Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
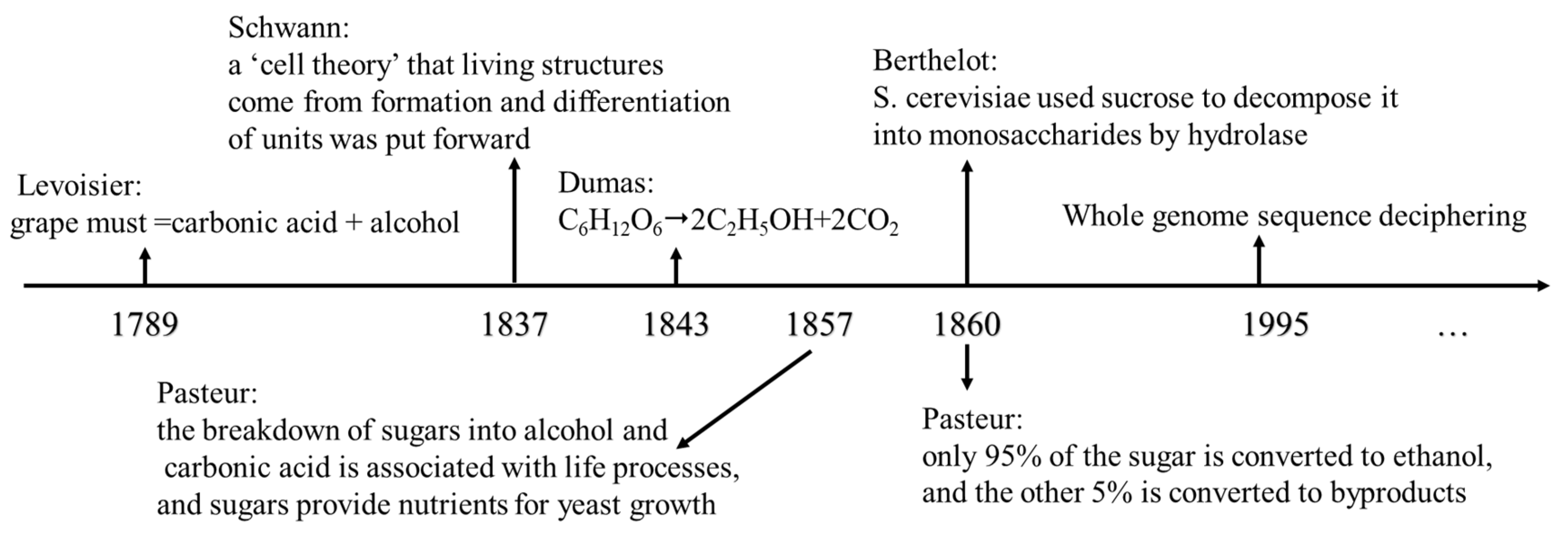
1. Introduction
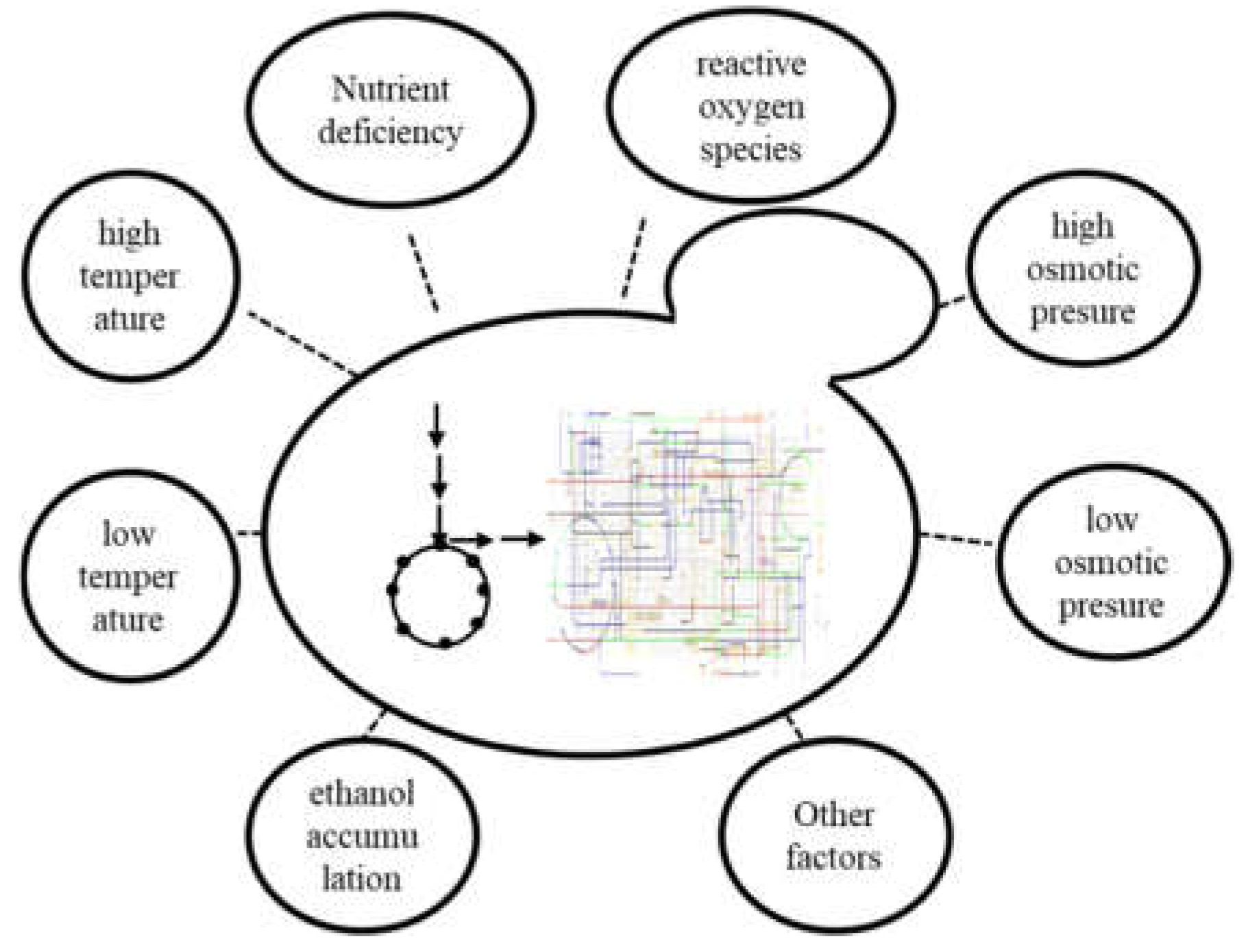
2. Ethanol Fermentation with Saccharomyces Cerevisiae
2.1. Ethanol Fermentation Based on Glucose and Sucrose
2.2. Ethanol Fermentation Based on Starch
2.3. Ethanol Fermentation Based on Molasses
3. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Competing interests
Consent for publication
Availability of data and materials
Ethics approval and consent to participate
References
- Samuel D. Investigation of ancient egyptian baking and brewing methods by correlative microscopy. Science 1996;273(5274):488-490. [CrossRef]
- Wang WY. Wu SH. Xie YH. Zhong M. Wei ML. Li ZY. Long XF. Niu FX. A high-throughput screening procedure (Py-Fe3+) for enhancing ethanol production by Saccharomyces cerevisiae using ARTP random mutagenesis. Processes. 2022, 10, 2186. [CrossRef]
- Barnett JA. Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiology 2003;149(3):557-567. [CrossRef]
- Lanska DJ. Encyclopedia of the neurological sciences. 2014;846-847. [CrossRef]
- Poethig RS. Life with 25000 genes. Genome Res 2001;11(3):313-316. [CrossRef]
- Marullo P, Durrens P, Peltier E, Bernard M, Dubourdieu D. Natural allelic variations of Saccharomyces cerevisiae impact stuck fermentation due to the combined effect of ethanol and temperature; a QTL-mapping study. BMC Genom 2019;20(1):680. [CrossRef]
- Khatun MM, Yu X, Kondo A, Bai F, Zhao X. Improved ethanol production at high temperature by consolidated bioprocessing using Saccharomyces cerevisiae strain engineered with artificial zinc finger protein. Bioresour Technol 2017;245:1447-1454. [CrossRef]
- Lambrechts M, Pretorius I. Yeast and its importance to wine aroma - a review. South African J. Enol Vitic 2000;21(1). [CrossRef]
- Park JI, Grant CM, Davies MJ, Dawes IW. The cytoplasmic Cu,Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress. Generation of free radicals during freezing and thawing. J Biol Chem 1998;273(36):22921-22928. [CrossRef]
- Diniz-Mendes L, Bernardes E, de Araujo PS, Panek AD, Paschoalin VM. Preservation of frozen yeast cells by trehalose. Biotechnol Bioeng 1999;65(5):572-578. [CrossRef]
- Verstrepen KJ, Iserentant D, Malcorps P, Derdelinckx G, Dijck PV, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR. Glucose and sucrose: hazardous fast-food for industrial yeast? Trends Biotechnol 2004;22(10):531-537. [CrossRef]
- Izawa S, Ikeda K, Miki T, Wakai Y, Inoue Y. Vacuolar morphology of Saccharomyces cerevisiae during the process of wine making and japanese sake brewing. Appl Microbio Biotechnol 2010;88(1):277-282. [CrossRef]
- Perrier-Cornet JM, Hayert M, Saurat E, Milesse C, Gervais P. Effect of osmotic stress on high pressure inactivation of Saccharomyces cerevisiae. High Press Biosci Biotechnol 1999;27-30. [CrossRef]
- Qin L, Dong S, Yu J, Ning X, Xu K, Zhang S-J, Xu L, Li B-Z, Li J, Yuan Y-J, Li C. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation. Metab Eng 2020;61:160-170. [CrossRef]
- Treu L, Campanaro S, Nadai C, Toniolo C, Nardi T, Giacomini A, Valle G, Blondin B, Corich V. Oxidative stress response and nitrogen utilization are strongly variable in Saccharomyces cerevisiae wine strains with different fermentation performances. Appl Microbiol Biotechnol 2014;98(9):4119-4135. [CrossRef]
- O'Brien D, Craig J. Ethanol production in a continuous fermentation/membrane pervaporation system. Appl Microbiol Biotechnol 1996;44:699-704. [CrossRef]
- Daugulis AJ, Axford DB, Ciszek B, Malinowski JJ. Continuous fermentation of high-strength glucose feeds to ethanol. Biotechnol Lett 1994;16:637-642. [CrossRef]
- Mathew AS, Wang J, Luo J, Yau ST. Enhanced ethanol production via electrostatically accelerated fermentation of glucose using Saccharomyces cerevisiae. Sci Rep 2015;5:15713. [CrossRef]
- Indra Neel P, Gedanken A, Schwarz R, Sendersky E. Mild sonication accelerates ethanol production by yeast fermentation. Energy Fuels 2012;26(4):2352-2356. [CrossRef]
- Kogtimas A, Gourdoupis C, Psarianos C. Kaliafas, A.; Kanellaki, M. Continuous potable alcohol production by immobilized Saccharomyces cerevisiae on mineral kissiris. Appl Biochem Biotechnol 1991;30:203-216. [CrossRef]
- Pacheco TF, Galvão de Morais Júnior W, Zanella Guidini C, Marquez LDS, Cardoso VL, Resende MM, Ribeiro EJ. Alcoholic Fermentation with Self-Flocculating Yeast in a Tower Upflow Reactor. Chem Eng Technol 2015;38(2):345-354. [CrossRef]
- Santos LD, Sousa MDB, Guidini CZ, De Resende M. M, Cardoso VL, Ribeiro EJ. Continuous ethanol fermentation in tower reactors with cell recycling using flocculent Saccharomyces cerevisiae. Process Biochem 2015;50(11):1725-1729. [CrossRef]
- Breisha, GZ. Production of 16% ethanol from 35% sucrose. Biomass Bioenergy 2010;34(8):1243-1249. [CrossRef]
- Abouzied MM, Reddy CA. Fermentation of starch to ethanol by a complementary mixture of an amylolytic yeast and Saccharomyces cerevisiae. Biotechnol Lett 1987;9:59-62. [CrossRef]
- Shin YC, Lee SY, Choe YK, Kim HS, Byunt SM. Ethanol fermentation of cassava starch pretreated with alkali. Biotechnol Bioeng. 1986;28(4):627-630. [CrossRef]
- Ping W, Singh V, Hua X, Johnston DB, Tumbleson ME. Comparison of raw starch hydrolyzing enzyme with conventional liquefaction and saccharification enzymes in dry-grind corn processing. Cereal Chem 2007;84(1):10-14. [CrossRef]
- Kaseno, Kokugan T. The effect of molasses pretreatment by ceramic microfiltration membrane on ethanol fermentation. J Biosci Bioeng 1997;83(6):577-582. [CrossRef]
- Rattanapan A, Limtong S, Phisalaphong M. Ethanol production by repeated batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons. Appl Energy 2011;88(12):4400-4404. [CrossRef]
- Wu R, Chen D, Cao S, Lu Z, Huang R. Enhanced ethanol production from sugarcane molasses by industrially engineered Saccharomyces cerevisiae via replacement of the PHO4 gene. RSC Adv 2020;10:2267-2276. [CrossRef]
- Javan F, Mobini-Dehkordi M. Application of alpha-amylase in biotechnology. J Biol 2012. [CrossRef]
- Knox AM, Preez J, Kilian SG. Starch fermentation characteristics of Saccharomyces cerevisiae strains transformed with amylase genes from Lipomyces kononenkoae and Saccharomycopsis fibuligera - ScienceDirect. Enzyme & Microbial Technology. 2004;34(5):453-460. [CrossRef]
- Wang X, Nie Y, Xu Y. Industrially produced pullulanases with thermostability: Discovery, engineering, and heterologous expression. Bioresour Technol 2019;278:360-371. [CrossRef]
- Bothast RJ, Schlicher MA. Biotechnological processes for conversion of corn into ethanol. Appl Microbiol Biotechnol 2005;67(1):19-25. [CrossRef]
- Robertson GH, Wong DWS, Lee CC, Wagschal K, Orts WJ. Native or raw starch digestion: a key step in energy efficient biorefining of grain. J Agric Food Chem 2006;54(2):353-365. [CrossRef]
- van Zyl WH, Bloom M, Viktor MJ. Engineering yeasts for raw starch conversion. Appl Microbiol Biotechnol 2012;95(6):1377-1388. [CrossRef]
- Af A, Aas A, Np B. Production of bioethanol from four species of duckweeds (Landoltia punctata, Lemna aequinoctialis, Spirodela polyrrhiza, and Wolffia arrhiza) through optimization of saccharification process and fermentation with Saccharomyces cerevisiae. Saudi J Biol Sci 2021;28(1):294-301. [CrossRef]
- Hu Z, Tan P, Pu G. Multi-objective optimization of cassava-based fuel ethanol used as an alternative automotive fuel in Guangxi, China. Appl Energy 2006;83(8):819-840. [CrossRef]
- Choi GW, Moon SK, Kang HW, Min J, Chung BW. Simultaneous saccharification and fermentation of sludge-containing cassava mash for batch and repeated batch production of bioethanol by Saccharomyces cerevisiae CHFY0321. J Chem Technol Biotechnol 2010;84(4):547-553. [CrossRef]
- Lee CJ, Yangcheng H, Cheng JJ, Jane J-l. Starch characterization and ethanol production of duckweed and corn kernel. Starke 2015;68(3-4):348-354. [CrossRef]
- Ülgen K Ö, Saygili B, Önsan Z.İ, Kirdar B. Bioconversion of starch into ethanol by a recombinant Saccharomyces cerevisiae strain YPG-AB. Process Biochem 2002;37(10):1157-1168. [CrossRef]
- Eksteen JM, Rensburg PV, Otero RRC, Pretorius IS. Starch fermentation by recombinant Saccharomyces cerevisiae strains expressing the alpha-amylase and glucoamylase genes from lipomyces kononenkoae and saccharomycopsis fibuligera. Biotechnol Bioeng 2003;84(6):639-646. [CrossRef]
- Nakamura Y, Kobayashi F, And MO, Sawada T. Alcohol fermentation of starch by a genetic recombinant yeast having glucoamylase activity. Biotechnol Bioeng 1997;53(1):21-25. [CrossRef]
- Haan RD, Kroukamp H, Mert M, Bloom M, Görgens J, Zyl W. Engineering Saccharomyces cerevisiae for next generation ethanol production. J Chem Technol Biotechnol 2013;88(6):983-991. [CrossRef]
- Cripwell RA, Rose SH, Favaro L, Zyl WHV. Construction of industrial Saccharomyces cerevisiae strains for the efficient consolidated bioprocessing of raw starch. Biotechnol Biofuels 2019;12:201. [CrossRef]
- Chotineeranat S, Wansuksri R, Piyachomkwan K, Chatakanonda P, Weerathaworn P, Sriroth K. Effect of calcium ions on ethanol production from molasses by Saccharomyces cerevisiae. Sugar Tech 2010;12:120-124. [CrossRef]
- Ergun, AT. Effect of zeolite nay and ca-montmorillonite on ethanol production using synthetic molasses. Appl Biochem Biotechnol 2008;144(2):161-168. [CrossRef]
- Abbott DA, Ingledew WM. Buffering capacity of whole corn mash alters concentrations of organic acids required to inhibit growth of Saccharomyces cerevisiae and ethanol production. Biotechnol Lett 2004;26(16):1313-1316. [CrossRef]
- Echegaray OF, Carvalho J, Fernandes A, Sato S, Aquarone E, Vitolo M. Fed-batch culture of Sacchoromyces cerevisiae in sugar-cane blackstrap molasses: invertase activity of intact cells in ethanol fermentation. Biomass Bioenergy 2000;19(1):39-50. [CrossRef]
- Borzani W, Hiss H, Santos T, Vairo M. Semicontinuous ethanol fermentation of sugar cane blackstrap molasses by pressed yeast. Biotechnol Lett 1992;14(10):981-984. [CrossRef]
- Hatano KI, Kikuchi S, Nakamura Y, Sakamoto H, Takigami M, Kojima Y. Novel strategy using an adsorbent-column chromatography for effective ethanol production from sugarcane or sugar beet molasses. Bioresour Technol 2009;100(20):4697-4703. [CrossRef]
- Johnson, R. Dialysis and ultrafiltration of molasses for fermentation enhancement. Sep Purif Technol 2001;22–23:239-245. [CrossRef]
- Ghorbani F, Younesi H, Sari A, Najafpour G. Cane molasses fermentation for continuous ethanol production in an immobilized cells reactor by Saccharomyces cerevisiae. Renew Energ 2011;36(2):503-509. [CrossRef]
- Babu NK, Satyanarayana B, Balakrishnan K, Rao TR, Rao GS. Study of sugarcane pieces as yeast supports for ethanol production from sugarcane juice and molasses using newly isolated yeast from toddy sap. Mycobiology 2012;40(1):35-41. [CrossRef]
- Qin L, Dong S, Yu J, Ning X, Li C. Stress-driven dynamic regulation of multiple tolerance genes improves robustness and productive capacity of Saccharomyces cerevisiae in industrial lignocellulose fermentation. Metab Eng 2020;61:160-170. [CrossRef]
- Shima J, Takagi H. Stress-tolerance of baker's-yeast (Saccharomyces cerevisiae) cells: stress-protective molecules and genes involved in stress tolerance. Biotechnol Appl Biochem 2009;53(3):155-164. [CrossRef]
| Substrate | Method | Achievement | Ref |
|---|---|---|---|
| Glucose | Coupled to a flat-plate membrane pervaporation unit to recover ethanol | ethanol productivities reached 7.8 g/L/h | [16] |
| Glucose | Extractive fermentation, aqueous feeds with 413 and 495 g/L glucose | Ethanol conversion efficiencies reached 90%-95% conversion | [17] |
| Glucose | electrostatic fermentation with 15 V | The consumption of glucose reached 98% in 20 h | [18] |
| Glucose | Fermentation with mild ultrasonication | 10 times faster than non-stirred fermentation. | [19] |
| sucrose | Continuous potable production by immobilized on mineral kissiris | ethanol productivities reached 10 g/L/h | [20] |
| sucrose | Fermentation with self-flocculating yeast in a tower upflow reactor | ethanol productivities reached 13.5 g/L/h | [21] |
| sucrose | Continuous ethanol fermentation in tower reactors with cell recycling | ethanol productivities reached 18 g/L/h | [22] |
| sucrose | Addition of air at a rate of 150 dm3 /min/m3 of reactor volume during the first 12 h | 35% sucrose is consumed and 16% ethanol is fermented | [23] |
| Starch | Co-cultured with amylolytic yeast | fermented unhydrolyzed starch to ethanol with conversion efficiencies over 90% of the theoretical maximum. | [24] |
| Starch | Starch Pretreated with 0.5 M HCl at 60℃, followed by combined actions of a-amylase and glucoamylase. | Ethanol conversion efficiencies reached 95% | [25] |
| Starch | a raw starch hydrolyzing enzyme was used that converts starch into dextrins at low temperatures, and hydrolyzes dextrins into sugars | Ethanol conversion efficiencies reached 88.4% | [26] |
| molasses | Batch and semicontinuous immobilized yeast fermentations together with a pervaporation method | Residual sugar was reduced 42%, ethanol produced increased 18.1% | [27] |
| molasses | batch and continuous fermentations of blackstrap molasses using immobilized yeast cells on thin-shell silk cocoons |
Ethanol concentration 11.5% higher than produced by free cells | [28] |
| molasses | A new candidate ethanol fermentation-related regulatory gene, PHO4, was replaced from a low ethanol yield but rapid growth strain to a high ethanol yield industrial strain. | Average high ethanol production reached 114.71 g/ L, ethanol production increased by 5.30% The fermentation time was 12.5% lower than that of the original strain. |
[29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





