Submitted:
08 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathological Features Of Neurodegenerative Diseases
3. Gut microbiome alterations in neurodegenerative diseases
4. Microbiota-gut-brain axis
5. Linking gut microbiome dysbiosis and neurodegenerative diseases
5.1. Gut microbiome interacts with hosts Subsection
5.2. Gut microbiome-mitochondria connection
5.3. Defective autophagy
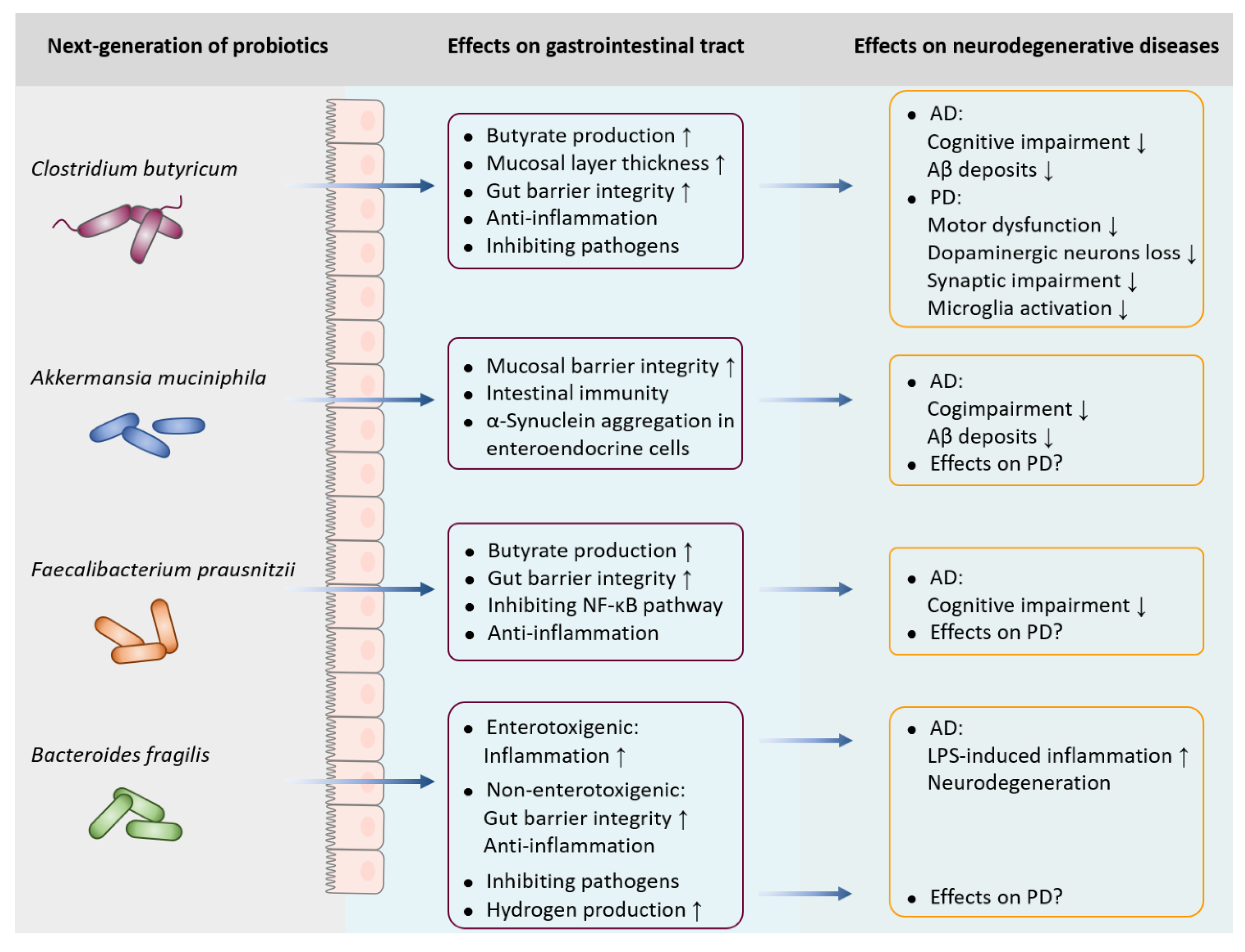
6. Next-generation of probiotics in neurodegenerative diseases
6.1. Clostridium butyricum
6.2. Akkermansia muciniphila
6.3. Faecalibacterium prausnitzii
6.4. Bacteroides fragilis
7. Perspectives and conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Bloem, B.R.; Okun, M.S. , Klein, C., Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chetelat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A. , Jones, D.T., Alzheimer disease. Nat Rev Dis Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Antonioli, L.; Colucci, R.; Blandizzi, C. , Fornai, M., Interplay among gut microbiota, intestinal mucosal barrier and enteric neuro-immune system: a common path to neurodegenerative diseases? Acta Neuropathol 2018, 136, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U. and Kayed, R., Amyloid beta, Tau, and alpha-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog Neurobiol 2022, 214, 102270. [Google Scholar] [CrossRef]
- Anis, E.; Xie, A.; Brundin, L. , Brundin, P., Digesting recent findings: gut alpha-synuclein, microbiome changes in Parkinson’s disease. Trends Endocrinol Metab 2022, 33, 147–157. [Google Scholar] [CrossRef]
- Travagli, R.A.; Browning, K.N. , Camilleri, M., Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 2020, 17, 673–685. [Google Scholar] [CrossRef]
- Jeremic, D.; Jimenez-Diaz, L. , Navarro-Lopez, J.D., Past, present and future of therapeutic strategies against amyloid-beta peptides in Alzheimer’s disease: a systematic review. Ageing Res Rev 2021, 72, 101496. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Bjorklund, T.; Wang, Z.Y.; Roybon, L.; Melki, R. , Li, J.Y., Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 2014, 128, 805–20. [Google Scholar] [CrossRef]
- Ahn, E.H.; Kang, S.S.; Liu, X.; Chen, G.; Zhang, Z.; Chandrasekharan, B.; Alam, A.M.; Neish, A.S.; Cao, X. , Ye, K., Initiation of Parkinson’s disease from gut to brain by delta-secretase. Cell Res 2020, 30, 70–87. [Google Scholar] [CrossRef]
- Jones, L.; Kumar, J.; Mistry, A.; Sankar Chittoor Mana, T.; Perry, G.; Reddy, V.P. , Obrenovich, M., The Transformative Possibilities of the Microbiota and Mycobiota for Health, Disease, Aging, and Technological Innovation. Biomedicines 2019, 7. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P. , Scheperjans, F., Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, X.; Xu, S.; Huang, P.; Li, B.; Du, J.; He, Y.; Su, B.; Xu, L.M.; Wang, L. , et al., Gut metagenomics-derived genes as potential biomarkers of Parkinson’s disease. Brain 2020, 143, 2474–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lu, Y.; Cai, M.; Zhu, C.; Tan, Y.L. , et al., Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J Alzheimers Dis 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R. , Wu, S., Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients With Alzheimer’s Disease. Front Cell Dev Biol 2020, 8, 634069. [Google Scholar]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H. , Chen, S., Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimers Dement 2019, 15, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K. , et al., Gut microbiome alterations in Alzheimer’s disease. Sci Rep 2017, 7, 13537. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K. , et al., Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L. , Nie, K., Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front Neurol 2020, 11, 137. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Hu, X.; Wang, T.; Liang, S.; Duan, Y.; Jin, F. , Qin, B., Structural changes of gut microbiota in Parkinson’s disease and its correlation with clinical features. Sci China Life Sci 2017, 60, 1223–1233. [Google Scholar] [CrossRef]
- Li, F.; Wang, P.; Chen, Z.; Sui, X.; Xie, X. , Zhang, J., Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci Lett 2019, 707, 134297. [Google Scholar] [CrossRef]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S. , et al., Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull Exp Biol Med 2017, 162, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A. , Schafer, K.H., Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 2016, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, F.; Kunstner, A.; Muller, S.H.; Kunzel, S.; Zeuner, K.E.; Margraf, N.G.; Deuschl, G.; Baines, J.F. , Kuhlenbaumer, G., Gut microbiota in Parkinson disease in a northern German cohort. Brain Res 2017, 1667, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T.; Wissemann, W.T.; Lewis, M.R.; Wallen, Z.D.; Peddada, S.D.; Factor, S.A.; Molho, E.; Zabetian, C.P. , et al., Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Zheng, W.; He, Y.; Tang, W.; Wei, X.; He, R.; Huang, W.; Su, Y.; Huang, Y.; Zhou, H. , et al., Gut microbiota in patients with Parkinson’s disease in southern China. Parkinsonism Relat Disord 2018, 53, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Yang, X.; Xu, S.; Wu, C.; Song, Y.; Qin, N.; Chen, S.D. , Xiao, Q., Alteration of the fecal microbiota in Chinese patients with Parkinson’s disease. Brain Behav Immun 2018, 70, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Martinez, G.; Chin, B.; Camarillo, C.; Herrera, G.V.; Yang, B.; Sarosiek, I. , Perez, R.G., A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J Parkinsons Dis 2020, 10, 185–192. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E. , Shannon, K.M., Colonic bacterial composition in Parkinson’s disease. Mov Disord 2015, 30, 1351–60. [Google Scholar] [CrossRef]
- Needham, B.D.; Kaddurah-Daouk, R. , Mazmanian, S.K., Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Sgritta, M.; Dooling, S.W.; Buffington, S.A.; Momin, E.N.; Francis, M.B.; Britton, R.A. , Costa-Mattioli, M., Mechanisms Underlying Microbial-Mediated Changes in Social Behavior in Mouse Models of Autism Spectrum Disorder. Neuron 2019, 101, 246–259. [Google Scholar] [CrossRef]
- Jaglin, M.; Rhimi, M.; Philippe, C.; Pons, N.; Bruneau, A.; Goustard, B.; Dauge, V.; Maguin, E.; Naudon, L. , Rabot, S., Indole, a Signaling Molecule Produced by the Gut Microbiota, Negatively Impacts Emotional Behaviors in Rats. Front Neurosci 2018, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.J.; Kim, J.; Lee, H.J.; Ryu, H.S.; Kim, K.; Lee, J.H.; Jung, K.W.; Kim, M.J.; Kim, M.J.; Kim, Y.J. , et al., Alpha-synuclein in gastric and colonic mucosa in Parkinson’s disease: Limited role as a biomarker. Mov Disord 2016, 31, 241–9. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, E.; Murphy, S. , Martinson, H.A., Alpha-Synuclein Pathology and the Role of the Microbiota in Parkinson’s Disease. Front Neurosci 2019, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K. , Hsiao, E.Y., Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015, 161, 264–76. [Google Scholar] [CrossRef] [PubMed]
- De Vadder, F.; Grasset, E.; Manneras Holm, L.; Karsenty, G.; Macpherson, A.J.; Olofsson, L.E. , Backhed, F., Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A 2018, 115, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V. , et al., Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Kibbie, J.J.; Dillon, S.M.; Thompson, T.A.; Purba, C.M.; McCarter, M.D. , Wilson, C.C., Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 2021, 226, 152126. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xu, B.; Wang, X.; Wan, W.H.; Lu, J.; Kong, D.; Jin, Y.; You, W.; Sun, H.; Mu, X. , et al., Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology 2022. [Google Scholar]
- Romero, E.; Ali, C.; Molina-Holgado, E.; Castellano, B.; Guaza, C. , Borrell, J., Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology 2007, 32, 1791–804. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, P.; Jiang, J.; Mou, T.; Li, Y.; Xi, C.; Wu, L.; Gao, X.; Zhang, D.; Chen, Y. , et al., New Evidence of Gut Microbiota Involvement in the Neuropathogenesis of Bipolar Depression by TRANK1 Modulation: Joint Clinical and Animal Data. Front Immunol 2021, 12, 789647. [Google Scholar] [CrossRef]
- Bi, M.; Kang, S.; Du, X.; Jiao, Q. , Jiang, H., Association between SNCA rs356220 polymorphism and Parkinson’s disease: A meta-analysis. Neurosci Lett 2020, 717, 134703. [Google Scholar] [CrossRef] [PubMed]
- Wallen, Z.D.; Stone, W.J.; Factor, S.A.; Molho, E.; Zabetian, C.P.; Standaert, D.G. , Payami, H., Exploring human-genome gut-microbiome interaction in Parkinson’s disease. NPJ Parkinsons Dis 2021, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Zhao, N.; Xu, X.; Xu, Y. , Zhu, B., Of genes and microbes: solving the intricacies in host genomes. Protein Cell 2018, 9, 446–461. [Google Scholar] [CrossRef] [PubMed]
- Franco-Obregon, A. and Gilbert, J.A., The Microbiome-Mitochondrion Connection: Common Ancestries, Common Mechanisms, Common Goals. mSystems 2017, 2. [Google Scholar]
- Tian, D. and Han, M., Bacterial peptidoglycan muropeptides benefit mitochondrial homeostasis and animal physiology by acting as ATP synthase agonists. Dev Cell 2022, 57, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Matheoud, D.; Cannon, T.; Voisin, A.; Penttinen, A.M.; Ramet, L.; Fahmy, A.M.; Ducrot, C.; Laplante, A.; Bourque, M.J.; Zhu, L. , et al., Intestinal infection triggers Parkinson’s disease-like symptoms in Pink1(-/-) mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C. , Sacks, O.W., Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol Scand 2004, 110, 267–9. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Candeias, E.; Esteves, A.R.; Magalhaes, J.D.; Ferreira, I.L.; Nunes-Costa, D.; Rego, A.C.; Empadinhas, N. , Cardoso, S.M., Microbial BMAA elicits mitochondrial dysfunction, innate immunity activation, and Alzheimer’s disease features in cortical neurons. J Neuroinflammation 2020, 17, 332. [Google Scholar] [CrossRef]
- Esteves, A.R.; Munoz-Pinto, M.F.; Nunes-Costa, D.; Candeias, E.; Silva, D.F.; Magalhaes, J.D.; Pereira-Santos, A.R.; Ferreira, I.L.; Alarico, S.; Tiago, I. , et al., Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria. Gut 2021. [Google Scholar]
- Nunes-Costa, D.; Magalhaes, J.D.; M, G.F.; Cardoso, S.M. , Empadinhas, N., Microbial BMAA and the Pathway for Parkinson’s Disease Neurodegeneration. Front Aging Neurosci 2020, 12, 26. [Google Scholar] [CrossRef]
- Kabat, A.M.; Pott, J. , Maloy, K.J., The Mucosal Immune System and Its Regulation by Autophagy. Front Immunol 2016, 7, 240. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Kimura, T.; Timmins, G.; Moseley, P.; Chauhan, S. , Mandell, M., Immunologic manifestations of autophagy. J Clin Invest 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Nighot, P.K.; Hu, C.A. , Ma, T.Y., Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem 2015, 290, 7234–46. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Nishitani, M.; Takakura, A.; Imai, Y.; Komatsu, M. , Kawashima, H., Autophagy Protects against Colitis by the Maintenance of Normal Gut Microflora and Secretion of Mucus. J Biol Chem 2015, 290, 20511–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, C.; Zhao, W.; He, C.; Ding, J.; Dai, R.; Xu, K.; Xiao, L.; Luo, L.; Liu, S. , et al., Impaired Autophagy in Intestinal Epithelial Cells Alters Gut Microbiota and Host Immune Responses. Appl Environ Microbiol 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O. , Eid, J., Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Ariyoshi, T.; Hagihara, M.; Takahashi, M. , Mikamo, H., Effect of Clostridium butyricum on Gastrointestinal Infections. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, M.; Ariyoshi, T.; Kuroki, Y.; Eguchi, S.; Higashi, S.; Mori, T.; Nonogaki, T.; Iwasaki, K.; Yamashita, M.; Asai, N. , et al., Clostridium butyricum enhances colonization resistance against Clostridioides difficile by metabolic and immune modulation. Sci Rep 2021, 11, 15007. [Google Scholar] [CrossRef]
- Imase, K.; Takahashi, M.; Tanaka, A.; Tokunaga, K.; Sugano, H.; Tanaka, M.; Ishida, H.; Kamiya, S. , Takahashi, S., Efficacy of Clostridium butyricum preparation concomitantly with Helicobacter pylori eradication therapy in relation to changes in the intestinal microbiota. Microbiol Immunol 2008, 52, 156–61. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, J.; Ju, Z.; Wu, J.; Wang, L.; Lin, H. , Sun, S., Clostridium butyricum Ameliorates Salmonella Enteritis Induced Inflammation by Enhancing and Improving Immunity of the Intestinal Epithelial Barrier at the Intestinal Mucosal Level. Front Microbiol 2020, 11, 299. [Google Scholar]
- Ariyoshi, T.; Hagihara, M.; Eguchi, S.; Fukuda, A.; Iwasaki, K.; Oka, K.; Takahashi, M.; Yamagishi, Y. , Mikamo, H., Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an Anti-inflammatory Effect on Antibiotic-Induced Intestinal Disorder. Front Microbiol 2020, 11, 587725. [Google Scholar] [CrossRef]
- Sun, J.; Li, H.; Jin, Y.; Yu, J.; Mao, S.; Su, K.P.; Ling, Z. , Liu, J., Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun 2021, 91, 703–715. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Hu, X.; Yang, C.; Xu, H.; Yao, Y. , Liu, J., Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J Agric Food Chem 2018, 66, 8415–8421. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z. , Liu, J., Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol Nutr Food Res 2020, 64, e1900636. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Wang, F.; Yu, X.; Ling, Z.; Li, H.; Zhang, H.; Jin, J.; Chen, W.; Pang, M. , et al., Neuroprotective Effects of Clostridium butyricum against Vascular Dementia in Mice via Metabolic Butyrate. Biomed Res Int 2015, 2015, 412946. [Google Scholar] [CrossRef]
- Sun, J.; Ling, Z.; Wang, F.; Chen, W.; Li, H.; Jin, J.; Zhang, H.; Pang, M.; Yu, J. , Liu, J., Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett 2016, 613, 30–5. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Du, J.; Wang, F.; Fang, R.; Yu, C.; Xiong, J.; Chen, W.; Lu, Z. , Liu, J., Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol Motil 2018, 30, e13260. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M. , de Vos, W.M., Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Zhang, T.; Li, Q.; Cheng, L.; Buch, H. , Zhang, F., Akkermansia muciniphila is a promising probiotic. Microb Biotechnol 2019, 12, 1109–1125. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Doring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B. , Wilmes, P., The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 2018, 33, 88–98. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P. , Wullner, U., Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naive Parkinson’s disease patients. Genome Med 2017, 9, 39. [Google Scholar] [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D. , Peng, Y., Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr Diabetes 2020, 10, 12. [Google Scholar] [CrossRef]
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; Gonzalez-Billault, C. , de Castro Fonseca, M., Akkermansia muciniphila induces mitochondrial calcium overload and alpha -synuclein aggregation in an enteroendocrine cell line. iScience 2022, 25, 103908. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Bourgonje, A.R.; Saeed, A.; Vich Vila, A.; Plomp, N.; Blokzijl, T.; Sadaghian Sadabad, M.; von Martels, J.Z.H.; van Leeuwen, S.S.; Weersma, R.K. , et al., Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium. Gut Microbes 2021, 13, 1993582. [Google Scholar] [CrossRef]
- Quevrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermudez-Humaran, L.G.; Pigneur, B. , et al., Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef]
- Martin, R.; Chain, F.; Miquel, S.; Lu, J.; Gratadoux, J.J.; Sokol, H.; Verdu, E.F.; Bercik, P.; Bermudez-Humaran, L.G. , Langella, P., The commensal bacterium Faecalibacterium prausnitzii is protective in DNBS-induced chronic moderate and severe colitis models. Inflamm Bowel Dis 2014, 20, 417–30. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y. , et al., Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm Bowel Dis 2018, 24, 1926–1940. [Google Scholar] [CrossRef]
- Ueda, A.; Shinkai, S.; Shiroma, H.; Taniguchi, Y.; Tsuchida, S.; Kariya, T.; Kawahara, T.; Kobayashi, Y.; Kohda, N.; Ushida, K. , et al., Identification of Faecalibacterium prausnitzii strains for gut microbiome-based intervention in Alzheimer’s-type dementia. Cell Rep Med 2021, 2, 100398. [Google Scholar] [CrossRef]
- Deng, H.; Yang, S.; Zhang, Y.; Qian, K.; Zhang, Z.; Liu, Y.; Wang, Y.; Bai, Y.; Fan, H.; Zhao, X. , et al., Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front Microbiol 2018, 9, 2976. [Google Scholar] [CrossRef]
- De Filippis, F.; Esposito, A. , Ercolini, D., Outlook on next-generation probiotics from the human gut. Cell Mol Life Sci 2022, 79, 76. [Google Scholar] [CrossRef]
- Lukiw, W.J. , Bacteroides fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front Microbiol 2016, 7, 1544. [Google Scholar] [CrossRef] [PubMed]
- Sofi, M.H.; Wu, Y.; Ticer, T.; Schutt, S.; Bastian, D.; Choi, H.J.; Tian, L.; Mealer, C.; Liu, C.; Westwater, C. , et al., A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD. JCI Insight 2021, 6. [Google Scholar]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A. , et al., Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One 2015, 10, e0142164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Ito, M.; Hamaguchi, T.; Mori, H.; Takeda, Y.; Baba, R.; Watanabe, T.; Kurokawa, K.; Asakawa, S.; Hirayama, M. , et al., Quantification of hydrogen production by intestinal bacteria that are specifically dysregulated in Parkinson’s disease. PLoS One 2018, 13, e0208313. [Google Scholar] [CrossRef]

| Subjects | Gut microbiome alterations | |
|---|---|---|
| Increased | Decreased | |
| AD patients |
Ruminococcaceae, Enterococcus, Streptococcus, Alistipes, Dorea, Collinsella, Eggerthella |
Faecalibacterium, Lachnospira, Roseburia, Coprococcus |
| PD patients |
Alistipes, Streptococcus, Ruminococcus, Enterobacter, Enterococcus, Verrucomicrobium, Desulfovibrio, Anaetroncus |
Faecalibacterium, Prevotella, Blautia, Lachnospira, Roseburia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




