1. Introduction
Antimicrobial resistance (AMR) has brought out a serious threat to the public health and economic development, and the COVID-19 pandemic has further accelerated this global problem [
1]. Thereby, new antimicrobial agents are desperately developed [
2,
3]. Most antibiotics would bring about some adverse reactions to human body during their treatment on bacterial infection, and eventually be also resistant to pathogenic bacteria after a period of use in clinic [
4]. However, some plant secondary metabolites not only have antimicrobial activities, and also show good safety for human body since they exist in all sorts of plant derived foods and beverages [
5,
6]. Among them, plant flavonoids have paid a close attention to [
7,
8,
9,
10,
11].
Flavonoids are an important class of secondary metabolites widely distributed in various plants, and approximately 10,000 compounds have been discovered so far. Many of them show different degrees of inhibitory activity to pathogenic bacteria especially gram-positive ones, and some of them can also enhance the inhibitory effect of some antimicrobial agents and/or even reverse the AMR [
12,
13]. Simultaneously, various antibacterial mechanisms were reported for plant flavonoids [
7,
9,
12], and which was involved the synthesis inhibitions to DNA, proteins and cell envelope, the damage of cell membrane, and so on. Recently, Yuan,
et al. confirmed that the cell membrane is the major site of plant flavonoids acting on the gram-positive bacteria, and which includes the damage of phospholipid bilayers and likely involves the inhibition of the respiratory chain, or some others [
14,
15]. Another, they pointed out that the antibacterial activities of plant flavonoids to the gram-positive bacteria are directly related to their lipophilicities, and present nonspecific characterization concluded from the antimicrobial quantitative relationships between the physicochemical parameters and the antimicrobial activities [
14,
15].
The antimicrobial mechanism of plant flavonoids damaging the phospholipid bilayers of gram-positive bacteria was confirmed as above, while other mechanisms acting on the cell membrane should be further explored. As Yuan
et al. pointed out [
14,
15], plant flavonoids present nonspecific antimicrobial mechanism. Thereby, the non-enzyme inhibitions of plant flavonoids to the respiratory chain of gram-positive bacteria were also our focus although some probable enzyme mechanisms were likely involved. The compositions of the respiratory chains for different bacteria are varied, while the quinone pool is always a center of electron transfer in the respiratory chain for most bacteria [
16,
17]. For gram-positive bacteria, the menaquinone (MK), together with its reducing form as methyl hydroquinone (MKH
2), is a sole quinones for the electron transfer in the respiratory chain for gram-positive bacteria [
16,
17,
18]. Inspired by the similar structural and antioxidant characters of plant flavonoids to MKH
2, we deduced that the quinone pool is a key target of plant flavonoids inhibiting gram-positive bacteria. To confirm this, here twelve compounds (
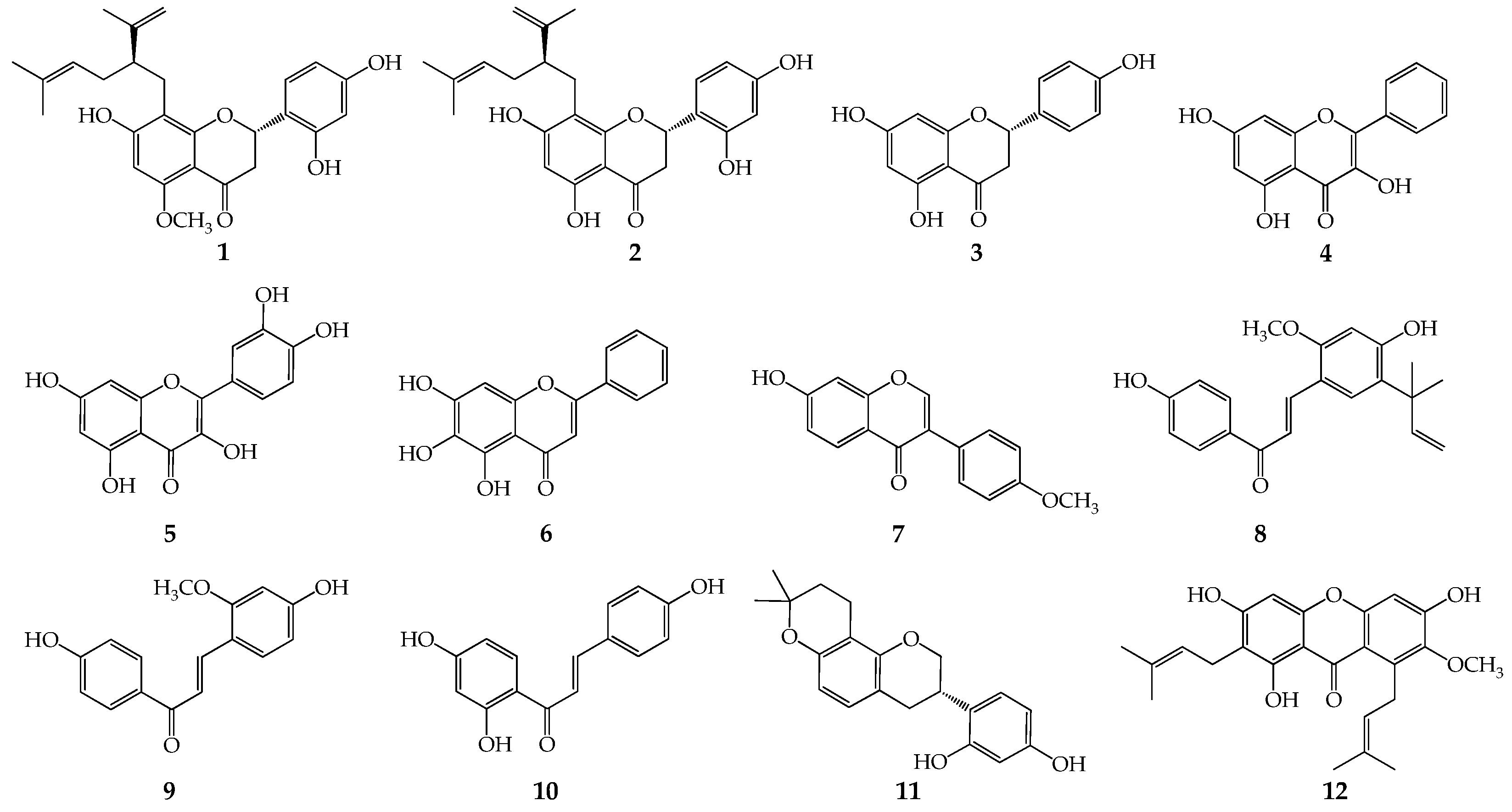
Figure 1), with seven structural subtypes and various degrees of inhibitory activities, were preliminary selected for determining the interference of MK-4 (or MK extract from
Staphylococcus aureus) on the inhibitory activities of flavonoids to gram-positive bacteria.
2. Results
2.1. Calculated and tested minimum inhibitory concentrations (MICs)
Twelve plant flavonoids (
Figure 1), with seven structural subtypes including dihydroflavones, flavonols, flavones, isoflavones, chalcones, flavanes and xanthones, were selected for verifying the inference that the quinone pool is a key target of plant flavonoids inhibiting gram-positive bacteria. Their average MICs (or MIC
90s) against gram-positive such as S. aureus, S. epidermidis and Bacillus subtilis were calculated according to equation (1) in section 4.3 [
15], and the results (
Table 1) showed that they had different degrees of inhibitory activities and can be used for further screening to obtain required flavonoids with different antibacterial activities.
Subsequently, The MICs of these plant flavonoids against S. aureus ATCC25923 were determined using broth microdilution method, and the results were also shown in
Table 1. From
Table 1, it indicated that these flavonoids present different degrees of inhibitory activity to S. aureus ATCC 25923, with the MIC values ranged from 2 to 4,096 (or more than 1,024) μg/mL. Considering that selecting plant flavonoids with various structural subtypes and different antimicrobial activities can enhance the scientificity and rationality of the verification experiments, nine plant flavonoids with six subtypes, including α-mangostin, sophoraflavanone G, licochalcone A, kurarinone, glabridin, isoliquiritigenin, baicalein, echinatin and quercetin, were selected for further experiments.
Another, observed from
Table 1, it also indicated that the larger the lipophilicities of plant flavonoids, the greater their antimicrobial activities. This confirmed again that the lipophilicity is a key factor of plant flavonoids against gram-positive bacteria. Another, according to the rules that the predicted MICs ranged from 1/4× to 4× the determined ones were acceptable [
14,
15] and those less than 1/8× or more than 8× the determined ones were completely unacceptable, the calculated MIC values of only one plant flavonoid as echinatin was unacceptable, and which once again confirmed the efficiency of equation (1) for predicting the MICs of plant flavonoids against gram-positive bacteria.
Table 1.
Minimum inhibitory concentrations (MICs) of twelve plant flavonoids against gram-positive bacteria.
Table 1.
Minimum inhibitory concentrations (MICs) of twelve plant flavonoids against gram-positive bacteria.
| Compounds |
LogP |
Calculated MICsa
|
Tested MICs (μg/mL)b
|
Compounds |
LogP |
Calculated MICs |
Tested MICs
(μg/mL) |
| μmol/L |
μg/mL |
μmol/L |
μg/mL |
| Kurarinone |
6.30 |
28.92 |
12.67 |
8 |
Formononetin |
3.15 |
549.61 |
147.35 |
˃1024 |
| Sophoraflavanone G |
6.52 |
16.37 |
6.95 |
2 ~ 4 |
Licochalcone A |
4.95 |
74.44 |
25.17 |
4 |
| Naringenin |
3.19 |
509.64 |
138.67 |
512 |
Echinatin |
3.23 |
472.20 |
127.54 |
˃1,024 |
| Galangin |
2.83 |
974.47 |
263.15 |
˃1,024 |
Isoliquiritigenin |
3.40 |
338.78 |
86.76 |
512 ~ 1,024 |
| Quercetin |
2.07 |
3,063.61 |
925.21 |
4,096 |
Glabridin |
4.39 |
74.74 |
24.38 |
8 ~ 16 |
| Baicalein |
3.31 |
404.47 |
109.23 |
512 ~ ˃1,024 |
α-Mangostin |
6.70 |
8.17 |
3.35 |
2 |
2.2. Influences of MK-4 on plant flavonoids against S. aureus
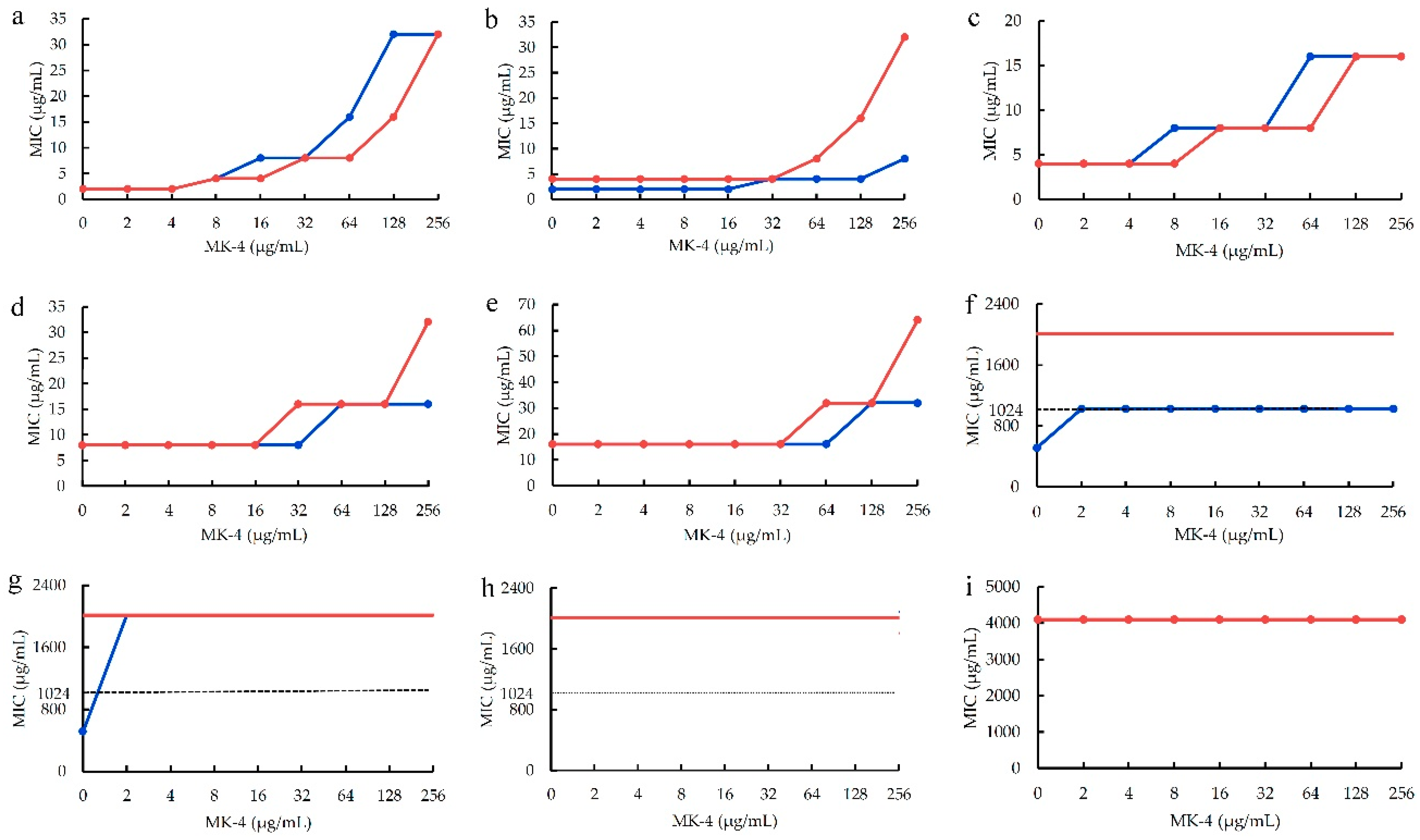
To verify our hypothesis, MK-4 (menaquinone-4) was selected as a simplified representative for preliminarily exploring the influences of MK-4 on plant flavonoids against S. aureus. The results (
Figure 2) showed that the antimicrobial activities of five plant flavonoids (α-mangostin, sophoraflavanone G, licochalcone A, kurarinone and glabridin) with their MICs ranged from 2 to 16 μg/mL obviously decreased along with the increase of the interfering concentrations (from 2 to 256 μg/mL) of MK-4 (
Figure 2a–e). However, those plant flavonoids with the MICs more than 512 μg/mL decreased a little or were unable to evaluate (
Figure 2f–h) for isoliquiritigenin, baicalein and echinatin, and even remianed unchanged for quercetin with the MICs of 4,098 μg/mL (
Figure 2i).
Another, the MIC changes of plant flavonoids against S. aureus ATCC 25923 after interfered with the MK-4 concentration of 256 μg/mL were list in
Table 2 for obtaining clearer and more intuitive MIC changes.
So, these above together indicated that the larger the interfering concentrations of MK are, the more remarkably the antibacterial activities of plant flavonoids decrease. Furthermore, it also indicated that the greater the antibacterial activities of plant flavonoids are, the more obvious the interferences of MK on their antibacterial activities are, and the more greatly their antibacterial activities decrease. Simultaneously, menaquinones are the sole quinones in the quinone pools of gram-positive bacteria, and which don’t contain ubiquinones. Thereby, it was inferred that plant flavonoids can target the quinone pools of gram-positive bacteria especially S. aureus.
2.3. Influences of MK extract on plant flavonoids against S. aureus
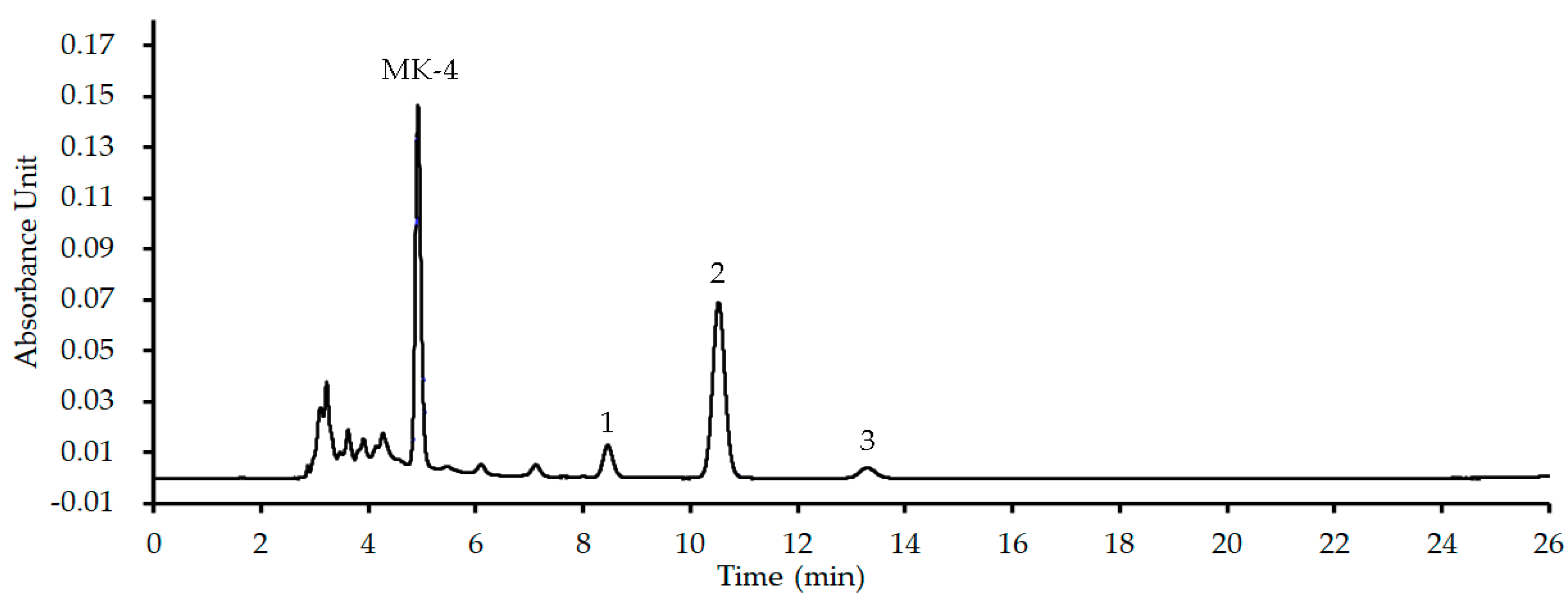
To further confirm the above inference, the MKs in the quinone pool of S. aureus ATCC 25923 was extracted accoring to the method in section 4.6.1, and marked as MK extarct. Using MK-4 as an internal standard, the HPLC-UV analyses for MK extract were performed according to the method [
19]. The representative HPLC profile was shown as
Figure 3, and its detailed HPLC-UV profile was shown as
Figure S1 in supplementary files. Compared with the UV spectroscopy (
Figure S1b) of MK-4 which the retention time is 4.944 min in the HPLC profile (
Figure S1a), the results (
Figure 3 or
Figure S1a) indicated that there are three menaquinones with the retention times respectively at 8.463 (peak 1), 10.535 (peak 2) and 13.318 (peak 3) min in MK extract. As S. aureus mainly contains MK-8 together with a little of MK-7 and -9 in the quinone pool and those MKs have similar physicochemical properties, main chromatographic peak 2 in the HPLC profile (
Figure 3) corresponded to MK-8, and those of peaks 1 and 3 to MK-7 and 9. Thereby, the menaquinones contained in MK extract were in accordance with those in the quinone pool of S. aureus, and can used for further interfered experiments.
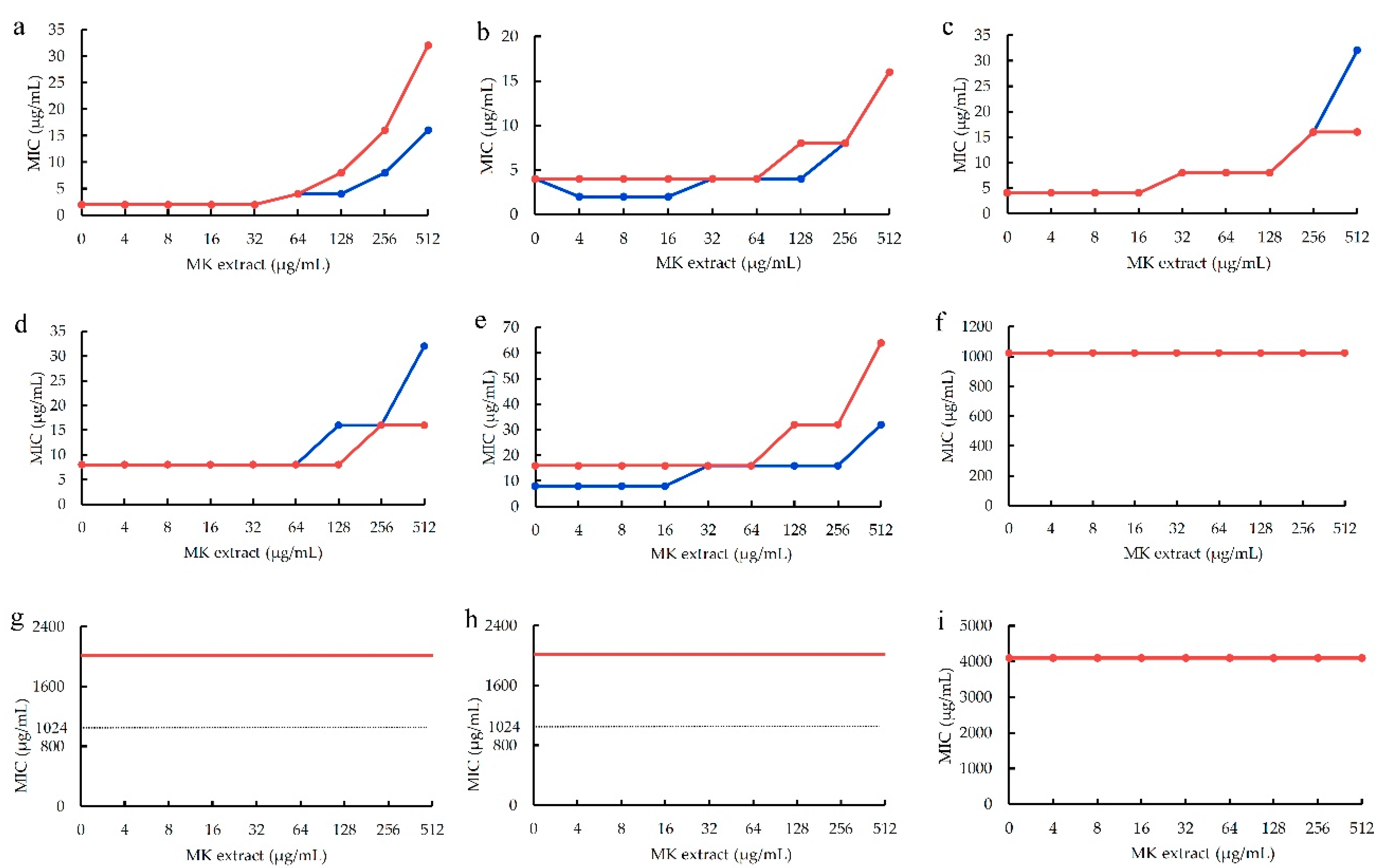
Using this MK extract, the influences of MK extract on plant flavonoids against S. aureus ATCC 25923 were determined. The results (
Figure 4) also showed that the antimicrobial activities of five plant flavonoids (α-mangostin, sophoraflavanone G, licochalcone A, kurarinone and glabridin) with their MICs ranged from 2 to 16 μg/mL obviously decreased along with the increase of the interfering concentrations of MK-4 from 4 to 512 μg/mL (
Figure 4a–e). However, those plant flavonoids with the MICs equal to or more than 1,024 μg/mL remianed unchanged, for isoliquiritigenin and quercetin respectively with the MICs of 1,024 and 4,098 μg/mL (
Figure 2f,i), or were unable to evaluate (
Figure 2g,h) for baicalein and echinatin. This indicated that the greater the antibacterial activities of plant flavonoids, the more greatly their antibacterial activities of plant flavonoids decrease along with the increase of the interfering concentrations of MK extract. Moreover, for those plant flavonoids with their MICs equal to or more than 1,024 μg/mL, their MIC values seemed to remained unchanged along with the increase from 4 to 512 μg/mL of the MK extract concentrations.
Another, the MIC changes of plant flavonoids against S. aureus ATCC 25923 after interfered with the MK-4 concentration of 512 μg/mL were also list in
Table 2 for obtaining clearer and more intuitive change information. From
Table 2, it indicated that similar results and rules were presented for the MIC changes of flavonoids inhibiting S. aureus whether interfered with MK extract or MK-4. Namely, the greater the antibacterial activities of plant flavonoids are, the more obvious the interferences of MK extract on their antibacterial activities are, and the more greatly their antibacterial activities decrease. Thereby, it was further confirmed that the quinone pool is a key target of plant flavonoids against S. aureus, since both above results of two interfered experiments were obtained from vaious structural subtypes with different antimicrobial activities.
3. Discussion
In our previous work, it had confirmed that the cell membrane is the main site of plant flavonoids against gram-positive bacteria, and which likely involves the damage to the phospholipid bilayers and the inhibition to the respiratory chain, etc. Inspired by the similar structural and antioxidant characters of plant flavonoids to MKH2, we had inferred that the quinone pool is a key target of plant flavonoids inhibiting gram-positive bacteria. Thereby, here twelve compounds, with seven structural subtypes and different antimicrobial activities, were selected for verifying this inference. The interfering experiments of MK-4 and MK extract from S. aureus confirmed that the quinone pool is a key target of plant flavonoids against S. aureus.
Similar to
S. aureus, the menaquinones (MKs) are the sole quinones for the electron transfer in the respiratory chain of gram-positive bacteria [
16,
17]. Simultaneously, the antimicrobial quantitative relationship between the parameters and the antimicrobial activities, together with many publications [
8,
13,
20], indicated that a certain flavonoid has similar antimicrobial activities to various gram-positive bacteria. Thereby, it can be inferred that plant flavonoids have similar mechanism targeting the quinone pools in the respiratory chains of gram-positive bacteria. For gram-negative bacteria, there are two quinones MKs and ubiquinones in the quinone pools of their respiratory chains [
17], and so which is in accordance with the fact that plant flavonoids show weak antimicrobial activities to gram-negative bacteria [
8,
13,
20]. Conversely, this further confirmed the reasonability of our initial inference.
During the interfering experiments of MK-4 and MK extract from
S. aureus, some precise MIC data of isoliquiritigenin, baicalein and echinatin were not obtained, and recorded as more than 1,024 μg/mL (
Figure 2 or/and
Figure 3) since these flavonoids present too poor solubility to be effectively determined. However, those of isoliquiritigenin (1,024 μg/mL) and quercetin (4,096 μg/mL), together with the precise MIC data of other plant flavonoids, already confirmed the MIC change trends of plant flavonoids against gram-positive bacteria along with the increase of the interfering concentrations of MK-4 or MK extract. Simultaneously, interfering with the MKs extracted from
S. aureus, it more powerfully confirmed that the MK pools is a key target of plant flavonoids against gram-positive bacteria. Furthermore, it is worth exploring that whether there are some other lipophilic components, except MKs and membrane phospholipid, with the potency of interference for plant flavonoids against gram-positive bacteria.
Totally, the antimicrobial activities of plant flavonoids decreased along with the interfering concentrations of MK-4 and MK extract. However, it showed that they presented a stepwise decrease (
Figure 2 and
Figure 3), not a complete dose-dependent
S-shaped curve. Thereby, the effects of plant flavonoids on the quinone pool of gram-positive bacteria likely involve multiple mechanisms including enzyme and non-enzyme inhibition. The enzyme mechanisms probably involved the inhibition to some enzymes on the respiratory chain [
21], while not on the synthase of MKs since which are the sole quinones in gram-positive bacteria. The non-enzyme mechanisms probably included the electron transfer, membrane potential, and/or reactive oxygen stress (ROS). However, the real ones should be further explored.
4. Materials and Methods
4.1. Materials, Chemicals and Reagents
Kurarinone (≥98%) were purchased from Wuhan ChemFaces Biochemical Co., Ltd. (Wuhan, China). Sophoraflavanone G (>98%), glabridin (99.8%) and echinatin (98%) were purchased from Shanghai TopScience Co., Ltd. (Shanghai, China). Isoliquirtigenin (98%), formononetin (98%), naringenin (97%), galangin (98%) and baicalin (98%) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Quercetin (97%) was purchased from Shanghai Meryer Co., Ltd. (Shanghai, China). Licochalcones A (˃98.0%) and α-mangostin (>98.0%) were purchased from Chengdu Push Bio-technology Co., Ltd. (Chengdou, China). All the compounds were stored at −20°C. The stock solutions of above plant flavonoids were prepared by dissolving in a certain volume of dimethyl sulfoxide (DMSO), and diluted with Mueller Hinton broth (MHB) to obtain a concentration of 4096 μg/mL. The stock solution was mixed well, and then diluted to the desired concentrations with MHB immediately before use. In another, the DMSO concentrations in all the test systems were kept to less than 5.0%, and all those in the blank controls were 5.0%.
MK-4 was purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol and petroleum ether used for the MK extract from S. aureus were obtained from Xilong Scientific Co., Ltd. (Shantou, China). Casein hydrolysate (Qingdao Hope Bio-Technology Co., Ltd., Qingdao, China), starch soluble (Xilong Scientific Co., Ltd., Shantou, China), beef extract and agar powder (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China) were used for preparing the media. DMSO was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Thiazolyl blue tetrazolium bromide was purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China), and 96-well plates were purchased from Shanghai Excell Biological Technology Co., Ltd. (Shanghai, China). All reagents were analytical or biochemical ones. All TopPette Pipettors (2~20 μL and 20~200 μL) were purchased from DLAB Scientific Co., Ltd., Beijing, China.
Mueller Hinton agar (MHA) consisted of casein hydrolysate 17.5 g/L, starch soluble 1.5 g/L, beef extract 3.0 g/L, and agar powder 17.0 g/L dissolving in purified water, and the pH value of 7.40 ± 0.20. MHB was prepared without agar powder according to the same composition and procedure to MHA.
4.2. Bacterial Strains and Growth Condition
S. aureus ATCC 25923 was purchased from American Type Culture Collection, Manassas, VA, USA, and this organism was stored in MicrobankTM microbial storages (PRO-LAB diagnostics, Toronto, Canada) at −20°C. Prior to use, S. aureus was cultured onto MHA plate at 37°C, and then pure colonies from the plate were inoculated into MHA at 37°C for 24 h on a rotary shaker (160 rpm). A 1:100 dilution of the overnight culture was made into fresh MHB, and then incubated at 37°C until the exponential phase for the following experiments. MHB was used for the antimicrobial susceptibility tests.
4.3. MIC Calculation
The physicochemical parameters LogP of tested plant flavonoids were calculated using software ACD/Labs 6.0. Then, the average MIC (or MIC
90) values of these compounds against gram-positive bacteria were predicted according to the following equation (1) [
15].
where y is the average MIC (or MIC
90) values of a certain flavonoid to gram-positive bacteria, mainly including S. aureus, S. epidermidis, and Bacillus subtilis; x is the physicochemical parameter LogP value of this compound.
4.4. Antimicrobial Susceptibility Assay
According to the standard procedure described by the Clinical and Laboratory Standards Institute (CLSI) [
22], the exponential phase culture was diluted with MHB to achieve an
S. aureus concentration approximately 1.0×10
6 CFU/mL, and then the susceptibility of plant flavonoids against
S. aureus ATCC 25923 was determined using the broth microdilution method on the 96-well plates (Shanghai Excell Biological Technology Co., Ltd., Shanghai, China) in triplicate [
4]. Referred to the calculated MIC values of plant flavonoids, the initial concentration of each compound was set. After the 96-well plate were incubated at 35°C for 24 h, a 20 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 4.0 mg/mL) was added into each well, shaking well, and stayed for 30 min at ambient temperature. The minimum inhibitory concentration (MIC), defined as the lowest concentration of compounds that completely inhibited bacterial growth in the micro-wells, was judged from no color change when the bacterial growth in blank wells was sufficient [
23].
4.5. Influences of MK-4 on plant flavonoids against S. aureus
Using the checkerboard method referring to our previous work [
4], the influences of MK-4 on plant flavonoids against S. aureus were evaluated from the combined antimicrobial effects of MK-4 and each compound. Briefly, a serial concentations from 8 to 1,024 μg/mL of test compounds (
Figure 1) and MK-4 respectively in the horizontal or vertical direction were prepared with MHB medium in a separate 96-well plate by twofold dilution method. Next, a 50 μL of test compound or MK-4 with different concentrations were correspondingly added into the designed wells on another plate to obtain different proportions with test compounds (
Figure 1) or MK-4 concentrations from 4 to 512 μg/mL, and then an 100 μL of bacterial suspension (approximately 1.0×10
6 CFU/mL) was added into each well. Differently, for compounds
8,
11 and
12 (
Figure 1), the final concentrations of test compounds in corresponding wells ranged from 8 to 1,024 μg/mL, and those of compound
6 in
Figure 1 ranged from 8 to 4,096 μg/mL.
Another, column 11 contained a serial concentrations from 2 to 256 μg/mL of MK-4 in MHB with 5 × 10
5 cfu/mL S. aureus isolate were used as negative controls. Column 12 contained a serial concentrations from 2 to 256 μg/mL for test compound (
1,
2,
10,
13 or
14), from 8 to 1,024 μg/mL for test compound (
8,
11 or
12), and from 32 to 4,096 μg/mL for compound
6 were used as accompanying controls, respectively. According to the the same procedure as
Section 4.4, the MICs of each flavonoid against S. aureus were determined under the interferences of different MK-4 concentrations.
4.6. Influences of MK extract on plant flavonoids against S. aureus
4.6.1. MK extract from S. aureus
Referred to the method reported by Schurig-Briccio,
et al. [
24], the MK was extracted from
S. aureus ATCC 25923. Briefly, a 3,000 mL of
S. aureus cells at exponential phase were collected by centrifugation at 3,000 rpm for 15 min. The pallet was resuspended with 30 mL purified water, and then the mixture was crushed by a SCIENTZ-IID ultrasound cell breaker (Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) for 12 min (2 s treatment and 3 s interval). Next, the mixture was centrifuging at 3,000 rpm for 15 min, and the pallet was resuspended with 3 mL water and extracted with 17.5 mL of methanol:petroleum ether (6:4,
v/v) by an vigorous vortex for 1 min (3 times). After stayed for 2 h, the mixture was eddied again for another 1 min, followed by centrifuging at 3,000 rpm for 10 min. The upper organic layer was transferred to a 10-mL centrifuge tube, and was then evaporated under a nitrogen stream to obtain a dried and oily residue (marked as the MK extract).
4.6.2. HPLC-UV analyses for the MK extract
A standard solution (35.0 μg/mL) of MK-4 and a sample solution (128.0 μg/mL) of MK extract were prepared using methanol:isopropanol (60:40,
v/
v). Simultaneously, both above solutions were mixed by equal volume to obtain a mixed solution of MK-4 plus MK extract, and among which MK-4 was used as an internal standard. Referred to our previous work [
19], the menaquinones contained in the MK extract were analyzed using a HPLC-UV method without methodological validation. Briefly, the HPLC-UV analyses were performed on a Waters e2695 separation system consisting of a model 2998 ultraviolet detector (Milford, MA, USA), and the detection wavelength was set at 247 nm. A Hypersil ODS2 (4.6 mm × 250 mm, 5.0 µm) (Dalian Elite Analytical Instruments Co., Ltd., Dalian, China) was used as the chromatographic column which was kept at room temperature throughout the experiments. Methanol/isopropanol (60:40,
v/v) was used as the mobile phase, and the flow rate was set at 1.0 mL/min, along with an injection volume of 20 μL. After injection into the HPLC system, main MKs in the MK extract were identified from the UV spectral characteristics of all chromatographic peaks in the HPLC profile of the mixed solution (
Figure 3 and
S1), according to our previous publication [
19]. In detailed, based on the UV spectral characteristics, MK analogs were identified if a chromatographic peak in the HPLC profile of the mixed solution has similar UV absorption curve to that of the MK-4.
4.6.3. MICs of plant flavonoids with the interference of the MK extract
According to the method and procedure in
Section 4.5., the MICs of plant flavonoids with the interference of the MK extract were determined using checkerboard method. Differently, a serial concentrations from 16 to 2,048 μg/mL of MK extract in the vertical direction were prepared with MHB medium in a separate 96-well plate by twofold dilution method, and the final concentrations of the MK extract were ranged from 4 to 512 μg/mL. Another, column 11 contained a serial concentrations from 4 to 512 μg/mL of MK extract in MHB with 5 × 10
5 cfu/mL S. aureus isolate were used as negative controls.
5. Conclusions
Based on our previous conclusion that the cell membrane is the major site of plant flavonoids acting on the gram-positive bacteria and which likely involves the inhibition of the respiratory, the quinone pool is a key target of plant flavonoids inhibiting gram-positive bacteria was deduced, inspired by the similar structural and antioxidant characters of plant flavonoids to MKH2. To verify this, twelve plant flavonoids with seven structural subtypes were selected for their MIC tests, and nine of them with six structural subtypes were eventually used for the determinations of their MICs against S. aureus, with the interferences of different concentrations of MK-4 and the MKs extracted from S. aureus. The results showed that the greater the antibacterial activities of plant flavonoids, the more greatly their antibacterial activities decreased along with the increase of the interfering concentrations of MK-4 and the MK extract, and while those with weaker antimicrobial activities decreased a little or remained unchanged. Especially, the MICs of α-mangostin with greatest inhibitory activity to S. aureus in these nine plant flavonoids, respectively increased by 16 times and 8 to16 times under the interference of MK-4 (256 μg/mL) and the MK extract (512 μg/mL). These above verified our hypothesis that the quinone pool is a key target of plant flavonoids inhibiting gram-positive bacteria, and which likely involves multiple mechanisms including some enzyme and non-enzyme inhibitions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, The supporting information includes Figure S1: The HPLC-UV profiles of representative MK extract from
S. aureus ATCC 25923.
Author Contributions
Conceptualization, G.Y.; methodology, G.Y.; software, L.Z., Y.Y., J.Z. and G.Y.; validation, G.Y., L.Z., Y.Y. and J.Z.; formal analysis, G.Y., L.Z., Y.Y., X.X. and J.Z.; investigation, L.Z., Y.Y., J.Z., X.X., G.Y., S.L., B.D. and X.L; resources, G.Y.; data curation, G.Y., L.Z., Y.Y., J.Z., X.X., S.L. and B.D.; writing—original draft preparation, G.Y., L.Z., Y.Y. and J.Z.; writing—review and editing, G.Y., L.Z., Y.Y., J.Z., X.X., S.L., B.D. and X.L.; visualization, G.Y., L.Z., J.Z., Y.Y. and X.L.; supervision, G.Y.; project administration, G.Y.; funding acquisition, G.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China, Grant numbers 81960636 and 82073745.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Authors are thankful to the financial supports from National Natural Science Foundation of China, Grant numbers 81960636 and 82073745.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Wool, P.R.E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [CrossRef]
- Laxminarayan, R.; Sridhar, D.; Blaser, M.; Wang, M.; Woolhouse, M. Achieving global targets for antimicrobial resistance. Science 2016, 353, 874–875. [CrossRef]
- Kurosu, M.; Siricilla, S.; Mitachi, K. Advances in MRSA drug discovery: where are we and where do we need to be? Exp. Opin. Drug Discov. 2013, 8, 1095–1116. [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other's mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 7237. [CrossRef]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with activity against methicillin-resistant Staphylococcus aureus. Phytomedicine 2022, 100, 154073. [CrossRef]
- Panda, L; Duarte-Sierra, A. Recent advancements in enhancing antimicrobial activity of plant-derived polyphenols by biochemical means. Horticulturae 2022, 8, 401. [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [CrossRef]
- Tan, Z.; Deng, J.; Ye, Q.; Zhang, Z. The antibacterial activity of natural-derived flavonoids. Curr. Top. Med. Chem. 2022, 22, 1009–1019. [CrossRef]
- Song, L.; Hu, X.; Ren, X.; Liu, J.; Liu, X. Antibacterial modes of herbal flavonoids combat resistant bacteria. Front. Pharmacol. 2022, 13, 873374. [CrossRef]
- Wu, S.C.; Yang, Z.Q.; Liu, F.; Peng, W.J.; Qu, S.Q.; Li, Q.; Song, X.B.; Zhu, K.; Shen, J.Z. Antibacterial effect and mode of action of flavonoids from licorice against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 2019, 10, 2489. [CrossRef]
- Zhou, K.; Yang, S.; Li, S.M. Naturally occurring prenylated chalcones from plants: structural diversity, distribution, activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2236–2260. [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [CrossRef]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Sci. Rep. 2021, 11, 10471. [CrossRef]
- Yuan, G.; Xia, X.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antimicrobial quantitative relationship and mechanism of plant flavonoids to gram-positive bacteria. Pharmaceuticals 2022, 15, 1190. [CrossRef]
- Paudel, A.; Hamamoto H.; Panthee S.; Sekimizu K. Menaquinone as a potential target of antibacterial agents. Drug Discov. Ther. 2016, 10, 123-128. [CrossRef]
- Kurosu, M.; Begari, E. Vitamin K2 in electron transport system: are enzymes involved in vitamin K2 biosynthesis promising drug targets? Molecules 2010, 15, 1531-1553. [CrossRef]
- Hamamoto, H.; Urai, M.; Ishii, K.; Yasukawa, J.; Paudel, A.; Murai, M.; Kaji, T.; Kuranaga, T.; Hamase, K.; Katsu, T.; Su, J.; Adachi, T.; Uchida, R.; Tomoda, H.; Yamada, M.; Souma, M.; Kurihara, H.; Inoue, M.; Sekimizu, K. Lysocin E is a new antibiotic that targets menaquinone in the bacterial membrane. Nat. Chem. Biol. 2015, 11, 127-133. [CrossRef]
- Cao, S.; Du, X.; Li, P.; Yuan, G.; Chen, S.; Chen, W.; Song, X.; Kuang, B. A chemical screening method for menaquinone-producing strains based on HPLC-UV technology. J. Microbiol. Methods 2020, 172, 105907. [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial effects of flavonoids and their structure-activity relationship study: a comparative interpretation. Molecules 2022, 27, 1149.
- Haraguchi, H.; Tanimoto, K.; Tamura, Y.; Mizutani, K.; Kinoshita, T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry 1998, 48, 125-129. [CrossRef]
- Clinical and Laboratory and Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; Approved Standards, CLSI document M07-A10; Clinical and Laboratory and Standards Institute: Wayne, PA, USA, 2015.
- Wu, X.; Xu, L.; Li, P.; Wang, Y.; Yuan, G. The anti-methicillin-resistant Staphylococcus aureus activities of azalomycin F derivatives and those of them combined with vitamin K3. Chin. J. Antibiot. 2016, 41, 584-589.
- Schurig-Briccio, L.A.; Yano, T.; Rubin, H.; Gennis, R.B. Characterization of the type 2 NADH:menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines. Biochim. Biophys. Acta. 2014, 1837, 954-963. [CrossRef]
Figure 1.
The chemical structures of twelve compounds with seven subtypes of plant flavonoids. 1, Kurarinone; 2, Sophoraflavanone G; 3, Naringenin; 4, Galangin; 5, Quercetin; 6, Baicalein; 7, Formononetin; 8, Licochalcone A; 9, Echinatin; 10, Isoliquiritigenin; 11, Glabridin; 12, α-Mangostin.
Figure 1.
The chemical structures of twelve compounds with seven subtypes of plant flavonoids. 1, Kurarinone; 2, Sophoraflavanone G; 3, Naringenin; 4, Galangin; 5, Quercetin; 6, Baicalein; 7, Formononetin; 8, Licochalcone A; 9, Echinatin; 10, Isoliquiritigenin; 11, Glabridin; 12, α-Mangostin.
Figure 2.
The influences of MK-4 on plant flavonoids against S. aureus ATCC 25923. a, α-Mangostin; b, Sophoraflavanone G; c, Licochalcone A; d, Kurarinone; e, Glabridin; f, Isoliquiritigenin; g, Baicalein; h, Echinatin; i, Quercetin; Each compound was tested twice, test 1 showed the red lines and test 2 showed the blue lines in the planes (sometimes the red and blue lines overlapped, and showed as the red lines), and those lines without data dots in f, g and h indicated that the MICs were more than 1024 μg/mL which values marked as dashed lines.
Figure 2.
The influences of MK-4 on plant flavonoids against S. aureus ATCC 25923. a, α-Mangostin; b, Sophoraflavanone G; c, Licochalcone A; d, Kurarinone; e, Glabridin; f, Isoliquiritigenin; g, Baicalein; h, Echinatin; i, Quercetin; Each compound was tested twice, test 1 showed the red lines and test 2 showed the blue lines in the planes (sometimes the red and blue lines overlapped, and showed as the red lines), and those lines without data dots in f, g and h indicated that the MICs were more than 1024 μg/mL which values marked as dashed lines.
Figure 3.
The HPLC profile of representative MK extarct from S. aureus ATCC 25923. MK-4 used as an internal standard, and peaks 1, 2, and 3 corresponded MK-7, -8 and -9, respectively.
Figure 3.
The HPLC profile of representative MK extarct from S. aureus ATCC 25923. MK-4 used as an internal standard, and peaks 1, 2, and 3 corresponded MK-7, -8 and -9, respectively.
Figure 4.
The influences of MK extract on plant flavonoids against S. aureus ATCC 25923. a, α-Mangostin; b, Sophoraflavanone G; c, Licochalcone A; d, Kurarinone; e, Glabridin; f, Isoliquiritigenin; g, Baicalein; h, Echinatin; i, Quercetin; Each compound was tested twice, test 1 showed the red lines and test 2 showed the blue lines in the plane (sometimes the red and blue lines overlapped, and showed as the red lines), and those lines without data dot in f, g and h indicated the MICs were more than 1,024 μg/mL which marked as dashed lines.
Figure 4.
The influences of MK extract on plant flavonoids against S. aureus ATCC 25923. a, α-Mangostin; b, Sophoraflavanone G; c, Licochalcone A; d, Kurarinone; e, Glabridin; f, Isoliquiritigenin; g, Baicalein; h, Echinatin; i, Quercetin; Each compound was tested twice, test 1 showed the red lines and test 2 showed the blue lines in the plane (sometimes the red and blue lines overlapped, and showed as the red lines), and those lines without data dot in f, g and h indicated the MICs were more than 1,024 μg/mL which marked as dashed lines.
Table 2.
The MIC changes of plant flavonoids against S. aureus after interfered with maximum test concentrations of MK-4 and MK extract.a.
Table 2.
The MIC changes of plant flavonoids against S. aureus after interfered with maximum test concentrations of MK-4 and MK extract.a.
| Compounds |
MICAlone
(μg/mL) |
MIC change (times) b
|
Compounds |
MICAlone
(μg/mL) |
MIC change (times) |
| MK-4 |
MK extract |
MK-4 |
MK extract |
| α-Mangostin |
2 |
16 |
8 ~ 16 |
Isoliquiritigenin |
512 ~ ˃1024 |
2 /-c
|
1 |
| Sophoraflavanone G |
2 ~ 4 |
4 ~ 8 |
4 ~ 8 |
Baicalein |
512 ~ ˃1024 |
- |
- |
| Licochalcone A |
4 |
4 |
4 ~ 8 |
Echinatin |
˃1024 |
- |
- |
| Kurarinone |
8 |
2 ~ 4 |
2 ~ 4 |
Quercetin |
4096 |
1 |
1 |
| Glabridin |
8 ~ 16 |
2 ~ 4 |
4 |
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).