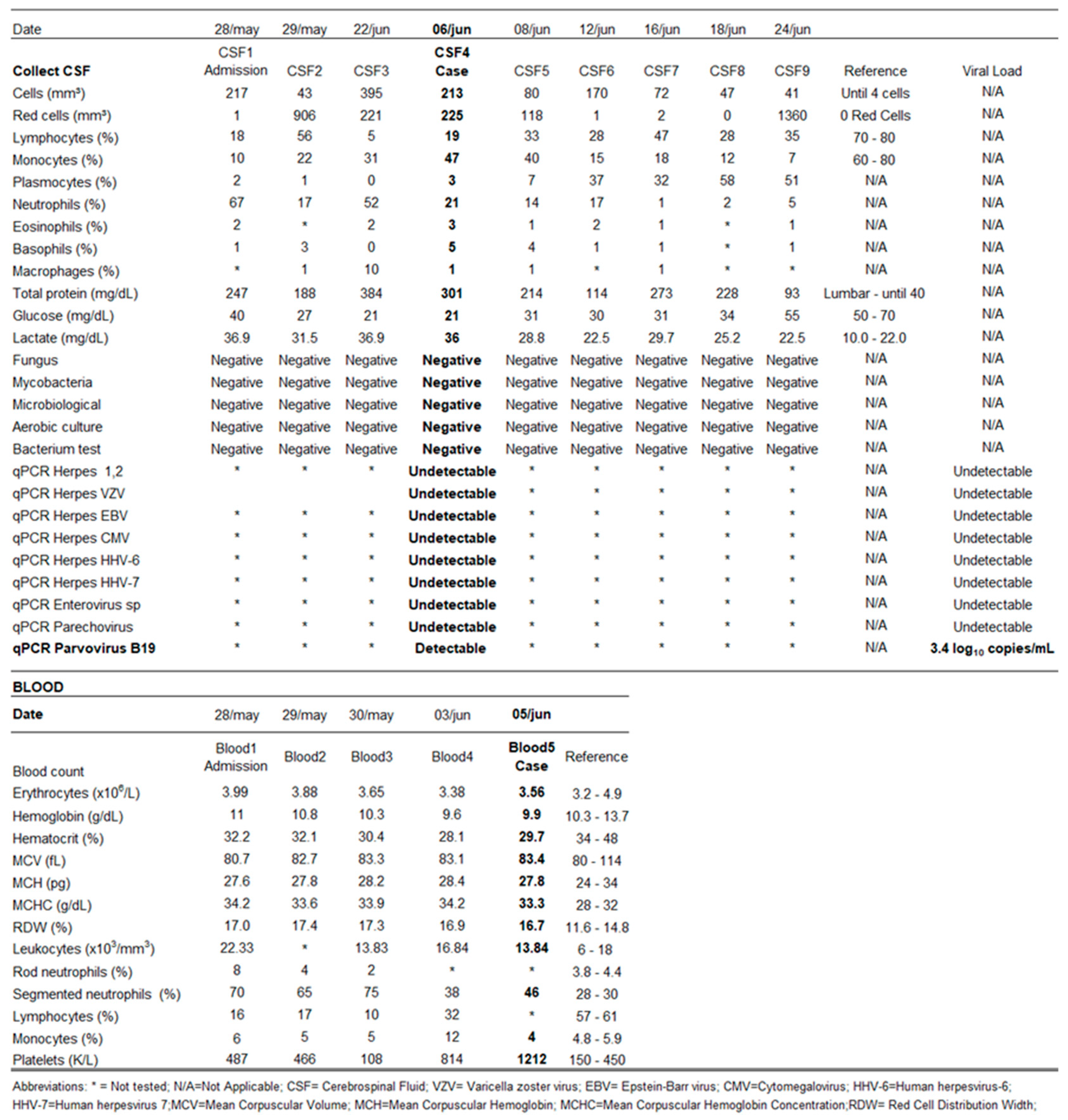
Routine analyses and cultures of CSF, blood, throat swabs and urine yielded no pathogenic growth. Antibiotic therapy was maintained for an additional one week after admission and was discontinued after negative laboratory tests were obtained. On admission to our hospital (nine days after symptoms onset) a CSF sample indicated pleocytosis (217 leucocytes/ml, with 18% lymphocytes and 67% neutrophils, monocytes 10%, plasma cells 2%, eosinophils 2%, basophils 1%), along with 1 red blood cell/ml, protein content 247 mg/ dL (60-80mg/dL), lactate level 36.9 mg/dL (10.0 to 22.0mg/dL) and glucose concentration 40 mg/dL (2/3 glycemia rate).
During her hospitalization (May 2019 to June 2019), other CSF samples were collected, revealing persistence of cytological changes, particularly an increase in the number of red blood cells. These alterations persisted for about 40 days after the onset of symptoms, in the absence of identification of any etiological agent. The patient’s initial CSF sample was subsequently subjected to a more complete etiological investigation.
Computer tomography performed 10 days after the onset of symptoms showed supratentorial hydrocephalus, diffuse cerebral edema, diffuse subarachnoid hemorrhage and multiple hypodense areas affecting the cortex and white matter of the frontal, temporal, parietal, and occipital lobes. An External Ventricular Drain (EVD) was inserted, and the patient remained hospitalized for three months for seizure control. She was discharged after stabilization of her general condition. During the clinical evolution, there was no skin rash described by the attending physicians or the patient´s relatives. A serological test for B19 was not performed.
Three years after the initial diagnosis of bacterial meningitis, the child is still being followed up at our hospital, with neurologic sequelae and a diagnosis of cerebral palsy.
3. Materials and Methods
3.1. Assessment of sample
Briefly, 500 µL of the CSF was centrifuged at 12.000 × g for 10 min, and total nucleic acids were extracted from 200 µl of the supernatant using the Extracta 96 Fast Kit (Loccus SP, SP, Brazil). Afterwards, cDNA synthesis was performed with Superscript IV Reverse transcription according to the manufacturer’s protocol (Thermo Fisher Scientific Inc.). A second strand of cDNA was obtained using DNA Polymerase I Large (Klenow) Fragment (Promega, WI, USA) and viral nucleic acid dosage was determined according to Quantus (Promega, WI, USA). Subsequently, the sample was submitted to a Nextera XT Sample Preparation Kit (Illumina, CA, USA) to construct a DNA library, identified by means of double barcodes. For size range selection, Pippin Prep (Sage Science, Inc.) was used to select a 400 bp insert (range 400-700 bp). The library was deep sequenced using the Nova Seq 6000 Sequencer (Illumina, CA, USA) with 250 bp ends.
The bioinformatic analysis was performed according to the protocol described by Deng et al [
23]. That shared a percent nucleotide identity of 95% or more were assembled from obtained sequence reads by reassembly. The complete B19 genome were mapped with Geneious R9.
4. Results
4.1. Laboratory and clinical epidemiological characterization
The CSF sample was negative for all other etiological agents tested as described above by the latex agglutination test, India ink staining, bacterial, fungi and tuberculosis cultures and real time PCR for Human Enterovirus, Parechovirus and Herpesvirus. Parvovirus B19 was detected with 3.4 log10 copies/mL by real time PCR.
Table 1 presents a summary of characteristics of the case and demographic data.
4.2. Metagenomic analysis of the CSF samples
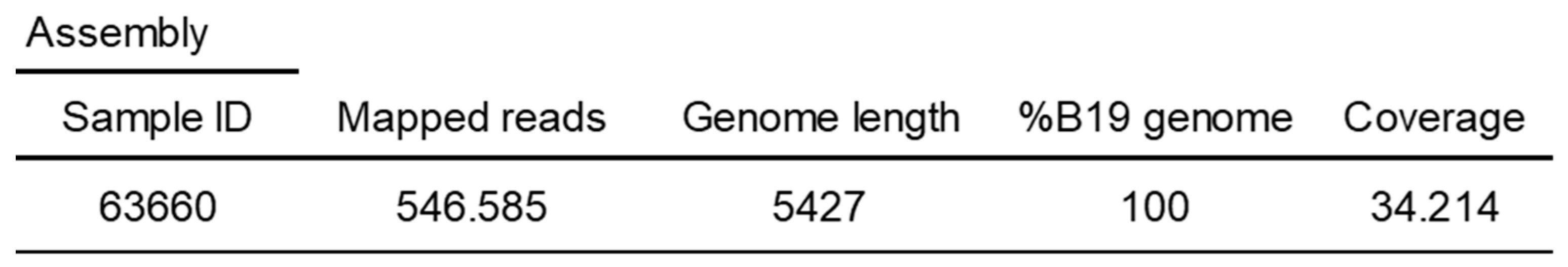
The Parvovirus B19 complete genome was recovered from the CSF sample by a metagenomic approach (
Table 2). This complete genome sequence (sample ID 63660) has been deposited in GenBank under the accession number pending.
4.3. Phylogenetic analysis
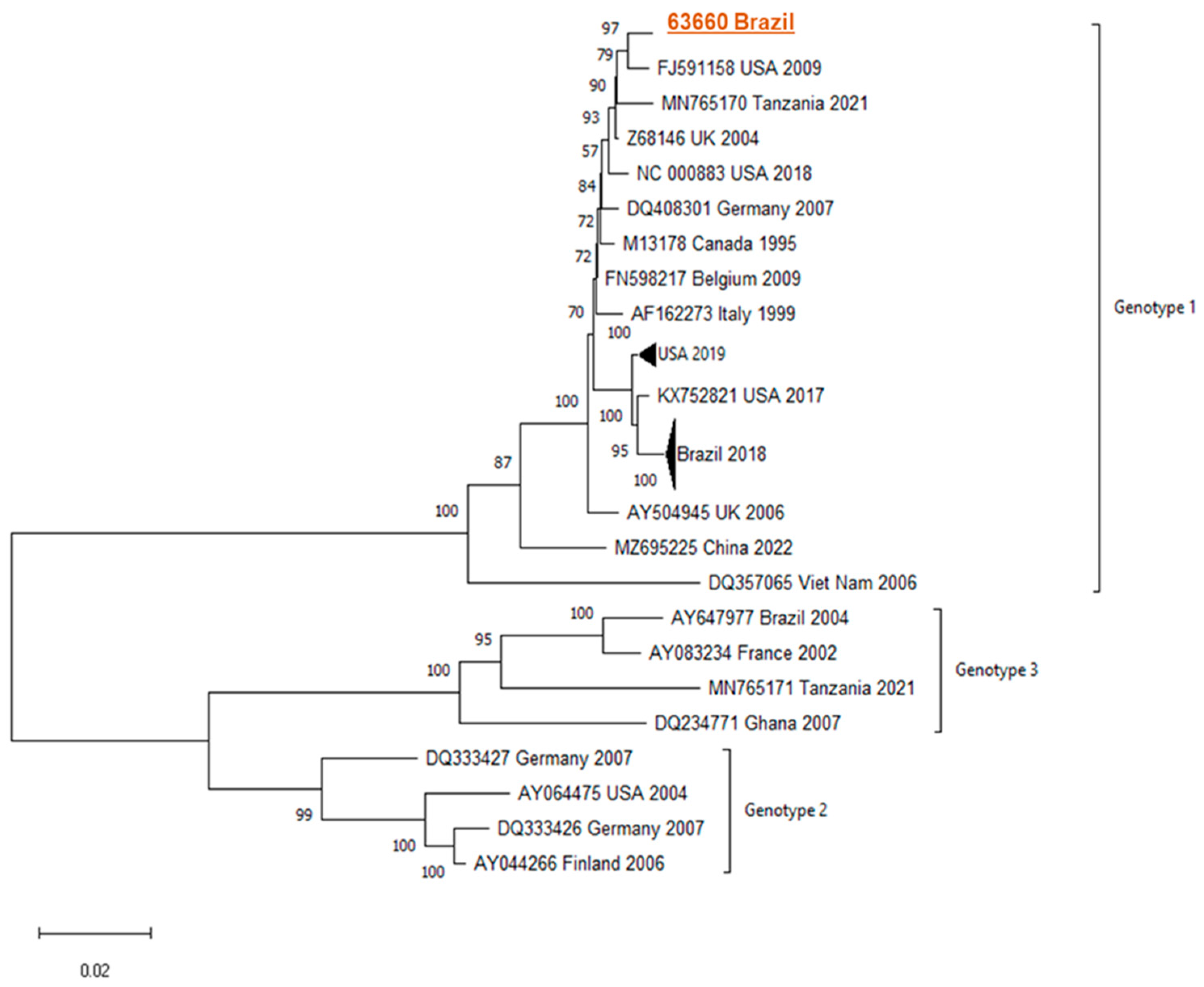
Phylogenetic analyses demonstrated similarity/clustering with genotype 1 B19 sequences compared to GenBank sequences.
Figure 1 presents the phylogenetic tree found.
5. Discussion
Parvovirus B19 is common worldwide and in addition to its classic manifestations, has been associated with a variety of neurological symptoms in children, including encephalitis and encephalopathy. However, B19 infection is not usually investigated in cases of symptomatic neurological diseases in newborns. In most reported cases, this virus was only sought after a negative finding of other pathogens commonly involved in meningoencephalitis.
In our series of 600 CSF samples from acute CNS infections B19 was identified in only a single sample (0.17%). In other case series, this virus has been identified as a pathogen associated with undiagnosed meningoencephalitis, reaching a prevalence rate of up to 4.3% [
14],[
26],[
27] , a frequency higher than that obtained in our series.
The present case report describes a patient of 38 days old with meningitis and encephalopathy associated with Haemophilus Influenzae and Human Parvovirus B19. Her CSF also revealed pleocytosis with increased protein and lactate, and normal glucose levels. These findings are in agreement with prior descriptions of Parvovirus B19 CNS infection [
14],[
28],[
29],[
30]. In blood tests, a decrease in hemoglobin with levels below 10 g/dL, and anisocytosis was observed , as reported in the literature [
14],[
31,
32]. The absence of a skin rash in this patient during the clinical course is consistent with previous reports in patients with neurological manifestations caused by B19 infection [
14].
Ten days after the onset of symptoms, imaging (CT) showed diffuse cerebral edema, diffuse subarachnoid hemorrhage, and multiple hypodense areas affecting the cortex and white matter in the frontal, temporal, parietal, and occipital lobes. All these alterations have been described among children acutely infected by either Parvovirus B19 or Haemophilus influenza [
29],[
33,
34]. Also, hydrocephalus and seizures are frequently described among the various neurological complications associated with both conditions [
33,
35].
The precise mechanism involved in the pathogenesis of B19 is still very controversial. Non-productive infection of B19 virus has been described in various tissues, including the CNS. It has been postulated that infection initiation stimulates cellular responses in the tissues against the virus which could culminate in a myriad of pathologies, such as meningoencephalitis [
3],[
36]. B19 infection or the expression of viral proteins could modulate the immune response in the infected tissues by dysregulation of cytokines and production of autoantibodies, or by the cytotoxic effect of the B19 viral nonstructural protein (NS1) damaging cerebrovascular endothelium [
9],[
37,
38,
39,
40,
41,
42,
43]
It has also been postulated that when patients are under immunosuppression or during concomitant infection with other pathogens, the B19 viral load increases, which could evoke a more potent inflammatory disease [
3],[
44]. In this regard the immature immune response of the newborn could have also contributed to the severe clinical outcome observed in the present case. The overlapping infections of Haemophilus influenzae and Parvovirus B19, as described in the present case, could also be a determining factor involved in the severity of neurological manifestations observed.
Parvovirus B19 concomitant infection with other infectious agents have been described, such as Influenza virus, staphylococcus, and mumps virus. All cases of B19 concomitant infection with other pathogens described in the literature have in common the fact that they are extremely severe and have evolved with serious sequelae or death.
To the best of our knowledge, this is the first case report of encephalopathy associated with Haemophilus Influenzae and Human Parvovirus B19.
It is relevant to comment on the possibility of vertical parvovirus B19 transmission during pregnancy. Parvovirus B19 infects 1-5% of pregnant women, generally with normal pregnancy outcomes [
3], [
45]. However, transmission of parvovirus B19 across the placenta can lead to fetal infection [
45]. The risk of fetal complications depends largely on the gestational age at the time of maternal infection with parvovirus B19. However, fetal abnormalities associated with parvovirus B19 are rare. Whenever present they may result from injuries to different fetal organs including the brain, and as a result may also cause neurodevelopmental problems.
In the case reported here, according to information from the child´s parents and medical records, pregnancy and delivery were uneventful, and the newborn developed normally until the 30th day of life, when Haemophilus influenzae meningitis was diagnosed. It is plausible to suppose that in case an asymptomatic parvovirus B19 infection occurred during pregnancy or just after delivery, the co-infection with Haemophilus influenzae may have triggered a more potent immune-mediated reaction which led to the observed neurological complications.
The Parvovirus B19 complete genome was recovered from the CSF and the genotypic showed, through phylogeny, sequences similar to B19 genotype 1. Genotype 1 is more prevalent in most parts of the world including Brazil [
12,
13],[
46,
47,
48,
49,
50], showing a close relationship with the American sequence (Genebank #FJ591158). The other Brazilian sequences available in the reconstructions were relatively distant, suggesting a distant ancestor. Assessing the nucleotide composition, we observed a variation of approximately 2% in sequences of the same genotype. This diversity is observed in single stranded DNA viruses due to their high mutation rate. According to our findings, there was no evidence of possible modifications in the analyzed Parvovirus B19 genome that would justify its presence in the CSF or the severity of the clinical manifestation. Further studies are necessary to evaluate the role of host's genetic factors or other viral factors which could be involved in the pathogenesis of Parvovirus B 19 in the CNS.
6. Conclusions
Based on the case analyzed here we hypothesize that: 1- Metagenomics analysis of a CSF sample would be useful in the diagnosis of severe meningoencephalitis cases as a complement to traditional diagnostic protocols; 2- B19 could be involved more frequently than has been described so far, since it is not regularly tested for in cases of acute neurological manifestations.
Author Contributions
Conceptualization, N.E.F and A.C.d.C.; investigation, N.E.F., L.H. and M.C.M.C.; funding acquisition, E.G.K., M.C.M.C., H.R.G.; project administration, C.G.T.S. and A.C.S.d.O.; methodology, N.E.F, A.C.d.C., S.H.L., M.C.M.C. and T.R.T.M.; sequencing analysis, N.E.F., A.C.d.C. and M.F.; writing—original draft preparation, N.E.F., and L.H.; data investigation, N.E.F and H.G.O.P.; writing—review and editing, T.R.T.M., S.S.W., M.C.M.C., D.V., S.F.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This case report was funded FAPESP (Research Support Foundation of the State of São Paulo-SP – Brazil -Process Number 2017/10264-6) and Abbott Diagnostics, Abbott Park, IL, USA.
Informed Consent Statement
Informed consent was obtained from the patient’s parents.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics
This study was approved by the local ethics committee (CONEP) , protocol No. CAAE: 67203417.0.0000.0068.
References
- Cotmore, S.F.; Agbandje-McKenna, M.; Canuti, M.; Chiorini, J.A.; Eis-Hubinger, A.M.; Hughes, J.; Mietzsch, M.; Modha, S.; Ogliastro, M.; Pénzes, J.J.; et al. ICTV Virus Taxonomy Profile: Parvoviridae. J Gen Virol 2019, 100, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Servant-Delmas, A.; Mercier, M.; Laperche, S.; Lefrère, J.J. [Genetic diversity of human erythroviruses. Consequences on infectious safety of plasma derivatives]. Transfus Clin Biol 2009, 16, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Söderlund-Venermo, M.; Young, N.S. Human Parvoviruses. Clin Microbiol Rev 2017, 30, 43–113. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.A.; Davis, J.S.; Schultz, W.H.; Ware, R.E. Subclinical parvovirus B19 infection in children with sickle cell anemia. J Pediatr Hematol Oncol 2003, 25, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.E.; Hibbs, J.R.; Gallinella, G.; Anderson, S.M.; Lehman, E.D.; McCarthy, P.; Young, N.S. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen). N Engl J Med 1994, 330, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Slavov, S.N.; Rodrigues, E.S.; Sauvage, V.; Caro, V.; Diefenbach, C.F.; Zimmermann, A.M.; Covas, D.T.; Laperche, S.; Kashima, S. Parvovirus B19 seroprevalence, viral load, and genotype characterization in volunteer blood donors from southern Brazil. J Med Virol 2019, 91, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Huatuco, E.M.; Durigon, E.L.; Lebrun, F.L.; Passos, S.D.; Gazeta, R.E.; Azevedo Neto, R.S.; Massad, E. Seroprevalence of human parvovirus B19 in a suburban population in São Paulo, Brazil. Rev Saude Publica 2008, 42, 443–449. [Google Scholar] [CrossRef]
- Vilmane, A.; Terentjeva, A.; Tamosiunas, P.L.; Suna, N.; Suna, I.; Petraityte-Burneikiene, R.; Murovska, M.; Rasa-Dzelzkaleja, S.; Nora-Krukle, Z. Human Parvoviruses May Affect the Development and Clinical Course of Meningitis and Meningoencephalitis. Brain Sci 2020, 10. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawashima, H. Acute encephalitis and encephalopathy associated with human parvovirus B19 infection in children. World J Clin Pediatr 2015, 4, 126–134. [Google Scholar] [CrossRef]
- Grillo, E.; da Silva, R.J. Childhood chorea-encephalopathy and unremarkable MRI: an association suggesting parvovirus B19 infection. Dev Med Child Neurol 2009, 51, 759–761. [Google Scholar] [CrossRef]
- Barah, F.; Whiteside, S.; Batista, S.; Morris, J. Neurological aspects of human parvovirus B19 infection: a systematic review. Rev Med Virol 2014, 24, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, N.; Mesquita, F.S.; Oliveira, D.B.L.; Villabona-Arenas, C.J.; Zaki Pour, S.; de Sousa-Capra, C.; Lopes, G.P.; Santana, R.A.F.; Pinho, J.R.R.; Balarini, K.; et al. An Outbreak of Human Parvovirus B19 Hidden by Dengue Fever. Clin Infect Dis 2019, 68, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Cnc Garcia, R.; Leon, L.A. Human parvovirus B19: a review of clinical and epidemiological aspects in Brazil. Future Microbiol 2021, 16, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, V.S.; Baía-da-Silva, D.C.; Silva, V.A.; Pivoto João, G.A.; Marinho, E.P.M.; Cubas-Vega, N.C.; Val, F.F.A.; Perez-Gomez, A.S.; Monte, R.L.; Mota, A.; et al. Neurological Manifestations Associated with Parvovirus B19 Infection in Immunocompetent Children: Case Series and Systematic Review. J Trop Pediatr 2021, 67. [Google Scholar] [CrossRef] [PubMed]
- Perlejewski, K.; Popiel, M.; Laskus, T.; Nakamura, S.; Motooka, D.; Stokowy, T.; Lipowski, D.; Pollak, A.; Lechowicz, U.; Caraballo Cortés, K.; et al. Next-generation sequencing (NGS) in the identification of encephalitis-causing viruses: Unexpected detection of human herpesvirus 1 while searching for RNA pathogens. J Virol Methods 2015, 226, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Shen, A.; Lv, X.; Yang, X.; Ren, H.; Zhao, Y.; Zhang, Y.; Gong, Y.; Ni, P.; Wu, H.; et al. Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing. J Neurovirol 2016, 22, 240–245. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, M.F.; Linhares, A.C.; Shirley, J.A. Fifth disease in children living in Belém, Brazil. Rev Inst Med Trop Sao Paulo 1989, 31, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.P.; Buckley, M.M.; Brown, K.E.; Cohen, B.J. The prevalence of antibody to human parvovirus B19 in Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo 1990, 32, 41–45. [Google Scholar] [CrossRef]
- Pabbaraju, K.; Wong, S.; Wong, A.A.; Tellier, R. Detection of enteroviruses and parechoviruses by a multiplex real-time RT-PCR assay. Mol Cell Probes 2015, 29, 81–85. [Google Scholar] [CrossRef]
- Verstrepen, W.A.; Kuhn, S.; Kockx, M.M.; Van De Vyvere, M.E.; Mertens, A.H. Rapid detection of enterovirus RNA in cerebrospinal fluid specimens with a novel single-tube real-time reverse transcription-PCR assay. J Clin Microbiol 2001, 39, 4093–4096. [Google Scholar] [CrossRef]
- Lima, L.R.; Silva, A.P.; Schmidt-Chanasit, J.; Paula, V.S. Diagnosis of human herpes virus 1 and 2 (HHV-1 and HHV-2): use of a synthetic standard curve for absolute quantification by real time polymerase chain reaction. Mem Inst Oswaldo Cruz 2017, 112, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.D.R.; Cubel Garcia, R.C.N.; Cruz, O.G.; Pinto, M.A.; Amado Leon, L.A. Quantitative real-time PCR for differential diagnostics of parvovirus B19 infection in acute liver failure patients. Expert Rev Mol Diagn 2019, 19, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucleic Acids Res 2015, 43, e46. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 1993, 10, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Barah, F.; Vallely, P.J.; Chiswick, M.L.; Cleator, G.M.; Kerr, J.R. Association of human parvovirus B19 infection with acute meningoencephalitis. Lancet 2001, 358, 729–730. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, C.; Prasad, P.; Sudarshan, S.; George, A.K.; Sreenivas, D.; Rasheed, R.; Ghosh, A.; Pal, A.; Hameed, S.K.S.; Bandyopadhyay, B.; et al. Identification and Genomic Characterization of Parvovirus B19V Genotype 3 Viruses from Cases of Meningoencephalitis in West Bengal, India. Microbiol Spectr 2022, 10, e0225121. [Google Scholar] [CrossRef]
- Erol, I.; Alehan, F.; Yalçin, K. Refractory status epilepticus owing to human parvovirus B19 encephalitis in a child. J Child Neurol 2006, 21, 820–822. [Google Scholar] [CrossRef]
- Greco, F.; Barbagallo, M.L.; Chiodo, D.C.; Guglielmino, R.; Sorge, G. Severe ataxia as a complication of human parvovirus B19 acute encephalitis in a child. J Child Neurol 2008, 23, 1078–1080. [Google Scholar] [CrossRef]
- Druschky, K.; Walloch, J.; Heckmann, J.; Schmidt, B.; Stefan, H.; Neundörfer, B. Chronic parvovirus B-19 meningoencephalitis with additional detection of Epstein-Barr virus DNA in the cerebrospinal fluid of an immunocompetent patient. J Neurovirol 2000, 6, 418–422. [Google Scholar] [CrossRef]
- Yetgin, S.; Cetin, M.; Yenicesu, I.; Ozaltin, F.; Uçkan, D. Acute parvovirus B19 infection mimicking juvenile myelomonocytic leukemia. Eur J Haematol 2000, 65, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J.; Sen, M. Parvovirus B19-induced thrombocytopenia and anemia in a child with fatal fulminant hepatic failure coinfected with hepatitis A and E viruses. J Trop Pediatr 2009, 55, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.F.; Myers, A.L. Changing Epidemiology of Haemophilus influenzae in Children. Infect Dis Clin North Am 2018, 32, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, S.; Sarnaik, S.A.; Becker, C.; Shurney, W.W.; Nigro, M.; Savaşan, S. Acute encephalopathy with parvovirus B19 infection in sickle cell disease. Arch Dis Child 2002, 87, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.O.; Torres, B.R.; Romanelli, R.M.C.; Rocha, F.S.V.; Viegas, E.C.C.; Diniz, L.M.O. Haemophilus influenzae Serotype a as a Cause of Meningitis in Children in Brazil. Pediatr Infect Dis J 2022, 41, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Adamson-Small, L.A.; Ignatovich, I.V.; Laemmerhirt, M.G.; Hobbs, J.A. Persistent parvovirus B19 infection in non-erythroid tissues: possible role in the inflammatory and disease process. Virus Res 2014, 190, 8–16. [Google Scholar] [CrossRef]
- Lunardi, C.; Tinazzi, E.; Bason, C.; Dolcino, M.; Corrocher, R.; Puccetti, A. Human parvovirus B19 infection and autoimmunity. Autoimmun Rev 2008, 8, 116–120. [Google Scholar] [CrossRef]
- Wagner, A.D.; Goronzy, J.J.; Matteson, E.L.; Weyand, C.M. Systemic monocyte and T-cell activation in a patient with human parvovirus B19 infection. Mayo Clin Proc 1995, 70, 261–265. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, X.Q. Acute viral encephalitis associated with human parvovirus B19 infection: unexpectedly diagnosed by metagenomic next-generation sequencing. J Neurovirol 2020, 26, 980–983. [Google Scholar] [CrossRef]
- Weigel-Kelley, K.A.; Yoder, M.C.; Srivastava, A. Alpha5beta1 integrin as a cellular coreceptor for human parvovirus B19: requirement of functional activation of beta1 integrin for viral entry. Blood 2003, 102, 3927–3933. [Google Scholar] [CrossRef]
- Cooling, L.L.; Koerner, T.A.; Naides, S.J. Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect Dis 1995, 172, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R.; Barah, F.; Chiswick, M.L.; McDonnell, G.V.; Smith, J.; Chapman, M.D.; Bingham, J.B.; Kelleher, P.; Sheppard, M.N. Evidence for the role of demyelination, HLA-DR alleles, and cytokines in the pathogenesis of parvovirus B19 meningoencephalitis and its sequelae. J Neurol Neurosurg Psychiatry 2002, 73, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, S.; Tanaka, N.; Tada, K.; Nose, M.; Nakamura, M.; Muraoka, O.; Hirano, T.; Sugamura, K. A cytotoxic nonstructural protein, NS1, of human parvovirus B19 induces activation of interleukin-6 gene expression. J Virol 1996, 70, 8485–8491. [Google Scholar] [CrossRef] [PubMed]
- Heegaard, E.D.; Brown, K.E. Human parvovirus B19. Clin Microbiol Rev 2002, 15, 485–505. [Google Scholar] [CrossRef]
- Ornoy, A.; Ergaz, Z. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res 2017, 109, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Hübschen, J.M.; Mihneva, Z.; Mentis, A.F.; Schneider, F.; Aboudy, Y.; Grossman, Z.; Rudich, H.; Kasymbekova, K.; Sarv, I.; Nedeljkovic, J.; et al. Phylogenetic analysis of human parvovirus b19 sequences from eleven different countries confirms the predominance of genotype 1 and suggests the spread of genotype 3b. J Clin Microbiol 2009, 47, 3735–3738. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.I.; Afonso, A.M.S.; Curti, S.P.; Silva, P.E.; Barbosa, T.F.; Silva, E.R.; Figueiredo, C.A. Genotype 1 of human parvovirus B19 in clinical cases. Rev Assoc Med Bras (1992) 2017, 63, 224–228. [Google Scholar] [CrossRef]
- Freitas, R.B.; Melo, F.L.; Oliveira, D.S.; Romano, C.M.; Freitas, M.R.; Macêdo, O.; Linhares, A.C.; de A Zanotto, P.M.; Durigon, E.L. Molecular characterization of human erythrovirus B19 strains obtained from patients with several clinical presentations in the Amazon region of Brazil. J Clin Virol 2008, 43, 60–65. [Google Scholar] [CrossRef]
- Conteville, L.C.; Zanella, L.; Marín, M.A.; Filippis, A.M.; Nogueira, R.M.; Vicente, A.C.; Mendonça, M.C. Parvovirus B19 1A complete genome from a fatal case in Brazil. Mem Inst Oswaldo Cruz 2015, 110, 820–821. [Google Scholar] [CrossRef]
- Oliveira, M.I.d.; Afonso, A.M.S.; Figueiredo, C.A.; Curti, S.P.; Klautau, G.B.; Sallum, M.A.M. Molecular characterization of human parvovirus B19 associated with neuromyelitis. Rev Pan-Amazonica de Saude 2011, 2, 71–74. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).