1. Introduction
Among social organisms, honey bees exhibit defensive mechanisms as a fundamental tool for their survival [
1]. This complex behavioral pattern (aggressive behavior) is expressed in
Apis mellifera L. and influenced by external and internal stimuli [
2,
3] which may vary in similar colonies in an apiary [
4,
5,
6]. The phenotypic expression of behavioral patterns in honeybee colonies could depend on both individual and colony characterization [
7,
8]. The survival and production of hive products in honeybee colonies depends on their behavioral output which is a key factor in animal ecology. For instance: the removal of diseased brood in honeybee colonies is attributed to hygienic behavior [
9,
10]; good communication network, pollen and honey storage are eased by wax and comb-building ability [
11]; protection of colony resources is aided by aggressive behavior [
1].
Aggressiveness in honey bees is not only beneficial to honeybee colonies but to humans as well. Although aggressive bees have been known to be a potential source of death to animals and human [
3], keeping significantly defensive honeybee colonies has become a conservation positive activity [
12]. Knowledge of honeybee aggressiveness has recently become very important regardless of public hazards associated with their painful stings. Honey bee stings are well known to deter a wide range of intruders (predators) around honeybee colonies. Crop raiding by elephants [
13,
14] is now known to be managed by using
Apis mellifera in beehive fences [
13,
15,
16,
17].
Aggression in honeybee colonies is affected by many factors. However, the release of stings spread pheromones that mitigate their defensive behavior to culminate in a massive attack [
18]. In another study, aggression is performed by worker bees defending their hives against intruders [
3,
19] and is facilitated by a massive response coordinated by many worker bees [
3]. Also, social information due to environmental changes could impact adult bee responsiveness to aggressive cues [
20] which further regulate individual phenotype displayed by nest mates [
21,
22,
23].
Aggressiveness in honey bees has been reported as a complicated behavior with well-defined components (environmental, maturity, and inherited) [
3,
24]. It is thought to be fully developed in bees older than 7 days [
25] and its phenotypic expression varies among species. For instance, Africanized bees were reported to be more defensive than European bees [
27,
28]. This trait was also found to be heritable [
29] and genetically dominant [
30,
31]. In the past decades, field investigations have been carried out to evaluate the defensive behavior in
Apis mellifera meanwhile it is necessary to have a better understanding of the phenotypic variability in aggressiveness among bred lines of honeybee colonies. Important drivers of aggressiveness (pursuit and stinging) that are highly linked to orientation flight are considered to determine this aspect.
The demand for defensive bees by man has called on the attention of honeybee breeders to select and breed aggressive bees for a purpose. However, defensive bees can pursue and sting any moving object upon disturbance [
32] without targeting. The use of chemical pheromones [
33] and small moving objects [
22,
33] are field assays adopted to evaluate aggressiveness in honey bees. However, knowledge of honeybee orientation aggressiveness towards small moving targeted objects, when disturbed, is scanty. Thus, bees that are capable of orientating their aggressiveness towards small targeted objects when disturbed are of great interest. To achieve this, Field evaluation of aggressiveness among bred lines of honey bees is required to overcome the challenges by practicing controlled breeding to maintain desirable traits [
34]. We bred
Apis mellifera L. in isolated mating stations and used field assays to simultaneously evaluate their aggressive responses. We repeatedly examined whether aggression is oriented and differs among bred lines as well as the minimum time within which honeybees’ response to this behavior is clearly visible. This study provides evidence that aggression and orientation varies among bred lines of honeybee colonies and that, aggressive bees oriented flight more than less-aggressive bees.
2. Materials and Methods
2.1. Breeding and placement of honey bee colonies at the experimental apiary
Honeybee colonies of
Apis mellifera L. were selected in early spring (March) 2022 from the experimental apiary (35.591° N, 126.278° E) of the honeybee breeding laboratory of the National Institute of Agricultural Sciences, Rural Development Administration, Wanju, Republic of Korea. The colonies were made up of five lineages of
Apis mellifera L. (lines A, B, C, D, and E). In spring (April to May 2022), a single strong queenright colony was selected from each lineage for drone and queen rearing. One empty built comb and one empty drone comb were marked and provided to each colony for the queen to deposit fertilized and unfertilized eggs respectively. These combs were inserted in-between broods and the presence of eggs was checked every 24 h. Within a period of 3 days when eggs are expected to hatch into larvae, the queen cell combs were removed to transfer the first-instar (≤20 h-old) larvae into artificial queen cell cups for queen rearing [
35]. Each queen cell cup attached to rearing frames contained one drop (5 ml syringe) of diluted royal jelly in water at a ratio of 1:1 (v/v).The queen bee was excluded with a queen excluder by making a second floor (supper) for rearing the larvae. Each colony was fed sugar syrup and pollen cake as food supplements for drone and queen rearing. Ten mating nuclei (mating hives) were prepared for each lineage. Each mating hive contained one brood comb with worker bees, one food comb (honeycomb), a feeder and 2 ̶ 3 capped queen cells. Regarding the life cycle of queen (16 days) and drone (25 days) bees, drones were reared and taken to mating stations before mating hives. Three days prior to queen emergence, 2 ̶ 3 capped queen cells were inserted into each mating hive and placed at mating stations. The presence of emerged queens was examined three days later. After monitoring mating hives at mating stations for fifteen days, we recorded the presence of eggs and larvae. Successfully mated queens were moved with nuclei hives to the experimental apiary. Each lineage was bred separately from another in a closed mating system. Although mating success was not 100%, mated colonies were placed at the experimental apiary according to lineage (bred lines). Colonies were fed sugar syrup and pollen cake for colony development after which experimental colonies were selected per bred line.
2.2. Chemical, physical and visual stimuli application (assays)
We applied a series of assays according to Collins et al. [
36] to test the aggressive behavior in honey bee colonies. Chemicals associated with the honey bee sting (alarm pheromones) were mixed and diluted in paraffin oil at a ratio of 1: 9 (v/v) as described by Blum et al. [
37] with the omission of n-decyl acetate (
Table 1). To provide a visual stimulus, a square of dark suede leather (5 by 5 cm) made from an animal skin (pig) (
Figure S1) was attached on movable objects and jiggled at the colonies entrance. Honey bee colonies were marbled from behind as a physical stimulus to elicit aggressiveness. A combination of these stimuli was provided to each colony for evaluating their aggressive behavior.
2.3. Assessment of colonies aggressiveness and orientation
Fifteen queenright colonies from five bred lines (three colonies per bred line) placed at the experimental apiary were selected and used for this study. Sampling was done in autumn (September and October, late flowering season), during the day, and at an interval of two days. The experiment was conducted in two parts: evaluation of aggressive behavior (part A), and determination of orientation aggressiveness (part B).
PART A: To evaluate the aggressive behavior in the honeybee colonies, three treatments were employed to test aggression in honey bees: T1, alarm pheromone (
Table 1); T2, chemical composition of isopentyl acetate and paraffin oil at a ratio of 1: 9 (v/v); and T3, no chemical application. Johnson et al. [
38] reported that isopentyl acetate (IPA) is the principal component of the alarm pheromone. T1 and T2 were sprayed on square suede while T3 was empty suede (control). Colonies were not disturbed (no marbling). Suede were attached to movable objects and held 40 cm perpendicular to the hive entrance. Movable objects with suede were jiggled vertically at 2 rounds per second (rps) to provide a visual cue to bees. Sampling was done once a day at 10 sec, 30 sec, 60 sec, and 90 sec using a stopwatch at an interval of two days and replicated four times. We recorded the time for the first attack on the suede, the culminating intensity and the number of stings left on the suede.
PART B: To determine whether honey bee colonies perform orientation aggressiveness, alarm pheromone (
Table 1) and paraffin oil were sprayed on separate square leather suede using a hand sprayer (APOLLO IND CO. LTD, kr). Both suede were attached to movable objects and held 40cm perpendicular to the hive entrance and 30 cm apart. Movable objects with suede were jiggled up and down simultaneously at 2 rps to provide a visual cue to bees. Paraffin was used as the control. The experiment was conducted in two rounds (marbled and non-marbled). We anticipated that the simultaneous introduction of alarm pheromone and paraffin will be a positive test to determine orientation when colonies are disturbed and when normal. Cues necessary for alerting, activating, recruiting, and attacking were provided to provoke a response. Colonies were marbled once during each sampling to allow colonies stability before the next sampling. Sampling was done in 10sec, 30sec, 60sec, and 90sec each replicated four times. The number of stings left on the suede was recorded in all instances.
2.4. Data analysis
Data were described using Descriptive Statistics. One-way analysis of variance (ANOVA) was used to compare the means of more than two groups, followed by the Tukey pos-hoc test. Two-tailed Student’s t-test and the Mann-Whitney test were used to compare the means of two groups. Pearson’s correlation was used to evaluate the relationship between the mean number of stings for alarm pheromone and paraffin mixed with IPA over the experimental period. The non-parametric Kruskal-Wallis test followed by multiple pairwise comparison of variance using Dunn’s procedure was used to compare the means of more than two groups that were not normally distributed. The XLSTAT statistical software version 2007.8.04 was used to conduct the analysis with levels of significance set at 5%.
3. Results
3.1. Effects of assay on time and intensity of recruitment of honey bees on the suede.
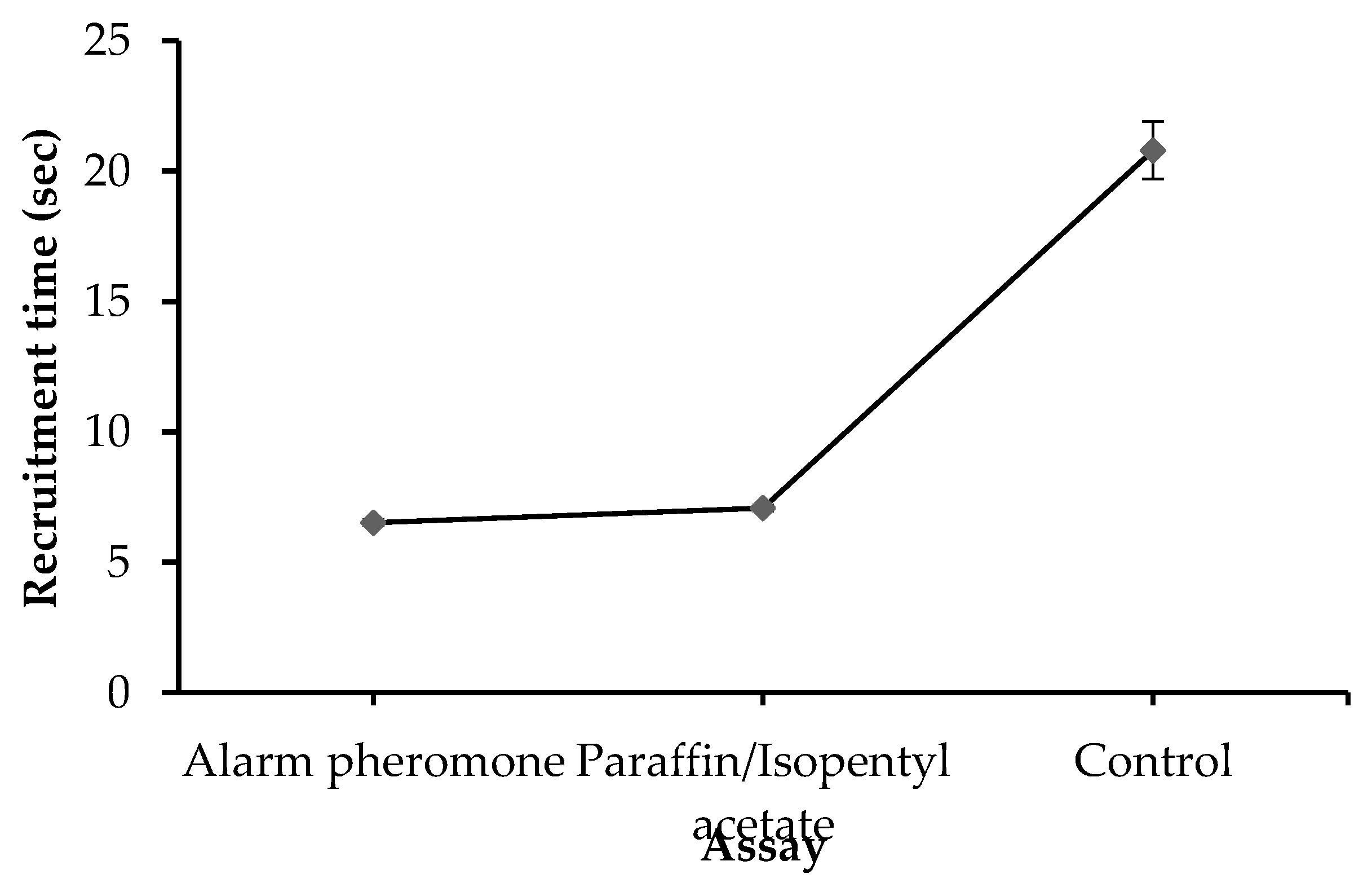
The recruitment time of honey bees towards an assay was used to describe the level at which respective assays could alert bees under the same visual cue and environmental conditions. The degree of the crowdedness of honey bees around assays showed the intensity of the alert. The intensity of recruitment varied from high (for alarm pheromone), average (for isopentyl acetate/paraffin) to low (for the empty suede) (
Table 2). Using the alarm pheromone test, the time of recruitment of honey bees was marginally significant among bred lines (F
4, 235 = 2.386, P = 0.052) (
Table 2). Meanwhile the time of recruitment varied significantly among bred lines when a mixture of paraffin and IPA was applied (F
4, 235 = 12.063, P < 0.0001) (
Table 2). The time of recruitment for the control (empty suede) did not differ significantly among bred lines (F
4, 92 = 0.105, P = 0.981) (
Table 2). Within bred lines, the time of recruitment equally differed significantly between the three assays: line A (F
2, 116 = 79.457, P < 0.0001); line B (F
2, 111 = 86.603, P < 0.0001); Line C (F
2, 116 = 62.636, P < 0.0001); line D (F
2, 105 = 56.180, P < 0.0001); and line E (F
2, 114 = 73.332, P < 0.0001).
Generally, the time of recruitment varied significantly among assays (F
2, 56 = 56.955, P < 0.0001). The mean time of recruitment for alarm pheromone, Paraffin mixed with IPA, and the control was 6.52 sec, 7.08 sec and 18.88 sec respectively (
Figure 1). Similarly, the time of recruitment recorded for alarm pheromone and paraffin mixed with IPA differed significantly (t = 1.965, P = 0.0004). However, the time difference in recruitment between alarm pheromone and paraffin mixed with IPA was relatively small (0.56 sec) compared to that of the control (12.36 sec) (
Figure S2).
3.2. Behavioral responses of honey bee colonies to different assays
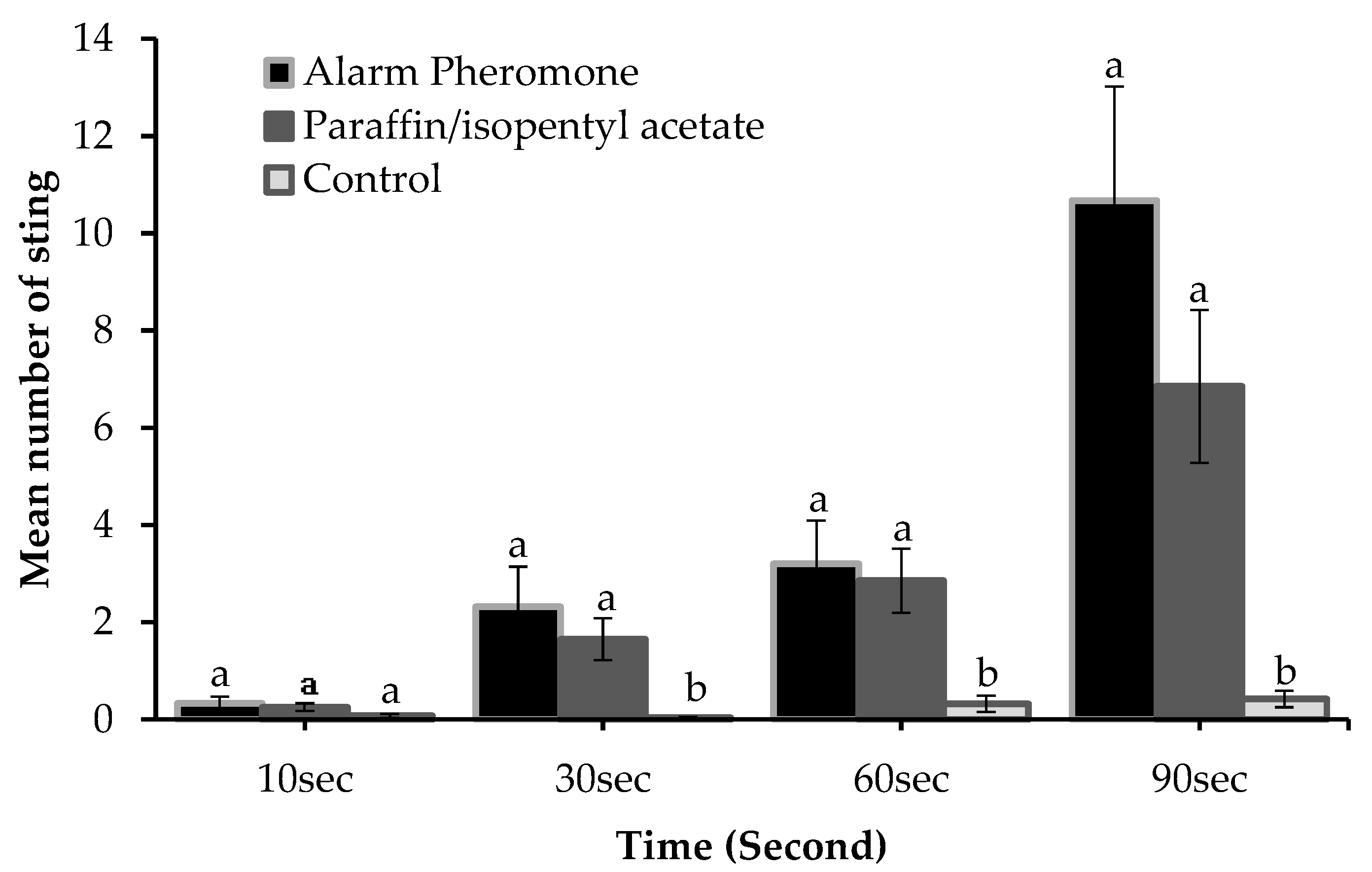
The behavioral response of honey bees which culminated in a sting varied according to assay and time of sampling (
Figure 2). The results indicated that the mean number of stings for the three assays did not differ significantly at 10 sec (F
2, 42 = 1.799, P = 0.178) but differed significantly at 30 sec (F
2, 42 = 4.577, P = 0.016), 60 sec (F
2, 42 = 5.082, P = 0.011), and 90 sec (F
2, 42 = 6.983, P = 0.002) (
Figure 2). Though no significant difference was found for the mean number of stings between alarm pheromone and paraffin mixed with IPA, it is evident that the mean number of stings was higher in alarm pheromone suede compared to paraffin mixed with IPA suede at all instances (
Figure 2). This indicates that honey bees responded more to the alarm pheromone suede than other suede. The duration at which assays were exposed to honey bee colonies influenced their stinging response as the number of stings increase with time of exposure (
Figure 2).
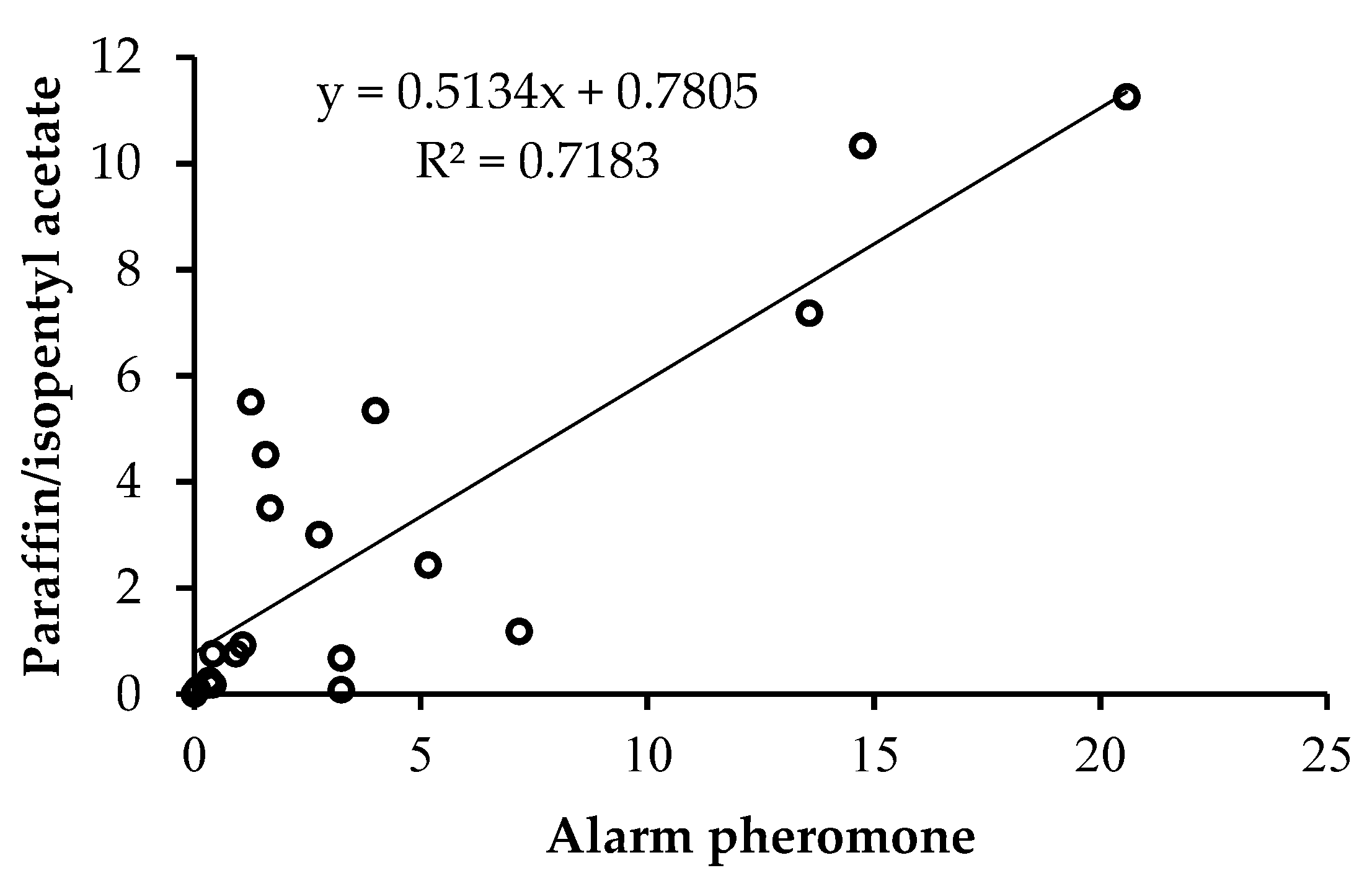
Honey bees responded positively to the two chemical assays but the rate of response was higher in alarm pheromone than paraffin mixed with IPA at all intervals (
Table S1). The mean number of stings for both chemical assays increased with increasing time of exposure. However, the degree of increase was higher in alarm pheromone suede compared to paraffin mixed with IPA (
Figure 3). A significantly positive correlation was recorded between honey bee response (number of stings) to the two chemical assays at different time of sampling (r = 0.848, P < 0.0001) (
Figure 3).
3.3. Variation in the aggressive behavior among honey bee bred lines.
The mean number of stings did not vary significantly in lines A, B, C and D but varied significantly in line E for both the alarm pheromone and paraffin mixed with IPA (
Table 3). In the control experiment, the mean number of stings per line did not differ significantly for all the five bred lines (
Table 3). We found a significant difference in the mean number of stings among bred lines for alarm pheromone and control but not for paraffin mixed with IPA (
Table 3). Generally, honey bee aggression was significantly higher in lines E and C compared to other bred lines across all three treatments. However, no significant difference was observed per bred line between alarm pheromone and paraffin mixed with IPA. This indicated that honey bee aggression which culminated in a sting was similar to the alarm pheromone and paraffin mixed with IPA. Though in most bred lines (80%), the mean number of stings was higher in the alarm pheromone compared to paraffin mixed with IPA, paraffin mixed with IPA is a chemical cue necessary to elicit an aggressive response in honey bees.
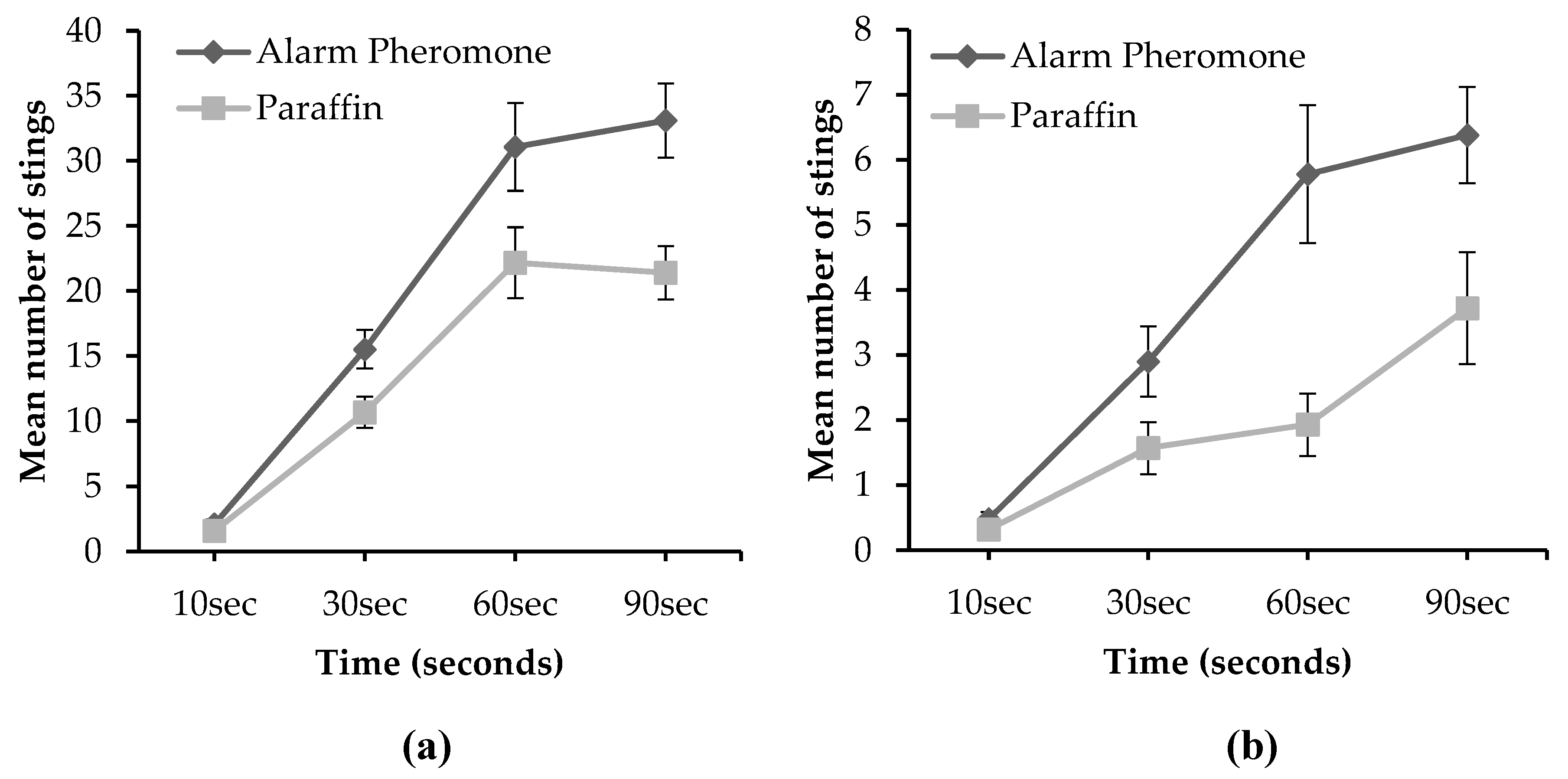
3.4. Orientation aggressiveness among bred lines of honey bee colonies.
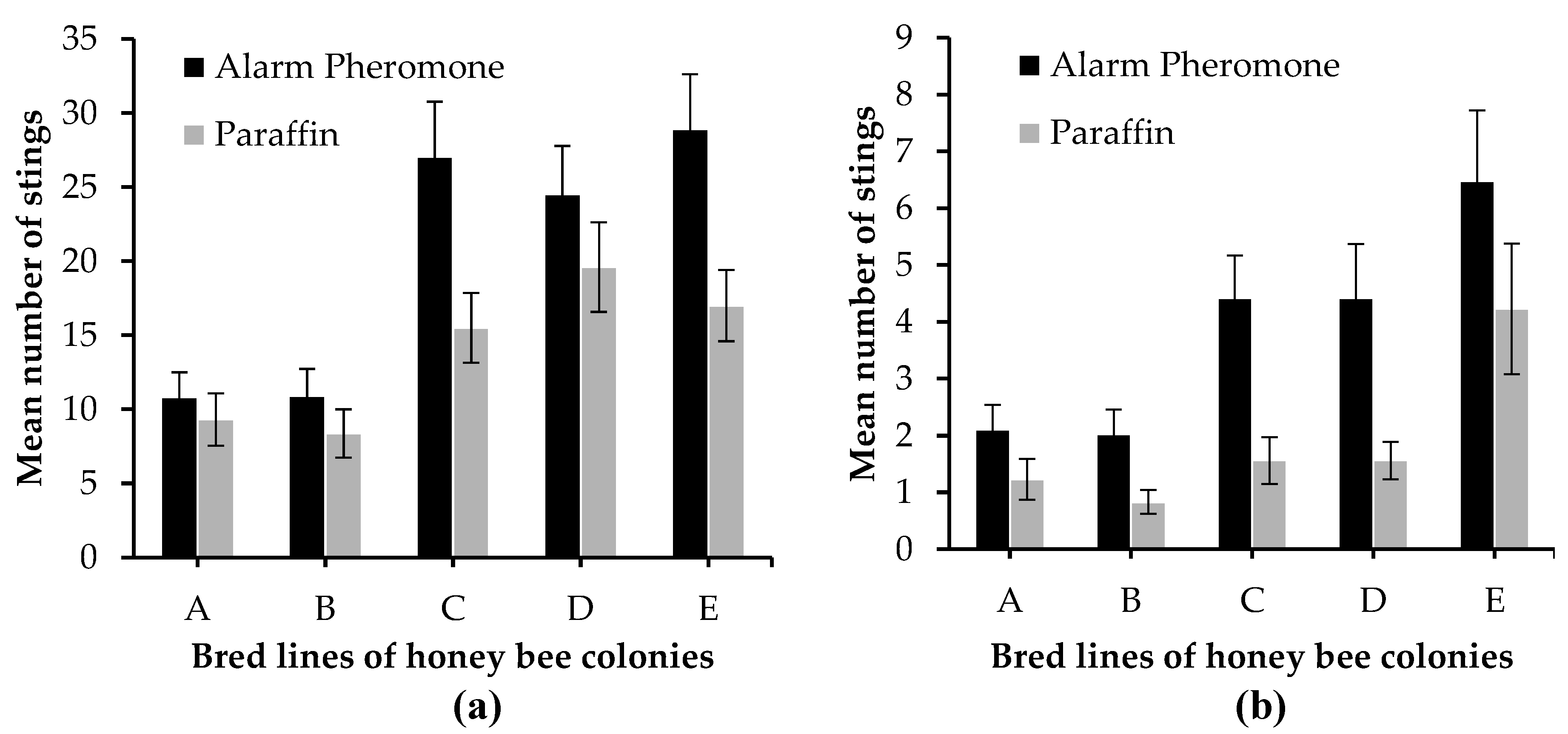
The mean number of honey bee stings in response to either alarm pheromone or paraffin suede was recorded when colonies were marbled and when colonies were not marbled (
Figure 4). Colonies C, D, and E were found to be the most aggressive and orientated their flight towards the alarm pheromone suede (
Figure 4). We found significant differences for the mean number of stings between alarm pheromone and paraffin when colonies were marbled (U = 33109, P = 0.004) and when colonies were not marbled (U = 36683, P < 0.0001). Generally, the mean number of stings for alarm pheromone was higher than that of paraffin when colonies were marbled (20.44 ± 1.42 and 13.96 ± 1.05 respectively) and when colonies were not marbled (3.89 ± 0.38 and 1.88 ± 0.28 respectively) (±SE). When colonies were marbled, the mean number of stings differed significantly among bred lines for alarm pheromone (K = 25.185, df = 4, P < 0.0001) and paraffin (K = 14.551, df = 4, P = 0.006). This did not follow the same scenario when colonies were not marbled because the mean number of stings varied significantly among bred lines for alarm pheromone (K = 16.178, df = 4, P = 0.003) but insignificant for paraffin (K = 9.211, df = 4, P = 0.056) (
Table S2).
In line A, there was no significant difference between the mean number of stings for alarm pheromone and paraffin when colonies were marbled (U = 1258, P = 0.438) and when colonies were not marbled (U = 1387, P = 0.063). The mean number of stings did not differ significantly between alarm pheromone and paraffin when colonies were marbled (U = 1275, P = 0.363) but differed significantly when colonies were not marbled (U = 1456, P = 0.016) in line B. We recorded significant differences in the mean number of stings between alarm pheromone and paraffin when colonies were marbled (U = 1467, P = 0.021) and when colonies were not marbled (U = 1611.5, P = 0.0004) in line C. In line D, the mean number of stings did not differ significantly between alarm pheromone and paraffin when colonies were marbled (U = 1280, P = 0.348) but differed significantly when colonies were not marbled (U = 1443.5, P = 0.027). The mean number of stings between alarm pheromone and paraffin in line E differed significantly when marbled (U = 1432, P = 0.04) and when not marbled (U = 1454, P = 0.024). However, no significant differences were observed between mean number of stings for alarm pheromone and paraffin at 60 sec and 90 sec (
Figure 5).
4. Discussion
Honey bee workers express behavioral responses to defend their hives against intruders (aggressive behavior) [
3] and equally remove dead broods, and parasites and limit the spread of some diseases in the colonies [
39].
Though we found a significant difference in the time of recruitment between alarm pheromone and paraffin mixed with IPA, a mixture of paraffin and IPA recruited honey bees within the average possible time (
Figure S3). Our results agree with those of Wager and Breed [
40] who found that IPA had a higher level of flight activity and recruited bees than other components of the alarm pheromone. The present study strongly supports the fact that IPA is a major component of the sting alarm pheromone [
41,
42]. It is left to investigate the different concentrations of IPA mixed with paraffin oil on the degree and intensity of recruitment of honey bees because Lensky et al. [
43] reported that increasing levels of stinging responses of isolated bees corresponds to the concentration of IPA used for stimulation.
Despite the combination of many chemicals to form the alarm pheromone, IPA showed the capacity to elicit aggressive responses (alerting, recruiting and attracting) in honey bees which also increased with time of exposure. Collins et al. [
36] reported that IPA is probably a stimulus to elicit aggressive behavior in honey bees by alerting, activating and attracting the bees. In 1989, Collins et al. [
42] found positive correlations between levels of IPA and defensive behavior in the field (number of stings) and in the laboratory. Collins and Kubasek [
33] documented that the mean number of bees responding to the chemical pheromone outside the colony entrance increases with time. The marginally significant difference in the number of stings between alarm pheromone and paraffin mixed with IPA could be attributed to the multi-component of the alarm pheromone which provided a complex signaling system to the bees.
In most bred lines (80%), the mean number of stings was higher in the alarm pheromone compared to paraffin mixed with IPA, paraffin mixed with IPA is a chemical cue necessary to elicit an aggressive response in honey bees. According to a model of honey bee defensive behavior [
36], IPA is probably a stimulus for alerting, activating, and attracting but not for culminating. In this study, it is evident that honey bee response to paraffin mixed with IPA culminated in a sting. In all apiaries and in the wild, honey bee colonies respond aggressively when disturbed or towards a stimulus, but the intensity of the response may differ from colony to colony [
21]. Aggressiveness in honey bee is a social behavior well known to be influenced by environmental, maturational and hereditary factors [
3,
24]. The variation in our study (among bred lines) is linked to hereditary because environmental and maturational factors were kept constant. We anticipated that the duration of exposure of honey bees to paraffin mixed with IPA could orientate their aggression and consequently the number of stings. Harrison et al. [
44] reported that long-term modulation of social cues induced changes in the phenotypic expression of aggression.
Aggressive bred lines showed high potential of performing orientation flight which culminated in a sting when colonies were disturbed than less aggressive bred lines. The simultaneous introduction of alarm pheromone and paraffin on suede at the hive entrance induced a cue that permitted bees to orientate their flight. Other methods like video recording are also used to track flight paths at hive entrance [
45]. Scheiner et al. [
46] used behavioral assays to induce a response by disturbing the hive. It is still undefined whether honey bees can orientate their flight by distinguishing two nearby cues when disturbed. Collins and Kubasek [
33] reported that 90% of honey bees responded to the alarm pheromone within an average time of 13.6 ± 8.95 sec. Boch et al. [
47] found that the capacity of the alarm pheromone to alert and attract bees at the hive entrance was 1.32 times greater than that of identical concentrations of IPA though both essays were not simultaneously introduced. This study indicates that honey bees orientated their aggressiveness towards the alarm pheromone more than the control (paraffin) in both cases (marbled and non-marbled). However, the degree of orientation varies with bred lines and aggressiveness where very aggressive bred lines performed orientation more than less aggressive lines. For instance, bred lines C, D and E expressed high level of aggression and orientated their aggressiveness towards a particular stimulus (alarm pheromone) compared to bred lines A and B with low level of aggression (
Table S2). Again, we recorded insignificant disparity for orientation aggressiveness within individual bred lines of honeybee colonies. Hunt et al. [
24] reported that aggressiveness in honey bees is influenced by hereditary factors. It is convincing though important to understand the extent to which the phenotypic expression of the hereditary factors varies among different bred lines of the same species. In another study conducted by Harrison et al. [
44], the phenotypic expression of aggressiveness in honey bees can be modulated due to differentiation in social cues by certain genes on the course of time. In our study, the phenotypic variation in the aggressive behavior among bred lines of honey bee colonies could be attributed to changes in inherited characteristics over time.
5. Conclusions
Honey bee aggression is a social behavior that can be influenced by internal and external cues. In this study, the results demonstrated that both alarm pheromone and IPA mixed with paraffin recruited bees to suede but the time of recruitment was lesser in alarm pheromone compared to IPA mixed with paraffin. Honey bee response to both pheromones culminated to a sting though higher in the alarm pheromone. The phenotypic expression of aggressiveness varied among bred lines of honey bee colonies. Honey bees performed orientation aggressiveness which varied among bred lines and was higher in more aggressive bred lines compared to less aggressive bred lines. Therefore, it is crucial to repeatedly evaluate aggressiveness and orientation at the colony level and among bred lines when selecting defensive and less defensive honey bee colonies for breeding. Further studies should be conducted to evaluate the different concentrations of IPA mixed in paraffin necessary to elicit aggression and orientation in honey bee colonies within the shortest possible time. The use of a single chemical to determine aggressive bees is less costly compared to the complex alarm pheromone if similar results could be obtained.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org, Figure S1: Dark leather suede used for evaluating aggressiveness in honey bees; Figure S2: Box plot for the time of recruitment of honey bee per assay; Figure S3: Variation in the recruitment time among bred lines of honey bee colonies; Table S1: Mean number of stings of honey bee in response to assays at different time of sampling; Table S2: Variation in the mean number of stings among bred lines when colonies were marbled and when not marbled.
Author Contributions
Conceptualization, P.N.A., B-S.P. and Y-S.C.; Methodology, P.N.A. and B-S.P.; Software, P.N.A.; Validation, D-W.K.; Formal Analysis, P.N.A.; Investigation, P.N.A., B-S.P.; Resources, Y-S.C.; Data Curation, P.N.A., B-S.P. and D-W.K.; Writing – Review & Editing, P.N.A., B-S.P.; Visualization, D-W.K. and Y-S.C.; Supervision, Y-S.C.; Project Administration, B-S.P. and Y-S.C.; Funding Acquisition, B-S.P. All authors have read and approved the final manuscript.
Funding
This research was funded by the Research Program for Agricultural Science and Technology Development, grant number PJ01418001, National Institute of Agricultural Sciences, Rural Development Administration, Republic of Korea.
Data Availability Statement
The data presented in this study are within the manuscript and available on request from the corresponding author.
Acknowledgments
We are grateful to the bee breeding laboratory, National Institute of Agricultural Science (NIAS), Rural Development Administration (RDA), Republic of Korea and the Institute of Agricultural Research for Development (IRAD), Republic of Cameroon for their collaboration during this study. We appreciate the technical assistance of beekeepers in the bee breeding laboratory at NIAS.
Conflicts of Interest
The authors declare no conflict of interests. The sponsors had no role in the design, execution, interpretation, or writing of the study, or in the decision to publish the results.
References
- Alcock, J. Animal behavior: An evolutionary approach, 9th ed.; Sinauer Associates, Sunderland, MA, US, 2009, pp 546. 2009.
- Collins, A.M.; Rinderer, T.E. Effect of empty comb on defensive behaviour of honeybees. J. Chem. Ecol. 1985, 11, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Breed, M.D.; Guzmán-Novoa, E.; Hunt, G.J. Defensive behavior of honey bees: Organization, Genetics, and Comparisons with Other Bees. Annu. Rev. Èntomol. 2004, 49, 271–298. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Rinderer, T.E.; Tucker, K.W. Colony defense of two honeybee types and their hybrid 1naturally mated queens. J Apic Res. 1988, 27, 137–140. [Google Scholar] [CrossRef]
- Villa, J.D. Defensive Behaviour of Africanized and European Honeybees at two Elevations in Colombia. J. Apic. Res. 1988, 27, 141–145. [Google Scholar] [CrossRef]
- Page, R.E.; Robinson, G.E.; Fondryk, M.K.; Nasr, M.E. Effects of worker genotypic diversity on honeybee colony development and behaviour Apis mellifera L. Behav. Ecol. Sociobiol. 1995, 36, 387–396. [Google Scholar] [CrossRef]
- Page, R.E.; Rueppell, O.; Amdam, G.V. Genetics of reproduction and regulationof honeybee (Apis mellifera L. ) social behavior. Annu. Rev. Genet. 2012, 46, 97 ̶ 119. [Google Scholar] [CrossRef]
- Avalos, A.; Fang, M.; Pan, H.; Lluch, A.R.; Lipka, A.E.; Zhao, S.D.; Giray, T.; Robinson, G.E.; Zhang, G.; Hudson, M.E. Genomic regions influencing aggressive behavior in honey bees are defined by colony allele frequencies. Proc. Natl. Acad. Sci. USA 2020, 117, 17135–17141. [Google Scholar] [CrossRef]
- Spivak, M.; Gilliam, M. Hygienic behavior of honey bees and its application for control of brood diseases and varroa. Bee World 1998, 79, 124 ̶ 134. [Google Scholar] [CrossRef]
- Harrison, J.W. Bees with Varroa Sensitive Hygiene preferentially remove mite infested pupae aged ≤ five days post capping. J. Apic. Res. 2007, 46, 134 ̶ 139. [Google Scholar] [CrossRef]
- Bogdanov, S. Beeswax: Production, Properties Composition and control. Bee Product Science 2016, 1, 1 ̶ 18. www.bee–hexagonnet. [Google Scholar]
- Russell, R. Beekeeping, poverty alleviation and conservation in Imadiala, Madagascar. Bees for Development Journal 2008, 84, 6–7. [Google Scholar]
- Ngama, S.; Korte, L.; Bindelle, J.; Vermeulen, C.; Poulsen, J.R. How Bees Deter Elephants: Beehive Trials with Forest Elephants (Loxodonta africana cyclotis) in Gabon. PLoS ONE 2016, 11, e0155690. [Google Scholar] [CrossRef] [PubMed]
- Mmbaga, N.E.; Munishi, L.K.; Treydte, A.C. How dynamics and drivers of land use/land cover change impact elephant conservation and agricultural livelihood development in Rombo, Tanzania. J. Land Use Sci. 2017, 12, 168–181. [Google Scholar] [CrossRef]
- Cook, R.; Parrini, F.; King, L.; Witkowski, E. ; Henley African honeybees as a mitigation method for elephant impact on trees. Biol. Conserv. 2018, 217, 329–336. [Google Scholar] [CrossRef]
- King, L.E.; Lala, F.; Nzumu, H.; Mwambingu, E.; Douglas-Hamilton, I. Beehive fences as a multidimensional conflict-mitigation tool for farmers coexisting with elephants. Conserv. Biol. 2017, 31, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Djoko, I.B.; Weladji, R.B.; Paré, P. Diurnality in the defensive behaviour of African honeybees Apis mellifera adansonii and implications for their potential efficacy in beehive fences. Oryx 2022, 57, 445–451. [Google Scholar] [CrossRef]
- Wright, M.G.; Spencer, C.; Cook, R.M.; Henley, M.D.; North, W.; Mafra-Neto, A. African bush elephants respond to a honeybee alarm pheromone blend. Curr. Biol. 2018, 28, R778–R780. [Google Scholar] [CrossRef]
- Winston, M.L. The biology of the honey bee. Harvard University Press, USA, 1987; pp, 294.
- Rittschof, C.C.; Coombs, C.B.; Frazier, M.; Grozinger, C.M.; Robinson, G.E. Early-life experience affects honey bee aggression and resilience to immune challenge. Sci. Rep. 2015, 5, 15572. [Google Scholar] [CrossRef]
- Alaux, C.; Sinha, S.; Hasadsri, L.; Hunt, G.J.; Guzmán-Novoa, E.; DeGrandi-Hoffman, G.; Uribe-Rubio, J.L.; Southey, B.R.; Rodriguez-Zas, S.; Robinson, G.E. Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 15400–15405. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Novoa, E.; Page. R.E. Genetic dominance and worker interactions affect honey bee colony defense. Behav. Ecol. 1994, 5, 91 ̶ 97. [CrossRef]
- Hunt, G.J.; Guzmán-Novoa, E.; Uribe-rubio, J.L.; Prieto-Merlos, D. Genotype-environment interactions in honeybee guarding behavior. Anim Bahav. 2003, 66, 459–467. [Google Scholar] [CrossRef]
- Hunt, G. Flight and fight: A comparative view of the neurophysiology and genetics of honey bee defensive behavior. J. Insect Physiol. 2007, 53, 399–410. [Google Scholar] [CrossRef]
- Burrell, D.; Smith, B.H. Age-related but not caste-related regulation of abdominal mechanisms underlying the sting reflex of the honey bee, Apis mellifera. J. Comp. Physiol. A. 1994, 174, 581 ̶ 592. [Google Scholar] [CrossRef]
- Collins, A.M.; Rinderer, T.E.; Harbo, J.R.; Bolten, A.B. Colony Defense by Africanized and European Honey Bees. Science 1982, 218, 72–74. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Page, R.E. Backcrossing Africanized honey bee (Apis mellifera L. ) queens to European drones reduces colony defensive behavior. Ann. Entomol. Soc. Am. 1993, 86, 352 ̶ 355. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Page. R.E. The impact of Africanized bees on Mexican beekeeping. Am. Bee. J. 1994, 134, 101 ̶ 106.
- Guzman-Novoa, E.; Hunt, G.J.; Page, R.E.; Fondrk, M.K. Genetic Correlations Among Honey Bee (Hymenoptera: Apidae) Behavioral Characteristics and Wing Length. Ann. Èntomol. Soc. Am. 2002, 95, 402–406. [Google Scholar] [CrossRef]
- Degrandi-Hoffman, G.; Collins, A.M.; Martin, J.H.; Schmidt, J.O.; Spangler, H.G. Nest defense behavior in colonies from crosses between Africanized and European honeybees (Apis melli(era L) (Hymenoptera: Apidae). J. Insect. Behav. 1998, 11, 37 ̶ 45. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Hunt, G.J.; Uribe, J.L.; Smith, C.; Arechavaleta-Velasco, M.E. Confirmation of QTL effects and evidence of genetic dominance of honey bee defensive behavior: results of colony and individual behavioral assays. Behav. Genet. 2002b, 32, 95 ̶ 102. [CrossRef]
- Winston, M.L. Killer bees: the Africanized honey bee in the Americas. Cambridge, Mass, Harvard University Press, USA, 1992; pp. 162.
- Collins, A.M.; Kubasek, K.J. Field Test of Honey Bee (Hymenoptera: Apidae) Colony Defensive Behavior1. Ann. Èntomol. Soc. Am. 1982, 75, 383–387. [Google Scholar] [CrossRef]
- Plate, M.; Bernstein, R.; Hppe, A.; Bienefelt, K. The importance of controlled mating in honey bee breeding. Genet. Sel. Evol. 2019, 51, 1 ̶ 14. [Google Scholar] [CrossRef]
- DOOLITTLE, G.M. Scientific queen-rearing as practically applied; being a method by which the best of queen-bees are reared in perfect accord with nature’s ways: for the amateur and veteran in beekeeping. 6th ed. Am bee J. Hamilton, Illinois, 1915; pp. 126.
- Collins, A.M.; Rinderer, T.E.; Tucker, K.W.; Sylvester, H.A.; Lackett. J.J. A model of honeybee defensive behavior. J. Apic. Res. 1980, 19, 224 ̶ 231.
- Blum, M.S.; Fales, H.M.; Tucker, K.W.; Collins, A.M. Chemistry of the Sting Apparatus of the Worker Honeybee. J. Apic. Res. 1978, 17, 218–221. [Google Scholar] [CrossRef]
- Johnson, L.K.; Haynes, L.W.; Carlson, M.A.; Fortnum, H.A.; Gorgas, D.L. Alarm substances of the stingless bee,Trigona silvestriana. J. Chem. Ecol. 1985, 11, 409–416. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, Individual, and Group Facilitation of Disease Resistance in Insect Societies. Annu. Rev. Èntomol. 2009, 54, 405–423. [Google Scholar] [CrossRef]
- Wager, B.R.; Breed, M.D. Does Honey Bee Sting Alarm Pheromone Give Orientation Information to Defensive Bees? Ann. Èntomol. Soc. Am. 2000, 93, 1329–1332. [Google Scholar] [CrossRef]
- Boch, R.; Shearer, D.A.; Stone, B.C. Identification of Iso-Amyl Acetate as an Active Component in the Sting Pheromone of the Honey Bee. Nature 1962, 195, 1018–1020. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Rinderer, T.E.; Daly, H.V. Alarm pheromone production by two honey bee (Apis mellifera) types. J. Chem. Ecol. 1989, 15, 1747 ̶ 1756. [Google Scholar] [CrossRef]
- Lensky, Y.; Cassier, P.; Tel-Zur, D. The setaceous membrane of honey bee (Apis mellifera L.) workers' sting apparatus: Structure and alarm pheromone distribution. J. Insect Physiol. 1995, 41, 589–595. [Google Scholar] [CrossRef]
- Harrison, J.W.; Palmer, J.H.; Rittschof, C.C. Altering social cue perception impacts honey bee aggression with minimal impacts on aggression-related brain gene expression. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Magnier, B.; Ekszterowicz, G.; Laurent, J.; Rival, M.; Pfister, F. Bee hive traffic monitoring by tracking bee flight paths. 13th Internal joint conference on computer vision, Imaging and Computer Graphics Theory and Applications, January 27 ̵ 29, in Funchal, Madeira, Portugal, 2018, 563 ̶ 571. [CrossRef]
- Scheiner, R.; Abramson, C.I.; Brodschneider, R.; Crailsheim, K.; farina, W.M.; Fuchs, S.; Grünewald, B.; Hahshold, S.; Karrer, M.; Koeniger, G.; Koeniger, N.; Menzel, R.; Mujagic, S.; Radspieler, G.; SchmickI, T.; Schneider, C.; Siegel, A.J.; Szopek, M.; Thenius, R. Standard methods for behavioral studies of Apis mellifera. J. Apic. Res. 2013, 52, 1 ̶ 58. [Google Scholar] [CrossRef]
- Boch, R.; Shearer, D.A.; Petrasovits, A. Efficacies of two alarm substances of the honeybee. J. Insect. Physiol. 1970, 16, 17 ̶ 24. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).