Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
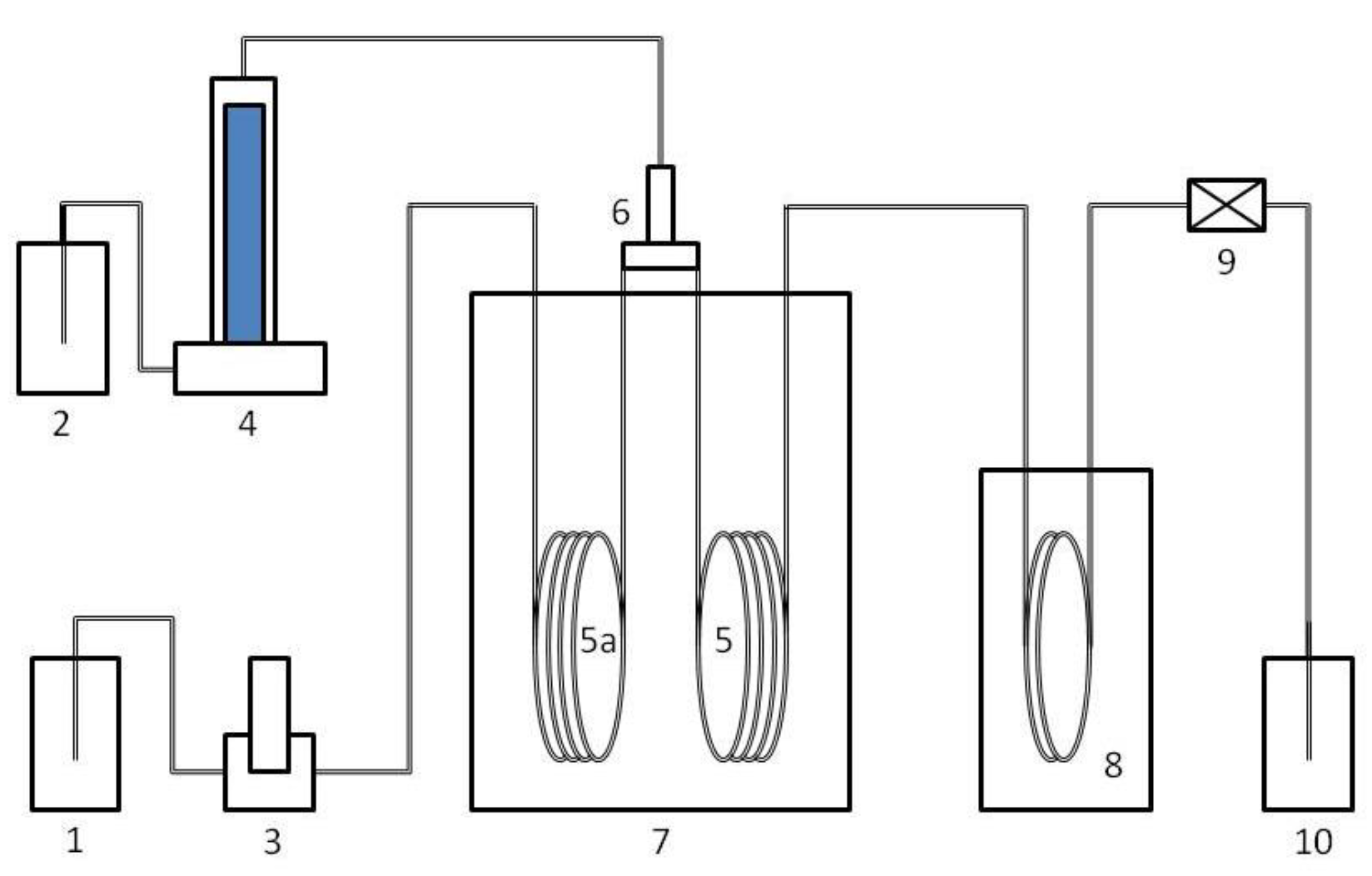
2.1. Synthesis Methods
2.2. Characterizations
2.3. Catalytic Tests
3. Results
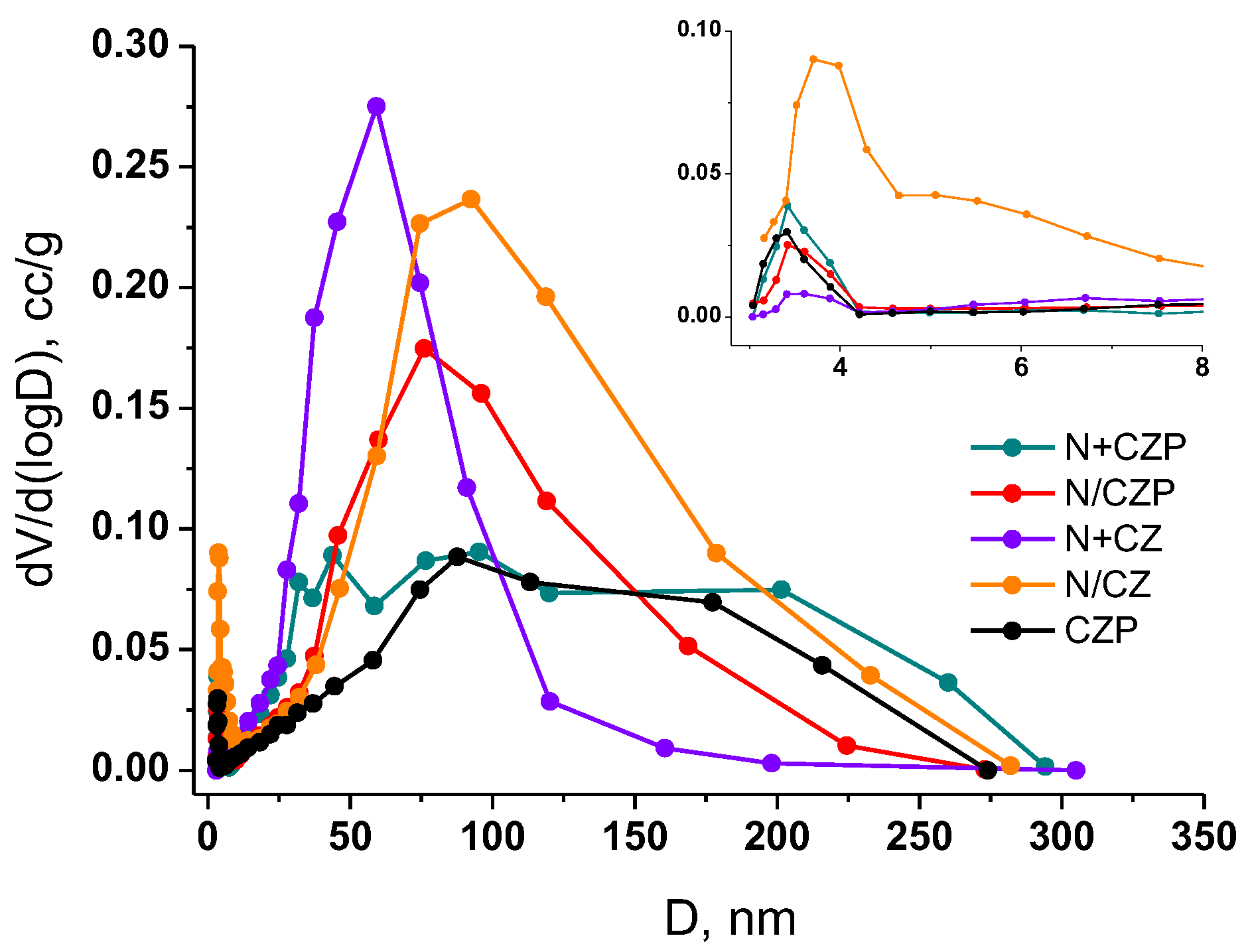
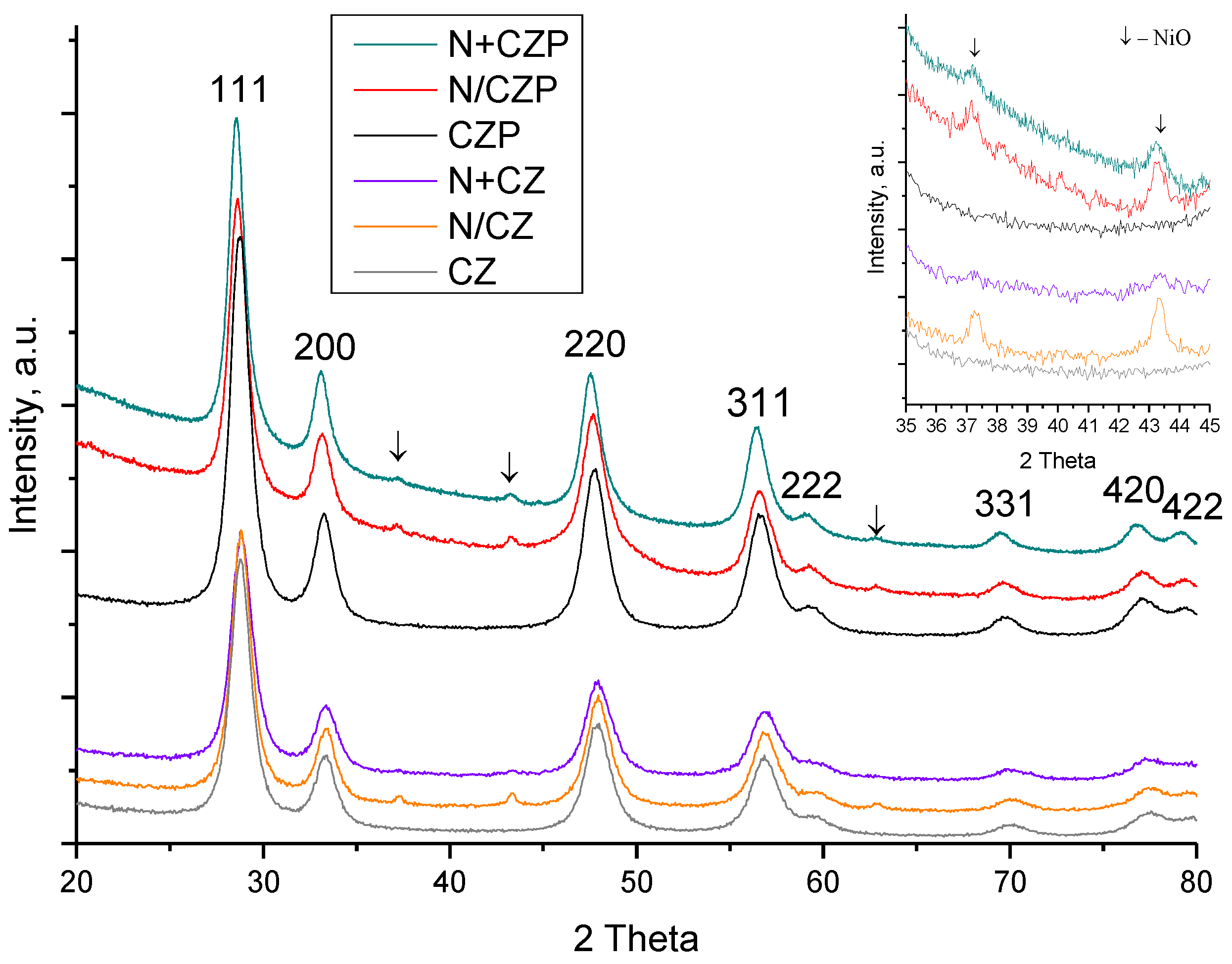
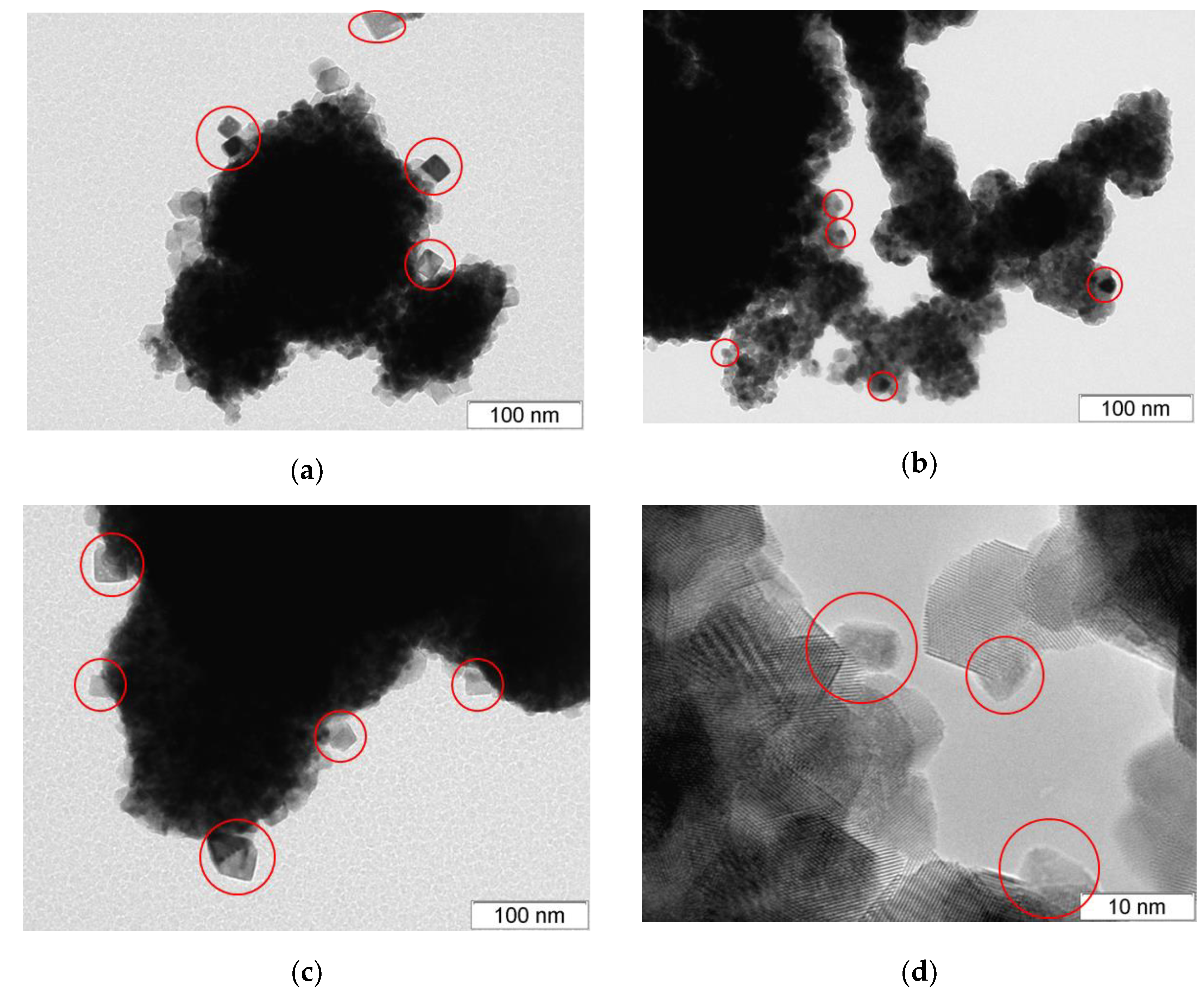
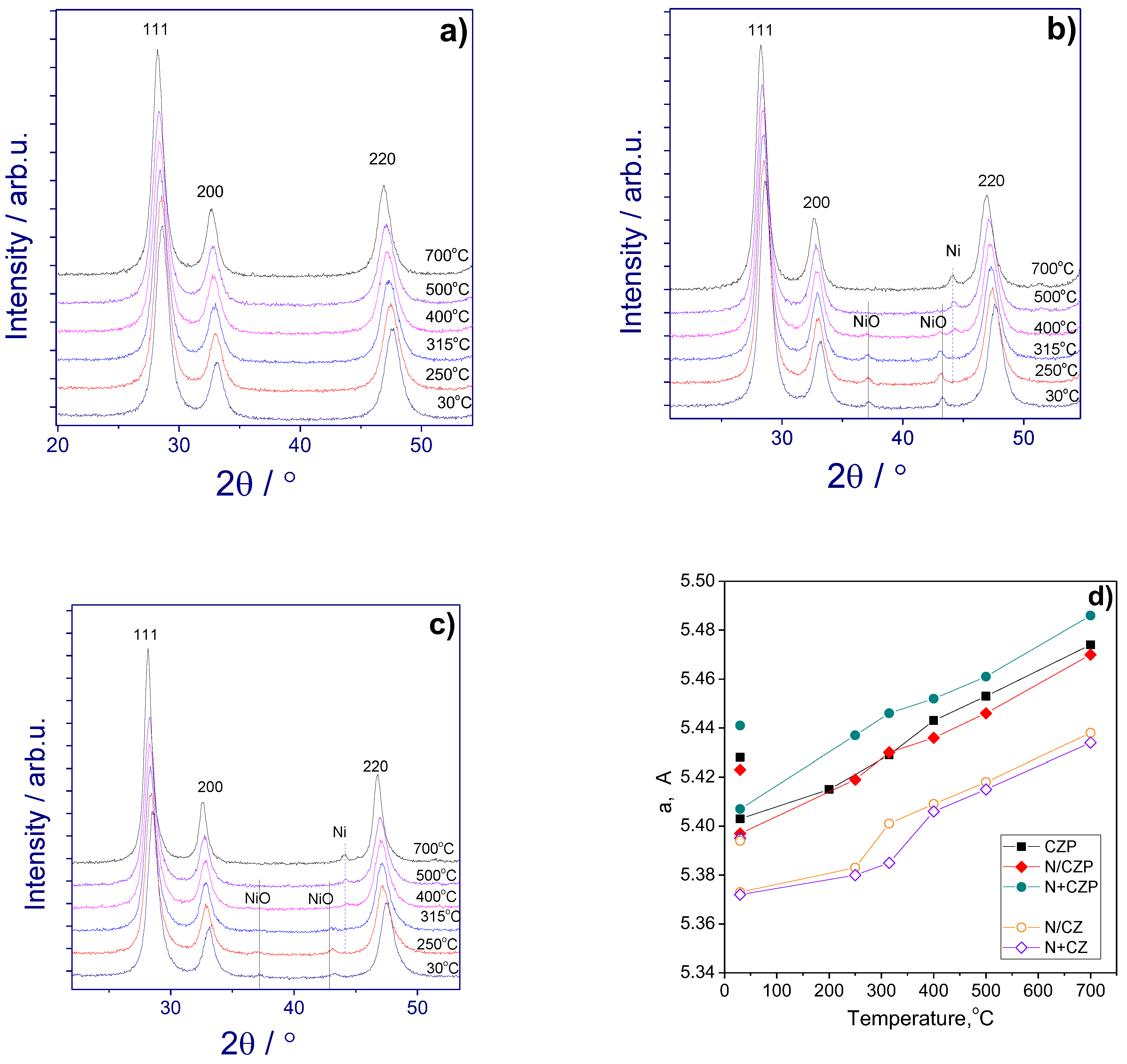
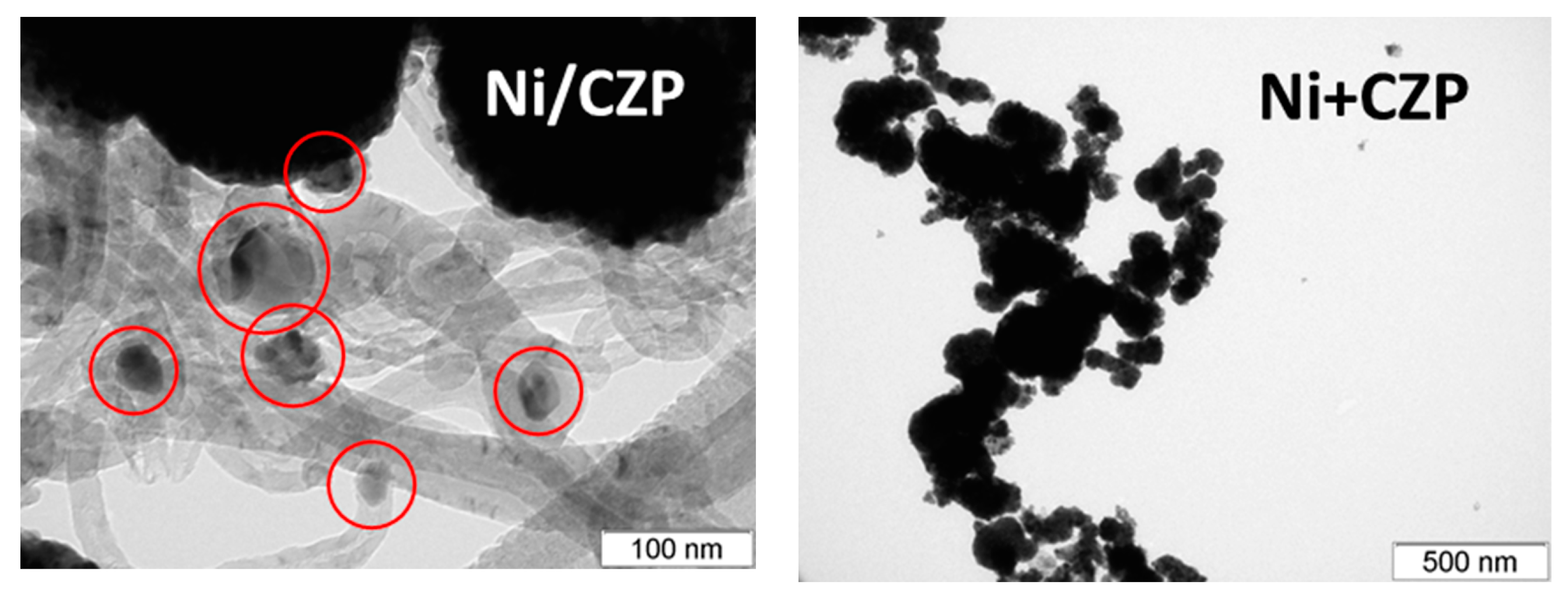
3.1. Textural and Structural Features
| Designation | Ni addition method | Composition | SSA, m2/g | a, Å |
|---|---|---|---|---|
| CZP | - | Ce0.75Zr0.15Pr0.1O2 | 14 | 5.403(1) |
| N/CZP | impregnation | 5%Ni/Ce0.75Zr0.15Pr0.1O2 | 9 | 5.397(1) |
| N+CZP | one-pot | 5%Ni+Ce0.75Zr0.15Pr0.1O2 | 14 | 5.407(1) |
| CZ | - | Ce0.75Zr0.25O2 | 29 | 5.369(1) |
| N/CZ | impregnation | 5%Ni/Ce0.75Zr0.25O2 | 21 | 5.373(1) |
| N+CZ | one-pot | 5%Ni+Ce0.75Zr0.25O2 | 14 | 5.372(1) |
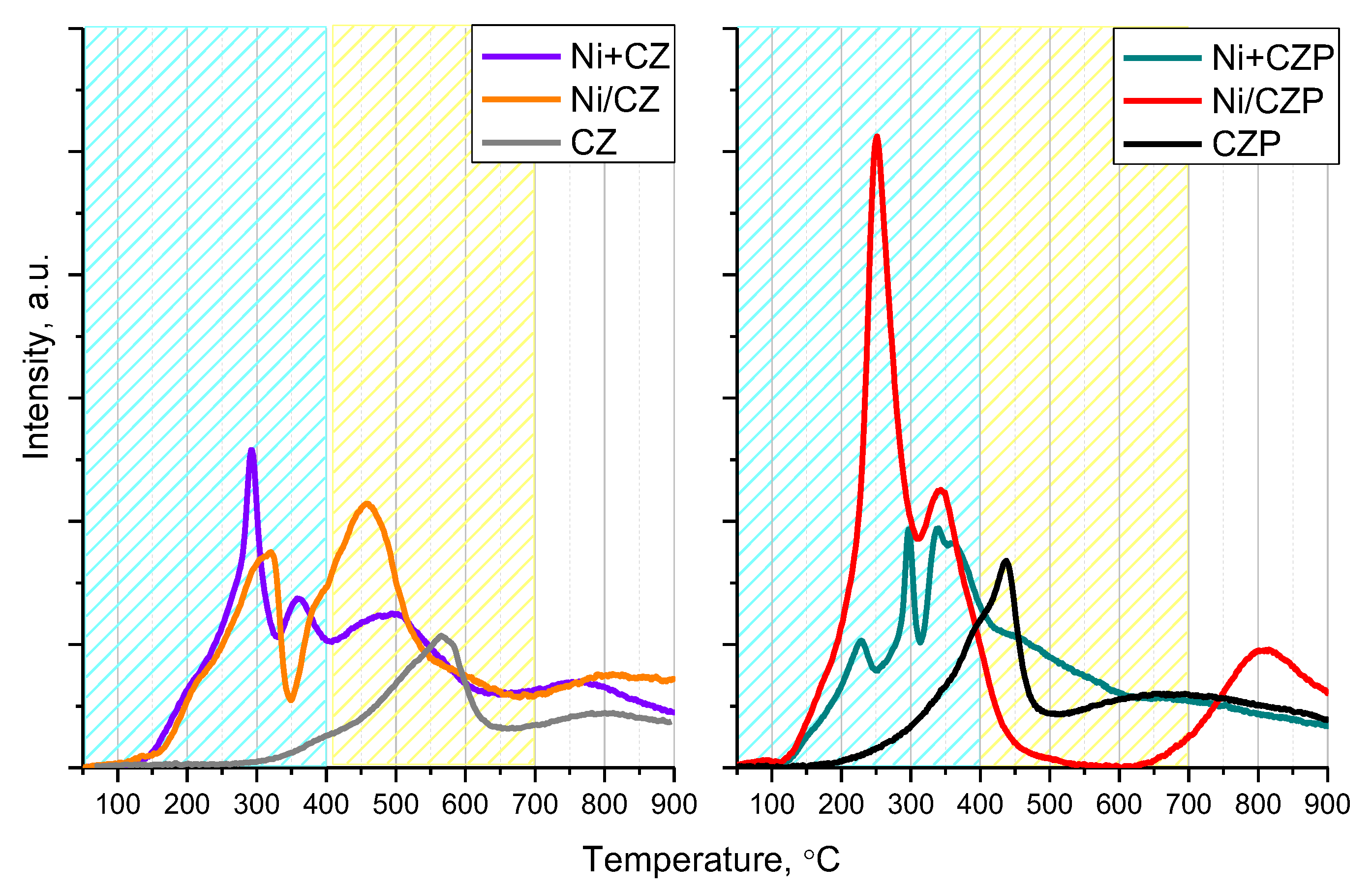
3.2. Reducibility and Oxygen Reactivity
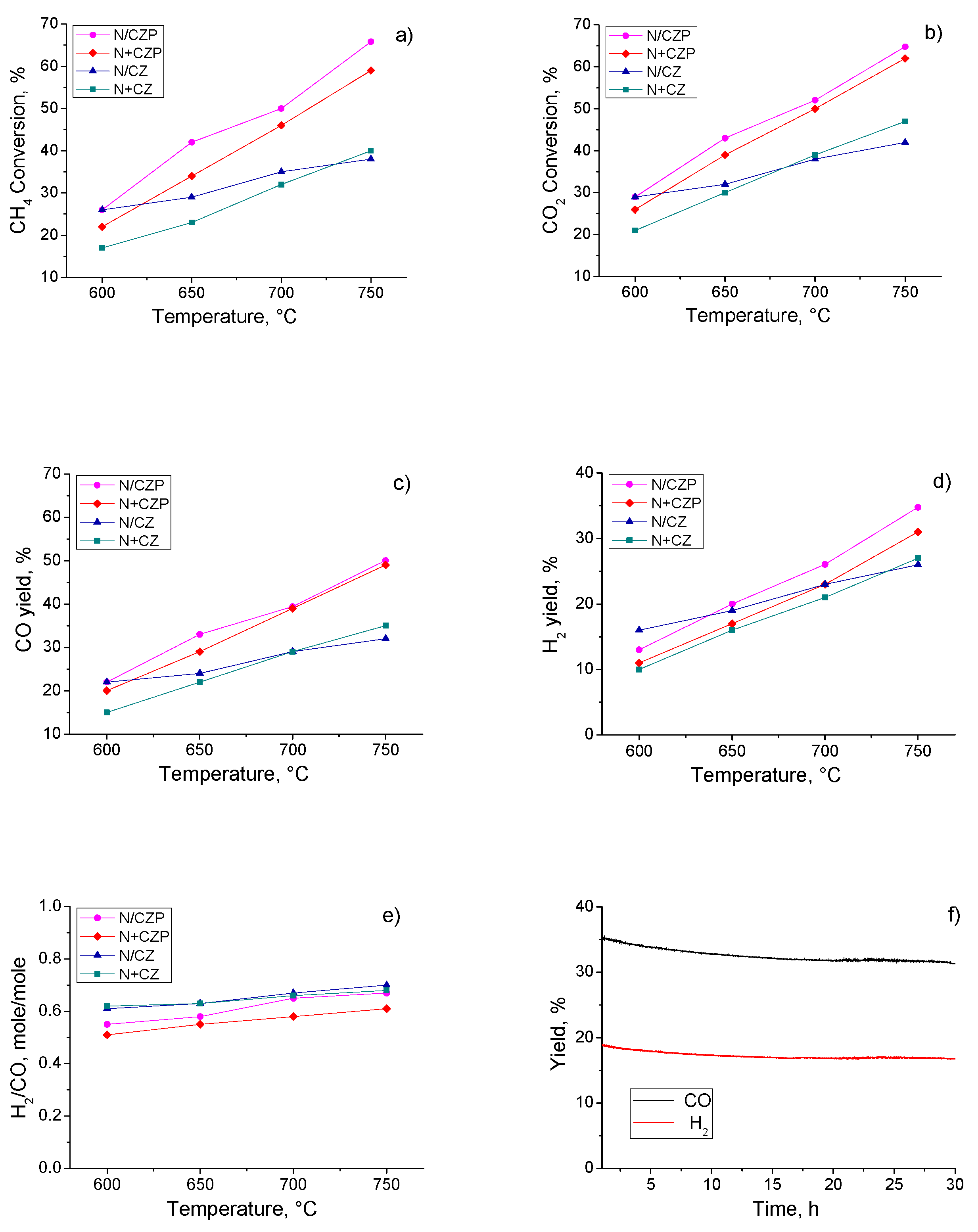
3.3. Catalytic Activity in MDR
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jang, W.-J. Shim, J.-O. Kim, H.-M. Yoo, S.-Y. Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catalysis Today 2019, 324, 15–26. [CrossRef]
- Aresta, M. , Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Transactions 2007, 2975-2992. [CrossRef]
- Verykios, X. E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen, International Journal of Hydrogen Energy 2003, 28, 10, 1045- 1063. [CrossRef]
- Su, B. , Wang, Y., Xu, Z., Han, W, Jin, H., Wang, H. Novel ways for hydrogen production based on methane steam and dry reforming integrated with carbon capture. Energy Conversion and Management 2022, 270, 116199. [Google Scholar] [CrossRef]
- Alipour, Z. , Borugadda, V.B., Wang, H., Dalai, A. K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. Chemical Engineering Journal 2023, 452, 3, 139416. [Google Scholar] [CrossRef]
- Abdulrasheed, A. , Jalil, A.A., Gambo, Y., Ibrahima, M., Hambali, H. U., Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renewable and Sustainable Energy Reviews 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Yentekakis, I. V. , Panagiotopoulou, P., Artemakis, G. A review of recent efforts to promote dry reforming of methane (DRM) to syngas production via bimetallic catalyst formulation. Applied Catalysis B: Environmental 2021, 296, 120210. [Google Scholar] [CrossRef]
- Rosli, S. N. A. , Abidin, S.Z., Osazuwa, O. U., Fan, X., Jiao, Y. The effect of oxygen mobility/vacancy on carbon gasification in nano catalytic dry reforming of methane: A review. Journal of CO2 Utilization 2022, 63, 102109. [Google Scholar] [CrossRef]
- Zhao, K. , Zhang, R., Gao, Y., Lin, Y., Liu, A., Wang, X., Zheng, A., Huang, Z., Zhao, Z. High syngas selectivity and near pure hydrogen production in perovskite oxygen carriers for chemical looping steam methane reforming. Fuel Processing Technology 2022, 236, 107398. [Google Scholar] [CrossRef]
- Li, R.-J. , Zhang, J.-P., Shi, J., Li, K.-Z., Liu, H.-L., Zhu, X. Regulation of metal-support interface of Ni/CeO2 catalyst and the performance of low temperature chemical looping dry reforming of methane. Journal of Fuel Chemistry and Technology 2022, 50, 11, 1458–1470. [Google Scholar] [CrossRef]
- K. Yu, L.-L. Lou, Sh. Liu, W. Zhou, Asymmetric Oxygen Vacancies: the Intrinsic Redox Active Sites in Metal Oxide Catalyst. Advanced Science 2020, 7, 1901970. [CrossRef]
- Yang, J. , Hu, S., Fang, Y., Hoang, S., Li, L., Yang, W., Liang, Z., Wu, J., Hu, J., Xiao, W., Pan, C., Luo, Z., Ding, J., Zhang, L., and Guo, Y. Oxygen Vacancy Promoted O2 Activation over Perovskite Oxide for Low-Temperature CO Oxidation. ACS Catalysis 2019, 9, 11, 9751–9763. [Google Scholar] [CrossRef]
- Wu, L. , Xie, X., Ren, H., Gao, X. A short review on nickel-based catalysts in dry reforming of methane: Influences of oxygen defects on anti-coking property. Materials Today: Proceedings 2021, 42, 153–160. [Google Scholar]
- Salcedo, A. , Lustemberg, P.G., Rui, N., Palomino, R. M., Liu, Z., Nemsak, S., Senanayake, S. D., Rodriguez, J. A., Ganduglia-Pirovano, M. V., Irigoyen, B. Reaction Pathway for Coke-Free Methane Steam Reforming on a Ni/CeO2 Catalyst: Active Sites and the Role of Metal−Support Interactions. ACS Catalysis 2021, 11, 8327−8337. [Google Scholar] [CrossRef]
- McFarland, E. W. , Metiu, H. Catalysis by Doped Oxides. Chemical Reviews 2013, 113, 6, 4391–4427. [Google Scholar] [CrossRef]
- Shi, J. , Li, H., Genest, A., Zhao, W., Qi, P., Wang, T., Rupprechter, G. High-performance water gas shift induced by asymmetric oxygen vacancies: Gold clusters supported by ceria-praseodymia mixed oxides. Applied Catalysis B: Environmental 2022, 301, 120789. [Google Scholar] [CrossRef]
- Vasiliades, M.A. , Djinovi’c, P., Davlyatova, L.F., Pintar, A., Efstathiou, A.M. Origin and reactivity of active and inactive carbon formed during DRM over Ni/ Ce0.38Zr0.62O2-δ studied by transient isotopic techniques. Catalysis Today 2018, 299, 201–211. [Google Scholar] [CrossRef]
- Puigdollers, A. R. , Schlexer, P., Tosoni, S., Pacchioni, G., Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catalysis 2017, 7, 6493−6513. [Google Scholar] [CrossRef]
- Teh, L.P. , Setiabudi, H.D., Timmiati, S.N., Aziz, M.A.A., Annuar, N.H.R., Ruslan, N.N. Recent progress in ceria-based catalysts for the dry reforming of methane: A review. Chemical Engineering Science 2021, 239, 116606. [Google Scholar] [CrossRef]
- Salaev, M.A. , Liotta, L.F., Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. International Journal of Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Makri, M.M. , Vasiliades, M.A., Petallidou, K.C., Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt%Ni/Ce1−xMxO2−I (M = Zr4+, Pr3+) catalysts. Catalysis Today 2015, 259, 150–164. [Google Scholar] [CrossRef]
- Zhang, F. , Liu, Z., Zhang, S., Akter, N., Palomino, R.M., Vovchok, D., Orozco, I., Salazar, D., Rodriguez, J.A., Llorca, J., Lee, J., Kim, D., Xu, W., Frenkel, A.I., Li, Y., Kim, T., Senanayake, S.D. In situ elucidation of the active state of Co–CeOx catalysts in the dry reforming of methane: the important role of the reducible oxide support and interactions with cobalt. ACS Catalysis 2018, 8, 3550–3560. [Google Scholar] [CrossRef]
- Lu, M. , Zhang, X., Deng, J., Kuboon, S., Faungnawakij, K., Xiao, S., Zhang, D. Coking resistant dry reforming of methane over BN–nanoceria interface-confined Ni catalysts. Catalysis Science & Technology 2020, 10, 4237–4244. [Google Scholar] [CrossRef]
- Boaro, M, Colussi, S and Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Frontiers in Chemistry 2019, 7, 28. [CrossRef] [PubMed]
- Liu, B. , Li, W., Song W., Liu, J. Carbonate-mediated Mars–van Krevelen mechanism for CO oxidation on cobalt-doped ceria catalysts: facet-dependence and coordination-dependence. Phys. Chem. Chem. Phys. 2018, 20, 16045–16059. [Google Scholar] [CrossRef] [PubMed]
- Sadykov, V.A. , Simonov, M.N., Mezentseva, N.V., Pavlova, S.N., Fedorova, Yu.E., Bobin, A.S., Bespalko, Yu.N., Ishchenko, A.V., Krieger, T.A., Glazneva, T.S., Larina, T.V., Cherepanova, S.V., Kaichev, V.V., Saraev, A.A., Chesalov, Y.A., Shmakov, A.N., Roger, A-C., Adamski, A. Ni-loaded nanocrystalline ceria-zirconia solid solutions prepared via modified Pechini route as stable to coking catalysts of CH4 dry reforming. Open Chem 2016, 14, 363–376. [Google Scholar] [CrossRef]
- Smirnova, M.Yu. , Pavlova, S.N., Krieger, T.A., Bespalko, Yu.N., Anikeev, V.I., Chesalov, Yu.A., Kaichev, V.V., Mezentseva, N.V., Sadykov, V.A. The synthesis of Ce1–xZrxO2 oxides in supercritical alcohols and catalysts for carbon dioxide reforming of methane on their basis. Russian Journal of Physical Chemistry B 2017, 12, 15–28. [Google Scholar] [CrossRef]
- Vasiliades, M.A. , Makri, M.M., Djinovic´, P., Erjavec, B., Pintar, A., Efstathiou, A.M. Dry reforming of methane over 5 wt% Ni/Ce1-xPrxO2-catalysts: Performance and characterisation of active and inactive carbon by transient isotopic techniques. Applied Catalysis B: Environmental 2016, 197, 168–183. [Google Scholar] [CrossRef]
- Sinev, M.Yu. , Graham, G.W., Haack, L.P., Shelef, M. Kinetic and structural studies of oxygen availability of the mixed oxides Pr1-xMxOy (M = Ce, Zr). J. Mater. Res. 1996, 11, 1960–1971. [Google Scholar] [CrossRef]
- Niu, G. , Zoellner, M.H., Schroeder, T., Schaefer, A., Jhang, J.H., Zielasek, V., B¨aumer, M., Wilkens, H., Wollschlager, J., Olbrich, R., Lammers, C., Reichling, M. Controlling the physics and chemistry of binary and ternary praseodymium and cerium oxide systems. Phys. Chem. Chem. Phys. 2015, 17, 24513–24540. [Google Scholar] [CrossRef]
- Kambolis, A. , Matralis, H., Trovarelli, A., Papadopoulou, Ch. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Hirano, M. , Hirai, K.Effect of hydrolysis conditions on the direct formation of nanoparticles of ceria–zirconia solid solutions from acidic aqueous solutions. J. Nanoparticle Res. 2003, 5, 147–156. [Google Scholar] [CrossRef]
- Pradeep, E. , Habu, T., Tooriyama, H., Ohtani, M., Kobiro K. Ultra-simple synthetic approach to the fabrication of CeO2–ZrO2 mixed nanoparticles into homogeneous, domain, and core–shell structures in mesoporous spherical morphologies using supercritical alcohols. J. Supercrit. Fluids 2015, 97, 217–223. [Google Scholar] [CrossRef]
- Montoya, J.A. , Romero-Pascual, E., Gimon, C., Del Angel, P., Monzón, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Luisetto, I. , Tuti, S., Romano, C., Boaro, M., Di Bartolomeo, E., Kesavan, J.K., Kumar, S.S., Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Kuznetsova, T.G. , Sadykov, V.A., Moroz, E.M., Trukhan, S.N., Paukshtis, E.A., Kolomiichuk, V.N., Burgina, E.B., Zaikovskii, V.I., Fedotov, M.A., Lunin, V.V., Kemnitz, E. Preparation of Ce-Zr-O composites by a polymerized complex method. Stud. Surf. Sci. Catal 2002, 143, 659–667. [Google Scholar] [CrossRef]
- Basile, F. , Mafessanti, R., Fasolini, A., Fornasari, G., Lombardi, E., Vaccari A. Effect of synthetic method on CeZr support and catalytic activity of related Rh catalyst in the oxidative reforming reaction. J. Eur. Ceram 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Cai, W. , Dong, J., Chen, Q., Xu, T., Zhai, S., Liu, X., Cui, L., Zhang S. One-pot microwave-assisted synthesis of Cu-Ce0.8Zr0.2O2 with flower-like morphology: Enhanced stability for ethanol dry reforming. Adv Powder Technol 2020, 31, 3874–3881. [Google Scholar] [CrossRef]
- Manjunatha, S. , Dharmaprakash, M.S., Thermal stability, optical and Photoluminescence properties of spherical CexZr1−xO2 (x = 0.05) crystalline blue-emitting nanophosphors synthesized by microwave method. Mater. Res. Express, 2018, 5, 035043. [Google Scholar] [CrossRef]
- Guo, J. , Xin, X., Zhang, X., Zhang, S. Ultrasonic-induced synthesis of high surface area colloids CeO2–ZrO2. J Nanopart Res 2009, 11, 737–741. [Google Scholar] [CrossRef]
- Khani, Y. , Bahadoran, F., Shariatinia, Z., Varmazyari, M., Safari, N. Synthesis of highly efficient and stable Ni/CexZr1-xGdxO4 and Ni/X-Al2O3 (X = Ce, Zr, Gd, Ce-Zr-Gd) nanocatalysts applied in methane reforming reactions. Ceram. Int. 2020, 46, 25122–25135. [Google Scholar] [CrossRef]
- Guo, T. , Du, J., Wu, J., Li, J. Enhanced properties of solid solution (CeZr)O2 modified with metal oxides for catalytic oxidation of low-concentration methane. Chin. J. Chem. Eng. 2017, 25, 187–192. [Google Scholar] [CrossRef]
- Lovell, E. , Horlyck, J., Scott, J., Amal, R. Flame spray pyrolysis-designed silica/ceria-zirconia supports for the carbon dioxide reforming of methane. Appl. Catal. 2017, 546, 47–57. [Google Scholar] [CrossRef]
- Veriansyah, B. , Park, H., Kim, J.D., Min, B.K., Shin, Y.H., Lee, Y.W., Kim, J. Characterization of surface-modified ceria oxide nanoparticles synthesized continuously in supercritical methanol. J Supercrit Fluids 2009, 50, 283–291. [Google Scholar] [CrossRef]
- Lustemberg, P.G. , Mao, Z., Salcedo, A., Irigoyen, B, Ganduglia-Pirovano, M.V., Campbell, C.T. Nature of the active sites on Ni/CeO2 catalysts for methane conversions. ACS Catal. 2021, 11, 10604−10613. [Google Scholar] [CrossRef] [PubMed]
- Li, M. , van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Applied Catalysis B: Environmental 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Anchieta, C.G. , Assaf, E.M., Assaf, J.M. Syngas production by methane tri-reforming: Effect of Ni/CeO2 synthesis method on oxygen vacancies and coke formation. Journal of CO2 Utilization 2022, 56, 101853. [Google Scholar] [CrossRef]
- Lyu, Y. , Jocz, J.N., Xu, R., Stavitski, E., Sievers, C. Nickel speciation and methane dry reforming performance of Ni/CexZr1-xO2 prepared by different synthesis methods. ACS Catal. 2020, 10, 11235–11252. [Google Scholar] [CrossRef]
- Bespalko, Y. , Smal, E., Simonov, M., Valeev, K., Fedorova, V., Krieger, T., Cherepanova, S., Ishchenko, A., Rogov, V., Sadykov, V. Novel Ni/Ce(Ti)ZrO2 catalysts for methane dry reforming prepared in supercritical alcohol media. Energies 2020, 13, 3365. [Google Scholar] [CrossRef]
- Simonov, M. , Bespalko, Y., Smal, E., Valeev, K., Fedorova, V., Krieger, T., Sadykov, V. Nickel-containing ceria-zirconia doped with Ti and Nb. Effect of support composition and preparation method on catalytic activity in methane dry reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. Journal of Applied Crystallography, 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Ballauri, S. , Sartoretti, E., Novara, C., Giorgis, F., Piumetti, M., Fino, D., Russo, N., Bensaid, S. Wide range temperature stability of palladium on ceria-praseodymia catalysts for complete methane oxidation. Catalysis Today 2022, 390-391, 185–197. [CrossRef]
- Makri, M.M. , Vasiliades, M.A., Petallidou, K.C., Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt%Ni/Ce1−xMxO2−δ (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Rajendran, M. , Mallick, K.K., Bhattacharya, A.K., Combustion synthesis, powder characteristics and crystal structure of phases in Ce–Pr–O system. J. Mater. Sci. 1998, 33, 5001–5006. [Google Scholar] [CrossRef]
- Iglesias, I. , Baronetti, G., Marino, F. Ni/Ce0.95M0.05O2-d (M = Zr, Pr, La) for methane steam reforming at mild conditions. Int J Hydrogen Energy 2017, 42, 29735–29744. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L. , Zarrou, H., D’Huysser, A. Hydrogen production at low temperature from methane on cerium and nickel based mixed oxides. Int J Hydrogen Energy 2008, 33, 5527–5534. [Google Scholar] [CrossRef]
- Takeguchi, T. , Furukawa, S.N., Inoue, M. Hydrogen spillover from NiO to the large surface area CeO2-ZrO2 solid solutions and activity of the NiO/CeO2-ZrO2 catalysts for partial oxidation of methane. J Catal 2001, 202, 14–24. [Google Scholar] [CrossRef]
- Smal, E. , Bespalko, Y., Arapova, M., Fedorova, V., Valeev, K., Eremeev, N., Sadovskaya, E., Krieger, T., Glazneva, T., Sadykov, V., Simonov, M. Carbon formation during methane dry reforming over Ni-containing ceria-zirconia catalysts. Nanomaterials 2022, 12, 3676. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





