1. Introduction
The phycobilisome (PBS) was first identified by Gantt and Conti (1966). PBSs are large chromophore-protein complexes located on the stromal side of thylakoid membranes, consisting of phycobilin, apoproteins and linker proteins. As a light-harvesting antenna system, PBSs absorb light between 450 and 650 nm and transmit it efficiently to the reaction centres of the photosynthetic systems of cyanobacteria and red algae.
The main bricks in the spatial arrangement of phycobilisomes are phycobiliproteins (PBPs), which have covalently attached linear open-chain tetrapyrroles called phycobilins, and linker proteins. With the linker proteins, PBPs form the structural backbone of the phycobilisome. Diversity in the number and spatial structure of lyase-catalysed phycobilin binding varies the spectral properties of the PBPs. Therefore, phycobilin synthesis, lyase catalysis, and self-assembly of apoproteins and the auxiliary linker proteins are essential for the assembly of phycobiliproteins and the structural and functional stability of the PBS.
The primary prerequisite for photosynthetic light reactions is the capture and efficient transfer of light energy. The unique structure of the phycobilisome determines its function in photosynthesis. The phycobiliproteins form the overall organisation from high to low energy in the phycobilisome and contribute to the rapid, efficient and unidirectional transfer of absorbed excitation energy to the chlorophylls in the reaction centre. The irreversible transfer of energy from the absorption of light energy by the PBS to photosystem II (PSII) or partially to photosystem I (PSI) may be achieved by the formation of the PBS-PSII-PSI complex. Linker proteins play a key role in the formation of the complex. To counteract photodamage, cyanobacterial cells have evolved non-photochemical quenching (NPQ) mediated by carotenoids. Moreover, chromatic acclimation (CA) enables PBSs to regulate the pigment composition to optimise light absorption for photosynthesis, thus adapting to the fickle light environment (Montgomery 2017).
In addition to their role in photosynthesis, the PBPs possess excellent spectral properties that can be used in numerous applications. The brightly coloured lustre and fluorescence of the PBP mean that it can be used as a fluorescent probe in bioscience research. Compared with traditional fluorescent probes, PBPs are highly hydrophilic and can maintain their fluorescence properties in the aqueous environment for a longer period. Among them, phycoerythrin (PE) is the most stable PBP and is, therefore, the most commonly used PBP fluorescent probe. PBPs are also safe and non-toxic natural products that can be used as a natural colouring for food, cosmetics and dyes (Mysliwa-Kurdziel and Solymosi 2017).
Phycobilisomes eliminate excess reactive oxygen species, activate antioxidant enzymes, increase antioxidant enzyme activity, and inhibit lipid peroxidation and DNA damage (Wu, Liu et al. 2016). Furthermore, phycobiliprotein has anti-inflammatory and anticancer effects (Leung, Lee et al. 2013), as well as therapeutic and preventive effects on a wide range of diseases in clinical trials (Jiang, Wang et al. 2018). Based on the antioxidant and optical properties of phycobiliproteins, phycocyanin has been used in various fields such as food, medicine and cosmetics.
Currently, phycobiliproteins and phycocyanobilin are mainly extracted and purified from Spirulina through complex steps. However, the heterologous biosynthesis of PBPs and phycobilins in chassis cells, including Escherichia coli and cyanobacteria, has attracted increasing attention. Researchers have achieved the efficient synthesis of phycocyanin in E. coli through combinatorial metabolic engineering (Mukougawa, Kanamoto et al. 2006, Ge, Li et al. 2013). Some groups have also studied the structure and function of lyase enzymes (Zhao, Höppner et al. 2017). Moreover, many investigations have revealed the pharmacological and biological properties of natural or recombinant PBPs, including antioxidant (Nakagawa, Ritcharoen et al. 2016), anti-inflammatory (Liu, Wang et al. 2015) and anti-tumour (Deniz, Ozen et al. 2016) activities. Synthetic biology and the genetic engineering of chassis cells have enabled the production of various recombinant PBPs, but the rational design of synthetic biological components for the assembly and uniform standardisation of PBPs still faces many challenges.
The structure, function and applications of PBPs have been extensively studied with particular effort being made on the heterologous biosynthesis and reconstitution of phycobiliproteins. Current hot issues in the PBS field mainly focus on the following aspects: (1) structure and function mining; (2) mechanisms of the excitation energy transfer within the PBS; (3) biosynthesis and dynamic assembly; (4) potential applications, which are critically discussed in this article.
2. Requirements for phycobilisome assembly
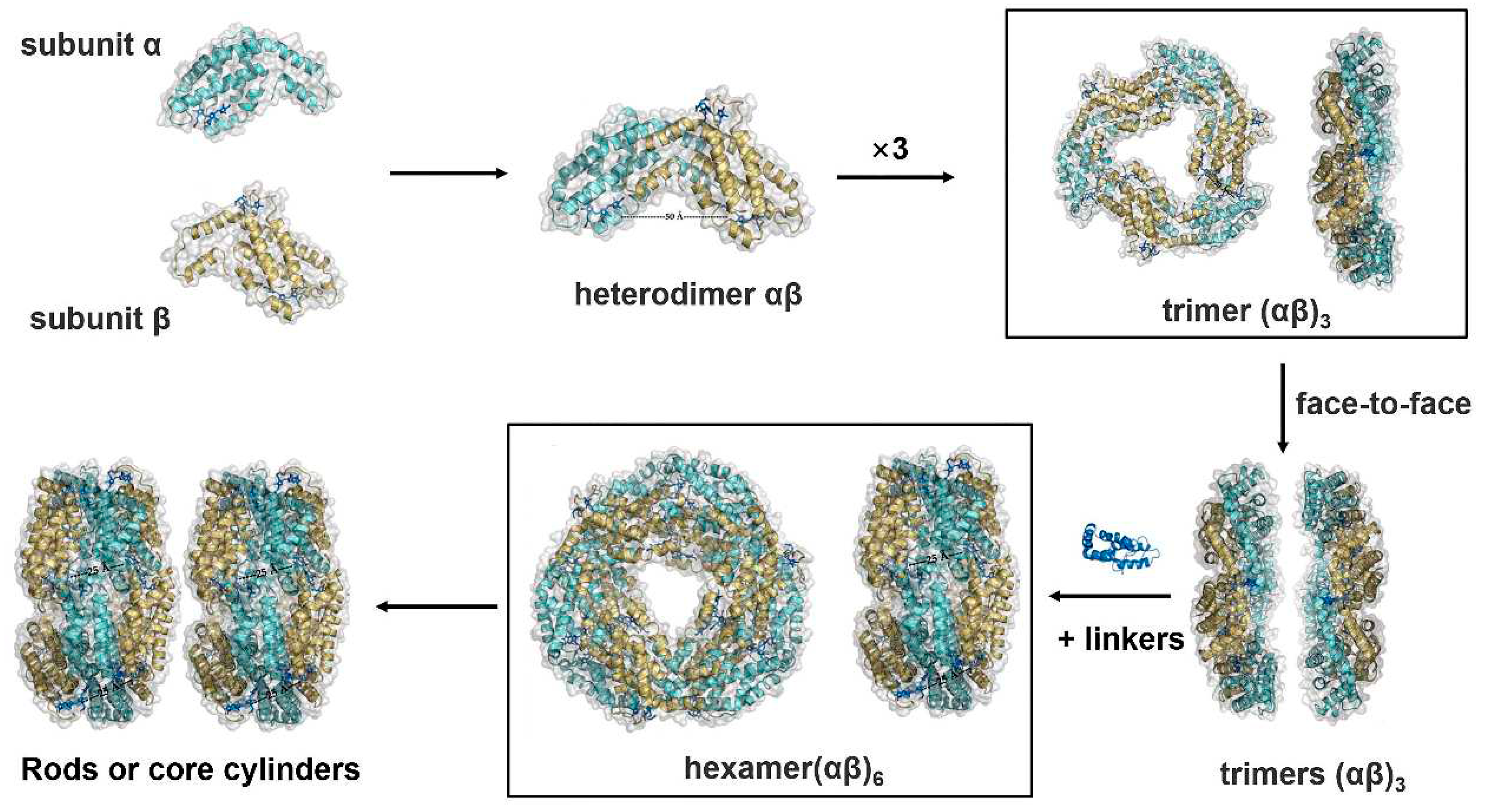
The PBS self-assembles from soluble homologous protein subunits that contain conserved cysteine residues to covalently anchor the phycobilins. The assembly process begins with the coupling of phycobilins and apoproteins to form phycobiliprotein subunits, in which the lyase enzyme makes the binding of phycobilins to apoproteins more accurate and catalytically efficient. This process is followed by the spontaneous aggregation of the subunits to form a trimer (αβ)
3. Next, the trimer binds to the linking polypeptide, which triggers the formation of the phycobilisome 'nucleus' and the binding of the 'rods' to form the final phycobilisome (Li, Su et al. 2019) (
Figure 1).
2.1. Phycobiliprotein and PBS structural organisation
Crystal structures of the PBSs from various cyanobacteria and red algae have been resolved (Ma, You et al. 2020, Domínguez-Martín, Sauer et al. 2022, Kawakami, Hamaguchi et al. 2022). PBPs, the main component of the PBS supercomplex, are a group of disk-shaped macromolecular proteins with covalently attached linear open-chain tetrapyrroles known as phycobilins (Apt, Collier et al. 1995). The basic building block of PBPs is a monomer comprising α- and β-subunits, each with a molecular mass of 15–20 kDa and 160–165 amino acids (Li, Su et al. 2019). The α- and β-subunits are encoded by the genes cpeA and cpeB, respectively. Each subunit carries heterogeneous phycobilins at conserved cysteine residues. Phycoerythrin subunits mainly use phycoerythrobilin (PEB) or phycourobilin (PUB) that contribute to the 560 nm light-absorbance. The heterogeneity of the spectral properties of PBPs enables the PBS to collect excitation energy from the rods and funnel it to the photosynthetic reaction centre.
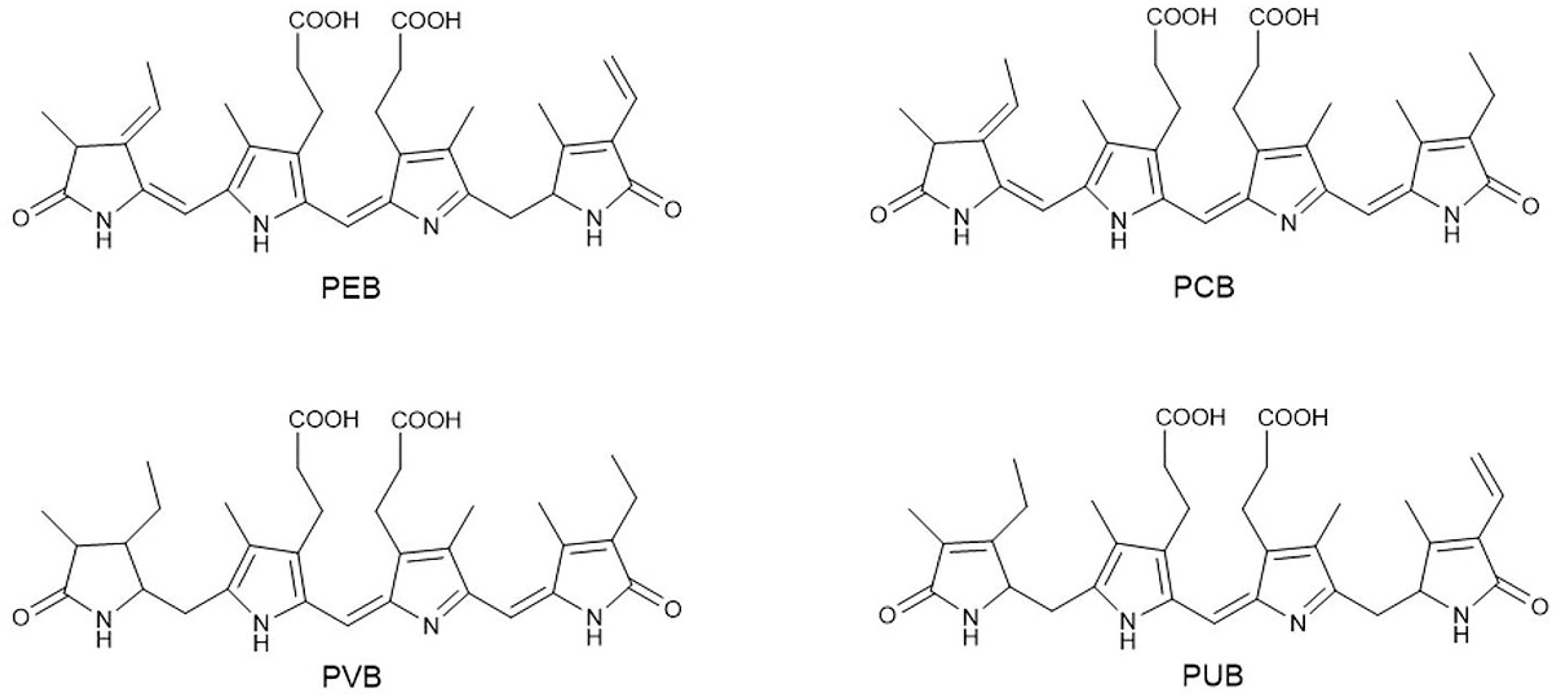
PBS consists of several peripheral rods that project radially from the central core subcomplex. Typically, the central core consists of a bundle of three core cylinders, each composed of two or four disks of allophycocyanin (APC) trimer. Six rods are bound to the central core and the bundle of core cylinders lies on the surface of the photosystem complexes. The peripheral rods of the PBS supercomplex are made up of phycobiliprotein hexamers together with colourless linker proteins and the bilin chromophores. Generally, the rod subcomplex consists of two or more disks of PBP hexamers, typically PE, phycocyanin (PC) or phycoerythrocyanin (PEC). These PBP hexamers are spatially arranged to allow directional energy transfer from PEC or PE to PC within the rod and then to APC in the core. PBP hexamers covalently bind assorted molecules of linear tetrapyrrole chromophores including PEB, phycocyanobilin (PCB), PUB and phycoviolobilin (PVB) (
Figure 2). Phycoerythrin disks associated with PEB or PUB are located in the distal part of the rod and PE absorbs blue–yellow light of 490–560 nm according to the chromophore composition.
In summary, the PBS consists of structurally related PBPs, and variations in the PBP-bilin pairs, as well as in the spatial arrangement, are precisely determined to optimise energy transfer.
2.2. The synthesis of phycobilin
In the photosynthetic light-harvesting complexes, the main chromophore is the tetrapyrrole derivative. The abundant light-harvesting pigment in plants is chlorophyll, a cyclic tetrapyrrole chromophore. However, in cyanobacteria, the pigments are open-chain tetrapyrrole chromophores called phycobilins. Eight types of phycobilins have been identified (Zhou, Gasparich et al. 1992), of which four (PEB, PCB, PUB and PVB) are common. The precursor substance of phycobilins is haem, which can be synthesised by haem oxidase (HO) and ferredoxin-dependent bilin reductases (FDBRs) (Dammeyer and Frankenberg-Dinkel 2008). Aminolevulinic acid (ALA) is converted to protoporphyrin, a precursor of phytochrome, through multistep enzymatic reactions. Protoporphyrin ring chelates Fe
2+ to produce haem. Subsequently, the haem unravels the closed-chain tetrapyrrole ring, catalysed by haem oxidase, and removes Fe
2+ to produce the open-chain tetrapyrrole molecule biliverdin IXα (BV), which is then transformed into phytochromobilins (in plants) or various phycobilins (in cyanobacteria and red algae) by FDBRs (
Figure 3).
Each subunit of PBP can bind one to four phycobilins at conserved cysteine sites (Blot, Wu et al. 2009). Most PBPs covalently couple with phycobilins through the sulphydryl group of cysteine, generally forming a thioether bond with C31 of the A-ring of PBP. In some phycobiliproteins, C31 of the A-ring and C181 of the D-ring of phycocyanin can form two thioether bonds with two cysteine sulphydryl groups simultaneously (Sonani, Roszak et al. 2018). In addition, there are PBP subunits, such as ApcE, that are coupled to PCB through non-covalent bonds (Miao, Ding et al. 2016). Normally, multiple conserved cysteine sites of PBPs provide anchorage for certain phycobilins. Due to variations in the PBP-bilin pairs, as well as in the spatial arrangements, PBPs exhibit a wide range of spectral properties. Phycocyanin absorbs red light (620–630 nm), PEC absorbs orange light (550–620 nm), and PE absorbs blue–yellow light (500–560 nm) according to the chromophore composition (Watanabe and Ikeuchi 2013).
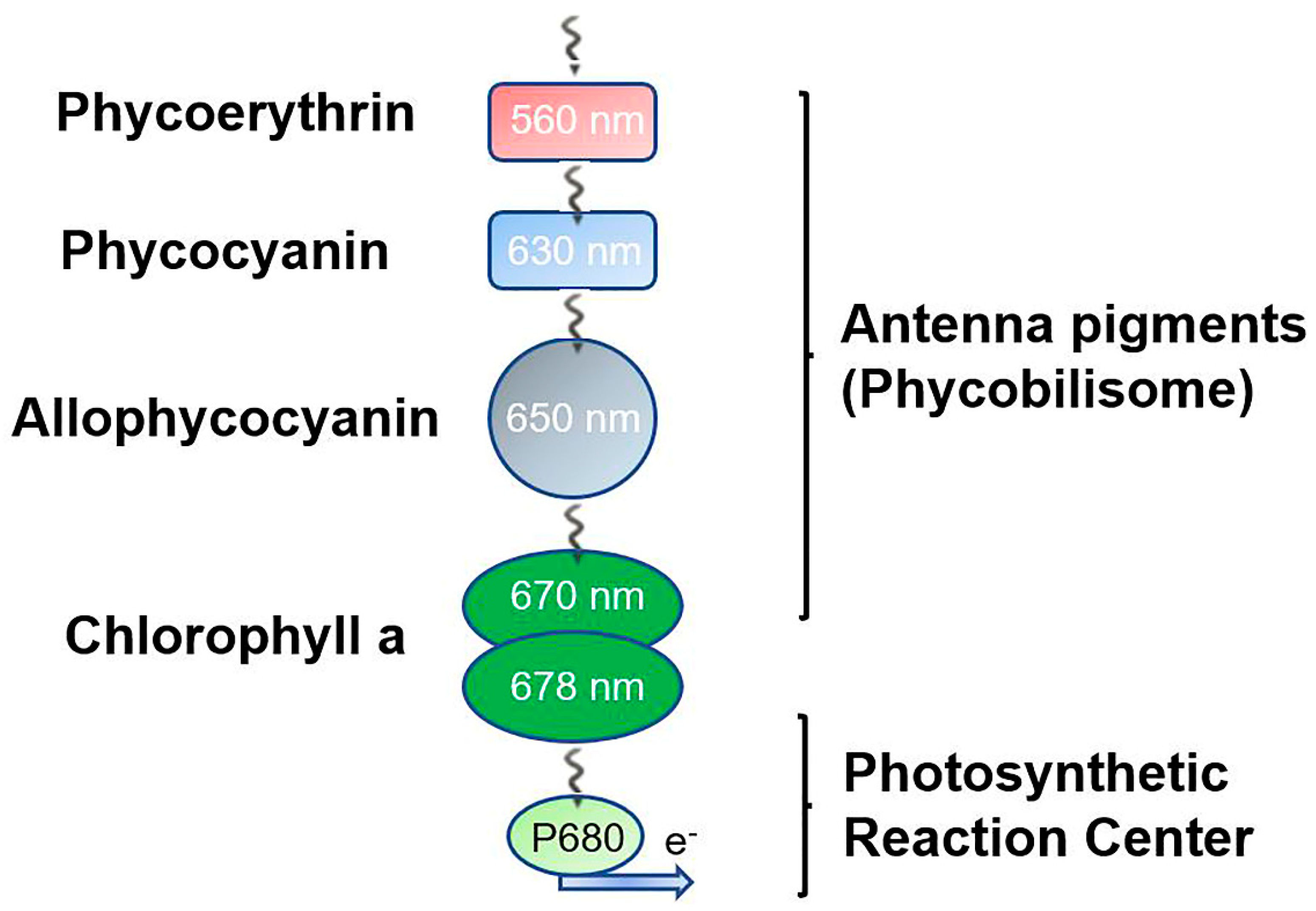
The light energy absorbed by phycobilins is converted to chemical energy. Energy is transferred through fluorescence resonance between subunits, then transmitted uniaxially to inferior PBP rods, and finally reaches the reaction centre located in the thylakoid. These PBPs are spatially arranged to allow directional energy transfer from PEC or PE to PC within the rod and then to APC in the core (Watanabe and Ikeuchi 2013). Chlorophylls capture the energy and transport it to the photosynthetic electron transport chain at an efficiency of higher than 95% (Sidler 1994, Zhang, Lambrev et al. 2015) (
Figure 4).
2.3. Lyases catalyse the binding of phycobilin to apoproteins
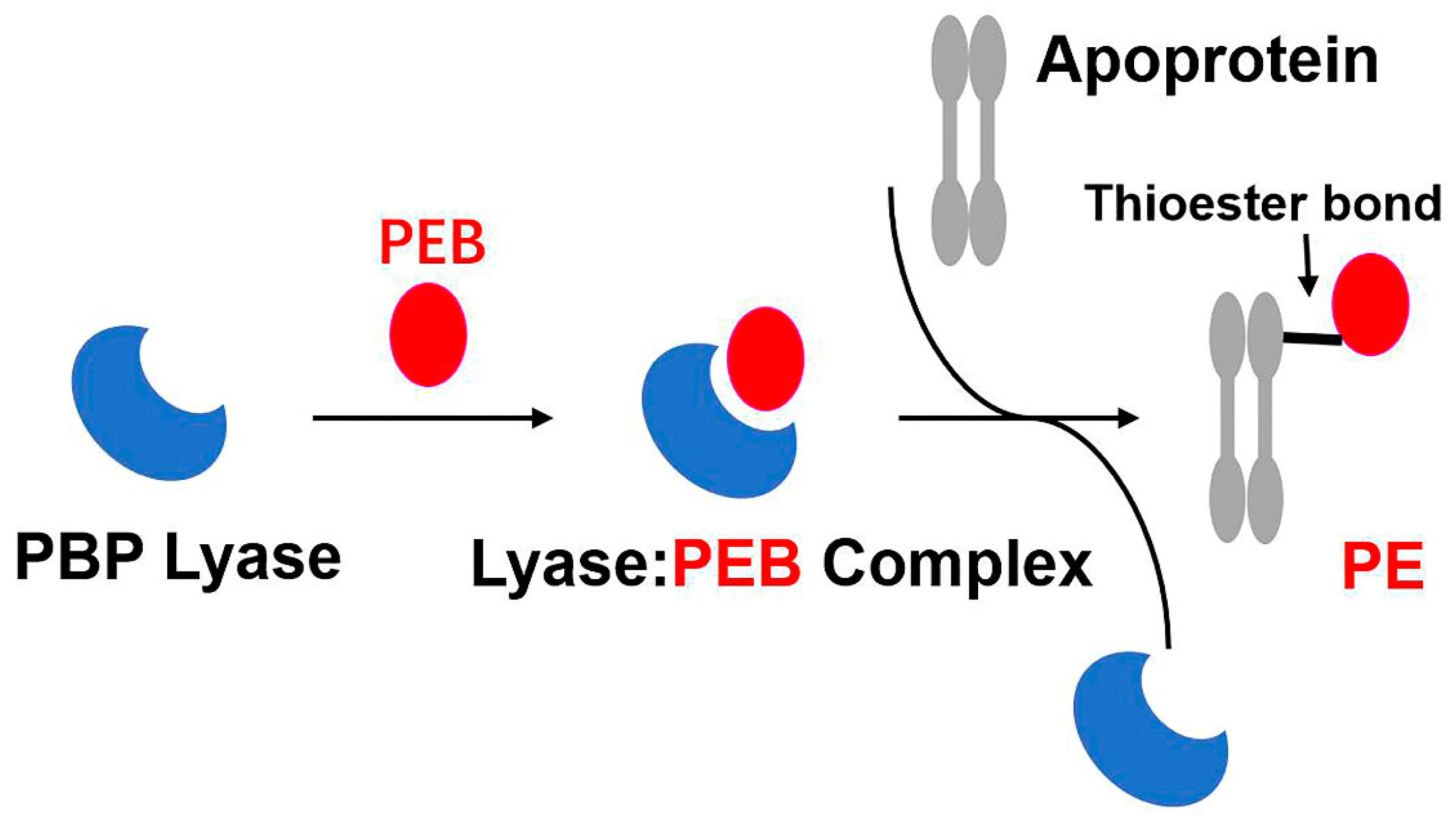
The attachment of phycobilins to PBPs needs to be catalysed by the corresponding lyase, while some apoproteins can autonomously link with phycobilins at conserved cysteine sites (Zolla, Bianchetti et al. 2002), such as the core-membrane linkers (ApcE or L
CM) that are capable of autocatalytically attaching to PCB (Zhao, Su et al. 2005). The covalent bond of phycobilins to phycobiliproteins may occur spontaneously both
in vivo and
in vitro. Nonetheless, specific PBP lyases enable the correct phycobilins to pair with the cysteine residues of PBP subunits appropriately and efficiently (
Figure 5).
To date, three major groups of phycobilin lyases have been characterised: S/U-type, T-type, and E/F-type (Fairchild, Zhao et al. 1992, Fairchild and Glazer 1994, Saunée, Williams et al. 2008). The crystal structures of the S/U- and T-type lyases are antiparallel β-barrel structures similar to lipocalin (Kronfel, Kuzin et al. 2013, Overkamp, Gasper et al. 2014). In contrast, the crystal structures of E/F-type lyases adopt a fully helical structure (Kumarapperuma, Joseph et al. 2022).
The first lyase to be discovered that catalyses the covalent linkage of phycocyanin and phycocyanobilin was CpcE/F (CpcE and CpcF co-catalysis). Fairchild et al. (1992) confirmed that CpcE/F catalyses PCB attachment to the cysteine at position 84 of the α-subunit of PC (Fairchild, Zhao et al. 1992, Fairchild and Glazer 1994). Amino acid homology studies revealed many other types of lyases and that CpcT is homologous to CpeT, which affects the synthesis of PE, and the analysis of PC from CpcT mutants revealed that CpcT can link PC to Cys-153 on the β-subunit of PC (Shen, Saunée et al. 2006). CpeF is a phycoerythrobilin lyase that ligates the double-chain PEB to β-haemoglobin in cyanobacteria (Kronfel, Hernandez et al. 2019).
The presence of a chaperone protein is sometimes required for lyase to catalyse the binding of phycobilin to phycobiliprotein. The activity of CpeT is greatly enhanced when the chaperone-like protein CpeZ is present (Nguyen, Joseph et al. 2020).
3. Heterologous biosynthesis of phycobiliproteins
In the process of heterologous PBP synthesis, the complete pathway can be divided into two steps. The first stage is the synthesis of phycobilins and apoproteins. The phycobilin synthesis is derived from haem, which is first broken down into BV by haem oxygenase, and then BV is reduced to other types of phycobilins by FDBRs. The second stage is the covalent attachment of bilins to apoproteins, a process that is either catalysed by specific lyases or occurs spontaneously (Biswas, Vasquez et al. 2010).
In 1985, the α- and β-subunit genes of allophycocyanin were isolated from the cyanobacteria genome and transferred into Escherichia coli to achieve heterologous expression (Bryant, de Lorimier et al. 1985). Since then, many researchers have introduced the cpcB/A gene into E. coli to explore the recombinant phycobiliprotein subunits (Yu, Li et al. 2016, Wu, Yang et al. 2018). The C-phycocyanin β-subunit gene was introduced into E. coli and its anticancer effect was studied. The results showed that the β-subunit could inhibit the proliferation of cancer cells and induce cell apoptosis, making it a promising cancer prevention or treatment agent (Wang, Liu et al. 2007). There have also been many attempts to synthesise phycocyanin in E. coli by genetic engineering (Mukougawa, Kanamoto et al. 2006, Ge, Li et al. 2013); however, the titre of reconstituted PCB in chassis cells remains low due to the poor catalytic efficiency of the synthetic enzyme and the lack of precursors and cofactors. In a recent study, the synthesis of PCB could be enhanced by assembling haem oxygenase and ferric reductase in appropriate proportions, expressing NAD kinase and adding NADPH (Zhao, Gao et al. 2022). In addition to heterologous expression in E. coli, direct expression of PC in mammalian cells was achieved in 2013 (Müller, Engesser et al. 2013).
To obtain complete recombinant PBPs, the phycobilins need to covalently bind with the apoproteins, a process catalysed by self-assembly or lyases. Correct and efficient binding of phycobilins to apoproteins is catalysed by lyases and, therefore, introducing highly efficient lyases into chassis cells is the key to the synthesis of complete PBPs in vivo. E/F-type lyases covalently bind pigments to the α-subunits of phycobiliproteins specifically, and a subclass of E/F-type lyases can chemically modify chromophores (Zhao, Höppner et al. 2017). Unlike other lyases, E/F lyase also has chromophore separation activity. CpcS/U specifically binds PCB to Cys-82 of CpcB and Cys-81 of ApcA and ApcB (Saunée, Williams et al. 2008). T-lyase is responsible for attaching pigments to Cys-155 of the phycobilin β-subunit (Shen, Saunée et al. 2006, Zhou, Ding et al. 2014).
Based on the genetic engineering studies above, it should be possible to achieve large-scale production of low-cost recombinant PBPs and obtain various types of PBPs with improved functions for future applications through molecular design and recombinant synthesis.
4. Photosynthetic function of phycobilisomes
4.1. Light energy capture and transfer in PBS
Phycobilisomes are large pigment-protein complexes located on the thylakoid of cyanobacteria and red algae that function as light-harvesting antennae, absorbing light energy through pigment molecules and transmitting it to the reaction centres of PSII (Sui 2021). The structure of an intact PBS in complex with PSII from Anabaena sp. strain PCC 7120 was unambiguously resolved using single-particle electron microscopy (Chang, Liu et al. 2015). Moreover, the research focusing on the in situ structures of PBS-PSII-PSI-LHC megacomplexes from the red alga Porphyridium purpureum provided interaction details between PBS, PSII and PSI at near-atomic resolution using cryogenic electron tomography. All these works contribute a solid structural basis for unravelling the mechanisms of the PBS-PSII-PSI-LHC megacomplex assembly, the efficient energy transfer from PBS to the two photosystems, and the regulation of energy distribution between PSII and PSI (You, Zhang et al. 2023).
Water presents a unique light environment and different species of small cyanobacteria and red algae can survive by absorbing a wide range of wavelengths. Phycobilisomes exhibit various and flexible absorption peaks to ensure the efficiency of capturing and transmitting light energy in deep water, in particular, the blue–green light that can penetrate deep water. In addition, PBSs form huge arrays on the thylakoid membranes as antennae. The arrangement of phycobilisomes on the thylakoid membranes is light intensity-dependent, and their arrangements may be both disordered and ordered. At a light intensity of 15 W.m-2 (medium light), the PBSs on the thylakoid membrane are clustered in discrete regions, while at a light intensity of 6 W.m-2 (low light), uniformly sized PBSs on the thylakoid membrane are orderly arranged in parallel (Folea, Zhang et al. 2008).
Interestingly, cyanobacteria exhibit a form of photomorphogenesis termed chromatic acclimation (CA), and one of the characteristics of CA is the regulation of the pigment composition of PBPs to optimise light absorption for photosynthesis, thus adapting to the light environment in water (Montgomery 2017). The proximity of chromophores in adjacent PBSs suggests that there might also be light energy transfer between PBSs (Zheng, Zheng et al. 2021).
In the PBS structure, the energy is first received by the PE or PEC at the distal part of the rod and transmitted to the APC core through the PC. Finally, it is transferred to PSII or PSI through the multidomain core-membrane linker (LCM) at the end of the core complex. There are two possible energy transfer pathways in this process: direct energy transfer from PBS to PSI (PBS→PSI transfer) and indirect transfer through PSII (PBS→PSII→PSI transfer) (Ueno, Aikawa et al. 2017). In terms of the energy level of light absorption, these four PBPs can be further divided into three types: high energy (PE and PEC), medium energy (PC) and low energy (APC). According to the arrangement of PBPs in the PBS, the PBPs form an overall organisation from high to low energy in PBS. Through this holistic organisation, the absorbed excitation energy can be transferred to the auxiliary chlorophyll of the photosystem quickly, efficiently and directionally. In addition, APC contributes to the excitation of energy from peripheral rods of the PBS or from directly absorbed red light to auxiliary chlorophyll in the photosystem (Soulier and Bryant 2021).
These large protein complexes capture incident sunlight and transfer the energy to PSII or partially to PSI. This process is achieved by forming PBS-PSII-PSI complexes. Linker proteins play a key role in the formation of modified complexes. ApcE and ApcF are responsible for forming protrusions at the base of the PBS core that fit with the pores on one side of the PSII cell membrane, allowing the PBS and PSII to be tightly connected, which is necessary for the transmission of light energy from PBS to PSII (Chang, Liu et al. 2015). The abundant aromatic amino acid benzene rings on the linker proteins can also form π-π interactions with the tetrapyrrole rings of surrounding pigment molecules, which are involved in regulating the energy state of the pigment molecules to ensure efficient unidirectional energy transfer (Ma, You et al. 2020).
4.2. Light acclimation of PBS
The structure and specialised function of the PBS allows the captured light energy to be transferred to the photosynthetic reaction centres with more than 95% efficiency (Zhang, Lambrev et al. 2015). However, excessive light harvesting can also cause damage to cyanobacteria, so cyanobacteria have evolved a photoprotection mechanism called non-photochemical quenching (NPQ) that rapidly converts excess excited energy into heat before it causes damage. However, this process leads to a reduction in the efficiency of light energy conversion. A photoprotection mode mediated by orange carotenoid protein (OCP) is known to change from OCPO to OCPR after absorbing redundant blue–green light, and then four OCPR form two dimers which are bound to PBS respectively, leading to NPQ. Notably, not every PBS is equally sensitive to NPQ, and only one of the three PBS conformational states reported associations with the OCPR (Domínguez-Martín, Sauer et al. 2022). The difference in the conformational state is generated by switching the position of the two rods, which regulates light harvesting.
To adapt to changes in environmental conditions, the composition and function of PBSs would change accordingly. Light intensity has the greatest influence on the composition of the rod and the ratio of PC:APC so as to yield a maximum production of PC under optimal photon flux. Other environmental factors also change the composition of the rods. For example, light colour and temperature can change the PC:PE ratio (Chenu, Keren et al. 2017). The photosynthetic electron transfer rate of PSII also affects the structure of the PBS. The ratio of PBS to chlorophyll protein content is influenced by the electron transfer rate when the required phytochrome is sufficient. Changes in copper ion concentration affect the stability of PBS, and changes in the structural stability of PBS follow the same trend as changes in the rate of electron transfer associated with copper ion concentration, and the structural stability of PBS decreases with the decrease of electron transfer rate, which may be related to structural changes in the rods. Iron deficiency inhibits PBS synthesis but does not affect the stability of PBS.
Degradation of PBS plays an important role in photoprotection, cell maintenance, growth and development in a constantly changing environment. During nitrogen limitation, PBSs in cells are degraded to avoid excessive light uptake and to allocate effective nitrogen to functions essential for growth and survival (Yoshihara and Kobayashi 2022). As a large nutrient reserve, the degradation of PBSs can provide essential amino acids for metabolic processes, and low levels of photosynthesis and loss of pigments are essential for cell survival during nitrogen, sulphur or phosphorus depletion. NblB and NblA are essential components for phycocyanin degradation under starvation conditions, during which NblB levels decrease approximately twofold and directly mediate pigment degradation through chromophore segregation. In contrast, NblA is highly expressed during starvation and may bind to one or two PBS complexes, destabilising the PBS complex and initiating proteochrome degradation (Nagarajan, Zhou et al. 2019). NblB-dependent PC degradation did not occur in the absence of NblA, so NblB has a dependent effect on NblA (Levi, Sendersky et al. 2018). Recent studies have found that NblD plays a critical role in the coordinated catabolism of PBSs and thus is a factor in the genetically programmed response to nitrogen starvation (Krauspe, Fahrner et al. 2021).
5. Prospects
In the past decades, great progress has been made in the understanding of PBSs and PBPs. High-resolution crystal structures of PBSs associated with the photosynthetic apparatus are now available. Phycobilisomes have a more complex structure and a more flexible type of assembly than other protein structures involved in photosynthesis. Although the interplay of multiple disciplines, including structural biology, biochemistry, genetic engineering and bioinformatics, gave us the molecular structures of PBSs and a pathway for recombinant PBPs, investigations on the dynamic assembly and energy transfer process of PBPs have progressed slowly because of the complex changes in the chromophore conformations and the intricate interactions among PBPs. With increasing knowledge of PBP assembly and the structural organisation of chromophores, it should be possible to simulate the assembly and energy-delivery route in the future. More in-depth studies on the structure and energy transfer of PBSs with techniques across the physical and life sciences would help not only to understand the process of PBP self-assembly but also to illustrate the spectroscopic properties of the phycobilin binding region. Research on the structure of PBPs would also deepen the understanding of their fine-tuned light-harvesting and energy-delivery capabilities. Zhang et al. (2017) reported the three-dimensional structure at near-atomic resolution of an intact PBS, which provides the basis for revealing the PBS assembly and light-transfer processes.
With the sequencing of the genome of algal species complete, more PBPs, linker proteins and chromophore lyases would be identified. Through the construction of genetically engineered chassis cells (bacteria or cyanobacteria), producing reconstituted PBPs, including recombinant PC or PE, at a large scale would be possible, thus broadening the scope of their application. For example, the biosynthesis and assembly of PE into the light-harvesting apparatus of other species may expand the wavelength range of absorbance and improve the efficiency of light-trapping devices under unique light conditions. The highly efficient utilisation of solar energy under low light might be accelerated by establishing artificial light-harvesting antennae. Research into genetically recombinant PBPs has also laid the material and technical foundations for the construction of PBP-based artificial solar energy capture devices.
Acknowledgments
This work was supported jointly by the National Key R&D Program of China (2021YFA0909600), the National Natural Science Foundation of China (32170138), the Natural Science Foundation of Henan Province (212300410024), the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (22IRTSTHN024), and the 111 Project (#D16014).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Apt, K.; Collier, J.L.; Grossman, A.R. Evolution of the Phycobiliproteins. Journal of Molecular Biology 1995, 248, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Vasquez, Y.M.; Dragomani, T.M.; Kronfel, M.L.; Williams, S.R.; Alvey, R.M.; Bryant, D.A.; Schluchter, W.M. Biosynthesis of cyanobacterial phycobiliproteins in Escherichia coli: Chromophorylation efficiency and specificity of all bilin lyases from Synechococcus sp. strain PCC 7002. Appl Environ Microbiol 2010, 76, 2729–2739. [Google Scholar] [PubMed]
- Blot, N.; Wu, X.J.; Thomas, J.C.; Zhang, J.; Garczarek, L.; Böhm, S.; Tu, J.M.; Zhou, M.; Plöscher, M.; Eichacker, L.; Partensky, F.; Scheer, H.; Zhao, K.H. Phycourobilin in trichromatic phycocyanin from oceanic cyanobacteria is formed post-translationally by a phycoerythrobilin lyase-isomerase. J Biol Chem 2009, 284, 9290–9298. [Google Scholar] [CrossRef] [PubMed]
- Bryant, D. A., R. de Lorimier, D. H. Lambert, J. M. Dubbs, V. L. Stirewalt, S. E. Stevens, Jr., R. D. Porter, J. Tam and E. Jay Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A 1985, 82, 3242–3246. [CrossRef] [PubMed]
- Chang, L.; Liu, X.; Li, Y.; Liu, C.C.; Yang, F.; Zhao, J.; Sui, S.F. Structural organization of an intact phycobilisome and its association with photosystem II. Cell Res 2015, 25, 726–737. [Google Scholar] [CrossRef]
- Chenu, A.; Keren, N.; Paltiel, Y.; Nevo, R.; Reich, Z.; Cao, J. Light Adaptation in Phycobilisome Antennas: Influence on the Rod Length and Structural Arrangement. J Phys Chem B 2017, 121, 9196–9202. [Google Scholar] [CrossRef]
- Dammeyer, T.; Frankenberg-Dinkel, N. Function and distribution of bilin biosynthesis enzymes in photosynthetic organisms. Photochem Photobiol Sci 2008, 7, 1121–1130. [Google Scholar] [CrossRef]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. The Journal of Supercritical Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Domínguez-Martín, M.A.; Sauer, P.V.; Kirst, H.; Sutter, M.; Bína, D.; Greber, B.J.; Nogales, E.; Polívka, T.; Kerfeld, C.A. Structures of a phycobilisome in light-harvesting and photoprotected states. Nature 2022, 609, 835–845. [Google Scholar] [CrossRef]
- Fairchild, C.D.; Glazer, A.N. Oligomeric structure, enzyme kinetics, and substrate specificity of the phycocyanin alpha subunit phycocyanobilin lyase. J Biol Chem 1994, 269, 8686–8694. [Google Scholar] [CrossRef]
- Fairchild, C.D.; Zhao, J.; Zhou, J.; Colson, S.E.; Bryant, D.A.; Glazer, A.N. Phycocyanin alpha-subunit phycocyanobilin lyase. Proc Natl Acad Sci U S A 1992, 89, 7017–7021. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Medina-Campos, O.N.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Pedraza-Chaverri, J. C-phycocyanin prevents cisplatin-induced nephrotoxicity through inhibition of oxidative stress. Food Funct 2014, 5, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Folea, I.M.; Zhang, P.; Aro, E.M.; Boekema, E.J. Domain organization of photosystem II in membranes of the cyanobacterium Synechocystis PCC6803 investigated by electron microscopy. FEBS Lett 2008, 582, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Gantt, E.; Conti, S.F. Phycobiliprotein localization in algae. Brookhaven Symp Biol 1966, 19, 393–405. [Google Scholar] [PubMed]
- Ge, B.; Li, Y.; Sun, H.; Zhang, S.; Hu, P.; Qin, S.; Huang, F. Combinational biosynthesis of phycocyanobilin using genetically-engineered Escherichia coli. Biotechnol Lett 2013, 35, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Liu, G.; Liu, H.; Zhu, F.; Ji, H.; Li, B. C-Phycocyanin exerts anti-cancer effects via the MAPK signaling pathway in MDA-MB-231 cells. Cancer Cell Int 2018, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Hamaguchi, T.; Hirose, Y.; Kosumi, D.; Miyata, M.; Kamiya, N.; Yonekura, K. Core and rod structures of a thermophilic cyanobacterial light-harvesting phycobilisome. Nat Commun 2022, 13, 3389. [Google Scholar] [CrossRef]
- Krauspe, V.; Fahrner, M.; Spät, P.; Steglich, C.; Frankenberg-Dinkel, N.; Maček, B.; Schilling, O.; Hess, W.R. Discovery of a small protein factor involved in the coordinated degradation of phycobilisomes in cyanobacteria. Proc Natl Acad Sci U S A 2021, 118. [Google Scholar] [CrossRef]
- Kronfel, C.M.; Hernandez, C.V.; Frick, J.P.; Hernandez, L.S.; Gutu, A.; Karty, J.A.; Boutaghou, M.N.; Kehoe, D.M.; Cole, R.B.; Schluchter, W.M. CpeF is the bilin lyase that ligates the doubly linked phycoerythrobilin on β-phycoerythrin in the cyanobacterium Fremyella diplosiphon. J Biol Chem 2019, 294, 3987–3999. [Google Scholar] [CrossRef]
- Kronfel, C.M.; Kuzin, A.P.; Forouhar, F.; Biswas, A.; Su, M.; Lew, S.; Seetharaman, J.; Xiao, R.; Everett, J.K.; Ma, L.C.; Acton, T.B.; Montelione, G.T.; Hunt, J.F.; Paul, C.E.; Dragomani, T.M.; Boutaghou, M.N.; Cole, R.B.; Riml, C.; Alvey, R.M.; Bryant, D.A.; Schluchter, W.M. Structural and biochemical characterization of the bilin lyase CpcS from Thermosynechococcus elongatus. Biochemistry 2013, 52, 8663–8676. [Google Scholar] [CrossRef]
- Kumarapperuma, I.; Joseph, K.L.; Wang, C.; Biju, L.M.; Tom, I.P.; Weaver, K.D.; Grébert, T.; Partensky, F.; Schluchter, W.M.; Yang, X. Crystal structure and molecular mechanism of an E/F type bilin lyase-isomerase. Structure 2022, 30, 564–574.e563. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M.; Smith, C.; Safford, M.; Terstappen, L.W.; Thomas, T.E. Single laser three color immunofluorescence staining procedures based on energy transfer between phycoerythrin and cyanine 5. Cytometry 1991, 12, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.O.; Lee, H.H.; Kung, Y.C.; Tsai, M.F.; Chou, T.C. Therapeutic effect of C-phycocyanin extracted from blue green algae in a rat model of acute lung injury induced by lipopolysaccharide. Evid Based Complement Alternat Med 2013, 2013, 2013, 916590. [Google Scholar] [CrossRef] [PubMed]
- Levi, M.; Sendersky, E.; Schwarz, R. Decomposition of cyanobacterial light harvesting complexes: NblA-dependent role of the bilin lyase homolog NblB. Plant J 2018, 94, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, H.N.; Pu, Y.; Chen, J.; Liu, L.N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol Adv 2019, 37, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Y.; Cao, M.; Pan, T.; Yang, Y.; Mao, H.; Sun, L.; Liu, G. Anti-allergic activity of R-phycocyanin from Porphyra haitanensis in antigen-sensitized mice and mast cells. International Immunopharmacology 2015, 25, 465–473. [Google Scholar] [CrossRef]
- Ma, J.; You, X.; Sun, S.; Wang, X.; Qin, S.; Sui, S.F. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature 2020, 579, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Ding, W.L.; Zhao, B.Q.; Lu, L.; Xu, Q.Z.; Scheer, H.; Zhao, K.H. Adapting photosynthesis to the near-infrared: Non-covalent binding of phycocyanobilin provides an extreme spectral red-shift to phycobilisome core-membrane linker from Synechococcus sp. PCC7335. Biochim Biophys Acta 2016, 1857, 1857, 688–694. [Google Scholar] [CrossRef]
- Montgomery, B.L. Seeing new light: Recent insights into the occurrence and regulation of chromatic acclimation in cyanobacteria. Current Opinion in Plant Biology 2017, 37, 18–23. [Google Scholar] [CrossRef]
- Mukougawa, K.; Kanamoto, H.; Kobayashi, T.; Yokota, A.; Kohchi, T. Metabolic engineering to produce phytochromes with phytochromobilin, phycocyanobilin, or phycoerythrobilin chromophore in Escherichia coli. FEBS Lett 2006, 580, 1333–1338. [Google Scholar] [CrossRef]
- Müller, K.; Engesser, R.; Timmer, J.; Nagy, F.; Zurbriggen, M.D.; Weber, W. Synthesis of phycocyanobilin in mammalian cells. Chem Commun (Camb) 2013, 49, 8970–8972. [Google Scholar] [CrossRef] [PubMed]
- Mysliwa-Kurdziel, B.; Solymosi, K. Phycobilins and Phycobiliproteins Used in Food Industry and Medicine. Mini Rev Med Chem 2017, 17, 1173–1193. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A.; Zhou, M.; Nguyen, A.Y.; Liberton, M.; Kedia, K.; Shi, T.; Piehowski, P.; Shukla, A.; Fillmore, T.L.; Nicora, C.; Smith, R.D.; Koppenaal, D.W.; Jacobs, J.M.; Pakrasi, H.B. Proteomic Insights into Phycobilisome Degradation, A Selective and Tightly Controlled Process in The Fast-Growing Cyanobacterium Synechococcus elongatus UTEX 2973. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Ritcharoen, W.; Sri-Uam, P.; Pavasant, P.; Adachi, S. Antioxidant properties of convective-air-dried Spirulina maxima: Evaluation of phycocyanin retention by a simple mathematical model of air-drying. Food and Bioproducts Processing 2016, 100, 292–302. [Google Scholar] [CrossRef]
- Nguyen, A.A.; Joseph, K.L.; Bussell, A.N.; Pokhrel, S.; Karty, J.A.; Kronfel, C.M.; Kehoe, D.M.; Schluchter, W.M. CpeT is the phycoerythrobilin lyase for Cys-165 on β-phycoerythrin from Fremyella diplosiphon and the chaperone-like protein CpeZ greatly improves its activity. Biochim Biophys Acta Bioenerg 2020, 1861, 1861, 148284. [Google Scholar] [CrossRef] [PubMed]
- Overkamp, K.E.; Gasper, R.; Kock, K.; Herrmann, C.; Hofmann, E.; Frankenberg-Dinkel, N. Insights into the biosynthesis and assembly of cryptophycean phycobiliproteins. J Biol Chem 2014, 289, 26691–26707. [Google Scholar] [CrossRef]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int J Biol Macromol 2013, 60, 393–398. [Google Scholar] [CrossRef]
- Saunée, N.A.; Williams, S.R.; Bryant, D.A.; Schluchter, W.M. Biogenesis of phycobiliproteins: II. CpcS-I and CpcU comprise the heterodimeric bilin lyase that attaches phycocyanobilin to CYS-82 OF beta-phycocyanin and CYS-81 of allophycocyanin subunits in Synechococcus sp. PCC 7002. J Biol Chem 2008, 283, 7513–7522. [Google Scholar] [CrossRef]
- Shen, G.; Saunée, N.A.; Williams, S.R.; Gallo, E.F.; Schluchter, W.M.; Bryant, D.A. Identification and characterization of a new class of bilin lyase: The cpcT gene encodes a bilin lyase responsible for attachment of phycocyanobilin to Cys-153 on the beta-subunit of phycocyanin in Synechococcus sp. PCC 7002. J Biol Chem 2006, 281, 17768–17778. [Google Scholar]
- Sidler, W.A. (1994). Phycobilisome and Phycobiliprotein Structures. The Molecular Biology of Cyanobacteria. D. A. Bryant. Dordrecht, Springer Netherlands: 139-216.
- Sonani, R.R.; Roszak, A.W.; de Percin Northumberland, C.O.; Madamwar, D.; Cogdell, R.J. An improved crystal structure of C-phycoerythrin from the marine cyanobacterium Phormidium sp. A09DM. Photosynth Res 2018, 135, 65–78. [Google Scholar] [CrossRef]
- Soulier, N.; Bryant, D.A. The structural basis of far-red light absorbance by allophycocyanins. Photosynth Res 2021, 147, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.F. Structure of Phycobilisomes. Annu Rev Biophys 2021, 50, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Aikawa, S.; Niwa, K.; Abe, T.; Murakami, A.; Kondo, A.; Akimoto, S. Variety in excitation energy transfer processes from phycobilisomes to photosystems I and II. Photosynth Res 2017, 133, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Gao, X.; Carter, C.L.; Liu, Z.R. The recombinant beta subunit of C-phycocyanin inhibits cell proliferation and induces apoptosis. Cancer Lett 2007, 247, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, C.; Li, B.; Liu, C.; He, P. [Photodynamic effect of two kinds of phycobiliproteins on human liver cancer cell line SMMC-7721 in vitro]. Sheng Wu Gong Cheng Xue Bao 2009, 25, 1417–1423. [Google Scholar]
- Watanabe, M.; Ikeuchi, M. Phycobilisome: Architecture of a light-harvesting supercomplex. Photosynth Res 2013, 116, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch Toxicol 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Yang, H.; Chen, Y.T.; Li, P.P. Biosynthesis of Fluorescent β Subunits of C-Phycocyanin from Spirulina subsalsa in Escherichia coli, and Their Antioxidant Properties. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Yoshihara, A.; Kobayashi, K. Photosynthesis and Cell Growth Trigger Degradation of Phycobilisomes during Nitrogen Limitation. Plant Cell Physiol 2022, 62, 189–199. [Google Scholar] [CrossRef]
- You, X.; Zhang, X.; Cheng, J.; Xiao, Y.; Ma, J.; Sun, S.; Zhang, X.; Wang, H.W.; Sui, S.F. In situ structure of the red algal phycobilisome-PSII-PSI-LHC megacomplex. Nature 2023, 616, 199–206. [Google Scholar] [CrossRef]
- Yu, P.; Li, P.; Chen, X.; Chao, X. Combinatorial biosynthesis of Synechocystis PCC6803 phycocyanin holo-α-subunit (CpcA) in Escherichia coli and its activities. Appl Microbiol Biotechnol 2016, 100, 5375–5388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lambrev, P.H.; Wells, K.L.; Garab, G.; Tan, H.S. Direct observation of multistep energy transfer in LHCII with fifth-order 3D electronic spectroscopy. Nat Commun 2015, 6, 7914. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Höppner, A.; Xu, Q.Z.; Gärtner, W.; Scheer, H.; Zhou, M.; Zhao, K.H. Structures and enzymatic mechanisms of phycobiliprotein lyases CpcE/F and PecE/F. Proc Natl Acad Sci U S A 2017, 114, 13170–13175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.H.; Su, P.; Bohm, S.; Song, B.; Zhou, M.; Bubenzer, C.; Scheer, H. Reconstitution of phycobilisome core-membrane linker, LCM, by autocatalytic chromophore binding to ApcE. Biochim Biophys Acta 2005, 1706, 1706, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, H.; Wang, Y.; Wang, Z.; Zhou, J. Efficient Synthesis of Phycocyanobilin by Combinatorial Metabolic Engineering in Escherichia coli. ACS Synth Biol 2022, 11, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zheng, Z.; Li, X.; Wang, G.; Zhang, K.; Wei, P.; Zhao, J.; Gao, N. Structural insight into the mechanism of energy transfer in cyanobacterial phycobilisomes. Nat Commun 2021, 12, 5497. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gasparich, G.E.; Stirewalt, V.L.; de Lorimier, R.; Bryant, D.A. The cpcE and cpcF genes of Synechococcus sp. PCC 7002. Construction and phenotypic characterization of interposon mutants. J Biol Chem 1992, 267, 16138–16145. [Google Scholar] [CrossRef]
- Zhou, W.; Ding, W.L.; Zeng, X.L.; Dong, L.L.; Zhao, B.; Zhou, M.; Scheer, H.; Zhao, K.H.; Yang, X. Structure and mechanism of the phycobiliprotein lyase CpcT. J Biol Chem 2014, 289, 26677–26689. [Google Scholar] [CrossRef]
- Zolla, L.; Bianchetti, M.; Rinalducci, S. Functional studies of the Synechocystis phycobilisomes organization by high performance liquid chromatography on line with a mass spectrometer. Eur J Biochem 2002, 269, 1534–1542. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).