1. Introduction
The utilization of the electrophysiological method is an important way for the understanding of the mechanisms of ion displacement to the epithelial tissues, which are analyzed as electronics circuits and promoted through two electrodes placed in certain parts of the heart [
1,
2]. The electrocardiogram (ECG) monitors the heartbeat rhythm, cardiac Frequency, and the identification of heart diseases by through an electrophysiological analysis [
3].
Arthropoda (Ecdysozoa, Arthropoda) possesses an open circulatory system, divided into two parts: the vascular and the lacunar. The vascular portion is located in the medial line of the arthropods and consists of a tubular heart as a central element, which is located right below the dorsal α-chitin cuticle and bombs hemolymph for their different body parts. The dorsal vessel is myogenic, but its rhythmicity is modulated by neuropeptides and neurotransmitters [
4]. The venous return occurs in the lacunar portion, where the hemolymph flows directly from the haemocoel to the vascular portion, passing by the ostia [
5]. The hemolymph circulation is crucial for promoting the transport of nutrients, neurohormones, molecules of circulation, immunological factors, and residual products [
6].
In bees (Hymenoptera, Apoidea), a taxon that provides important ecosystem services, mainly pollination [
7,
8], the vascular portion consists of the heart (located in the abdomen) and the aorta (located in the thorax), in which its diameter is much smaller [
9,
10]. The bee heart is tubular, located below the abdominal wall; it begins in the first abdominal ostia and extends up to the posterior half of the VI abdominal segment, and possesses several openings (ostioles) in the wall, which allows the entrance of hemolymph from the lacunar portion. In the anterior part, it connects with the aorta; the hemolymph flows from the final part of the heart, in the abdomen, in the direction of the thorax. The heart has a muscular wall that pumps the hemolymph to the aorta, which is a thinner vessel that pumps the hemolymph to the heart; in this area it possesses an opening in its extremity, below the heart [
10,
11]. The bee circulatory system has contractions both in the dorsal vessel and in pulsating organs that provide a flow for the extremity [
12]. These contractions are caused by diaphragms, which cause the hemolymph to return from the haemocoel for the heart, via the ostioles [
10].
Studies about the cardiac function and blood (hemolymph) circulation in bees are relatively scarce, and were performed mostly in the honeybee,
Apis mellifera and in a lesser extent, in the bumblebee
Bombus terrestris. These studies are mostly descriptive, while more recent focused on the effects of stressors, mainly pesticides in the heart and other functions [
12,
13,
14,
15,
16,
17,
18,
19,
20]. From these, few used ECG for measuring the bees’ heartbeats [
18,
19].
In stingless bees [
21], a taxon of eusocial bees consisting of more than 600 species [
22], there are no studies on their cardiac function. Stingless bees are important pollinators [
7], which are exposed to several stressors, such as thermal stress caused by global climatic changes [
23], by natural foraging behavior [
24], and by pesticides [
25]. A recent study showed that the pesticide effects, in case, the dimetoate, does not equally affect stingless bees and
A. mellifera [
26]; however, see [
27] for a rebuttal. Thus, base studies in Meliponini, including the cardiac function, are important for the understanding of their physiology, and how they could answer to different stressors. For example, in
A. mellifera, it was shown that the acaricide amitraz, used to control
Varroa destructor, provokes an acceleration on the heartbeats of workers, compared with the control treatment [
18].
Thus, in this study we studied the cardiac function, via ECG, in the Amazonian stingless bee species
Melipona flavolineata Friese, 1900 [
28], an important pollinator and visitor of native and crop plants [
7,
29] and used as a model species for studies on the reproduction and supplemental feeding of stingless bees´ colonies [
30,
31,
32]. We evaluated the heartbeat in the studied species, and the effect of contention stress in bees across the time. We measured the heartbeats for 30 minutes, observing the amplitude, cardiac frequency, energy intensity, R-R, QT intervals, and the duration of QRS complex during the restraint period. We used isoflurane as an anesthetic, which has been used successfully in insects [
34] and bees, causing few long-term alterations in their physiology [
34,
35].
2. Materials and Methods
2.1. Studied species
We used eight
M. flavolineata worker bees, a species that inhabits the Brazilian states Pará, Maranhão and Tocantins [
28], whose colonies were maintained in the meliponary of the Laboratory of Biology and Ecology of Bees of the Federal University of Pará, Belém, Brazil. We chose young worker bees from the nest that weren’t still in forager activity. These bees were brought to the Laboratory of Pharmacology and Toxicology of Natural Products (Laboratório de Farmacologia e Toxicologia de Produtos Naturais) UFPA-ICB, to an environment with a regulated temperature (25-28
ºC) and they were used in the experiments described below.
2.2. Animal preparation for the experiment
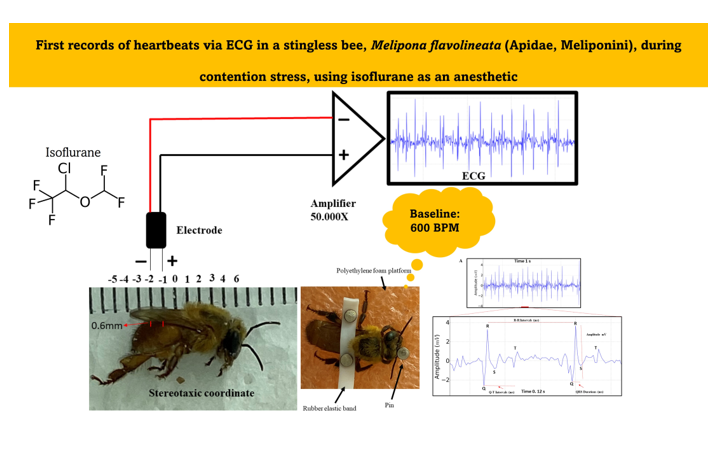
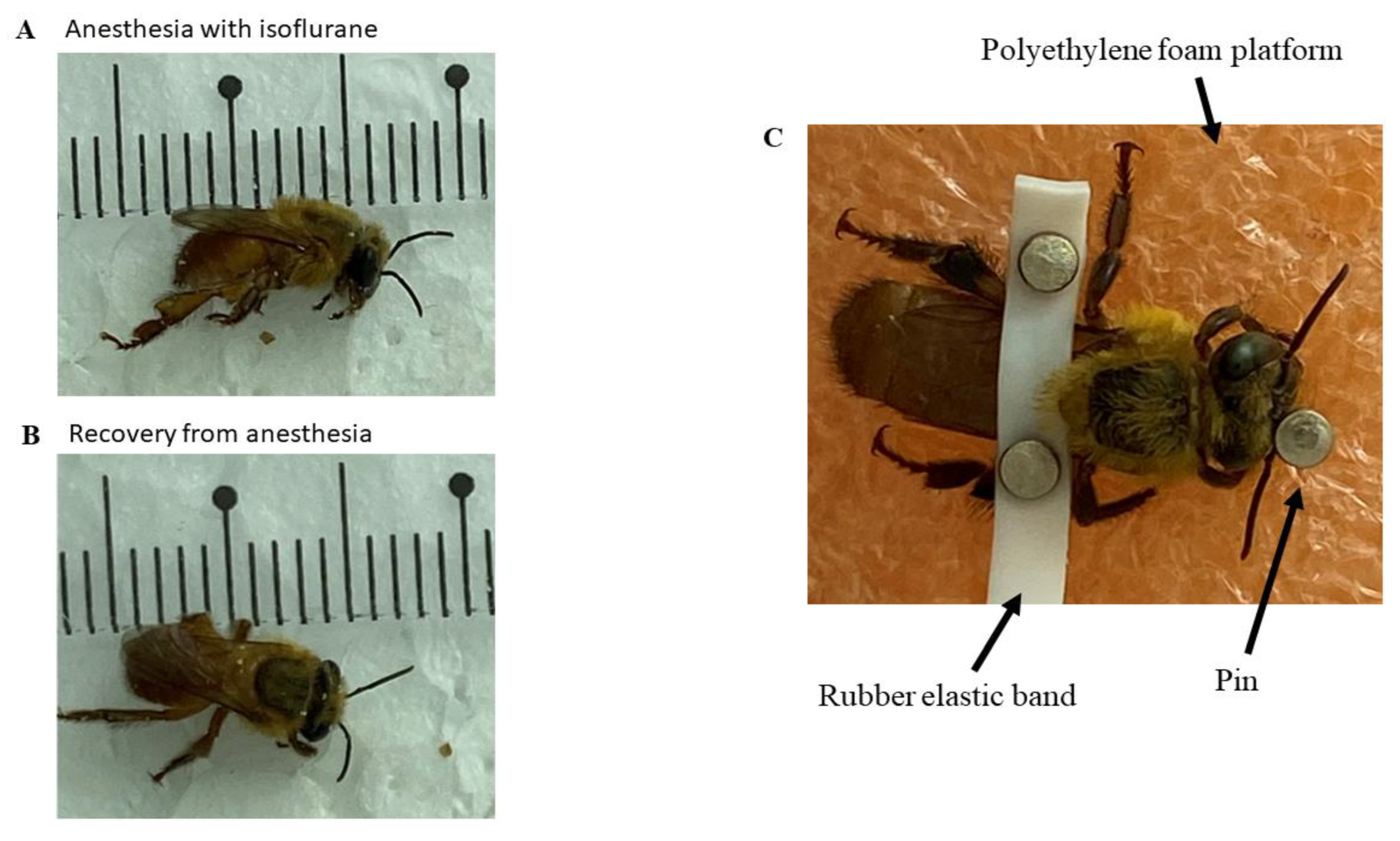
Bees were previously anesthetized with isoflurane at a room temperature of 24°C, with a 1.5 ml volume of the drug soaked in cotton, and they were kept in contact with the anesthetic within a 150 x 20 mm Petri dish glass until the loss of the postural reflex onwards (
Figure 1A). The mean time for induction was 57.75 ± 8.44 seconds and the mean time for recovery of the posture reflex was 2,645 ± 33.56 seconds (
Figure 1B). This procedure was necessary to allow the fixation of the bees upon a polyethylene foam platform with a small rubber band between the bees´ thorax region and the abdomen. Thus, it was possible to fix the electrodes for the ECG (
Figure 1C). Pins prevented the bee from moving too much and interfering with the measurements. In this work, the method by Kaiser [
37] was used with modifications, to capture the data with the available equipment and materials.
2.3. Manufacture of electrodes, implantation and obtaining of Electrocardiographic (ECG) recordings
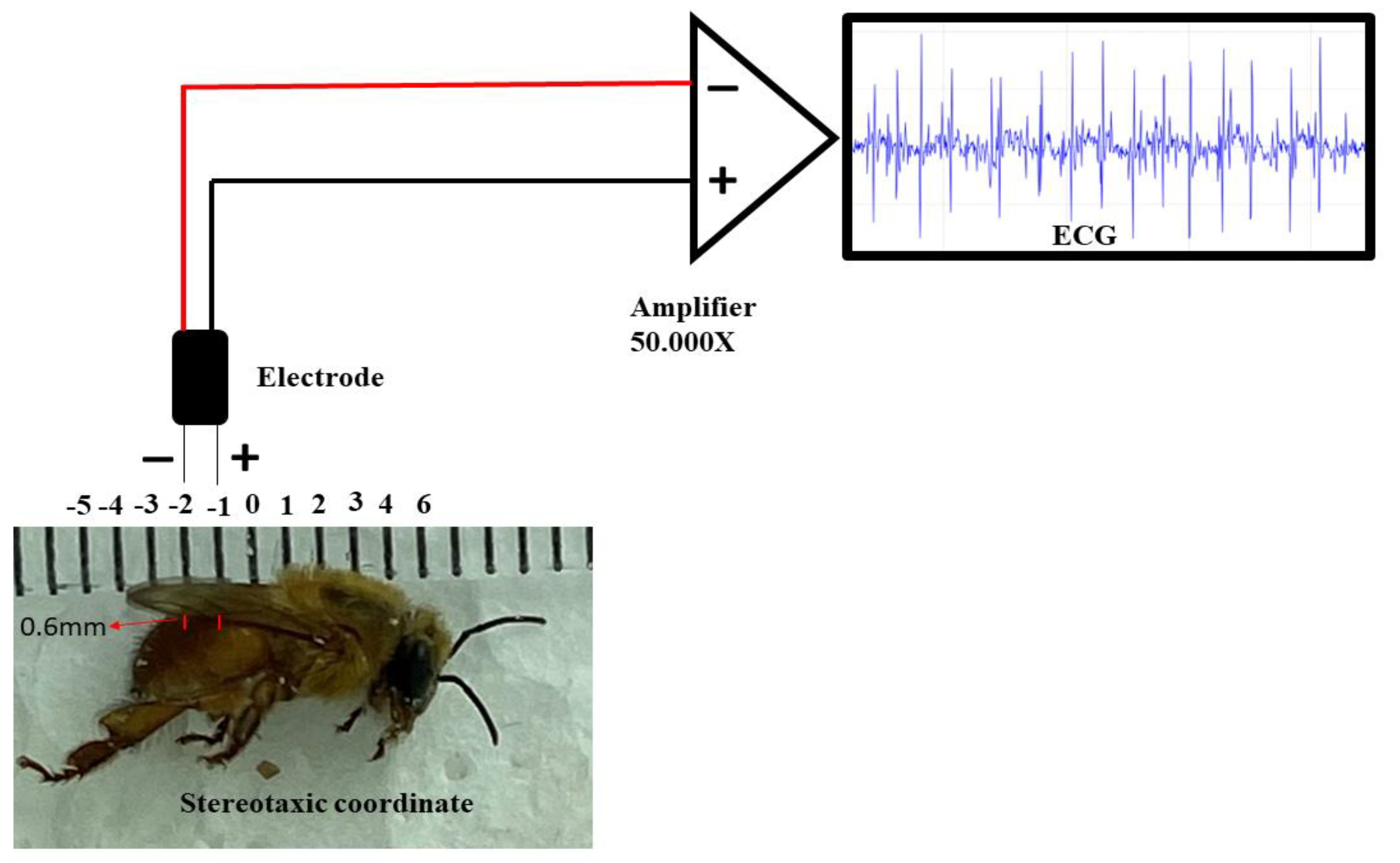
Regarding the ECG, the electrodes were made from JST SM cables with 2 Jack pins 13 cm long. The nickel-chromium wire electrodes (Morelli ortodontia), conjugated at a distance of 1 mm, with 0.2 mm in diameter and 2 mm in length. They were insulated with a liquid insulator and after drying the material, the electrode was fixed in a stereotactic device. After the bee fixation, the following coordinates were obeyed, considering the recording electrode as a parameter: the zero point was at the intersection between thorax-abdomen in the midsagittal line, with an anteroposterior coordinate of 1 mm, and dorsoventral coordinate of 0.6 mm (
Figure 2). After the procedure, the ECG was recorded. The entire procedure for obtaining the record was performed inside a metal screen Faraday cage. The electrodes were connected to a high-impedance amplifier (Grass Technologies, P511) with signal amplification of 50,000X, monitored by an oscilloscope (Protek, 6510).
All records lasted 30 minutes for each bee. Fragments were taken from each record every 5 minutes for analysis to compare the effects of stress on hemolymph pumping. Thus, we analyzed the last second of each individual’s cardiac activity in six different periods: A = 299-300s, B= 599-600s, C=899-900s, D= 1199-1200s, E= 1499-1500s and F=1799 -1800s. This delimitation of periods was necessary due to the large database generated, which would be difficult to visualize if shown continuously.
2.4. Statistical analyses
For statistical analysis, normality and homogeneity tests were used for data variations through the Kolmogorov-Smirnov and Levene tests, respectively. Data are presented as
mean ± standard deviation (SD) and
F and
p values are included, when relevant. Significance levels of *p<0.05, **p<0.01, ***p<0.001 were considered for all analyses. Comparisons between analyzed periods were based on a two-way ANOVA followed by Tukey’s test for multiple comparisons. Statistical analyses to identify and remove outliers were performed using GraphPad Prism, version 8 (Graph-Pad Software Inc., San Diego, CA, USA). The parameters analyzed in the records were: Heart rate (BPM), Amplitude (mV), R-R Interval (ms), QRS complex duration (ms) and Q-T interval (ms) (
Figure 3B).
3. Results
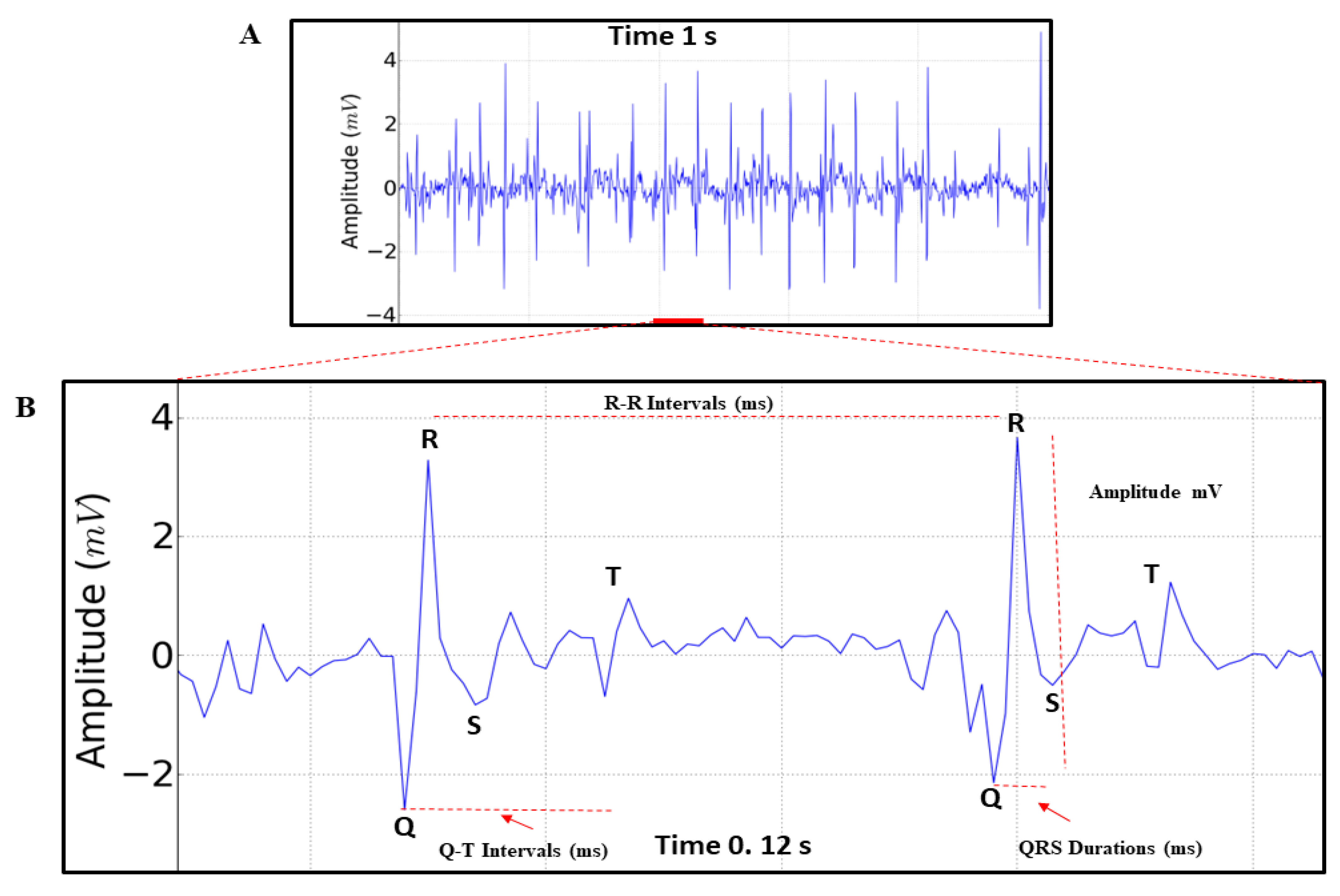
We observed that the triggering of impulses maintains the rhythm and that it can undergo rapid variations. When analyzing the records, we only identified the QRS complex, which represents the contraction of the structure, and the T wave, which represents the repolarization of the heart (
Figure 3A,B).
The electrocardiographic recording demonstrating the cardiac function of M. flavolineata (n=8) in the first five minutes of recording during restraint showed a mean heart rate of 596 ± 81.91 BPM, with an amplitude of 1,735 ± 0,559 mV (magnification of 50,000X). The R-R interval, which represents the positive deflagration in the contraction, lasted 101.6 ± 13.17 ms. The contraction cycle to boost the hemolymph which is represented by the QRS duration averaged 6.15 ± 0.2268 ms and the Q-T interval representing the complete cycle with depolarization and repolarization of the pulsatile structure averaged 18.50 ± 2.33 ms.
The ECG recordings lasting 30 minutes during M. flavolineata containment demonstrated amplitude variation (Figure 4A). Fragments were taken from each record for analysis at the end of every 5 minutes of recording to compare the effects of stress on hemolymph pumping. During the first five minutes, there was an increase in the amplitude of the recording (A), followed by a progressive decrease until the 10th minute of recording (B). The amplitude increased again at the 15th minute (C) and remained with little variation in the fragments of the 20th (D), 25th (E) and 30th (F) minutes. The spectrogram demonstrates an increase in the level of circulating energy in the period from 300 to 600s (Figure 4B).
During the 30-minute cardiac recording, a difference in the average linear power of the records could be observed, with an average power of 6.606 ± 0.7461 mV2/ Hz x 10-3 being obtained in the recording period of 0-300s, which was significantly lower than in periods of 300-600s (33.15 ± 3.365 mV2/ Hz x 10-3), 600-900s (15.03 ± 1.641 mV2/ Hz x 10-3) and 900-1200s (12.36 ± 2.116 mV2/ Hz x 10- 3). However, it did not present a significant difference between the periods of 1200-1500 (5.602 ± 0.9207 mV2/ Hz x 10-3; p= 0.9018) and 1500-1800s (4.600 ± 1.571 mV2/ Hz x 10-3; p= 0.3179). The period of greatest power was 300-600s, which was significantly higher than the other groups. In the periods of 600-900s and 900-1200s, the power remained constant and later showed a decrease in the periods of 1200-1500s and 1500-1800s (
Figure 4C).
For each alteration of the tracing during the stress of containment, a pattern of cardiac activity behavior was analyzed (
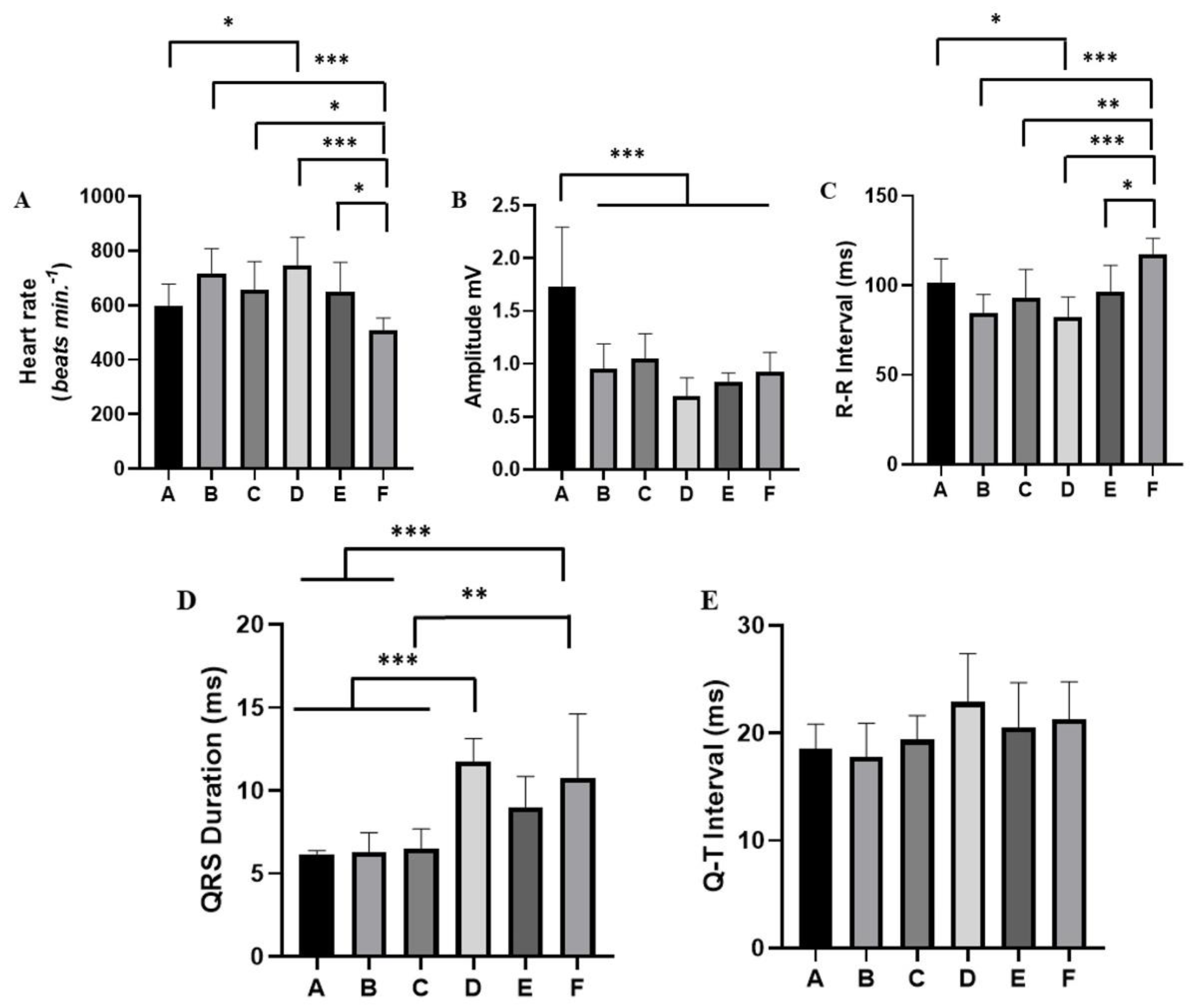
Figure 5). Thus, the heart rate in period A (mean of 596 ± 81.91 bpm) did not differ from periods B (718.3 ± 90.00 bpm; p=0.1006) and C (656.8 ± 103.6 bpm; p=0.7656), but it was lower than in period D (747.1 ± 102.8 bpm, p=0.001). Period A showed no difference for groups E (649.8± 108.3 bpm; p=0.8483) and for group F (508.4 ± 45.52 bpm; p=0.4037). Period F showed a decrease in cardiac activity, which was lower than in periods B, C, D and E (Figure 6A).
The QRS amplitude varied during the period of restraint stress. During period A, an increase in amplitude (1.735 ± 0.559 mV) was observed, which was significantly lower than the other periods: B (0.955 ± 0.2324 mV), C (1.045 ± 0.238 mV), D (0.694 ± 0.1768 mV), E (0.832 ± 0.08043 mV) and F (0.9218 ± 0.185 mV) (Figure 6B).
For the R-R interval in period A (mean of 101.6 ± 13.17 ms), there was no significant difference for periods B (84.63 ± 10.31 ms; p=0.1041) and C (92.75 ± 16.13 ms; p=0.7318). However, it was higher than in period D (82.0 ± 11.55 ms). Period A did not differ from period E (96.25± 14.98 ms; p=0.9573) and from period F (117.5 ± 8.864 ms; p=0.1507). Period F showed a decrease in cardiac activity and the R-R interval was greater than periods B, C, D and E (Figure 6C).
During the worker bees’ restraint stress, there was an increase in the QRS duration from period D onwards (mean: 11.75 ± 1.38 ms), which was significantly lower than in periods A (6.15 ± 0.2268 ms), B (6.275 ± 1.185 ms) and C (6.50 ± 1.395 ms), but showed no significant difference for periods E (9.00 ± 1.852 ms; p=0.0789) and F (10.75 ± 3.882 ms) (p=0.9105). Period E did not differ from periods A, B and C. However, during period F, there was an increase in the QRS distance, being greater than in periods A, B and C (Figure 6D).
During the registration period, the QT interval did not vary significantly. In period A, the average was 18.50 ± 2330 ms; B: 17.75 ± 3.151 ms, C: 19.88 ± 2.264 ms, D: 22.88 ± 4.518 ms, E: 20.50 ± 4.209 ms, and F: 21.25 ± 3.536 ms (F
(5, 42)= 2.399, p=0.0531;
Figure 6E).
4. Discussion
Our study was the first to register in detail the cardiac function, via ECG, in a meliponine bee, and to use isoflurane as an anesthetic, instead of CO2 or cold. We showed in this study that the bee restraint caused variations in the heart activity of worker bees, with the highest potencies observed between 300 to 600 seconds; it suggests a higher effort for the haemolymph pumping in this period. The alterations in the bees´ heartbeats were higher according to the contention time; the other parameters of cardiac activity also changed in a similar way, mainly the amplitude, which varied during the 30-minute register. The experiment allowed us to evaluate the cardiac activity at rest, which is around 600 bpm, together with other factors.
The stress caused by a period of 30 minutes of restraint allowed us to verify alterations on M. flavolineata cardiac activity; the main functions that pointed to stress were the cardiac frequency, the amplitude, and the parameter of contractile activity, which is related to the QRS duration (the speed of heart contraction). However, the Q-T interval did not have a significant difference during the contention stress.
In
A. mellifera, in vivo experiments showed that cold-anesthetized workers exposed to amitraz, an acaricide used to control infestations of
Varroa destructor (Arachnida: Parasitiformes), had a significant increase (about 135%) on their heartbeat by several hours, showed by ECG [
18]. A similar pattern was also found in other study, which also showed that, due to the interaction between amitraz and the octopamine receptors, there is a reduction of the tolerance of newly-emerged workers to viral infections [
16]. In this case, the intrinsic activities of the toxic element (amitraz) do not allow to evaluate the cardiac parameters caused by the manipulation of bees. In our study, the contention stress allowed us to show the cardiac acidity of the workers without the interference of any toxic agent. Thus, these studies showed that the cardiac function is an indicative parameter of physiological stress in bees [
16,
18]. Other stressors, such as pesticides provoke noxious effects in stingless bees; however, the cardiac function has not been yet included as a measured parameter in this group [
37].
Another approached aspect of our study, also not tested in stingless bees, was the anesthetic effect of isoflurane. It has been previously tested in other bee’s species, presenting few lasting effects on thir physiology [
34,
35]. In
M. flavolineata, after the one minute’s anesthesia induction, workers returned to motor activity about four to five minutes; after this period, they resumed motor activity (visualized by the head and antennal movement). Only after this period we measured their cardiac activity. Our results are promising, since they showed that workers anesthetized with isoflurane “awoke” rapidly after the procedure and moved similarly to bees non-anesthetized.
Since several studied showed that other anesthetics, like CO
2- and cold-induced narcosis provokes undesired short and long-term effects in
A. mellifera workers [
34,
38,
39,
40,
41], future studied must compare the effects of different anesthetics, including isoflurane, on stingless bees´ physiology. Therefore, our study provided a baseline that will allow the use of cardiac function as a proxy variable related with different stressors in stingless bees. Data on the cardiac function in other Meliponini species must also be obtained, for comparisons.
Author Contributions
Felipe Contrera: Conceptualization, Methodology, Writing- Original draft preparation, Writing- Reviewing and Editing. Moisés Hamoy: Conceptualization, Methodology, Data Curation, Visualization, Investigation, Formal Analysis, Software, Writing- Original draft preparation, Writing- Reviewing and Editing. Bárbara Lopes: Conceptualization, Methodology, Investigation, Writing- Reviewing and Editing. Clarissa Paz, Maria Hamoy, Murilo Santos, Gabriela Barbosa, Anthony Amaral, Luiz Pinho: Formal Analysis, Writing- Original draft preparation.
Funding
We thank the National Council for Scientific and Technological Development (CNPq) for productivity grant to Felipe Contrera (process 310112/2022-2), and the Coordination for the Improvement of Higher Education Personnel (CAPES, Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior – Finance Code 001) for a Ph.D scholarship to Bárbara Lopes (process number 88887.703093/2022-00) and FARMABIO coordination by the Ph.D scholarship to Clarissa Paz.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We also thanks Lucas Martins Bernardes for assistance on bee colonies management during the experiments, and the Laboratory of Pharmacology and Toxicology of Natural Products from the Federal University of Pará (UFPA) for support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bakker, R. 1985. Electrophysiology of fish intestine. In Transport Processes, Iono-and Osmoregulation. Proceedings in life sciences. Gilles R., Gilles-Baillien, M., Eds.; Springer: Berlin Heidelberg, Germany, 1985; pp. 240–250. [Google Scholar] [CrossRef]
- Dubin, M.D. Interpretação Rápida do ECG, 3rd ed.; Ed. de Publicações Científicas: Rio de Janeiro, Brazil, 1996; 295p. [Google Scholar]
- Berkaya, S.K.; Uysal, A.K.; Gunal, E.S.; Ergin, S.; Gunal, S.; Gulmezoglu, M.B. A survey on ECG analysis. Biomed. Signal Process. Control, 2018; 43, 216–235. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect heart rhythmicity is modulated by evolutionarily conserved neuropeptides and neurotransmitters. Curr. Op. Ins. Sci. 2018, 29, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Minelli, A.; Boxshall, G.; Fusco, G. 2013. An Introduction to the Biology and Evolution of Arthropods. In Artrophod Biology and Evolution. Minelli, A., Boxshall, G., Fusco, G. Eds.; Springer: Berlin, Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Klowden, M.J. Physiological Systems in Insects. Academic Press: USA, 2013, 682 p.
- Borges, R.C.; Padovani, K.; Imperatriz-Fonseca, V.L.; Giannini, T.C.A. Dataset of multi-functional ecological traits of Brazilian bees. Sci. Data 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Ollerton, J. Pollinator Diversity: Distribution, Ecological Function, and Conservation. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 353–376. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: structure and function, 4th. ed.; Cambridge University Press: UK, 1998; 788p. [Google Scholar]
- Cruz-Landim, C. Abelhas – Morfologia e função de sistemas. Editora UNESP: Brasil, 2009. 408p. [CrossRef]
- Carreck, N.L. , Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; Englesdorp, D.V. Standard methods for Apis mellifera anatomy and dissection. J. Apic. Res. 2013, 52(4), 1–40. [Google Scholar] [CrossRef]
- O’Neal, S.; Anderson, T. Dissection and Observation of Honey Bee Dorsal Vessel for Studies Cardiac Function. J. Vis. Exp. 2016, 118, 55029. [Google Scholar] [CrossRef]
- Cruz-Landim, C. 1976. Degenerative changes in the heart muscle from senescent honeybee workers (Apis mellifera adansonii). J. Invertebr. Pathol. 1976, 27, 1–5. [Google Scholar] [CrossRef]
- Kuusik, A.; Martin, A-J. ; Mänd, M.; Hiiesaar, K.; Metspalu, L.; Tartes, U. 2002. Interrelations of gas exchange cycles, body movements and heartbeats in the foragers of bumblebee Bombus terrestris (Hymenoptera: Apidae) al low temperatures. Eur. J. Entomol. 2002, 99, 209–214. [Google Scholar] [CrossRef]
- Mänd, M.; Kuusik, A.; Martin, A-J. ; Williams, I.H.; Luik, A.; Karise, R.; Metspalu, L.; Hiesaar, K. 2006. Regular periods of abdominal contractions recorded from larvae of the bumblebee, Bombus terrestris (Hymenoptera: Apidae). Eur. J. Entomol. 2006, 103, 319–322. [Google Scholar] [CrossRef]
- O’Neal, S.; Brewster, C.C.; Bloomquist, J.F.; Anderson, T. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J. Invertebr. Pathol. 149, 119–126. [CrossRef]
- O’Neal, S.; Swale, D.R.; Bloomquist, J.F.; Anderson, T. ATP-sensitive inwardly rectifying potassium channel modulators alter cardiac function in honey bees. J. Ins. Physiol. 99, 95–100. [CrossRef]
- Papaefthimiou, C.; Papachristoforou, A.; Theophilidis, G. Biphasic responses of the honeybee heart to nanomolar concentrations of amitraz. Pestic. Biochem. Physiol. 2013, 107, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Schwab, E.R.; Chilson, R.A.; Eddleman, C.D. Heartbeat rate modulation mediated by the ventral nerve cord in the honey bee, Apis mellifera. J. Comp. Physiol B. 1991, 161, 602–610. [Google Scholar] [CrossRef]
- Yelkovan, S.; Arıkan, H.; Çakıcı, Ö. Caste and age related changes in circulatory hemocytes of honey bee, Apis mellifera anatolica (Hymenoptera: Apidae). J. Apic. Res. 2021, 60(3), 512–521. [Google Scholar] [CrossRef]
- Michener, C.D. The bees of the world. 2nd ed.; John Hopkins University Press, Baltimore, USA, 2007; 953p.
- Roubik, D.W. Stingless Bee (Apidae: Apinae: Meliponini) Ecology. Annu. Rev. Entomol. 2023, 68, 231–56. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Cobos, M.E.; Jaramillo, J.; Ospina, R. Climate change will reduce the potential distribution ranges of Colombia’s most valuable pollinators. Perspect. Ecol. Evol. 2001, 19(2), 195–206. [Google Scholar] [CrossRef]
- Maia-Silva, C.; da Silva Pereira, J.; Freitas, B. M.; Hrncir, M. Don’t stay out too long! Thermal tolerance of the stingless bees Melipona subnitida decreases with increasing exposure time to elevated temperatures. Apidologie 2021, 52, 218–229. [Google Scholar] [CrossRef]
- Cham, K.O.; Nocelli, R.C.F.; Borges, L.O.; Viana-Silva, F.E.C.; Tonelli, C.A.M.; Malaspina, O.; Menezes, C.; Rosa-Fontana, A.S.; Blochtein, B.; Freitas, B.M.; Pires, C.S.S.; Oliveira, F.F.; Contrera, F.A.L.; Torezani, K.R.S.; Ribeiro, M.F.; Siqueira, M.A.L.; Rocha, M.C.L.S.A. Pesticide Exposure Assessment Paradigm for Stingless Bees. Env. Entomol. 2019, 48, 36–48. [Google Scholar] [CrossRef]
- Lourencetti, A.P.S. , Azevedo, P., Miotelo, L., Malaspina, O., Nocelli, R.C.F. Surrogate species in pesticide risk assessments: Toxicological data of three stingless bees species. Environ. Pollut. 2023, 318, 120842. [Google Scholar] [CrossRef]
- Thompson, L. , Santos, G., Cione, A. Letter to the editor regarding Lourencetti et al. Surrogate species in pesticide risk assessments: Toxicological data of three stingless bees species. Environ. Pollut. 2023, 319, 121011. [Google Scholar] [CrossRef]
- Pedro, S.R.M. The stingless bee fauna in Brazil (Hymenoptera: Apidae). Sociobiol. 2014, 61, 348–354. [Google Scholar] [CrossRef]
- Pimentel, A.D.A.; de Abreu, V.H.R.; Krug, C.; Miranda, I.S. 2023. A review of pollen types foraged by Melipona in the Brazilian Amazon. Palynol. 2023. [Google Scholar] [CrossRef]
- Costa, L.; Venturieri, G.C. Diet impacts on Melipona flavolineata workers (Apidae, Meliponini). J. Apic. Res. 2009, 48(1), 38–45. [Google Scholar] [CrossRef]
- Teixeira, J.; Queiroz, A.C.; Veiga, J.; Leão, K.; Contrera, F.; Domingues, F.; Fontes, J. E.; Lopes, T.; Marsaioli, A.; Menezes, C. Soy extract as protein replacement to feed Melipona flavolineata Friese (Hymenoptera, Apidae, Meliponini), J. Apic. Res. 2020, 59, 104–114. [Google Scholar] [CrossRef]
- Veiga, J.C.; Leão, K.L.; Coelho, B.W.; Queiroz, A.C.M.; Menezes, C.; Contrera, F.A.L. The Life Histories of the “Uruçu Amarela” Males (Melipona flavolineata, Apidae, Meliponini). Sociobiol. 2018, 65(4), 780–783. [Google Scholar] [CrossRef]
- Mcmillan, H.A.; Nørgard, M.; Maclean, H.J.; Overgaard, J.; Williams, C.J.A. A critical test of Drosophila anaesthetics: Isoflurane and sevoflurane are benign alternatives to cold and CO2. J. Ins. Physiol. 2017, 101, 97–106. [Google Scholar] [CrossRef]
- Gooley, Z.C.; Gooley, A.C. 2021. Metabolic effects of anesthetics (cold, CO2, and isoflurane) and captivity conditions in isolated honey bee (Apis mellifera) foragers under different ambient temperatures. J. Apic. Res. [CrossRef]
- Ludin, N.M.; Cheeseman, J.F.; Merry, A.F.; Millar, C.D.; Warman, G.R. The effects of the general anaesthetic isoflurane on the honey bee (Apis mellifera) circadian clock. Chronobiol. Int. 2016, 33, 128–133. [Google Scholar] [CrossRef]
- Kaiser, W.; Weber, T.; Otto, D.; Miroschnikow, A. Oxygen supply of the heart and electrocardiogram potentials with reversed polarity in sleeping and resting honey bees. Apidologie 2014, 45, 73–87. [Google Scholar] [CrossRef]
- Lima, M.A.P.; Martins, G.F.; Oliveira, E.E.; Guedes, R.N.C. Agrochemical-induced stress in stingless bees: peculiarities, underlying basis, and challenges. J. Comp. Physiol. A. 2016, 202, 733–747. [Google Scholar] [CrossRef]
- 38. Chen, Y-M.; Fu, Y.; He, J.; Wang, J-H. Effects of cold narcosis on memory acquisition, consolidation and retrieval in honeybees (Apis mellifera). Zool. Res. [CrossRef]
- Groening, J.; Venini, D.; Srinivasan, M.V. Effects of cold anaesthesia on the defensive behaviour of honeybees. Ins. Soc. 2018, 65, 359–366. [Google Scholar] [CrossRef]
- Stec, D.; Kuszewska, K. 2020. CO2 narcosis influences the memory of honey bees. J. Apic. Res. 2020, 59, 663–668. [Google Scholar] [CrossRef]
- Tutun, H.; Sevin, S.; Çetintav, B. 2020. Effects of different chilling procedures on honey bees (Apis mellifera) for anestesia. Ank. Univ. Vet. Fak. Derg. 2020, 67, 289–294. [Google Scholar] [CrossRef]
Figure 1.
(A) Characteristics of Melipona flavolineata worker anesthetized with isoflurane and with loss of posture reflex; (B) Recovery of posture reflex after anesthesia; (C) animal restrained for implantation of electrodes by stereotaxis.
Figure 1.
(A) Characteristics of Melipona flavolineata worker anesthetized with isoflurane and with loss of posture reflex; (B) Recovery of posture reflex after anesthesia; (C) animal restrained for implantation of electrodes by stereotaxis.
Figure 2.
Components for the recording: Stereotaxic coordinates used in the animals for the acquisition of the electrocardiographic record, positioning of the conjugated electrodes at a distance of 1 mm, high impedance amplifier (50,000 X of signal amplification). The characteristic signal was observed on a monitor.
Figure 2.
Components for the recording: Stereotaxic coordinates used in the animals for the acquisition of the electrocardiographic record, positioning of the conjugated electrodes at a distance of 1 mm, high impedance amplifier (50,000 X of signal amplification). The characteristic signal was observed on a monitor.
Figure 3.
(A) Electrocardiogram of Melipona flavolineata, represented by 1 second of recording, in the first 5 minutes of restraint. (B) amplification of 0.12 seconds of the ECG trace demonstrating the analyzed parameters that allow the evaluation of Heart Rate (BPM), Amplitude of record (mV), R-R Interval (ms), QRS Duration (ms) and Q-T Interval (ms).
Figure 3.
(A) Electrocardiogram of Melipona flavolineata, represented by 1 second of recording, in the first 5 minutes of restraint. (B) amplification of 0.12 seconds of the ECG trace demonstrating the analyzed parameters that allow the evaluation of Heart Rate (BPM), Amplitude of record (mV), R-R Interval (ms), QRS Duration (ms) and Q-T Interval (ms).
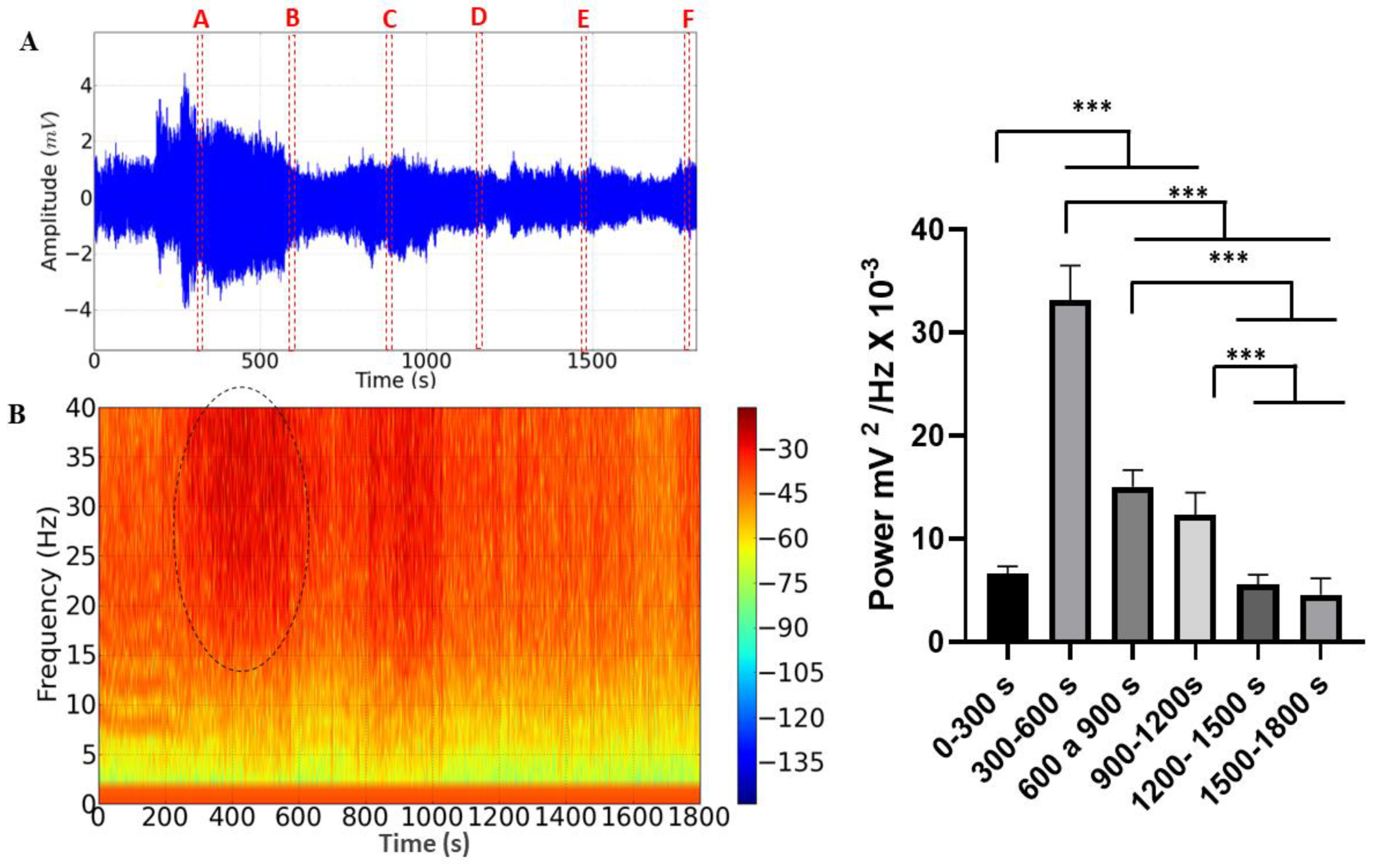
Figure 4.
Electrocardiographic tracing represented by 30 minutes of recording, demonstrating the areas of the recording that were analyzed (dotted in red): A = 299-300s, B= 599-600s, C=899-900s, D= 1199-1200s, E=1499-1500s and F=1799-1800s. (A); Energy distribution spectrogram demonstrating areas with different cardiac energy intensities during restraint stress. The area demarcated by the dotted circle indicates the highest energy intensity in the record. (B); The linear power graph of cardiac activity during restraint stress in Melipona flavolineata. (After ANOVA followed by Tukey; ***p<0.001; n=8).
Figure 4.
Electrocardiographic tracing represented by 30 minutes of recording, demonstrating the areas of the recording that were analyzed (dotted in red): A = 299-300s, B= 599-600s, C=899-900s, D= 1199-1200s, E=1499-1500s and F=1799-1800s. (A); Energy distribution spectrogram demonstrating areas with different cardiac energy intensities during restraint stress. The area demarcated by the dotted circle indicates the highest energy intensity in the record. (B); The linear power graph of cardiac activity during restraint stress in Melipona flavolineata. (After ANOVA followed by Tukey; ***p<0.001; n=8).
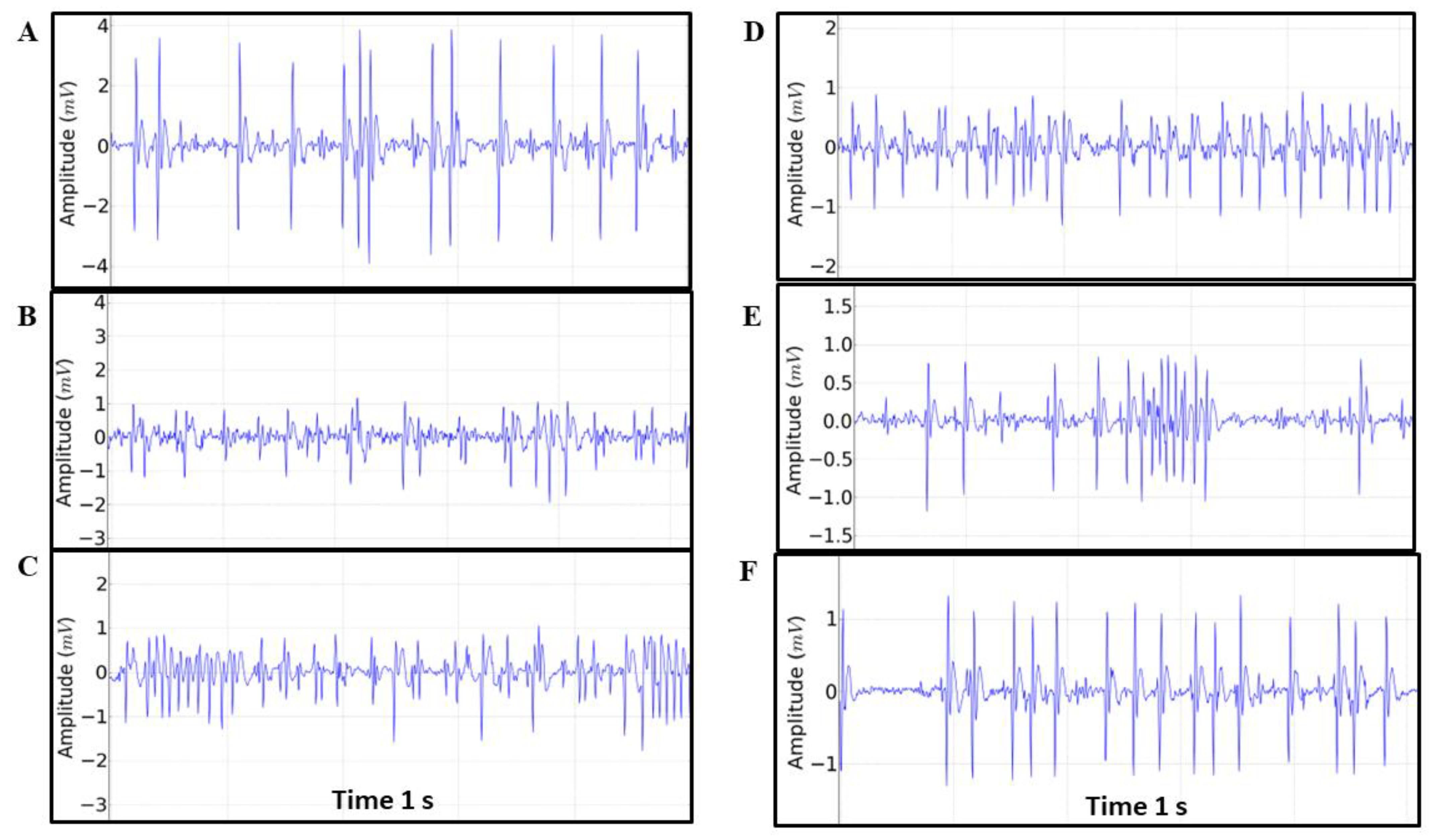
Figure 5.
Tracing patterns found during the 30-minute recording obtained every 5 minutes referring to the patterns: A = 299-300s, B= 599-600s, C=899-900s, D= 1199-1200s, E= 1499 -1500s and F=1799-1800s. In each tracing, the Heart Rate (BPM), Amplitude (mV), R-R Interval, QRS Duration and Q-T Interval were analyzed.
Figure 5.
Tracing patterns found during the 30-minute recording obtained every 5 minutes referring to the patterns: A = 299-300s, B= 599-600s, C=899-900s, D= 1199-1200s, E= 1499 -1500s and F=1799-1800s. In each tracing, the Heart Rate (BPM), Amplitude (mV), R-R Interval, QRS Duration and Q-T Interval were analyzed.
Figure 6.
Evaluation of the cardiac activity of Melipona flavolineata during physical restraint: (A) Heart rate bpm; (B) QRS amplitude (mV); (C) R-R Interval (ms); (D) QRS duration (ms); and (E) Q-T interval. After ANOVA followed by Tukey. *p<0.05, **p<0.01, ***p<0.001 (n=8).
Figure 6.
Evaluation of the cardiac activity of Melipona flavolineata during physical restraint: (A) Heart rate bpm; (B) QRS amplitude (mV); (C) R-R Interval (ms); (D) QRS duration (ms); and (E) Q-T interval. After ANOVA followed by Tukey. *p<0.05, **p<0.01, ***p<0.001 (n=8).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).