Submitted:
07 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Animal Housing
2.3. Photoperiods
2.4. Sperm Recovery and Analysis
2.5. Sperm Morphology
2.6. Fertility
2.7. Statistical analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Correa, L.M.; Fernández, J.L. Influence of Melatonin on the physiology and the conduct of ungulates. Rev. Investig. Altoandin. 2017, 19(3), 337-350. [CrossRef]
- Zarazaga, L.A.; Gatica, M.C.; Celi, I.; Guzmán, J.L.; Malpaux, B. Effect of artificial long days and/or melatonin treatment on the sexual activity of Mediterranean bucks. Small Rumin. Res. 2010, 93, 110–8. [CrossRef]
- Chemineau, P. Medio Ambiente y Reproducción Animal. World Animal Review 1993, 77(1), 2–14. Retrieved from http://www.fao.org/ag/Aga/AGAP/FRG/FEEDback/War/v1650b/v1650b04.htm.
- Lincoln, G.A. Photoperiod-pineal-hypothalamic relay in sheep. Anim. Reprod. Sci. 1992, 28(1–4), 203–217. [CrossRef]
- Prendergast, B.J.; Nelson, R.J.; Zucker, I. Mammalian Seasonal Rhythms. In Hormones, Brain and Behavior. Pfaff, D.W., Arnold, A.P., Etgen, A.M., Fahrbach, S.E., Rubin, R.T. Eds., 2002, pp. 93–156. [CrossRef]
- Nakane, Y.; Yoshimura, T. 2019. Photoperiodic regulation of reproduction in vertebrates. Annu. Rev. Anim. Biosci. 2019, 7, 173-194. [CrossRef]
- Santiago-Moreno, J.; Gómez-Brunet, A.; González-Bulnes, A.; Toledano-Díaz, A.; Malpaux, B.; López-Sebastián, A. Differences in reproductive pattern between wild and domestic rams are not associated with inter-specific annual variations in plasma prolactin and melatonin concentrations. Domest. Anim. Endocrinol. 2005, 28(4), 416–29. [CrossRef]
- Bauer, B.; Womastek, I.; Dittami, J.; Huber, S. The effects of early environmental conditions on the reproductive and somatic development of juvenile guinea pigs (Cavia aperea f. porcellus). Gen. Comp. Endocrinol. 2008, 155, 680–5. [CrossRef]
- Guenther, A.; Palme, R.; Dersen, M.; Kaiser, S.; Trillmich, F. Photoperiodic effects on reproductive development in male cavies (Cavia aperea). Physiol. Behav. 2014, 123, 142-147. [CrossRef]
- Trillmich, F.; Mueller, B.; Kaiser, S.; Krause, J. Puberty in female cavies (Cavia aperea) is affected by photoperiod and social conditions. Physiol. Behav. 2009, 96(3), 476–80. [CrossRef]
- Maroto-Morales, A.; Ramón, M.; García-Álvarez, O.; Soler, A.J.; Esteso, M.C.; Martínez-Pastor, F.; Pérez-Guzmán, M.D.; Garde, J.J. Characterization of ram (Ovis aries) sperm head morphometry using the Sperm-Class Analyzer. Theriogenology 2010, 73(4), 437-448. [CrossRef]
- Yániz, J.L.; Capistrós, S.; Vicente-Fiel, S.; Soler, C.; de Murga, J.N.; Santolaria, P. Study of nuclear and acrosomal sperm morphometry in ram using a computer-assisted sperm morphometry analysis fluorescence (CASMA-F) method. Theriogenology 2014, 82(6), 921-924. [CrossRef]
- Kim, K.S.; Foster, J.A.; Gerton, G.L. Differential release of guinea pig sperm acrosomal components during exocytosis. Biol. Reprod. 2001, 64(1), 148-56. [CrossRef]
- Cabeza, U.; Ordoñez, C.; Meza, A.; Cucho, H. Morphological and morphometric characterization of the Guinea pig (Cavia porcellus). Spermova 2021, 10(2), 94-101. [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 2010, 160(7), 1577-79. [CrossRef]
- Everitt, J.I.; Gross, E.A. Euthanasia and Necropsy. In The Laboratory Rat. 2nd Ed., Suckow, M.A., Weisbroth, S.H., Franklin, C.L. Eds., Elsevier Academic Press, 2006 665–678. [CrossRef]
- Quispe, H.A.; Ciprián, R.; Ordoñez, C.; Ampuero, E.; Cucho, H. Hypoosmotic swelling test in alpaca (Vicugna pacos) spermatozoa recovered the vas deferens. Spermova 2015, 5(1), 10-14. [CrossRef]
- Ugarelli, A.; Evangelista-Vargas, S.; Santiani, A. Evaluation of acrosome integrity in epididimal alpaca sperm by flow cytometry. Rev. de Investig. Vet. del Perú 2017, 28(1), 130-40. [CrossRef]
- Heideman, P.D.; Sylvester, C.J. Reproductive photoresponsiveness in unmanipulated male Fisher 344 laboratory rats. Biol. Reprod. 1997, 57, 134–138. [CrossRef]
- Sancho, S.; Pinart, E.; Briz, M.; Garcia-Gil, N.; Badia, E.; Bassols, J.; Kádár, E.; Pruneda, A.; Bussalleu, E.; Yeste, M.; Coll, M.G.; Bonet, S. Semen quality of postpubertal boars during increasing and decreasing natural photoperiods. Theriogenology 2004, 62(7), 1271-1282. [CrossRef]
- Ayala Guanga, L.E.; Rodas Carpio, R.; Almeida, A.; Torres Inga, C.S.; Nieto Escandón, P.E. Influence of penis spicule of cavy (Cavia porcellus) on their sexual behavior, Fertility and sperm quality. Rev. Prod. Anim. 2017, 29(3), 36-42. http://scielo.sld.cu/pdf/rpa/v29n3/rpa06317.pdf.
- Rodríguez, G.J.; Barrios-Arpi, M.; Huanca, L.W.; Rodríguez, G.A.; Revuelta, L. Levels of glucose and triglycerides in seminal plasma ans spermatic motility in guinea pig fed with 10% more in digestible energy. Rev. Complut. Cienc. Vet. 2016, 10(1), 75-82. [CrossRef]
- Ferdinand, N.; Christiane, N.S.; Augustave, K.; D´Alex, T.C.; Raphael K.J.; … Joseph, T. Effect of Guava (Psidium guajava) leaves essential oil on some reproductive parameters in male guinea pig (Cavia porcellus). Biol. Syst. Open Access 2014, 3, 25. [CrossRef]
- Meza, A.; Huanca, N.; Aragón, S.; Cucho, H. Domestic guinea pig semen collection protocol (Cavia porcellus) by electroejaculation method. Spermova 2018, 8(1), 94.
- Benavides, F.; Sutovsky, P.; López, V.; Kennedy, C.; Echevarría, L. Semen parameters of fertile guinea pigs (Cavia porcellus) collected by transrectal electroejaculation. Animals 2020, 10, 767. [CrossRef]
- Kozioł, K.; Broda, D.; Romerowicz-Misielak, M.; Nowak, S.; Koziorowski, M. Melatonin concentration in peripheral blood and melatonin receptors (MT1 and MT2) in the testis and epididymis of male roe deer during active spermatogenesis. Theriogenology 2020, 149, 25-37. [CrossRef]
- Ngoula, F.; Tekam, M.G.; Kenfack, A.; Tchingo, C.D.A.T.; Nouboudem, S.; Ngoumtsop, H., ...Tchoumboue, J. Effects of heat stress on some reproductive parameters of male cavie (Cavia porcellus) and mitigation strategies using guava (Psidium guajava) leaves essential oil. J. Therm. Biol. 2017, 64, 67-72. [CrossRef]
- Durairajanayagam, D.; Sharma, R.K.; du Plessis, S.S.; Agarwal, A. Testicular heat stress and sperm quality. In Male Infertility, 1st ed.; du Plessis, S., Agarwal, A., Sabanegh, Jr. E.S., Eds.; Springer New York, NY, USA, 2014, 105-125. [CrossRef]
- Sabés-Alsina, M.; Tallo-Parra, O.; Mogas, M.T.; Morrell, J.M.; Lopez-Bejar, M. Heat stress has an effect on motility and metabolic activity of rabbit spermatozoa. Anim. Reprod. Sci. 2016, 173, 18-23. [CrossRef]
- Yucra, A. 2013. Características microscópicas del semen y morfometría del espermatozoide del cuy doméstico (Cavia porcellus) y silvestre (Cavia tschudii). Thesis of bachelor, Universidad Nacional de San Antonio Abad del Cusco, Cusco-Peru, 2013.
- Ball, B.A.; Mohammed, H.O. Morphometry of stallion spermatozoa by computer-assisted image analysis. Theriogenology 1995, 44, 367–377. [CrossRef]
- Hidalgo, M.; Rodríguez, I.; Dorado, J. Influence of staining and sampling procedures on goat sperm morphometry using the Sperm Class Analyzer. Theriogenology 2006, 66, 996-1003. [CrossRef]
- Verstegen, J.; Iguer-Ouada, M.; Onclin, K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology 2022, 57, 149–179. [CrossRef]
- Yániz, J.L.; Soler, C.; Santolaria, P. Computer assisted sperm morphometry in mammals: a review. Anim. Reprod. Sci. 2015, 156, 1-12. [CrossRef]
- Malo, A.F.; Gomendio, M.; Garde, J.; Lang-Lenton, B.; Soler, A.J.; Roldan, E.R. Sperm design and sperm function. Biol. Lett. 2006, 2(2), 246-249. [CrossRef]
- Soler, C.; Sancho, M.; García, A.; Fuentes, M.C.; Núñez, J.; Cucho, H. Ejaculate fractioning effect on llama sperm head morphometry as assessed by the ISAS® CASA system. Reprod. Domest. Anim. 2014, 49(1), 71-78. [CrossRef]
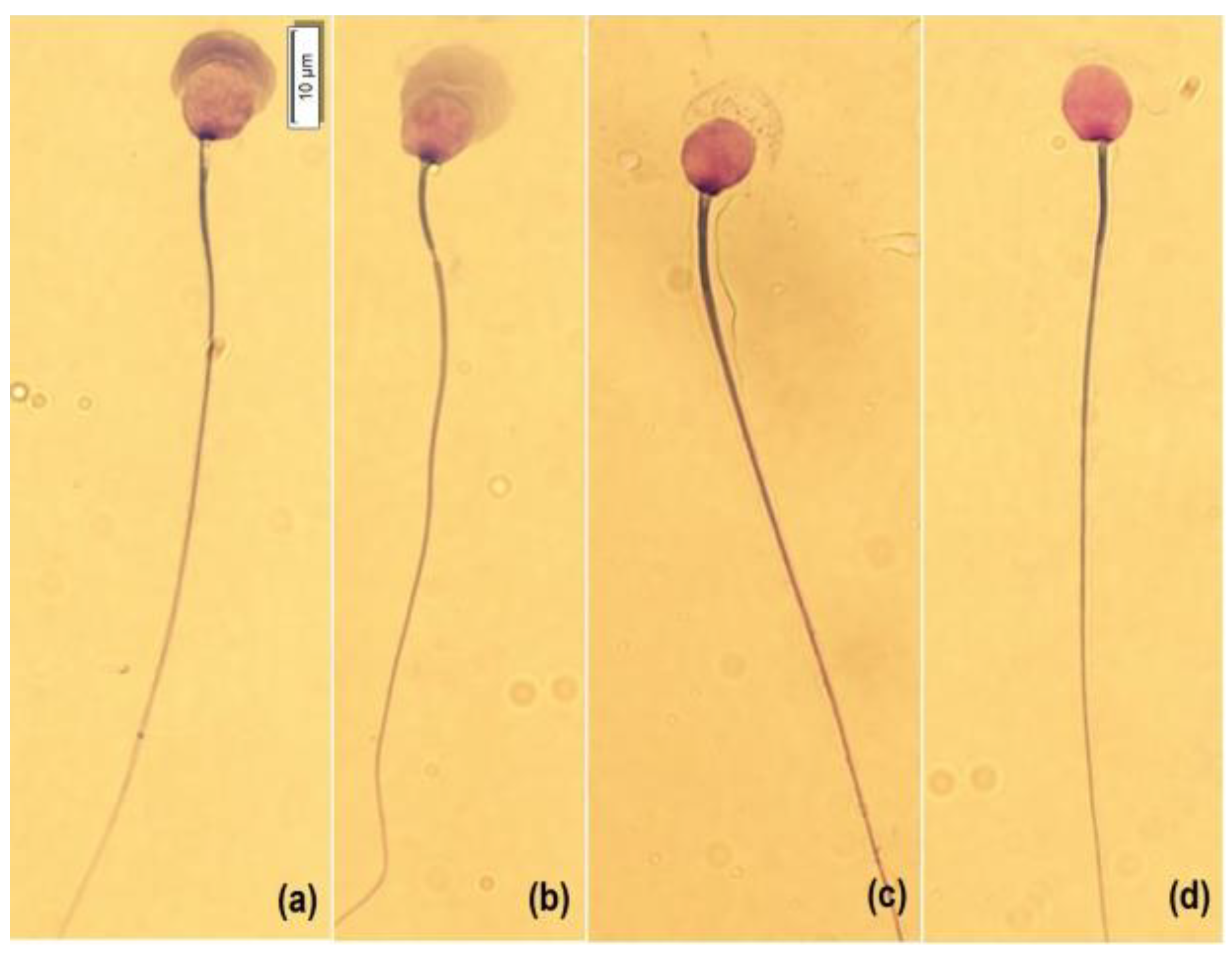
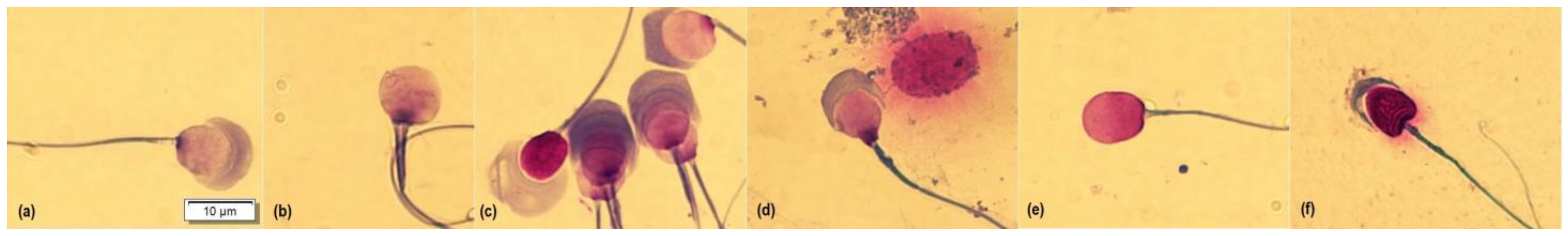
| Variable | FT0 | FT1 | FT2 | p-valor | SEM | CV |
|---|---|---|---|---|---|---|
| N | 6 | 5 | 5 | |||
| Live weight (g) | 1163.18 | 1264.38 | 1036.82 | 0.14 | 43.15 | 14.94 |
| Testicle weight (g) | 12.24 | 14.72 | 13.70 | 0.60 | 0.87 | 25.88 |
| Concentration (M/mL) | 915.23ab | 1151.10a | 456.19b | 0.04* | 108.40 | 51.28 |
| Progressive motility (%) | 29.44 | 22.88 | 15.61 | 0.27 | 3.32 | 57.61 |
| Non progressive motility (%) | 50.19a | 48.49a | 13.89b | 0.02* | 6.10 | 63.65 |
| Total motility (%) | 79.63a | 71.37a | 29.50b | 0.01* | 8.07 | 52.56 |
| Traits | FT0 | FT1 | FT2 | Test K-W |
SEM | CV |
|---|---|---|---|---|---|---|
| N | 314 | 280 | 344 | |||
| Core length (µm) | 8.25a | 8.25a | 8.16b | <0.01** | 0.013 | 4.72 |
| Core width (µm) | 7.34 | 7.37 | 7.36 | 0.83 | 0.011 | 4.49 |
| Core perimeter (µm) | 26.10b | 26.40a | 26.21b | <0.01** | 0.033 | 3.86 |
| Core area (µm2) | 48.93b | 49.94a | 49.16b | <0.01** | 0.123 | 7.64 |
| Ellipticity | 1.125a | 1.121a | 1.110b | <0.01** | 0.002 | 5.28 |
| Rugosity | 0.901a | 0.899b | 0.898c | <0.01** | 0.000 | 1.05 |
| Elongation | 0.058a | 0.056a | 0.051b | <0.01** | 0.000 | 47.83 |
| Regularity | 0.972a | 0.957c | 0.961b | <0.01** | 0.000 | 2.48 |
| Acrosome perimeter (µm) | 33.83 | 33.98 | 33.56 | 0.05 | 0.113 | 8.35 |
| Acrosome area (µm2) | 79.11 | 79.92 | 77.98 | 0.05 | 0.569 | 18.04 |
| Head length (µm) | 12.25 | 12.31 | 12.31 | 0.68 | 0.035 | 6.41 |
| Midpiece length (µm) | 11.45a | 11.17b | 11.14b | <0.01** | 0.024 | 6.64 |
| Midpiece width (µm) | 0.63c | 0.65b | 0.72a | <0.01** | 0.003 | 17.97 |
| Tail length (µm) | 89.66a | 88.72b | 89.43b | <0.01** | 0.289 | 8.05 |
| Category | FT0 | FT1 | FT2 | p-valor | SEM | CV |
|---|---|---|---|---|---|---|
| N | 2903 | 2043 | 2004 | - | - | - |
| Type 1 (%) | 62.22 | 47.67 | 38.87 | 0.06 | 5.12 | 40.66 |
| Type 2 (%) | 7.27a | 11.53ab | 26.16b | 0.04* | 3.93 | 108.49 |
| Type 3 (%) | 11.81 | 11.97 | 9.67 | 0.62 | 1.54 | 54.94 |
| Type 4 (%) | 18.70 | 28.83 | 25.30 | 0.50 | 3.88 | 64.79 |
| Abnormal (%) | 1.84 | 1.64 | 1.50 | 0.81 | 0.55 | 131.56 |
| Traits | Type 1 | Type 2 | Type 3 | Type 4 | Test K-W | SEM | CV |
|---|---|---|---|---|---|---|---|
| N | 447 | 194 | 101 | 196 | |||
| Core length (µm) | 8.211ab | 8.170b | 8.199ab | 8.279a | 0.03* | 0.013 | 4.72 |
| Core width (µm) | 7.301b | 7.424a | 7.375a | 7.406a | <0.01** | 0.011 | 4.49 |
| Core perimeter (µm) | 26.081b | 26.342a | 26.313ab | 26.426a | <0.01** | 0.033 | 3.86 |
| Core area (µm2) | 48.730b | 49.709a | 49.578ab | 50.131a | <0.01** | 0.123 | 7.64 |
| Ellipticity | 1.126a | 1.102b | 1.113ab | 1.119a | <0.01** | 0.002 | 5.28 |
| Rugosity | 0.899 | 0.899 | 0.899 | 0.901 | 0.15 | 0.000 | 1.05 |
| Elongation | 0.059a | 0.048b | 0.053ab | 0.056a | <0.01** | 0.001 | 47.83 |
| Regularity | 0.967a | 0.959b | 0.959b | 0.962b | <0.01** | 0.001 | 2.48 |
| Acrosome perimeter (µm) | 33.283b | 35.209a | 29.629c | - | <0.01** | 0.113 | 8.35 |
| Acrosome area (µm2) | 76.446b | 86.124a | 58.584c | - | <0.01** | 0.569 | 18.04 |
| Head length (µm) | 12.105b | 12.766a | 11.212c | 8.279d | <0.01** | 0.035 | 6.41 |
| Midpiece length (µm) | 11.297a | 11.318a | 11.254ab | 11.086b | <0.01** | 0.024 | 6.64 |
| Midpiece width (µm) | 0.665 | 0.673 | 0.685 | 0.666 | 0.41 | 0.004 | 17.97 |
| Tail length (µm) | 89.201 | 88.715 | 89.342 | 90.011 | 0.06 | 0.289 | 8.05 |
| Type 1 | Type 2 | Type 3 | Type 4 | Abnormalities | |
|---|---|---|---|---|---|
| Live weight | 0.00 | 0.00 | 0.08 | -0.03 | -0.07 |
| Testicular weight | -0.24 | 0.46 | 0.10 | -0.11 | -0.19 |
| Concentration | 0.22 | -0.01 | 0.00 | -0.28 | 0.14 |
| Progressive motility | 0.29 | -0.07 | -0.16 | -0.26 | 0.06 |
| Non Progressive motility | -0.07 | 0.08 | 0.23 | -0.08 | 0.05 |
| Total motility | 0.07 | 0.03 | 0.11 | -0.17 | 0.06 |
| Type 1 | -0.61* | -0.38 | -0.56* | -0.21 | |
| Type 2 | 0.11 | -0.26 | 0.25 | ||
| Type 3 | -0.01 | 0.63* | |||
| Type 4 | -0.24 |
| Variable | FT0 | FT1 | FT2 | p-valor |
|---|---|---|---|---|
| N | 4 | 4 | 5 | |
| Pregnancy rate (%) | 100.0a | 50.0b | 0.0c | 0.01* |
| N° offspring | 1.5 | 3.0 | - | 0.13 |
| Calving age (days) | 152.5±4.2b | 129.5±4.2a | - | <0.01** |
| Probable mating age (days) | 84.5±4.2b | 61.5±4.2a | - | <0.01** |
| Pregnancy rate | N° offspring | Mating age | |
|---|---|---|---|
| Live weight | 0.38 | 0.83* | -0.71 |
| Testicular weight | -0.12 | 0.65 | -0.99** |
| Concentration | 0.33 | 0.70 | -0.19 |
| Progressive motility | 0.65* | -0.54 | -0.01 |
| Non Progressive motility | 0.67* | 0.88* | -0.82* |
| Total motility | 0.73** | -0.10 | -0.51 |
| Type 1 | 0.25 | -0.33 | 0.63 |
| Type 2 | -0.43 | -0.21 | -0.15 |
| Type 3 | 0.06 | 0.08 | -0.12 |
| Type 4 | 0.08 | 0.57 | -0.83* |
| Abnormalities | 0.10 | -0.55 | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





