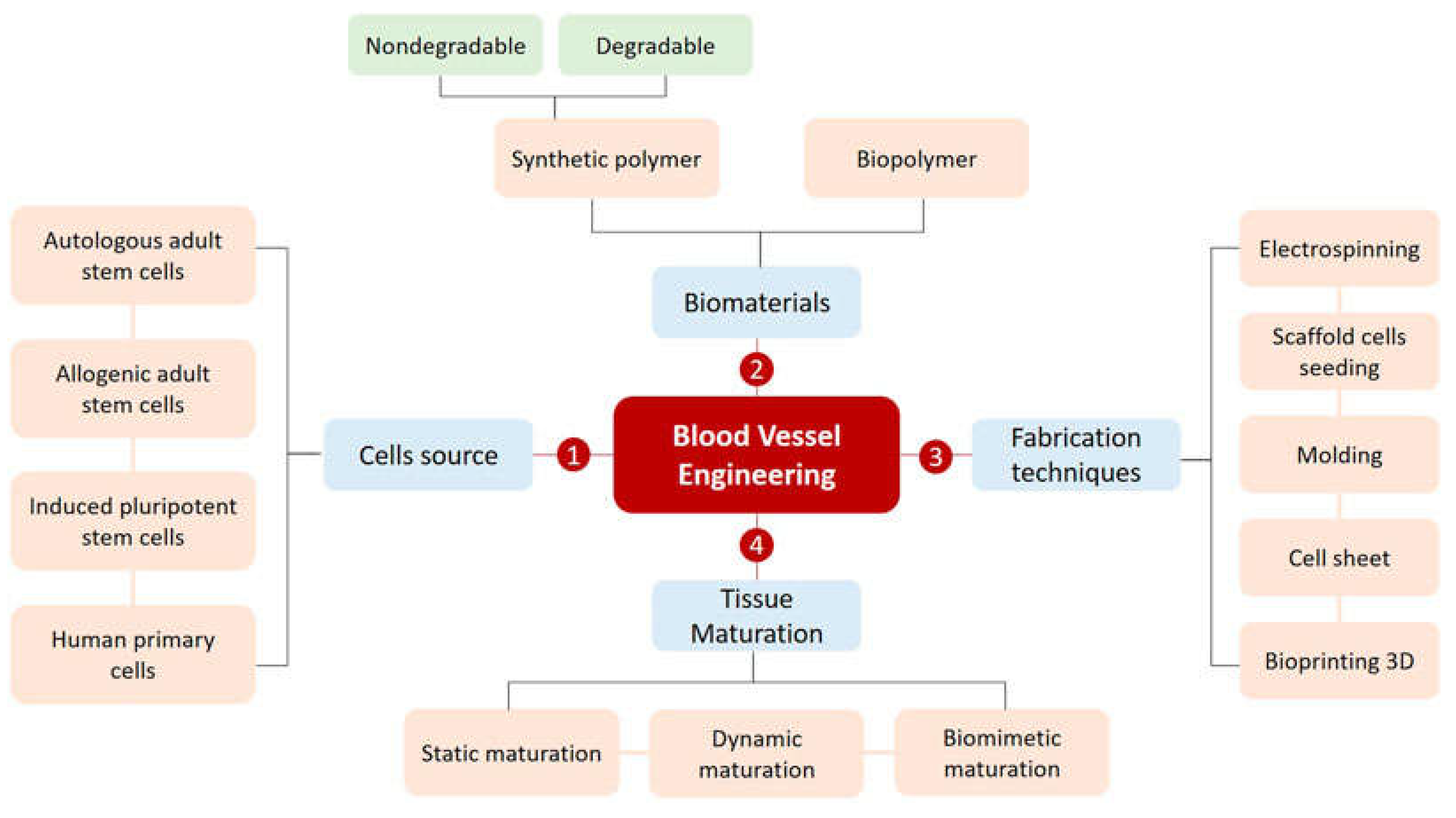
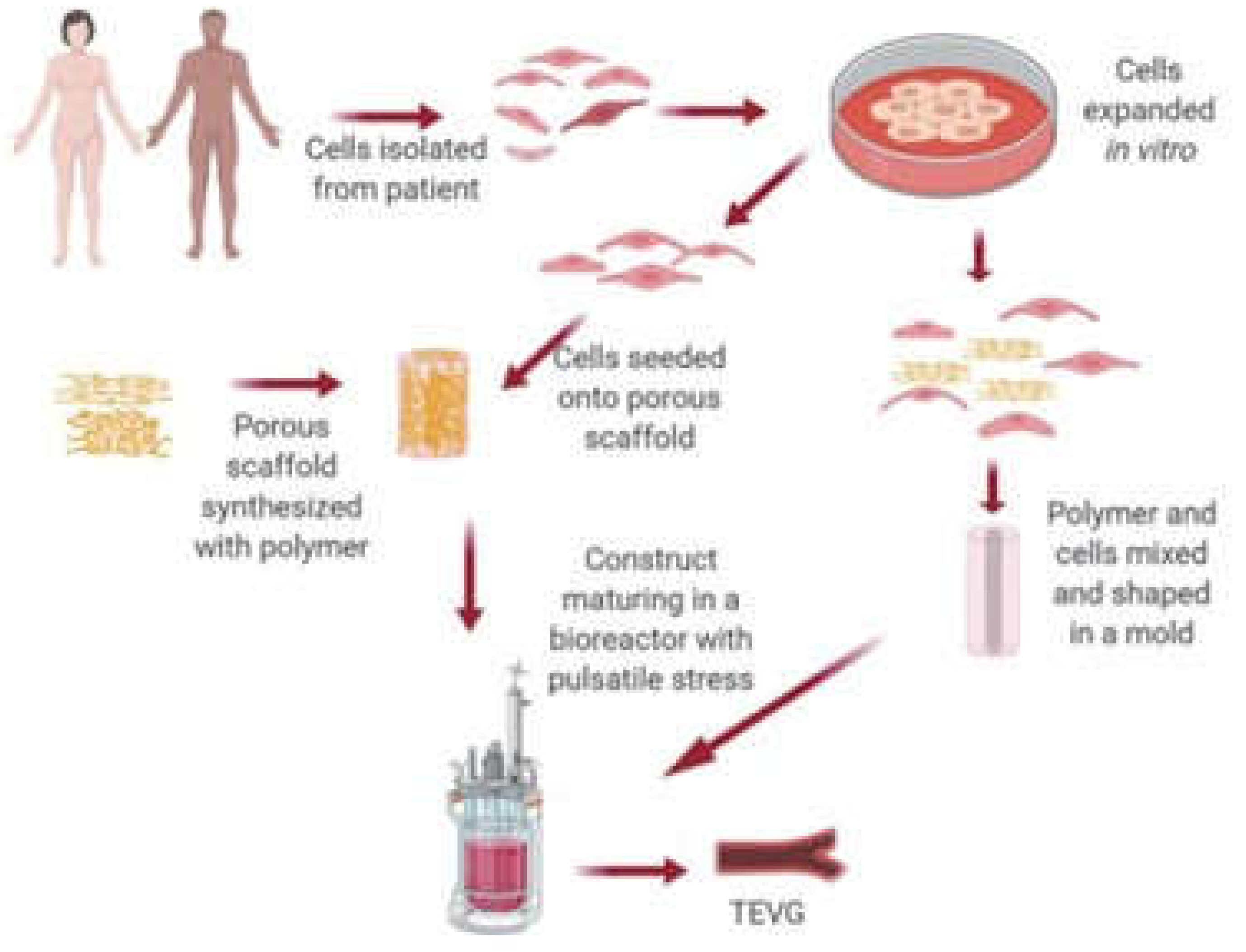
Statement of Significance
Synthetic grafts fail up to 75% within three years of implantation. This failure rate indicates that the current clinical strategies for managing cardiovascular disease utilizing small-diameter vessel-bypassing systems need improvement. In vascular tissue engineering and regenerative medicine, researchers intend to improve this critical clinical issue using bio-fabrication techniques that combine additive manufacturing and biomaterials science. However, several aspects of tissue-engineered vascular grafts (TEVGs) must be improved, such as cell availability, biocompatibility, mechanical properties, the safety of supplemented materials, and growth potential. Although recent advances in TEVG fabrication are promising, more research is needed to generate optimal TEVGs.
1. Introduction
According to the American Heart Association, cardiovascular disease (CVD) reported 18.6 million deaths in 2019, steadily rising as a foremost global reason for morbidity and mortality [
1,
2]. Yet, thousands die yearly due to vascular diseases, congestive heart failure, stroke, myocardial infarction, and valvular heart disease. Thus far, in the United States, millions of Americans live with coronary heart disease and vascular diseases necessitating surgical involvement via settling vascular grafts to repair vascular damage. CVD increases mortality and is predicted to increase to 23.3 million annually worldwide by 2030 [
3,
4]. The increasing number of vascular bypass surgeries in the United States has led to a higher demand for vascular grafts, which has broadened its application prospects.
There are three types of vascular grafts: allogeneic, autologous, and synthetic. Autologous vascular grafts, such as the internal thoracic artery and saphenous vein, are the best choices for coronary artery bypass grafting patients because allogeneic vascular grafts often lead to immune rejection [
5,
6]. When patients do not have suitable blood vessels for transplantation, synthetic vascular grafts made of polylactic acid, polyglycolic acid, and expanded polytetrafluoroethylene can be used as alternatives to autologous vessels [
7]. However, synthetic vascular grafts have biocompatibility and vascular compliance limitations due to their cytotoxicity and degradation rate[
8].
As a result, tissue engineering has emerged as a field capable of producing different vascular grafts to overcome these limitations. As a result, tissue engineering has gained interest in biomedical research, particularly in the cardiovascular field, to prepare vascular grafts with good biological activity and biocompatibility [
9]. Tissue-engineered vascular grafts (TEVGs) are classified into three categories based on their diameter: large-diameter, medium-diameter, and small-diameter vascular grafts. Synthetic materials have successfully produced tissue-engineered vascular grafts (TEVGs) with large or medium diameters. However, in small-diameter vascular grafts, platelet attachment and thrombus formation increase due to a mismatch in mechanical properties between the graft and natural blood vessels. This leads to excessive proliferation of smooth muscle cells and new intima hyperplasia[
8].
Figure 1.
The basic strategies for productively designing a blood vessel. Reprinted from ref. [
10].
Figure 1.
The basic strategies for productively designing a blood vessel. Reprinted from ref. [
10].
As a result, modern tissue-engineering methods, such as the decellularization procedure and 3D additive bioprinting methods, have been proposed to increase the availability of bioengineered vascular grafts to manage CVD better. Vascular grafting has many applications in CVD. For example, many experiments have focused on the scaffold and cell base for treating CVDs (
Figure 1). Specifically, small-diameter vascular grafts are in high demand for coronary artery bypass grafting, often necessary due to coronary artery obstruction. In addition, recent advancements in engineering cardiac tissue have improved techniques such as stem cell isolation and culture in bioreactors[
11]. The primary principle is to produce living cells through cellularized grafts that can grow and replace damaged cells using a combination of cells, scaffolds, and growth factors[
12].
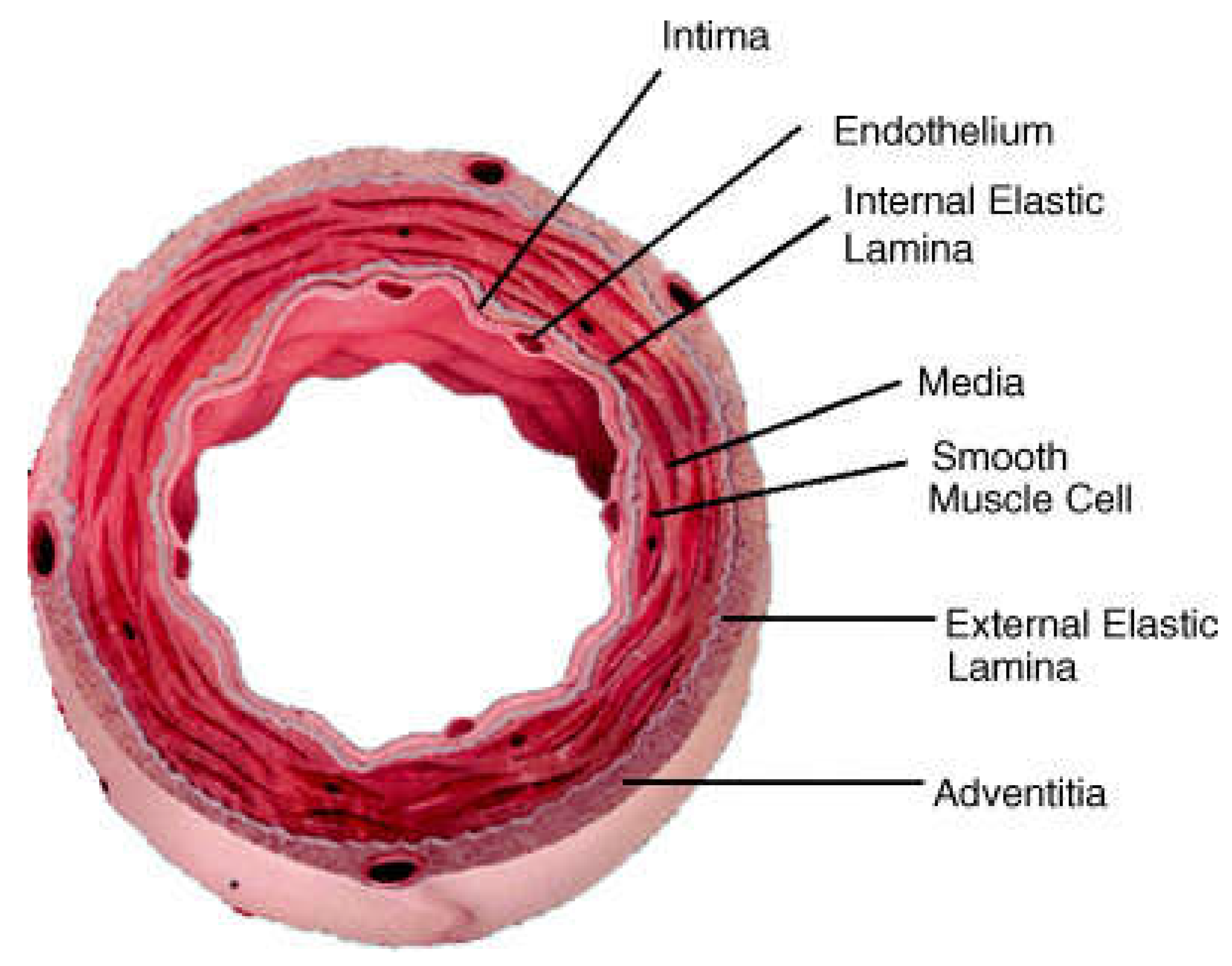
2. Structures of Blood Vessels
Reproducing vascular tissue depends entirely on the functions of blood vessels from different compositions at the bimolecular level. The arterial wall is composed of three distinct layers, i.e., the tunica, a membranous covering of a separate layer of the wall of a blood vessel (
Figure 2).
2.1. Tunica Intima
The tunica intima is the innermost, thinnest layer and comprises endothelial cells (ECs) maintained by the subepithelial layer of connective tissue and supportive cells. ECs regulate biological processes, such as blood flow regulation and extracellular matrix (ECM) component synthesis. In addition, ECs allow material in and out of the bloodstream and white blood cells. A thin membrane of elastic fibers parallel to the blood vessels encloses the Tunica Intima [
14].
2.2. Tunica Media
The tunica media includes smooth muscle, elastin fibers, and connective tissue organized in concentric layers. The smooth muscle function has contractile phenotypes that open and close blood vessels. In addition, it works independently and involuntarily. The thicker area, essential for maintaining blood pressure, consists of more elastic fibers in the arteries than in the veins [
15].
2.3. Tunica Adventitia
The tunica adventitia is the outer layer of blood vessels that comprises connective tissue and fibroblasts embedded in a loose collagen matrix. Additionally, most collagen types are type I and II, providing blood vessel strength or resistance to dilation due to excessive blood pressure [
14].
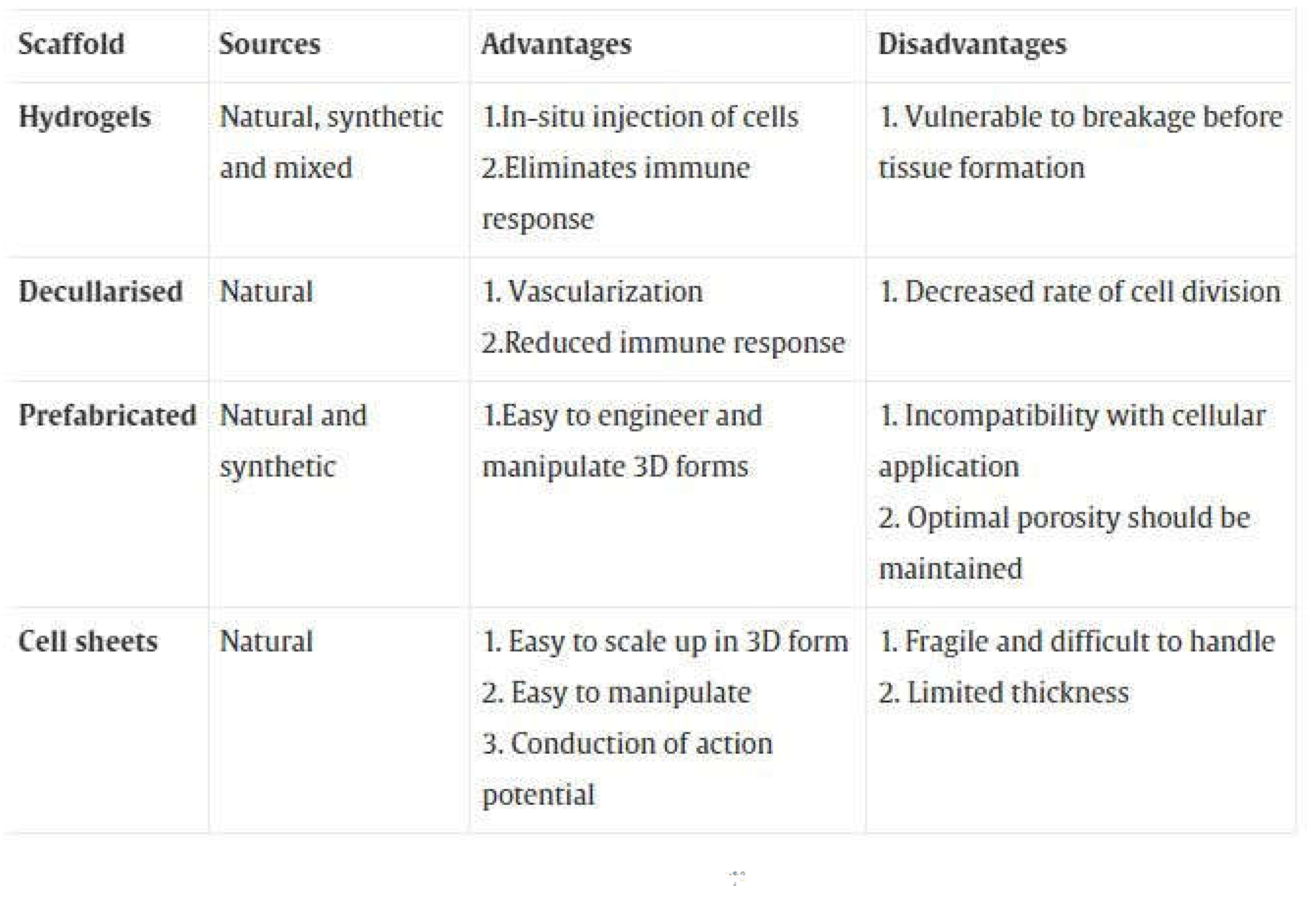
3. Scaffold Types and Approaches
Many researchers have been dedicated to scaffold-guided vascular reconstruction, which means understanding how to use "scaffolds" to help rebuild blood vessel tissue. Scaffolds are 3D structures that help support attaching cells and growing tissue. The ideal scaffold should break down naturally after the tissue has formed, so there is no need to remove it, and the breakdown products should not be harmful to the cells. Scaffolds can be made from either synthetic or natural materials [
16]. Scaffolds for tissue engineering should be non-immunogenic, non-toxic, and flexible, with a good surface for cell attachment and proliferation and 3D structures for extracellular matrix (ECM). Tissue-engineered vascular grafts (TEVGs) can be fabricated using different methods, including biodegradable polymer-based, decellularized ECM-based, cell sheet-based, and 3D bioprinting approaches. The first two methods are scaffold-guided, while the cell sheet-based approach is self-assembled without synthesized materials. 3D bioprinting, including the spheroid-based technique, is a promising approach for TEVG fabrication with diverse potential applications [
17,
18,
19].
Table 1 shows the different types of scaffolds.
3.1. Biodegradable Polymers
TEVGs are engineered grafts used for surgical procedures and must withstand the blood pressure in the body. Biodegradable polymers, such as polyglycolic acid (PGA), polylactic acid (PLA), and poly(ε-caprolactone) (PCL), are commonly used as temporary scaffolds to support seeded vascular cells in the fabrication of tissue-engineered vascular grafts (TEVGs)[
20]. These materials degrade over time and provide mechanical support until the implanted cells secrete and organize their own extracellular matrices (ECMs). The degradation rate of the polymer-based scaffolds can be manipulated by changing their size, surface area, and composition, which affects their mechanical properties and ability to withstand blood pressure. However, an imbalance between material degradation and neotissue formation can lead to mechanical mismatch and subsequent graft failure[
21]. The process of creating implantable TEVGs can take weeks or months, making them more appropriate for planned or elective surgeries rather than emergency situations. However, decellularized materials may be a more readily available option for emergency situations, as they can be cryopreserved until needed.
In 1988, researchers seeded rat vascular smooth muscle cells (SMCs) on biodegradable polyurethane-based scaffolds and implanted them in the rat aorta. After two days, multilayered SMCs were observed, which had become thicker by one week after implantation. In addition, the neotissues showed endothelial cell-like cells on the luminal side, indicating the successful integration of the seeded cells and the formation of new tissue[
22].
Researchers have explored using biodegradable polymer-based tissue-engineered vascular grafts (TEVGs) for patient implantation. One such approach involves a bilayered scaffold developed by Vorp's research group in 2009, which consists of a highly porous inner layer for cell integration and growth and an external reinforcing fibrous layer [
23]. Human skeletal muscle-derived stem cells were seeded on the inner surface of the scaffold using a rotational vacuum seeding system, which may have the potential for use in regenerative medicine[
24].
Hoerstrup's research group implanted biodegradable polymer-based tissue-engineered vascular grafts seeded with autologous myofibroblasts and endothelial cells (ECs) into growing lambs, which showed potential for growth and remodeling. The TEVGs were incubated in a pulsatile bioreactor for 21 days before implantation as main pulmonary artery replacements and followed up for two years after implantation. The implanted grafts showed increased diameter and length, and imaging tests revealed no evidence of complications [
25,
26].
Electrospinning is a technique that can create a nanofibrous scaffold – a fragile and lightweight structure [
27,
28,
29]. This kind of scaffold interacts with cells differently from standard synthetic grafts, with beneficial uses in medical applications [
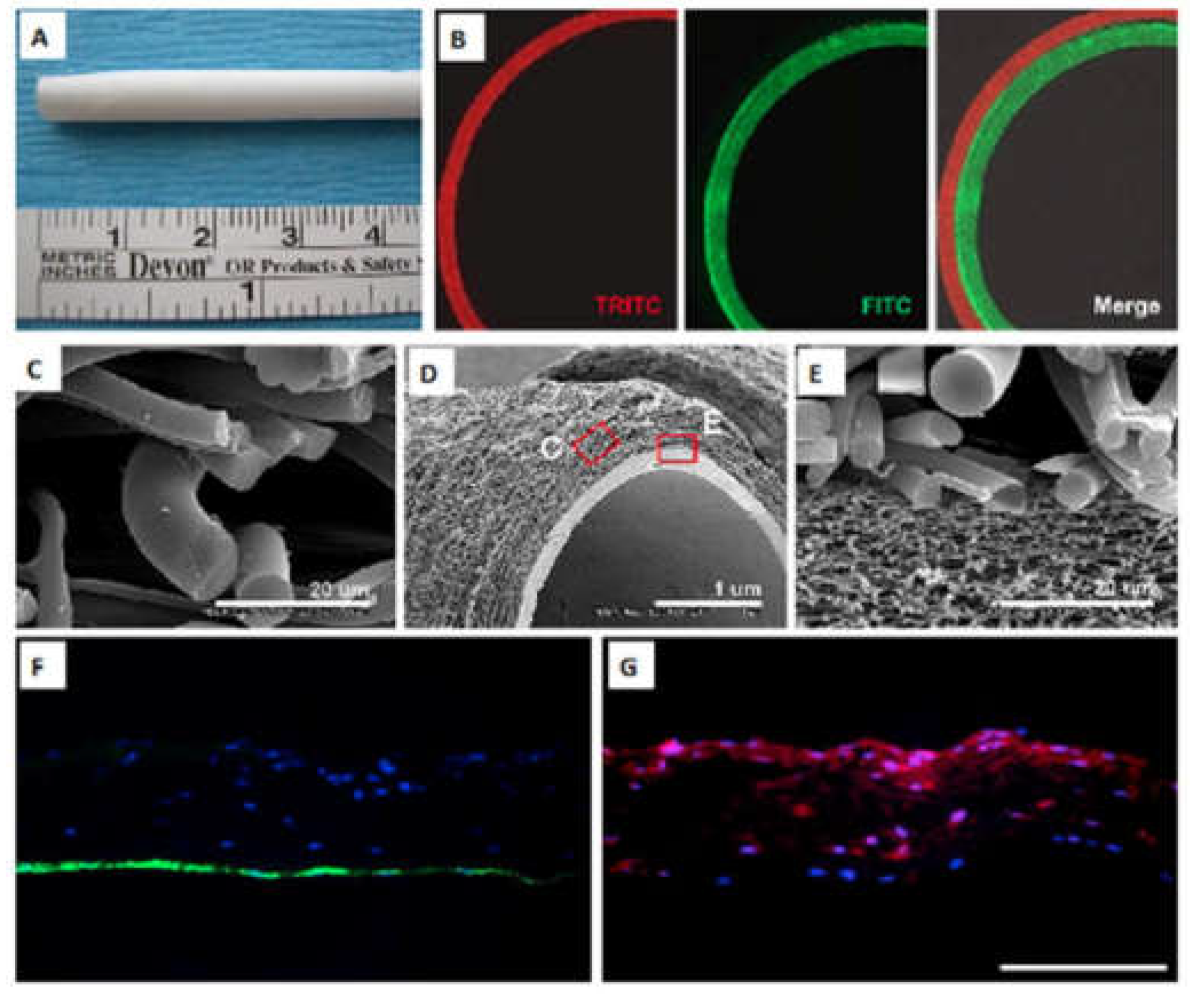
30]. The electrospinning technique exposes the polymers to a very high electric current, causing those polymers to form fibers, eventually creating a scaffold. Changing various microstructural parameters, such as the fiber size, porosity, and alignment, can control the scaffolds’ mechanical properties. Additionally, manipulating the scaffolds’ mechanical properties can regulate cell survival, migration, and proliferation when forming tissue. For example, Ju et al. (2010) used a particular technique to produce a PCL/collagen bilayer scaffold with an outer layer that allowed for easier smooth muscle cell infiltration and an inner layer for easier endothelial cell attachment (
Figure 3A–G) [
31].
Electrospinning can use either degradable (e.g., PLA, PGA, and polyurethane/silk fibroin) or natural materials to create scaffolds and tubular conduits, such as vascular grafts [
32]. For example, combining poly (lactic-co-glycolic acid) with collagen type I and elastin can improve scaffolds’ mechanical properties for creating electrospun blood vessels [
33,
34]. Alternatively, naturally derived materials like collagen, gelatin, and fibronectin can enhance cellular functions like adhesion, growth, and differentiation by providing more Arginylglycylaspartic acid binding sites [
31]. For example, scientists create artificial blood vessels using electrospun tubular scaffolds with controlled release of vascular endothelial growth factor [
35,
36]. This approach can improve cell adhesion and proliferation in the vessel wall, leading to better anti-thrombogenic properties and avoiding common problems post-implantation [
37]. However, using native tissue as a scaffold is sometimes very limiting because getting the right size, caliber, and length is challenging. Therefore, scientists developed a synthetic biomaterial with a naturally derived substance to make it more consistent with fabricating [
38].
3.1.1. Decellularised Scaffolds
Decellularization is a process used to treat tissues, using physical, chemical, and enzymatic methods to remove the cells while keeping the tissue architecture intact [
39]. The decellularized vascular matrix is the non-cellular part of the blood vessel wall, which is more than a supporting structure, but a vital microenvironment for cell life. These sources are constantly being updated through experimental techniques. Decellularized ECM-based approach involves using grafts from different animal and human sources that have been stripped of their cells to reduce the risk of rejection. It consists of three main components: structural proteins (collagen and elastin), linked/adhesive proteins (integrin, fibronectin, and laminin), and invisible gelatinous structures (proteoglycan and hyaluronic acid). The content of these components can vary depending on the developmental stages of blood vessels and their functions. The first decellularized vascular grafts were derived from bovine blood vessels and ureters. Since then, tissue engineering technology has evolved, producing vascular grafts such as Artecraft®, Solcograft®, and ProCol® using these decellularized materials[
40]. Scientists showed that these types of grafts are effective for hemodialysis access with similar success rates as traditional ePTFE grafts but with lower complication rates such as infection and thrombosis. Chemla and Morsy conducted a study on SynerGraft® for hemodialysis grafts. They found that decellularized grafts had similar success rates to conventional prosthetic grafts regarding patency and freedom from infection after 12 months[
41].
In 1966, Rosenberg et al. tried decellularization with bovine carotid artery but faced occlusion of vascular graft postoperatively[
42]. In 1995, Sandusky et al. implanted porcine small intestine submucosa (SIS) as a carotid artery interposition graft in dogs with equal patency rate in both the vascular graft and autogenous saphenous vein[
43].
In 2003, Dahl and colleagues tested three different methods to remove cells from pig carotid arteries. The methods utilized were as follows: in Treatment A, which involves submerging vessels in a nonionic detergent solution with a chelator to inhibit enzymes and deoxynuclease for 24 hours; in Treatment B, involves submerging vessels in a hypotonic solution for 11 hours followed by transfer to a hypertonic solution for 11 hours; in Treatment C, vessels were submerged in a zwitterionic detergent solution. Among these methods, They found that treatment C was the most effective in eliminating cell nuclei while maintaining mechanical properties similar to native vessels [
44].
In 2011, Dahl et al. created tissue-engineered vascular grafts (TEVGs) using a bio-degradable scaffold-based decellularization approach. They seeded the scaffolds with cells from an aortic artery and allowed them to grow for several weeks in a machine that stretched them. Then they removed all the cells so that only the scaffold remained, which had high-pressure resistance even after being stored at low temperatures for up to one year. Also, the resulting TEVGs were seeded with autologous ECs (endothelial cells) shortly before implantation into baboons as arteriovenous conduits. These implanted TEVGs maintained 88% patency without aneurysmal formation for up to six months in the baboon models.[
45].
In 2017, Daugs et al. used a decellularization technique successfully applied to bovine carotid arteries using different chemical solutions. The histological analysis showed good preservation of the extracellular matrix structure, and mechanical tests proved that the decellularized arteries have appropriate biomechanical properties [
46]. The decellularized vascular matrix has unique advantages for small-diameter tissue-engineered vascular grafts, including complete retention of the extracellular matrix, superior biocompatibility, and non-immunogenicity. These advantages could replace the patient’s vessels in coronary artery bypass grafting.
3.1.2. Natural Scaffolds
In the field of bioengineering, collagen is a material that is often used in tissue engineering. Collagen is the main structural protein in blood vessels and is produced by cells as precursor procollagens. Procollagens are turned into collagens using procollagen proteinases, forming collagen fibers[
47]. Collagen is the main component of the extracellular matrix (ECM), which plays a role in signaling and maintaining blood pressure and smooth muscle cell function. It also promotes cell adhesion and proliferation and has low antigenicity, making it biocompatible [
48,
49]. When using collagen in TEVGs, it is essential to consider how platelets and coagulation proteins can attach to the integrin binding sites, leading to thrombus formation [
50]. To address this issue, researchers have used a collagen-rich construct derived from small intestine submucosa and type I bovine collagen that was cross-linked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride and treated with heparin-benzalkonium chloride complex to reduce its tendency for thrombosis [
51]. These findings suggest that using many collagens in tissue-engineered blood vessels (TEVGs) makes them stronger and helps attract endothelial cells, but it can also make the TEVG more likely to cause blood clots. Therefore, controlling this risk is essential before implanting the TEVG[
52].
Elastic fibers are made up of elastin and microfibrils. Tropoelastin is a precursor to elastin secreted by cells, which then gets cross-linked with lysyl oxidase to form elastic fibers that cannot be dissolved[
53]. Elastic fibers differ from collagen fibers and can only be made in the body during early development [
54]. Once they are damaged, they don't usually repair themselves. Some studies have found increased levels of elastin when smooth muscle cells (SMCs) were used to create tissue-engineered blood vessels [
55]. Creating functional layered elastic fibers in vitro is currently challenging.
Elastin is essential for regulating the thickness of blood vessel walls by preventing excessive growth of smooth muscle cells (SMCs). Mutations in the elastin gene can cause SMCs to grow too much, leading to blockages in arteries. Elastin also affects cell functions such as adhesion and chemotaxis[
56]. Elastin also contributes to ECM signaling and helps maintain elasticity and stiffness. Elastin is a material used in the body to maintain the elasticity of blood vessels, allowing blood pressure to withstand. It does this by having cell adhesion sites and allowing for flexibility. This contributes to the mechanical properties of arteries [
57,
58].
Silk fibroin is a biocompatible and biodegradable material commonly used in medical applications [
59,
60]. It is less likely to cause an immune response in humans and can promote tissue growth. Due to its thermal stability can be used in various forms, such as non-woven fabrics, sponges, or coatings. In a study, a bilayer vascular graft with sponge-coated and woven silk fibroin was used to evaluate a common carotid artery bypass in a canine. The sponge layer of the graft was mainly degraded and replaced by fibrous tissue after one year[
61].
Chitosan is a material recently studied for use as a tissue-engineered vascular graft due to its antibacterial and high hemostatic effects[
62]. However, it has a low molecular weight and degrades rapidly in vitro and in vivo, making it challenging to use as a single material for cardiovascular tissue[
63]. Instead, it is often combined with other materials to facilitate cellular infiltration and vascular formation [
64]. Chitosan is a widely used polymer in regenerative and tissue engineering applications due to its excellent biocompatibility and biodegradability. It is particularly useful in skin tissue engineering because of its structural similarity to skin tissue. Cardoso et al. developed chitosan hydrogels containing Nano encapsulated Phenytoin for cutaneous wound healing [
65]. Huang et al. developed a type of hydrogel from a combination of Cellulose nanocrystals and Carboxymethyl chitosan that can promote painless and scarless tissue regeneration for burn wound healing. This hydrogel has self-healing abilities and can dissolve easily. The hydrogel's high equilibrium swelling ratio (350%) is due to the dialdehyde-modified cellulose nanocrystal [
66].
3.1.3. Hydrogels Scaffolds
Hydrogels are a scaffold used in cardiac tissue engineering[
67]. They capture cells within a gel matrix formed by cross-linking water-soluble polymers, which can be natural or synthetic materials. Anchoring materials like RGD (arginine–glycine–aspartic acid) peptides help the cells adhere to the scaffold and multiply. As cells grow, scaffolds lose water and become compressed. Initially used for cell multiplication outside the body, hydrogels can now be injected into patients to deliver cells directly where needed [
68]. Injectable hydrogels are a type of material that can be delivered to the body using catheters. They are made by mixing liquid hydrogels and do not require surgery or drugs for delivery, making them an attractive option in tissue engineering[
69]. Some types of hydrogels, like chitosan and alginate-based ones, show desirable biocompatibility properties, making them precisely useful for tissue engineering purposes [
70,
71]. Hydrogels can be used in tissue engineering as scaffolds that mimic natural tissues. They provide bulk and mechanical structures for cells to adhere to or suspend within. Incorporating appropriate peptide moieties, such as RGD adhesion peptides, can significantly increase cellular attachment and improve cellular migration, proliferation, growth, and organization in tissue regeneration applications. Many different types of cells have been shown to bind favorably to these modified hydrogel scaffolds, including endothelial cells (ECs), fibroblasts, and smooth muscle cells [
72,
73]. Hydrogels have become popular as scaffold materials because they are similar to natural tissues and provide a good environment for cell growth. Scientists have found ways to control the shape, size, and other properties of hydrogel scaffolds so they can be used more effectively in tissue engineering applications, like creating blood vessels or complex tissues made up of multiple types of cells. Hydrogels have properties that make them suitable for use as supporting matrices in cardiac tissue engineering because they are soft, viscoelastic, and similar to natural tissues. Hydrogels are used to support and deliver cells into damaged heart tissue. They help maintain cell survival, functioning, and restore the wall stress of the heart [
74,
75]. Scientists have recently developed many types of hydrogels to help repair damaged heart tissue. For example, a group of researchers created an injectable gel made from pig heart cells that helped support the growth and survival of different types of heart cells in rats. Another study involved implanting hollow tubes filled with immature heart cells into adult rats' arteries. These studies are promising but need further testing on hearts affected by myocardial infarction[
75].
Wang et al. developed a hydrogel made of PEG derivative and a-cyclodextrin to encapsulate bone marrow MSCs. At the same time, Walker et al. implanted PET mesh-reinforced PHEMA hydrogel into the canine epicardium without significant fibrosis or thickening for 12 months after implantation. However, trace calcification was observed, raising concerns about biocompatibility over time [
76,
77,
78].
The use of hydrogel scaffolds for tissue engineering still faces significant challenges. Some of these include poor cell penetration and irregular cell seeding, difficulties in engineering complex tissues with multiple cell types, the limited mechanical properties of hydrogels which restrict their application to soft tissues only, and a lack of microvasculature leading to loss of function and viability due to insufficient nutrient transportation.
4. Cell Sources for Tissue-Engineered Vascular Grafts (TEVGs)
Blood vessels are composed of different layers that perform specific functions, primarily elasticity and anti-inflammatory/anti-thrombogenic roles. Smooth muscle cells (SMCs) and endothelial cells (ECs) are the key components of these layers, respectively, while fibroblasts make up the outer layer that anchors the vessel to surrounding tissue [
79]. Therefore, in designing and creating TEVGs, considering the role of these various cell types and their origin will avoid graft rejection in implantation [
79]. Although autologous cells are the ideal approach to creating vascular grafts, it is not always possible in patients with widespread arterial disease. Additionally, adult cells have limited ability to grow and regenerate. Therefore, researchers are exploring using mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) as sources of cells for TEVGs [
80]. During the past decade, considerable interest has been directed toward using stem cells in TEVGs, because stem cells and endothelial progenitor cells (EPCs) [
81,
82] can differentiate into vascular lineages, and the result can restore vascular systems.
4.1. Endothelial cells (ECs)
ECs are essential cells in TEVGs because they help prevent thrombosis and bacterial infections [
52]. When TEVGs contain multiple layers of ECs, they are less likely to experience these complications, improving their success as a graft. In addition, ECs can regulate various significant physiological processes. For example, ECs can sense blood flow-induced shear stress, which stimulates them to produce nitric oxide and regulate vascular tone and blood coagulation. ECs can also induce pro-inflammatory or anti-inflammatory responses depending on whether or not the blood flow is disturbed [
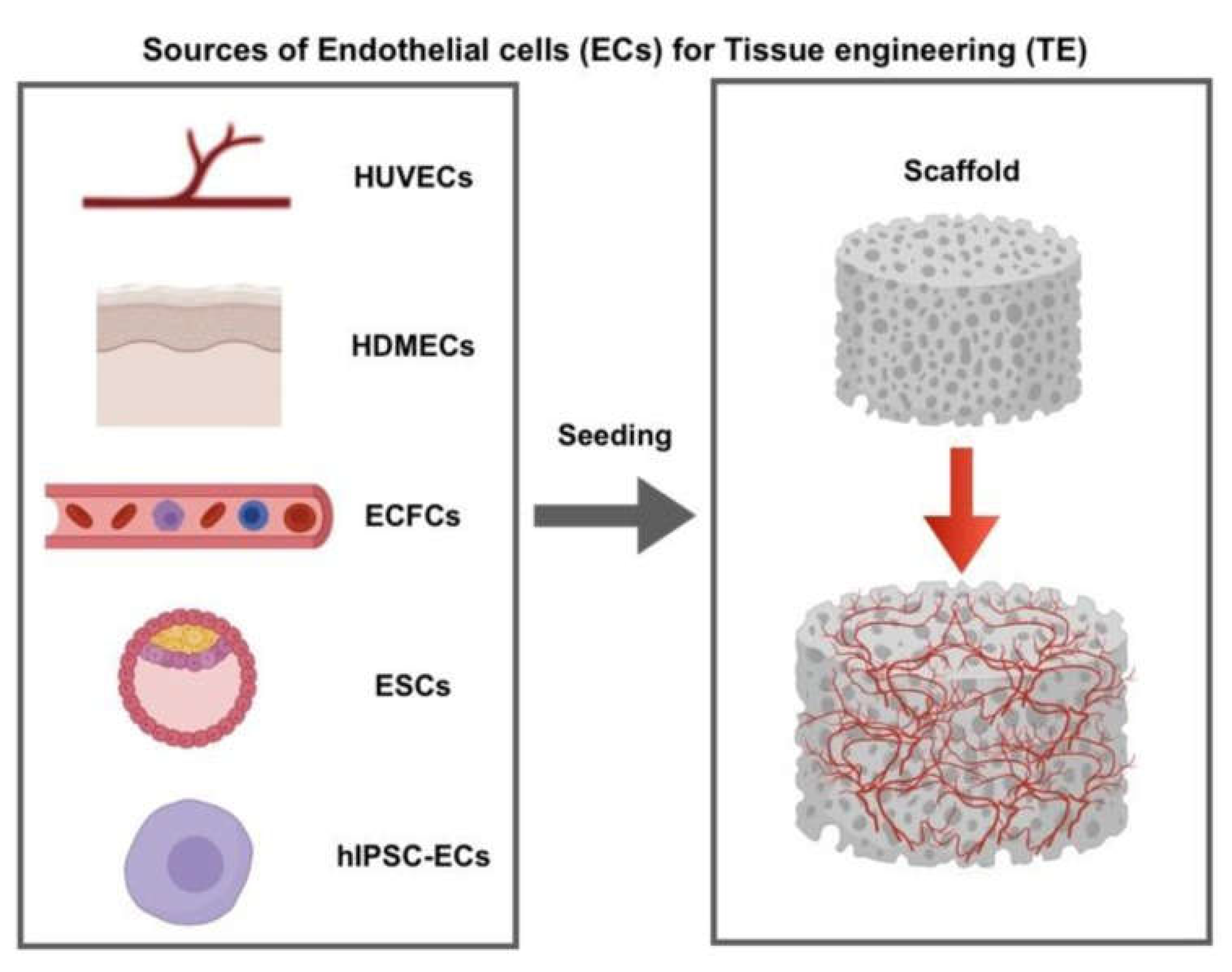
83]. In most studies, the cells used are sources of ECs are embryonic stem cells, human umbilical vein, microvascular ECs, human Dermal, and endothelial colony-forming cells (
Figure 4). Non-all ECs need the same efficient properties attributable to giver unpredictability and tissue site disease. Nevertheless, quite a few in vivo and in vitro experiments demonstrated good quality efficient phenotypes, greater vascular concentration, and further steady blood vessels as soon as ECs were co-cultured with different cell kinds, e.g., fibroblasts linked to ECs only [
84,
85].
Consequently, due to countless restrictions of ECs, the alteration has been concerned with other cell bases such as pluripotent stem cells, EPCs, and MSCs [
79,
80,
87,
88]. These cells expand quickly in vitro environments with greatly determined angiogenic assets and are excellent resources for medical purposes. Cell-based therapy for vascular regeneration is promising since most cells significantly influence the process of vascular regeneration and, ultimately, induce vessel-like network formation in the ischemic tissues.
4.2. Pluripotent Stem Cells
Pluripotent stem cells (PSCs) can replicate and grow indefinitely, differentiating into the kinds of cells found in all three layers of an embryo. This transformation makes these cells attractive for medically treating various diseases and injuries. Two types of PSCs are found in humans: embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). While both can differentiate into vascular cells, iPSC-derived endothelial cells (iPSC-ECs) have shown a different capacity to form capillaries than human umbilical vein endothelial cells (HUVECs) [
89]. However, hPSCs must reach a particular differentiation stage for therapeutic use to avoid teratoma formation [
90].
Luo and colleagues (2020) created artificial blood vessels using vascular SMCs derived from human iPSCs [
87]. The cells were first differentiated into vascular SMCs, seeded onto a scaffold, and incubated to form the TEVGs. Next, human-induced pluripotent stem cells were turned into vascular SMCs using an embryoid body-based approach that takes 26 days to obtain mature vascular SMCs [
87]. Pluripotent stem cells allow researchers to create models of human heart development and diseases in vitro, providing a foundation for heart disease treatment utilizing a patient's cardiac cells. However, directing cell differentiation into desired cell types requires understanding the stages of cardiovascular development, which research in model organisms can inform. Even when researchers use specific methods to turn stem cells into a particular cell type, it is still challenging to get a pure population. More research must improve these methods to get the desired cell populations [
91].
4.3. Endothelial Progenitor Cells
EPCs are a promising stem cell resource for vascular regeneration. They are naturally obtained from adult stem cells involving peripheral blood [
92], cord blood [
93], and bone marrow [
94]. However, the debate over these cells’ source, isolation, and cellular role remains a prospective issue. Asahara et al. (1997) were the first to use adult stem cells to regenerate blood vessels [
95]. They assumed pre-existing blood vessels originating from postnatal neovascularization were exclusively based on fully differentiated ECs. However, Asahara revealed that supposed hematopoietic precursors cells (CD34+, Flk-1+/KDR+) from human adult circulating blood cells might segregate into ECs in vitro and be known as EPCs [
92]. Yet, several markers distinguish these cells. However, a powerful argument about the proper sort of EPCs and the specificity of these markers exists [
94]. EPCs may also evaluate the possibility of vascular disease [
94].
4.4. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are a promising source for creating tissue-engineered blood vessels because they can regenerate, regulate immune responses, and prevent clotting [
96]. MSCs can be obtained from various sources, such as bone marrow or fat tissues [
97]. They have a high rate of cell division compared to other adult cells. They can interact with different types of immune cells by reducing inflammation while increasing suppressive cytokines that help control rejection after transplantation[
98]. MSCs can prevent blood clotting because of the heparan sulfate proteoglycans on their cell surfaces. They can also recruit endothelial cells through angiogenic factors they secrete. A study by Zhao et al. showed successful implantation of TEVG from MSC sheets in rats, resulting in complete endothelialization after four weeks [
88]. Mesenchymal stem cells (MSCs) can be turned into smooth muscle cells (SMCs) and endothelial cells (ECs). Researchers Gong and Niklason used this process to create tissue-engineered vascular grafts by seeding undifferentiated MSCs onto biodegradable scaffolds, adding TGFβ1 to induce differentiation into SMCs exposing them to pulsatile flow[
99]. MSCs can be differentiated into various types of cells, including SMCs and ECs, making them a promising source for creating tissue-engineered vascular grafts (TEVGs) [
100]. MSCs are a minor subpopulation of bone marrow [
101]. MSCs discovered by Friedenstein et al. adhered to plastic and had a fibroblast-like morphology [
102]. Cell-based therapy and bone marrow-derived MSCs have embraced the potential for regenerating neovessels that sustain blood flows in the ischemic blood vessels [
103]. In addition, MSCs are a prospective therapy in regenerative medicine due to their ability to expand and secrete paracrine factors with antigenic effects and are robust in low oxygen circumstances [
104]. Using MSCs at the position of limb ischemia considerably improved pain at the ankle-brachial index as a measurement of vascular function [
105,
106]. Clinical trials using mononuclear cells from bone marrow or peripheral blood have also shown promising results. Accordingly, alternate beneficial cell types are preferred for treating peripheral artery disease.
MSCs have been extensively researched because they are present in many tissues in the body and can turn into many kinds of cells [
107]. In addition, scientists have discovered that when introduced into the body, these cells move to areas of inflammation, produce substances that can reduce inflammation, and the body's immune system does not reject MSCs. Research conducted in vitro has also indicated that these cells can potentially treat problems related to clotting (thrombosis) in implants [
108].
5. Cell Sheet Approach
Despite improved techniques, using materials outside the body in scaffold-guided tissue engineering techniques can still interfere with compatibility. Living cells ideally remodel TEVGs based on their surrounding environments. In scaffold-based TEVGs, the breakdown of products of biodegradable materials and the denatured proteins of the decellularized cellular matrix (ECM) may disrupt the remodeling process. Using self-assembled approaches to create TEVGs is advantageous because it allows cells to form connections with each other and build natural networks without using scaffolds [
109].
L’Heureux et al. (1998) generated the first cell sheet-based TEVGs without synthetic or external biomaterials [
110]. They used human umbilical vein smooth muscle cells (SMCs) and human skin fibroblasts cultured with ascorbic acid for four weeks to enhance collagen synthesis. The resulting TEVGs produced abundant ECMs. The SMC layers were manually peeled and wrapped around a mandrel to mimic the tunica media, and the constructs were cultured in a bioreactor with pulsatile flow. Fibroblast sheets were manufactured and wrapped around the SMC constructs to mimic the tunica adventitia. These constructs were cultured for an additional seven weeks before human umbilical vein ECs were seeded to the inner side of each construct. Each layer was fused one week after the ECs were seeded, and the entire process took four months from start to implantation [
110]. The burst pressure of 2594 ± 501 mmHg was comparable to that of the human saphenous vein. Implantation in the canine femoral artery revealed that the grafts were well-sutured and could withstand blood pressure in vivo. However, the graft patency was 50% seven days after implantation due to clotting.
Endothelial cell (EC) seeding was omitted in the animal study to avoid acute rejection [
111]. The inspiration for this strategy came from a medical treatment used on severe burns called epithelial cell sheets [
112]. This procedure involves using heat to separate fibroblasts and other cells into thin layers, creating a "sheet" or "cell sheets." These cell sheets are then placed into a tube-shaped scaffold and cultured in a bioreactor. Following the procedure, these artificial blood vessels have high burst pressures, similar proteins to natural tissue, and strong suture strength with functional endothelialization. In addition, engineered blood vessels have high primary patency and functional flow in a canine model [
113]. In another study, researchers took cells from elderly patients with advanced CVD and created TEVGs using a unique technique called self-assembled cell sheets. These TEVGs were tested in rats for up to 180 days [
110,
114].
Researchers have developed cell sheet engineering to avoid the limitations of tissue reconstruction using biodegradable scaffolds or single-cell suspension injection. Cell sheets prepared at 37°C allow various cells to adhere and proliferate. In addition, scientists found that when the temperature is below 32°C, the cells can detach without using proteolytic enzymes. This approach to constructing cell sheets has been used for various tissue reconstructions, including cardiac patches and periodontal ligaments [
115].
The cell sheet must be carefully removed from the surface of its growing container. Removal can be difficult without damaging the sheet by excessive mechanical stress [
113]. Fortunately, the sheet can be detached from the surface without external forces. Using a temperature-sensitive chemical material, the surface of the container becomes hydrophobic at a regular temperature, allowing the cells to multiply and form the sheet. When the temperature [
116,
117,
118,
119] is lowered, the surface of the container becomes hydrophilic and allows the cells to detach from the surface without the risk of tearing or damage [
120]. This process enables the removal of the sheet without damaging the intercellular connections. The limitations of this process are an extended culture period and specialized equipment to create a 3-dimensional structure [
120].
A faster technique that does not require specialized equipment is a non-adhesive agarose template to form the tissue [
121]. This technique results in a cohesive tissue ring formed by the cells settling and aggregating. The mechanical properties could be better than the cell-sheet-based approach. Though the results are promising, this process still takes a long time due to the maturation process in the bioreactor [
122]. However, growing the vascular tissue directly in the patient’s body can speed up this process.
Self-assembled cell-sheet-based techniques are a promising approach for TEVG. This technique uses a cell sheet composed of a single layer of cells to form a vascular graft. This technique can create a vascular graft that closely mimics the native tissue’s structure and function. Additionally, this technique can reduce the risk of transmitting pathogens from the donor to the recipient.
6.3. D Bioprinting
3D printing was invented to create three-dimensional molds and supports from various materials [
18]. 3D bioprinting is a newer technique that utilizes cells as a bio-ink to create 3D structures. Common bio-inks include hydrogels that provide a scaffold for supporting cells and spheroids, which allow cells to form cell-cell junctions and assemble their own ECM. While bioprinted TEVGs are not yet available for clinical use, the technology is rapidly improving. This technology holds great promise in recreating the native vascular anatomy of living tissues [
19].
3D models are based on cell culture, meaning scientists replicate many natural properties with cell-made structures [
123,
124]. This approach allows them to study natural cellular behaviors, morphology, and functions, which cannot be done with traditional 2D cell cultures [
125]. For example, spheroids - multicellular structures - will enable scientists to emulate how different cell types interact [
124]. However, the bio-inks used to create these structures are currently limited in their ability to replicate the designs of native tissues due to their printability, mechanical properties, and ability to deposit a high density of cells into complex architectures [
126,
127]. In addition, bioprinting must place materials and cells in specific locations for complex systems, which helps scientists study structures and functions in greater detail. For example, researchers have been working on using 3D bioprinting to create cardiac patches, which are tissue-engineered materials to replace a damaged muscle. They use a layer-by-layer approach involving special "bio-inks" made from biomaterials [
126,
127] such as alginate, collagen, gelatin, hyaluronic acid, and decellularized ECM scaffolds [
128].
Nakayama's research group created implantable bioprinted TEVGs without scaffolds using spheroids made up of human umbilical vein ECs (40%), human aortic SMCs (10%), and human dermal fibroblasts (50%) [
92].In addition, they used a needle array to construct the tubular-shaped TEVGs. After incubating for four days, the needle array was removed, and the tubular construct was perfused in a bioreactor for an additional four days. The resulting TEVGs were successfully implanted in the abdominal aortas of nude rats, where they maintained patency for five days. In addition, the TEVGs were histologically analyzed and exhibited endothelialization after implantation [
129].
Extrusion-based bioprinting creates anatomically relevant vessels similar to our circulatory system’s veins and arteries. Despite some success, these scaffold-based patches can degenerate quickly. However, more advanced printing technologies such as selective laser sintering, stereolithography [
123], digital light processing, and 2-photon polymerization are more suitable for printing smaller vessels, like capillaries [
130].
7. Approaches to Building Tissues Vascular Grafts
Tissue engineering vascular grafts is a technology used to create a new blood vessel. Traditional tissue engineering has four main steps, including isolating cells and seeding them onto an appropriate scaffold with appropriate chemicals and factors to improve the physiological and mechanical properties (
Figure 5). The first step is to isolate the cells from the host. After that, the cells are grown and multiplied in culture. Next, the cells must be placed onto a scaffold and given the right combination of hormones and proteins to help them grow and stay healthy. The fourth step is to implant the tissue back into the host to test tissue functionality.
Vascular cells, mainly ECs, are the cells that cover the lumen of the vessels and prevent the blood from clotting. In addition, ECs can integrate into the embedded scaffold and develop vascular self-construction native vessels in vivo [
132]. Smooth muscle cells (SMCs) are also important because they involve the vessel's contractility and eventually control blood volume [
133,
134,
135]. Most cell types from donor vessels or stem cells exhibited updated progress of cell-based TEVGs from in vitro and in vivo to preclinical evaluation [
136].
When implanting vascular grafts into an animal, those cells would not be able to adapt to the high-pressure and high-flow environment, and ultimately, the cells would fall off. Therefore, bioreactors [
137] preconditioned and trained them to adhere to their surface
. Niklason et al. (1999) conducted one of the first studies of bioreactors for vascular grafts [
138]. They explained the critical contribution of flow-inducing shear stress for smooth muscle cell migration via a vascular structure scaffold. The material should be biocompatible, safe, and have physical properties like blood vessels. Bioreactors help mature the cells. In the end, those cells would further mature and become a more tissue-like structure within five to ten days to implant in vivo. The tissue and vessel survive, function normally, and eventually develop into tissue. For example, researchers used the sheep carotid artery model [
132,
139] by exposing the carotid artery, removing 5 cm of the carotid artery, replacing the vessel construct, and following the sheep for six months to monitor patency. The maintained blood flow was comparable to arterial blood flow. Finally, a computerized tomography scan ensured the implanted vessel maintained its original shape and size [
140].
In tissue engineering, cells are supposed to create an ECM, during which the polymer is reduced, slowly producing the proposed tissue. For example, researchers have accomplished significant progress in remodeling the tissue-engineered heart valve constructs like the native blood vessels [
141], as depicted in (
Figure 6).
Researchers developed a system many years ago to secure those cells from peripheral blood. Scientists have developed an extracorporeal cellular affinity column that hooks into an artery, and the other side connects to the vein [
142]. Therefore, the blood would flow through the queue and into the column. First, several speeds are conjugated with antibodies specific to endothelial progenitor cells (EPCs) [
143]. Then, when the blood flows through the column, those EPCs would bind to the antibody [
144] and be selected while returning the entire blood volume to the patients [
145]. This approach has successfully obtained many cells compared to a conventional method. Additionally, that system worked very well to get and isolate EPCs and SMCs.
More advanced techniques could be found to shorten the time to prepare and deliver the necessary blood vessels to patients using a cell-free vascular graft.
8. Cell-free Vascular Graft
Cell-based technologies help make tissue-engineered vascular grafts (TEVGs). However, making TEVGs is lengthy and expensive and can be more complicated when working with primary adult cells or stem cells from older individuals with limited expansion potential. To help solve these issues, some researchers have created completely acellular vascular grafts that can be accessed more quickly and replace blood vessels to treat cardiovascular diseases. Of the many approaches, one method uses conjugate heparin [
146,
147,
148], an anti-thrombogenic agent, onto the luminal surface of the vascular scaffold to prevent blood clotting. Scientists have recently reported developing cell-free grafts implanted successfully in a large, preclinical animal model (sheep carotid artery) [
132] and following the animals for six months. Researchers revealed that the conjugate-heparin approach helped the implanted vessels maintain their patency and normal blood flow. Consequently, by the conjugating method, heparin [
148] could support its function, and the body can mobilize those necessary cells over time and try to repair themselves.
SMCs are not required for the medial layer of the graft if the appropriate biomaterial is used [
149]. Additionally, the properties of the biomaterial (like porosity) [
150] become more critical for graft patency and remodeling [
151]. Other studies have used a biodegradable elastomer (a type of flexible polymer) to create a new artery in rat models. Instead of using endothelial cells (ECs), other studies used growth factors and antibodies to attract host circulating EPCs [
152]. Finally, Zhou et al. used heparin and a vascular endothelial growth factor protein to replace the endothelial lining and successfully implanted it in a canine arterial model [
152]. Since free-cell vascular grafts form a functional confluent endothelium, are populated by host smooth muscle cells, and remodel within the host, they overcome many of the issues associated with cells.
9. Clinical Translation of TEVGs
Clinical trials have employed different methods of producing TEVGs, including using a biodegradable polymer for venous shunts in children with congenital heart disease and small-diameter TEVGs for arteriovenous shunts in adults undergoing hemodialysis. Despite positive results, complications like intimal hyperplasia and thrombosis are still common [
153].
Shin'oka and his team (2001) developed the Biodegradable Polymer-Based Approach for Venous Shunt [
154]. It involves removing vascular cells from an autologous peripheral vein and planting them on a scaffold made of a 50:50 copolymer of PLA and PCL. The TEVG with autologous vascular cells is then implanted into a low-pressure, high blood flow large-diameter venous system. Although the initial operation was successful, cell expansion required eight weeks. However, in their subsequent clinical trial, no significant complications were observed after 11 years of follow-up except for graft stenosis, where 28% of the patients required balloon angioplasty [
155]
.
Decellularized grafts from animal and human veins may substitute for hemodialysis access. Clinical trials have tested commercially available grafts from different sources, demonstrating similar patency rates as conventional grafts made of ePTFE but with lower rates of complications like infection and thrombosis [
41,
156].
A clinical trial involved a Cell Sheet-Based Approach for Arteriovenous Shunt. Researchers took autologous fibroblasts and ECs from the skin and superficial veins of ten patients with end-stage renal disease and generated TEVGs containing three layers [
157]. Then, a bioreactor applied a pulsatile strain to the TEVGs. The total generation time ranged from six to nine months. Three of the ten patients experienced early graft failure, another was withdrawn due to gastrointestinal bleeding, and one died of unrelated causes. The grafts in the other five patients functioned for 6-20 months, with a primary patency of 78% at one month and 60% at six months after implantation [
158].
A new method for creating TEVGs with an organized elastic fiber structure is vital because long-term use of vascular grafts is susceptible to graft stenosis, related to compliance mismatch between implanted grafts and native vessels. The new approach involves coating SMCs with fibronectin and gelatin and repeating the process to create a layered elastic fiber structure. In addition, mechanical stimulation, including hydrostatic pressure, is also discussed as an essential factor in TEVG development [
158,
159].
9.1. Clinical Translation of TEVGs in Heart Valve
Many research groups have attempted to create tissue-engineered heart valves [
160], but the best design remains to be determined. In addition, the timing of the transplantation is essential, as is the regenerative ability to replace it with a functional native valve over time. Heart valve dysfunction is another critical issue that increases as people age. Approximately eight million Americans are diagnosed with valve disease; at least 60,000 people will have either valve repair or replacement surgeries [
161]. Heart valves come in two types: mechanical and the other uses bio-prostheses for these procedures. Still, bio-prostheses valves have advantages and disadvantages, requiring replacement roughly every ten years. Currently, available mechanical or biological therapies are limited, which has increased interest in producing self-remodeling bio prostheses by seeding them with autologous valve endothelial or interstitial substitutes.
Scientists initially applied the tissue engineering concept to build heart valve tissue. First, the valve tissue donor’s cellular components are removed, leaving the extracellular matrix intact. Then the EPCs are obtained to coat the heart valve with these cells. Next, they place those cells into the bioreactor to train and adhere to produce a functional monolayer endothelial cell covering the valvular surface and leaflets. Without complications with infected cells, the seeding process or the bioreactor will take approximately six months [
162]
. As indicated, many resources, time, and work are required.
Therefore, they developed a way to shorten and simplify the process by conjugating the valve scaffold with a CD -133 antibody [
163]. Moreover, that antibody is a specific cell marker for EPCs. EPCs bind antibodies so that as the blood flows through the valve, antibodies capture those free-floating cells and eventually produce a self-seeding heart valve. When implanted into a sheep model [
132,
164,
165,
166] and compared to different methods, even at two weeks, they could see the endothelial cell progenitor cells covering the surface of the heart valve [
163]. Furthermore, the cellularity increased, and progenitor cells penetrated after a month and differentiated into fully matured ECs.
More importantly, biomechanically, the conjugated heart valve is like a standard valve with evidence of tissue remodeling over time. As indicated, conjugation is more accessible and consistent and takes less time than seeded valves. In addition, endothelial cell coverage is quicker and superior with a conjugated valve containing myofibroblast interstitial cells. Even though results are promising, this method of conjugating the valve scaffold with an antibody still needs to be completed, possibly since it is essential to produce the best quantity of autologous ECs and their growth. Additionally, confirming the longstanding adherence of ECs to valve surfaces is another inspiring mission.
10. Promising Clinical Outlook
In this review, we reported the current state of tissue engineering using scaffolds and cell-based approaches in the cardiovascular field. Even though all the disciplines are developing and improving rapidly, they face many challenges.
The field of generating TEVGs is constantly evolving with the introduction of new technologies such as iPSCs and 3D bioprinting. In addition, clinical trials are being conducted to advance this field further.
Future endeavors require innovative and improved methods for coupling biodegradation scaffolds with translational innovations to challenge the problematic remodeling of vascular grafts (
Figure 7). Promising results await with new material combinations that will degrade but can create native blood vessels properly.
On the other hand, electrospinning is currently the most used manufacturing method, offering standardization and industrialization of the TEVGs. Nonetheless, more studies are still required for manufacturing to reach an ideal fiber thickness and porosity for the grafts since cell migration can be affected while maintaining long-term graft mechanical properties.
Strategies such as decellularized blood vessels offer a suitable alternative for TEVGs since their mechanical properties are very similar to the native tissues and are successful in the regeneration process. However, standardization and industrialization are highly complex and require multiple commercial steps. Further advances in TEVG technology can result in favorable outcomes for patients with cardiovascular diseases.
Author Contributions
S.S. wrote —the original manuscript; T.W. —reviewed and edited; S.T.; supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- E.J. Benjamin, P. Muntner, A. Alonso, M.S. Bittencourt, C.W. Callaway, A.P. Carson, A.M. Chamberlain, A.R. Chang, S. Cheng, S.R. Das, F.N. Delling, L. Djousse, M.S.V. Elkind, J.F. Ferguson, M. Fornage, L.C. Jordan, S.S. Khan, B.M. Kissela, K.L. Knutson, T.W. Kwan, D.T. Lackland, T.T. Lewis, J.H. Lichtman, C.T. Longenecker, M.S. Loop, P.L. Lutsey, S.S. Martin, K. Matsushita, A.E. Moran, M.E. Mussolino, M. O'Flaherty, A. Pandey, A.M. Perak, W.D. Rosamond, G.A. Roth, U.K.A. Sampson, G.M. Satou, E.B. Schroeder, S.H. Shah, N.L. Spartano, A. Stokes, D.L. Tirschwell, C.W. Tsao, M.P. Turakhia, L.B. VanWagner, J.T. Wilkins, S.S. Wong, S.S. Virani, E. American Heart Association Council on, C. Prevention Statistics, S. Stroke Statistics, Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association, Circulation 139(10) (2019) e56-e528. [CrossRef]
- S.L. Dahl, J.L. Blum, L.E. Niklason, Bioengineered vascular grafts: can we make them off-the-shelf?, Trends Cardiovasc Med 21(3) (2011) 83-9. [CrossRef]
- C.D. Mathers, D. Loncar, Projections of global mortality and burden of disease from 2002 to 2030, PLoS Med 3(11) (2006) e442. [CrossRef]
- G.A. Mensah, G.A. Roth, V. Fuster, The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond, J Am Coll Cardiol 74(20) (2019) 2529-2532.
- G. Konig, T.N. McAllister, N. Dusserre, S.A. Garrido, C. Iyican, A. Marini, A. Fiorillo, H. Avila, W. Wystrychowski, K. Zagalski, M. Maruszewski, A.L. Jones, L. Cierpka, L.M. de la Fuente, N. L'Heureux, Mechanical properties of completely autologous human tissue engineered blood vessels compared to human saphenous vein and mammary artery, Biomaterials 30(8) (2009) 1542-50. [CrossRef]
- P.F. Sanchez, E.M. Brey, J.C. Briceno, Endothelialization mechanisms in vascular grafts, J Tissue Eng Regen Med 12(11) (2018) 2164-2178. [CrossRef]
- W. Jia, M. Li, H. Weng, G. Gu, Z. Chen, Design and comprehensive assessment of a biomimetic tri-layer tubular scaffold via biodegradable polymers for vascular tissue engineering applications, Mater Sci Eng C Mater Biol Appl 110 (2020) 110717. [CrossRef]
- X. Ren, Y. Feng, J. Guo, H. Wang, Q. Li, J. Yang, X. Hao, J. Lv, N. Ma, W. Li, Surface modification and endothelialization of biomaterials as potential scaffolds for vascular tissue engineering applications, Chem Soc Rev 44(15) (2015) 5680-742.
- M.W. Curtis, B. Russell, Cardiac tissue engineering, J Cardiovasc Nurs 24(2) (2009) 87-92.
- C.D. Devillard, C.A. Marquette, Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel, Front Bioeng Biotechnol 9 (2021) 721843. [CrossRef]
- J. Leor, Y. Amsalem, S. Cohen, Cells, scaffolds, and molecules for myocardial tissue engineering, Pharmacol Ther 105(2) (2005) 151-63. [CrossRef]
- E. Avolio, M. Caputo, P. Madeddu, Stem cell therapy and tissue engineering for correction of congenital heart disease, Front Cell Dev Biol 3 (2015) 39. [CrossRef]
- C. Davis, J. Fischer, K. Ley, I.J. Sarembock, The role of inflammation in vascular injury and repair, J Thromb Haemost 1(8) (2003) 1699-709. [CrossRef]
- M.K. Pugsley, R. Tabrizchi, The vascular system. An overview of structure and function, J Pharmacol Toxicol Methods 44(2) (2000) 333-40.
- Y. Naito, T. Shinoka, D. Duncan, N. Hibino, D. Solomon, M. Cleary, A. Rathore, C. Fein, S. Church, C. Breuer, Vascular tissue engineering: towards the next generation vascular grafts, Adv Drug Deliv Rev 63(4-5) (2011) 312-23. [CrossRef]
- A. García, M.V. Cabañas, J. Peña, S. Sánchez-Salcedo, Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials, Pharmaceutics 13(11) (2021). [CrossRef]
- K.A. Athanasiou, G.G. Niederauer, C.M. Agrawal, Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers, Biomaterials 17(2) (1996) 93-102.
- B. Mosadegh, G. Xiong, S. Dunham, J.K. Min, Current progress in 3D printing for cardiovascular tissue engineering, Biomed Mater 10(3) (2015) 034002. [CrossRef]
- A.V. Borovjagin, B.M. Ogle, J.L. Berry, J. Zhang, From Microscale Devices to 3D Printing: Advances in Fabrication of 3D Cardiovascular Tissues, Circ Res 120(1) (2017) 150-165.
- L. Xue, H.P. Greisler, Biomaterials in the development and future of vascular grafts, J Vasc Surg 37(2) (2003) 472-80. [CrossRef]
- H. Sun, L. Mei, C. Song, X. Cui, P. Wang, The in vivo degradation, absorption, and excretion of PCL-based implant, Biomaterials 27(9) (2006) 1735-40. [CrossRef]
- X. Yue, B. van der Lei, J.M. Schakenraad, G.H. van Oene, J.H. Kuit, J. Feijen, C.R. Wildevuur, Smooth muscle cell seeding in biodegradable grafts in rats: a new method to enhance the process of arterial wall regeneration, Surgery 103(2) (1988) 206-12.
- L. Soletti, Y. Hong, J. Guan, J.J. Stankus, M.S. El-Kurdi, W.R. Wagner, D.A. Vorp, A bilayered elastomeric scaffold for tissue engineering of small diameter vascular grafts, Acta Biomater 6(1) (2010) 110-22. [CrossRef]
- L. Soletti, A. Nieponice, J. Guan, J.J. Stankus, W.R. Wagner, D.A. Vorp, A seeding device for tissue engineered tubular structures, Biomaterials 27(28) (2006) 4863-70. [CrossRef]
- S.P. Hoerstrup, I. Cummings Mrcs, M. Lachat, F.J. Schoen, R. Jenni, S. Leschka, S. Neuenschwander, D. Schmidt, A. Mol, C. Gunter, M. Gossi, M. Genoni, G. Zund, Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model, Circulation 114(1 Suppl) (2006) I159-66.
- I. Cummings, S. George, J. Kelm, D. Schmidt, M.Y. Emmert, B. Weber, G. Zund, S.P. Hoerstrup, Tissue-engineered vascular graft remodeling in a growing lamb model: expression of matrix metalloproteinases, Eur J Cardiothorac Surg 41(1) (2012) 167-72.
- A. Hasan, A. Memic, N. Annabi, M. Hossain, A. Paul, M.R. Dokmeci, F. Dehghani, A. Khademhosseini, Electrospun scaffolds for tissue engineering of vascular grafts, Acta Biomater 10(1) (2014) 11-25. [CrossRef]
- S. Ramakrishna, K. Fujihara, W.-E. Teo, T. Yong, Z. Ma, R. Ramaseshan, Electrospun nanofibers: solving global issues, Materials Today 9(3) (2006) 40-50. [CrossRef]
- X. Wang, B. Ding, G. Sun, M. Wang, J. Yu, Electro-spinning/netting: A strategy for the fabrication of three-dimensional polymer nano-fiber/nets, Prog Mater Sci 58(8) (2013) 1173-1243. [CrossRef]
- H. Wu, J. Fan, C.C. Chu, J. Wu, Electrospinning of small diameter 3-D nanofibrous tubular scaffolds with controllable nanofiber orientations for vascular grafts, J Mater Sci Mater Med 21(12) (2010) 3207-15. [CrossRef]
- Y.M. Ju, J.S. Choi, A. Atala, J.J. Yoo, S.J. Lee, Bilayered scaffold for engineering cellularized blood vessels, Biomaterials 31(15) (2010) 4313-21. [CrossRef]
- X. Wang, B. Ding, B. Li, Biomimetic electrospun nanofibrous structures for tissue engineering, Mater Today (Kidlington) 16(6) (2013) 229-241. [CrossRef]
- T. Asakura, T. Tanaka, R. Tanaka, Advanced Silk Fibroin Biomaterials and Application to Small-Diameter Silk Vascular Grafts, ACS Biomater Sci Eng 5(11) (2019) 5561-5577. [CrossRef]
- A. Dhasmana, L. Singh, P. Roy, N.C. Mishra, Silk fibroin protein modified acellular dermal matrix for tissue repairing and regeneration, Mater Sci Eng C Mater Biol Appl 97 (2019) 313-324. [CrossRef]
- M.T. Poldervaart, H. Gremmels, K. van Deventer, J.O. Fledderus, F.C. Oner, M.C. Verhaar, W.J. Dhert, J. Alblas, Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture, J Control Release 184 (2014) 58-66. [CrossRef]
- L. Zentilin, S. Tafuro, S. Zacchigna, N. Arsic, L. Pattarini, M. Sinigaglia, M. Giacca, Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels, Blood 107(9) (2006) 3546-54. [CrossRef]
- K.A. Rocco, M.W. Maxfield, C.A. Best, E.W. Dean, C.K. Breuer, In vivo applications of electrospun tissue-engineered vascular grafts: a review, Tissue Eng Part B Rev 20(6) (2014) 628-40. [CrossRef]
- K. Kinoshita, M. Iwase, M. Yamada, Y. Yajima, M. Seki, Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms, Biotechnol J 11(11) (2016) 1415-1423. [CrossRef]
- G.H. Borschel, Y.C. Huang, S. Calve, E.M. Arruda, J.B. Lynch, D.E. Dow, W.M. Kuzon, R.G. Dennis, D.L. Brown, Tissue engineering of recellularized small-diameter vascular grafts, Tissue Eng 11(5-6) (2005) 778-86. [CrossRef]
- S. Pashneh-Tala, S. MacNeil, F. Claeyssens, The Tissue-Engineered Vascular Graft-Past, Present, and Future, Tissue Eng Part B Rev 22(1) (2016) 68-100.
- E.S. Chemla, M. Morsy, Randomized clinical trial comparing decellularized bovine ureter with expanded polytetrafluoroethylene for vascular access, Br J Surg 96(1) (2009) 34-9. [CrossRef]
- N. Rosenberg, A. Martinez, P.N. Sawyer, S.A. Wesolowski, R.W. Postlethwait, M.L. Dillon, Jr., Tanned collagen arterial prosthesis of bovine carotid origin in man. Preliminary studies of enzyme-treated heterografts, Ann Surg 164(2) (1966) 247-56. [CrossRef]
- G.E. Sandusky, G.C. Lantz, S.F. Badylak, Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery, J Surg Res 58(4) (1995) 415-20. [CrossRef]
- S.L.M. Dahl, J. Koh, V. Prabhakar, L.E. Niklason, Decellularized Native and Engineered Arterial Scaffolds for Transplantation, Cell Transplant 12(6) (2003) 659-666. [CrossRef]
- S.L. Dahl, A.P. Kypson, J.H. Lawson, J.L. Blum, J.T. Strader, Y. Li, R.J. Manson, W.E. Tente, L. DiBernardo, M.T. Hensley, R. Carter, T.P. Williams, H.L. Prichard, M.S. Dey, K.G. Begelman, L.E. Niklason, Readily available tissue-engineered vascular grafts, Sci Transl Med 3(68) (2011) 68ra9. [CrossRef]
- A. Daugs, B. Hutzler, M. Meinke, C. Schmitz, N. Lehmann, A. Markhoff, O. Bloch, Detergent-Based Decellularization of Bovine Carotid Arteries for Vascular Tissue Engineering, Ann Biomed Eng 45(11) (2017) 2683-2692. [CrossRef]
- K.M. Pawelec, S.M. Best, R.E. Cameron, Collagen: a network for regenerative medicine, J Mater Chem B 4(40) (2016) 6484-6496.
- C.H. Lee, A. Singla, Y. Lee, Biomedical applications of collagen, Int J Pharm 221(1-2) (2001) 1-22. [CrossRef]
- K. Jakab, C. Norotte, B. Damon, F. Marga, A. Neagu, C.L. Besch-Williford, A. Kachurin, K.H. Church, H. Park, V. Mironov, R. Markwald, G. Vunjak-Novakovic, G. Forgacs, Tissue engineering by self-assembly of cells printed into topologically defined structures, Tissue Eng Part A 14(3) (2008) 413-21. [CrossRef]
- L. Badimon, J.J. Badimon, V.T. Turitto, S. Vallabhajosula, V. Fuster, Platelet thrombus formation on collagen type I. A model of deep vessel injury. Influence of blood rheology, von Willebrand factor, and blood coagulation, Circulation 78(6) (1988) 1431-42. [CrossRef]
- T. Huynh, G. Abraham, J. Murray, K. Brockbank, P.O. Hagen, S. Sullivan, Remodeling of an acellular collagen graft into a physiologically responsive neovessel, Nat Biotechnol 17(11) (1999) 1083-6. [CrossRef]
- D. Radke, W. Jia, D. Sharma, K. Fena, G. Wang, J. Goldman, F. Zhao, Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development, Adv Healthc Mater 7(15) (2018) e1701461. [CrossRef]
- H. Yanagisawa, J. Wagenseil, Elastic fibers and biomechanics of the aorta: Insights from mouse studies, Matrix Biol 85-86 (2020) 160-172. [CrossRef]
- A. Waterhouse, S.G. Wise, M.K. Ng, A.S. Weiss, Elastin as a nonthrombogenic biomaterial, Tissue Eng Part B Rev 17(2) (2011) 93-9. [CrossRef]
- A.H. Huang, J.L. Balestrini, B.V. Udelsman, K.C. Zhou, L. Zhao, J. Ferruzzi, B.C. Starcher, M.J. Levene, J.D. Humphrey, L.E. Niklason, Biaxial Stretch Improves Elastic Fiber Maturation, Collagen Arrangement, and Mechanical Properties in Engineered Arteries, Tissue Eng Part C Methods 22(6) (2016) 524-33. [CrossRef]
- M.E. Curran, D.L. Atkinson, A.K. Ewart, C.A. Morris, M.F. Leppert, M.T. Keating, The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis, Cell 73(1) (1993) 159-68. [CrossRef]
- S. Laurent, P. Boutouyrie, P. Lacolley, Structural and genetic bases of arterial stiffness, Hypertension 45(6) (2005) 1050-5. [CrossRef]
- W.F. Daamen, J.H. Veerkamp, J.C. van Hest, T.H. van Kuppevelt, Elastin as a biomaterial for tissue engineering, Biomaterials 28(30) (2007) 4378-98.
- A.H.P. Chan, E.C. Filipe, R.P. Tan, M. Santos, N. Yang, J. Hung, J. Feng, S. Nazir, A.J. Benn, M.K.C. Ng, J. Rnjak-Kovacina, S.G. Wise, Altered processing enhances the efficacy of small-diameter silk fibroin vascular grafts, Sci Rep 9(1) (2019) 17461. [CrossRef]
- G.H. Altman, F. Diaz, C. Jakuba, T. Calabro, R.L. Horan, J. Chen, H. Lu, J. Richmond, D.L. Kaplan, Silk-based biomaterials, Biomaterials 24(3) (2003) 401-416.
- D. Aytemiz, W. Sakiyama, Y. Suzuki, N. Nakaizumi, R. Tanaka, Y. Ogawa, Y. Takagi, Y. Nakazawa, T. Asakura, Small-diameter silk vascular grafts (3 mm diameter) with a double-raschel knitted silk tube coated with silk fibroin sponge, Adv Healthc Mater 2(2) (2013) 361-8. [CrossRef]
- H. Zhang, S.H. Neau, In vitro degradation of chitosan by a commercial enzyme preparation: effect of molecular weight and degree of deacetylation, Biomaterials 22(12) (2001) 1653-8. [CrossRef]
- Y.M. Yang, W. Hu, X.D. Wang, X.S. Gu, The controlling biodegradation of chitosan fibers by N-acetylation in vitro and in vivo, J Mater Sci Mater Med 18(11) (2007) 2117-21. [CrossRef]
- T. Fukunishi, C.A. Best, T. Sugiura, T. Shoji, T. Yi, B. Udelsman, D. Ohst, C.S. Ong, H. Zhang, T. Shinoka, C.K. Breuer, J. Johnson, N. Hibino, Tissue-Engineered Small Diameter Arterial Vascular Grafts from Cell-Free Nanofiber PCL/Chitosan Scaffolds in a Sheep Model, PLoS One 11(7) (2016) e0158555. [CrossRef]
- A.M. Cardoso, E.G. de Oliveira, K. Coradini, F.A. Bruinsmann, T. Aguirre, R. Lorenzoni, R.C.S. Barcelos, K. Roversi, D.R. Rossato, A.R. Pohlmann, S.S. Guterres, M.E. Burger, R.C.R. Beck, Chitosan hydrogels containing nanoencapsulated phenytoin for cutaneous use: Skin permeation/penetration and efficacy in wound healing, Mater Sci Eng C Mater Biol Appl 96 (2019) 205-217. [CrossRef]
- W. Huang, Y. Wang, Z. Huang, X. Wang, L. Chen, Y. Zhang, L. Zhang, On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing, ACS Appl Mater Interfaces 10(48) (2018) 41076-41088. [CrossRef]
- M. Tallawi, E. Rosellini, N. Barbani, M.G. Cascone, R. Rai, G. Saint-Pierre, A.R. Boccaccini, Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: a review, J R Soc Interface 12(108) (2015) 20150254. [CrossRef]
- A.H. Nguyen, P. Marsh, L. Schmiess-Heine, P.J. Burke, A. Lee, J. Lee, H. Cao, Cardiac tissue engineering: state-of-the-art methods and outlook, J Biol Eng 13 (2019) 57. [CrossRef]
- Y.-K.A. Wu, J. Yu, The role of tissue engineering in cellular therapies for myocardial infarction: a review, Journal of Materials Chemistry B 3(31) (2015) 6401-6410. [CrossRef]
- I.Y. Galaev, B. Mattiasson, 'Smart' polymers and what they could do in biotechnology and medicine, Trends Biotechnol 17(8) (1999) 335-40.
- M.M. Al-Rooqi, M.M. Hassan, Z. Moussa, R.J. Obaid, N.H. Suman, M.H. Wagner, S.S.A. Natto, S.A. Ahmed, Advancement of chitin and chitosan as promising biomaterials, Journal of Saudi Chemical Society 26(6) (2022) 101561. [CrossRef]
- H. Shin, S. Jo, A.G. Mikos, Biomimetic materials for tissue engineering, Biomaterials 24(24) (2003) 4353-64. [CrossRef]
- U. Hersel, C. Dahmen, H. Kessler, RGD modified polymers: biomaterials for stimulated cell adhesion and beyond, Biomaterials 24(24) (2003) 4385-415. [CrossRef]
- J.M. Singelyn, J.A. DeQuach, S.B. Seif-Naraghi, R.B. Littlefield, P.J. Schup-Magoffin, K.L. Christman, Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering, Biomaterials 30(29) (2009) 5409-16. [CrossRef]
- R.K. Birla, G.H. Borschel, R.G. Dennis, D.L. Brown, Myocardial engineering in vivo: formation and characterization of contractile, vascularized three-dimensional cardiac tissue, Tissue Eng 11(5-6) (2005) 803-13.
- T. Wang, X.J. Jiang, Q.Z. Tang, X.Y. Li, T. Lin, D.Q. Wu, X.Z. Zhang, E. Okello, Bone marrow stem cells implantation with alpha-cyclodextrin/MPEG-PCL-MPEG hydrogel improves cardiac function after myocardial infarction, Acta Biomater 5(8) (2009) 2939-44. [CrossRef]
- S. Thussu, M.A. Fiala, Walker et al.: Diversity in Clinical Trials, J Gen Intern Med 38(5) (2023) 1314-1315.
- A.S. Walker, M.A. Blue, T.A. Brandon, J. Emmanual, E.J. Guilbeau, Performance of a hydrogel composite pericardial substitute after long-term implant studies, ASAIO J 38(3) (1992) M550-4. [CrossRef]
- C. Macedo, E.A. Orkis, I. Popescu, B.D. Elinoff, A. Zeevi, R. Shapiro, F.G. Lakkis, D. Metes, Contribution of naive and memory T-cell populations to the human alloimmune response, Am J Transplant 9(9) (2009) 2057-66. [CrossRef]
- S. Sundaram, J. One, J. Siewert, S. Teodosescu, L. Zhao, S. Dimitrievska, H. Qian, A.H. Huang, L. Niklason, Tissue-engineered vascular grafts created from human induced pluripotent stem cells, Stem Cells Transl Med 3(12) (2014) 1535-43. [CrossRef]
- N. Alobaid, M.E. Alnaeb, K.M. Sales, A.M. Seifalian, D.P. Mikhailidis, G. Hamilton, Endothelial progenitor cells and their potential clinical applications in peripheral arterial disease, Endothelium 12(5-6) (2005) 243-50. [CrossRef]
- Y. Fujita, A. Kawamoto, Stem cell-based peripheral vascular regeneration, Adv Drug Deliv Rev 120 (2017) 25-40. [CrossRef]
- P.H. Stone, P. Libby, W.E. Boden, Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review, JAMA Cardiol 8(2) (2023) 192-201.
- J.M. Melero-Martin, Z.A. Khan, A. Picard, X. Wu, S. Paruchuri, J. Bischoff, In vivo vasculogenic potential of human blood-derived endothelial progenitor cells, Blood 109(11) (2007) 4761-8. [CrossRef]
- S. Kale, J. Hanai, B. Chan, A. Karihaloo, G. Grotendorst, L. Cantley, V.P. Sukhatme, Microarray analysis of in vitro pericyte differentiation reveals an angiogenic program of gene expression, FASEB J 19(2) (2005) 270-1. [CrossRef]
- A. Palladino, I. Mavaro, C. Pizzoleo, E. De Felice, C. Lucini, P. de Girolamo, P.A. Netti, C. Attanasio, Induced Pluripotent Stem Cells as Vasculature Forming Entities, J Clin Med 8(11) (2019). [CrossRef]
- J. Luo, L. Qin, L. Zhao, L. Gui, M.W. Ellis, Y. Huang, M.H. Kural, J.A. Clark, S. Ono, J. Wang, Y. Yuan, S.M. Zhang, X. Cong, G. Li, M. Riaz, C. Lopez, A. Hotta, S. Campbell, G. Tellides, A. Dardik, L.E. Niklason, Y. Qyang, Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs, Cell Stem Cell 26(2) (2020) 251-261 e8. [CrossRef]
- J. Zhao, L. Liu, J. Wei, D. Ma, W. Geng, X. Yan, J. Zhu, H. Du, Y. Liu, L. Li, F. Chen, A novel strategy to engineer small-diameter vascular grafts from marrow-derived mesenchymal stem cells, Artif Organs 36(1) (2012) 93-101. [CrossRef]
- J.R. Bezenah, Y.P. Kong, A.J. Putnam, Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures, Sci Rep 8(1) (2018) 2671. [CrossRef]
- D. Klein, iPSCs-based generation of vascular cells: reprogramming approaches and applications, Cell Mol Life Sci 75(8) (2018) 1411-1433.
- F. Zhang, F. Citra, D.A. Wang, Prospects of induced pluripotent stem cell technology in regenerative medicine, Tissue Eng Part B Rev 17(2) (2011) 115-24. [CrossRef]
- T. Asahara, A. Kawamoto, H. Masuda, Concise review: Circulating endothelial progenitor cells for vascular medicine, Stem Cells 29(11) (2011) 1650-5. [CrossRef]
- D.T. Harris, I. Rogers, Umbilical cord blood: a unique source of pluripotent stem cells for regenerative medicine, Curr Stem Cell Res Ther 2(4) (2007) 301-9. [CrossRef]
- M. Peichev, A.J. Naiyer, D. Pereira, Z. Zhu, W.J. Lane, M. Williams, M.C. Oz, D.J. Hicklin, L. Witte, M.A. Moore, S. Rafii, Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors, Blood 95(3) (2000) 952-8. [CrossRef]
- T. Asahara, T. Murohara, A. Sullivan, M. Silver, R. van der Zee, T. Li, B. Witzenbichler, G. Schatteman, J.M. Isner, Isolation of putative progenitor endothelial cells for angiogenesis, Science 275(5302) (1997) 964-7. [CrossRef]
- M. Abedin, Y. Tintut, L.L. Demer, Mesenchymal stem cells and the artery wall, Circ Res 95(7) (2004) 671-6. [CrossRef]
- S.P. Bruder, N. Jaiswal, S.E. Haynesworth, Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation, J Cell Biochem 64(2) (1997) 278-94. [CrossRef]
- S. Aggarwal, M.F. Pittenger, Human mesenchymal stem cells modulate allogeneic immune cell responses, Blood 105(4) (2005) 1815-22. [CrossRef]
- Z. Gong, L.E. Niklason, Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs), FASEB J 22(6) (2008) 1635-48. [CrossRef]
- C. Han, X. Luo, D. Zou, J. Li, K. Zhang, P. Yang, N. Huang, Nature-inspired extracellular matrix coating produced by micro-patterned smooth muscle and endothelial cells endows cardiovascular materials with better biocompatibility, Biomater Sci 7(7) (2019) 2686-2701. [CrossRef]
- C. Brown, C. McKee, S. Bakshi, K. Walker, E. Hakman, S. Halassy, D. Svinarich, R. Dodds, C.K. Govind, G.R. Chaudhry, Mesenchymal stem cells: Cell therapy and regeneration potential, J Tissue Eng Regen Med 13(9) (2019) 1738-1755. [CrossRef]
- A.J. Friedenstein, J.F. Gorskaja, N.N. Kulagina, Fibroblast precursors in normal and irradiated mouse hematopoietic organs, Exp Hematol 4(5) (1976) 267-74.
- P.K. Raghav, Z. Mann, S. Ahlawat, S. Mohanty, Mesenchymal stem cell-based nanoparticles and scaffolds in regenerative medicine, Eur J Pharmacol 918 (2022) 174657. ttps://doi.org/10.1016/j.ejphar.2021.174657.
- T. Kinnaird, E. Stabile, M.S. Burnett, C.W. Lee, S. Barr, S. Fuchs, S.E. Epstein, Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms, Circ Res 94(5) (2004) 678-85. [CrossRef]
- J. Yang, M. Ii, N. Kamei, C. Alev, S.M. Kwon, A. Kawamoto, H. Akimaru, H. Masuda, Y. Sawa, T. Asahara, CD34+ cells represent highly functional endothelial progenitor cells in murine bone marrow, PLoS One 6(5) (2011) e20219. [CrossRef]
- Z. Yang, F. Cao, H. Li, S. He, T. Zhao, H. Deng, J. Li, Z. Sun, C. Hao, J. Xu, Q. Guo, S. Liu, W. Guo, Microenvironmentally optimized 3D-printed TGFbeta-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep, Acta Biomater 150 (2022) 181-198.
- S.T. Ji, H. Kim, J. Yun, J.S. Chung, S.M. Kwon, Promising Therapeutic Strategies for Mesenchymal Stem Cell-Based Cardiovascular Regeneration: From Cell Priming to Tissue Engineering, Stem Cells Int 2017 (2017) 3945403. [CrossRef]
- P. Silva Couto, M.C. Rotondi, A. Bersenev, C.J. Hewitt, A.W. Nienow, F. Verter, Q.A. Rafiq, Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds, Biotechnol Adv 45 (2020) 107636. [CrossRef]
- S.P. Higgins, A.K. Solan, L.E. Niklason, Effects of polyglycolic acid on porcine smooth muscle cell growth and differentiation, J Biomed Mater Res A 67(1) (2003) 295-302. [CrossRef]
- N. L'Heureux, S. Paquet, R. Labbe, L. Germain, F.A. Auger, A completely biological tissue-engineered human blood vessel, FASEB J 12(1) (1998) 47-56.
- R.W. Grauss, M.G. Hazekamp, F. Oppenhuizen, C.J. van Munsteren, A.C. Gittenberger-de Groot, M.C. DeRuiter, Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods, Eur J Cardiothorac Surg 27(4) (2005) 566-71. [CrossRef]
- N. L'Heureux, N. Dusserre, G. Konig, B. Victor, P. Keire, T.N. Wight, N.A. Chronos, A.E. Kyles, C.R. Gregory, G. Hoyt, R.C. Robbins, T.N. McAllister, Human tissue-engineered blood vessels for adult arterial revascularization, Nat Med 12(3) (2006) 361-5. [CrossRef]
- D.G. Seifu, A. Purnama, K. Mequanint, D. Mantovani, Small-diameter vascular tissue engineering, Nat Rev Cardiol 10(7) (2013) 410-21. [CrossRef]
- N. L'Heureux, N. Dusserre, A. Marini, S. Garrido, L. de la Fuente, T. McAllister, Technology insight: the evolution of tissue-engineered vascular grafts--from research to clinical practice, Nat Clin Pract Cardiovasc Med 4(7) (2007) 389-95. [CrossRef]
- M. Yamato, T. Okano, Cell sheet engineering, Materials Today 7(5) (2004) 42-47.
- H.J. Choi, J.J. Lee, Y.J. Park, J.W. Shin, H.J. Sung, J.W. Shin, Y. Wu, J.K. Kim, MG-63 osteoblast-like cell proliferation on auxetic PLGA scaffold with mechanical stimulation for bone tissue regeneration, Biomater Res 20 (2016) 33. [CrossRef]
- J. Ko, N.K. Mohtaram, F. Ahmed, A. Montgomery, M. Carlson, P.C. Lee, S.M. Willerth, M.B. Jun, Fabrication of poly (ϵ-caprolactone) microfiber scaffolds with varying topography and mechanical properties for stem cell-based tissue engineering applications, J Biomater Sci Polym Ed 25(1) (2014) 1-17. [CrossRef]
- G. Wei, P.X. Ma, Partially nanofibrous architecture of 3D tissue engineering scaffolds, Biomaterials 30(32) (2009) 6426-34. [CrossRef]
- D. Xu, S. Chen, C. Xie, Q. Liang, X. Xiao, Cryogenic 3D printing of modified polylactic acid scaffolds with biomimetic nanofibrous architecture for bone tissue engineering, J Biomater Sci Polym Ed 33(4) (2022) 532-549. [CrossRef]
- J.A. Hubbell, Bioactive biomaterials, Curr Opin Biotechnol 10(2) (1999) 123-9.
- C. Norotte, F.S. Marga, L.E. Niklason, G. Forgacs, Scaffold-free vascular tissue engineering using bioprinting, Biomaterials 30(30) (2009) 5910-7. [CrossRef]
- H. Mertsching, J. Hansmann, Bioreactor technology in cardiovascular tissue engineering, Adv Biochem Eng Biotechnol 112 (2009) 29-37.
- M. Alonzo, S. AnilKumar, B. Roman, N. Tasnim, B. Joddar, 3D Bioprinting of cardiac tissue and cardiac stem cell therapy, Transl Res 211 (2019) 64-83. [CrossRef]
- J. Jang, J.Y. Park, G. Gao, D.W. Cho, Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics, Biomaterials 156 (2018) 88-106. [CrossRef]
- J. Kim, J.S. Kong, W. Han, B.S. Kim, D.W. Cho, 3D Cell Printing of Tissue/Organ-Mimicking Constructs for Therapeutic and Drug Testing Applications, Int J Mol Sci 21(20) (2020). [CrossRef]
- H.W. Kang, S.J. Lee, I.K. Ko, C. Kengla, J.J. Yoo, A. Atala, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity, Nat Biotechnol 34(3) (2016) 312-9. [CrossRef]
- J. Zhang, E. Wehrle, M. Rubert, R. Muller, 3D Bioprinting of Human Tissues: Biofabrication, Bioinks, and Bioreactors, Int J Mol Sci 22(8) (2021).
- Q. Gao, B.S. Kim, G. Gao, Advanced Strategies for 3D Bioprinting of Tissue and Organ Analogs Using Alginate Hydrogel Bioinks, Mar Drugs 19(12) (2021). [CrossRef]
- M. Itoh, K. Nakayama, R. Noguchi, K. Kamohara, K. Furukawa, K. Uchihashi, S. Toda, J. Oyama, K. Node, S. Morita, Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae, PLoS One 10(9) (2015) e0136681.
- D.L. Coutu, A.M. Yousefi, J. Galipeau, Three-dimensional porous scaffolds at the crossroads of tissue engineering and cell-based gene therapy, J Cell Biochem 108(3) (2009) 537-46. [CrossRef]
- S.G. Chen, F. Ugwu, W.C. Li, N.M. Caplice, E. Petcu, S.P. Yip, C.L. Huang, Vascular Tissue Engineering: Advanced Techniques and Gene Editing in Stem Cells for Graft Generation, Tissue Eng Part B Rev 27(1) (2021) 14-28. [CrossRef]
- M. Ahmed, G. Hamilton, A.M. Seifalian, The performance of a small-calibre graft for vascular reconstructions in a senescent sheep model, Biomaterials 35(33) (2014) 9033-40. [CrossRef]
- S.H. Bhang, S. Lee, J.Y. Shin, T.J. Lee, B.S. Kim, Transplantation of cord blood mesenchymal stem cells as spheroids enhances vascularization, Tissue Eng Part A 18(19-20) (2012) 2138-47. [CrossRef]
- L. Buttafoco, P. Engbers-Buijtenhuijs, A.A. Poot, P.J. Dijkstra, I. Vermes, J. Feijen, Physical characterization of vascular grafts cultured in a bioreactor, Biomaterials 27(11) (2006) 2380-9. [CrossRef]
- M.C. Galmiche, V.E. Koteliansky, J. Briere, P. Herve, P. Charbord, Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway, Blood 82(1) (1993) 66-76. [CrossRef]
- J.W. Shin, D.W. Lee, M.J. Kim, K.S. Song, H.S. Kim, H.O. Kim, Isolation of endothelial progenitor cells from cord blood and induction of differentiation by ex vivo expansion, Yonsei Med J 46(2) (2005) 260-7. [CrossRef]
- N. Plunkett, F.J. O'Brien, Bioreactors in tissue engineering, Technol Health Care 19(1) (2011) 55-69.
- L.E. Niklason, J. Gao, W.M. Abbott, K.K. Hirschi, S. Houser, R. Marini, R. Langer, Functional arteries grown in vitro, Science 284(5413) (1999) 489-93. [CrossRef]
- D. Shum-Tim, U. Stock, J. Hrkach, T. Shinoka, J. Lien, M.A. Moses, A. Stamp, G. Taylor, A.M. Moran, W. Landis, R. Langer, J.P. Vacanti, J.E. Mayer, Jr., Tissue engineering of autologous aorta using a new biodegradable polymer, Ann Thorac Surg 68(6) (1999) 2298-304; discussion 2305. [CrossRef]
- G. De Visscher, L. Mesure, B. Meuris, A. Ivanova, W. Flameng, Improved endothelialization and reduced thrombosis by coating a synthetic vascular graft with fibronectin and stem cell homing factor SDF-1alpha, Acta Biomater 8(3) (2012) 1330-8. [CrossRef]
- W. He, A. Nieponice, L. Soletti, Y. Hong, B. Gharaibeh, M. Crisan, A. Usas, B. Peault, J. Huard, W.R. Wagner, D.A. Vorp, Pericyte-based human tissue engineered vascular grafts, Biomaterials 31(32) (2010) 8235-44.
- B.W. Tillman, S.K. Yazdani, R.L. Geary, M.A. Corriere, A. Atala, J.J. Yoo, Efficient recovery of endothelial progenitors for clinical translation, Tissue Eng Part C Methods 15(2) (2009) 213-21. [CrossRef]
- R. Raina, J. Wang, A. Sharma, R. Chakraborty, Extracorporeal Therapies in the Treatment of Focal Segmental Glomerulosclerosis, Blood Purif 49(5) (2020) 513-523. [CrossRef]
- F. Li, N. Vijayasankaran, A.Y. Shen, R. Kiss, A. Amanullah, Cell culture processes for monoclonal antibody production, MAbs 2(5) (2010) 466-79.
- K. Hsia, C.L. Yao, W.M. Chen, J.H. Chen, H. Lee, J.H. Lu, Scaffolds and Cell-Based Tissue Engineering for Blood Vessel Therapy, Cells Tissues Organs 202(5-6) (2016) 281-295. [CrossRef]
- J.P. Cuenca, H.J. Kang, M.A.A. Fahad, M. Park, M. Choi, H.Y. Lee, B.T. Lee, Physico-mechanical and biological evaluation of heparin/VEGF-loaded electrospun polycaprolactone/decellularized rat aorta extracellular matrix for small-diameter vascular grafts, J Biomater Sci Polym Ed 33(13) (2022) 1664-1684. [CrossRef]
- C. Devine, B. Hons, C. McCollum, Heparin-bonded Dacron or polytetrafluoroethylene for femoropopliteal bypass grafting: a multicenter trial, J Vasc Surg 33(3) (2001) 533-9. [CrossRef]
- K.R. Tardalkar, T.B. Marsale, N.C. Bhamare, J.R. Kshersagar, J.K. Patil, A. Adnaik, M.G. Joshi, Heparin coated decellularized xenogeneic small diameter vascular conduit for vascular repair with early luminal reendothelialization, Cell Tissue Bank (2022). [CrossRef]
- M. Peck, D. Gebhart, N. Dusserre, T.N. McAllister, N. L'Heureux, The evolution of vascular tissue engineering and current state of the art, Cells Tissues Organs 195(1-2) (2012) 144-58. [CrossRef]
- A.V. Sterpetti, W.J. Hunter, R.D. Schultz, C. Farina, Healing of high-porosity polytetrafluoroethylene arterial grafts is influenced by the nature of the surrounding tissue, Surgery 111(6) (1992) 677-82.
- S. Row, H. Peng, E.M. Schlaich, C. Koenigsknecht, S.T. Andreadis, D.D. Swartz, Arterial grafts exhibiting unprecedented cellular infiltration and remodeling in vivo: the role of cells in the vascular wall, Biomaterials 50 (2015) 115-26. [CrossRef]
- M.T. Koobatian, S. Row, R.J. Smith, Jr., C. Koenigsknecht, S.T. Andreadis, D.D. Swartz, Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model, Biomaterials 76 (2016) 344-58. [CrossRef]
- W. Wystrychowski, L. Cierpka, K. Zagalski, S. Garrido, N. Dusserre, S. Radochonski, T.N. McAllister, N. L'Heureux, Case study: first implantation of a frozen, devitalized tissue-engineered vascular graft for urgent hemodialysis access, J Vasc Access 12(1) (2011) 67-70. [CrossRef]
- T. Shin'oka, G. Matsumura, N. Hibino, Y. Naito, M. Watanabe, T. Konuma, T. Sakamoto, M. Nagatsu, H. Kurosawa, Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells, J Thorac Cardiovasc Surg 129(6) (2005) 1330-8. [CrossRef]
- T. Sugiura, G. Matsumura, S. Miyamoto, H. Miyachi, C.K. Breuer, T. Shinoka, Tissue-engineered Vascular Grafts in Children With Congenital Heart Disease: Intermediate Term Follow-up, Semin Thorac Cardiovasc Surg 30(2) (2018) 175-179. [CrossRef]
- H.E. Katzman, M.H. Glickman, A.F. Schild, R.M. Fujitani, J.H. Lawson, Multicenter evaluation of the bovine mesenteric vein bioprostheses for hemodialysis access in patients with an earlier failed prosthetic graft, J Am Coll Surg 201(2) (2005) 223-30. [CrossRef]
- T.N. McAllister, M. Maruszewski, S.A. Garrido, W. Wystrychowski, N. Dusserre, A. Marini, K. Zagalski, A. Fiorillo, H. Avila, X. Manglano, J. Antonelli, A. Kocher, M. Zembala, L. Cierpka, L.M. de la Fuente, N. L'Heureux, Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study, Lancet 373(9673) (2009) 1440-6. [CrossRef]
- N. L'Heureux, T.N. McAllister, L.M. de la Fuente, Tissue-engineered blood vessel for adult arterial revascularization, N Engl J Med 357(14) (2007) 1451-3. [CrossRef]
- M. Matsusaki, K. Kadowaki, E. Adachi, T. Sakura, U. Yokoyama, Y. Ishikawa, M. Akashi, Morphological and histological evaluations of 3D-layered blood vessel constructs prepared by hierarchical cell manipulation, J Biomater Sci Polym Ed 23(1-4) (2012) 63-79. [CrossRef]
- H.M. Nugent, E.R. Edelman, Tissue engineering therapy for cardiovascular disease, Circ Res 92(10) (2003) 1068-78. [CrossRef]
- A. Vijayan, Tackling AKI: prevention, timing of dialysis and follow-up, Nat Rev Nephrol 17(2) (2021) 87-88.
- E.R. Ochoa, J.P. Vacanti, An overview of the pathology and approaches to tissue engineering, Ann N Y Acad Sci 979 (2002) 10-26; discussion 35-8.
- J.D. Vossler, Y. Min Ju, J.K. Williams, S. Goldstein, J. Hamlin, S.J. Lee, J.J. Yoo, A. Atala, CD133 antibody conjugation to decellularized human heart valves intended for circulating cell capture, Biomed Mater 10(5) (2015) 055001. [CrossRef]
- T. Shinoka, C.K. Breuer, R.E. Tanel, G. Zund, T. Miura, P.X. Ma, R. Langer, J.P. Vacanti, J.E. Mayer, Jr., Tissue engineering heart valves: valve leaflet replacement study in a lamb model, Ann Thorac Surg 60(6 Suppl) (1995) S513-6.
- T. Shinoka, P.X. Ma, D. Shum-Tim, C.K. Breuer, R.A. Cusick, G. Zund, R. Langer, J.P. Vacanti, J.E. Mayer, Jr., Tissue-engineered heart valves. Autologous valve leaflet replacement study in a lamb model, Circulation 94(9 Suppl) (1996) II164-8.
- G. Zund, C.K. Breuer, T. Shinoka, P.X. Ma, R. Langer, J.E. Mayer, J.P. Vacanti, The in vitro construction of a tissue engineered bioprosthetic heart valve, Eur J Cardiothorac Surg 11(3) (1997) 493-7.
- W. Tan, P. Boodagh, P.P. Selvakumar, S. Keyser, Strategies to counteract adverse remodeling of vascular graft: A 3D view of current graft innovations, Front Bioeng Biotechnol 10 (2022) 1097334. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).