Introduction
In the last decade, scientific research has focused on new and more advantageous methods for the extraction of bioactive molecules, such as polyphenolic compounds and vitamins, from food waste [
1,
2,
3,
4,
5]. The main reasons for the growing interest in these matrices are in their properties, namely they provide the food industry with an efficient and environmentally friendly solution for waste disposal and can represent a rich and economical source of useful molecules, if properly treated.
Apple (
Malus domestica) is a type of fruit available all year round, typical of temperate zones, although widespread throughout the world for economic and cultural reasons [
7,
8,
9]. In 2020, according to the Food and Agriculture Organization report, world apple production was estimated at ~86 million tons, i.e.,~28% less than that of bananas, with 120 million tons, but ~8% and 10% more than grapes and oranges, respectively. Among the leading producing countries, China ranks with a production of ~40.5 million tons. Approximately 18% of global production is transformed into drinks, syrups, jams, purees, etc., generating large quantities of by-products whose disposal can constitute an environmental problem and add additional costs to food producers [
9,
10]. However, due to the high content of natural compounds important for human health, apple waste is an important resource, which can be used to obtain products of greater economic and nutritional value [
10,
11,
12,
13,
14,
15,
16].
Apple peel is a rich source of dietary fiber [
15,
17], minerals [
17], flavonoids [
15,
18] and phenolic acids [
15,
18]. Polyphenols and dietary fiber exert a positive influence on the prevention of various diseases and have a positive effect on human health [
2,
16,
19,
20]. The distribution of polyphenols, molecules important to balance oxidative stress and inflammatory processes [
2,
18,
19], varies between the peel and its flesh, being the first richer in flavonoids, such as quercetin glycosides and cyanidin glycosides [
2,
12].
Nutritionists have detected that the apple is also an essential source of vitamins, such as C, B1, B2, B3, and A [
17,
21,
22]. Various reports indicate that, like the polyphenols, the content of vitamin C is higher (1.5- to 3.3-fold greater), in the peel compared to its flesh. Vitamin C is a powerful antioxidant with a dietary reference intake of 25-90 mg/day. Its content in the peel ranged from 99.2 ± 10.0 to 300.9 ± 10.8 mg/100g [
21,
22].
Therefore, given the potentially high value of apple waste and the need to ensure a healthy environment for a sustainable economy, the development of analytical methods capable of valorizing industrial by-products in an environmentally sustainable way is in high demand. In fact, new methods are currently available in the literature for the best use of this source [
1,
2,
5,
10,
12,
16]. Previously, conventional extraction techniques (maceration and Soxhlet) were the only ones used in the extraction of phytocompounds from apple. The extracts were obtained by treating fresh or dried (oven-dried or freeze-dried) peels with solvents of different polarity, such as aqueous acetone or ethanol solutions. Although acetone proved to be more suitable than the oven-dried/ethanol combination for the extraction of polyphenols from apple peels, the latter procedure was preferred because it was more suitable for food uses [
16]. Unfortunately, conventional methods are not environmentally friendly and easily applicable to large-scale samples, such as industrial ones.
Recently, green extraction technologies are preferred to conventional methods for transforming apple waste into high value-added products [
1,
2,
10].
Supercritical fluid extraction (SFE) constitutes a more cost-effective alternative to conventional procedures because it is simple, eco-friendly and can be applied to systems on different scales. In fact, it can be used in the laboratory for analytical (from few hundred milligrams to a few grams of sample) or for preparative purposes (a few hundred grams of sample), but also to treat quantities of raw material in the order of kilograms (pilot systems) up to tons (industrial sector) [
2,
16].
In this work, carbon dioxide (CO2) was used as supercritical fluid with ethanol as modifier, for the simultaneously extraction of the bioactive molecules from apple peel. The waste apple peels were obtained from three among of the most common cultivars in Italy from organic (Royal Gala and Stark) and non-organic farming (Gala).
Each SC-CO
2 extract was characterized in terms of total phenolic content, flavonoid, anthocyanin, vitamin C and antioxidant activity. In addition, the antioxidant activity of each extract was assayed in in vitro tests on mitochondria from the human embryonic kidney cell line (HEK293) [
23] to estimate NADH-ubiquinone oxidoreductase (Complex I) activity in presence of the investigated extracts.
Several mitochondrial diseases but also some cancers [
24], and more in general mitochondrial dysfunction associated to ageing, may depend on Complex I impairment, since cataracts, diabetic mellitus, short stature, neuromuscular degeneration, cardiomyopathy, sensorineural hearing loss, and kidney failure, etc. [
25,
26], thus, it becomes crucial to find small molecules able to stimulate Complex I activity and/or mitochondrial function.
In this context, the aim of this preliminary work is to quantify the antioxidant fractions present in the investigated apple peel extracts and to assay Complex I activity in presence of the investigated extracts for estimating their abilities in stimulating the oxidation of NADH and mitochondrial function for possible future pharmacological applications.
2. Materials and Methods
2.1. Apple Collection and Apple Peel Preparation
Apples from Stark, Gala, and Royal Gala cultivar were acquired by local suppliers in the period August-September 2021.
The fruits were washed with tap water and thin peels were obtained from each cultivar and then cut into small pieces, put on Petri plates, weighed, and immediately covered with paper to avoid light exposure. Samples were frozen at -20 °C for 24 hours and finally freeze-dried for 48 hours in a ScanVac cool safe lyophilizer supplied by Chemie (BA, Italy), operating at a temperature of -50 °C and a pressure of 0.5 torr. Freeze-dried peel samples were stored into a dryer at room temperature in the dark, until the time of use.
2.2. Supercritical Fluid Extraction
SC-CO
2 extraction was then carried out according to Aresta et al [
4], with modifications. The SC-CO
2 system was a Spe-ed SFE from
Applied Separations (Allentown, PA, USA). One gram of each freeze-dried peel sample was mixed with a small quantity of a dispersing material, Ottawa sand (O
2Si, 50-70 mesh particle size,
Applaied Separation), to facilitate the contact between the supercritical fluid and the sample particles. The mixture was placed into a 10 mL extraction cell, which was sealed under pressure before being introduced into the oven at 60 °C.
Carbon dioxide (supercritical fluid grade, SCF) from Rivoira (Milan, Italy) was used as extraction solvent with 20 % ethanol (liquid chromatographic grade, LC; Sigma-Aldrich, Milan, Italy) as co-solvent. Before each extraction, the manual purging of the co-solvent pump was necessary both to ensure the correct flow at the same stage and to prevent the pump from undergoing a pressure stroke during extraction. Then, a static period of 3 minutes was applied before starting the extraction by activating the co-solvent pump. Subsequently, the dynamic extraction process was carried out at 60 °C and 250 bar for 15 minutes, with carbon monoxide and ethanol (0.4 mL/min) at a total flow rate of 2 ml/min. The extracts were collected in amber vials using the heated micrometric valve, set at 70 ° C. At the end of each extraction cycle, an ethanol cleaning cycle was carried out using a stainless-steel by-pass instead of the jar, for 3 minutes, to remove all residues.
The extract sample was then filtered through a syringe filter device with a 25 mm diameter and 0.45 μm PTFE (polytetrafluoroethylene) membrane (Sigma-Aldrich), before to be subjected to analytical analysis.
2.3. Total Antioxidant Capacity (TAC) Assay
The assay was performed according to slightly modified previous literature protocols [
27]. An aliquot (10 μL) of SC-CO
2 extract (S) was transferred into a test tube containing 0.99 mL of 0.1 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH;
Sigma Aldrich), or ethanol (EtOH). The solutions were kept in the dark for 1 h at room temperature. The absorbances were read at 517 nm against EtOH using a UV/Visible spectrophotometer (UV-1650 PC;
Shimadzu, Kyoto, Japan). The radical scavenging activity (RSA) was expressed as the percentage of the DPPH consumed, which was calculated using the following formula:
where
AS is the absorbance of the extract with DPPH,
AEtOH is the absorbance of the extract without DPPH, and
ADPPH is the absorbance of the DPPH solution. The calibration curve was prepared with standard solutions of (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox;
Sigma Aldrich) in the range 1-100 mM. The TAC was expressed as Trolox equivalent antioxidant capacity (TEAC) (mmole of Trolox /100 g of f.d.m) [
28].
2.4. Quantification of Antioxidant Compounds
2.4.1. Total Phenols (TPs) Determination
The TPs content was evaluated by using 4-benzoylamino-2,5-dimethoxybenzenediazonium chloride hemi [zinc chloride] salt, namely Fast Blue BB diazonium salt (FB BB D;
Sigma Aldrich), according to Medina et al. protocol [
28]. Briefly, 0.1 mL of 0.1 % (w/v) FB BB D reagent in 0.1 % ethanol was added at 1 mL of SC-CO
2 extract previously diluted 1:10 in water. Samples were accurately mixed and 0.1 mL of 20 % (w/v) Na
2CO
3 in 1 M NaOH was added to each test tube. The absorbances of samples were measured at 420 nm at 0 and 30 min and the difference between two measurements was used for the estimation. Gallic acid (GA;
Sigma Aldrich) was used as the standard compound (calibration curve in the range 0.02-20 μg/mL) for quantification of TPs, expressed as mg of GA/100 g of freeze-dried material (fdm). Each measurement was performed in triplicate.
2.4.2. Total Flavonoids (TFs) Determination
The content of TFs was carried out following the method proposed by Zhishen et al. [
29], with a few modifications. Briefly, 50 μL of SC-CO
2 extract was dried in a test tube and 0.5 mL of distilled water (suitable for HPLC;
Sigma-Aldrich) was added to the residue. After accurate mixing, 37.5 mL of 5% (w/v) NaNO₂ (
Sigma-Aldrich) was added to each test tube, which was then kept for 5 min at room temperature. Subsequently, 37.5 mL of 10 % (w/v) AlCl
3 (
Sigma-Aldrich) was added. After 6 min, 250 mL of 1 M NaOH was added, mixed and then each tube received other 300 mL of distilled water. The absorbance at 510 nm of the mixture was finally measured, against a blank sample obtained in the same mode. GA was used as the standard compound for quantification of TFs, expressed as mg of GA/100 g of fdm. Each measurement was performed in triplicate.
2.4.3. Total Anthocyanins (TAs) Determination
The monomeric anthocyanin content of the SC-CO
2 extract from apple peel was measured using a spectrophotometric pH differential protocol [
30]. An aliquot (0.1mL) of apple peel extract was mixed thoroughly with 0.9 mL of 0.025 M KCl pH 1 buffer. The absorbance of the mixture was measured at 515 and 700 nm against a distilled water blank. Another aliquot was combined similarly with 25 mM sodium acetate buffer pH 4.5, and the absorbance of the solution was measured at the same wavelengths. Finally, the differences between (A
515nm - A
700nm) pH 1 and (A
515nm - A
700nm) pH 4.5 were used for the determination of total anthocyanins. Cyanidin-3-glucoside chloride (CG, Reference Substance;
Sigma-Aldrich) was used as the standard compound for quantification of anthocyanin content in each apple peel extract. Total anthocyanins were expressed as mg of CG/100 g of fdm. Each measurement was performed in triplicate.
2.4.4. Ascorbic Acid (AA) Determination
The quantification of AA was performed according to Kschonsek et al. [
18]. Briefly
, an aliquot (0.2 mL) of SC-CO
2 extracts, previously diluted 1:10 with ultra-pure water, was mixed with 0.3 mL of trichloroacetic acid (0.31 M;
Sigma Aldrich) and centrifuged (5 min, 17,000 g). Then, 0.3 mL of supernatant was mixed with 0.1 mL of DNP reagent [one volume of thiourea solution (0.83 M in distilled water), one volume of copper sulphate solution (24 mM in distilled water) and 20 volumes of 2,4-dinitrophenylhydrazine solution (0.11 M in 4.5 M sulfuric acid). All reagents were provided by
Sigma Aldrich]. The mixture was heated in a thermomixer for 1 h at 60 °C. The samples were cooled in an ice bath for 5 min. Then, 0.4 mL of sulfuric acid (9 M;
Sigma Aldrich) was added and mixed. The samples were kept in the dark for 20 min. Finally, each sample was transferred into a semi-micro cuvette and measured at 520 nm. Each measurement was performed in triplicate. In the same way, a calibration line was obtained with six standard solutions of AA (Reference Standard;
Sigma Aldrich) covering the concentration range of 0.06 to 6 mg/mL.
2.5. UHPLC–DAD Analysis
Chromatographic analyses were performed using an UHPLC system (LC20ADXR; Shimadzu,
Milan, Italy) equipped with a binary pump (LC20ADXR; Shimadzu) , an auto sampler (SIL20ACXR; Shimadzu) and a UV-diode array detector (PDA-1; Shimadzu) with a flow cell (10 mm, 1/1600, steel, 2.4 mL, LWL). The chromatographic column was an Accucore XLC18 (150x4.6 mm, 4 μm; Thermo Scientific, Milan, Italy) equipped with an Accucore XLC18 precolumn (10x4 mm, 4 μm; Thermo Scientific).
The mobile phase used was (A) 0.1% formic acid in water, (B) acetonitrile (Sigma Aldrich) with a flow rate of 1.2 mL/ min. The gradient elution program was as follows: B (8%) (2 min), 8% B to 15% B (3 min), 15% B to 25% B (5 min), 25% B to 35% (5min), 35% B to 50% B (5 min), B (50%) (15 min), and re-equilibration of the column, (5 min). The total chromatographic run time was 45 min. Injection volume was 20 μL.
Spectra were acquired in the range of 220–500 nm, while meticulous composite chromatograms were obtained using the Max Plot software of the PDA-1 detector, which automatically selects and stores the maximum absorbances for each peak, thus providing the maximum signal for all compounds detected. The phenolic compounds were identified by comparing their retention times, UV–visible with those obtained from standard compounds (10 μg/mL; all Sigma-Aldrich), when available. Otherwise, compounds were tentatively identified comparing the obtained information with available data reported in the literature.
An enzymatic deconjugation was also performed as follows: 0.1 mL of enzymatic solution for glycosides hydrolysis [obtained by dissolving 10 mg of β-Glucuronidase from bovine liver (type B-1, ≥1,000,000 units/g solid; Sigma Aldrich) in 13 mL of 0.1 M acetate buffer, pH 5.0] was added to 0.05 mL of SC-CO2 extract and the resulting mixture was immediately incubated overnight (i.e. approximately 17 hours) at 37 °C. Before being subjected to chromatographic analysis, the mixture was filtered through a syringe filter device with 13 mm diameter (0.45 μm PTFE ; Sigma-Aldrich),
The experiments were performed in triplicate.
2.6. Evaluating Biological Activity
2.6.1. Cell Cultures
HEK293 cells were obtained from the American Type Culture Collection (
https://www.atcc.org/products/crl-1573). For the proposed analyses, HEK293 were mantained in Dulbecco’s Modified Eagle’s Medium (DMEM) high glucose with sodium pyruvate and stable glutamine (
Euroclone, Pero (MI), Italy), supplemented with 10 % (v/v) fetal bovine serum (FBS;
Euroclone ECS0180L, Pero, Milan, Italy), and 1 % (v/v) penicillin-streptomycin (
Euroclone, ECM0010, Pero, Milan, Italy ). The cells were cultured in a humidified atmosphere with 5 % CO
2 and 37 °C.
2.6.2. Mitochondrial Isolation
Cells were grown in T75 flasks.At confluence cells were detached by trypsin and centrifuged at 125 g for 5 min, the supernatant was discarded, and the pelleted cells were resuspended in Ringer NaCl buffer (135 mM NaCl, 20 mM HEPES, 0.8 mM MgSO4, 3 mM KCl, 1.8 mM CaCl2, 11 mM D-glucose, pH = 7.5) according to Palacino et al [
31]. Afterwards cells were centrifuged at 125 g for 5 min and the obtained pellet was suspended in 2 mL of A buffer (sucrose 320 mM, Tris-HCl 5 mM, EGTA 2 mM, pH = 7.4), and homogenized with a glass–Teflon grinder kept in ice. Thus, the homogenate underwent a centrifugation step at 4 ◦C for 6 min at 2000 g to remove nuclei and tissue particles, while the supernatant was collected for a further centrifugation at 4 ◦C for 15 min at 12,000 g to pellet mitochondria. The entire isolation procedure of subcellular fractions was carried out according to Palacino et al. [
31]. The mitochondria amount was determined in according to Bradford methods [
32].
2.4.3. Complex I Activity Measurements
Rotenone-sensitive NADH (β-Nicotinamide adenine dinucleotide) ubiquinone oxidoreductase (Complex I) activity was measured spectrophotometrically following the decrease of NADH absorbance at 340 nm as described by Spinazzi at al. [
33], with some modifications. It was used a reaction mix containing 25 μg sample mitochondria, TNS buffer 3 % (w/v), 50 mM potassium phosphate buffer pH 7.5, 600 mM KCN (
Sigma Aldrich), and 90 mM NADH (≥97% pure, grade HPLC;
Sigma Aldrich). The mitochondria were incubated for 2 min with apple peel extracts diluted 1:100, 1:1000 or 1:10000 in H
2O or with only ethanol used as a control (at the same dilutions in water) before starting the reaction. The reaction was started by adding 30 mM Decilubiquinone (≥97% pure, grade HPLC;
Sigma Aldrich). NADH (β-Nicotinamide adenine dinucleotide) ubiquinone oxidoreductase (Complex I) specific activity was checked by adding an excess of Rotenone 100 μM. In parallel, as a further control test, it was prepared a cuvette containing the same quantity of extract (ethanol) dilutions and the mitochondrial sample, in presence of 100 μM Rotenone (≥95% pure;
Sigma Aldrich), which is a Complex I selective inhibitor.
3. Results and Discussion
3.1. Supercritical Fluid Extraction
The parameters applied during supercritical fluid extraction (SFE) were found in the literature [
1,
2,
3,
4,
34]. Having a low polarity, ethanol was selected as extraction co-solvent to help carbon dioxide for vitamin C extraction, while a percentage of 20% was chosen to favor the extraction of polyphenols. Besides, a mild temperature (60°C) was selected to avoid thermal degradation of the compounds and preserve the antioxidant properties of the extracts [
4,
34]. Given these observations, the samples were prepared according to the protocol optimized by Aresta et al. for the determination of polyphenols and vitamins in winemaking by-products by extraction with SC-CO
2 [
4].
3.2. Quantification of Antioxidant Compounds
The specific spectrophotometric assays for the determination of the total antioxidant capacity and the quantification of the antioxidant compounds present in the extracts of Stark, Gala and Gala Royal apple peel obtained by SFE (2.2 paragraph), have been selected from the literature [
28] considering specific features, such as sensitivity, specificity, and ease of execution. TPs were detected using the FB BB D reagent which, unlike the more common Folin-Ciocalteu, is not sensitive to reducing sugars which can be naturally present in extracts and determine overestimates with Folin-Ciocalteu [
28].
Table 1 shows the average values of the complete set of determinations (TAC, TPs, TFs, TAs and AA) performed on each apple peel SC-CO
2 extract. The data obtained were in good agreement with the existing literature for all the analytes except for ascorbic acid, which was found in all extracts at concentration levels lower than expected [
17,
18].
T-test showed some statistically significant differences between the extracts of the three cultivars in the five assays (p<0.05). Stark extracts were higher in TP, TA, and AA than the other two. Instead, it showed no significant differences in comparison with Royal Gala for TAC. Gala and Royal Gala were similar in TP and TA. On the other hand, vitamin C contents, were very different between the three extracts. In particular, the Gala apples were characterized by lower levels and lower antioxidant activity. Then, phytochemicals can contribute in different ways to the antioxidant activity of the extracts, and the poor correlation between the estimated amounts of the analytes and the antioxidant power of the samples.
3.3. UHPLC–DAD Analysis
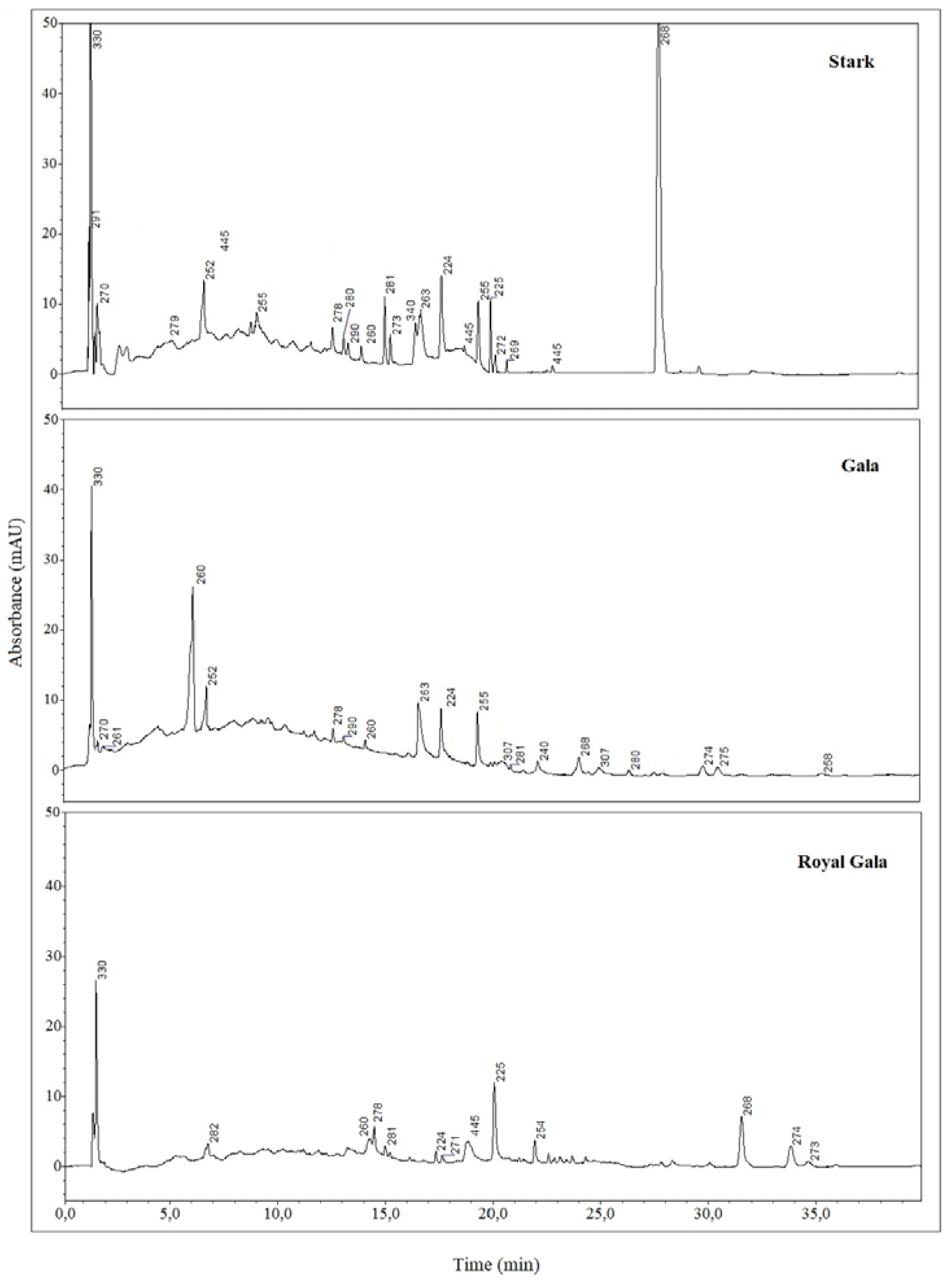
Chromatographic analyses confirmed the existence of differences between samples deriving from apple waste of different origin.
Figure 1 shows chromatograms relevant to the three SC-CO
2 extracts (Stark, Gala and Royal Gala
Some polyphenols were identified by comparing both the chromatographic retention times and the UV-visible spectra taken at the apex of each peak with those obtained from standard compounds analyzed under the same conditions.
Table 2 lists the standards that have been subjected to chromatographic analysis, their retention times (RT, min), the λ
max recorded by the detector and the presence or absence in the considered cultivar.
Rutin, is a glycoside of the flavonol quercetin, in which the aglycone is bound to the disaccharide α-L-rhamnopyranosyl-(1
→6)-β-D-glucopyranose. It is found in a wide variety of plants and apple peel and is believed to be a rich source of flavonoids [
7,
35]. However, it was unexpectedly detected only in Stark extracts.
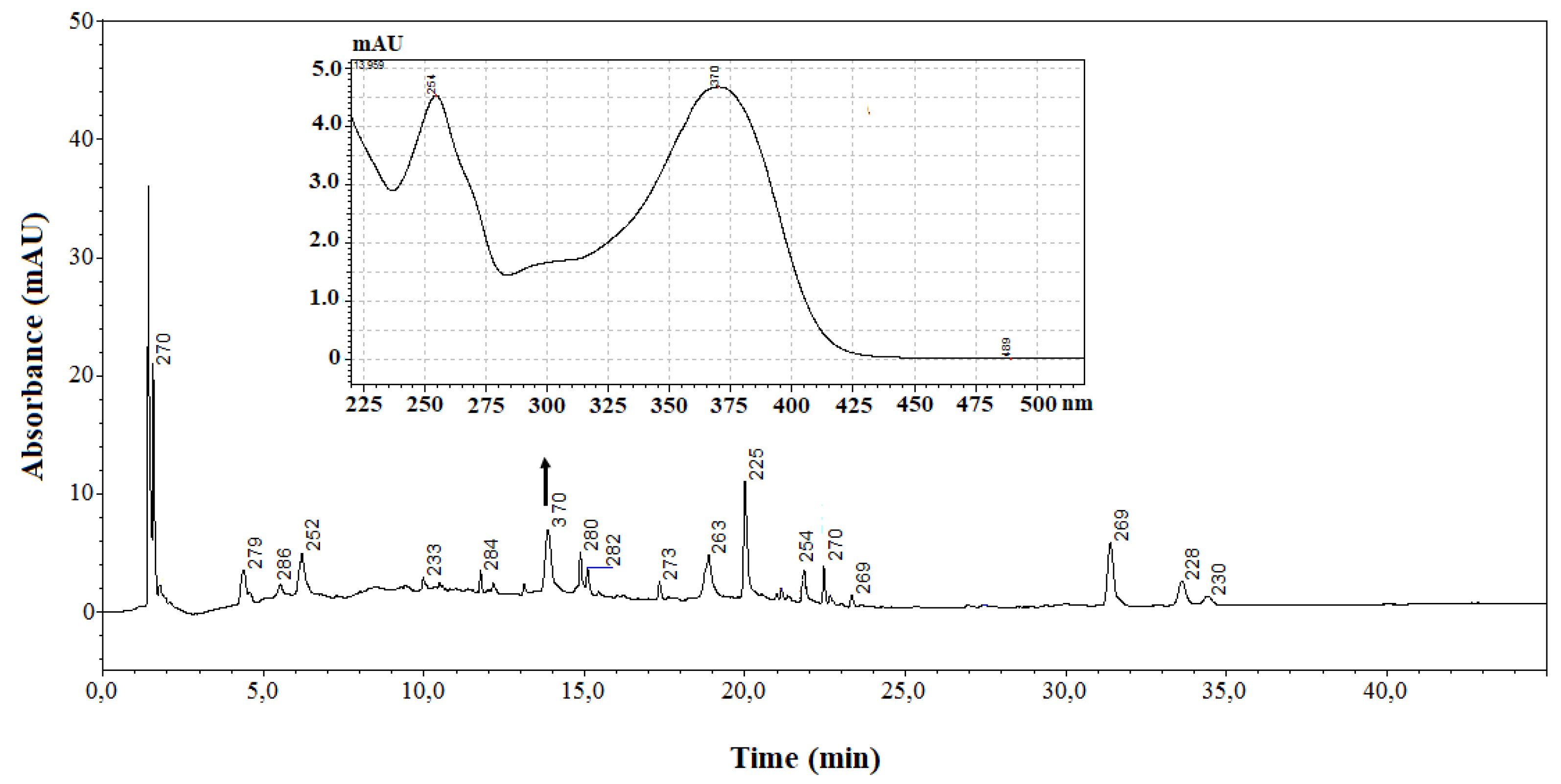
Then, all the SC-CO
2 extracts were subjected to enzymatic hydrolysis, to obtain the respective aglycones. The glycosylated deconjugates were then subjected to LC analysis in order to check their presence.
Figure 2 shows, for instance, an LC chromatogram of a deconjugate Stark sample. Both the retention time and the UV-visible spectrum of the peak eluting at 13.9 minutes were found clearly comparable to those of the standard of quercetin analysed using the same chromatographic conditions. On the contrary, analysis of the deconjugates related to the other two cultivars confirmed the absence of the flavonol.
The careful inspection of retention times and spectra and the provisional identification, also comparing the obtained information with the literature data showed the probable presence of catechins (λ
max 280 nm), caffeic acid (238 nm), cyanidins (278 nm), chlorogenic acid (324 nm), isoflavones (260 nm), retinoid (340 nm) and carotenoids (445 nm) [
36].
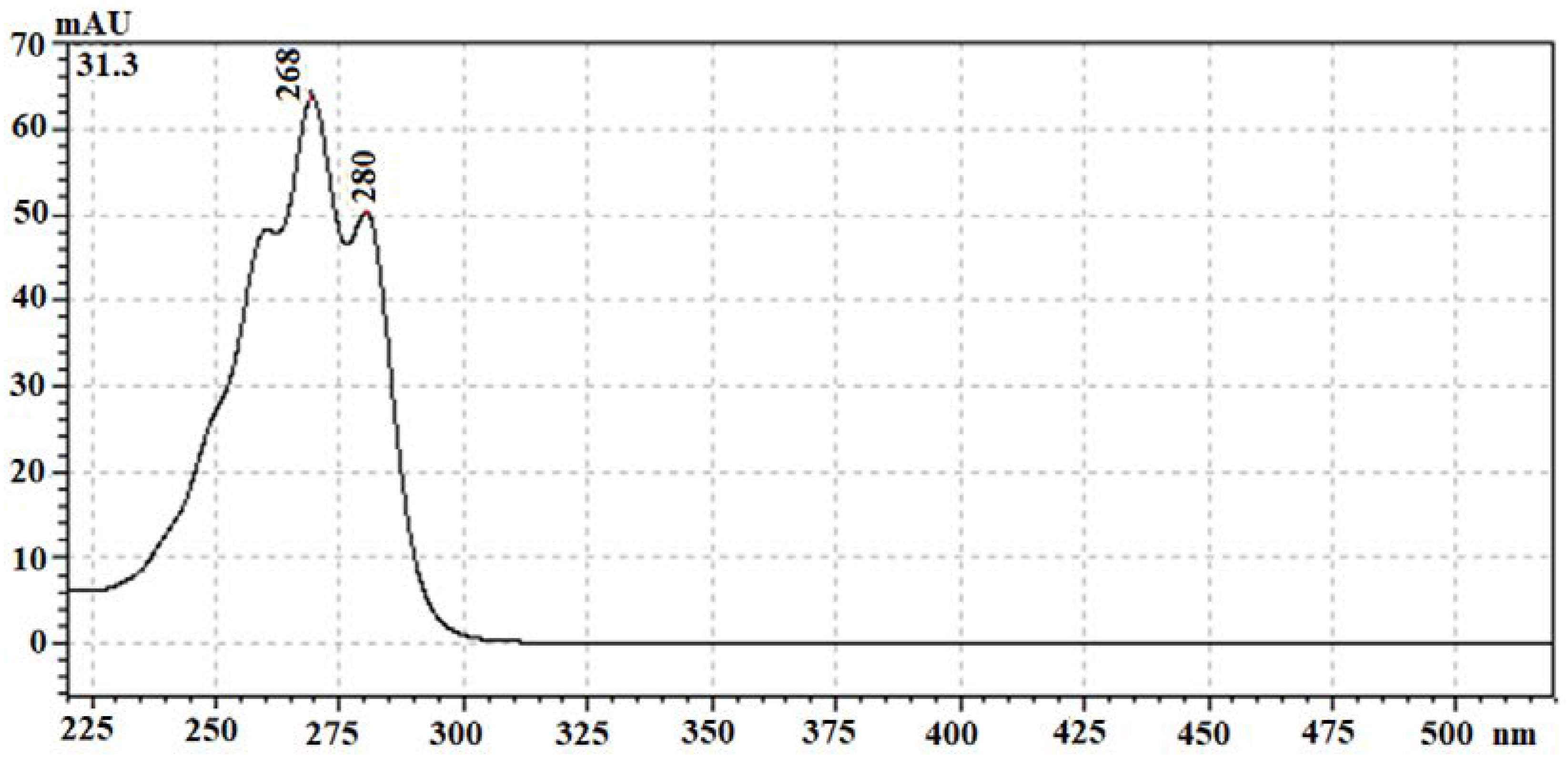
In the chromatograms relative to the SC-CO
2 extracts of Sark samples, it is evident the presence of a very abundant peak at the retention time of 31.3 min, whose UV-visible spectrum is reported in
Figure 3. Interestingly, this spectrum is shared with other peaks of the same chromatogram, namely the one with RT to 18.9, 22.5 and 23.4 minutes. Comparing the chromatographic data relevant to this extract with those of the other cultivar analysed, only one peak in the chromatogram relevant to the Gala Royal extract showed a peak eluting at 18.9 min, also characterized by the same UV-visible spectrum. It is worth noting that Gala Royal and Stark cultivars are both from organic farming.
The spectra of these unknown compounds are similar to those of some lipophilic compounds. Carotenoids are pigments synthesized by all plants, many bacteria, and some fungi, widespread in nature, with more than 700 species, very important for human health. Some carotenoids are precursors of vitamin A, which exerts multiple functions: during embryonic development, the immune response and in the visual cycle [
37].
3.3. Evaluating Biological Activity
To examine the effect of each apple peel extract on mitochondria, the NADH oxidoreductase activity was measured on mitochondria isolated from HEK293 cells in presence of different dilutions of the investigated extracts.
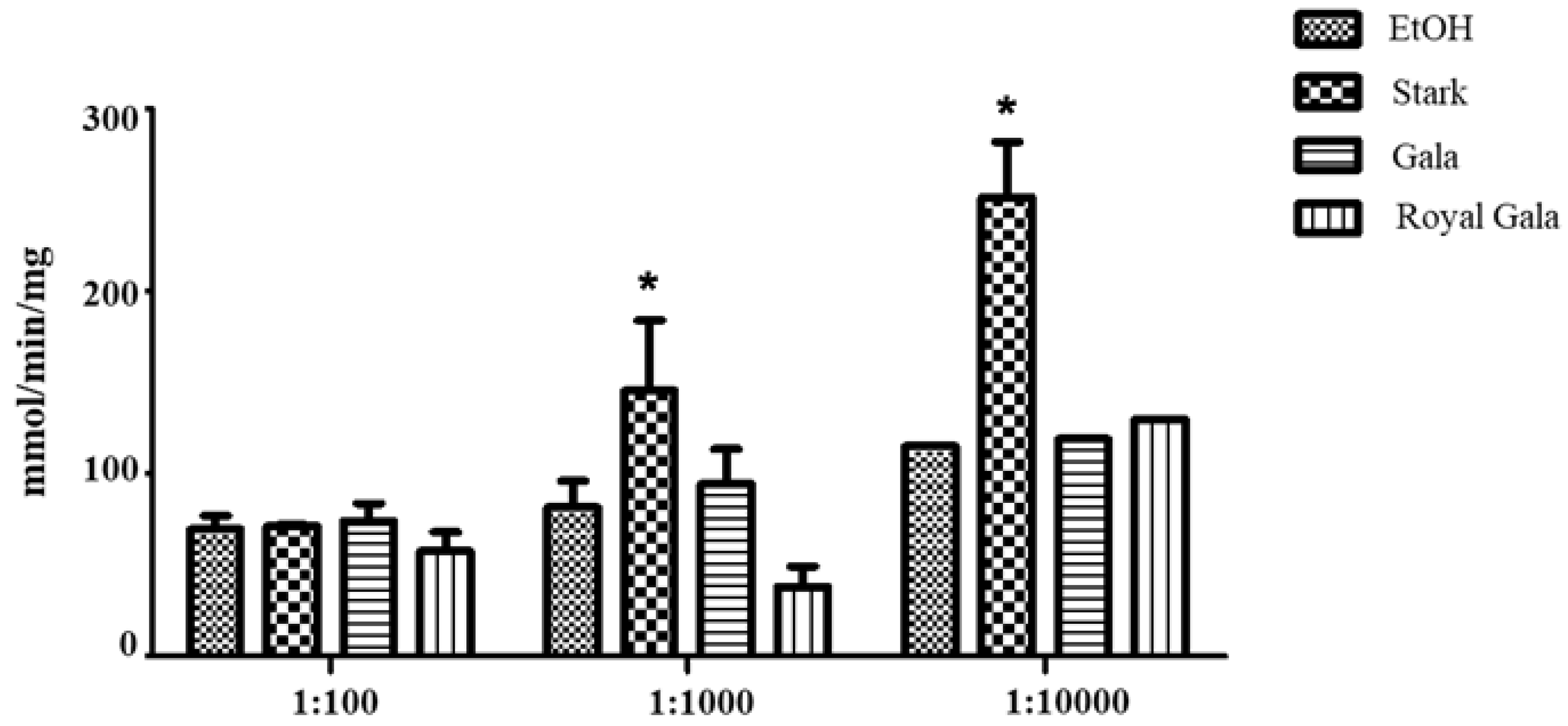
Firstly, it was noticed that the complex I activity measured from mitochondria extracted from the cultured selected cells after the addition of the three apple peel extracts at 1:100 dilution was unchanged with respect the ethanol control. It was instead observed an increase of complex I activity in presence of the Stark apple peel extract at 1:1000 and above all at 1:10000 dilution, with respect to the ethanol control at the same dilution, whereas the Complex I activity in presence of Gala and Royal Gala apple peel extracts was unchanged (
Figure 4).
While at the employed dilutions, it appears that ethanol presence does not affect mitochondrial function, coherently with Tempio et al. [
38], and that SC-CO
2 extracts from the apple peel of Stark cultivar at the investigated increasing dilution are able to stimulate Complex I activity. It is observed that Stark extracts are richer in bioactive molecules than the other two extracts. Above all, it is observed that Stark extracts contains almost twice the concentration of AA, TPs and TAs compared to the other two investigated extracts.
While it is observed that the most concentrated Stark extracts are relatively well tolerated by mitochondria (comparable to the other investigated extracts), more diluted Stark extracts produce a significant stimulation of Complex I activity. The latter observation can be ascribed either to the minimum concentration of bioactive molecules able to produce a positive effect without causing any toxicity, or to the fact that the specific combination of AA, TPs and TAs in Stark extracts at the assayed increasing dilutions is able to exert a more efficient protective effect on mitochondria, compared to the other two extracts. Indeed, it is known that an excess of antioxidant molecules, while may counteract ROS formation into mitochondria, can alter the endogenous ROS signaling between the cytosol and mitochondria, and can even result in toxicity [
39,
40]. Indeed, it is known that apple peel are enriched in vitamins (i.e., vitamin E and vitamin K [
41,
42]) that can be structurally related to ubiquinone that can be able to target either mitochondrial proteins of the inner membrane or other FAD/NADH/ubiquinone dependent dehydrogenases widely expressed in other cell-compartments [
39].
More in general, our speculations about the protective role of the tested extracts on mitochondrial function are coherent with Carrasco-Pozo et al. hypothesis [
43], about a possible protective role for dietary polyphenols on mitochondria. The authors also showed that quercetin, resveratrol and rutin protected Caco-2 cells against indomethacin-induced mitochondrial dysfunction. About this, the hypothesized protective role was related to the ability of some antioxidant molecules to enter cells and accumulate in mitochondria. In addition, a structural similarity between quercetin and ubiquinone has been proposed by authors, probably responsible for the protective effect [
43]. Similarly, Skemiene et al. [
44], proposed that anthocyanins can behave as electron acceptors in complex I, resulting in increased ATP production in pathological conditions, such as after ischemia.
Based on the experimental results obtained, coherent with analogous findings described in literature, it is evident that Stark apple peel extracts appear to be a potentially advantageous dietary supplement for human health. For this reason, soon, it will be the subject of further studies aimed at determining, at a qualitative and quantitative level, the individual polyphenols concentration. The role of each compound, in terms of antioxidant capacity and, above all, in terms of protective activity on mitochondria in presence of pro-apoptotic molecules will be explored by performing mitochondrial respiration assays on mitochondria extracted from different cell-lines.
Author Contributions
Conceptualization, A.A. and C.Z.; Methodology, A.A., P.C., C.L.P.; Validation, A.A., P.C. and C.L.P.; Investigation, A.A., P.C., L.T; Resources, A.A.; Data Curation, A.A.; Writing—Original Draft Preparation, A.A. and C.L.P.; Writing—Review & Editing, A.A.; Supervision, C.Z.; Funding Acquisition, A.A. and C.Z. All authors have read and agreed to the published version of the manuscript.