Submitted:
09 May 2023
Posted:
10 May 2023
You are already at the latest version
Abstract
Keywords:
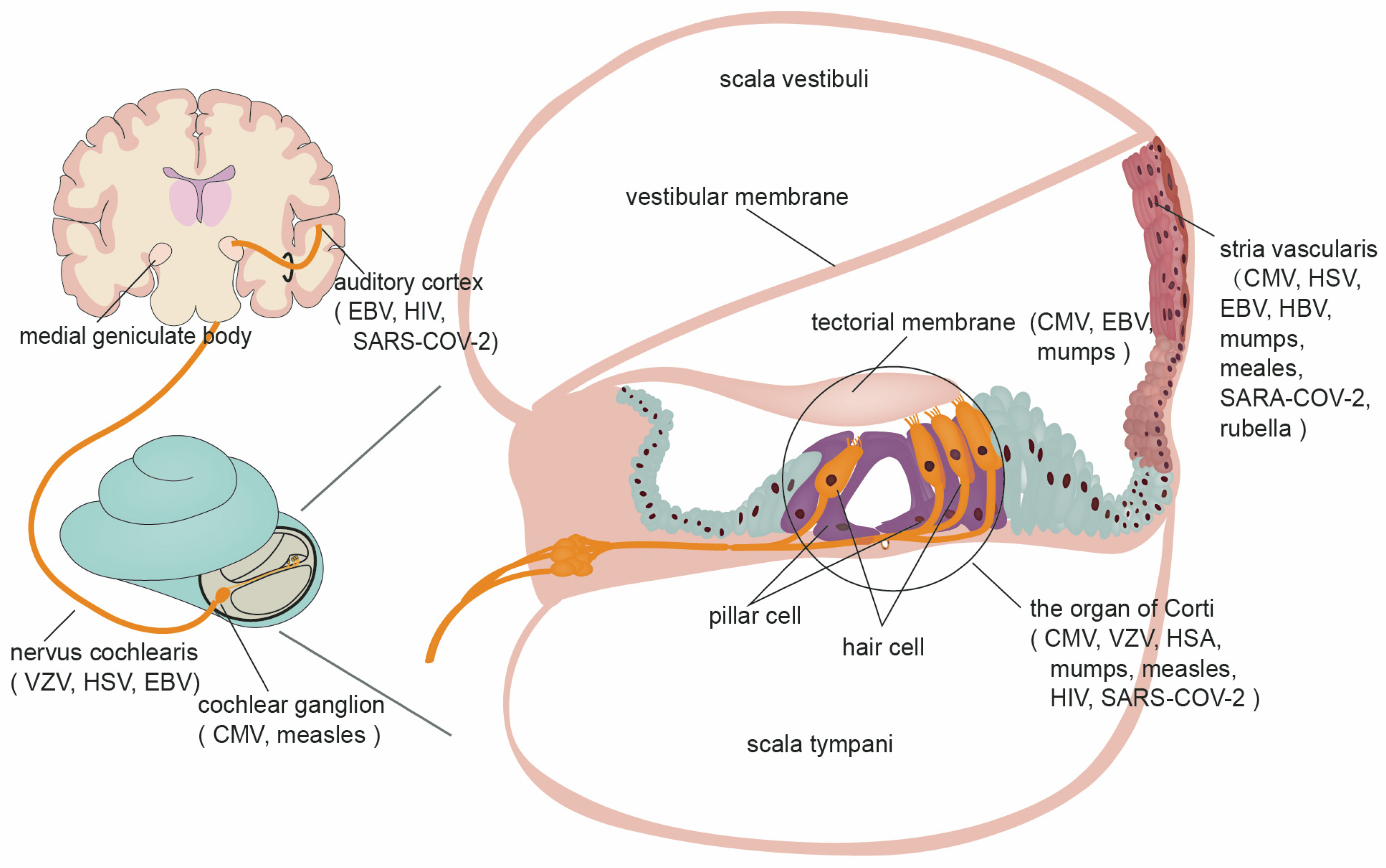
1. Introduction
2. Cytomegalovirus
Inflammatory response

Inner ear development
Inner ear homeostasis
3. Severe acute respiratory syndrome coronavirus 2
4. Herpes simplex virus
5. Varicella-zoster virus
6. Epstein-Barr virus
7. Hepatitis B virus
8. Human immunodeficiency virus
9. Rubella virus
10. Zika virus
11. West Nile virus
12. Human enterovirus
13. Lassa virus
14. Influenza A virus
15. Mumps virus
16. Measles virus
17. Lymphocytic choriomeningitis mammarenavirus
18. Toscana virus
19. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull World Health Organ 2021, 99. [Google Scholar] [CrossRef] [PubMed]
- Cohen, B.E.; Durstenfeld, A.; Roehm, P.C. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Lyall, H. Congenital cytomegalovirus - who, when, what-with and why to treat? J Infect 2017, 74 Suppl 1, S89–S94. [Google Scholar] [CrossRef]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, X.; Chen, S.; Xiang, J.; Peng, Z.; Sun, Y. Analysis of the Results of Cytomegalovirus Testing Combined with Genetic Testing in Children with Congenital Hearing Loss. J Clin Med 2022, 11. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, S.; Sun, Y. Research Progress on Cytomegalovirus Infection-Related Congenital Deafness. Chinese Journal of Otology 2021, 19, 982–986. [Google Scholar] [CrossRef]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries With Universal Screening: A Systematic Review and Meta-analysis. JAMA Netw Open 2021, 4, e2120736. [Google Scholar] [CrossRef]
- Lanzieri, T.M.; Chung, W.; Leung, J.; Caviness, A.C.; Baumgardner, J.L.; Blum, P.; Bialek, S.R.; Demmler-Harrison, G. Hearing Trajectory in Children with Congenital Cytomegalovirus Infection. Otolaryngol Head Neck Surg 2018, 158, 736–744. [Google Scholar] [CrossRef]
- Davis, L.E.; Johnsson, L.G.; Kornfeld, M. Cytomegalovirus labyrinthitis in an infant: morphological, virological, and immunofluorescent studies. J Neuropathol Exp Neurol 1981, 40. [Google Scholar] [CrossRef]
- Bradford, R.D.; Yoo, Y.-G.; Golemac, M.; Pugel, E.P.; Jonjic, S.; Britt, W.J. Murine CMV-induced hearing loss is associated with inner ear inflammation and loss of spiral ganglia neurons. PLoS Pathog 2015, 11, e1004774. [Google Scholar] [CrossRef]
- Harris, J.P.; Fan, J.T.; Keithley, E.M. Immunologic responses in experimental cytomegalovirus labyrinthitis. Am J Otolaryngol 1990, 11, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Schachtele, S.J.; Mutnal, M.B.; Schleiss, M.R.; Lokensgard, J.R. Cytomegalovirus-induced sensorineural hearing loss with persistent cochlear inflammation in neonatal mice. J Neurovirol 2011, 17, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.Y.W.; Seleme, M.C.; Payne, S.; Jonjic, S.; Hirose, K.; Britt, W. Virus-induced cochlear inflammation in newborn mice alters auditory function. JCI Insight 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhang, S.; Yang, J. Coexistence of IL-6 -572C/G and ICAM-1 K469E Polymorphisms among Patients with Sudden Sensorineural Hearing Loss. Tohoku J Exp Med 2018, 245. [Google Scholar] [CrossRef]
- Zhuang, W.; Wang, C.; Shi, X.; Qiu, S.; Zhang, S.; Xu, B.; Chen, M.; Jiang, W.; Dong, H.; Qiao, Y. MCMV triggers ROS/NLRP3-associated inflammasome activation in the inner ear of mice and cultured spiral ganglion neurons, contributing to sensorineural hearing loss. Int J Mol Med 2018, 41, 3448–3456. [Google Scholar] [CrossRef]
- Xia, W.; Yan, H.; Zhang, Y.; Wang, C.; Gao, W.; Lv, C.; Wang, W.; Liu, Z. Congenital Human Cytomegalovirus Infection Inducing Sensorineural Hearing Loss. Front Microbiol 2021, 12, 649690. [Google Scholar] [CrossRef]
- Halenius, A.; Gerke, C.; Hengel, H. Classical and non-classical MHC I molecule manipulation by human cytomegalovirus: so many targets—but how many arrows in the quiver? Cell Mol Immunol 2015, 12, 139–153. [Google Scholar] [CrossRef]
- Huang, S.-N.; Zhou, Y.-P.; Jiang, X.; Yang, B.; Cheng, H.; Luo, M.-H. Hearing Loss Caused by HCMV Infection through Regulating the Wnt and Notch Signaling Pathways. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Natale, F.; De Curtis, M.; Bizzarri, B.; Orlando, M.P.; Ralli, M.; Liuzzi, G.; Caravale, B.; Franco, F.; Gaeta, A.; Giancotti, A.; et al. Isolated auditory neuropathy at birth in congenital cytomegalovirus infection. Ital J Pediatr 2020, 46, 3. [Google Scholar] [CrossRef]
- Carraro, M.; Almishaal, A.; Hillas, E.; Firpo, M.; Park, A.; Harrison, R.V. Cytomegalovirus (CMV) Infection Causes Degeneration of Cochlear Vasculature and Hearing Loss in a Mouse Model. J Assoc Res Otolaryngol 2017, 18, 263–273. [Google Scholar] [CrossRef]
- Teissier, N.; Bernard, S.; Quesnel, S.; Van Den Abbeele, T. Audiovestibular consequences of congenital cytomegalovirus infection. Eur Ann Otorhinolaryngol Head Neck Dis 2016, 133, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, L.; Bonasoni, M.P.; Santini, D.; Piccirilli, G.; Chiereghin, A.; Guerra, B.; Landini, M.P.; Capretti, M.G.; Lanari, M.; Lazzarotto, T. Human fetal inner ear involvement in congenital cytomegalovirus infection. Acta Neuropathol Commun 2013, 1, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shi, X.; Wang, C.; Niu, H.; Zeng, L.; Qiao, Y. Cochlear Spiral Ganglion Neuron Apoptosis in Neonatal Mice with Murine Cytomegalovirus-Induced Sensorineural Hearing Loss. J Am Acad Audiol 2016, 27, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.L.; Gyamfi-Bannerman, C. Diagnosis and antenatal management of congenital cytomegalovirus infection. Am J Obstet Gynecol 2016, 214. [Google Scholar] [CrossRef] [PubMed]
- Naing, Z.W.; Scott, G.M.; Shand, A.; Hamilton, S.T.; van Zuylen, W.J.; Basha, J.; Hall, B.; Craig, M.E.; Rawlinson, W.D. Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol 2016, 56. [Google Scholar] [CrossRef] [PubMed]
- Jückstock, J.; Rothenburger, M.; Friese, K.; Traunmüller, F. Passive Immunization against Congenital Cytomegalovirus Infection: Current State of Knowledge. Pharmacology 2015, 95, 209–217. [Google Scholar] [CrossRef] [PubMed]
- 方峰. 新生儿巨细胞病毒感染及疾病的诊治. 中国实用儿科杂志 2011, 26, 6–8. [Google Scholar]
- Ji, C. Expert Consensus on Vaccination for Children with Special Health Status XXV - Cytomegalovirus Infection in Infants and Vaccination. Chinese Journal of Practical Pediatrics 2019, 34, 808–809. [Google Scholar] [CrossRef]
- Ross, S.A.; Michaels, M.G.; Ahmed, A.; Palmer, A.L.; Sánchez, P.J.; Bernstein, D.I.; Feja, K.; Stewart, A.; Boppana, S.B.; Fowler, K.B. Contribution of Breastfeeding to False-Positive Saliva Polymerase Chain Reaction for Newborn Congenital Cytomegalovirus Screening. J Infect Dis 2018, 217, 1612–1615. [Google Scholar] [CrossRef]
- Revello, M.G.; Tibaldi, C.; Masuelli, G.; Frisina, V.; Sacchi, A.; Furione, M.; Arossa, A.; Spinillo, A.; Klersy, C.; Ceccarelli, M.; et al. Prevention of Primary Cytomegalovirus Infection in Pregnancy. EBioMedicine 2015, 2, 1205–1210. [Google Scholar] [CrossRef]
- Shahar-Nissan, K.; Pardo, J.; Peled, O.; Krause, I.; Bilavsky, E.; Wiznitzer, A.; Hadar, E.; Amir, J. Valaciclovir to prevent vertical transmission of cytomegalovirus after maternal primary infection during pregnancy: a randomised, double-blind, placebo-controlled trial. Lancet 2020, 396, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Permar, S.R.; Schleiss, M.R.; Plotkin, S.A. Advancing Our Understanding of Protective Maternal Immunity as a Guide for Development of Vaccines To Reduce Congenital Cytomegalovirus Infections. J Virol 2018, 92. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Qi, Y. Guidelines for screening and clinical intervention for congenital cytomegalovirus infection. Chinese Journal of Practical Gynecology and Obstetrics 2019, 35, 417–423. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Lin, C.-Y.; Sánchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Kiell, J.M.; et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr 2003, 143, 16–25. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med 2015, 372, 933–943. [Google Scholar] [CrossRef]
- Pasternak, Y.; Ziv, L.; Attias, J.; Amir, J.; Bilavsky, E. Valganciclovir Is Beneficial in Children with Congenital Cytomegalovirus and Isolated Hearing Loss. J Pediatr 2018, 199, 166–170. [Google Scholar] [CrossRef]
- Asselah, T.; Durantel, D.; Pasmant, E.; Lau, G.; Schinazi, R.F. COVID-19: Discovery, diagnostics and drug development. J Hepatol 2021, 74, 168–184. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10. [Google Scholar] [CrossRef]
- Violi, F.; Pastori, D.; Cangemi, R.; Pignatelli, P.; Loffredo, L. Hypercoagulation and Antithrombotic Treatment in Coronavirus 2019: A New Challenge. Thromb Haemost 2020, 120, 949–956. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, H.S. Sudden sensorineural hearing loss after COVID-19 vaccination. Int J Infect Dis 2021, 113, 341–343. [Google Scholar] [CrossRef]
- Chern, A.; Famuyide, A.O.; Moonis, G.; Lalwani, A.K. Bilateral Sudden Sensorineural Hearing Loss and Intralabyrinthine Hemorrhage in a Patient With COVID-19. Otol Neurotol 2021, 42, e10–e14. [Google Scholar] [CrossRef]
- Chirakkal, P.; Al Hail, A.N.; Zada, N.; Vijayakumar, D.S. COVID-19 and Tinnitus. Ear Nose Throat J 2021, 100, 160S–162S. [Google Scholar] [CrossRef] [PubMed]
- Trecca, E.M.C.; Gelardi, M.; Cassano, M. COVID-19 and hearing difficulties. Am J Otolaryngol 2020, 41, 102496. [Google Scholar] [CrossRef] [PubMed]
- Guigou, C.; Schein, A.D.; Blanchard, C.; Folia, M. Sudden sensorineural hearing loss and SARS-CoV-2: Don’t forget the standard work-up! Eur Ann Otorhinolaryngol Head Neck Dis 2021, 138, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Fancello, V.; Hatzopoulos, S.; Corazzi, V.; Bianchini, C.; Skarżyńska, M.B.; Pelucchi, S.; Skarżyński, P.H.; Ciorba, A. SARS-CoV-2 (COVID-19) and audio-vestibular disorders. Int J Immunopathol Pharmacol 2021, 35, 20587384211027373. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Hearing Loss, Tinnitus, and Dizziness in COVID-19: A Systematic Review and Meta-Analysis. Can J Neurol Sci 2022, 49, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.M.C.; Cruz, T.L.G. Otologic and vestibular symptoms in COVID-19: A scoping review. World J Otorhinolaryngol Head Neck Surg 2022. [Google Scholar] [CrossRef]
- Dusan, M.; Milan, S.; Nikola, D. COVID-19 caused hearing loss. Eur Arch Otorhinolaryngol 2022, 279, 2363–2372. [Google Scholar] [CrossRef]
- Meng, X.; Wang, J.; Sun, J.; Zhu, K. COVID-19 and Sudden Sensorineural Hearing Loss: A Systematic Review. Front Neurol 2022, 13, 883749. [Google Scholar] [CrossRef]
- Saniasiaya, J. Hearing Loss in SARS-CoV-2: What Do We Know? Ear Nose Throat J 2021, 100, 152S–154S. [Google Scholar] [CrossRef]
- Karimi-Galougahi, M.; Naeini, A.S.; Raad, N.; Mikaniki, N.; Ghorbani, J. Vertigo and hearing loss during the COVID-19 pandemic - is there an association? Acta Otorhinolaryngol Ital 2020, 40, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Touret, F.; de Lamballerie, X. Of chloroquine and COVID-19. Antiviral Res 2020, 177, 104762. [Google Scholar] [CrossRef] [PubMed]
- Marcink, T.C.; Kicmal, T.; Armbruster, E.; Zhang, Z.; Zipursky, G.; Golub, K.L.; Idris, M.; Khao, J.; Drew-Bear, J.; McGill, G.; et al. Intermediates in SARS-CoV-2 spike-mediated cell entry. Sci Adv 2022, 8, eabo3153. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Flannery, W.; Mostert, C. Novel ENT triad of anosmia, ageusia and hearing impairment in COVID-19. Intern Med J 2020, 50, 1155. [Google Scholar] [CrossRef] [PubMed]
- Cure, E.; Cumhur Cure, M. Comment on "Hearing loss and COVID-19: A note". Am J Otolaryngol 2020, 41, 102513. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.M.; Hooper, J.E.; Mostafa, H.H.; Stewart, C.M. SARS-CoV-2 Virus Isolated From the Mastoid and Middle Ear: Implications for COVID-19 Precautions During Ear Surgery. JAMA Otolaryngol Head Neck Surg 2020, 146, 964–966. [Google Scholar] [CrossRef]
- Mustafa, M.W.M. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol 2020, 41, 102483. [Google Scholar] [CrossRef]
- Jeong, M.; Ocwieja, K.E.; Han, D.; Wackym, P.A.; Zhang, Y.; Brown, A.; Moncada, C.; Vambutas, A.; Kanne, T.; Crain, R.; et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun Med (Lond) 2021, 1, 44. [Google Scholar] [CrossRef]
- De Luca, P.; Scarpa, A.; Ralli, M.; Tassone, D.; Simone, M.; De Campora, L.; Cassandro, C.; Di Stadio, A. Auditory Disturbances and SARS-CoV-2 Infection: Brain Inflammation or Cochlear Affection? Systematic Review and Discussion of Potential Pathogenesis. Front Neurol 2021, 12, 707207. [Google Scholar] [CrossRef]
- Kandimalla, R.; Chakraborty, P.; Vallamkondu, J.; Chaudhary, A.; Samanta, S.; Reddy, P.H.; De Feo, V.; Dewanjee, S. Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Wichova, H.; Miller, M.E.; Derebery, M.J. Otologic Manifestations After COVID-19 Vaccination: The House Ear Clinic Experience. Otol Neurotol 2021, 42, e1213–e1218. [Google Scholar] [CrossRef] [PubMed]
- Formeister, E.J.; Chien, W.; Agrawal, Y.; Carey, J.P.; Stewart, C.M.; Sun, D.Q. Preliminary Analysis of Association Between COVID-19 Vaccination and Sudden Hearing Loss Using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngol Head Neck Surg 2021, 147, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Yanir, Y.; Doweck, I.; Shibli, R.; Najjar-Debbiny, R.; Saliba, W. Association Between the BNT162b2 Messenger RNA COVID-19 Vaccine and the Risk of Sudden Sensorineural Hearing Loss. JAMA Otolaryngol Head Neck Surg 2022, 148, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, F.; Cambria, F.; Colizza, A.; Ralli, M.; Greco, A.; de Vincentiis, M.; Petrella, C.; Fiore, M.; Minni, A.; Barbato, C. Sudden Sensorineural Hearing Loss after Third Dose Booster of COVID-19 Vaccine Administration. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Aasfara, J.; Hajjij, A.; Bensouda, H.; Ouhabi, H.; Benariba, F. A unique association of bifacial weakness, paresthesia and vestibulocochlear neuritis as post-COVID-19 manifestation in pregnant women: a case report. Pan Afr Med J 2021, 38, 30. [Google Scholar] [CrossRef] [PubMed]
- Narozny, W.; Tretiakow, D.; Skorek, A. In Reference to The Challenges of Pharmacotherapy of SARS-CoV-2 Infection in Patients With Sudden Sensorineural Hearing Loss Due to COVID-19. Laryngoscope 2021, 131, E2335. [Google Scholar] [CrossRef]
- Halford, W.P.; Kemp, C.D.; Isler, J.A.; Davido, D.J.; Schaffer, P.A. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J Virol 2001, 75, 6143–6153. [Google Scholar] [CrossRef]
- Cooper, I.D.; Crofts, C.A.P.; DiNicolantonio, J.J.; Malhotra, A.; Elliott, B.; Kyriakidou, Y.; Brookler, K.H. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Heart 2020, 7. [Google Scholar] [CrossRef]
- Zeng, H.; Xu, C.; Fan, J.; Tang, Y.; Deng, Q.; Zhang, W.; Long, X. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia. JAMA 2020, 323, 1848–1849. [Google Scholar] [CrossRef]
- Salem, D.; Katranji, F.; Bakdash, T. COVID-19 infection in pregnant women: Review of maternal and fetal outcomes. Int J Gynaecol Obstet 2021, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Panahi, L.; Amiri, M.; Pouy, S. Risks of Novel Coronavirus Disease (COVID-19) in Pregnancy; a Narrative Review. Arch Acad Emerg Med 2020, 8, e34. [Google Scholar] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Lei, D.; Fang, C.; Li, C.; Wang, M.; Liu, Y.; Bao, Y.; Sun, Y.; Huang, J.; Guo, Y.; et al. Perinatal Transmission of 2019 Coronavirus Disease-Associated Severe Acute Respiratory Syndrome Coronavirus 2: Should We Worry? Clin Infect Dis 2021, 72, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Zarchi, M.; Neamatzadeh, H.; Dastgheib, S.A.; Abbasi, H.; Mirjalili, S.R.; Behforouz, A.; Ferdosian, F.; Bahrami, R. Vertical Transmission of Coronavirus Disease 19 (COVID-19) from Infected Pregnant Mothers to Neonates: A Review. Fetal Pediatr Pathol 2020, 39, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Bwire, G.M.; Njiro, B.J.; Mwakawanga, D.L.; Sabas, D.; Sunguya, B.F. Possible vertical transmission and antibodies against SARS-CoV-2 among infants born to mothers with COVID-19: A living systematic review. J Med Virol 2021, 93, 1361–1369. [Google Scholar] [CrossRef]
- Oskovi-Kaplan, Z.A.; Ozgu-Erdinc, A.S.; Buyuk, G.N.; Sert-Dinc, U.Y.; Ali-Algan, C.; Demir, B.; Sahin, D.; Keskin, H.L.; Tayman, C.; Moraloglu-Tekin, Ö. Newborn Hearing Screening Results of Infants Born To Mothers Who Had COVID-19 Disease During Pregnancy: A Retrospective Cohort Study. Ear Hear 2022, 43, 41–44. [Google Scholar] [CrossRef]
- Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection. Ultrasound Obstet Gynecol 2021, 57, 232–241. [CrossRef]
- Yilmaz, M.; Aksin, Ş.; Balsak, D.; Avci, F.; Özdoğru, O.; Helvacıoğlu, B.; Erdemoğlu, M.; Aboalhasan, Y.; Doğan, G. Comparison of Perinatal, Newborn, and Audiometry Results of COVID-19 Pregnant Women. Int J Clin Pract 2022, 2022, 2699532. [Google Scholar] [CrossRef]
- Alan, M.A.; Alan, C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int J Pediatr Otorhinolaryngol 2021, 146, 110754. [Google Scholar] [CrossRef] [PubMed]
- AlMukdad, S.; Harfouche, M.; Farooqui, U.S.; Aldos, L.; Abu-Raddad, L.J. Epidemiology of herpes simplex virus type 1 and genital herpes in Australia and New Zealand: systematic review, meta-analyses and meta-regressions. Epidemiol Infect 2023, 151, e33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Whitley, R.; Baines, J. Clinical management of herpes simplex virus infections: past, present, and future. F1000Res 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 2007, 120, 898–921. [CrossRef] [PubMed]
- Westerberg, B.D.; Atashband, S.; Kozak, F.K. A systematic review of the incidence of sensorineural hearing loss in neonates exposed to Herpes simplex virus (HSV). Int J Pediatr Otorhinolaryngol 2008, 72, 931–937. [Google Scholar] [CrossRef]
- Kaga, K.; Kaga, M.; Tamai, F.; Shindo, M. Auditory agnosia in children after herpes encephalitis. Acta Otolaryngol 2003, 123, 232–235. [Google Scholar] [CrossRef]
- Rabinstein, A.; Jerry, J.; Saraf-Lavi, E.; Sklar, E.; Bradley, W.G. Sudden sensorineural hearing loss associated with herpes simplex virus type 1 infection. Neurology 2001, 56, 571–572. [Google Scholar] [CrossRef]
- Stokroos, R.J.; Albers, F.W.; Schirm, J. The etiology of idiopathic sudden sensorineural hearing loss. Experimental herpes simplex virus infection of the inner ear. Am J Otol 1998, 19, 447–452. [Google Scholar]
- Nomura, Y.; Kurata, T.; Saito, K. Cochlear changes after herpes simplex virus infection. Acta Otolaryngol 1985, 99, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Stokroos, R.J.; Albers, F.W.; Schirm, J. Therapy of idiopathic sudden sensorineural hearing loss: antiviral treatment of experimental herpes simplex virus infection of the inner ear. Ann Otol Rhinol Laryngol 1999, 108, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Veltri, R.W.; Wilson, W.R.; Sprinkle, P.M.; Rodman, S.M.; Kavesh, D.A. The implication of viruses in idiopathic sudden hearing loss: primary infection or reactivation of latent viruses? Otolaryngol Head Neck Surg 1981, 89, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Koide, J.; Yanagita, N.; Hondo, R.; Kurata, T. Serological and clinical study of herpes simplex virus infection in patients with sudden deafness. Acta Otolaryngol Suppl 1988, 456, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Yamauchi, S.; Shinkawa, A.; Horiuchi, M.; Sakai, M. Immunological and virological study of sudden deafness. Auris Nasus Larynx 1996, 23, 63–68. [Google Scholar] [CrossRef] [PubMed]
- García Berrocal, J.R.G.; Ramírez-Camacho, R.; Portero, F.; Vargas, J.A. Role of viral and Mycoplasma pneumoniae infection in idiopathic sudden sensorineural hearing loss. Acta Otolaryngol 2000, 120, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Jaiyeoba, O.; Amaya, M.I.; Soper, D.E.; Kilby, J.M. Preventing neonatal transmission of herpes simplex virus. Clin Obstet Gynecol 2012, 55, 510–520. [Google Scholar] [CrossRef] [PubMed]
- van Oorschot, D.; Vroling, H.; Bunge, E.; Diaz-Decaro, J.; Curran, D.; Yawn, B. A systematic literature review of herpes zoster incidence worldwide. Hum Vaccin Immunother 2021, 17, 1714–1732. [Google Scholar] [CrossRef]
- Patil, A.; Goldust, M.; Wollina, U. A Review of Clinical Manifestations and Management. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Cobelli Kett, J. Perinatal varicella. Pediatr Rev 2013, 34, 49–51. [Google Scholar] [CrossRef]
- Tien, C.-T.; Young, Y.-H. Sudden Sensorineural Hearing Loss in 6 Patients Following Dental Procedure. Ear Nose Throat J 2021, 100, 304S–308S. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Xiong, G.; Xiang, G.; Xu, S.; Zheng, Y.; Zhang, L. Sudden deafness as an initial presentation of varicella: case report and literature review. Ann Palliat Med 2021, 10, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Psillas, G.; Arnaoutoglou, M.; Gatsios, T.; Rizos, D.; Koutsouraki, E.; Vital, V. Autoimmune recurrent facial palsy and bilateral sudden sensorineural hearing loss following Ramsay Hunt-like syndrome. Auris Nasus Larynx 2012, 39, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Kim, B.-R.; Shin, J.E.; Kim, C.-H. Clinical manifestations in patients with herpes zoster oticus. Eur Arch Otorhinolaryngol 2016, 273, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, J.; Moon, I.S.; Lee, H.-K.; Lee, W.-S. Statistical analysis of pure tone audiometry and caloric test in herpes zoster oticus. Clin Exp Otorhinolaryngol 2008, 1, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Choi, H.; Shin, J.E. Characteristics of hearing loss in patients with herpes zoster oticus. Medicine (Baltimore) 2016, 95, e5438. [Google Scholar] [CrossRef] [PubMed]
- Jahr, S.H.; Wahl, M.S.; Majid, B.; Samuelsen, E. Herpes zoster oticus. Tidsskr Nor Laegeforen 2021, 141. [Google Scholar] [CrossRef] [PubMed]
- Proctor, L.; Perlman, H.; Lindsay, J.; Matz, G. Acute vestibular paralysis in herpes zoster oticus. Ann Otol Rhinol Laryngol 1979, 88, 303–310. [Google Scholar] [CrossRef]
- Zajtchuk, J.T.; Matz, G.J.; Lindsay, J.R. Temporal bone pathology in herpes oticus. Ann Otol Rhinol Laryngol 1972, 81, 331–338. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Yanagihara, N.; Kurata, T. Middle ear mucosa in Ramsay Hunt syndrome. Ann Otol Rhinol Laryngol 1990, 99, 359–362. [Google Scholar] [CrossRef]
- Kaberos, A.; Balatsouras, D.G.; Korres, S.G.; Kandiloros, D.; Economou, C. Audiological assessment in Ramsay Hunt syndrome. Ann Otol Rhinol Laryngol 2002, 111, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Wayman, D.M.; Pham, H.N.; Byl, F.M.; Adour, K.K. Audiological manifestations of Ramsay Hunt syndrome. J Laryngol Otol 1990, 104, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Abramovich, S.; Prasher, D.K. Electrocochleography and brain-stem potentials in Ramsay Hunt syndrome. Arch Otolaryngol Head Neck Surg 1986, 112, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-H.; Jeong, K.-H.; Ahn, S.H.; Shin, D.H.; Kim, Y.W.; Shin, J.E. Vibration- and hyperventilation-induced nystagmus in patients with Ramsay Hunt syndrome with vertigo. Otolaryngol Head Neck Surg 2015, 152, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Toda, N.; Takahashi, M.; Azuma, T.; Nakamura, K.; Takao, S.-I.; Harada, M.; Takeda, N. Vestibular and cochlear neuritis in patients with Ramsay Hunt syndrome: a Gd-enhanced MRI study. Acta Otolaryngol 2013, 133, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Mizuno, T.; Naganawa, S.; Sugiura, M.; Yoshida, T.; Teranishi, M.; Sone, M.; Nakashima, T. 3D-FLAIR MRI in facial nerve paralysis with and without audio-vestibular disorder. Acta Otolaryngol 2010, 130, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, H.; Iwasaki, S.; Ushio, M.; Takeuchi, N.; Murofushi, T. The lesion site of vestibular dysfunction in Ramsay Hunt syndrome: a study by click and galvanic VEMP. J Vestib Res 2006, 16, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Beutner, K.R.; Friedman, D.J.; Forszpaniak, C.; Andersen, P.L.; Wood, M.J. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother 1995, 39, 1546–1553. [Google Scholar] [CrossRef]
- Maximova, N.; Antonio, P.; Marilena, G.; Rovere, F.; Tamaro, P. Complete remission of VZV reactivation treated with valganciclovir in a patient with total lymphocyte depletion and acute kidney injury after allogeneic bone marrow transplantation. APMIS 2015, 123, 77–80. [Google Scholar] [CrossRef]
- Shiraki, K.; Yasumoto, S.; Toyama, N.; Fukuda, H. Amenamevir, a Helicase-Primase Inhibitor, for the Optimal Treatment of Herpes Zoster. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Thorley-Lawson, D.A. EBV Persistence--Introducing the Virus. Curr Top Microbiol Immunol 2015, 390, 151–209. [Google Scholar] [CrossRef] [PubMed]
- Nowalk, A.; Green, M. Epstein-Barr Virus. Microbiol Spectr 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. Epstein-Barr virus (EBV) reactivation and therapeutic inhibitors. J Clin Pathol 2019, 72, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I. Epstein-Barr virus infection. N Engl J Med 2000, 343, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Karagöz, E.; Beköz, H.S.; Ceylan, B.; Mert, A. Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis presenting with acute sensorineural hearing loss: a case report and review of the literature. Infez Med 2017, 25, 277–280. [Google Scholar] [PubMed]
- Williams, L.L.; Lowery, H.W.; Glaser, R. Sudden hearing loss following infectious mononucleosis: possible effect of altered immunoregulation. Pediatrics 1985, 75, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Erzurum, S.; Kalavsky, S.M.; Watanakunakorn, C. Acute cerebellar ataxia and hearing loss as initial symptoms of infectious mononucleosis. Arch Neurol 1983, 40, 760–762. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Lossos, A.; Lossos, I.S. Sudden hearing loss following acute hepatitis. Postgrad Med J 1999, 75, 309–312. [Google Scholar] [CrossRef]
- Buza, N.; Bálint, I.; Schneider, T.; Koltai, L.; Orosz, Z. Unusual clinical manifestation of virus-associated hemophagocytic syndrome. Pathol Res Pract 2003, 199, 755–759. [Google Scholar] [CrossRef]
- Wong, H.; Hwang, Y.-Y.; Leung, R.Y.Y.; Chan, G.S.W.; Khong, P.-L.; Kwong, Y.-L. Unilateral hearing loss due to lymphocytosis and a contralateral putamen lesion. Ann Hematol 2015, 94, 703–704. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q. A case of severe sensorineural deafness due to EBV infection. In Proceedings of the The 23rd National Conference on Integrating Chinese and Western Medicine in Pediatrics, Shenyang, Liaoning Province, China; 2019; pp. 132–133. [Google Scholar]
- Schuknecht, H.F.; Donovan, E.D. The pathology of idiopathic sudden sensorineural hearing loss. Arch Otorhinolaryngol 1986, 243. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Bi, S.; Yang, W.; Wang, L.; Cui, G.; Cui, F.; Zhang, Y.; Liu, J.; Gong, X.; Chen, Y.; et al. Evaluation of the impact of hepatitis B vaccination among children born during 1992-2005 in China. J Infect Dis 2009, 200, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Chen, E.Q.; Cui, Y.L.; Zeng, L.; Wang, Y.J.; Tang, H. Seroprevalence of hepatitis B virus markers in individuals for physical examination in West China Hospital, China. Eur Rev Med Pharmacol Sci 2011, 15, 592–596. [Google Scholar] [PubMed]
- Negahdari, B.; Darvishi, M.; Saeedi, A.A. Gold nanoparticles and hepatitis B virus. Artif Cells Nanomed Biotechnol 2019, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-T.; Fang, K.-H.; Yang, Y.-H.; Lin, M.-H.; Chen, P.-C.; Tsai, M.-S.; Hsu, C.-M. Risk of developing sudden sensorineural hearing loss in patients with hepatitis B virus infection: A population-based study. Ear Nose Throat J 2018, 97, E19–E27. [Google Scholar] [PubMed]
- Nasab, M.S. Association between hepatitis B and hearing status. Oman Med J 2012, 27, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.P. Association between hepatitis B and hearing status. Eur Rev Med Pharmacol Sci 2017, 21, 922–927. [Google Scholar] [PubMed]
- Gholami Parizad, E.; Gerami Matin, H.; Gholami Parizad, E.; Khosravi, A. The Prevalence of Hearing Loss in Patients with Hepatitis B Infection Compared with Healthy Volunteers. Iran J Otorhinolaryngol 2017, 29, 127–132. [Google Scholar]
- Azizul Islam, S.; Chung, J.W.; Lee, Y.-S.; Cho, H.; Moon, S.-S. Negative Association of Hepatitis B Virus With Hearing Impairment. Am J Audiol 2018, 27, 324–332. [Google Scholar] [CrossRef]
- Sood, A.B.; O'Keefe, G.; Bui, D.; Jain, N. Vogt-Koyanagi-Harada Disease Associated with Hepatitis B Vaccination. Ocul Immunol Inflamm 2019, 27, 524–527. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; van Zonneveld, M.; van Nunen, A.B.; Niesters, H.G.M.; Schalm, S.W.; de Man, R.A. Polyarteritis nodosa associated with hepatitis B virus infection. The role of antiviral treatment and mutations in the hepatitis B virus genome. Eur J Gastroenterol Hepatol 2004, 16, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Feng, S. A case of sudden deafness complicated by viral hepatitis. Medical & Pharmaceutical Journal of Chinese People's Liberation Army 1995, 126. [Google Scholar]
- Huang, C.-C.; Lin, W.-B.; Chang, P.-H.; Chan, K.-C.; Lee, T.-J. Sudden deafness as a presenting symptom of chronic hepatitis B with acute exacerbation. Otolaryngol Head Neck Surg 2009, 141, 659–660. [Google Scholar] [CrossRef] [PubMed]
- In Danger: UNAIDS Global AIDS Update 2022 [EN/RU]. Available online: https://reliefweb.int/report/world/danger-unaids-global-aids-update-2022-enru (accessed on.
- Wang, J.; Sung, V.; Carew, P.; Burt, R.A.; Liu, M.; Wang, Y.; Afandi, A.; Wake, M. Prevalence of Childhood Hearing Loss and Secular Trends: A Systematic Review and Meta-Analysis. Acad Pediatr 2019, 19, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Dawood, G.; Klop, D.; Olivier, E.; Elliott, H.; Pillay, M.; Grimmer, K. Nature and extent of hearing loss in HIV-infected children: A scoping review. Int J Pediatr Otorhinolaryngol 2020, 134, 110036. [Google Scholar] [CrossRef]
- Chao, C.K.; Czechowicz, J.A.; Messner, A.H.; Alarcón, J.; Kolevic Roca, L.; Larragán Rodriguez, M.M.; Gutiérrez Villafuerte, C.; Montano, S.M.; Zunt, J.R. High prevalence of hearing impairment in HIV-infected Peruvian children. Otolaryngol Head Neck Surg 2012, 146, 259–265. [Google Scholar] [CrossRef]
- Walsh, H.; Zuwala, J.; Hunter, J.; Oh, Y. Congenital Cytomegalovirus and Human Immunodeficiency Virus: Effects on Hearing, Speech and Language Development, and Clinical Outcomes in Children. Front Pediatr 2021, 9, 771192. [Google Scholar] [CrossRef]
- Hrapcak, S.; Kuper, H.; Bartlett, P.; Devendra, A.; Makawa, A.; Kim, M.; Kazembe, P.; Ahmed, S. Hearing Loss in HIV-Infected Children in Lilongwe, Malawi. PLoS One 2016, 11, e0161421. [Google Scholar] [CrossRef]
- Cai, T.; McPherson, B. Hearing loss in children with otitis media with effusion: a systematic review. Int J Audiol 2017, 56, 65–76. [Google Scholar] [CrossRef]
- Torre, P.; Hoffman, H.J.; Springer, G.; Cox, C.; Young, M.A.; Margolick, J.B.; Plankey, M. Hearing loss among HIV-seropositive and HIV-seronegative men and women. JAMA Otolaryngol Head Neck Surg 2015, 141, 202–210. [Google Scholar] [CrossRef]
- Matas, C.G.; Leite, R.A.; Magliaro, F.C.L.; Gonçalves, I.C. Audiological and electrophysiological evaluation of children with acquired immunodeficiency syndrome (AIDS). Braz J Infect Dis 2006, 10, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Maro, I.I.; Fellows, A.M.; Clavier, O.H.; Gui, J.; Rieke, C.C.; Wilbur, J.C.; Chambers, R.D.; Jastrzembski, B.G.; Mascari, J.E.; Bakari, M.; et al. Auditory Impairments in HIV-Infected Children. Ear Hear 2016, 37, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Pappas, D.G.; Chandra f1p4ar, H.K.; Lim, J.; Hillman, D.E. Ultrastructural findings in the cochlea of AIDS cases. Am J Otol 1994, 15, 456–465. [Google Scholar] [PubMed]
- Wolters, P.L.; Brouwers, P.; Civitello, L.; Moss, H.A. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS 1997, 11, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Xu, J.; Ank, B.; Watts, D.H.; Camarca, M.; Mofenson, L.M.; Pilotto, J.H.; Joao, E.; Gray, G.; Theron, G.; et al. Congenital Cytomegalovirus and HIV Perinatal Transmission. Pediatr Infect Dis J 2018, 1016–1021. [Google Scholar] [CrossRef]
- Simdon, J.; Watters, D.; Bartlett, S.; Connick, E. Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: a report of 3 possible cases and review of the literature. Clin Infect Dis 2001, 32, 1623–1627. [Google Scholar] [CrossRef]
- Benki-Nugent, S.; Wamalwa, D.; Langat, A.; Tapia, K.; Adhiambo, J.; Chebet, D.; Okinyi, H.M.; John-Stewart, G. Comparison of developmental milestone attainment in early treated HIV-infected infants versus HIV-unexposed infants: a prospective cohort study. BMC Pediatr 2017, 17, 24. [Google Scholar] [CrossRef]
- Thompson, K.M.; Simons, E.A.; Badizadegan, K.; Reef, S.E.; Cooper, L.Z. Characterization of the Risks of Adverse Outcomes Following Rubella Infection in Pregnancy. Risk Anal 2016, 36, 1315–1331. [Google Scholar] [CrossRef]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef]
- rubella. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/rubella (accessed on.
- Bento, R.F.; Castilho, A.M.; Sakae, F.A.; Andrade, J.Q.; Zugaib, M. Auditory brainstem response and otoacoustic emission assessment of hearing-impaired children of mothers who contracted rubella during pregnancy. Acta Otolaryngol 2005, 125, 492–494. [Google Scholar] [CrossRef]
- Anderson, H.; Barr, B.; Wedenberg, E. Genetic disposition--a prerequisite for maternal rubella deafness. Arch Otolaryngol 1970, 91, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Töndury, G.; Smith, D.W. Fetal rubella pathology. J Pediatr 1966, 68, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bowden, D.S. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev 2000, 13, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Immunization coverage. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/immunization-coverage (accessed on.
- Smith, R.J.H.; Bale, J.F.; White, K.R. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Gubler, D.J. Zika Virus. Clin Microbiol Rev 2016, 29, 487–524. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Cao-Lormeau, V.M.; Gubler, D.J. Zika virus: following the path of dengue and chikungunya? Lancet 2015, 386, 243–244. [Google Scholar] [CrossRef] [PubMed]
- de Fatima Vasco Aragao, M.; van der Linden, V.; Brainer-Lima, A.M.; Coeli, R.R.; Rocha, M.A.; Sobral da Silva, P.; Durce Costa Gomes de Carvalho, M.; van der Linden, A.; Cesario de Holanda, A.; Valenca, M.M. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ 2016, 353, i1901. [Google Scholar] [CrossRef] [PubMed]
- Carteaux, G.; Maquart, M.; Bedet, A.; Contou, D.; Brugières, P.; Fourati, S.; Cleret de Langavant, L.; de Broucker, T.; Brun-Buisson, C.; Leparc-Goffart, I.; et al. Zika Virus Associated with Meningoencephalitis. N Engl J Med 2016, 374, 1595–1596. [Google Scholar] [CrossRef]
- Barbi, L.; Coelho, A.V.C.; Alencar, L.C.A.d.; Crovella, S. Prevalence of Guillain-Barré syndrome among Zika virus infected cases: a systematic review and meta-analysis. Braz J Infect Dis 2018, 22, 137–141. [Google Scholar] [CrossRef]
- Leal, M.C.; Muniz, L.F.; Ferreira, T.S.A.; Santos, C.M.; Almeida, L.C.; Van Der Linden, V.; Ramos, R.C.F.; Rodrigues, L.C.; Neto, S.S.C. Hearing Loss in Infants with Microcephaly and Evidence of Congenital Zika Virus Infection - Brazil, November 2015-May 2016. MMWR Morb Mortal Wkly Rep 2016, 65, 917–919. [Google Scholar] [CrossRef]
- C Lage, M.-L.; Carvalho, A.L.d.; Ventura, P.A.; Taguchi, T.B.; Fernandes, A.S.; Pinho, S.F.; Santos-Junior, O.T.; Ramos, C.L.; Nascimento-Carvalho, C.M. Clinical, Neuroimaging, and Neurophysiological Findings in Children with Microcephaly Related to Congenital Zika Virus Infection. Int J Environ Res Public Health 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.C.d.; Muniz, L.F.; Maciel, R.J.; Ramos, D.S.; Albuquerque, K.M.G.d.; Leão, Â.M.C.; Mendonça, M.V.d.; Leal, M.d.C. Hearing and communicative skills in the first years of life in children with congenital Zika syndrome. Braz J Otorhinolaryngol 2022, 88, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Thawani, A.; Sammudin, N.H.; Reygaerts, H.S.; Wozniak, A.N.; Munnamalai, V.; Kuhn, R.J.; Fekete, D.M. Zika virus can directly infect and damage the auditory and vestibular components of the embryonic chicken inner ear. Dev Dyn 2020, 249, 867–883. [Google Scholar] [CrossRef]
- Musso, D.; Roche, C.; Nhan, T.-X.; Robin, E.; Teissier, A.; Cao-Lormeau, V.-M. Detection of Zika virus in saliva. J Clin Virol 2015, 68, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Epidemiological Alert - Neurological Syndrome, Congenital Malformations, and Zika Virus Infection: Implications for Public Health in the Americas - 1 December 2015. Available online: https://reliefweb.int/report/world/epidemiological-alert-neurological-syndrome-congenital-malformations-and-zika-virus (accessed on.
- Hinckley, A.F.; O'Leary, D.R.; Hayes, E.B. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics 2007, 119, e666–e671. [Google Scholar] [CrossRef] [PubMed]
- Alpert, S.G.; Fergerson, J.; Noël, L.P. Intrauterine West Nile virus: ocular and systemic findings. Am J Ophthalmol 2003, 136, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Petersen, L.R.; Marfin, A.A. West Nile virus: a primer for the clinician. Ann Intern Med 2002, 137, 173–179. [Google Scholar] [CrossRef]
- McBride, W.; Gill, K.R.S.; Wiviott, L. West Nile Virus infection with hearing loss. J Infect 2006, 53, e203–e205. [Google Scholar] [CrossRef]
- Casetta, I.; Ciorba, A.; Cesnik, E.; Trevisi, P.; Tugnoli, V.; Bovo, R. West Nile virus neuroinvasive disease presenting with acute flaccid paralysis and bilateral sensorineural hearing loss. J Neurol 2011, 258, 1880–1881. [Google Scholar] [CrossRef]
- Szatmary, G.; Leis, A.A. Concurrent West Nile virus infection in pneumococcal meningitis: clinical and MRI features. J Neuroimaging 2015, 25, 312–315. [Google Scholar] [CrossRef]
- Weatherhead, J.E.; Miller, V.E.; Garcia, M.N.; Hasbun, R.; Salazar, L.; Dimachkie, M.M.; Murray, K.O. Long-term neurological outcomes in West Nile virus-infected patients: an observational study. Am J Trop Med Hyg 2015, 92, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Parrino, D.; Brescia, G.; Trimarchi, M.V.; Tealdo, G.; Sasset, L.; Cattelan, A.M.; Bovo, R.; Marioni, G. Cochlear-Vestibular Impairment due to West Nile Virus Infection. Ann Otol Rhinol Laryngol 2019, 128, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Jamison, S.C.; Michaels, S.R.; Ratard, R.; Sweet, J.M.; Deboisblanc, B.P. A 41-year-old HIV-positive man with acute onset of quadriplegia after West Nile virus infection. South Med J 2007, 100, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
- West Nile virus. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/west-nile-virus (accessed on.
- Jubelt, B.; Lipton, H.L. Enterovirus/picornavirus infections. Handb Clin Neurol 2014, 123, 379–416. [Google Scholar] [CrossRef] [PubMed]
- Schattner, A.; Halperin, D.; Wolf, D.; Zimhony, O. Enteroviruses and sudden deafness. CMAJ 2003, 168, 1421–1423. [Google Scholar] [PubMed]
- Tekin, B.; Boire, N.; Shah, K.; Hanson, J.; Bridges, A.G. Viral panniculitis in a patient with disseminated opportunistic Enterovirus infection. J Cutan Pathol 2021, 48, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Mentel, R.; Kaftan, H.; Wegner, U.; Reissmann, A.; Gürtler, L. Are enterovirus infections a co-factor in sudden hearing loss? J Med Virol 2004, 72, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Wolf, D.G.; Elidan, J.; Eliashar, R. Enterovirus, cytomegalovirus, and Epstein-Barr virus infection screening in idiopathic sudden sensorineural hearing loss. Audiol Neurootol 2007, 12, 179–182. [Google Scholar] [CrossRef]
- Kadambari, S.; Braccio, S.; Ribeiro, S.; Allen, D.J.; Pebody, R.; Brown, D.; Cunney, R.; Sharland, M.; Ladhani, S. Enterovirus and parechovirus meningitis in infants younger than 90 days old in the UK and Republic of Ireland: a British Paediatric Surveillance Unit study. Arch Dis Child 2019, 104, 552–557. [Google Scholar] [CrossRef]
- Yang, T.-T.; Huang, L.-M.; Lu, C.-Y.; Kao, C.-L.; Lee, W.-T.; Lee, P.-I.; Chen, C.-M.; Huang, F.-Y.; Lee, C.-Y.; Chang, L.-Y. Clinical features and factors of unfavorable outcomes for non-polio enterovirus infection of the central nervous system in northern Taiwan, 1994-2003. J Microbiol Immunol Infect 2005, 38, 417–424. [Google Scholar]
- Bachor, E.; Karmody, C.S. Neural hearing loss in a child with poliomyelitis: a histopathological study. J Laryngol Otol 2001, 115, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.D.A. The coxsackievirus and adenovirus receptor: virological and biological beauty. FEBS Lett 2020, 594, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.D.A.; Avenarius, M.R.; Hansen, M.R.; Kimberling, W.J.; Najmabadi, H.; Smith, R.J.H.; Zabner, J. The Coxsackievirus and Adenovirus Receptor: a new adhesion protein in cochlear development. Hear Res 2006, 215, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Happi, A.N.; Happi, C.T.; Schoepp, R.J. Lassa fever diagnostics: past, present, and future. Curr Opin Virol 2019, 37, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Houlihan, C.; Behrens, R. Lassa fever. BMJ 2017, 358, j2986. [Google Scholar] [CrossRef] [PubMed]
- Yun, N.E.; Walker, D.H. Pathogenesis of Lassa fever. Viruses 2012, 4, 2031–2048. [Google Scholar] [CrossRef] [PubMed]
- Mateer, E.J.; Huang, C.; Shehu, N.Y.; Paessler, S. Lassa fever-induced sensorineural hearing loss: A neglected public health and social burden. PLoS Negl Trop Dis 2018, 12, e0006187. [Google Scholar] [CrossRef] [PubMed]
- Cummins, D.; McCormick, J.B.; Bennett, D.; Samba, J.A.; Farrar, B.; Machin, S.J.; Fisher-Hoch, S.P. Acute sensorineural deafness in Lassa fever. JAMA 1990, 264, 2093–2096. [Google Scholar] [CrossRef]
- Yun, N.E.; Ronca, S.; Tamura, A.; Koma, T.; Seregin, A.V.; Dineley, K.T.; Miller, M.; Cook, R.; Shimizu, N.; Walker, A.G.; et al. Animal Model of Sensorineural Hearing Loss Associated with Lassa Virus Infection. J Virol 2015, 90, 2920–2927. [Google Scholar] [CrossRef]
- Maruyama, J.; Reyna, R.A.; Kishimoto-Urata, M.; Urata, S.; Manning, J.T.; Harsell, N.; Cook, R.; Huang, C.; Nikolich-Zugich, J.; Makishima, T.; et al. CD4 T-cell depletion prevents Lassa fever associated hearing loss in the mouse model. PLoS Pathog 2022, 18, e1010557. [Google Scholar] [CrossRef]
- Garnett, L.E.; Strong, J.E. Lassa fever: With 50 years of study, hundreds of thousands of patients and an extremely high disease burden, what have we learned? Curr Opin Virol 2019, 37, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Okokhere, P.O.; Ibekwe, T.S.; Akpede, G.O. Sensorineural hearing loss in Lassa fever: two case reports. J Med Case Rep 2009, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, T.S.; Okokhere, P.O.; Asogun, D.; Blackie, F.F.; Nwegbu, M.M.; Wahab, K.W.; Omilabu, S.A.; Akpede, G.O. Early-onset sensorineural hearing loss in Lassa fever. Eur Arch Otorhinolaryngol 2011, 268, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; Poon, L.L.M.; Guan, Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol 2009, 45, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Simsolo, C. Acute unilateral sensorineural hearing loss due to H1N1 infection. Isr Med Assoc J 2010, 12, 450. [Google Scholar]
- Alsanosi, A.A. Influenza A (H1N1): a rare cause of deafness in two children. J Laryngol Otol 2012, 126, 1274–1275. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Huang, C.-C.; Hsueh, P.-Y.; Lee, T.-J. Bilateral sudden deafness following H1N1 vaccination. Otolaryngol Head Neck Surg 2010, 143, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Goldman, R.D. Novel influenza A(H1N1): clinical presentation, diagnosis, and management. Pediatr Emerg Care 2009, 25, 791–796. [Google Scholar] [CrossRef]
- Hviid, A.; Rubin, S.; Mühlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Richardson, M.; Elliman, D.; Maguire, H.; Simpson, J.; Nicoll, A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J 2001, 20, 380–391. [Google Scholar] [CrossRef]
- Hashimoto, H.; Fujioka, M.; Kinumaki, H. An office-based prospective study of deafness in mumps. Pediatr Infect Dis J 2009, 28, 173–175. [Google Scholar] [CrossRef] [PubMed]
- Everberg, G. Deafness following mumps. Acta Otolaryngol 1957, 48, 397–403. [Google Scholar] [CrossRef]
- Vuori, M.; Lahikainen, E.A.; Peltonen, T. Perceptive deafness in connectionwith mumps. A study of 298 servicemen suffering from mumps. Acta Otolaryngol 1962, 55, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Fujiwara, K.; Fukuda, A.; Fukuda, S.; Nishio, S.-Y.; Kitoh, R.; Hato, N.; Ikezono, T.; Ishikawa, K.; Kaga, K.; et al. The clinical features and prognosis of mumps-associated hearing loss: a retrospective, multi-institutional investigation in Japan. Acta Otolaryngol 2017, 137, S44–S47. [Google Scholar] [CrossRef] [PubMed]
- El-Badry, M.M.; Abousetta, A.; Kader, R.M.A. Vestibular dysfunction in patients with post-mumps sensorineural hearing loss. J Laryngol Otol 2015, 129, 337–341. [Google Scholar] [CrossRef]
- Davis, L.E.; Johnson, R.T. Experimental viral infections of the inner ear. I. Acute infections of the newborn hamster labyrinth. Lab Invest 1976, 34, 349–356. [Google Scholar] [PubMed]
- Tanaka, K.; Fukuda, S.; Suenaga, T.; Terayama, Y. Experimental mumps virus-induced labyrinthitis. Immunohistochemical and ultrastructural studies. Acta Otolaryngol Suppl 1988, 456. [Google Scholar] [CrossRef]
- Lindsay, J.R.; Davey, P.R.; Ward, P.H. Inner ear pathology in deafness due to mumps. Ann Otol Rhinol Laryngol 1960, 69, 918–935. [Google Scholar] [CrossRef]
- Smith, G.A.; Gussen, R. Inner ear pathologic features following mumps infection. Report of a case in an adult. Arch Otolaryngol 1976, 102, 108–111. [Google Scholar] [CrossRef]
- Brief report: update: mumps activity--United States, January 1-October 7, 2006. MMWR Morb Mortal Wkly Rep 2006, 55, 1152–1153.
- Noda, T.; Kakazu, Y.; Komune, S. Cochlear implants for mumps deafness: two paediatric cases. J Laryngol Otol 2015, 129 Suppl 2, S38–S41. [Google Scholar] [CrossRef]
- Stevens, G.A.; Bennett, J.E.; Hennocq, Q.; Lu, Y.; De-Regil, L.M.; Rogers, L.; Danaei, G.; Li, G.; White, R.A.; Flaxman, S.R.; et al. Trends and mortality effects of vitamin A deficiency in children in 138 low-income and middle-income countries between 1991 and 2013: a pooled analysis of population-based surveys. Lancet Glob Health 2015, 3, e528–e536. [Google Scholar] [CrossRef] [PubMed]
- Remington, P.L.; Hall, W.N.; Davis, I.H.; Herald, A.; Gunn, R.A. Airborne transmission of measles in a physician's office. JAMA 1985, 253, 1574–1577. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Perry, R.T.; Halsey, N.A. The clinical significance of measles: a review. J Infect Dis 2004, 189 Suppl 1, S4–16. [Google Scholar]
- Ojala, A. On changes in the cerebrospinal fluid during measles. Ann Med Intern Fenn 1947, 36, 321–331. [Google Scholar]
- McKenna, M.J. Measles, mumps, and sensorineural hearing loss. Ann N Y Acad Sci 1997, 830, 291–298. [Google Scholar] [CrossRef]
- Niparko, J.K. Pathology of the ear, Second Edition. By Harold F. Schuknecht, Lea & Febiger, Malvern, Pennsylvania, 1993, 672 pp, $149.50. Head & Neck 1994, 16, 298–298. [Google Scholar] [CrossRef]
- Sagar, P.R.; Shah, P.; Bollampally, V.C.; Alhumaidi, N.; Malik, B.H. Otosclerosis and Measles: Do Measles Have a Role in Otosclerosis? A Review Article. Cureus 2020, 12, e9908. [Google Scholar] [CrossRef]
- Bellini, W.J.; Helfand, R.F. The challenges and strategies for laboratory diagnosis of measles in an international setting. J Infect Dis 2003, 187 Suppl 1, S283–S290. [Google Scholar] [CrossRef]
- Asatryan, A.; Pool, V.; Chen, R.T.; Kohl, K.S.; Davis, R.L.; Iskander, J.K. Live attenuated measles and mumps viral strain-containing vaccines and hearing loss: Vaccine Adverse Event Reporting System (VAERS), United States, 1990--2003. Vaccine 2008, 26, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Semaan, M.T.; Gehani, N.C.; Tummala, N.; Coughlan, C.; Fares, S.A.; Hsu, D.P.; Murray, G.S.; Lippy, W.H.; Megerian, C.A. Cochlear implantation outcomes in patients with far advanced otosclerosis. Am J Otolaryngol 2012, 33, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Fornůsková, A.; Hiadlovská, Z.; Macholán, M.; Piálek, J.; de Bellocq, J.G. New Perspective on the Geographic Distribution and Evolution of Lymphocytic Choriomeningitis Virus, Central Europe. Emerg Infect Dis 2021, 27, 2638–2647. [Google Scholar] [CrossRef]
- Lymphocytic choriomeningitis virus infection in organ transplant recipients--Massachusetts, Rhode Island, 2005. MMWR Morb Mortal Wkly Rep 2005, 54, 537–539.
- Parker, J.C.; Igel, H.J.; Reynolds, R.K.; Lewis, A.M.; Rowe, W.P. Lymphocytic choriomeningitis virus infection in fetal, newborn, and young adult Syrian hamsters (Mesocricetus auratus). Infect Immun 1976, 13, 967–981. [Google Scholar] [CrossRef]
- Bonthius, D.J. The Arenaviruses. In Neurotropic Viral Infections: Volume 1: Neurotropic RNA Viruses, Reiss, C.S., Ed.; Springer International Publishing: Cham, 2016; pp. 149–174. [Google Scholar]
- Lewis, J.M.; Utz, J.P. Orchitis, parotitis and meningoencephalitis due to lymphocytic-choriomeningitis virus. N Engl J Med 1961, 265, 776–780. [Google Scholar] [CrossRef]
- Barton, L.L.; Mets, M.B.; Beauchamp, C.L. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am J Obstet Gynecol 2002, 187, 1715–1716. [Google Scholar] [CrossRef]
- Wilson, M.R.; Peters, C.J. Diseases of the central nervous system caused by lymphocytic choriomeningitis virus and other arenaviruses. Handb Clin Neurol 2014, 123, 671–681. [Google Scholar] [CrossRef]
- Lymphocytic Choriomeningitis (LCM). Available online: https://www.cdc.gov/vhf/lcm/index.html (accessed on.
- Hickerson, B.T.; Westover, J.B.; Jung, K.-H.; Komeno, T.; Furuta, Y.; Gowen, B.B. Effective Treatment of Experimental Lymphocytic Choriomeningitis Virus Infection: Consideration of Favipiravir for Use With Infected Organ Transplant Recipients. J Infect Dis 2018, 218, 522–527. [Google Scholar] [CrossRef]
- Herring, S.; Oda, J.M.; Wagoner, J.; Kirchmeier, D.; O'Connor, A.; Nelson, E.A.; Huang, Q.; Liang, Y.; DeWald, L.E.; Johansen, L.M.; et al. Inhibition of Arenaviruses by Combinations of Orally Available Approved Drugs. Antimicrob Agents Chemother 2021, 65. [Google Scholar] [CrossRef]
- Ayhan, N.; Charrel, R.N. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin Microbiol Infect 2020, 26, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, F.A.; Moreno-Docón, A.; Segovia-Hernández, M.; Fernández-Barreiro, A. [Deafness as a sequela of Toscana virus meningitis]. Med Clin (Barc) 2008, 130, 639. [Google Scholar] [PubMed]
- Howell, B.A.; Azar, M.M.; Landry, M.L.; Shaw, A.C. Toscana virus encephalitis in a traveler returning to the United States. J Clin Microbiol 2015, 53, 1445–1447. [Google Scholar] [CrossRef] [PubMed]
- Kilic, O.; Kalcioglu, M.T.; Cag, Y.; Tuysuz, O.; Pektas, E.; Caskurlu, H.; Cetın, F. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis 2020, 97, 208–211. [Google Scholar] [CrossRef]
| Genbank common name | Genome type | Genome size | Order | Family | Genus |
|---|---|---|---|---|---|
| Cytomegalovirus | dsDNA | 240 kb | Herpesvirales | Herpesviridae | Cytomegalovirus |
| Severe acute respiratory syndrome coronavirus 2 | +ssRNA | 29.9 kb | Nidovirales | Coronaviridae | Betacoronavirus |
| Herpes simplex virus | dsDNA | 150 kb | Herpesvirales | Herpesviridae | Simplexvirus |
| Varicella-zoster virus | dsDNA | 120–130 kb | Herpesvirales | Herpesviridae | Varicellovirus |
| Epstein-Barr virus | dsDNA | 172 kb | Herpesvirales | Herpesviridae | Lymphocryptovirus |
| Hepatitis B virus | dsDNA | 3.2 kb | Blubervirales | Hepadnaviridae | Orthohepadnavirus |
| Human immunodeficiency virus | +ssRNA | 9.18 kb | Ortervirales | Retroviridae | Lentivirus |
| Rubella virus | +ssRNA | 9.7 kb | Hepelivirales | Matonaviridae | Rubivirus |
| Zika virus | +ssRNA | 10.8 kb | Amarillovirales | Flaviviridae | Flavivirus |
| West Nile virus | +ssRNA | 10–11 kb | Amarillovirales | Flaviviridae | Flavivirus |
| Human enterovirus | +ssRNA | 7.4 kb | Picornavirales | Picornaviridae | Enterovirus |
| Lassa virus | -ssRNA | 10.7 kb | Bunyavirales | Arenaviridae | Mammarenavirus |
| Influenza virus | -ssRNA | 13.6 kb | Articulavirales | Orthomyxoviridae | |
| Mumps orthorubulavirus | -ssRNA | 15.3 kb | Mononegavirales | Paramyxoviridae | Orthorubulavirus |
| Measles virus | -ssRNA | 16 kb | Mononegavirales | Paramyxoviridae | Morbillivirus |
| Lymphocytic choriomeningitis mammarenavirus | -ssRNA | 11 kb | Bunyavirales | Arenaviridae | Mammarenavirus |
| Toscana virus | -ssRNA | 12.5 kb | Bunyavirales | Phenuiviridae | Phlebovirus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





