Submitted:
10 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
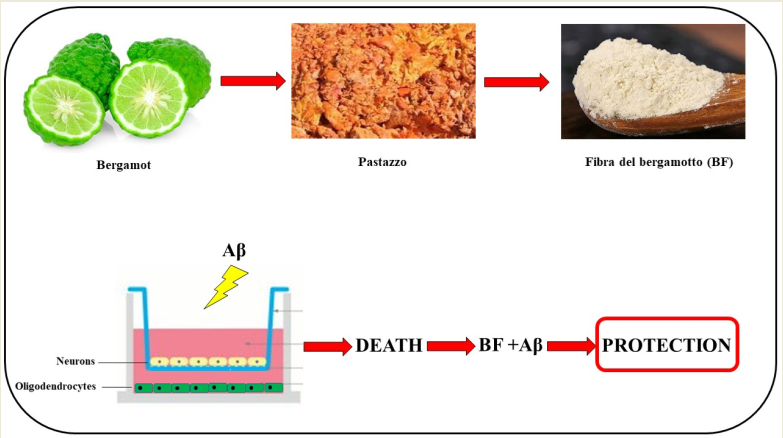
1. Introduction
2. Materials and methods
2.1. Plant material and extraction procedure
- 1).
- bergamot juice or bergamot oil;
- 2).
- pastazzo;
2.2. Oxygen Radical Absorbance Capacity (ORAC)
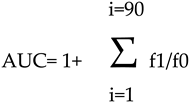
2.3. Measurement of total polyphenols by the Folin-Ciocalteu assay
2.4. Measurement of flavonoids content
2.5. Antioxidant activity through the DPPH assay
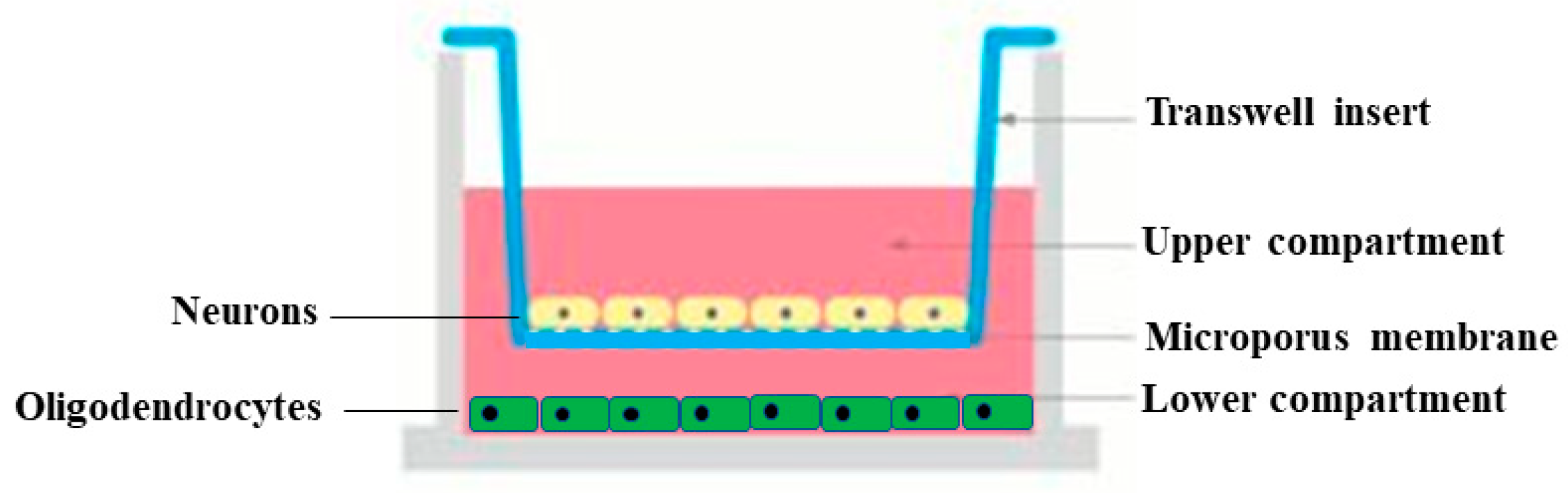
2.6. Cell cultures
2.7. Proliferation assay and cytotoxicity study
2.8. Measurement of in vitro Reactive Oxygen Species
2.9. Cell Lysis and Immunoblot Analysis
2.10. Annexin V Staining
2.11. Immunofluorescence
3. Results
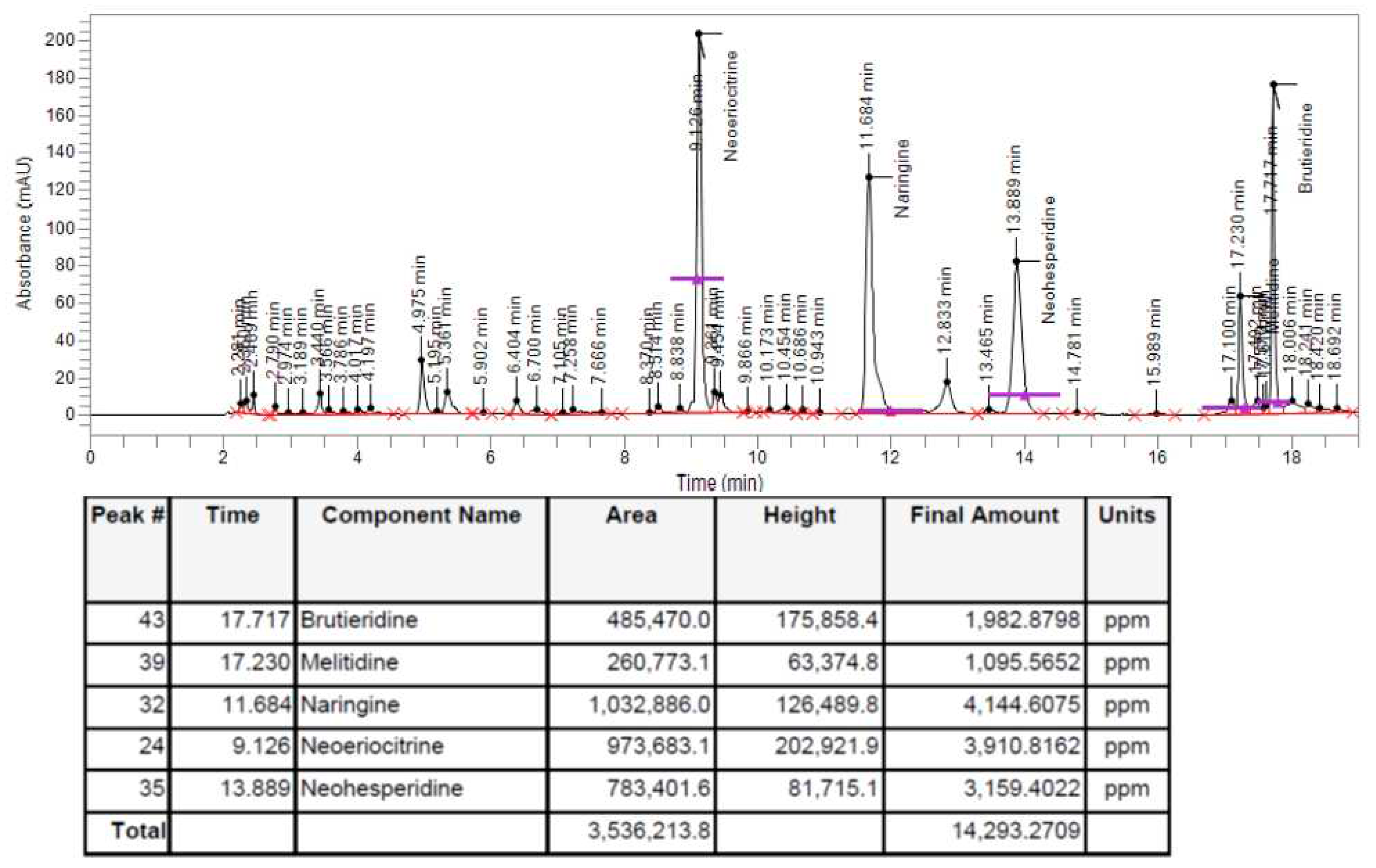
3.1. HPLC analysis of BF.
3.2. Total Polyphenols and Flavonoids
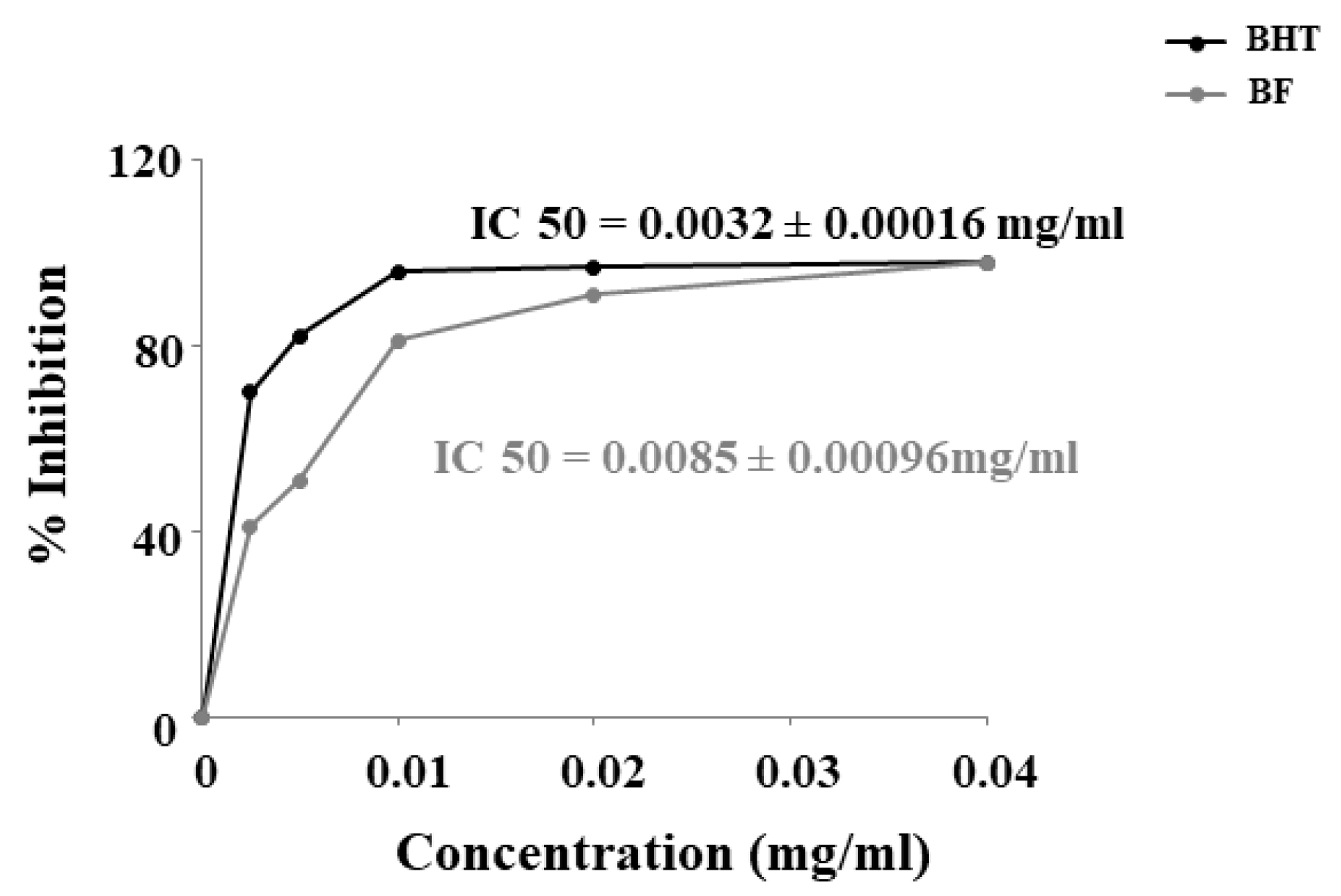
3.3. DPPH Free Radical Scavenging Activity
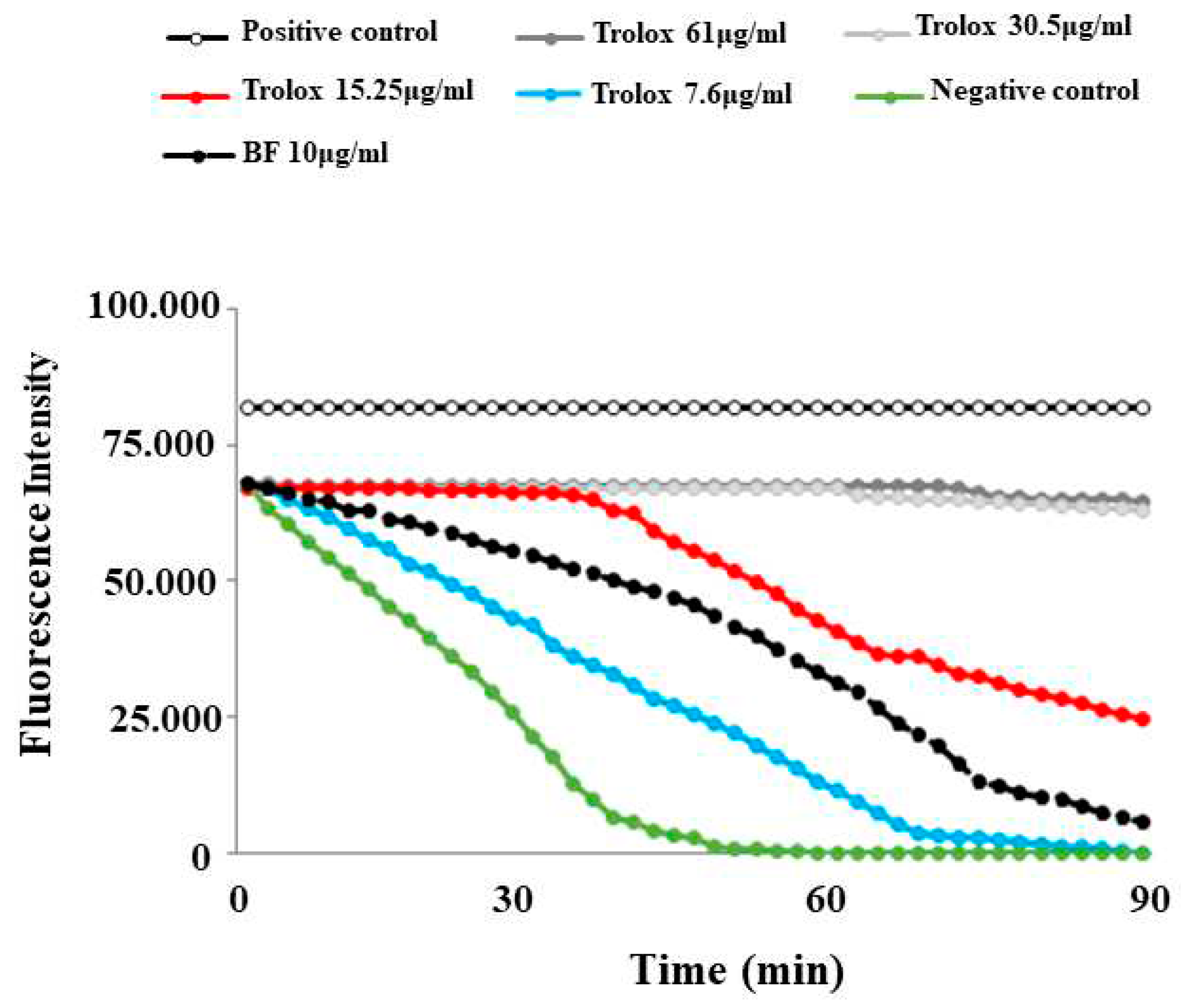
3.4. Antioxidant in vitro Activity
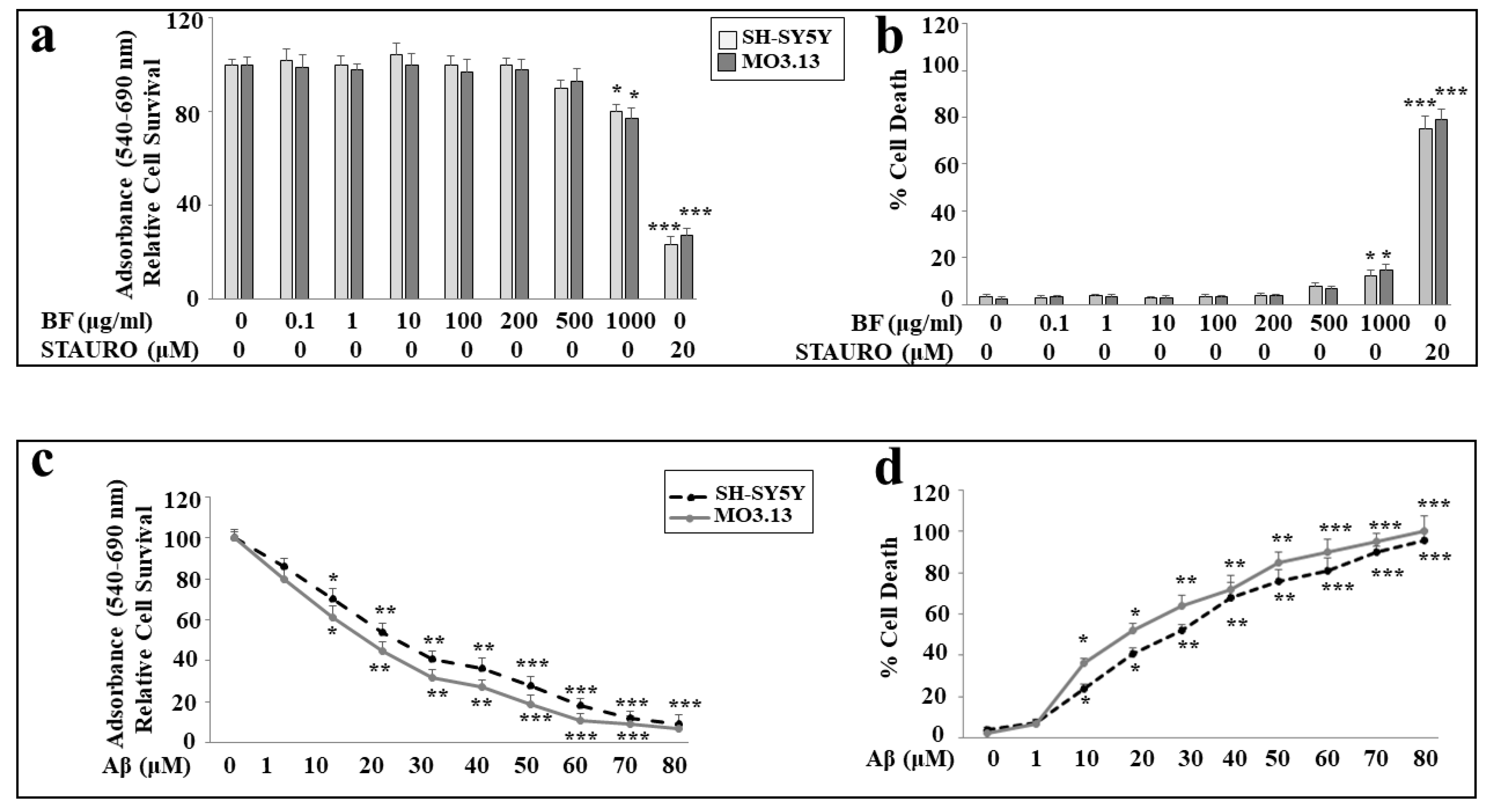
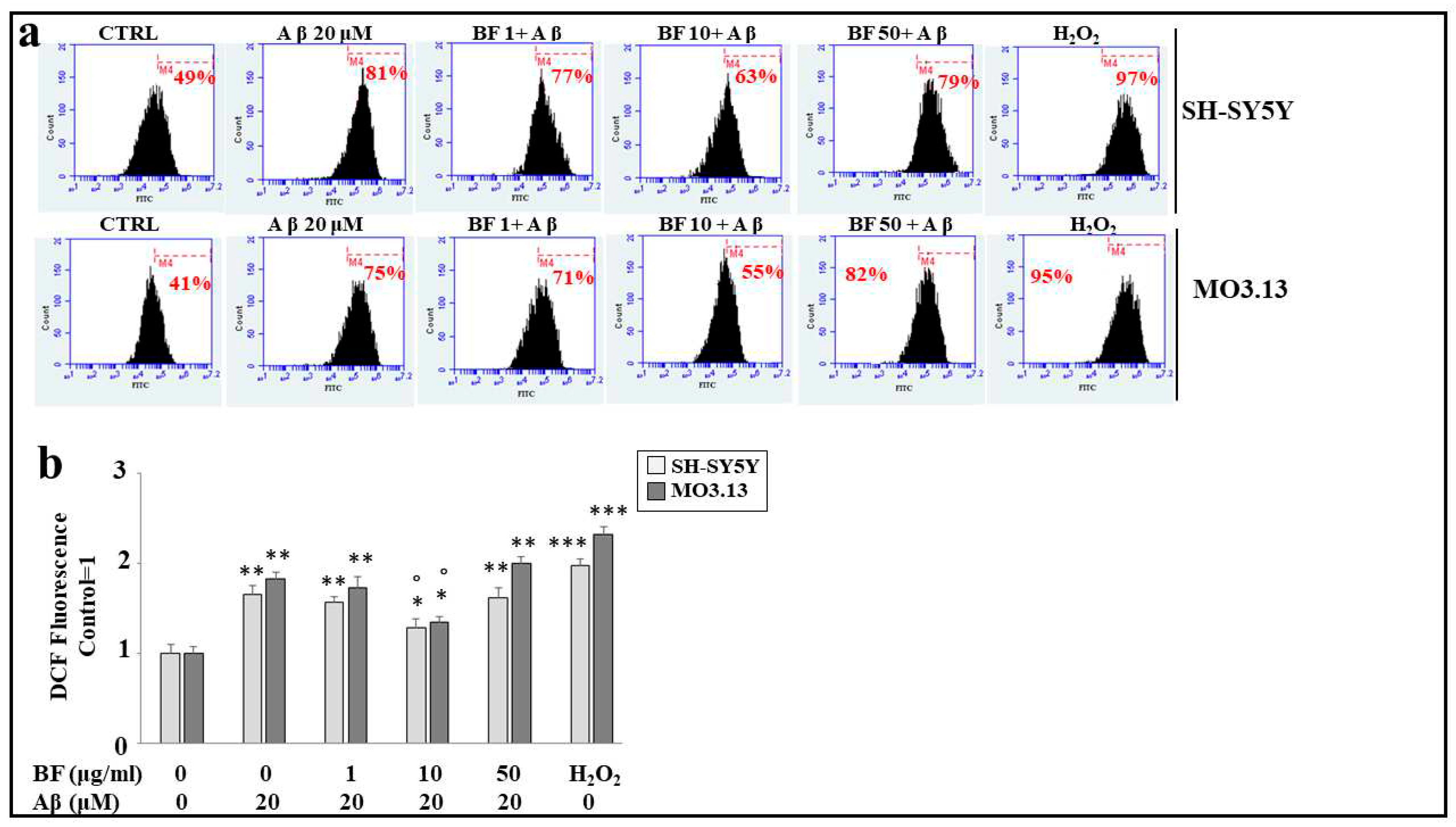
3.5. Effects of BF and Aβ on viability of neurons and oligodendrocytes
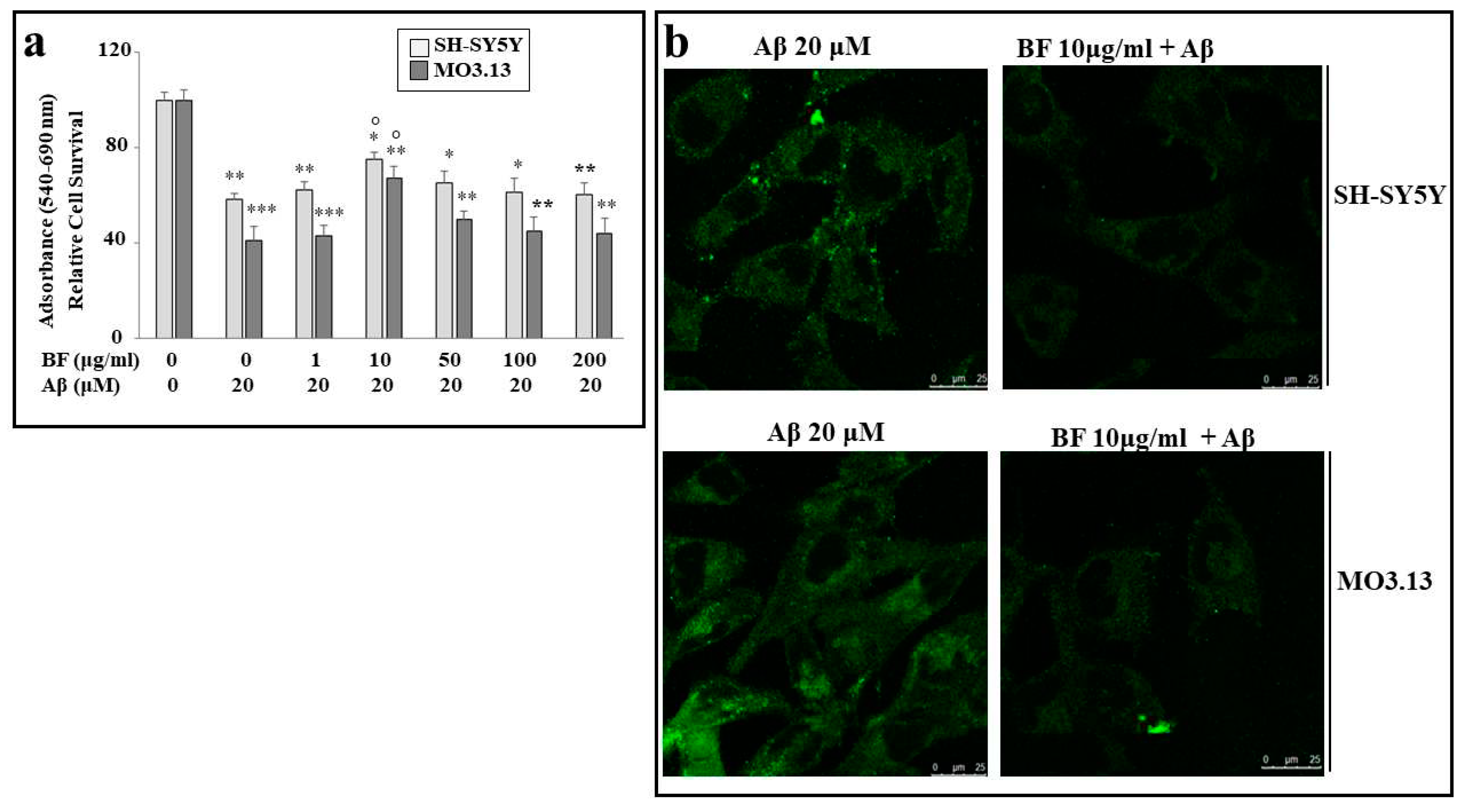
3.6. BF significantly protects against damage induced by Aβ
3.7. Antioxidant role of BF
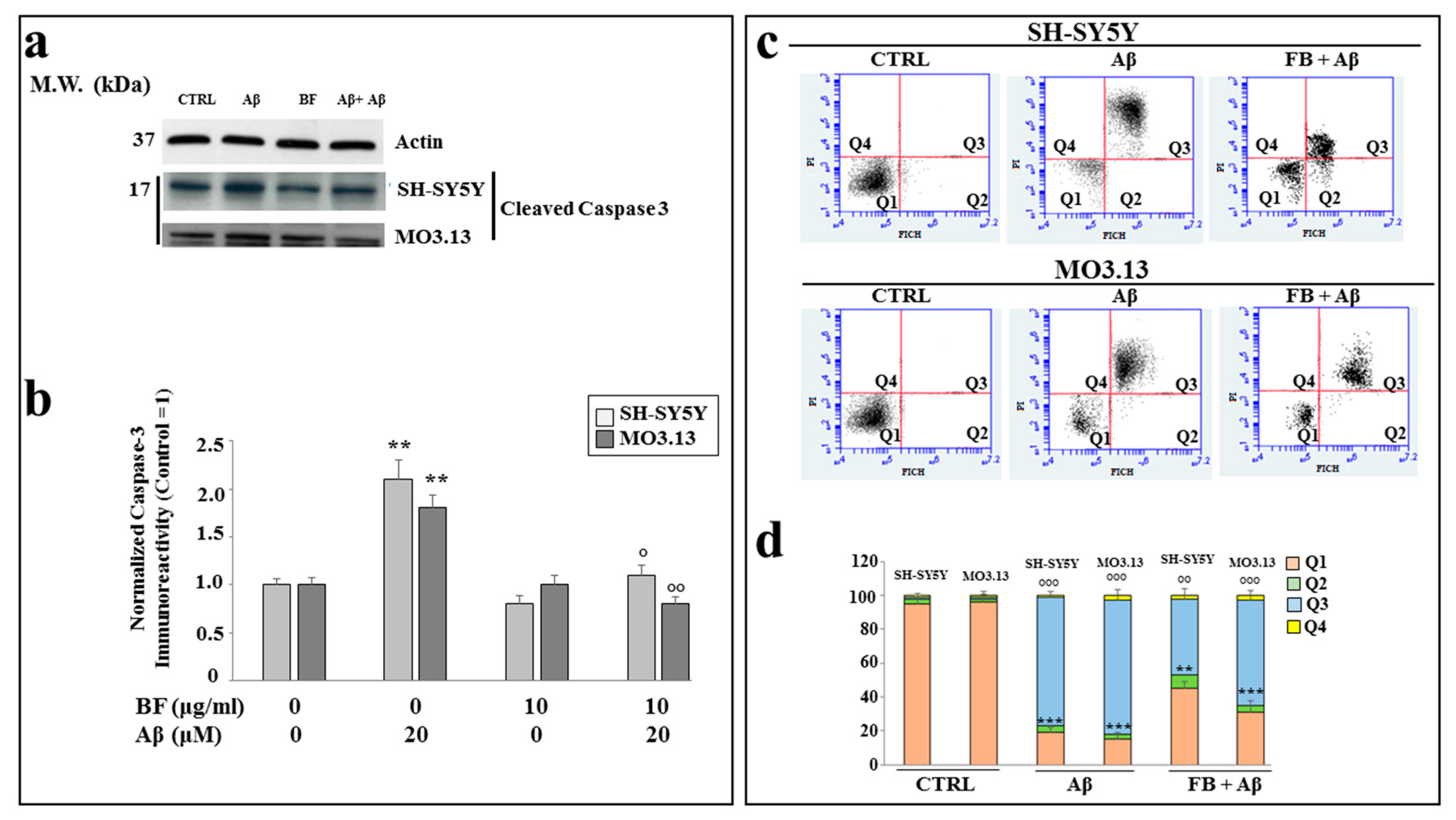
3.8. BF protects against apoptotic death induced by Aβ
4. Discussion and conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Veiga, M.; Costa, E.M.; Silva, S.; Pintado, M. Impact of plant extracts upon human health: A review. Crit Rev Food Sci Nutr. 2020, 60, 873–886. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and Health Benefits of Phytochemicals. Forsch Komplementmed. 2016, 23, 69–74. [Google Scholar] [CrossRef]
- Zhai, K.; Siddiqui, M.; Abdellatif, B.; Liskova, A.; Kubatka, P.; Büsselberg, D. Natural Compounds in Glioblastoma Therapy: Preclinical Insights, Mechanistic Pathways, and Outlook. Cancers (Basel). 2021, 13, 2317. [Google Scholar] [CrossRef]
- López-Gil, J.F.; Tárraga-López, P.J. Research on Diet and Human Health. Int J Environ Res Public Health. 2022, 19, 6526. [Google Scholar] [CrossRef]
- Leporini, M.; Loizzo, M.R.; Sicari, V.; Pellicanò, T.M.; Reitano, A.; Dugay, A.; Deguin, B.; Tundis, R. Citrus _ Clementina Hort. Juice Enriched with Its By-Products (Peels and Leaves): Chemical Composition, In Vitro Bioactivity, and Impact of Processing. Antioxidants 2020, 9, 298. [Google Scholar] [CrossRef]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-Industrial Potential of Exotic Fruit Byproducts as a Source of Food Additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Burò, I.; Consoli, V.; Castellano, A.; Vanella, L.; Sorrenti, V. Beneficial Effects of Standardized Extracts from Wastes of Red Oranges and Olive Leaves. Antioxidants (Basel). 2022, 11, 1496. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Apostolis, K. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Karam, M.C.; Petit, J.; Zimmer, D.; Elie; Joël, S. Effects of drying and grinding in production of fruit and vegetable powders: A review. J. Food Eng. 2016, 188, 32–49. [Google Scholar] [CrossRef]
- Bas-Bellver, C.; Barrera, C.; Betoret, N.; Seguí, L. Turning Agri-Food Cooperative Vegetable Residues into Functional Powdered Ingredients for the Food Industry. Sustainability 2020, 12, 1284. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Remize, F.; Poucheret, P. Fruits and Vegetables, as a Source of Nutritional Compounds and Phytochemicals: Changes in Bioactive Compounds during Lactic Fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Maugeri, A.; Ferlazzo, N.; Gangemi, S.; Calapai, G.; Schumacher, U.; Navarra, M. Anticancer Potential of Citrus Juices and Their Extracts: A Systematic Review of Both Preclinical and Clinical Studies. Front. Pharmacol. 2017, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Cirmi, S.; Navarra, M.; Woodside, J.V.; Cantwell, M.M. Citrus fruits intake and oral cancer risk: A systematic review and meta-analysis. Pharmacol. Res. 2018, 133, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; Yu, J. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Giuffrè, AM. Bergamot (Citrus bergamia, Risso): The Effects of Cultivar and Harvest Date on Functional Properties of Juice and Cloudy Juice. Antioxidants (Basel) 2019, 8, 221. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and Safety of Citrus spp. Essential Oils. Int J Mol Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Marotta, S.M.; Giarratana, F.; Parco, A.; Neri, D.; Ziino, G.; Giuffrida, A.; Panebianco, A. Evaluation of the Antibacterial Activity of Bergamot Essential Oils on Different Listeria Monocytogenes Strains. Italian journal of food safety. 2016, 5(4), 6176. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Ferlazzo, N.; Cirmi, S.; Mandalari, G.; Navarra, M. In vitro effect of bergamot (Citrus bergamia) juice against cagA-positive and-negative clinical isolates of Helicobacter pylori. BMC complementary and alternative medicine. 2015, 15, 256. [Google Scholar] [CrossRef]
- Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; Testai, L.; Calderone, V.; Pistelli, L.; Braca, A.; Martini, C. Antioxidant and Antisenescence Effects of Bergamot Juice. Oxid Med Cell Longev. 2018, 2018, 9395804. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.E.; Cirmi, S.; Musumeci, L.; Pergolizzi, S.; Maugeri, A.; Russo, C.; Mannucci, C.; Calapai, G.; Navarra, M. Mechanisms Underlying the Anti-Inflammatory Activity of Bergamot Essential Oil and Its Antinociceptive Effects. Plants (Basel) 2020, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Nauman, M.C.; Johnson, J.J. Clinical application of bergamot (Citrus bergamia) for reducing high cholesterol and cardiovascular disease markers. Integr Food Nutr Metab. 2019, 6, 10.15761/IFNM.1000249. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Luo, Y.; Hu, X.; Song, L.; Yang, J.; Zhu, J.; Wen, Y.; Yu, R. Isolation, structural characterization, and immunostimulatory activity of a new water-soluble polysaccharide and its sulfated derivative from Citrus medica L. var. sarcodactylis. Int J Biol Macromol. 2019, 123, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Rosano, G.M.C.; Anker, S.D.; Coats, A.J.S.; Seferovic, P.; Mollace, R.; Tavernese, A.; Gliozzi, M.; Musolino, V.; Carresi, C.; et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients. 2021, 13, 257. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Musolino, V.; Gliozzi, M.; Maiuolo, J.; Mollace, R.; Nucera, S.; Maretta, A.; Sergi, D.; Muscoli, S.; Gratteri, S.; et al. Anti-oxidant effect of bergamot polyphenolic fraction counteracts doxorubicin-induced cardiomyopathy: Role of autophagy and c-kitposCD45negCD31neg cardiac stem cell activation. J Mol Cell Cardiol. 2018, 119, 10–18. [Google Scholar] [CrossRef]
- Maiuolo, J.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Nucera, S.; Scicchitano, M.; Bosco, F.; et al. Effects of Bergamot Polyphenols on Mitochondrial Dysfunction and Sarcoplasmic Reticulum Stress in Diabetic Cardiomyopathy. Nutrients. 2021, 13, 2476. [Google Scholar] [CrossRef]
- Carresi, C.; Scicchitano, M.; Scarano, F.; Macrì, R.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.C.; Mollace, R.; Guarnieri, L.; Coppoletta, A.R.; Gliozzi, M.; Musolino, V.; Maiuolo, J.; Palma, E.; Mollace, V. The Potential Properties of Natural Compounds in Cardiac Stem Cell Activation: Their Role in Myocardial Regeneration. Nutrients. 2021, 13, 275. [Google Scholar] [CrossRef]
- Baron, G.; Altomare, A.; Mol, M.; Garcia, J.L.; Correa, C.; Raucci, A.; Mancinelli, L.; Mazzotta, S.; Fumagalli, L.; Trunfio, G.; et al. Analytical Profile and Antioxidant and Anti-Inflammatory Activities of the Enriched Polyphenol Fractions Isolated from Bergamot Fruit and Leave. Antioxidants (Basel). 2021, 10, 141. [Google Scholar] [CrossRef]
- Lascala, A.; Martino, C.; Parafati, M.; Salerno, R.; Oliverio, M.; Pellegrino, D.; Mollace, V.; Janda, E. Analysis of proautophagic activities of Citrus flavonoids in liver cells reveals the superiority of a natural polyphenol mixture over pure flavones. J Nutr Biochem. 2018, 58, 119–130. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; Scicchitano, M.; et al. The effect of bergamot polyphenolic fraction on lipid transfer protein system and vascular oxidative stress in a rat model of hyperlipemia. Lipids Health Dis. 2019, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Carresi, C.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Scarano, F.; Bosco, F.; Nucera, S.; Maiuolo, J.; Macrì, R.; Ruga, S.; Oppedisano, F.; et al. The Effect of Natural Antioxidants in the Development of Metabolic Syndrome: Focus on Bergamot Polyphenolic Fraction. Nutrients 2020, 12, 1504. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, J.; Bava, I.; Carresi, C.; Gliozzi, M.; Musolino, V.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. The Effects of Bergamot Polyphenolic Fraction, Cynara cardunculus, and Olea europea L. Extract on Doxorubicin-Induced Cardiotoxicity. Nutrients. 2021, 13, 2158. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Carresi, C.; Maiuolo, J.; Mollace, R.; Bosco, F.; Scarano, F.; Scicchitano, M.; Maretta, A.; Palma, E.; et al. Lipid-lowering effect of bergamot polyphenolic fraction: role of pancreatic cholesterol ester hydrolase. J Biol Regul Homeost Agents. 2017, 31, 1087–1093. [Google Scholar] [PubMed]
- Algieri, C.; Bernardini, C.; Oppedisano, F.; La Mantia, D.; Trombetti, F.; Palma, E.; Forni, M.; Mollace, V.; Romeo, G.; Nesci, S. Mitochondria Bioenergetic Functions and Cell Metabolism Are Modulated by the Bergamot Polyphenolic Fraction. Cells. 2022, 11, 1401. [Google Scholar] [CrossRef]
- Laganà, V.; Giuffrè, A.M.; De Bruno, A.; Poiana, M. Formulation of Biscuits Fortified with a Flour Obtained from Bergamot By-Products (Citrus bergamia, Risso). Foods 2022, 11, 1137. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, R.; Yin, L.; Zhang, N. Novel blasting extrusion processing improved the physicochemical properties of soluble dietary fiber from soybean residue and in vivo evaluation. J Food Eng. 2014, 120, 1–8. [Google Scholar] [CrossRef]
- Zhu, Y.; Chu, J.; Lu, Z.; Lv, F.; Bie, X.; Zhang, C. Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J Cereal Sci. 2018, 79, 456–461. [Google Scholar] [CrossRef]
- Mucke, L.; Selkoe, D.J. Neurotoxicity of amyloid β-protein: synaptic and network dysfunction. Cold Spring Harb Perspect Med. 2012, 2, a006338. [Google Scholar] [CrossRef]
- Gu, Y.; Vorburger, R.; Scarmeas, N.; Luchsinger, J.A.; Manly, J.J.; Schupf, N. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav Immun. 2017, 65, 150–160. [Google Scholar] [CrossRef]
- McCarrey, A.C.; Pacheco, J.; Carlson, O.D.; Egan, J.M.; Thambisetty, M.; An, Y. Interleukin-6 is linked to longitudinal rates of cortical thinning in aging. Transl Neurosci. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Hung, A.Y.; Citron, M.; Teplow, D.B.; Selkoe, D.J. beta-Amyloid, protein processing and Alzheimer's disease. Arzneimittelforschung. 1995, 45(3A), 398–402. [Google Scholar]
- Sadick, J.S.; O'Dea, M.R.; Hasel, P.; Dykstra, T.; Faustin, A.; Liddelow, S.A. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer's disease. Neuron. 2022, 110, 1788–1805. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, HM. Omega-3 Polyunsaturated Fatty Acids and Oxylipins in Neuroinflammation and Management of Alzheimer Disease. Adv Nutr (Bethesda, Md). 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Champ, C.; Day, J.; Aarts, E.; Bahr, B.A.; Bakker, M. Poor cognitive ageing: vulnerabilities, mechanisms and the impact of nutritional interventions. Ageing Res Rev. 2018, 42, 40–55. [Google Scholar] [CrossRef]
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, Aging and Alzheimer's disease. Nutrients. 2017, 9, 670. [Google Scholar] [CrossRef]
- van de Rest, O.; Berendsen, A.A.; Haveman-Nies, A.; de Groot, L.C. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer's disease. Ann Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef]
- Wu, L.; Sun, D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology. 2018, 90, e1789–e1798. [Google Scholar] [CrossRef] [PubMed]
- Luciano, M.; Corley, J.; Cox, S.R.; Valdés Hernández, M.C.; Craig, L.C.A.; Dickie, D.A. Mediterranean-type diet and brain structural change from 73 to 76 years in a Scottish cohort. Neurology. 2017, 88, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Kosalec, I.; Bakmaz, M.; Pepeljnjak, S.; Vladimir-Knezevic, S. Quantitative Analysis of the Flavonoids in Raw Propolis from Northern Croatia. Acta Pharm. 2004, 54, 65–72. [Google Scholar] [PubMed]
- Maiuolo, J.; Macrì, R.; Bava, I.; Gliozzi, M.; Musolino, V.; Nucera, S.; Carresi, C.; Sicchitano, M.; Bosco, F.; Scarano, F.; et al. Myelin disturbances produced by sub-toxic concentration of heavy metals: The role of oligodendrocyte dysfunction. Int. J. Mol. Sci. 2019, 20, 4554. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Navarra, M.; Maiuolo, J.; Rotiroti, D.; Bagetta, G.; Corasaniti, M.T. 17beta-estradiol protects SH-SY5Y Cells against HIV-1 gp120-induced cell death: evidence for a role of estrogen receptors. Neurotoxicology. 2005, 26, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Poudineh, M.; Ghotbi, T.; Azizi, F.; Karami, N.; Zolfaghari, Z.; Gheisari, F.; Hormozi, M.; Poudineh, S. Neuropharmaceutical Properties of Naringin Against Alzheimer's and Parkinson's Diseases: Naringin Protection Against AD and PD. Galen Med J. 2022, 11, e2337. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer's Disease. Curr Nutr Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef]
- Kuşi, M.; Becer, E.; Vatansever, H.S.; Yücecan, S. Neuroprotective Effects of Hesperidin and Naringin in SK-N-AS Cell as an In Vitro Model for Alzheimer's Disease. J Am Nutr Assoc. 2022, 1–9. [Google Scholar] [CrossRef]
- Emran, T.B.; Islam, F.; Nath, N.; Sutradhar, H.; Das, R.; Mitra, S.; Alshahrani, M.M.; Alhasaniah, A.H.; Sharma, R. Naringin and Naringenin Polyphenols in Neurological Diseases: Understandings from a Therapeutic Viewpoint. Life (Basel). 2022, 13, 99. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J Neural Transm (Vienna). 2012, 119, 891–910. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer's Disease. Mol Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: stuck in the middle of neurodegeneration. J Alzheimers Dis. 2010, Suppl 2, S357–367. [Google Scholar] [CrossRef]
- Singh, A.K.; Bissoyi, A.; Kashyap, M.P.; Patra, P.K.; Rizvi, S.I. Autophagy Activation Alleviates Amyloid-β-Induced Oxidative Stress, Apoptosis and Neurotoxicity in Human Neuroblastoma SH-SY5Y Cells. Neurotox Res. 2017, 32, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Krishtal, J.; Bragina, O.; Metsla, K.; Palumaa, P.; Tõugu, V. In situ fibrillizing amyloid-beta 1-42 induces neurite degeneration and apoptosis of differentiated SH-SY5Y cells. PLoS One. 2017, 12, e0186636. [Google Scholar] [CrossRef] [PubMed]
- Takada, E.; Okubo, K.; Yano, Y.; Iida, K.; Someda, M.; Hirasawa, A.; Yonehara, S.; Matsuzaki, K. Molecular Mechanism of Apoptosis by Amyloid β-Protein Fibrils Formed on Neuronal Cells. ACS Chem Neurosci. 2020, 11, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Mao, J.; Yuan, X.; Lin, Z.; Li, Y. Caspase-3-mediated cyclic stretch-induced myoblast apoptosis via a Fas/FasL-independent signaling pathway during myogenesis. J Cell Biochem. 2009, 107, 834–844. [Google Scholar] [CrossRef]
- Mahib, M.R.; Hosojima, S.; Kushiyama, H.; Suda, T.; Tsuchiya, K. Caspase-7 mediates caspase-1-induced apoptosis independently of Bid. Microbiol Immunol. 2020, 64, 143–152. [Google Scholar] [CrossRef]
- Nakano, H.; Shinohara, K. Time sequence analysis of caspase-3-independent programmed cell death and apoptosis in X-irradiated human leukemic MOLT-4 cells. Cell Tissue Res. 2002, 310, 305–311. [Google Scholar] [CrossRef]
- Alvarez, S.; Blanco, A.; Fresno, M.; Muñoz-Fernández, M.Á. TNF-α contributes to caspase-3 independent apoptosis in neuroblastoma cells: role of NFAT. PLoS One. 2011, 6, e16100. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent Trends on the Valorization Strategies for the Management of Citrus By-Products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, S.; Yang, Y. Production of Bacterial Cellulose from Byproduct of Citrus Juice Processing (Citrus Pulp) by Gluconacetobacter Hansenii. Cellulose 2018, 25, 6977–6988. [Google Scholar] [CrossRef]
- Russo, C.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
| Extract | Polyphenols (mg Naringin/g Extract) | Flavonoids (mg EQ/g Extract) |
|---|---|---|
| BF | 48.8 ± 5 mg | 6,21 ± 2.9 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





