Submitted:
08 May 2023
Posted:
09 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Structure of Skin
3. Issues Related to the Study and Management of Wound Healing
4. Vertebrates and Wound Healing
5. Wound Healing Modalities
Wounds can Heal in Three Different Ways:
6. Gross Stages of Wound Healing
7. Acute Inflammatory Reaction
8. Summary of Principal Events in Wound Healing
| Stages of wound healing | ||
|---|---|---|
| Phases | Time | Event |
| Hemostasis | A few minutes | Formation of the fibrin clot |
| Inflammatory | 3-12 days | Vasodilation, diapedesis, inflammatory response, phagocytosis. |
| Proliferation | 3 days to 12 days | Angiogenesis, granulation tissue formation, epithelialization |
| Maturation | 3 days to 6 months | Wound contraction |
9. Differences with Fetal Wound Healing
10. Recent Advances about the Principal Cells Involved in Wound Healing
10.1. Platelets
10.2. Mast Cells
10.3. Neutrophils
10.4. Macrophages
10.5. Dendritic Cells
10.6. Plasmacytoid Dendritic Cells
10.7. Lymphocytes
10.8. Keratinocytes
10.9. Endothelial Cells
10.10. Pericytes
10.11. Fibroblasts
10.12. Myofibroblasts
10.13. Central Role of Mast Cells in Wound Healing: A Hypothesis
| Wound healing | ||||
| Phases | Time | Cells | Main bioactive factors secreted by cells involved in wound healing | Functions |
| Hemostasis | A few minutes | Platelets: |
CYTOKINES: TNFalpha GROWTH FACTORS: PDGF, TGFbeta, TGFalfa, FGF, IGF-1, VEGF CHEMOKINES: CXCL8, CXCL1, CXCL2 |
Initiation of inflammatory responses, angiogenesis |
| Inflammatory | 3-12 minutes to 3 days | Mast Cells |
BIOGENIC AMMINE: Histamine CYTOKINES: TNFalpha, IL4, IL6, IL8 GROWTH FACTORS: VEGF, FGF |
Vasodilation, Inflammatory response Production of ECM |
| As above | As above | Neutrophils |
CYTOKINES: IL1beta, IL6, IL8, TNFalpha CHEMOKINES: CXCL1, CXCL2, CXCL8 GROWTH FACTORS: IGF, VEGF |
Inflammatory response, keratinocyte proliferation, fibroblast proliferation Angiogenesis, collagen synthesis, endothelial cell activation, |
| As above | As above | Macrophages |
CYTOKINES: IFNgamma, IL1beta, IL6, IL8, IL10, TNFalpha CHEMOKINES: Rantes GROWTH FACTORS: EGF, FGF, IGF, PGDF, TGFbeta, VEGF |
Inflammatory response Fibroblast proliferation, fibroblast chemotaxis, angiogenesis, ECM deposition |
| As above | As above |
Dendritic cells, plasmacytoid dendritic cells |
GROWTH FACTORS: TGFbeta CYTOKINES: IFN gamma |
Inflammatory response |
| As above | As above | Lymphocytes |
CYTOKINES: IFNgamma, IL2, IL4, IL10 CHEMOKINES: MCP, RANTES, MIP, Lymphotactin |
Inflammatory response, decrease in collagen synthesis, synthesis of MMPS Inflammatory response |
| Proliferation | 3 days to 6 months | Keratinocytes |
CYTOKINES: IL1, IL6, IL8, IL10, IL18, IL20, TNFalpha GROWTH FACTORS: TGFbeta, VEGF, EGF, PGDF, SCF CHEMOKINES: RANTES, MCP or MIP-1 |
Proliferation of keratinocytes Angiogenesis, proliferation of keratinocytes Inflammatory response |
| As above | As above | Endothelial cells |
GROWTH FACTORS: CTGF, FGF, IGF, TGFbeta, PGDF, VEGF |
Proliferation of fibroblasts and keratinocytes, differentiation of keratinocytes, angiogenesis |
| As above | As above | Fibroblasts |
CHEMOKINES: CXCL1, CX3CL1, CCL2 CYTOKINES: IL6, IL8, IL12 GROWTH FACTORS: FGF, IGF, KGF, VEGF |
Chemotaxis of inflammatory cells, proliferation of fibroblasts, fibroblast differentiation |
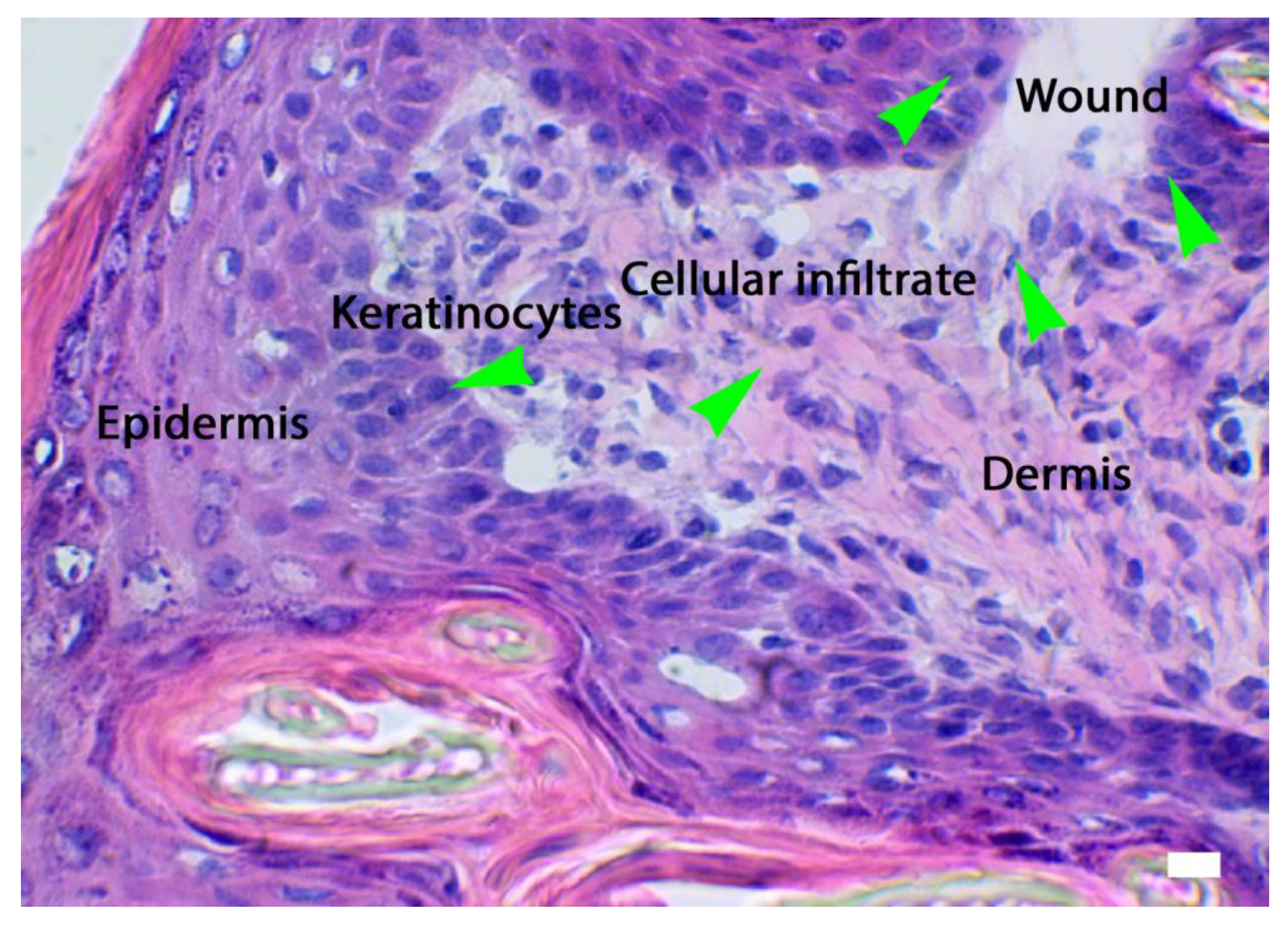
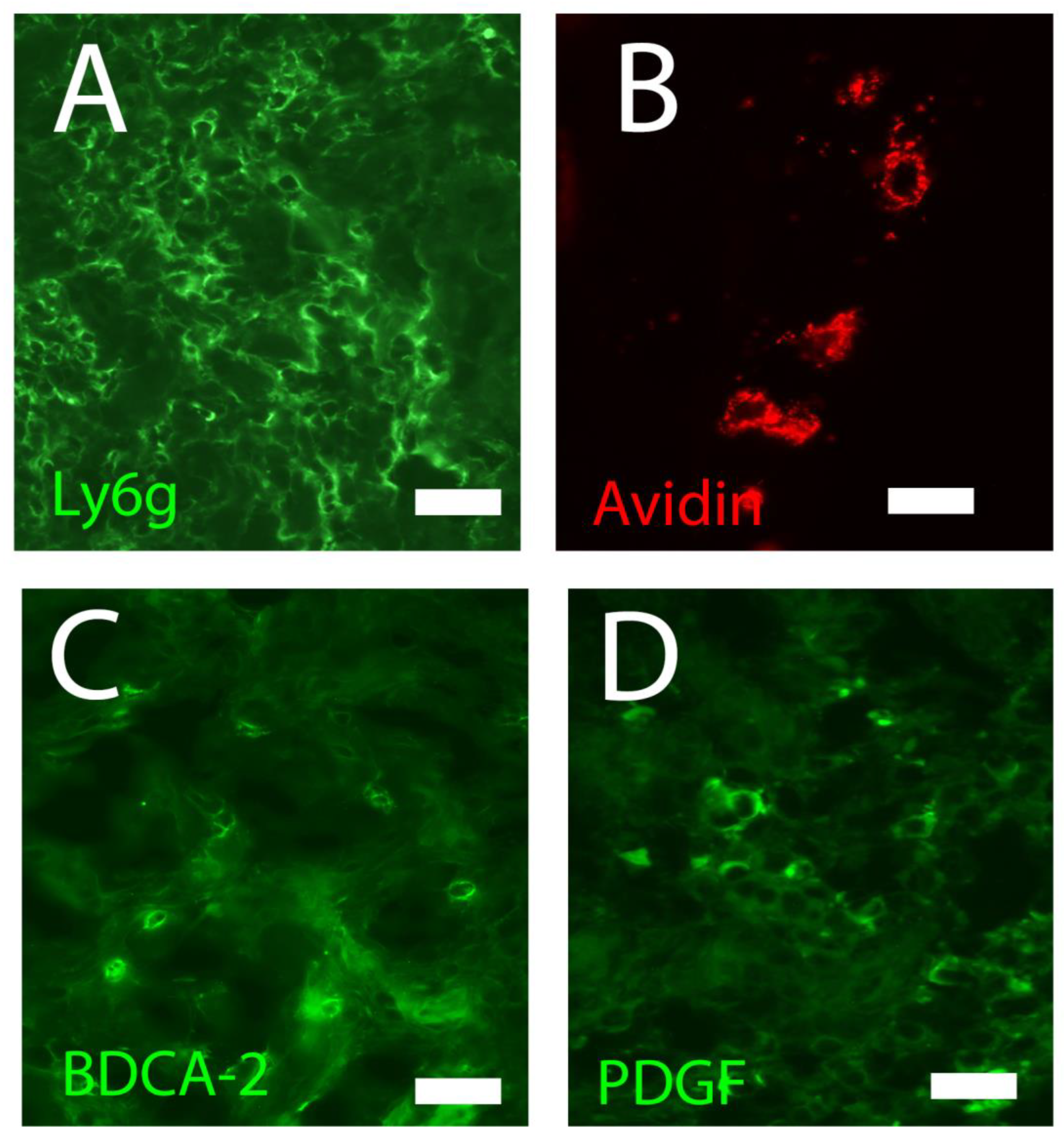
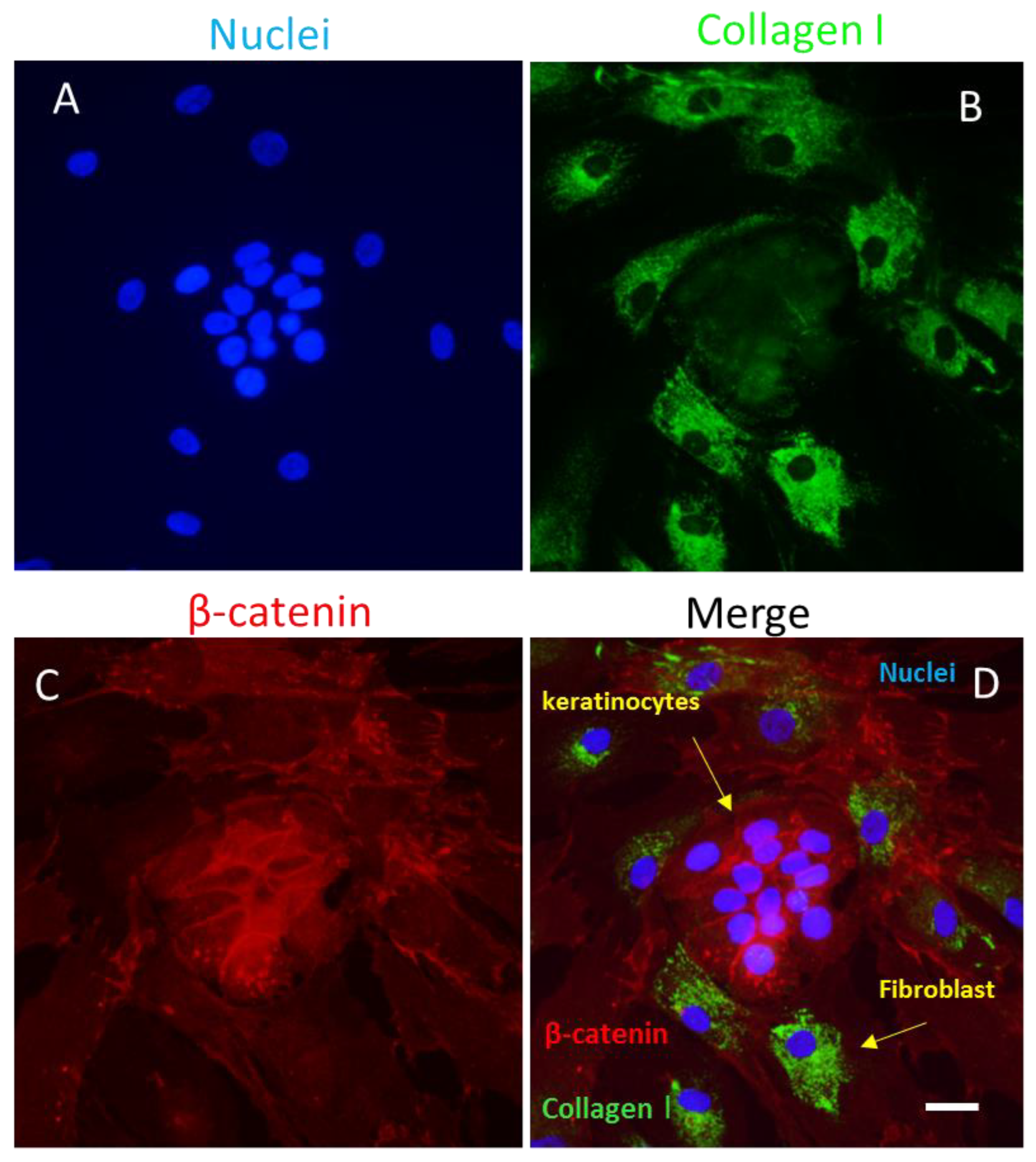
11. Recent Advances about the Molecular Biology of Wound Healing
11.1. Molecular Processes in the Hemostasis Phase
11.2. Molecular Processes in the Inflammatory Phase
11.3. Molecular Processes in the Proliferative Phase
11.4. Molecular Processes in the Maturation Phase
12. Chronic Wounds
13. Fibrosis: Hypertrophic Scarring and Keloids Associated with Wound Healing Phases
13.1. Hypertrophic Scarring
13.2. Keloids
14. Impairment of Wound Healing and Recent Therapeutic Strategies
14. Future Perspective and Current Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Denomination | Acronym |
| Actin Alpha 2 | ACTA 2 |
| Activator Protein 1 | AP1 |
| Angiopoietin 1 | ANG1 |
| Adrenergic Receptor | AR |
| Calcitonin Gene Related Peptide | CGRP |
| Caseinphosphopeptides | CPP |
| Chemokine (C-X-C motif) ligand | CXCL |
| Chemokine (C--C motif) ligand | CCL |
| Chemokine (C--C motif) receptor | CXCR |
| Chronic Wounds | CW |
| Connective tissue chemokine activating peptide | CTAP |
| Connective tissue growth factor | CTGF |
| Damage Associated Molecular Patterns | DAMPS |
| Dendritic cells | DCs |
| Exendin-4 | Exe4 |
| Extracellular matrix | ECM |
| Epithelial mesenchymal transaction | EMT |
| Epidermal Growth factor | EGF |
| Epidermal stem cellsETS-related gene | ESCERG |
| Extracellular Matrix | ECM |
| Fibroblast growth factor | FGF |
| Glucagon-Like Peptide-1 | GLP-1 |
| Growth-Related Oncogene | GRO |
| Heat shock protein | HSP |
| Hematoxylin Eosin | HE |
| IL | Interleukin |
| Insulin-like growth factor | IGF |
| Interferon | IFN |
| Interferon-g-inducible protein 10 | IP10 |
| Interferon-g-Induced Monokine | MIG |
| Interferon- inducible T cell alpha chemoattractant | ITAC |
| Keratinocyte growth factor | KGF |
| Light-emitting diodes | LED |
| Low Level Light Therapy | LLLT |
| Major Histocompatibility Complex | MHC |
| Mast Cells | MCs |
| Matrix Metalloproteinases | MMPs |
| Macrophage inflammatory protein | MIP |
| Macrophage-derived-chemokine | MDC |
| Metal–organic frameworks | MOFs |
| Mesenchymal stem cellsMonocyte chemoattractant proteins | MSCMCP |
| Monokine-induced gamma interferon | MIG |
| Natural Killer | NK |
| Nerve Growth Factor | NGF |
| Neurokinin A | NKA |
| Neuropeptide Y | NPY |
| Neutrophil-Activating Peptide 2 | NAP-2 |
| Nucleoid-Associated Protein | NAP |
| Non-steroidal anti-inflammatory drugs | NSAID |
| Pathogen-Associated Molecular Patterns | PAMPs |
| Pattern Recognition Receptors | PRR |
| Peroxidase | POD |
| Peroxisome Proliferator Activated Receptors | PPARs |
| Prostaglandine E2 | PGE2 |
| Plasmacytoid dendritic cells | PDCs |
| Platelet-Derived Growth Factor | PDGF |
| Poly(butyl cyanoacrylate) | PBCA |
| Protein Gene Product 9.5 | PGP 9.5 |
| Regulated Upon activation normal T-cell expressed and secreted | RANTES |
| Reactive oxygen species | ROS |
| Signal Transducer and Activator of Transcription 3 | STAT 3 |
| Smooth Muscle Actin | SMA |
| Stem Cell Factor | SCF |
| Stromal-derived growth factor | SDF |
| Substance P | SP |
| Tissue Inhibitor of Metalloproteinases | TIMP |
| Toll-like receptor | TLR |
| Transforming Growth Factor | TGF |
| Thrombospondin | TSP |
| Tumor Necrosis Factor | TNF |
| Ulex Europaeus Agglutinin | UEA |
| Vascular Endothelial Growth Factor | VEGF |
| Vasoactive intestinal peptide | VIP |
| Wound Healing | WH |
References
- Han, G. and Ceilley, R., Chronic wound healing: a review of current management and treatments. Adv Ther, 2017. 34(3): p. 599-610. [CrossRef]
- Sen, C.K. , Human wounds and its burden: an updated compendium of estimates. Adv Wound Care (New Rochelle), 2019. 8(2): p. 39-48. [CrossRef]
- D’Arcy, C. and Kiel, C., Cell adhesion molecules in normal skin and melanoma. Biomolecules, 2021. 11(8). p. 1213. [CrossRef]
- Bacci, S. , Cellular mechanisms and therapies in wound healing: looking toward the future. Biomedicines, 2021. 9(11). p.1611. [CrossRef]
- Gupta, S. , et al., Management of chronic wounds: diagnosis, preparation, treatment, and follow-up. Wounds, 2017. 29(9): p. S19-S36.
- Babalska, L. Z, et al., Wound antiseptics and european guidelines for antiseptic application in wound treatment. Pharmaceuticals 2021. 14(12): p. 1253. [CrossRef]
- Takeo, M. , Lee, W., and Ito, M., Wound healing and skin regeneration. Cold Spring Harb Perspect Med, 2015. 5(1): p. a023267. [CrossRef]
- Braiman-Wiksman, L. , et al., Novel insights into wound healing sequence of events. Toxicol Pathol, 2007. 35(6): p. 767-79. [CrossRef]
- Chen, L. , et al., Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2018. 9(6): p. 7204-7218. [CrossRef]
- Nguyen, A.V. and Soulika, A.M., The dynamics of the skin’s immune system. Int J Mol Sci, 2019. 20(8). p.1611. [CrossRef]
- Canedo-Dorantes, L. and Canedo-Ayala, M., Skin acute wound healing: a comprehensive review. Int J Inflam, 2019. 2019: p. 3706315. [CrossRef]
- Gold, M.H. , et al., Updated international clinical recommendations on scar management: part 2--algorithms for scar prevention and treatment. Dermatol Surg, 2014. 40(8): p. 825-31.
- Etulain, J. , Platelets in wound healing and regenerative medicine. Platelets, 2018. 29(6): p. 556-568. [CrossRef]
- Bacci, S. , Fine regulation during wound healing by mast cells, a physiological role not yet clarified. Int J Mol Sci, 2022. 23(3): p.1820. [CrossRef]
- Tyavambiza, C. , Meyer, M., and Meyer, S., Cellular and molecular events of wound healing and the potential of silver based nanoformulations as wound healing agents. Bioengineering (Basel), 2022. 9(11). p.712. [CrossRef]
- Krzyszczyk, P. , et al., The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing Phenotypes. Front Physiol, 2018. 9: p. 419. [CrossRef]
- Bacci, S. , et al., Immunohistochemical analysis of dendritic cells in skin lesions: correlations with survival time. Forensic Sci Int, 2014. 244: p. 179-85. [CrossRef]
- Han, Z. , et al., MiR-21/PTEN Axis Promotes Skin Wound Healing by Dendritic Cells Enhancement. J Cell Biochem, 2017. 118(10): p. 3511-3519. [CrossRef]
- Bordon, Y. , Dendritic cells: pDCs play off scratch. Nat Rev Immunol, 2011. 11(1): p. 8. [CrossRef]
- Nosbaum, A. , et al., Cutting edge: regulatory t cells facilitate cutaneous wound healing. J Immunol, 2016. 196(5): p. 2010-4. [CrossRef]
- Giantulli, S. , et al., Effect of 1-MHz ultrasound on the proinflammatory interleukin-6 secretion in human keratinocytes. Sci Rep, 2021. 11(1): p. 19033. [CrossRef]
- Piipponen, M. , Li, D., and Landen N.X., The immune functions of keratinocytes in skin wound healing. Int J Mol Sci, 2020. 21(22). [CrossRef]
- Morbidelli, L. , Genah, S., and Cialdai, F., Effect of microgravity on endothelial cell function, angiogenesis, and vessel remodeling during wound healing. Front Bioeng Biotechnol, 2021. 9: p. 720091. [CrossRef]
- Johnson, K.E. and Wilgus,T.A., Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous ound repair. Adv Wound Care (New Rochelle), 2014. 3(10): p. 647-661. [CrossRef]
- Bodnar, R.J. , et al., Perycites: a newly recognized player in wound healing. Wound Repair Regent, 2016. 24(2): p.204-14. [CrossRef]
- Cialdai, F. , Risaliti, C., and Monici, M., Role of fibroblasts in wound healing and tissue remodeling on earth and in space. Front Bioeng Biotechnol, 2022. 10: p. 958381. [CrossRef]
- Monika, P. , et al., Myofibroblast progeny in wound biology and wound healing studies. Wound Repair Regen, 2021. 29(4): p. 531-547. [CrossRef]
- Schultz, G.S. , et al., Principles of wound healing, in mechanisms of vascular disease: A reference book for vascular specialists, R. Fitridge and M. Thompson, Editors. 2011: Adelaide (AU).Available from: https://www.ncbi.nlm.nih.gov/books/NBK534261.
- Qing, C. , The molecular biology in wound healing & non-healing wound. Chin J Traumatol, 2017. 20(4): p. 189-193. [CrossRef]
- Olson, T.S. and Ley, K., Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol, 2002. 283(1): p. R7-28. [CrossRef]
- Brandt, E. , et al., The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol, 2000. 67(4): p. 471-8. [CrossRef]
- Gillitzer, R. and Goebeler, M., Chemokines in cutaneous wound healing. J Leukoc Biol, 2001. 69(4): p. 513-21.
- Murdoch, C. and Finn, A., Chemokine receptors and their role in inflammation and infectious diseases. Blood, 2000. 95(10): p. 3032-43.
- Yamamoto, T. , et al., Monocyte chemoattractant protein-1 enhances gene expression and synthesis of matrix metalloproteinase-1 in human fibroblasts by an autocrine IL-1 alpha loop. J Immunol, 2000. 164(12): p. 6174-9. [CrossRef]
- Rennekampff, H.O. , et al., Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res, 2000. 93(1): p. 41-54. [CrossRef]
- Engelhardt, E. , et al., Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol, 1998. 153(6): p. 1849-60. [CrossRef]
- Kulke, R. , et al., The CXC receptor 2 is overexpressed in psoriatic epidermis. J Invest Dermatol, 1998. 110(1): p. 90-4. [CrossRef]
- Goebeler, M. , et al., The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol, 1997. 108(4): p. 445-51. [CrossRef]
- Strieter, R.M. , et al., The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem, 1995. 270(45): p. 27348-57. [CrossRef]
- Belperio, J.A. , et al., CXC chemokines in angiogenesis. J Leukoc Biol, 2000. 68(1): p. 1-8.
- Randolph, G.J. and Furie, M.B., A soluble gradient of endogenous monocyte chemoattractant protein-1 promotes the transendothelial migration of monocytes in vitro. J Immunol, 1995. 155(7): p. 3610-8.
- Yoshida, M. , et al., TGF-beta-operated growth inhibition and translineage commitment into smooth muscle cells of periodontal ligament-derived endothelial progenitor cells through Smad- and p38 MAPK-dependent signals. Int J Biol Sci, 2012. 8(7): p. 1062-74. [CrossRef]
- Li, C. , et al., Notch signal regulates corneal endothelial-to-mesenchymal transition. Am J Pathol, 2013. 183(3): p. 786-95. [CrossRef]
- Bacci, S. and Bani, D., The epidermis in microgravity and unloading conditions and their effects on wound healing. Front Bioeng Biotechnol, 2022. 10: p. 666434. [CrossRef]
- Raziyeva, K. , et al., Immunology of acute and chronic wound healing. Biomolecules, 2021. 11(5). [CrossRef]
- Falanga, K. , et al., Chronic wounds. Nat Rev Dis Primers. 2022. 8(1):p. 50. [CrossRef]
- Kadam, S. , et al., Bioengineered platforms for chronic wound infection studies: how can we make them more human-relevant? Front Bioeng Biotechnol, 2019. 7: p. 418. [CrossRef]
- Grandi, V. , et al., Cellular mechanisms in acute and chronic wounds after PDT therapy: An update. Biomedicines, 2022. 10(7):p.1624. [CrossRef]
- Tottoli, E.M. , et al., Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics, 2020. 12(8): p.735. [CrossRef]
- Andrews, J.P. , et al., Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol, 2016. 51: p. 37-46. [CrossRef]
- Huang, J. , et al., Combined analyses of RNA-sequence and Hi-C along with GWAS loci-A novel approach to dissect keloid disorder genetic mechanism. PLoS Genet, 2022. 18(6): p. e1010168. [CrossRef]
- Lingzhi, Z. , Meirong, L., and Xiaobing, F., Biological approaches for hypertrophic scars. Int Wound J, 2020. 17(2): p. 405-418. [CrossRef]
- Jiang, D. and Rinkevich, Y., Scars or regeneration?-dermal fibroblasts as drivers of diverse skin wound responses. Int J Mol Sci, 2020. 21(2). p. 617. [CrossRef]
- Berman, B. , Maderal, A., and Raphael, B., Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol Surg, 2017. 43 Suppl 1: p. S3-S18. [CrossRef]
- Leszczynski, R. , et al., Laser therapy for treating hypertrophic and keloid scars. Cochrane Database Syst Rev 2022. 26 (9):p.CD011642. [CrossRef]
- Alghamdi, M.A. , et al., Identification of differentially methylated CpG sites in fibroblasts from keloid scars. Biomedicines, 2020. 8(7). p. 181. [CrossRef]
- Yang, R. , et al., Epidermal stem cells in wound healing and regeneration. Stem cells International, 2020. p.9148310. [CrossRef]
- Isakson, M. , et al., Mesenchymal stem cells and cutaneous wound healing: current evidence and future potential. Stem cells International, 2015. p. 831095. [CrossRef]
- Kua, J. , et al., Human umbilical cord lining-derived epithelial cells: a potential source of non-native epithelial cells that accelerate healing in a porcine cutaneous wound model. Int J Mol Sci, 2022. 23(16): p. 8918. [CrossRef]
- Khalil, H. , et al., Medications affecting healing: an evidence-based analysis. Int Wound J, 2017. 14(6): p.1340-1345. [CrossRef]
- Bacci, S. , et al., The pro-healing effect of exendin-4 on wounds produced by abrasion in normoglycemic mice. Eur J Pharmacol, 2015. 764: p. 346-352. [CrossRef]
- Paroli, G. , et al., The role of mast cells in cellular modifications evoked by Exendin-4 in treated wounds: a preclinical study. J Wound Care, 2022. 31(8): p.701-708. [CrossRef]
- Fernández Guarino, M. , et al., Methyl aminolaevulinic acid versus aminolaevulinic acid photodynamic therapy of actinic keratosis with low doses of red-light LED Illumination: results of long-term follow-up. Biomedicines 2022. 10 :p 3218. [CrossRef]
- Franca, C.M. , et al., Photobiomodulation in wound healing: what are we not considering? Photomed Laser Surg 2016. 34(2):p51-52,2016. [CrossRef]
- Cicchi, R. , et al., Observation of an improved healing process in superficial skin wounds after irradiation with a blue-LED haemostatic device. J Biophotonics 2016. 9(6): p.645-655. [CrossRef]
- Magni, G. , et al., Blue LED light modulates inflammatory infiltrate and improves the healing of superficial wounds. Photodermatol Photoimmunol Photomed. 2020. 36(2):p.166-168. [CrossRef]
- Magni, G. , et al., Blue-LED-light photobiomodulation of inflammatory responses and new tissue formation in mouse-skin wounds. Life (Basel). 2022.12(10): p.1564. [CrossRef]
- Nguyen, J.K. , et al., A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials 2019. 20(1):p.432. [CrossRef]
- Polak, A. , et al., A randomized, controlled clinical study to assess the effect of anodal and cathodal electrical stimulation on periwound skin blood flow and pressure ulcer size reduction in persons with neurological injuries. Ostomy Wound Manage. 2018. 64(2):p. 10-29.
- Alkahtani, S. A. , et al., Ultrasound-based techniques as alternative treatments for chronic wounds: a comprehensive review of clinical applications. Cureus. 24(12): p.e1952. [CrossRef]
- Bora, K. , et al., High-voltage electrical stimulation versus ultrasound in the treatment of pressure ulcers. Adv Skin Wound Care. 2017;30(12):p.565-570. [CrossRef]
- Bianchi, E. , et al.,Electrospun scaffolds based on poly(butyl cyanoacrylate) for tendon tissue engineering. Int J Mol Sci 2023. 24 (4): p. 3172. [CrossRef]
- Lin, C. , et al., Different dimensional copper-based metal–organic frameworks with enzyme-mimetic activity for antibacterial therapy. Int J Mol Sci 2023. 24:p. 3173. [CrossRef]
- Bachor, U., et al.,The in vitro impact of isoxazole derivatives on pathogenic biofilm and cytotoxicity of fibroblast cell line. Int J Mol Sci. 2023. 24, 2997. Int J Mol Sci. 2023, 24, 2997. [CrossRef]
- Di Lodovico, S., et al., Antimicrobial combined action of graphene oxide and light emitting diodes for chronic wound management. Int J Mol Sci. 2022, 23, 6942. [CrossRef] [PubMed]
- Cialdai, F., et al., Optimization of an ex-vivo human skin/vein model for long-term wound healing studies: ground prepa ratory activities for the ‘Suture in Space’ experiment onboard the international space station. Int J Mol Sci. 2022, 23, 14123. [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





