1. Introduction
Camelina [
Camelina sativa (L.) Crantz] is an ancient oilseed crop belonging to the Brassicaceae family, to the tribe Camelineae.
Camelina sativa, also called by different names such as false flax or gold of pleasure, has been grown as a crop since the Iron Age in European countries and Russia, where the center of origin is located. It was abandoned after the Second World War for more profitable crops [
1,
2,
3].
The average height of the plants is between 30 and 90 cm. The stems are branched, with alternate lanceolate leaves. Inflorescences are racemes composed of small yellow flowers with four petals. The siliques are smooth, leathery, 7 to 9 mm long and usually contain 5-15 golden/brown seeds. Seeds are 2 to 3 mm long and the weight of 1000 seeds is 0.8 to 2.0 grams [
4]. It is an interesting resilient plant with a fast growth cycle of about 85–100 days, which can be used in different low-input agronomic systems. especially in the increasingly topical perspective of climate change.
Nowadays, the interest in camelina has increased because of the crop’s great potential and the numerous uses, for examples as a new source of polyunsaturated fatty acids and proteins for feed, food and bio-based products. Camelina oil is attractive for its high content in the seeds (up to 40%), and for the high proportion of unsaturated fatty acids (30-40% alpha linolenic acid fraction, 15-25% linoleic acid fraction, 15% oleic acid fraction and about 15% eicosenoic acid fraction).
Although camelina oil has excellent nutritional and functional characteristics and the protein cake resulting from the pressing of the seeds has an excellent protein content, it also contains anti-nutritional compounds, such as glucosinolates, synapin, phytic acid and condensed tannins [
5]. However, the major antinutritional compounds are the glucosinolates (GSL), which are the main secondary metabolites present in
Brassicaceae and limit their use especially as feed [
6].
Among the agronomic traits that have to be considered in the selection of new varieties the yield, that can be expressed as size of the seed and competitive capacity (e.g. wider leaves), the concentration of anti-nutritional compounds, and the resistance to herbicides are of paramount importance. In the field of classical genetic improvement there are difficulties in breeding programs on
Camelina sativa as this species is allohexaploid (chromosome number 2n=40, genome size 750 Mbp) [
7] and does not present much variability [
8].
In this work, 8 spring varieties of Camelina sativa and a synthetic population, generated by crossing two spring-type parents, were studied in two different cultivation periods, spring and winter. The aim of the study was to evaluate the performances of these varieties through the comparison of yield and other agronomic parameters, bromatological analysis and antinutritional content in two cultivation periods.
2. Materials and Methods
2.1. Plant Materials
Eight different spring lines and a synthetic population of
Camelina sativa (
Table 1) were tested by low input farming, in the University of Milan experimental field, situated in Landriano (PV), North Italy (45°19′N 9°16′E), 88 m a.s.l. The screening trial aimed at performing an agronomic comparison between spring and winter cultivation.
The Calena variety was kindly provided by Dr. Incoronata Galasso, IBBA-CNR, Milan, Italy. Omich and Madalina varieties are registered in CPVO (Community Plant Variety Office). All the other experimental materials are advanced breeding lines provided by the germplasm bank at DISAA, Department of Agricultural and Environmental Sciences—Production, Landscape and Agroenergy, University of Milan, Italy.
2.2. Constitution of synthetic population
The synthetic population was developed starting from spring lines of Camelina sativa.
In particular the constitution of the synthetic population started with a cross between an experimental material of the University of Milan (C1199) and the Omich CPVO variety (C1204) performed in 2018. The breeding system used was a bulk method, in which after the initial cross, each generation was advanced by collecting and propagating the material by self-pollination, for 6 agronomic cycles. The synthetic population used in this study is the F6 generation.
2.3. Field experimentation
The camelina plants were grown in the North Italy area, at the experimental farm of the University of Milan in Landriano (PV). In
Table 2 the characteristics of the soil are reported.
The experiment was carried out by evaluating the spring-type pure lines and synthetic population of camelina at two different sowing times, in spring (from sowing in April 2021 to harvest in July 2021) (accessions C1201, C1204, C1202, C1999, C1200, C1215) and in winter (from sowing in October 2021 to harvest in May 2022) (accessions C1201, C1204, C1202, C1999, C1200, C1207, C1208, C1209, C1215).
Table 3 shows the mean temperature and rainfall for the months of interest for the two experimental fields.
No irrigation was provided in either of the cycles of cultivation. The experiment was laid out in randomized blocks in both the spring and winter fields. In the experimental field each accession (8 pure varieties and one synthetic population) was cultiva ted in three plots for a total of 27 plots. The size of each plot was about 6 m2 (5 m x 1.2 m). The sowing was carried out on tilled soil in rows spaced 0.2 m, using a Earthway 1001B manual seeder, with seed disc 1002-5, at a density of 8 kg/ha. Periodic monitoring was conducted during the crop cycle. At maturity, the harvest was carried out manually by cutting at ground level. The plants were collected and left to dry well. Once the drying was completed, the siliques were opened by applying pressure to the dry plants and the seeds were collected using a sieve.
2.4. Agronomic parameters
At maturity, in each plot, some agronomic traits were measured: plant height (from ground level to the tip of the plant), branch height (from ground level to the first branch node on the stem), number of branches (number of nodes present on the stem), stem diameter (measured below the first branch node from ground level), number of siliques per plant, silique size (length and width), number of seeds per siliqua, weight of 1000 seeds. To calculate the parameter weight of 1000 seeds, 3 samples were measured for each accession and for all the other parameter at least 10 samples were analysed. In each plot, one square meter was harvested, the number of plants was counted and the yield calculated.
2.5. Bromatological analysis
Analysis was conducted by bulking 100 g of seed per plot for each variety. 5 g of the bulked seeds were sampled for the bromatological analysis. The Camelina sativa seeds were analysed in triplicate in terms of principal nutrients: dry matter (DM), ash content (AC), ether extract (EE), crude proteins (CP), crude fibre (CF) (AOAC, 2019). DM was obtained by drying seeds in a forced air oven at 65 °C for 24 h (AOAC method 930.15). AC was obtained by incinerating samples in a muffle furnace at 550 °C (AOAC method 942.05). EE was determined using ether extraction in a Soxtec system (SER 148 Series Solvent Extractor, Velp Scientifica Srl, Usmate, Italy; AOAC method 2003.05). CP was determined through the Kjeldahl method (AOAC method 2001.11). CF was determined by the filter bag method (AOCS method Ba 6a-05) (AOCS, 2009).
2.6. Glucosinolates quantification
As reported in section 2.5. Bromatological analysis, 5g of bulked seeds were used for glucosinolates analyses.
Seeds were ground with mortar and pestle in liquid Nitrogen, kept frozen to avoid endogenous myrosinase action, and stored at -80°C until analysis. For glucosinolate extraction, 200 mg of ground seeds were resuspended in 5 ml of 80% Methanol (Sigma-Aldrich) and incubated at 70°C for 30 minutes. After centrifugation at 9,000 rpm for 20 minutes, 4 ml of the extract were transferred, and the solvent was evaporated under vacuum with an Eppendorf concentrator plus (Eppendorf). Glucosinolate quantification was performed by resuspending the desiccated material in 1 ml of 50 mM Citrate Buffer pH 6. After centrifugation 200 µl were added with 0.3 U of Thioglucosidase from Sinapis alba (white mustard) seed (Sigma-Aldrich) in a final volume of 500 µl. Enzymatic reaction was performed at 25°C for 20 min. Finally, Glucose released by Thioglucosidase from Glucosinolate was measured with Enzytec™ Generic D-Glucose / D-Fructose / Sucrose kit (R-biopharm) following manufacturer’s instructions. As negative control, free glucose quantification was performed for each sample without the addition of Thioglucosidase; the obtained value was subtracted to the relative glucose quantification. Each experiment was performed in duplicate.
2.7. Informatic tools
Microsoft Excel® was used to collect data, and the PAST program (Paleontological Statistics, version 4.12) was used to perform statistical analysis. Results are presented as least square means’ standard deviation. Statistically significant differences are considered for p ≤ 0.05.
3. Results
3.1. Comparison among varieties cultivated at two different sowing times
The agronomic comparison of all the plant materials under study (
Table 1) was carried out by sowing at two different times: spring cultivation and winter cultivation. The experimental design was randomized blocks and each genetic line was cultivated as reported in Materials and Methods.
The first experimental field (spring field) was sown in April 2021 and harvested in July 2021 (accessions C1201, C1204, C1202, C1999, C1200, C1215). The second experimental field (winter field) was sown in October 2021 and harvested in May 2022 (accessions C1201, C1204, C1202, C1999, C1200, C1207, C1208, C1209, C1215).
At maturity, at least 10 plants were collected, and the parameters of interest were measured. The results obtained are reported in
Figure 1.
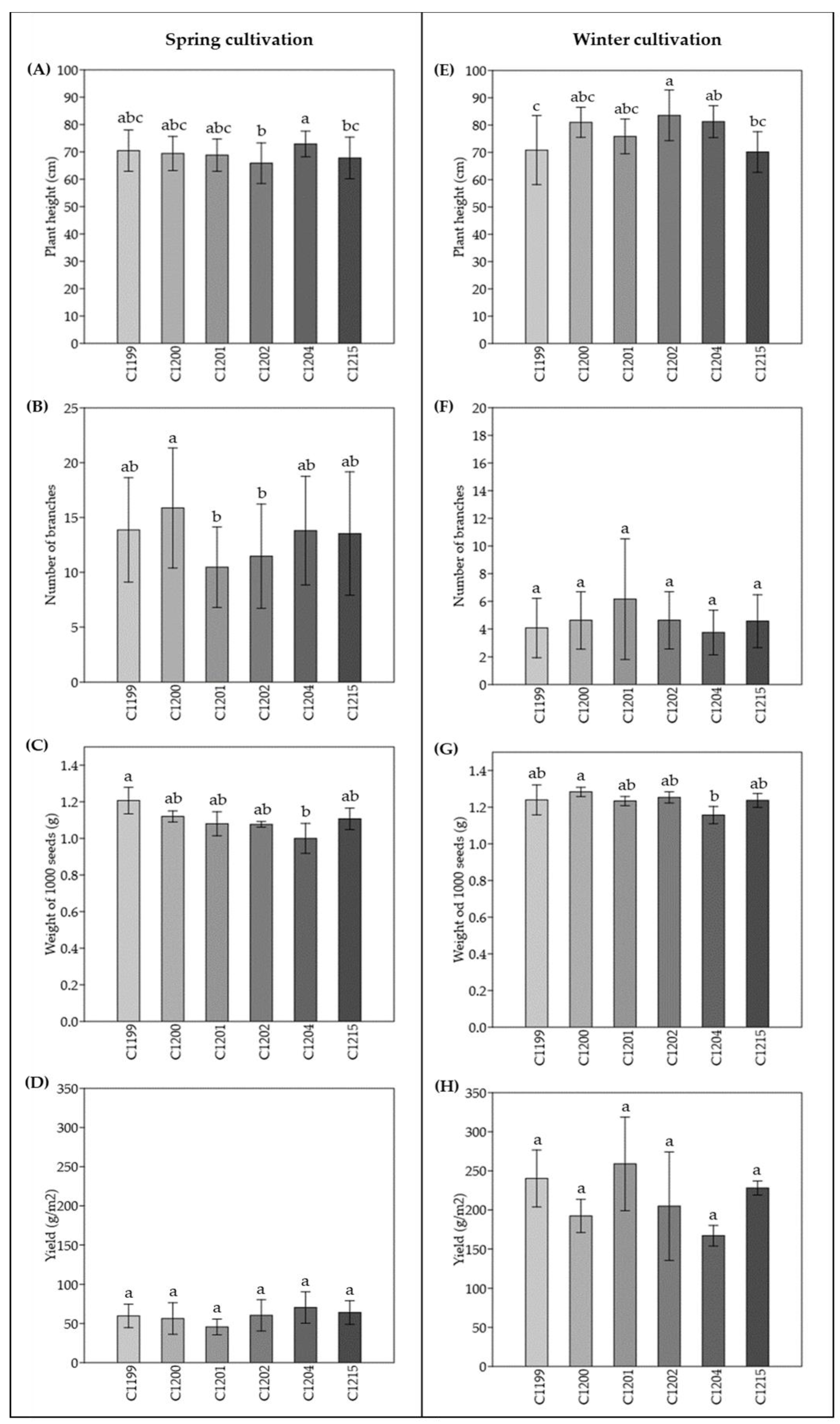
The agronomic traits measured for each genetic line were plant height, number of branches, weight of 1000 seeds and the yield. Starting from the plant height in the spring field, the variety with the highest average was C1204 (72.90 ± 4.69 cm) which was statistically different (p < 0.05) from C1202 (65.87 ± 7.43 cm) and from C1215 (67.80 ± 7.62 cm) the synthetic population. C1215 was also different from C1202 (
Figure 1A). In the winter field, the tallest height was recorded for the C1202 variety (83.55 ± 9.28 cm) which was statistically different from the C1199 variety with the lowest height (70.85 ± 12.66 cm). The variety C1204 (81.25 ± 5.82 cm) was also different from C1199. The synthetic population C1215 with height of 70.14 ± 7.45 cm was different from C1202 (
Figure 1E). Regarding the number of branches, in the spring field the varieties C1201 and C1202 were statistically different from C1200 (p < 0.05;
Figure 1B); in the winter field no statistically significant differences were found (
Figure 1F). The weight of 1000 seeds were also evaluated, in the spring field the weight was higher for C1199 (1.21 ± 0.07 g) variety and lower for C1204 (1.00 ± 0.08 g), between these two varieties the difference was statistically significant (p < 0.05;
Figure 1C). In the winter field, the statistically significant difference was between C1200 and C1204. The variety with the highest 1000 seeds weight was C1200 (1.28 ± 0.03 g) (
Figure 1G). Lastly, concerning the estimated yield in the spring field there were no statistically significant differences between the varieties, and the average of the estimated yields of all varieties was about 600 kg/ha (
Figure 1D). Also in the winter field, there are no statistically significant differences and the estimated average yield value for the varieties was about 2020 kg/ha (
Figure 1H). No correlation was found among the different traits studied.
Table 4 reports the yields compared among varieties within the same cultivation season, and the comparison between the spring cultivation (S) and the winter cultivation (W).
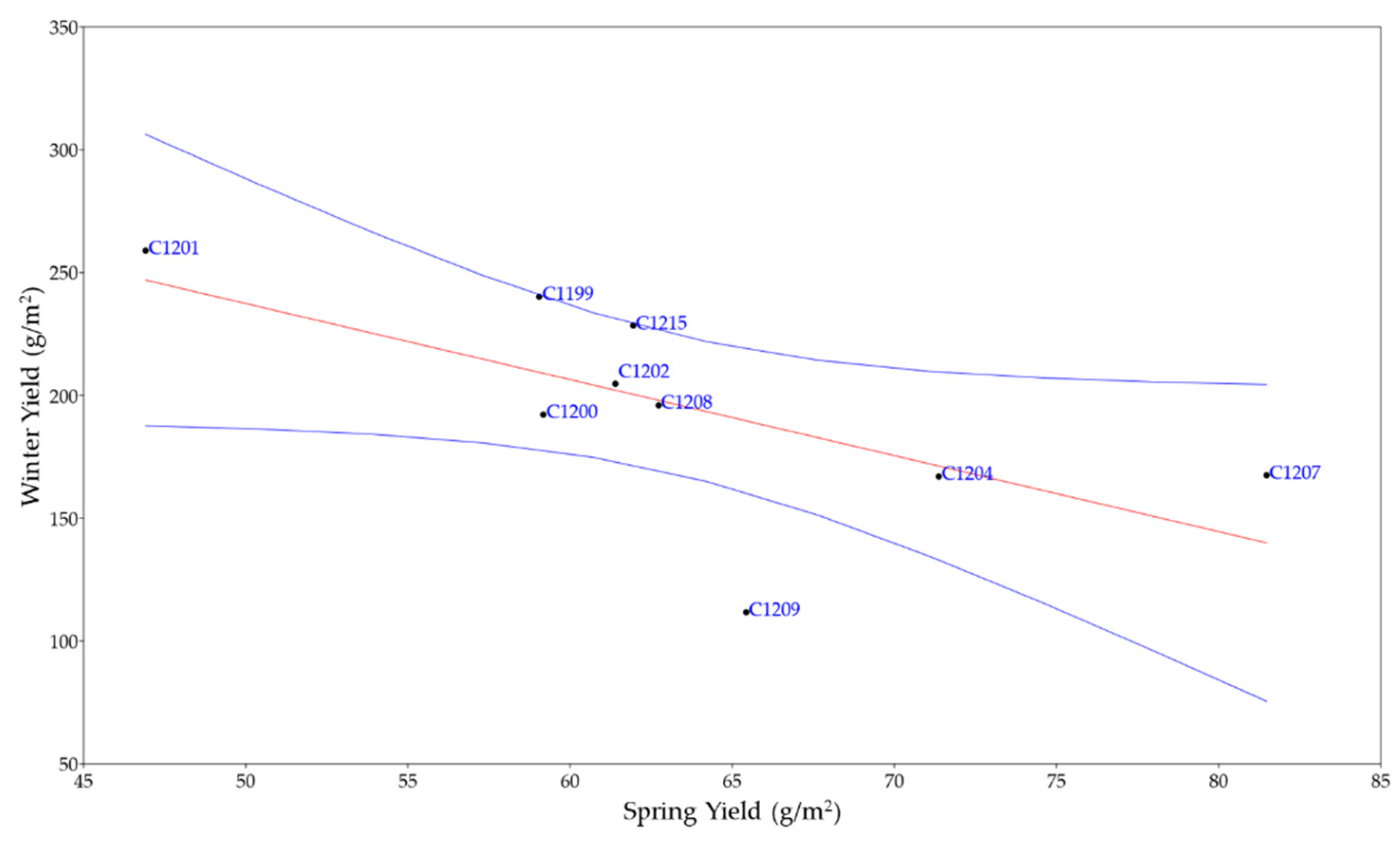
All genetic materials showed statistically different yields between spring field (S) and winter field (W). In
Figure 2 is shown the regression curve (r = -0.65565; p = 0.0552) between these two different sowing times.
3.2. Dissection of agronomic parameters involved in yield
During the growth of the winter crop, the agronomic comparison of all the plant materials under study (
Table 1) was carried out on the main parameters involved in yield required by the CPVO (Community Plant Variety Office). The agronomic traits measured for each genetic line were plant height (PH), number of branches (NB), branch height (BH), stem diameter (SD), number of siliques per plant (NSP), number of seeds per silique (NSS), silique length (SL), silique width (SW), weight of 1000 seeds (W1000), number of plants per m
2 (NP) and the yield (Y) (
Table 5).
The experimental design was randomized blocks and each genetic line was cultivated as reported in Materials and Methods. At maturity, at least 10 plants were collected, and some parameters were measured. The results obtained are reported in
Table 5.
Starting from the plant height, the tallest variety was C1202 (83.55 ± 9.28 cm); for this phenotypic trait there are no statistically significant differences between materials. Regarding the number of branches, the variety C1201 with the greater presence of ramifications on the stem was statistically different from C1209 which produced only a third of the number of branches (p < 0.05); while none of the others are statistically different from these varieties. However, the branch height was lower in the synthetic population with a value of 31.71 ± 10.93 cm and was statistically different from all other materials. The stem diameter measurements did not show statistically significant differences between lines, nor did the estimated number of siliques per plant. The number of seeds per silique was higher in varieties C1208 and C1209, respectively 17.80 ± 2.20 and 16.78 ± 2.78, these two varieties were statistically different from all the genetic lines except C1207. Two further traits considered concern the size of the siliques. The silique length was greater for varieties C1200 and C1209 and these were statistically different from C1199 and C1201. Regarding the width of the siliques, no big differences were recorded (average value 3 mm) apart from variety C1201 in which the average width of the siliques was 2 mm.
Another important trait that provides an estimate of yield was the weight of 1000 seeds, which was higher in the C1207 variety (1.44 ± 0.04 g) and lower in the C1209 variety (1.11 ± 0.02 g). The number of plants per m2 was highest in C1202 (421.67 ± 85.20), statistically different from C1209 (185 ± 56.35) (p < 0.05).
Lastly, the estimated yield was lower for the variety C1209, at about 1118 kg/ha, which is statistically different from the estimated yield of the variety C1199 and the synthetic population C1215 which recorded the highest yields, respectively about 2402 kg/ha and 2284 kg/ha.
3.3. Bromatological analyses and glucosinolates content
The comparison among different pure lines and the synthetic population showed that no big differences were recorded about dry matter and ash. For crude fiber, the highest value was observed in C1199 variety (24.49%) while the lowest was in C1200 variety (20.01%). The crude protein percentage ranged from 26.12% (C1201) to 30.75% (C1204). Lastly, the varieties with the highest ether extract were C1201 and C1207, respectively 31.43% and 31.15% while the lowest content was in C1209 (23.58%) (
Table 6).
The main antinutritional factors present in camelina seed, are glucosinolates. These molecules limit the utilization of the protein cake used as feed. For this reason, the analyses of the glucosinolates content present in the camelina seeds varieties was performed.
As shown in
Table 7, the lower glucosinolate content was found in the synthetic population C1215 (16.92 mmol/kg), while the variety with the highest content was C1204 (26.52 mmol/kg).
3.4. Correlation among agronomic and chemical traits
A correlation was performed on all the agronomic parameters collected with the bromatological data and the glucosinolates content. The results shown in
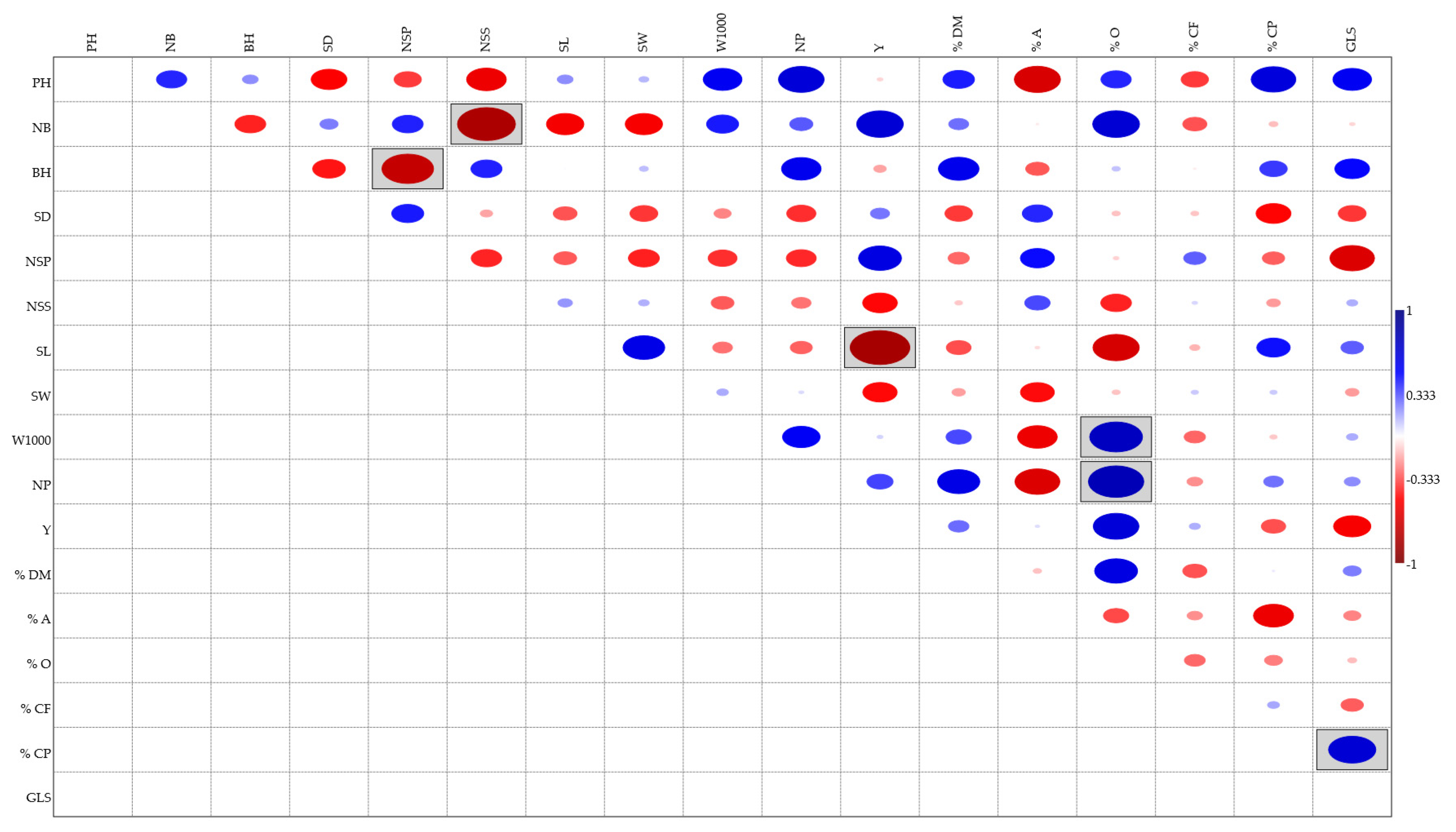
Figure 3 highlight (gray boxes) three positive correlations and three negative correlations which are statistically significant (p < 0.05);
Figure 4 shows the regressions referring to statistically significant correlations.
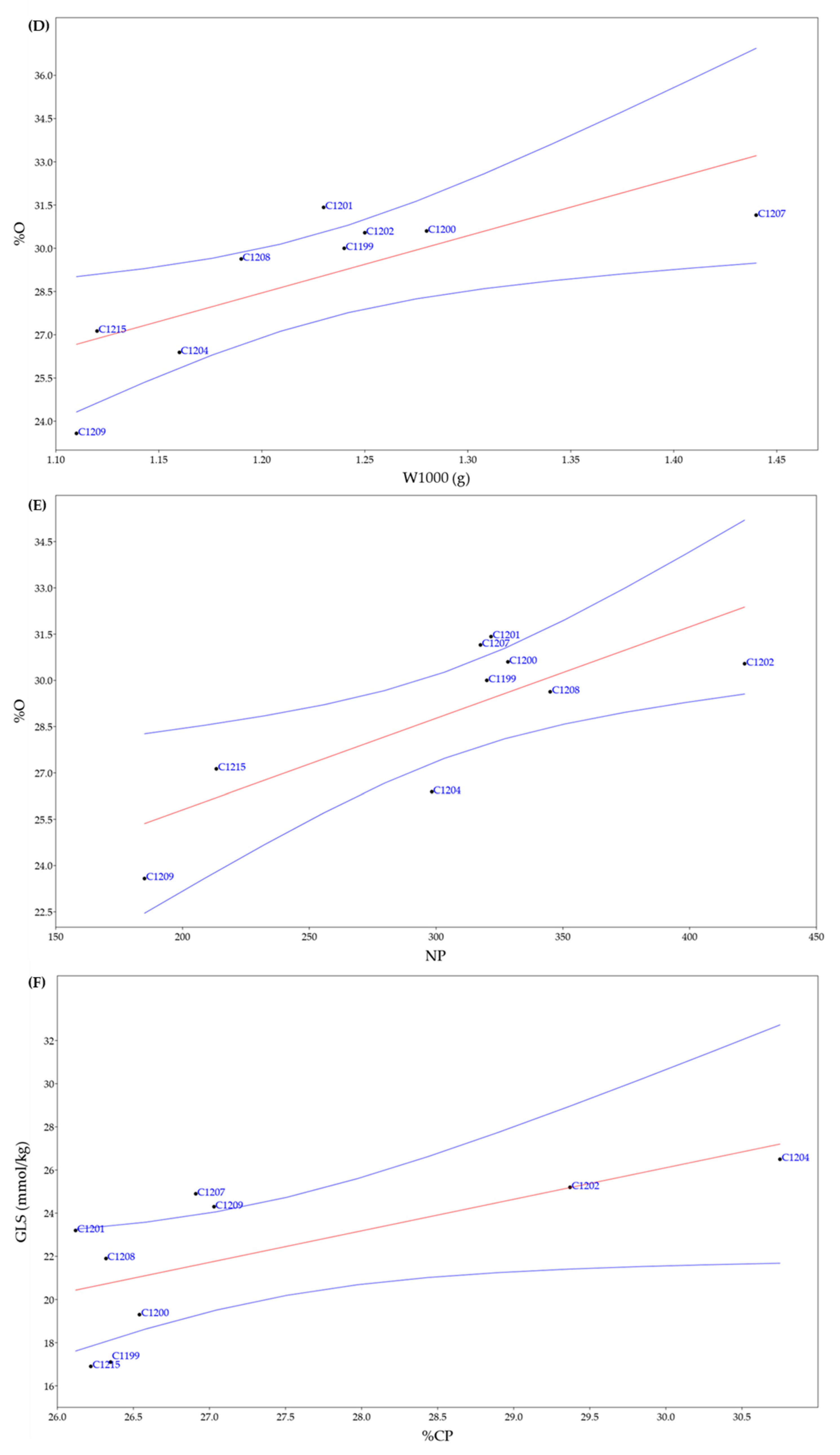
As regards the positive correlations, two of these refer to the percentage of oil in seeds (%O), which was correlated to the weight of the 1000 seeds (W1000) and to the number of plants per m2 (NP); while the other positive correlation was found between the content of glucosinolates (GLS) and the percentage of crude proteins (%CP).
Furthermore, negative correlations were found between agronomic parameters. The first was between the number of siliques per plant (NSP) and the height of the first branch above the ground (BH), the second between the number of seeds per siliqua (NSS) and the number of branches (NB) and the last and most significant between the yield (Y) and the length of the siliques (SL).
Plant height (PH), number of branches (NB), branch height (BH), stem diameter (SD), number of siliqua per plant (NSP), number of seeds per siliqua (NSS), silique length (SL), silique width (SW), weight of 1000 seeds (W1000), number of plants per m2 (NP), yield (Y), dry matter (%DM), ash (%A), crude fibre (%CF), crude protein (%CP), oil (%O), glucosinolates content (GLS).
Figure 5.
Regression plots of statistically significant correlations. (D) weight of 1000 seeds (W1000) and % Oil (%O) (r = 0.74559; p = 0.021102) (E) number of plants per m2 (NP) and % Oil (%O) (r = 0.78185; p = 0.012803); (F) % crude protein (%CP) and glucosinolates content (GLS) (r = 0.66743; p = 0.049508). In blue confidence band for the regression, in red regression line.
Figure 5.
Regression plots of statistically significant correlations. (D) weight of 1000 seeds (W1000) and % Oil (%O) (r = 0.74559; p = 0.021102) (E) number of plants per m2 (NP) and % Oil (%O) (r = 0.78185; p = 0.012803); (F) % crude protein (%CP) and glucosinolates content (GLS) (r = 0.66743; p = 0.049508). In blue confidence band for the regression, in red regression line.
3.5. Multivariate analyses
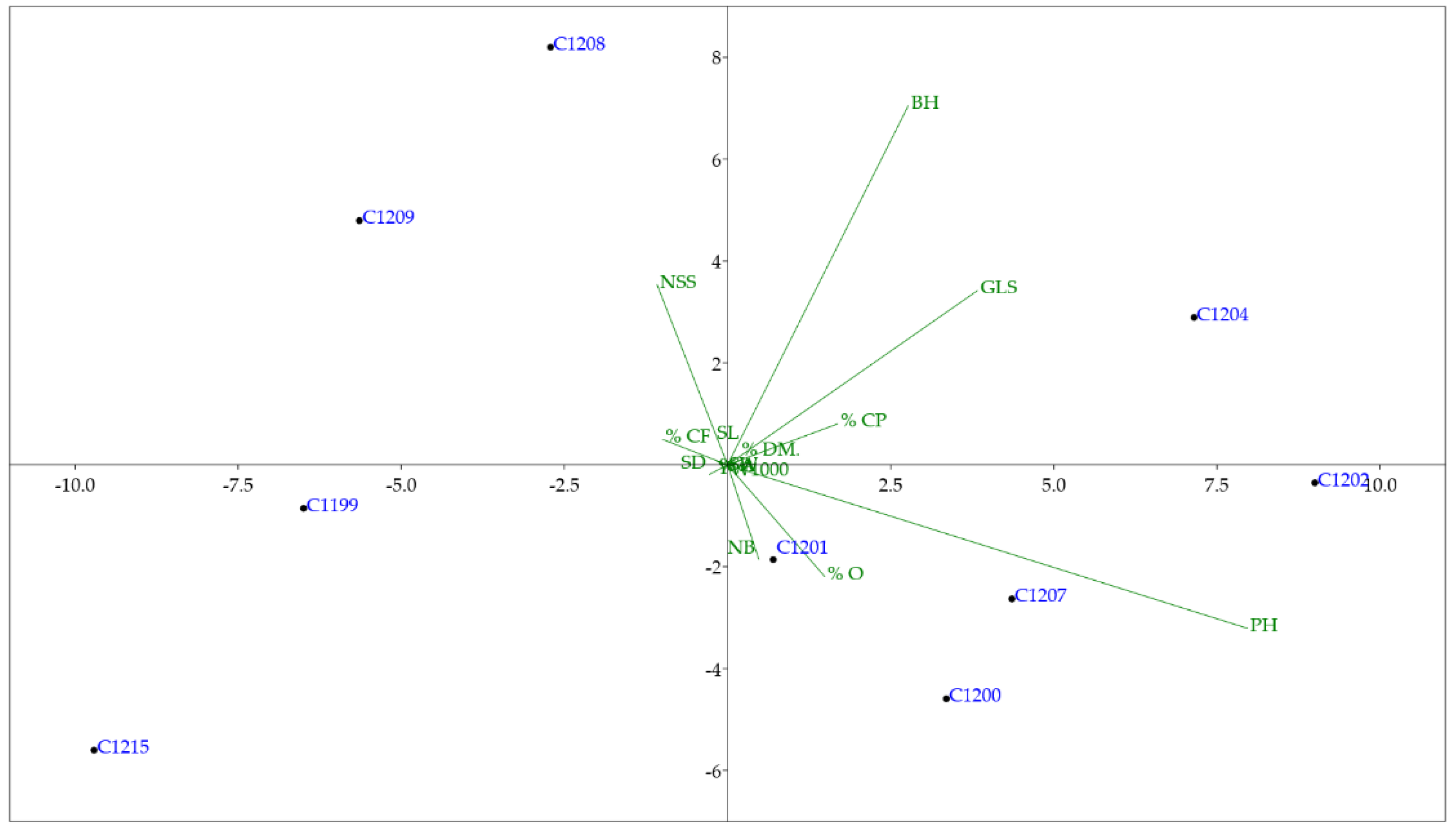
A multivariate analysis was carried out on the parameters of
Figure 3.
In the clustering analysis by imposing k means equal to 2, the two clusters are composed of C1202, C1207, C1200, C1204, C1201 and C1199, C1215, C1209, C1208. Instead, with k means equal to 3, in the first cluster there are C1204, C1202, C1207 and C1201, in the second C1209, C1199, C1215, C1208, while in the third there is the variety C1200.
Figure 6.
Principal components analysis (PCA) obtained using parameters of
Figure 3. In green the main determinants of the clustering.
Figure 6.
Principal components analysis (PCA) obtained using parameters of
Figure 3. In green the main determinants of the clustering.
4. Discussion
After the Second World War, the use of more productive hybrids and the advent of mechanization in agriculture led to a gradual disappearance of secondary crops which were less productive, among these
Camelina sativa. This ancient crop was largely used since the Neolithic age as suggested from archeological evidence [
10].
Nowadays, after a progressive abandonment and replacement with other more profitable oilseed crops such as canola (
Brassica napus L. var. oleifera), camelina is being rediscovered in agricultural production, especially thanks to its adaptability to unfavourable cultivation conditions; this crop, in fact has low need for agricultural inputs, and tolerates and resists cold and drought well; it is also resistant to brassicaceae diseases [
7,
11,
12]. It can also be grown on marginal and poor lands, bringing income thanks to the high value of its oil, rich in polyunsaturated fatty acids with long chains and the protein cake which is a sustainable raw material for diverse applications [
13]. According to Von Cossel et al., 96% of this crop is considered suitable for marginal lands in the Mediterranean area, where other more profitable crops would not be economically sustainable [
14].
Furthermore, in this area, a winter cultivation of camelina performs the useful function of a cover crop, providing agroecological benefits such as soil protection from erosion and prevention of leaching and nitrate runoff [
15]. Moreover, camelina is considered a melliferous plant, and its autumn-winter cultivation with spring flowering at the end of March makes it an early flowering crop useful for bees [
16].
In this scenario, this work aims to evaluate the agronomic performances of eight different genetic lines and a synthetic population in the northern Italian area, comparing two different sowing periods. The data reported in this work regarding yield, do not show statistical differences among the pure line varieties and the synthetic population when cultivated in the spring or winter periods (
Figure 1). However, some differences were registered regarding plant height, number of branches and weight of 1000 seeds. While considering the comparison of yields between spring and winter cultivations, statistically significant differences (p < 0.05) were found among all the materials suggesting that winter cultivation as a cover crop would be the best choice in the northern Italian agro-ecosystem (
Table 4,
Figure 2).
As reported in Angelini and co-authors’ work, in the Mediterranean area, with autumn sowing the crop encounters relatively milder temperatures during seed filling compared to spring sowing, thus favoring the production of seeds with better chemical characteristics [
17]. Many scientific papers state that camelina adapts well to different environments and sowing periods: however, the environmental conditions that most influence the yield and quality of the seeds are temperatures and water. Yield may be limited in years characterized by lower rainfall and/or high temperatures causing thermal stress during the reproductive phase [
18,
19]. Some previous studies have documented the reduction of yield with spring sowing, due to fewer siliques per plant, lower seed weight and reduced branching. Instead, with an autumn sowing, camelina accumulates more biomass thanks to the longer growing season and cooler temperatures [
7].
In Angelini and co-authors’ experiments, the cumulative rainfall received by winter crops was about 460 mm, and by spring crops about 170 mm (2017-2018). In these two years, the rainfall was below the long-term average, however, the amount of water received in the winter harvest exceeded the needs of the camelina.
In fact, the yields in the two different sowing periods resulted in the average production of the crop being respectively 1.6 t/ha in the spring field and 1.9 t/ha in the winter field. Comparing with our results, during the spring field the cumulative rainfall recorded was 147 mm and the yield was 0.6 t/ha, while during the winter field the cumulative rainfall was 404 mm and 2 t/ha the yield (
Table 3). Although the winter yields of Angelini's work were comparable with those obtained in this experiment, the spring yields were from our experiment were lower. These results are probably due to the very low water intake caused by the extremely dry season. Other agronomic differences found such as heights could also be due to the scarcity of water during the periods considered. However, the weights of 1000 seeds are comparable for both seasons (about 1.00 ± 0.03).
With the aim of dissecting yield agronomic parameters useful for future camelina breeding programs we measured: plant height, number of branches, branch height, stem diameter, number of siliques per plant, number of seeds per silique, silique length, silique width, weight of 1000 seeds, number of plants per m
2, reported in
Table 5.
In addition, bromatological analysis on dry matter, ash, crude fibre, crude protein, oil (
Table 6) and glucosinolate analysis (
Table 7) were carried out.
As regards the bromatological analyses, the protein content rate found was about 26% in the varieties C1199, C1200, C1201, C1208 and in the synthetic population C1215, while the highest values were found in C1204 (30.7%) and in C1202 (29.4%). The oil content, on the other hand, recorded the lowest value in the C1209 variety (23.6%) and the highest value in the C1201 variety (31.4%) (
Table 6).
The protein content found agrees with that reported in the literature, in fact, in the work of Pepera and co-authors, the range of protein content is between 20-30%, while the oil content is between 35-43% [
20]. In their work, the oil content can probably be traced to drought and high temperatures, as reported in the work of Brock and co-authors in 2020, who demonstrated that seed oil content can differ within species based on growing conditions, particularly temperature [
21].
Regarding the quantification of glucosinolates (GLS), the genetic materials in our study showed values from 17 to 26.5 mmol/kg (
Table 7), while in the work of Russo et al. the range reported for the species
Camelina sativa was 23−44 mmol/g [
22].
As showed in Jiang and co-authors’ work, the GLS content in camelina is highly dependent on the soil sulfur and nitrogen concentrations. It has been shown how the application of sulfur results in a significant increase of the total GLS while the application of nitrogen was negatively correlated with the GLS seed quantities [
19]. These results suggest that GLS in camelina can be manipulated by cultural management practices such as the application of nitrogen and sulfur.
Taking together all these data, a correlation analysis was performed, and the correlation plot showed six statistically significant correlations (p < 0.05) (
Figure 3).
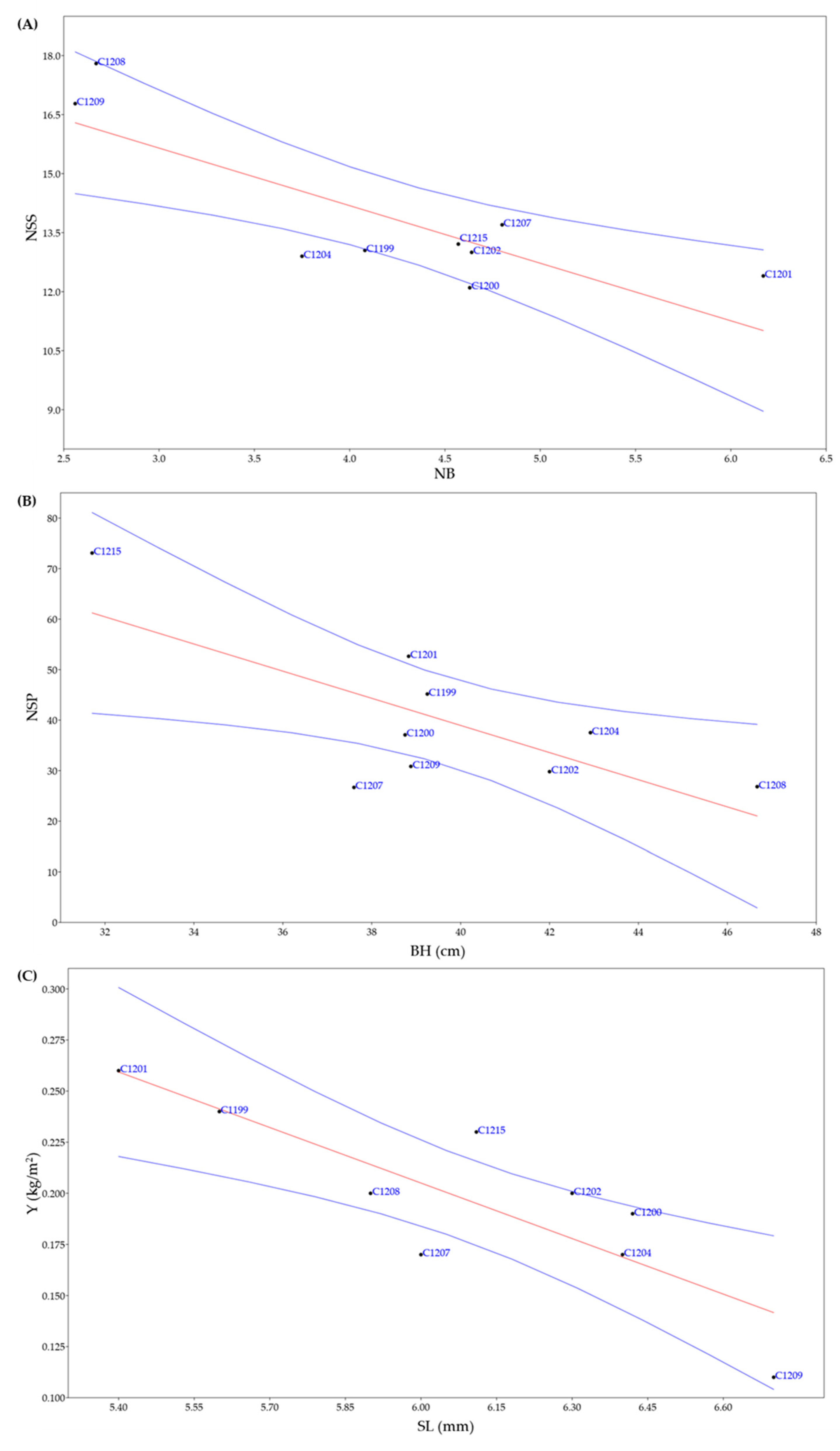
Three of these correlations were negative: the number of branches and the number of seeds per siliqua (r = - 0.81761; p = 0.007103) (
Figure 4A), the branch height and the number of siliques per plant (r = - 0.72895; p = 0.025873) (
Figure 4B), and the silique length and yield (r = - 0.8231; p = 0.0064183) (
Figure 4C).
Regarding the strongest correlation, between the length of the siliqua and the yield (
Figure 4C), Li and co-authors found 8 QTLs (Quantitative trait locus) associated with siliqua length [
23]. In their work, the genetic variation of Camelina sativa was evaluated using 161301 SNPs (Single nucleotide polymorphism) generated by whole genome resequencing, and out of a worldwide collection of 222 accessions it was confirmed that within the species the genetic variability is moderate/low. Furthermore, GWAS (genome-wide association studies) supplemented by linkage mapping, used an RIL (Recombinant inbred line) population consisting of 257 lines and allowed them to identify QTLs associated with several characteristics, in particular with siliqua length. Indeed, as reposted in the Supplementary Materials of Li and co-authors work, QTLs associated with siliqua length were found on the following chromosomes: Chr7 (SNP loc. 14318121; p=6.37E-11; MAF 0.217; effect 0.135), Chr12 (SNP loc. 4754022; p=4.05E-10; MAF 0.481; effect 0.122), Chr13 (SNP loc. 16869038; p=7.30E-10; MAF 0.222; effect -0.192), Chr5 (SNP loc. 16896860; p=3.01E-09; MAF 0.147; effect 0.187), Chr6 (SNP loc. 13707922; p=1.13E-08; MAF 0.451; effect 0.109), Chr16 (SNP loc. 26230101; p=2.10E-08; MAF 0.112; effect 0.189), Chr9 (SNP loc. 7139766; p=1.00E-07; MAF 0.157; effect -0.160) and Chr8 (SNP loc. 10452428; p=1.37E-07; MAF 0.126; effect 0.177). It can be hypothesized that among the 8 QTLs associated with siliqua length there is one or more genes that are linked with the trait yield [
23].
The other three positive correlations found are between: the weight of 1000 seeds and the oil content (r = 0.74559; p = 0.021102) (
Figure 5D), the number of plants per m
2 and oil content (r = 0.78185; p = 0.012803) (
Figure 5E) and the crude protein and glucosinolates content (GLS) (r = 0.66743; p = 0.049508) (
Figure 5F). The first positive correlation reported in
Figure 5D, regarding the percentage of oil in the seeds with the weight of 1000 seeds confirms one of the most important traits to look for in a breeding program to improve yield which is the size of the seed, especially to increase the oil content. Another positive correlation was found between glucosinolates and protein content (
Figure 5F). Glucosinolates are sulfur compounds whose biosynthetic pathway starts from sulfur amino acids, this could explain the correlation found; moreover, in the work of Williams and co-authors, Epithiospecific proteins (ESPs) in broccoli were studied and these non-catalytic cofactors of myrosinase were found to determine the proportion of nitriles produced by hydrolysis of glucosinolates. Their content is related to the content of glucosinolates [
24].
After the correlations analysis, all the collected data were used to perform a multivariate analysis and the main determinants describing clustering that have been highlighted are plant height and glucosinolates content (
Figure 6).
Glucosinolates (sulfur glycosides) are antinutritional compounds that limit the use of camelina cake left over from oil extraction in Europe and the United States. The limits of use are indicated in the European regulation EC Directive 2013/1275, and although in camelina the content of glucosinolates is lower (23−44 mmol/g) compared to other Brassicaceae crops, the quantity exceeds European legislation [
22]. For this reason, breeding programs are trying to lower the content of glucosinolates. In this work, the results obtained from the analysis carried out on these compounds identified, among the plant materials under study, the synthetic population (C1215) as the one with the lowest content (about 17 mmol/kg) as well as the C1199 variety which is a parent of the crossing which originated the synthetic population.
To our knowledge, this is the first work in which a synthetic population of Camelina sativa has been developed and tested. The results obtained, such as the low glucosinolate content, and the intermediate yield in both seasons in which it was tested suggest that the use of the synthetic population is functional in the cultivation of this hexaploid species with low genetic variability in different contexts.
By exploiting the adaptive potential of the synthetic population, the cultivation of camelina could be extended to marginal and/or mountainous areas since it shows tolerance to unfavourable conditions, contributing to yield stability and to abiotic and biotic stresses resistance.
Figure 1.
The plant height (A), number of branches (B), weight of 1000 seeds (C) and the estimated yield (D) of camelina varieties in the spring field. The plant height (E), number of branches (F), weight of 1000 seeds (G) and the estimated yield (H) of camelina varieties in the winter field. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05).
Figure 1.
The plant height (A), number of branches (B), weight of 1000 seeds (C) and the estimated yield (D) of camelina varieties in the spring field. The plant height (E), number of branches (F), weight of 1000 seeds (G) and the estimated yield (H) of camelina varieties in the winter field. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05).
Figure 2.
Regression plot between the yield of spring and winter cultivation (r = -0.65565; p = 0.0552). In blue confidence band for the regression, in red regression line.
Figure 2.
Regression plot between the yield of spring and winter cultivation (r = -0.65565; p = 0.0552). In blue confidence band for the regression, in red regression line.
Figure 3.
Correlation plot. Negative correlations in red, positive correlations in blue. Gray box on statistically significant correlations p< 0.05.
Figure 3.
Correlation plot. Negative correlations in red, positive correlations in blue. Gray box on statistically significant correlations p< 0.05.
Figure 4.
Regression plots of statistically significant negatives correlations. (A) Number of branches (NB) and number of seeds per siliqua (NSS) (r = - 0.81761; p = 0.007103); (B) branch height (BH) and number of siliques per plant (NSP) (r = - 0.72895; p = 0.025873); (C) silique length (SL) and yield (Y) (r = - 0.8231; p = 0.0064183). In blue confidence band for the regression, in red regression line.
Figure 4.
Regression plots of statistically significant negatives correlations. (A) Number of branches (NB) and number of seeds per siliqua (NSS) (r = - 0.81761; p = 0.007103); (B) branch height (BH) and number of siliques per plant (NSP) (r = - 0.72895; p = 0.025873); (C) silique length (SL) and yield (Y) (r = - 0.8231; p = 0.0064183). In blue confidence band for the regression, in red regression line.
Table 1.
Camelina sativa genotype and their respective ID codes tested in the study.
Table 1.
Camelina sativa genotype and their respective ID codes tested in the study.
| Name varieties |
Genetic constitution |
Code |
| Calena |
Pure line |
C1201 |
| Omich |
Pure line |
C1204 |
| Madalina |
Pure line |
C1202 |
| Experimental material |
Pure line |
C1199 |
| Experimental material |
Pure line |
C1200 |
| Experimental material |
Pure line |
C1207 |
| Experimental material |
Pure line |
C1208 |
| Experimental material |
Pure line |
C1209 |
| Experimental material |
Synthetic population
(C1199 X C1204) |
C1215 |
Table 2.
Soil analysis of the experimental field used for this study. SD are shown (n=3). (Modified from Landoni et al. 2020).
Table 2.
Soil analysis of the experimental field used for this study. SD are shown (n=3). (Modified from Landoni et al. 2020).
| Parameters |
Values |
| pH (H2O) |
6.5 ± 0.01 |
| Organic matter |
14.2 ± 0.12 g/kg |
| Sand |
56.2 ± 0.37% |
| Coarse Silt |
14.6 ± 0.52% |
| Fine Silt |
22.6 ± 1.53% |
| Clay |
6.6 ± 0.63% |
| P available (Bray/Kurtz) |
136 ± 1.44 mg/kg |
| Cation Exchange Capacity |
10.6 ± 0.24 cmol/kg |
| K exchangeable |
432 ± 8.16 mg/kg |
| Mg exchangeable |
229 ± 9.40 mg/kg |
| Ca exchangeable |
1331 ± 22.6 mg/kg |
| Estimated water holding capacity |
24.3 ± 1.59% |
Table 3.
Cumulative rainfall and average monthly temperatures relating to the spring cultivation and the winter cultivation.
Table 3.
Cumulative rainfall and average monthly temperatures relating to the spring cultivation and the winter cultivation.
| Year |
Month |
Monthly rainfall (mm) |
Mean monthly temperature (°C) |
| 2021 |
April |
70.6 |
13.22 |
| May |
60.8 |
18.67 |
| June |
15.8 |
26.03 |
| 2021 |
October |
52 |
14.12 |
| November |
165.6 |
9.49 |
| December |
46 |
3.11 |
| 2022 |
January |
28 |
3.18 |
| February |
16 |
7.59 |
| March |
9.8 |
9.13 |
| April |
25 |
14.44 |
| May |
62 |
22.47 |
Table 4.
Comparison of yields between spring cultivation (S) and winter cultivation (W) of Camelina sativa benchmark (C1199, C1200, C1201 and C1204) and the synthetic population (C1215). For each parameter measured, the asterisk indicates statistically significant differences of the trait between the spring field and the winter field (Tukey’s test, p < 0.05).
Table 4.
Comparison of yields between spring cultivation (S) and winter cultivation (W) of Camelina sativa benchmark (C1199, C1200, C1201 and C1204) and the synthetic population (C1215). For each parameter measured, the asterisk indicates statistically significant differences of the trait between the spring field and the winter field (Tukey’s test, p < 0.05).
| ID code |
Cultivation period |
Trait |
| |
|
Yield
(g/m2) |
Estimated Yield (kg/ha) |
|
| C1199 |
S |
59.68 ± 15.01 |
596.83 ± 150 |
|
| W |
240.18 ± 36.46 * |
2401.83 ± 365 * |
|
| C1200 |
S |
56.39 ± 20.14 |
563.92 ± 201 |
|
| W |
192.18 ± 21.20 * |
1921.83 ± 212 * |
|
| C1201 |
S |
45.64 ± 10.06 |
456.38 ± 101 |
|
| W |
258.93 ± 59.93 * |
2589.33 ± 599 * |
|
| C1202 |
S |
60.47 ± 20.01 |
604.66 ± 200 |
|
| W |
204.77 ± 69.43 * |
2047.67 ± 694 * |
|
| C1204 |
S |
70.46 ± 20.02 |
704.55 ± 200 |
|
| W |
167.02 ± 13.19 * |
1670.17 ± 132 * |
|
| C1215 |
S |
63.98 ± 15.10 |
639.83 ± 151 |
|
| W |
228.42 ± 9.03 * |
2284.20 ± 903 * |
|
| Total |
S |
59.43 ± 16.36 |
594.36 ± 164 |
|
| W |
202.22 ± 58.57 * |
2022.22 ± 586 * |
|
Table 5.
Agronomic characterization of Camelina sativa benchmark (C1199, C1200, C1201, C1202, C1204, C1207, C1208 and C1209) and the synthetic population (C1215) in winter cultivation. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Plant height (PH), number of branches (NB), branch height (BH), stem diameter (SD), number of siliques per plant (NSP), number of seeds per silique (NSS), silique length (SL), silique width (SW), weight of 1000 seeds (W1000), number of plants per m2 (NP) and the yield (Y).
Table 5.
Agronomic characterization of Camelina sativa benchmark (C1199, C1200, C1201, C1202, C1204, C1207, C1208 and C1209) and the synthetic population (C1215) in winter cultivation. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Plant height (PH), number of branches (NB), branch height (BH), stem diameter (SD), number of siliques per plant (NSP), number of seeds per silique (NSS), silique length (SL), silique width (SW), weight of 1000 seeds (W1000), number of plants per m2 (NP) and the yield (Y).
| Traits |
ID code |
| |
C1199 |
C1200 |
C1201 |
C1202 |
C1204 |
C1207 |
C1208 |
C1209 |
C1215 |
| PH (cm) |
70.85 ± 12.66 a
|
81.00 ± 5.50 a
|
75.83 ± 6.37 a
|
83.55 ± 9.28 a
|
81.25 ± 5.82 a
|
80.18 ± 12.64 a
|
71.10 ± 20.44 a
|
69.78 ± 9.31 a
|
70.14 ± 7.45 a
|
| NB |
4.08 ± 2.14 ab
|
4.63 ± 2.07 ab
|
6.17 ± 4.36 a
|
4.64 ± 2.06 ab
|
3.75 ± 1.60 ab
|
4.80 ± 1.99 ab
|
2.67 ± 1.32 ab
|
2.56 ± 1.81 b
|
4.57 ± 1.90 ab
|
| BH (cm) |
39.25 ± 9.76 a
|
38.75 ± 5.39 a
|
38.83 ± 9.06 a
|
42.00 ± 10.73 a
|
42.92 ± 10.15 a
|
37.60 ± 12.84 a
|
46.67 ± 6.71 a
|
38.88 ± 6.10 a
|
31.71 ± 10.93 b
|
| SD (mm) |
2.74 ± 0.41 a
|
2.25 ± 0.63 a
|
2.76 ± 0.82 a
|
2.08 ± 0.62 a
|
1.97 ± 0.73 a
|
2.05 ± 0.47 a
|
1.90 ± 0.45 a
|
2.66 ± 1.02 a
|
2.36 ± 0.59 a
|
| NSP |
45.17 ± 6.85 a
|
37.08 ± 4.09 a
|
52.64 ± 12.18 a
|
29.80 ± 10.10 a
|
37.52 ± 2.96 a
|
26.68 ± 3.75 a
|
26.82 ± 3.75 a
|
30.84 ± 3.67 a
|
41.57 ± 20.96 a
|
| NSS |
13.05 ± 3.34 b
|
12.10 ± 3.11 b
|
12.40 ± 3.20 b
|
13.00 ± 2.62 b
|
12.90 ± 3.73 b
|
13.70 ± 2.45 ab
|
17.80 ± 2.20 a
|
16.78 ± 2.78 a
|
13.21 ± 3.05 b
|
| SL (mm) |
5.60 ± 1.01 b
|
6.42 ± 0.81 a
|
5.40 ± 0.70 b
|
6.30 ± 0.95 ab
|
6.40 ± 0.52 ab
|
6.00 ± 0.29 ab
|
5.90 ± 0.74 ab
|
6.70 ± 0.81 a
|
6.11 ± 0.89 ab
|
| SW (mm) |
3.05 ± 0.22 c
|
3.30 ± 0.46 a
|
2.30 ± 0.48 b
|
3.00 ± 0.11 ac
|
2.90 ± 0.35 c
|
3.00 ± 0.11 ac
|
3.00 ± 0.15 ac
|
3.00 ± 0.14 c
|
2.85 ± 0.47 c
|
| W1000 (g) |
1.24 ± 0.08 cd
|
1.28 ± 0.03 d
|
1.23 ± 0.03 cd
|
1.25 ± 0.03 cd
|
1.16 ± 0.05 bc
|
1.44 ± 0.04 a
|
1.19 ± 0.03 bd
|
1.11 ± 0.02 b
|
1.24 ± 0.04 cd
|
| NP |
320 ± 77.62 ab
|
328.33 ± 35.11 ab
|
321.67 ± 79.43 ab
|
421.67 ± 85.20 a
|
298.33 ± 46.46 ab
|
317.50 ± 29.30 ab
|
345.00 ± 50.00 ab
|
185 ± 56.35 b
|
213.33 ± 65.26 b
|
| Y (g/m2) |
240.18 ± 36.46 a
|
192.18 ± 21.20 ab
|
258.93 ± 59.93 a
|
204.77 ± 69.43 ab
|
167.02 ± 13.19 ab
|
167.48 ± 25.83 ab
|
195.98 ± 27.41 ab
|
111.77 ± 13.30 b
|
228.42 ± 9.03 a
|
| Y (kg/ha) |
2401.83 ± 365 a
|
1921.83 ± 212 ab
|
2589.33 ± 599 a
|
2047.67 ± 694 ab
|
1670.17 ± 132 ab
|
1674.83 ± 258 ab
|
1959.83 ± 274 ab
|
1117.67 ± 133 b
|
1502.50 ± 758 a
|
Table 6.
Compositional analyses carried out on Camelina sativa seeds. The nutrient composition is expressed on a dry matter (DM) basis. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Dry matter (%DM), ash (%A), crude fibre (%CF), crude protein (%CP), oil (%O).
Table 6.
Compositional analyses carried out on Camelina sativa seeds. The nutrient composition is expressed on a dry matter (DM) basis. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Dry matter (%DM), ash (%A), crude fibre (%CF), crude protein (%CP), oil (%O).
| ID Code |
%DM |
%AC |
%CF |
%CP |
%O |
| C1199 |
95.21 ± 0.95 a
|
4.27 ± 0.02 bd
|
24.49 ± 1.31 a
|
26.35 ± 1.47 b |
29.99 ± 1.00 ac |
| C1200 |
95.56 ± 0.22 a |
4.37 ± 0.02 ab
|
20.01 ± 0.52 b
|
26.54 ± 1.06 b
|
30.61 ± 0.58 a
|
| C1201 |
95.61 ± 0.31 a |
4.55 ± 0.04 a
|
20.22 ± 0.77 b
|
26.12 ± 1.54 b |
31.43 ± 0.78 a |
| C1202 |
95.31 ± 0.30 a
|
4.25 ± 0.05 b
|
21.05 ± 1.33 ab |
29.37 ± 0.26 ab |
30.54 ± 1.37 a |
| C1204 |
95.48 ± 0.20 a |
4.28 ± 0.06 bc |
24.04 ± 1.70 a
|
30.75 ± 1.26 a
|
26.39 ± 0.78 bd |
| C1207 |
95.39 ± 0.29 a |
4.28 ± 0.12 bc |
21.79 ± 0.19 ab
|
26.91 ± 1.16 b
|
31.15 ± 0.30 a
|
| C1208 |
95.62 ± 0.27 a |
4.46 ± 0.07 acd |
22.74 ± 1.82 ab
|
26.32 ± 0.21 b |
29.63 ± 1.49 ac |
| C1209 |
95.05 ± 0.85 a
|
4.54 ± 0.03 a
|
21.16 ± 0.47 ab
|
27.03 ± 1.87 b |
23.58 ± 1.70 b
|
| C1215 |
95.08 ± 0.35 a |
4.55 ± 0.12 a |
23.91 ± 1.75 a
|
26.22 ± 1.55 b
|
27.13 ± 0.88 cd |
Table 7.
Glucosinolates analyses carried out on Camelina sativa seeds. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Glucosinolates content (GLS).
Table 7.
Glucosinolates analyses carried out on Camelina sativa seeds. For each parameter measured, different letters indicate statistically significant differences (Tukey’s test, p < 0.05). Glucosinolates content (GLS).
| ID Code |
GLS (mmol/kg) |
| C1199 |
17.10 ± 0.03 bc
|
| C1200 |
19.25 ± 0.07 c
|
| C1201 |
23.21 ± 0.19 de
|
| C1202 |
25.15 ± 1.22 ad
|
| C1204 |
26.52 ± 0.35 a
|
| C1207 |
24.88 ± 1.50 ad
|
| C1208 |
21.90 ± 0.40 e
|
| C1209 |
24.29 ± 0.11 ad
|
| C1215 |
16.92 ± 1.22 b
|