Submitted:
09 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Research Design and Methods
2.1. Study Design and Setting
2.2. Population and Data Collection
2.3. Microbiological Definitions
2.4. Statistical Analysis:
2.5. Ethical Considerations
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Epidemiology of Candida Species
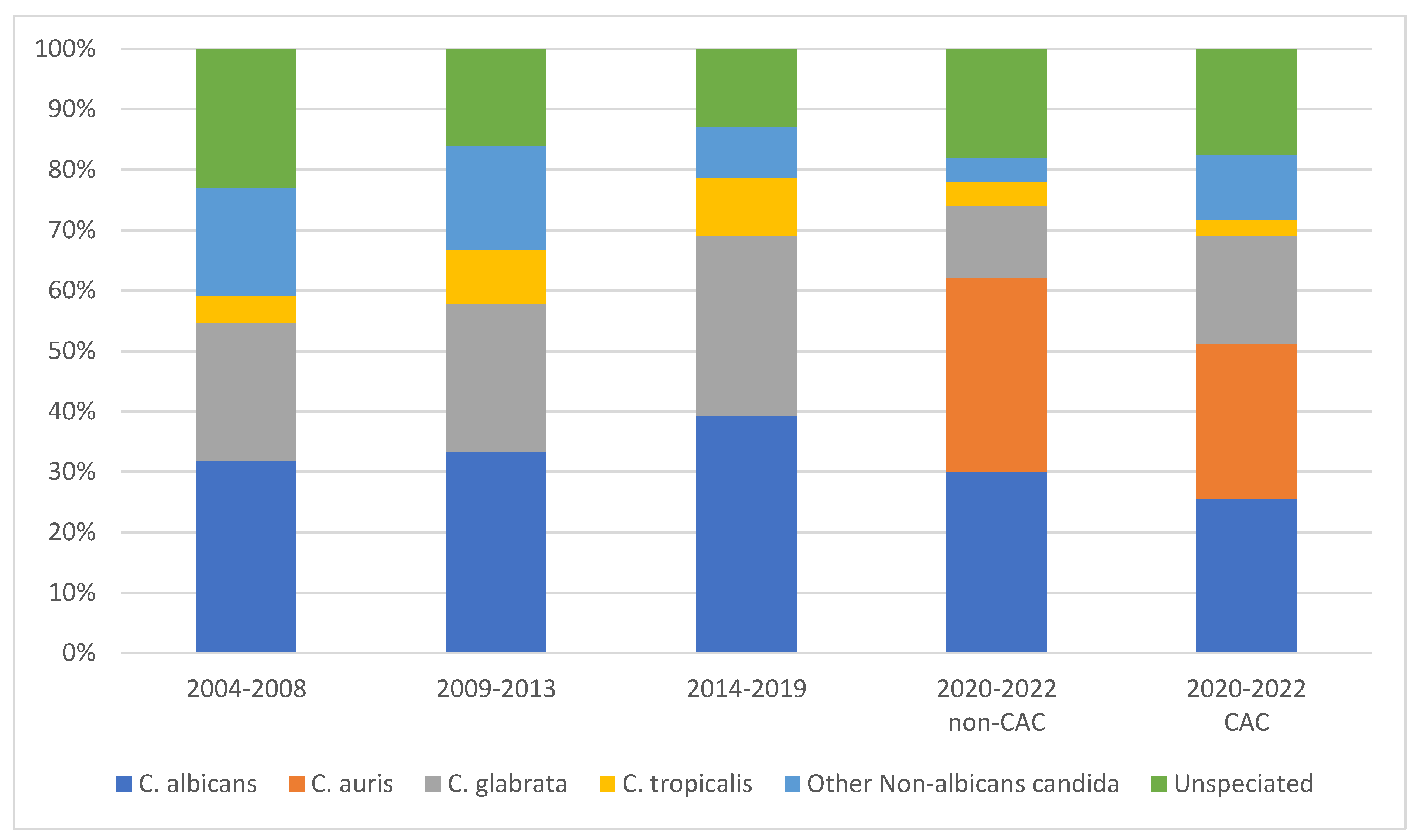
3.3. Antifungal Susceptibility
3.4. Outcomes of CAC and non-CAC
3.5. EQUAL Score Analysis
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Paiva, J.-A.; Pereira, J.M.; Tabah, A.; Mikstacki, A.; de Carvalho, F.B.; Koulenti, D.; Ruckly, S.; Çakar, N.; Misset, B.; Dimopoulos, G.; et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: data from the EUROBACT study. Crit. Care 2016, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kmeid, J.; Jabbour, J.-F.; Kanj, S.S. Epidemiology and burden of invasive fungal infections in the countries of the Arab League. J. Infect. Public Heal. 2019, 13, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, A.E., et al., Predominance of Candida glabrata among non-albicans Candida species in a 16-year study of candidemia at a tertiary care center in Lebanon. Pathogens, 2021. 10(1): p. 82. [CrossRef]
- Silva, L.N.; de Mello, T.P.; Ramos, L.d.S.; Branquinha, M.H.; Roudbary, M.; dos Santos, A.L.S. Fungal Infections in COVID-19-Positive Patients: A Lack of Optimal Treatment Options. Curr. Top. Med. Chem. 2020, 20, 1951–1957. [Google Scholar] [CrossRef]
- Allaw, F.; Haddad, S.F.; Habib, N.; Moukarzel, P.; Naji, N.S.; Kanafani, Z.A.; Ibrahim, A.; Zahreddine, N.K.; Spernovasilis, N.; Poulakou, G. COVID-19 and C. auris: A Case-Control Study from a Tertiary Care Center in Lebanon. Microorganisms 2022, 10, 1011. [Google Scholar] [CrossRef]
- Arastehfar, A.; Shaban, T.; Zarrinfar, H.; Roudbary, M.; Ghazanfari, M.; Hedayati, M.-T.; Sedaghat, A.; Ilkit, M.; Najafzadeh, M.J.; Perlin, D.S. Candidemia among Iranian Patients with Severe COVID-19 Admitted to ICUs. J. Fungi 2021, 7, 280. [Google Scholar] [CrossRef]
- Omrani, A.S.; Koleri, J.; Ben Abid, F.; Daghfel, J.; Odaippurath, T.; Peediyakkal, M.Z.; Baiou, A.; Sarsak, E.; Elayana, M.; Kaleeckal, A.; et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med Mycol. 2021, 59, 1262–1266. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Ridolfo, A.; Gervasoni, C.; Antinori, S. Invasive Fungal Infections Complicating COVID-19: A Narrative Review. J. Fungi 2021, 7, 921. [Google Scholar] [CrossRef]
- Avkan-Oğuz, V.; Çelİk, M.; Eren-Kutsoylu, O.Ö.; Nazli, A.; Uğur, Y.L.; Taylan, A.; Ergan, B.; Irmak, Ç.; Duğral, E.; Özkütük, A.A. Fungal colonization and infections in patients with COVID-19 in intensive care units: A real-life experience at a tertiary-care hospital. Respiratory Medicine and Research 2022, 82, 100937. [Google Scholar] [CrossRef]
- Routsi, C.; Meletiadis, J.; Charitidou, E.; Gkoufa, A.; Kokkoris, S.; Karageorgiou, S.; Giannopoulos, C.; Koulenti, D.; Andrikogiannopoulos, P.; Perivolioti, E.; et al. Epidemiology of Candidemia and Fluconazole Resistance in an ICU before and during the COVID-19 Pandemic Era. Antibiotics 2022, 11, 771. [Google Scholar] [CrossRef]
- Boxer, L.A. How to approach neutropenia. Hematology 2010, the American Society of Hematology Education Program Book 2012, 2012, 174–182. [Google Scholar] [CrossRef]
- CLSI, C. Performance standards for antimicrobial susceptibility testing. Clinical Lab Standards Institute 2016, 35, 16–38. [Google Scholar]
- CDC, N. Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection). Center for Disease Control Atlanta, GA: 2017.
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M.; Petermann, G.W. 2015 infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adultsa. Clinical infectious diseases 2015, 61, e26–e46. [Google Scholar] [CrossRef]
- Ghazi, S.; Rafei, R.; Osman, M.; El Safadi, D.; Mallat, H.; Papon, N.; Dabboussi, F.; Bouchara, J.-P.; Hamze, M. Ghazi, S.; Rafei, R.; Osman, M.; El Safadi, D.; Mallat, H.; Papon, N.; Dabboussi, F.; Bouchara, J.-P.; Hamze, M. The epidemiology of Candida species in the Middle East and North Africa. 2019, 29, 245–252. [CrossRef]
- Zahreddine, N.K.; Haddad, S.F.; Kerbage, A.; Kanj, S.S. Challenges of coronavirus disease 2019 (COVID-19) in Lebanon in the midst of the economic collapse. Antimicrob. Steward. Heal. Epidemiology 2022, 2, e67. [Google Scholar] [CrossRef]
- Allaw, F.; Zahreddine, N.K.; Ibrahim, A.; Tannous, J.; Taleb, H.; Bizri, A.R.; Dbaibo, G.; Kanj, S.S. First Candida auris Outbreak during a COVID-19 Pandemic in a Tertiary-Care Center in Lebanon. Pathogens 2021, 10, 157. [Google Scholar] [CrossRef]
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H.; et al. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2018, 68, 15–21. [Google Scholar] [CrossRef]
- Machado, M.; Estévez, A.; Sánchez-Carrillo, C.; Guinea, J.; Escribano, P.; Alonso, R.; Valerio, M.; Padilla, B.; Bouza, E.; Muñoz, P. Incidence of Candidemia Is Higher in COVID-19 versus Non-COVID-19 Patients, but Not Driven by Intrahospital Transmission. J. Fungi 2022, 8, 305. [Google Scholar] [CrossRef] [PubMed]
- Tsay, S.; Welsh, R.M.; Adams, E.H.; Chow, N.A.; Gade, L.; Berkow, E.L.; Poirot, E.; Lutterloh, E.; Quinn, M.; Chaturvedi, S. Notes from the field: ongoing transmission of Candida auris in health care facilities—United States, June 2016–May 2017. Morbidity and Mortality Weekly Report 2017, 66, 514. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Lu, P.-L.; Wang, Y.-L.; Chen, T.-C.; Chang, K.; Lin, S.-Y. Usefulness of EQUAL Candida Score for predicting outcomes in patients with candidaemia: a retrospective cohort study. Clin. Microbiol. Infect. 2020, 26, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Rahme, D.; Ayoub, M.; Shaito, K.; Saleh, N.; Assaf, S.; Lahoud, N. First trend analysis of antifungals consumption in Lebanon using the World Health Organization collaborating center for drug statistics methodology. BMC Infect. Dis. 2022, 22, 1–9. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S.; Al-Sweih, N.; Mokaddas, E.; Al-Banwan, K.; Alfouzan, W.; Al-Obaid, I.; Al-Obaid, K.; Varghese, S. Increasing Trends of Reduced Susceptibility to Antifungal Drugs Among ClinicalCandida glabrataIsolates in Kuwait. Microb. Drug Resist. 2020, 26, 982–990. [Google Scholar] [CrossRef]
- Habibzadeh, A.; Lankarani, K.B.; Farjam, M.; Akbari, M.; Kashani, S.M.A.; Karimimoghadam, Z.; Wang, K.; Imanieh, M.H.; Tabrizi, R.; Ahmadizar, F. Prevalence of Fungal Drug Resistance in COVID-19 Infection: a Global Meta-analysis. Curr. Fungal Infect. Rep. 2022, 16, 154–164. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Rizk, N.A.; Moghnieh, R.; Haddad, N.; Rebeiz, M.-C.; Zeenny, R.M.; Hindy, J.-R.; Orlando, G.; Kanj, S.S. Challenges to Antimicrobial Stewardship in the Countries of the Arab League: Concerns of Worsening Resistance during the COVID-19 Pandemic and Proposed Solutions. Antibiotics 2021, 10, 1320. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: a one health perspective. Antimicrobial resistance in bacteria from livestock and companion animals 2018, 521–547. [Google Scholar] [CrossRef]
- Kanj, S.S.; Ramirez, P.; Rodrigues, C. Beyond the Pandemic: The Value of Antimicrobial Stewardship. Front. Public Heal. 2022, 10, 902835. [Google Scholar] [CrossRef]
- El Zakhem, A.; El Eid, R.; Istambouli, R.; Tamim, H.; Kanj, S.S. The Utility of EQUAL Candida Score in Predicting Mortality in Patients with Candidemia. J. Fungi 2022, 8, 238. [Google Scholar] [CrossRef]
- Breazzano, M.P.; Day, H.R.; Bloch, K.C.; Tanaka, S.; Cherney, E.F.; Sternberg, P.; Donahue, S.P.; Bond, J.B. Utility of ophthalmologic screening for patients with Candida bloodstream infections: a systematic review. JAMA ophthalmology 2019, 137, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.; Arikan-Akdagli, S. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clinical Microbiology and Infection 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Phongkhun, K.; Pothikamjorn, T.; Srisurapanont, K.; Manothummetha, K.; Sanguankeo, A.; Thongkam, A.; Chuleerarux, N.; Leksuwankun, S.; Meejun, T.; Thanakitcharu, J.; et al. Prevalence of Ocular Candidiasis and Candida Endophthalmitis in Patients With Candidemia: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2023, 76, 1738–1749. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Impact of Treatment Strategy on Outcomes in Patients with Candidemia and Other Forms of Invasive Candidiasis: A Patient-Level Quantitative Review of Randomized Trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Gaur, S.; Bal, A. Candidaemia in the non-neutropenic patient: A critique of the guidelines. Int. J. Antimicrob. Agents 2013, 42, 294–300. [Google Scholar] [CrossRef]
- Nucci, M.; Braga, P.R.; Nouér, S.A.; Anaissie, E. Time of catheter removal in candidemia and mortality. Braz. J. Infect. Dis. 2018, 22, 455–461. [Google Scholar] [CrossRef]
- Ashong, C.N.; Hunter, A.S.; Mansouri, M.D.; Cadle, R.M.; Hamill, R.J.; Musher, D.M. Adherence to clinical practice guidelines for the treatment of candidemia at a Veterans Affairs Medical Center. 2017, 11, 18–23.
- Koehler, P.; Stecher, M.; Cornely, O.; Vehreschild, M.; Bohlius, J.; Wisplinghoff, H.; Vehreschild, J. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin. Microbiol. Infect. 2019, 25, 1200–1212. [Google Scholar] [CrossRef]
- Morgan, J.; Meltzer, M.I.; Plikaytis, B.D.; Sofair, A.N.; Huie-White, S.; Wilcox, S.; Harrison, L.H.; Seaberg, E.C.; Hajjeh, R.A.; Teutsch, S.M. Excess Mortality, Hospital Stay, and Cost Due to Candidemia: A Case-Control Study Using Data From Population-Based Candidemia Surveillance. Infect. Control. Hosp. Epidemiology 2005, 26, 540–547. [Google Scholar] [CrossRef]
- Ohki, S.; Shime, N.; Kosaka, T.; Fujita, N. Impact of host- and early treatment-related factors on mortality in ICU patients with candidemia: a bicentric retrospective observational study. J. Intensiv. Care 2020, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen 2018, 7, e00578. [Google Scholar] [CrossRef] [PubMed]
- E Seagle, E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; A Czaja, C.; Kayalioglu, H.; et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2021, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- I Blot, S.; Vandewoude, K.H.; A Hoste, E.; A Colardyn, F. Effects of nosocomial candidemia on outcomes of critically ill patients. Am. J. Med. 2002, 113, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, R.; Luan, Z.; Ma, X. Risk of invasive candidiasis with prolonged duration of ICU stay: a systematic review and meta-analysis. BMJ Open 2020, 10, e036452. [Google Scholar] [CrossRef]
- Dongelmans, D.A.; Termorshuizen, F.; Brinkman, S.; Bakhshi-Raiez, F.; Arbous, M.S.; de Lange, D.W.; van Bussel, B.C.T.; de Keizer, N.F.; Verbiest, D.P.; Velde, L.F.T.; et al. Characteristics and outcome of COVID-19 patients admitted to the ICU: a nationwide cohort study on the comparison between the first and the consecutive upsurges of the second wave of the COVID-19 pandemic in the Netherlands. Ann. Intensiv. Care 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Acosta, R.A.H.; Garrigos, Z.E.; Marcelin, J.R.; Vijayvargiya, P. COVID-19 Pathogenesis and Clinical Manifestations. Infect. Dis. Clin. North Am. 2022, 36, 231–249. [Google Scholar] [CrossRef]
- Alkofide, H.; Almohaizeie, A.; Almuhaini, S.; Alotaibi, B.; Alkharfy, K.M. Tocilizumab and Systemic Corticosteroids in the Management of Patients with COVID-19: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2021, 110, 320–329. [Google Scholar] [CrossRef]
| 2004-2008 | 2009-2013 | 2014-2019 | 2020-2022 (non-CAC) | 2020-2022 (CAC) | Total | |
|---|---|---|---|---|---|---|
| C. albicans | ||||||
| Fluconazole | 1/1 | 6/6 | 13/16 | 5/5 | 8/8 | 33/36 |
| Voriconazole | - | 3/4 | 14/17 | 5/5 | 8/8 | 30/34 |
| Amphotericin B | - | 3/3 | 17/17 | 5/5 | 8/8 | 33/33 |
| Caspofungin | - | 1/1 | 6/6 | 5/5 | 8/8 | 20/20 |
| C. tropicalis | ||||||
| Fluconazole | 2/2 | - | 3/3 | - | 1/1 | 6/6 |
| Voriconazole | - | 4/5 | 7/7 | 1/1 | 2/2 | 14/15 |
| Amphotericin B | - | 1/1 | 7/7 | 1/1 | 2/2 | 11/11 |
| Caspofungin | - | 1/1 | 1/1 | 1/1 | 2/2 | 5/5 |
| C. glabrata | ||||||
| Fluconazole | 3/4 | 3/10 | 17/23 | 2/6 | 4/7 | 29/50 |
| Voriconazole | 0/1 | 7/8 | 18/23 | 6/6 | 7/7 | 38/45 |
| Amphotericin B | - | 1/1 | 22/23 | 6/6 | 6/6 | 35/36 |
| Caspofungin | - | - | 9/9 | 3/6 | 2/7 | 14/22 |
| C. parapsilosis | ||||||
| Fluconazole | 2/2 | - | 3/3 | - | 1/1 | 6/6 |
| Voriconazole | - | - | 3/3 | - | 1/1 | 4/4 |
| Amphotericin B | - | - | 3/3 | - | 1/1 | 4/4 |
| Caspofungin | - | - | - | - | 1/1 | 1/1 |
| C. auris | ||||||
| Fluconazole | - | - | - | 0/3 | 2/9 | 2/12 |
| Voriconazole | - | - | - | 1/3 | 5/9 | 6/12 |
| Amphotericin B | - | - | - | 0/3 | 0/7 | 0/10 |
| Caspofungin | - | - | - | 8/8 | 3/3 | 11/11 |
| Total N = 64 |
CAC N = 32 (50.0%) |
Non-CAC N = 32 (50.0%) |
p-value | |
|---|---|---|---|---|
| Age* | 73 (19) | 75 (18) | 72 (18) | 0.14 |
| Male | 38 (59.4%) | 20 (62.5%) | 18 (56.3%) | 0.61 |
| Diabetes mellitus | 29 (45.3%) | 18 (56.3%) | 11 (34.4%) | 0.07 |
| ESRD on HD | 26 (40.6%) | 12 (37.5%) | 14 (43.8%) | 0.61 |
| AKI requiring HD | 2 (3.1%) | 1 (3.1%) | 1 (3.1%) | 1.00 |
| Hematologic malignancy | 8 (12.5%) | 5 (15.6%) | 3 (9.4%) | 0.70 |
| Solid organ malignancy | 16 (25.0%) | 5 (15.6%) | 11 (34.4%) | 0.08 |
| Recent chemotherapy | 14 (22.2%) | 4 (12.5%) | 10 (32.3%) | 0.05 |
| Recent immunotherapy | 4 (6.3%) | 2 (6.3%) | 2 (6.3%) | 1.00 |
| Neutropenia | 4 (6.3%) | 1 (3.1%) | 3 (9.4%) | 0.61 |
| Recent abdominal surgery** | 6 (9.4%) | 2 (6.3%) | 4 (12.5%) | 0.67 |
| Recent antibiotics** | 62 (96.9%) | 30 (93.8%) | 32 (100.0%) | 0.49 |
| Recent antifungals** | 22 (34.4%) | 12 (37.5%) | 10 (31.3%) | 0.59 |
| Mechanical ventilation | 49 (76.6%) | 26 (81.3%) | 23 (71.9%) | 0.37 |
| CVC | 55 (85.9%) | 27 (84.4%) | 28 (87.5%) | 1.00 |
| Persistent candidemia | 10 (24.4%) | 3 (15.8%) | 7 (31.8%) | 0.29 |
| Source of candidemia CLABSI UTI GI tract Unknown Others*** |
18 (28.1%) 20 (31.3%) 14 (21.9%) 15 (23.4%) 11 (17.5%) |
8 (25.0%) 12 (37.5%) 6 (18.8%) 9 (28.1%) 4 (12.9%) |
10 (31.2%) 8 (25.0%) 8 (25.0%) 6 (18.8%) 7 (21.9%) |
0.57 0.28 0.54 0.37 0.34 |
| Species C. albicans C. auris NAC other than C. auris |
17 (26.6%) 19 (29.7%) 28 (43.8%) |
9 (28.1%) 9 (28.1%) 14 (43.8%) |
8 (25.1%) 10 (31.3%) 14 (43.8%) |
0.94 |
| Total N = 64 |
CAC N = 32 (50.0%) |
Non-CAC N = 32 (50.0%) |
p-value | |
|---|---|---|---|---|
| Speciation | 51 (79.7%) | 26 (81.3%) | 25 (78.1%) | 0.75 |
| Susceptibility testing | 28 (48.3%) | 15 (51.7%) | 13 (44.8%) | 0.59 |
| Echocardiography | 15 (24.2%) | 9 (29.0%) | 6 (19.4%) | 0.37 |
| Ophthalmic examination | 11 (17.5%) | 5 (15.6%) | 6 (19.4%) | 0.69 |
| Empiric antifungal agent | ||||
| Fluconazole | 3 (5.1%) | 2 (6.7%) | 1 (3.4%) | 1.00 |
| Caspofungin | 36 (61.0%) | 25 (83.3%) | 11 (37.9%) | <0.001 |
| Anidulafungin | 14 (23.7%) | 5 (16.7%) | 9 (31.0%) | 0.19 |
| Micafungin | 1 (1.7%) | 0 (0.0%) | 1 (3.4%) | 0.49 |
| Lipid formulation of Amphotericin B | 5 (8.5%) | 1 (3.3%) | 4 (13.8%) | 0.19 |
| Voriconazole | 7 (11.9%) | 3 (10.0%) | 4 (13.8%) | 0.71 |
| Targeted antifungal agent |
0.054 |
|||
| Fluconazole | 44 (47.3%) | 3 (23.1%) | 41 (51.2%) | |
| Voriconazole | 14 (15.1%) | 3 (23.1%) | 11 (13.8%) | |
| Caspofungin | 13 (14%) | 2 (15.4%) | 11 (13.8%) | |
| Anidulafungin | 10 (10.8%) | 4 (30.8%) | 6 (7.5%) | |
| Micafungin | 4 (4.3%) | 1 (7.7%) | 3 (3.8%) | |
| Lipid formulation of Amphotericin B | 8 (8.6%) | 0 (0.0%) | 8 (10.0%) | |
| Empirical echinocandin | 48 (81.4%) | 28 (93.3%) | 20 (69.0%) | 0.016 |
| Step-down to fluconazole | 4 (7.1%) | 1 (3.3%) | 3 (11.5%) | 0.32 |
| Daily blood culture until negative | 2 (4.2%) | 1 (4.2%) | 1 (4.2%) | 1.00 |
| Completed 14 days of antifungals | 9 (17.0%) | 4 (14.8%) | 5 (19.2%) | 0.72 |
| 30-day mortality | 49 (76.6%) | 24 (75.0%) | 25 (78.1%) | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





