1. Introduction
Between 2009 and 2050, world food needs will increase by 70% and this is following the perpetual increase of the world human population [
1]. Moreover, jerky climate changes have been taking hold for quite some time. Thus, the global temperature is increased by 0.7°C which led to an increase in the frequency of natural climatic disasters. All of this has had a negative impact on food security and caused fluctuations in the supply of both human and animal food. On the other hand, the intensification of agriculture has deepened disorders in ecosystems such as depletion of fresh water resources, deforestation, deterioration of the organic and biological quality of cultivated soils, CO2 emissions increase, pressure on animal selection limiting biodiversity by focusing on animal breeds that are highly productive but very fragile and often unsuited to the microclimate of certain countries.
The challenge for both developed and developing countries is to reduce the degradation of the environment, and reach to sustainable increases in crop and livestock productions to secure present and future food supplies for both humans and animals [
2,
3]. This sustainability is crucially needed to limit poverty in the world, to preserve natural resources and to consecrate all efforts to maintain peace and prosperity for all [
4].
To face this environmental degradation, an Eco vigilance has formed and civil awareness has developed in different societies which prompted governments to take the problem of environment degradation seriously. Thus, international organizations such as FAO [
5], ICARDA [
6], CIRAD [
7] have set up research projects to promote new environment-friendly farming techniques in order to “protect the existing and repair the damaged” [
4].
On this regard, the world can only seek ways of sustainable food supply to face the continued pressure and the growing global increase in food.
Ecoagronomy is a sustainable development process that we can attempt in order to achieve this objective. Indeed, ecoagriculture, with all its versions, guarantees a relatively satisfactory and sufficient supply of food while preserving natural resources.
Conservation agriculture (CONS A) or regenerative agriculture is a sustainable model which does not disturb the ecosystem and preserves natural resources. Thus, it contributes to the preservation of the physico-biological proprieties of the soil and its microfauna; which has a positive impact on its fertility and its productivity.
Furthermore, CONS A protects the soil from erosion and, thanks to the presence of a permanent vegetal cover, it reduces the evaporation of water. On the other hand, it decreases the release of CO2 gases from the ground, reducing then global warming [
8,
9,
10,
11].
In addition to the benefits of CONS A, it is demonstrated that small ruminant livestock can provide food security, alleviate poverty and it is well integrated in the world sustainable nutrition development [
12]. Moreover, it was shown that the breeding of small ruminants, particularly sheep, can be carried out in conservation agriculture with success and good productivity [
13] and it was proved that there is an efficiency of crop-livestock production systems under CONS A with the guarantee of a sustainable food security in Tunisian dry areas [
14]. As far as it could be ascertained, there are no published studies on the impact of CONS A on sheep digestive parasitism. The main objective of this study is to identify the impact of conservation agriculture on the digestive parasitism of sheep by comparing it to that of conventional agriculture during grazing cycles.
At the same time, we seek to follow the weight variation of the animals as well as that of the hematological parameters.
2. Materials and Methods
2.1. Study farm
The present study was carried out in a private farm located in Krib locality, Siliana district, North west Tunisia (Latitude: 36.374471 E; Longitude: 9.175250 N) (
Figure 1).
Krib locality has a Köppen BSk climate type with an average annual rainfall between 250 and 600 mm and a mean winter and summer temperatures of 17.8 and 35°C, respectively [
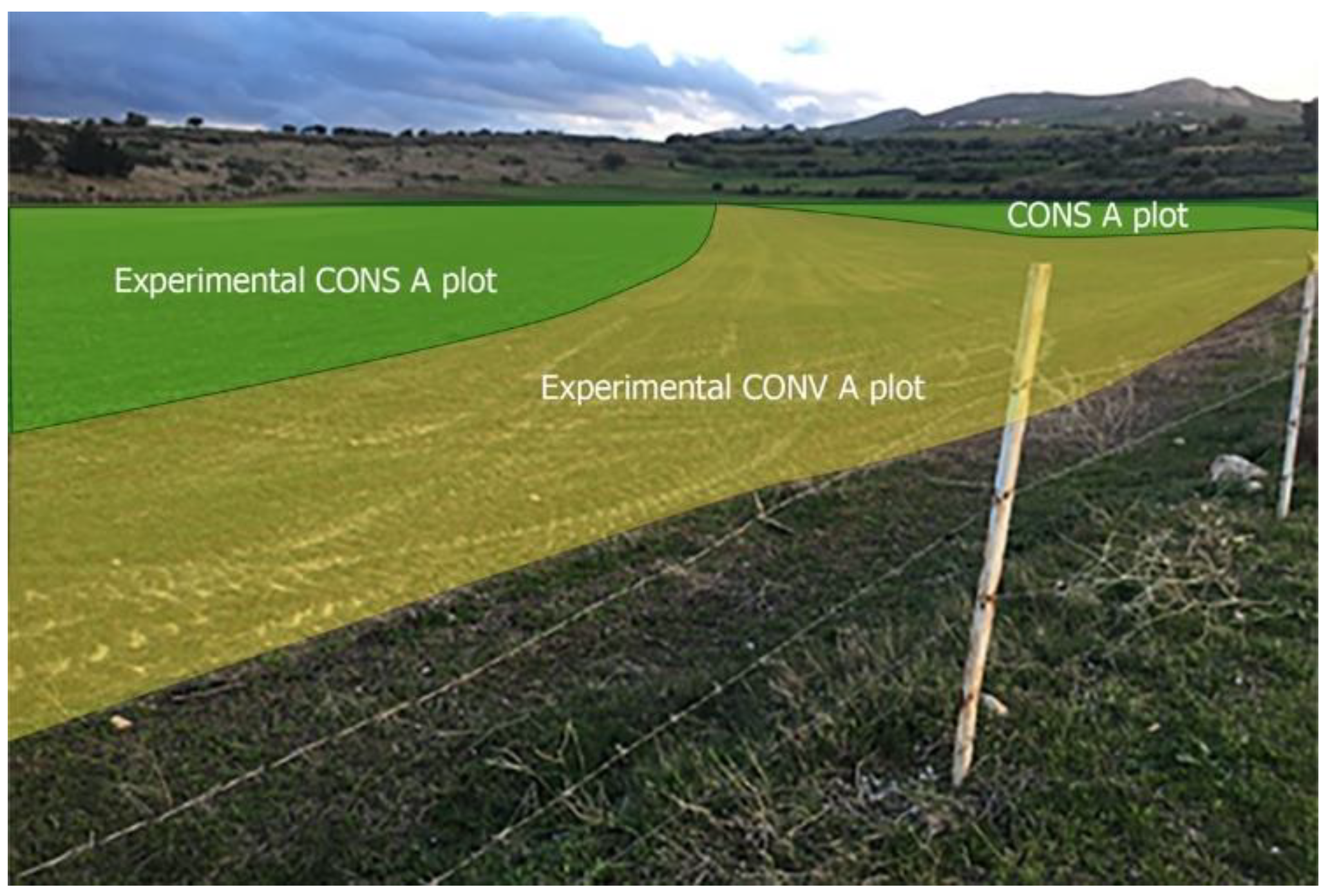
15]. The study land consists of two contiguous plots, one used for conservation agriculture (CONS A) and the other for conventional agriculture (CONV A) (
Figure 2). Agricultural activities were similar and performed at the same time in both plots. Both of them were planted with oats (Avena sativa), vetch (Vicia sativa), sulla (Hedysarum coronarium) and alfalfa (Medicago sativa).
2.2. Animals
Two batches of six male lambs each, were randomly selected from a herd of 130 Noir de Thibar, Queue Fine de l’Ouest and cross-breed sheep. At the inclusion date, lambs were aged between 5 and 9 months, their mean live weight was 24 kg (range: 16 - 32 kg). Animals were vaccinated against enterotoxaemia (Ovipan F®, MCI Santé Animale, Morocco) (subcutaneous injection of 2 ml/animal) and were drenched with 7 mg/kg albendazole (Dalben® 1.9, CEVA, France) during late January 2021. Each lambs’ batch was randomly divided into two groups of 3 lambs each and maintained in two separate boxes (
Figure 3). The two groups were randomly placed on pastures for two months, one on conservation agriculture (CONS A) plot and the other on conventional agriculture (CONV A) plot. Each batch of lambs pasture daily during 3 days in 25 m2 plot during 6 to 7 hours except during raining days where they are kept in their boxes. At the end of the day, lambs were fed with oat vetch hay and approximately 200 g of concentrate for each animal.
2.3. Sampling
Lambs were clinically examined, weighted and sampled (5 ml of blood in EDTA tubes, at least 10 gr of faeces) each two weeks.
Red blood cell count (RBC) (109/mL), haematocrit (Ht) (%) and haemoglobin (Hb) (g/dL) were estimated using an Auto Haematology analyser BC-2800Vet® (Shenzen Mindray Bio-Medical Electronics Co., Ltd, China).
All faecal samples were checked for the presence of gastro-intestinal parasites qualitatively (flotation technique) and quantitively (Mc Master technique). The later allowed the estimation of infection intensity that was expressed as egg per gram (epg) of gastro-intestinal nematodes, coccidian oocysts and whipworms.[
16].
The lambs were slaughtered after two months of pasturing. Immediately after being slaughtered, the gastrointestinal tract, lungs, liver and epiploon were removed and each carcass was weighed. The organs were thoroughly examined and dissected for the presence of lesions. Each portion of the gastrointestinal tract was separated and longitudinally opened. The digestive mucosa was thoroughly washed and collected in a bucket. All nematodes were collected and conserved in identified tubes containing 70% ethanol and stored at +4°C until analysed. Nematodes and segments of adult cestodes were counted and identified according to the key of Euzeby [
17].
2.4. Parasitological indicators
The following parasitological indicators were estimated [
18].
Total Worm Count (TWC) = total number of a nematode species found in one examined gastro-intestinal tract. Natural logarithm plus one (Ln(n+1)) was used for the presentation of the figures.
Infestation prevalence = 100 × number of infested lambs/number of examined lambs.
Infestation intensity = Number of worms in the gastro-intestinal tract/number of infested lambs. Infestation abundance = Number of worms in the gastro-intestinal tract/number of examined lambs.
2.5. Statistical analyses
The mean relative variation was used to compare the variation of lambs’ weight, haematocrit, haemoglobin and blood cell count during visits. The relative variation was estimated as follows: Mean relative variation (%) = 100 x (value at visit (n+1) - value at visit (n))/value at visit (n).
The comparison of the infestation prevalence rate between the two lamb groups was performed with the Fisher exact test.
The infestation intensity and abundance between the two groups of lambs was performed using Wilcoxon-Mann-Whitney and Krusskall-Wallys tests. All tests were considered significant at 5% threshold [
19].
3. Results
3.1. Relative variation of lambs’ liveweights
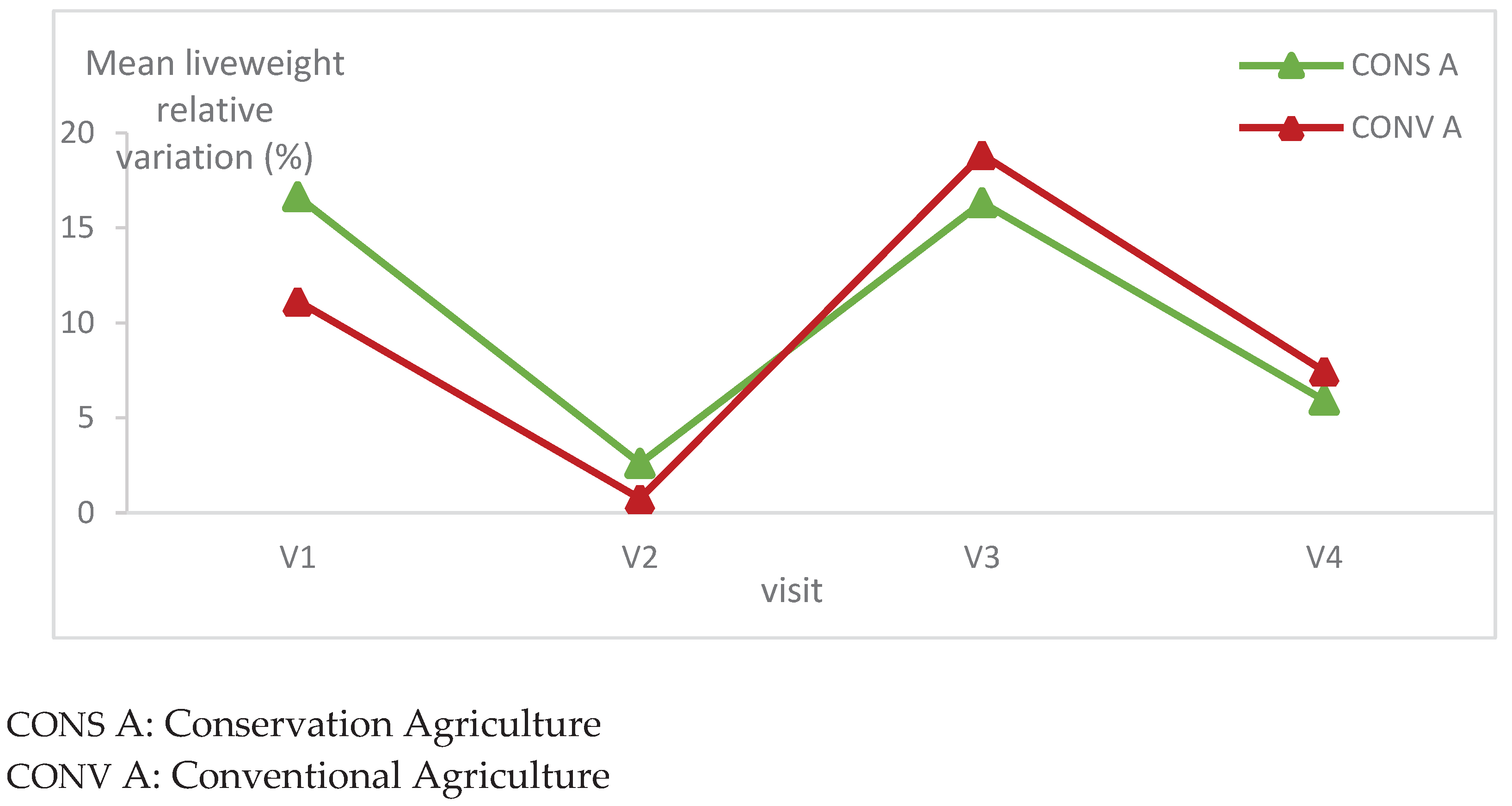
The mean relative variation of lambs’ liveweight had exactly the same trend in both animal groups. It decreased considerably at the second visit. There was not statistically significant difference between the liveweights in the two animal groups (
Table 1;
Figure 4).
It’s worth mentioning that there is a significant statistical difference in liveweight relative variation in each batch during all visits (
Table 1) (p=0.01 for both lambs batches).
The carcass yield was low for both types of agriculture and did not exceed the lower limit of the range of carcass yield in sheep (44.5 and 45.3% for conservation and conventional agriculture, respectively). No statistically significant variation was recorded for the carcass yield between the two batches of lambs (P = 0.39).
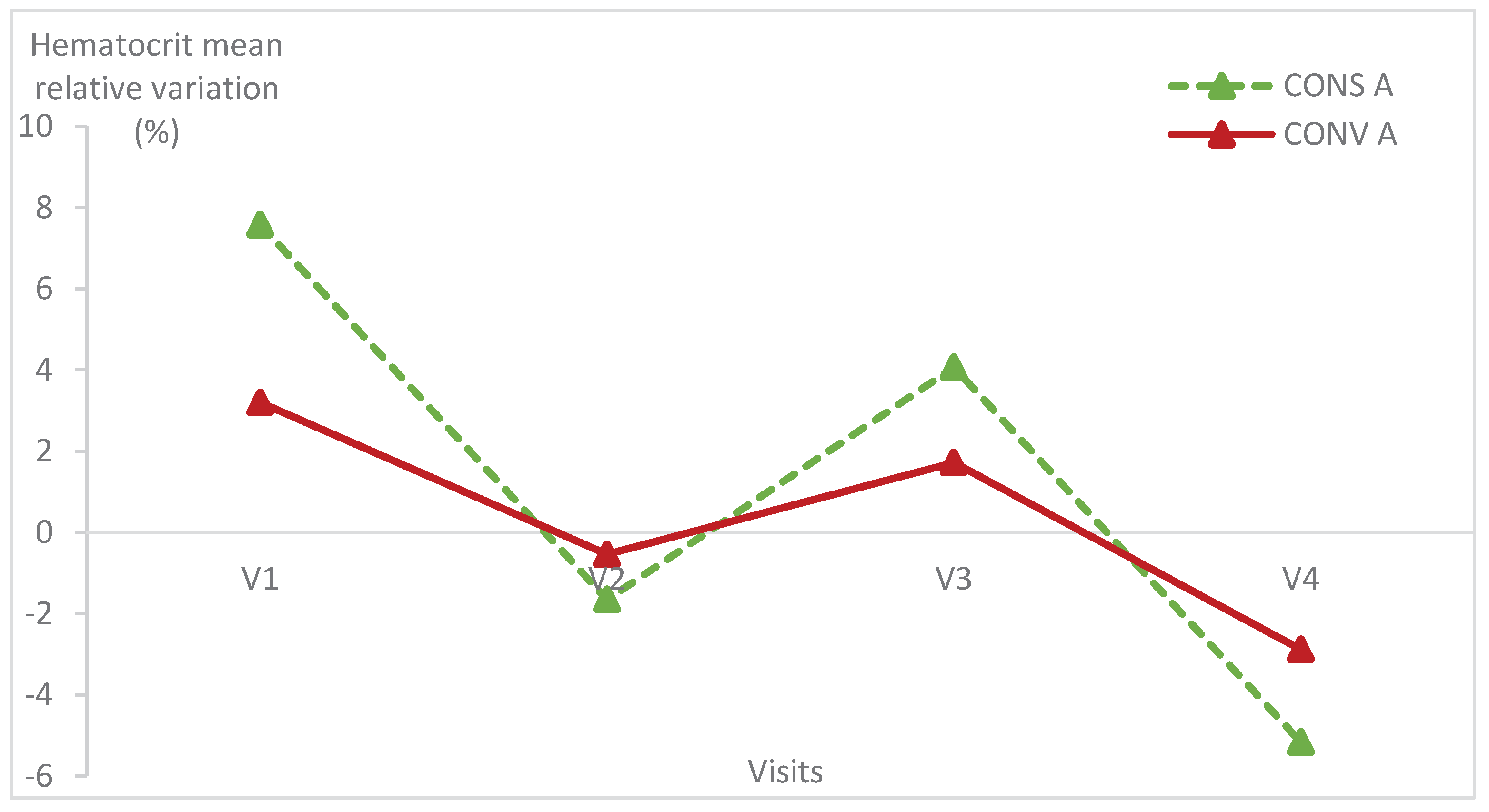
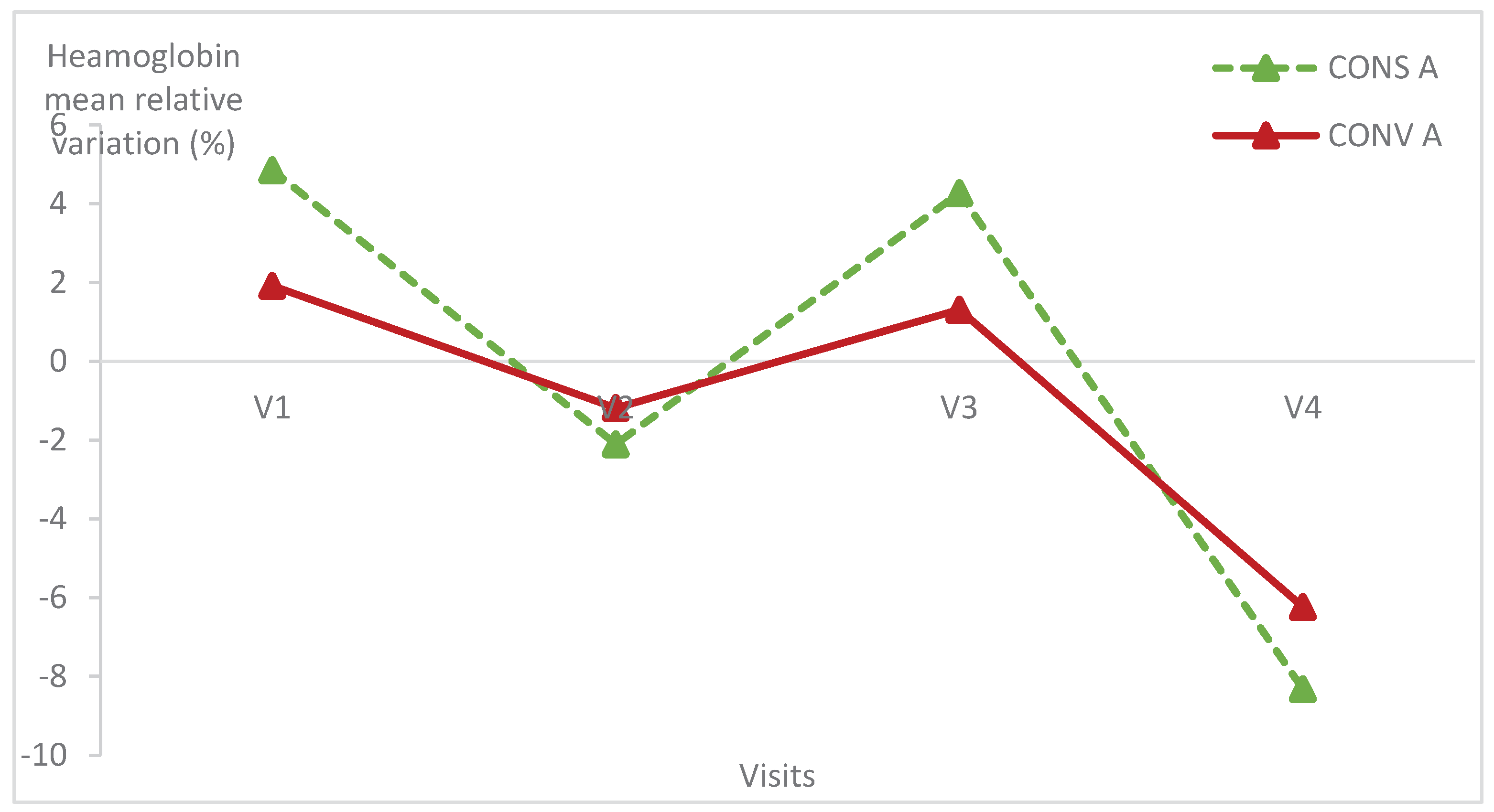
3.2. Relative variation of haematological parameters
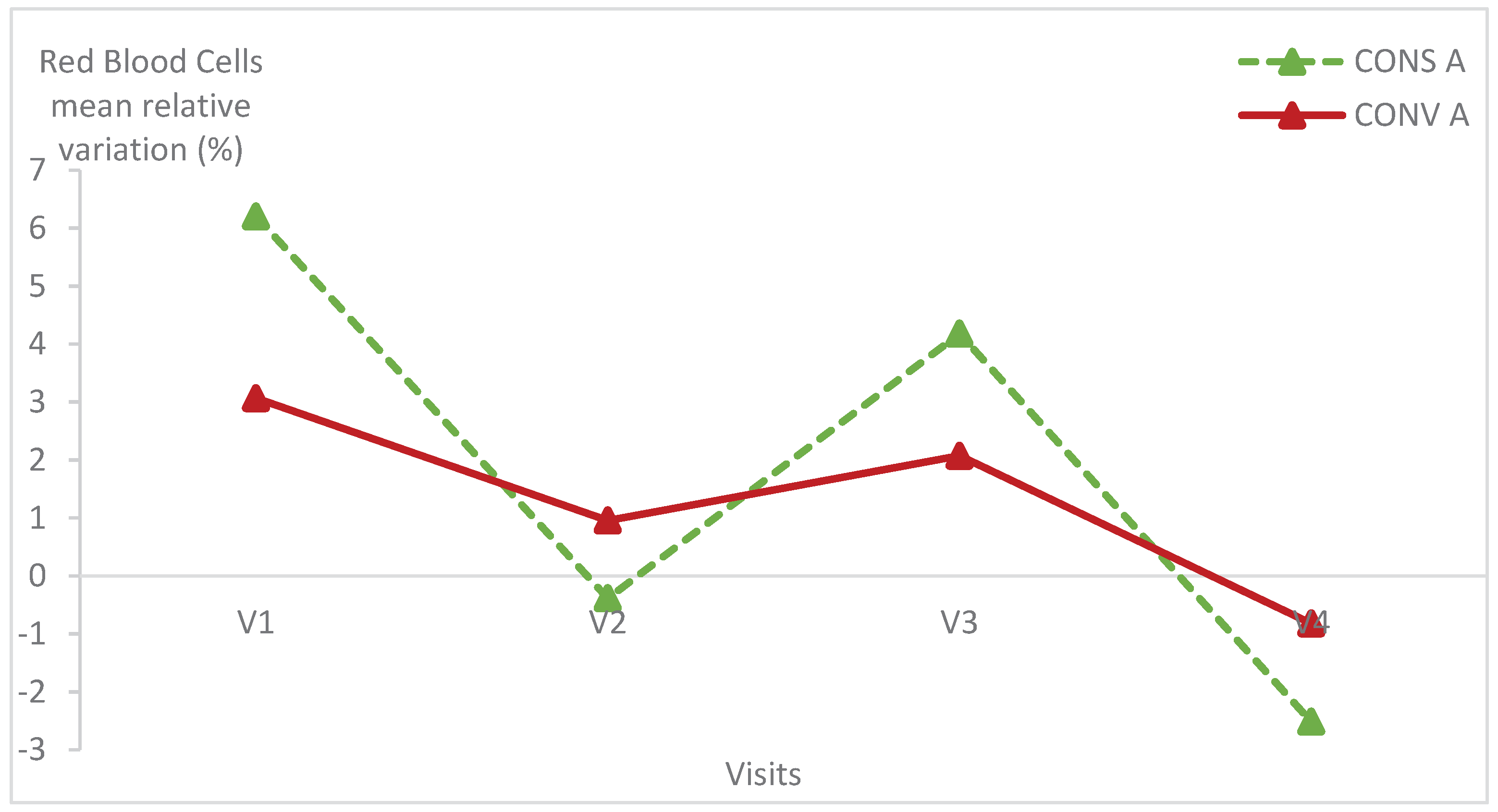
Haematological parameters were within the normal values of lamb blood parameters in all animals of both groups [
20,
21]. The haematological parameters had the same variation in the two lamb groups (
Figure 5,
Figure 6 and
Figure 7;
Table 1) (p>0.05).
There was no statistically significant difference within each group of lambs except the haemoglobin relative variation in lambs kept in CONS A (p = 0.04) (
Table 1). Indeed, the haemoglobin level of lambs in CONS A increased from the first to the fourth visit then decreased at the last visit.
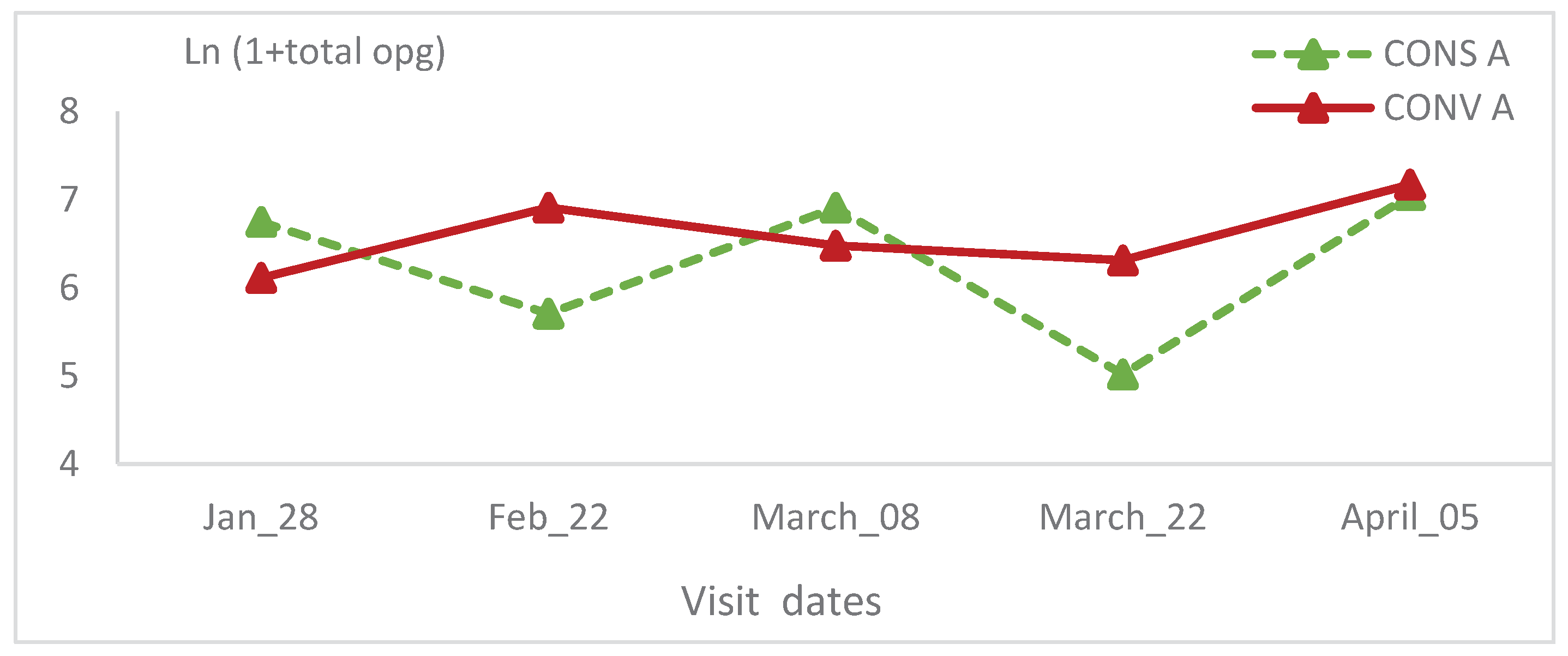
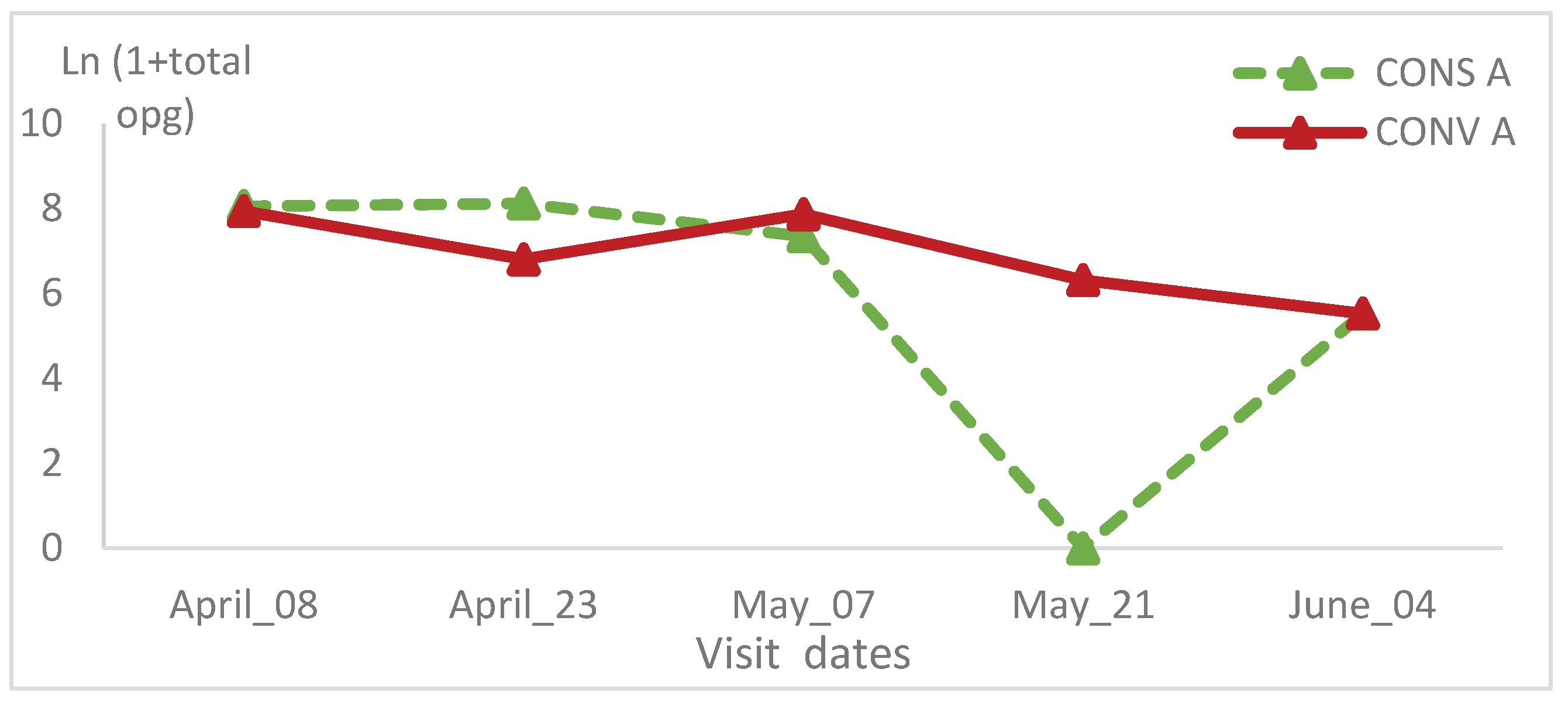
3.3. Coproscopic results
The total oocysts count, didn’t show a significant change in CONV A lambs’ group of the first batch (
Figure 8). In the CONS A group, this value decreased at the second and the fourth visit (
Figure 8). Within this group, the total oocyst counts showed a significant statistical variation (p=0.01) (
Table 1). The opg was high during the first visits, then it decreased at the third and fourth visits, finally it increased slightly at the last visit. The total oocysts count of the CONV A lambs’ group, showed the same trend and no statistical significant variation was recorded (
Figure 9).
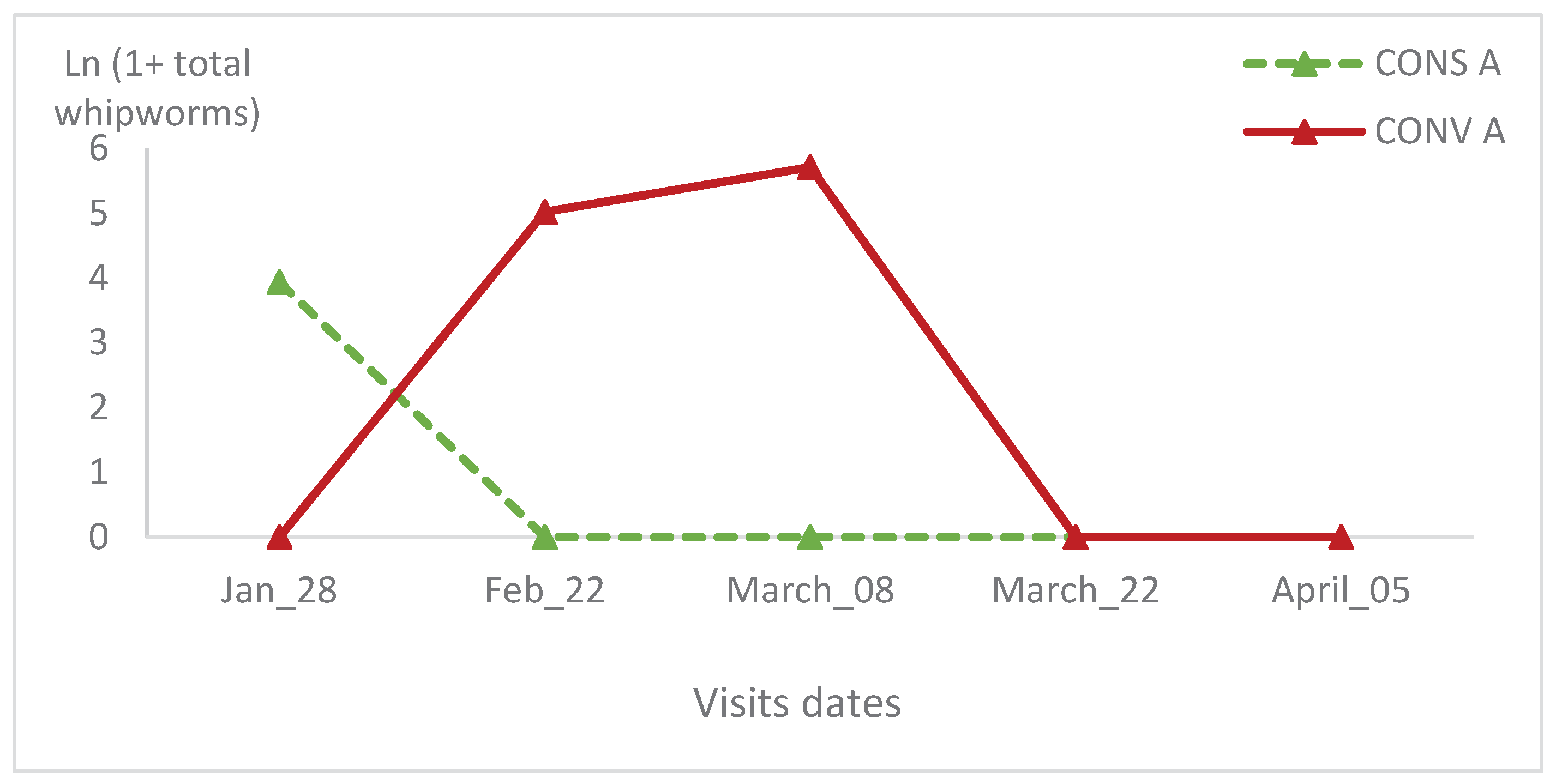
The whipworms relative variation in the CONV A lambs’ group of the first batch peaked during the third visit and then it reached naught at the last visit (
Figure 10). This value was naught from the second visit in the CONS A lambs’ group (
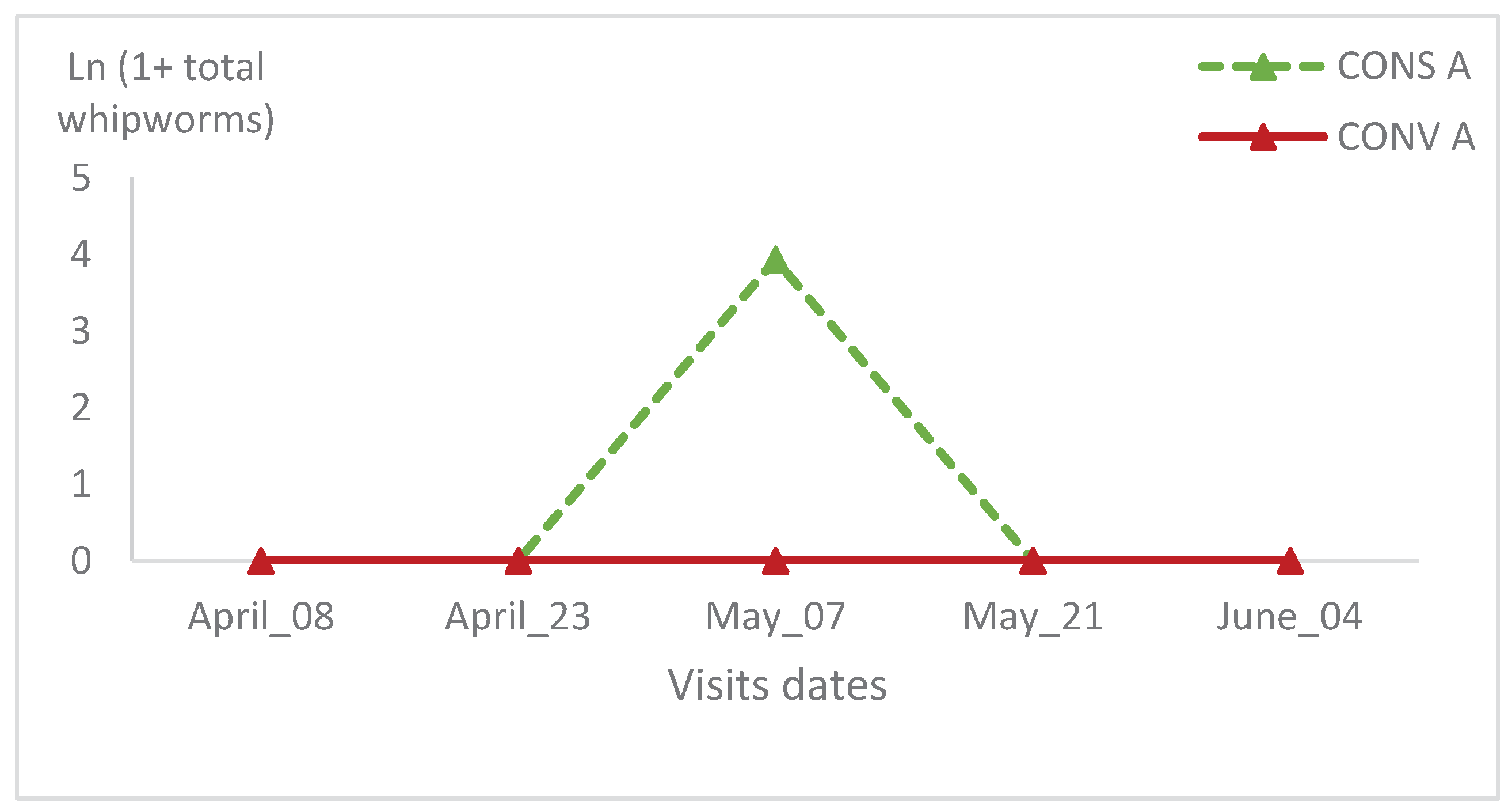
Figure 10). In the second batch, the whipworms relative variation was naught throughout the visits in the CONV A lambs’ group (
Figure 11). There was no significant statistical variation between lambs in the two groups during all visits and in the same batch (
Table 2).
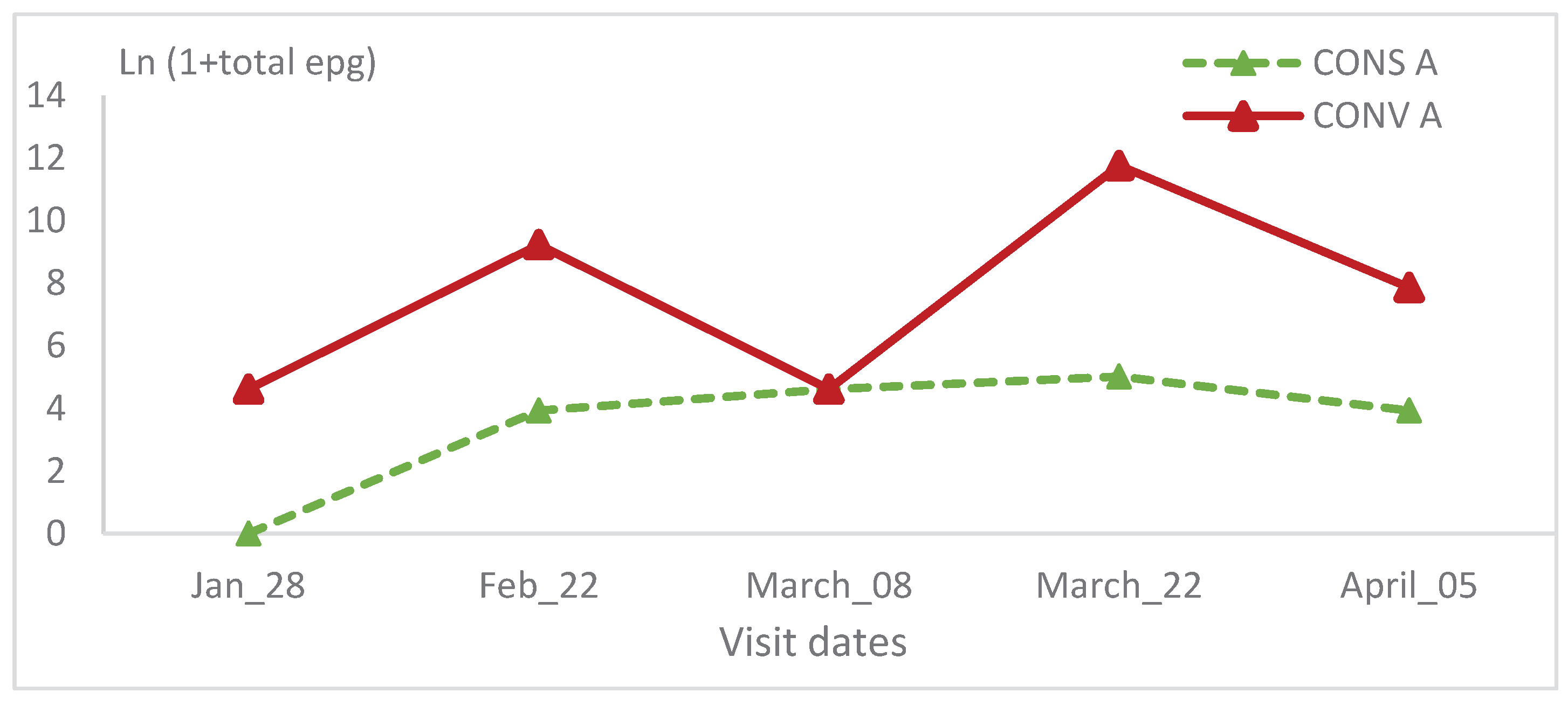
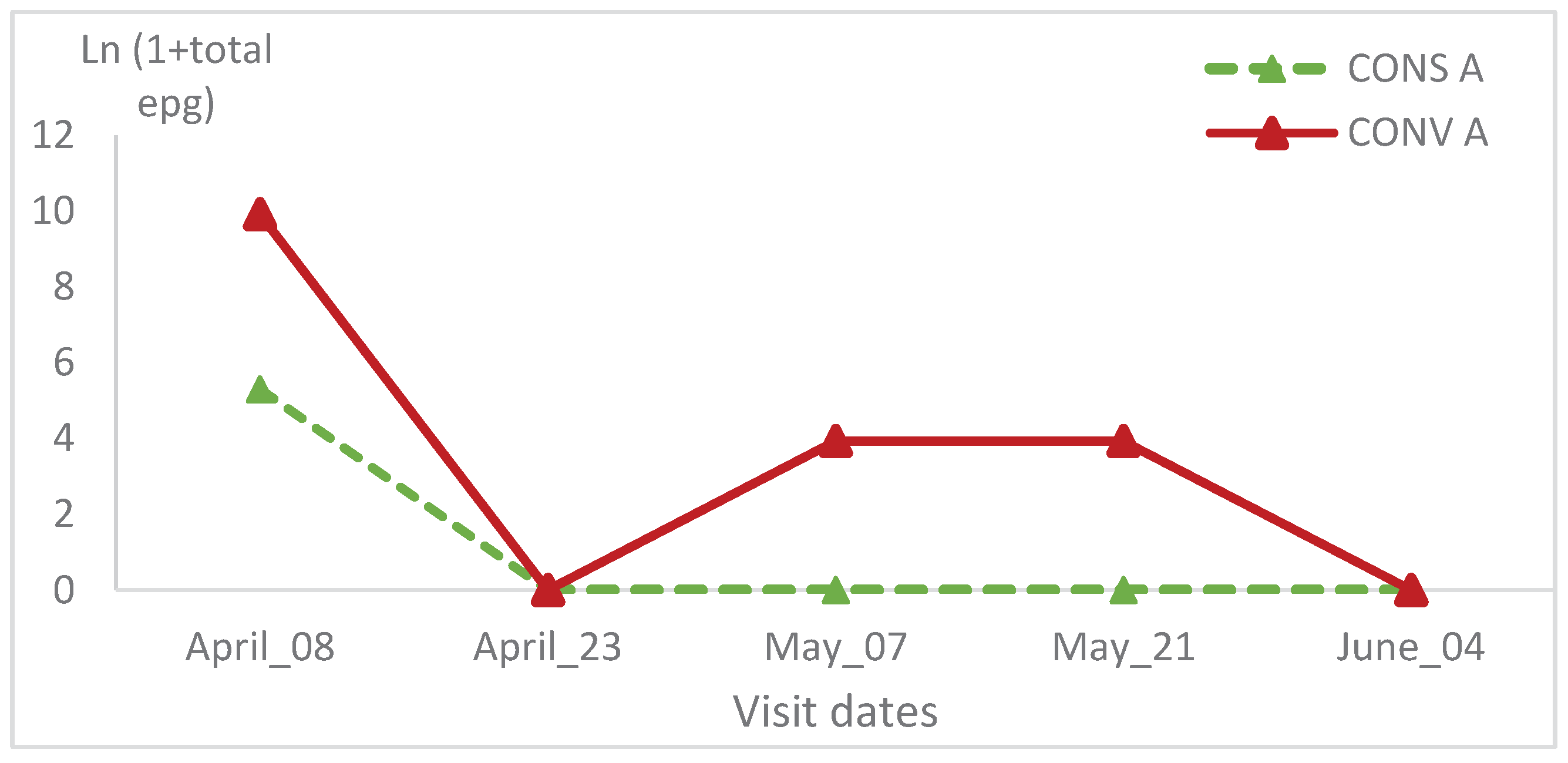
The epg relative variation wasn’t statistically significant between the two lambs’ groups and in the same group during all visits (
Table 1 and
Table 2;
Figure 12 and
Figure 13).
The prevalence rate of tapeworms did not change during all visits and no statistically significant variation was observed (p>0.99) (
Table 2).
3.4. Helminthologic necropsy
A total number of 905 parasites were collected from 12 lambs, among them abomasum nematodes were predominant, mainly Ostertagia sp. which was collected from all the lambs of both groups (
Figure 13), it represented 94.25% of the total number of parasites (853 worms). The Total Worms Count (TWC) varied between 9 and 190 worms per lamb.
There was no statistically difference between both infestation intensity, and abundance in the two lamb groups (
Table 3).
4. Discussion
Environmental benefits of conservation agriculture, especially regarding climate change and land preservation are very high. As part of the combination of crops with livestock, particularly in semi-arid areas, CONS A and sheep farming can be combined harmoniously [
13].
As far as we know, this is the first study investigating the effect of CONS A on sheep digestive parasitism. We found here in that mean relative variation of lambs’ liveweight decreases in the second visit in the two animal groups. This was probably due to the impact of adaptation period. The absence of statistical difference between lambs’ liveweights in the two batches means that there is no negative impact of CONS A pastures on their growth rate. A statistically significant variation of liveweights was observed in both animal groups during the five visits. This is due to the presence of a physiological high gain weight gain during this age period [
22]. Moreover, the mean carcass yield of both lamb groups was slightly lower than the normal yield values for fattening lambs. There was no statistically significant difference between the two animal batches (between 450 and 600 g/kg of body weight) [
23].
The parasitological status did not show any statistically significant difference regarding infection by Eimeria, whipworms, digestive strongyle eggs and tapeworms in the two lambs’ batches. This result confirms that pasturing on CONS A crops has no negative impact on digestive parasitism of lambs. Furthermore, no statistically significant difference was reported between the two lamb groups concerning infestation prevalence, intensity and abundance of all found parasites.
Eimeria faecal elimination during the grazing period showed the same trend in both types of agricultures with a higher infection intensity during the wet period. This result is in agreement with that reported by De Souza in grazing sheep on semiarid areas in Brazil [
24]. There was a statistically significant variation in total oocyst count in lambs grazing on CONS A pasture. The progressive decrease in the total oocyst count in the two batches of CONS A lambs’ infection intensity could be explained by a progressive installation of a specific anti-Eimeria immunity. This variation could be explained by the separation of experimental lambs from the rest of the sheep herd that stopped their contamination from carrier adult sheep [
25]. The relative increase in total oocyst count at the last visit could be explained by an increase of ambient humidity and temperature during the last visit.
The prevalence rate of worms varied between 16.67 and 66.7% in CONS A lamb’s batch and CONV A lamb batches. Therefore, the two lamb batches showed the same trend. Yan et al (2021) reported higher prevalence rate in sheep reaching 96.9% in China [
26]. This relatively low prevalence rate is probably related to the absence of promiscuity of the studied flock with others and a good management of pastures. Worms collected from the digestive tract of lambs were mainly represented by abomasum parasites, this is in agree with the two studies conducted on sheep gastrointestinal parasites in North Tunisia [
27,
28]. We found herein that Ostertagia spp. was the predominant nematode genus (94.25%) unlike the two studies cited above which reported a predominance of Teladorsagia sp. with an infection prevalence reaching 91.25 and 90.03% respectively. This is probably related to the rainfall and ambient temperature which constitute the two main factors conditioning survival of the outdoor parasite stages in soil.
5. Conclusions
We conclude that grazing on CONS A plots has no impact on the sheep digestive parasitism compared to those grazing in CONV A. Similarly, we showed that there is almost no difference in lamb growth rate, carcass yield as well as haematological parameters between lambs kept in the two pasture types. Further studies are needed to support these findings especially on a larger animal sample and to explore the impact of CONS A on other parasites and other domestic animal species.
Author Contributions
Conceptualization, S.E, M.G., M.R. and H.C-M.; methodology, M.G. and S.E.; validation, M.G., M.R. and H.CM.; formal analysis, S.E., S.L. and M.D.; investigation, S.E., L.S. and M.D.; resources, S.E.; data curation, S.E.; writing—original draft preparation, S.E.; writing—review and editing, S.E. and M.G.; supervision, M.G.; project administration, M.G.; funding acquisition, M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the ICARDA in the frame of CLCA project (BUS200341).
Institutional Review Board Statement
This study was approved by the Animal Ethics Committee - National School of Veterinary Medicine, AEC/IACUC, ENMV- Sidi Thabet, Tunisia (Approval Code: CEEA-ENMV 65/22).
Data Availability Statement
Data available from the corresponding author upon request.
Acknowledgments
We thank ICARDA for funding this work and providing all the material necessary to complete this study in good conditions. Special thanks are addressed to Mr. Adnen ABED RABBAH, the owner of the sheep herd as well as the plots of conservation and conventional agriculture. we commend his continued efforts to preserve and promote this mode of eco-agriculture since the 2000s.
Conflicts of Interest
Authors declare no conflict of interest.
References
- FAO-High Level Expert Forum How to Feed the World 2050 - Global Agriculture towards 2050 Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf (accessed on 8 December 2020).
- Kaasschieter, G.A.; De Jong, R.; Schiere, J.B.; Zwart, D.; Zwart1, D. Veterinary Quarterly Towards a Sustainable Livestock Production in Developing Countries and the Importance of Animal Health Strategy Therein TOWARDS A SUSTAINABLE LIVESTOCK PRODUCTION IN DEVELOPING COUNTRIES AND THE IMPORTANCE OF ANIMAL HEALTH STRATEGY TH. Vet Q 1992, 14, 66–75. [CrossRef]
- Naab, J.B.; Mahama, G.Y.; Yahaya, I.; Prasad, P.V.V. Conservation Agriculture Improves Soil Quality, Crop Yield, and Incomes of Smallholder Farmers in North Western Ghana. Front Plant Sci 2017, 8, 1–15. [CrossRef]
- UN The 2030 Agenda for Sustainable Development. Arsenic Research and Global Sustainability - Proceedings of the 6th International Congress on Arsenic in the Environment, AS 2016 2016, 1–41. [CrossRef]
- Sandra Corsi and Hafiz Muminjanov; FAO Conservation Agriculture Training Guide for Extension Agents and Farmers in Eastern Europe and Central Asia; 2019; ISBN 978-92-5-131456-2.
- Kassam, A.; Friedrich, T.; Derpsch, R.; Lahmar, R.; Mrabet, R.; Basch, G.; González-Sánchez, E.J.; Serraj, R. Conservation Agriculture in the Dry Mediterranean Climate. Field Crops Res 2012, 132, 7–17. [CrossRef]
- Giller, K.E.; Andersson, J.A.; Corbeels, M.; Kirkegaard, J.; Mortensen, D.; Erenstein, O.; Vanlauwe, B. Beyond Conservation Agriculture. Front Plant Sci 2015, 6.
- FAO Regards Sur l’agriculture de Conservation En Afrique de l’ouest et Du Centre et Ses Perspectives. Congrès mondial d’agriculture de conservation 2005, 114.
- Kassam, A.; Friedrich, T.; Shaxson, F.; Pretty, J. The Spread of Conservation Agriculture: Justification, Sustainability and Uptake. Int J Agric Sustain 2009, 7, 292–320. [CrossRef]
- Bayala, I.; Andrieu, N.; Bayala, I.; Mkomwa, S.; Essecofy, G.; Bougoum, H.; Zerbo, I.; Ganou, S.; Andrieu, N. Acquis et Défis de l’agriculture de Conservation. 2014, 2013–2014.
- Colley, T.A.; Olsen, S.I.; Birkved, M.; Hauschild, M.Z. Delta Life Cycle Assessment of Regenerative Agriculture in a Sheep Farming System. Integr Environ Assess Manag 2020, 16, 282–290. [CrossRef]
- Sargison, N.D. The Critical Importance of Planned Small Ruminant Livestock Health and Production in Addressing Global Challenges Surrounding Food Production and Poverty Alleviation. N Z Vet J 2020, 68, 136–144. [CrossRef]
- Abidi, S.; Benyoussef, S.; Ben Salem, H. Foraging Behaviour, Digestion and Growth Performance of Sheep Grazing on Dried Vetch Pasture Cropped under Conservation Agriculture. J Anim Physiol Anim Nutr (Berl) 2021, 105, 51–58. [CrossRef]
- Dhehibi, B.; Souissi, A.; Fouzai, A.; Frija, A.; Abdel Adhim, M.; Rekik, M. Efficiency of Crop-Livestock Production Systems Under Conservation Agriculture : Scope for Sustainable System Transformation to Achieving Food Security in Rain-Fed Drylands of Tunis ... Efficiency of Crop – Livestock Production Systems Under Conservation. In Proceedings of the Colloque international LESOR’2022 « Les territoires difficiles à l’épreuve des modèles de développement : acquis, défis et perspectives; 2022; pp. 1–15.
- Smaoui, A. Bioclimat et Végétation de La Tunisie et Des Régions Prospectées Pendant Le 12ème ITER Mediterraneum de OPTIMA. Bocconea 2015, 27, 13–20. [CrossRef]
- Raynaud, J. Etude de l’efficacité d’une Technique de Coproscopie Quantitative Pour Le Diagnostic de Routine et Le Contrôle Des Infestations Parasitaires Des Bovins, Ovins, Équins et Porcins. Ann Parasitol Hum Comp 45, 321_42.
- Taylor, M.A.; COOP, R.L.; WALL, R.L. Veterinary Parasitology; Blackwell Publishing Inc., 2007; ISBN 9781405119641.
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited; 1997; Vol. 83;.
- Schwartz, D. Méthodes Statistiques à L’usage Des Médecins et Des Biologistes; Flammarion, M.-S., Ed.; 4th Editio.; Paris, 1993; ISBN 2257103262.
- Adili, N.; Melizi, M.; Belabbas, H. Species Determination Using the Red Blood Cells Morphometry in Domestic Animals. Vet World 2016, 9, 960–963. [CrossRef]
- Ahmadi-hamedani, M.; Ghazvinian, K.; Atyabi, N.; Khanalizadeh, P.; Masoum, M.A.; Ghodrati, M.S. Hematological Reference Values of Healthy Adult Sangsari Sheep (Iranian Fat-Tailed Sheep) Estimated by Reference Value Advisor. Comp Clin Path 2016, 25, 459–464. [CrossRef]
- Saeid Bathaei, S.; Leroy, P.L. Genetic and Phenotypic Aspects of the Growth Curve Characteristics in Mehraban Iranian Fat-Tailed Sheep. Small Ruminant Research 1998, 29, 261–269. [CrossRef]
- Abidi, S.; Benyoussef, S.; Ben Salem, H. Foraging Behaviour, Digestion and Growth Performance of Sheep Grazing on Dried Vetch Pasture Cropped under Conservation Agriculture. J Anim Physiol Anim Nutr (Berl) 2021, 105, 51–58. [CrossRef]
- deSouza, L.E.B.; da Cruz, J.F.; Neto, M.R.T.; Albuquerque, G.R.; Melo, A.D.B.; Tapia, D.M.T. Epidemiology of Eimeria Infections in Sheep Raised Extensively in a Semiarid Region of Brazil. Revista Brasileira de Parasitologia Veterinaria 2015, 24, 410–415. [CrossRef]
- Carrau, T.; Silva, L.M.R.; Pérez, D.; Failing, K.; Martínez-Carrasco, C.; Macías, J.; Taubert, A.; Hermosilla, C.; de Ybáñez, R.R. Associated Risk Factors Influencing Ovine Eimeria Infections in Southern Spain. Vet Parasitol 2018, 263, 54–58. [CrossRef]
- Yan, X.; Liu, M.; He, S.; Tong, T.; Liu, iyong; Ding, K.; Deng, H.; Wang, P. An Epidemiological Study of Gastrointestinal Nematode and Eimeria Coccidia Infections in Different Populations of Kazakh Sheep. PLoS One 2021, 16, 1–17. [CrossRef]
- Akkari, H.; Gharbi, M.; Darghouth, M.A. Dynamics of Infestation of Tracers Lambs by Gastrointestinal Helminths under a Traditional Management System in the North of Tunisia. Parasite 2012, 19, 407–415. [CrossRef]
- Rouatbi, M.; Romdhane, R.; Bouaicha, F.; Saddem, R.; Sassi, L.; Dhibi, M.; Rekik, M.; Haile, A.; Mwacharo, J.M.; Rischkowsky, B.; et al. Individual Variability among Autochthonous Sheep in Northern Tunisia to Infection by Abomasum Nematodes and Babesia/Theileria Parasites. Vet Med Sci 2020, 6, 834–845. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).