1. Introduction
Rice (Oryza sativa L.) is a major crop that provides food to more than half of the global population. The global food crisis has become a serious issue and is predicted to worsen with the increasing global population over the next 20 years (Beukert et al. 2017; Zhao et al. 2015). Hybrid breeding is an effective way to manage the world food crisis, and hybrid rice breeding has been very successful in the past several decades, producing rice varieties with the potential to yield ~20% more than the inbred varieties in field production (Tu et al. 2000). Two hybrid production systems are used in rice breeding, namely the two-line system and three-line system, and they are widely used in hybrid seed production (Cheng et al. 2007; Kim and Zhang 2018). In general, elite restorers are beneficial for the F1 generation in hybrid breeding; therefore, identification and creation of excellent restorer cultivars plays an important role in hybrid seed production, which directly influences the final grain yield (Ouyang et al. 2022; Hussain et al. 2022).
Nitrogen is an important macronutrient essential for plant growth and development and has a strong influence on crop productivity (Singh et al. 2016; Iqbal et al. 2020). To improve crop grain yield, the application of synthetic N fertilizers has increased in recent decades, but the utilization of the applied N by crops is lower than 50% because of the low N use efficiency (NUE) (Ahrens et al. 2010; Zhu et al. 2020). Improving crop NUE is crucial for hybrid seed production and would be beneficial for farmers as well as for rice breeding (Ahrens et al. 2010; Chen et al. 2014; Dobermann and Cassman 2005; Guo et al. 2017; Xu et al. 2012). Guaranteeing food security and sustainable agricultural development using resource-saving and environmentally friendly methods has become a strategic concern over the past decades (Yu et al. 2022). Notably, “Green Super Rice” (GSR) was proposed for rice breeding and production based on rice functional genomics research (Zhang 2007; Yu et al. 2022). Many rice cultivars with different green traits have been identified and developed in the past few years, providing a solid foundation for modern rice breeding and production. Therefore, improving crop NUE while maintaining crop productivity has several economic and environmental benefits (Shin et al. 2018).
Genome-wide expression analysis is a powerful approach for analyzing complex traits such as NUE. RNA sequencing (RNA-seq), a high-throughput sequencing technology, is the best approach used in studies on rice, as high-quality and convenient genome information is available for rice (Wang et al. 2009; Mandal et al. 2022). In recent years, many studies investigated the rice transcriptome under various N conditions using large-scale datasets to identify N-responsive differential genes and pathways (Bi et al. 2009; Yang et al. 2015; Sinha et al. 2018; Cai et al. 2012). However, these studies were mostly limited to some transgenic lines with key genes identified in previous studies and focused on ordinary rice materials, which did not provide an accurate description of transcriptome-wide responses to changes in N availability. Our previous study carried out screening and primary identification of different rice cultivar resources in China, the results showed that different rice cultivars exhibit various characteristics respond to nitrate using NO3- as nitrogen source in hydroponic condition (Xiao et al. 2016). Among these cultivars, Nanhui511 (NH511) was characterized as a sensitive cultivar and Minghui23 (MH23) is an insensitive cultivar under different nitrogen supplies (Xiao et al. 2016). In the present study, we further investigated the physiological changes in two rice cultivars, NH511 and MH23, under different nitrogen supply conditions and combined multiple RNA-seq analyses to assess their transcriptomic variations, focusing on two indica restorer cultivars that responded to high (HN) and low nitrogen (LN) supplies. Our combined physiological and transcriptomic analysis of rice restorers revealed their N-responsive characteristics, improved our understanding of the regulatory activities involved in the N response, and provided new insights into high NUE breeding using elite restorers in rice.
2. Materials and methods
2.1. Rice materials and growth conditions
The seeds of two rice cultivars (Oryza sativa cv. Nanhui511 (NH511) and Minghui23 (MH23)) were germinated in Murashige & Skoog (MS) media and then transferred to tap water before being transferred into a hydroponic solution (1.8 mM KCl, 0.36 mM CaCl2, 0.54 mM MgSO4, 0.32 mM KH2PO4, 40μM Fe (II)-EDTA, 18.8μM H3BO3, 13.4 μM MnCl2, 0.32μM CuSO4, 0.3μM ZnSO4, 0.03μM Na2MoO4 and 1.6 mM Na2SiO3) supplied with KNO3 as the only N source. Then, the seedlings were divided into two groups and transferred to HN and LN conditions for six days of cultivation. The seedlings were cultivated in a growth chamber under a photoperiod of 12 h/12 h (light/dark) (~230 μmol m-2 s-1) at 28 °C/25 °C, and the solution was renewed every 3 d. For the LN and HN treatment assays, the seedlings were transferred to LN (0.2 mM KNO3) and HN solutions (5 mM KNO3), respectively. For NUE analysis, the seedlings were transferred to non-nitrogen fertilizer (0mM KNO3) and nitrogen fertilizer treatment (5 mM KNO3) respectively.
2.2. RNA isolation and transcriptomic analysis
Total cellular RNA was isolated from the samples treated for 3 h and grown under HN and LN conditions using the TRIzol method (Invitrogen, USA), with three biological replications used for this assay. Subsequently, RNA samples from across replications were pooled in equimolar concentration and then delivered to Biomarker Technologies (Beijing, China) for library construction. Separate libraries for each nitrogen treatment (HN and LN), variety (NH511 and MH23) was constructed using Truseq RNA Sample prep kit (Illumina, Woodslang, Singapore) according to the manufacturer’s protocol. Thus, a total of four libraries were constructed and each library was represented by three biological replications. The libraries were sequenced using paired end Illumina (HiseqTM 2500) sequencing technology. Differential expression analyses of the two conditions were performed using the DESeq R package (version 1.10.1). DESeq provides statistical routines for determining differential expression in digital gene expression data, using a model based on a negative binomial distribution. The resulting P values were adjusted using Benjamini and Hochberg’s approach to control for the false discovery rate. Genes with an adjusted p<0.05 found by DESeq were assigned as differentially expressed.
2.3. Quantitative RT-PCR
A total of 5 μg of RNA were isolated and analyzed firstly. After checking the quality and spectrophotometric quantification, 2μg RNA were converted into cDNA using M-MLV Reverse Transcriptase (Invitrogen, USA) following the manufacture’s protocol. Each sample was analyzed in triplicate using at least two cDNA preparations by qRT-PCR and TaKaRa SYBR Pre-mix Ex-TaqII reagent kits were employed to real-time PCR and the data obtained by Roche Light Cycler 480 system (Roche, Switzerland). The specificity of the reactions was verified by carrying out melting (dissociation) curve analysis. The expression variation in the N-related genes of NH511and MH23 were calculated and
ACTIN1 were used as endogenous control. The primers used in this experiment are listed in
Supplementary Table S1.
2.4. Enzyme activity, nitrogen content, and NUE analysis
Fifteen-day-old hydroponically grown seedlings were used for the nitrate reductase (NR) and glutamine synthase (GS) activity assays. The maximal in vitro activity of nitrate reductase was measured according to a previously reported method (Ferrario-Mery et al. 1998), and an enzyme assay kit (Grace Biotechnology, Suzhou, China) was used to analyze GS activity. The total N content was measured by Grace Biotechnology, and the NUE was calculated using the following equation (Shen et al. 2021):
NUE (%) = (TNF-TN0)/N × 100
where TN0 is the total nitrogen content of plants in the non-nitrogen fertilizer treatment group, TNF is the total nitrogen content of plants in the nitrogen fertilizer treatment group, and N is the total amount of nitrogen applied.
2.5. Chlorate sensitivity assay
The seedlings grown in 2 mM KNO3 Kimura B solution for 5 d were treated with 2 mM chlorate for 3 d and subsequently recovered in 2 mM KNO3 provided in Kimura B solution for 5 d. The survival rate of the control and treated seedlings was then calculated (Zhang et al. 2022).
2.6. Field trial of rice cultivars
In 2019 and 2020, NH511 and MH23 plants were grown in Chongqing (E106°, N29°) for field tests using different N supplies. The plants were planted in 10 rows, with 30 plants per row in each plot, and three replicates were used for each N condition. Urea was used as the N fertilizer at the concentrations of 80 kg N hm-2 for the low N (LN) and 500 kg N hm-2 for the high N treatment (HN). The plants at the edge of each plot were omitted from the analyses to avoid margin effects.
2.7. Agronomic trait analyses
Agronomic traits, including plant height, tiller numbers, 1,000-grain weight, and grain yield were measured according to a previous study (Hu et al. 2015).
3. Results
3.1. Morphological variations in NH511 and MH23 grown under HN and LN conditions
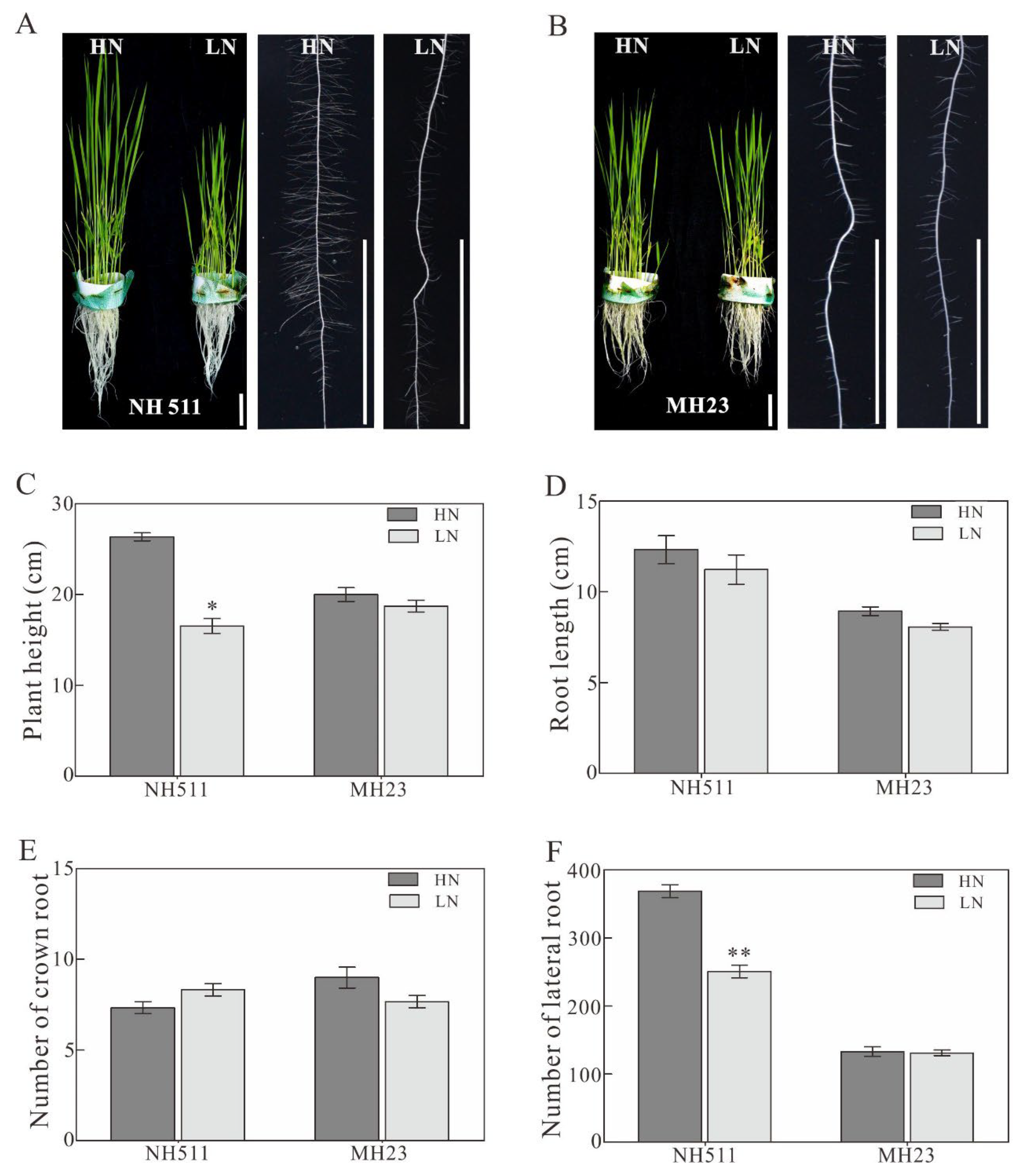
Our previous study showed that different rice materials exhibit different morphological and physiological variations when grown under different N supplies. In the present study, based on previous large-scale screening and preliminary results, we selected two typical rice restorers, namely NH511 and MH23, for further analysis. To fully understand their different growth responses to different N supplies, we first germinated them and cultivated them under LN conditions for seven days. Then, the seedlings were divided into two groups and transferred to HN and LN conditions for six days of cultivation. We found that NH511 is more sensitive than MH23 to HN supply conditions. Notably, compared to, NH511 exhibited faster growth and increased lateral root number and length under HN conditions; however, the MH23 phenotype showed no obvious differences in these characteristics (
Figure 1A and B). Moreover, our statistical data also showed a significant difference in plant height and number of lateral roots between NH511 plants treated with HN and those treated with LN (
Figure 1C and F), but not in crown root number and length (
Figure 1D and E). Taken together, these results demonstrated that unlike MH23, NH511 is a nitrogen-responsive restorer, which promotes its seedling growth by regulating lateral development and plant height.
3.2. NH511 shows better nitrogen absorption efficiency based on chlorate assays
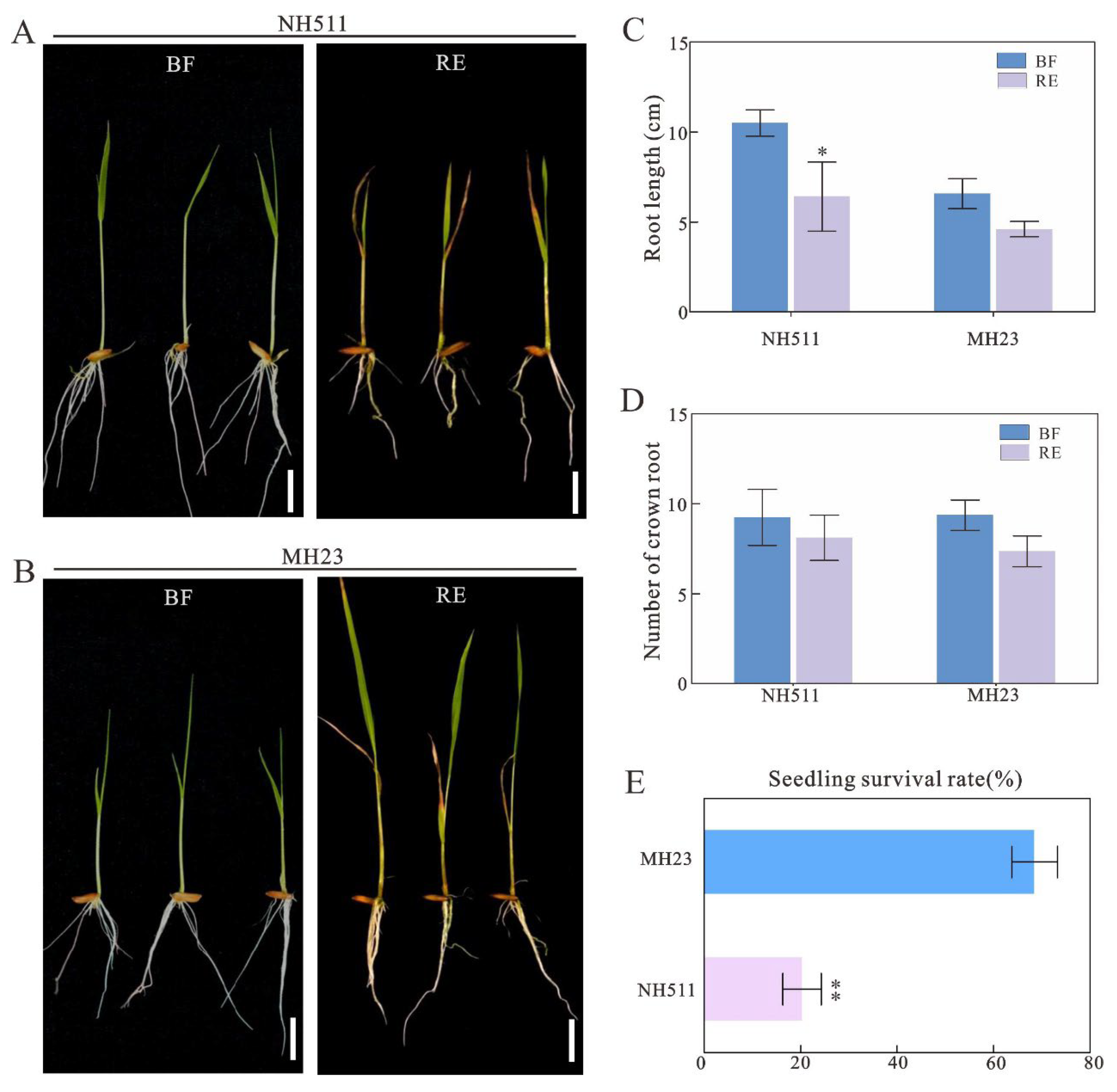
The above results of growth response to nitrate prompted us to further analyze whether NH511 modulates nitrate uptake and assimilation more efficiently than that of MH23 under the same conditions. To analyze this, we first germinated the seeds of NH511and MH23 and grown them under normal nitrogen conditions for five days. Subsequently, we transferred the seedlings into a chlorate-containing hydroponic solution for the treatment assay. When the plants were transferred to normal conditions for recovery growth, we found that NH511 exhibited retarded growth compared to that of MH23 (
Figure 2A and B). Furthermore, we analyzed the root length, which was significantly different in NH511 before and after treatment (
Figure 2C), but the number of roots did not change under the same conditions (
Figure 2D). Additionally, survival assay data showed significant difference between two cultivars that NH511 exhibited a lower survival rate (~20%) than that of MH23 (~70%) (
Figure 2E). Overall, the chlorate assay data suggested that unlike MH23, NH511 has excellent nitrogen absorption efficiency for seedling growth, which broadens our understanding of the differences among rice restorer cultivars and provides a basis for high NUE rice breeding.
3.3. Effect of different N supplies on nitrogen-metabolizing enzymes
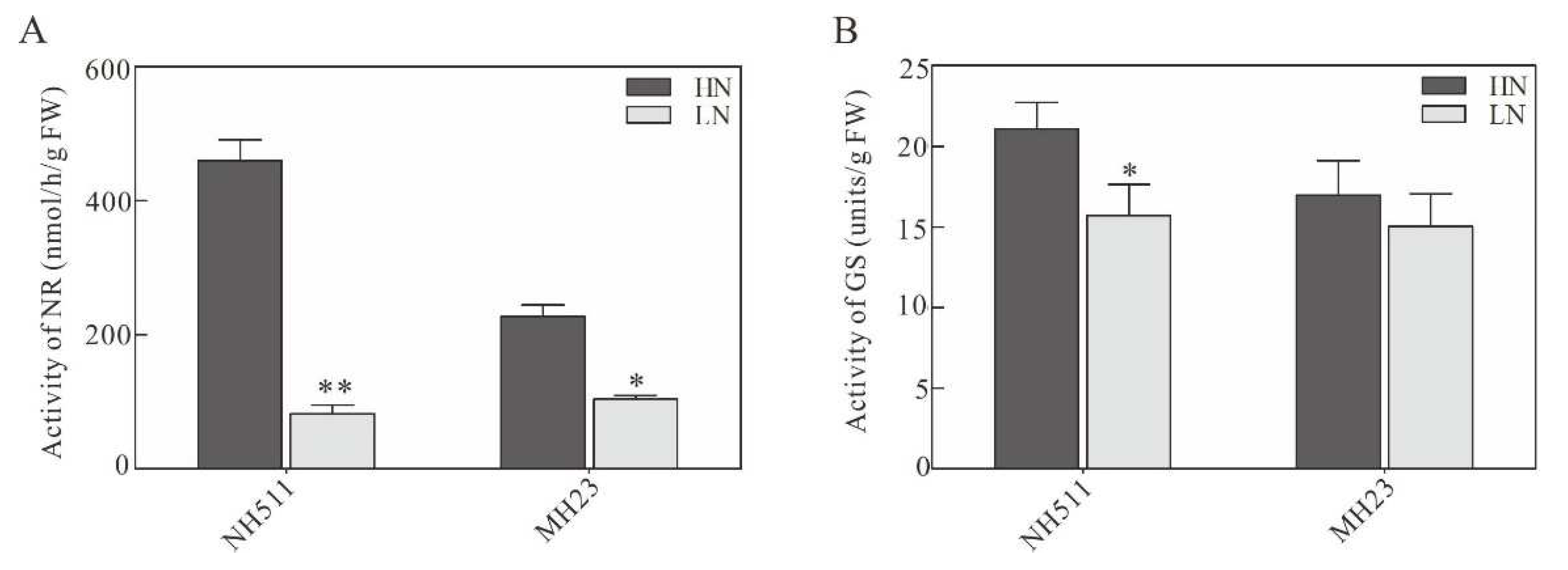
The nitrogen use efficiency in rice depends on the activity of enzymes related to nitrogen utilization. To understand whether the activity of these enzymes increases under HN conditions in NH511, we further analyzed the activity of nitrate reductase (NR) and glutamine synthase (GS) in NH511 and MH23 under HN and LN conditions. Results of the enzyme activity analysis showed that both NH511 and MH23 had a low activity of NR under LN conditions, which improved significantly under HN conditions, with NH511 having a higher NR activity in the HN treatment than in the LN treatment (
Figure 3A). Similarly, in NH511, GS activity also significantly increased under HN conditions compared to that under LN conditions; however, there was no significant change in the GS activity of MH23 between the two treatments (
Figure 3B). Furthermore, soluble protein content was not significantly different under different N supplies in both NH511 and MH23 (
Figure S1). These results implied that NH511 also improved its N assimilation by increasing nitrogen-metabolizing enzymes under HN conditions.
3.4. Nitrogen content and NUE under different N supply concentrations
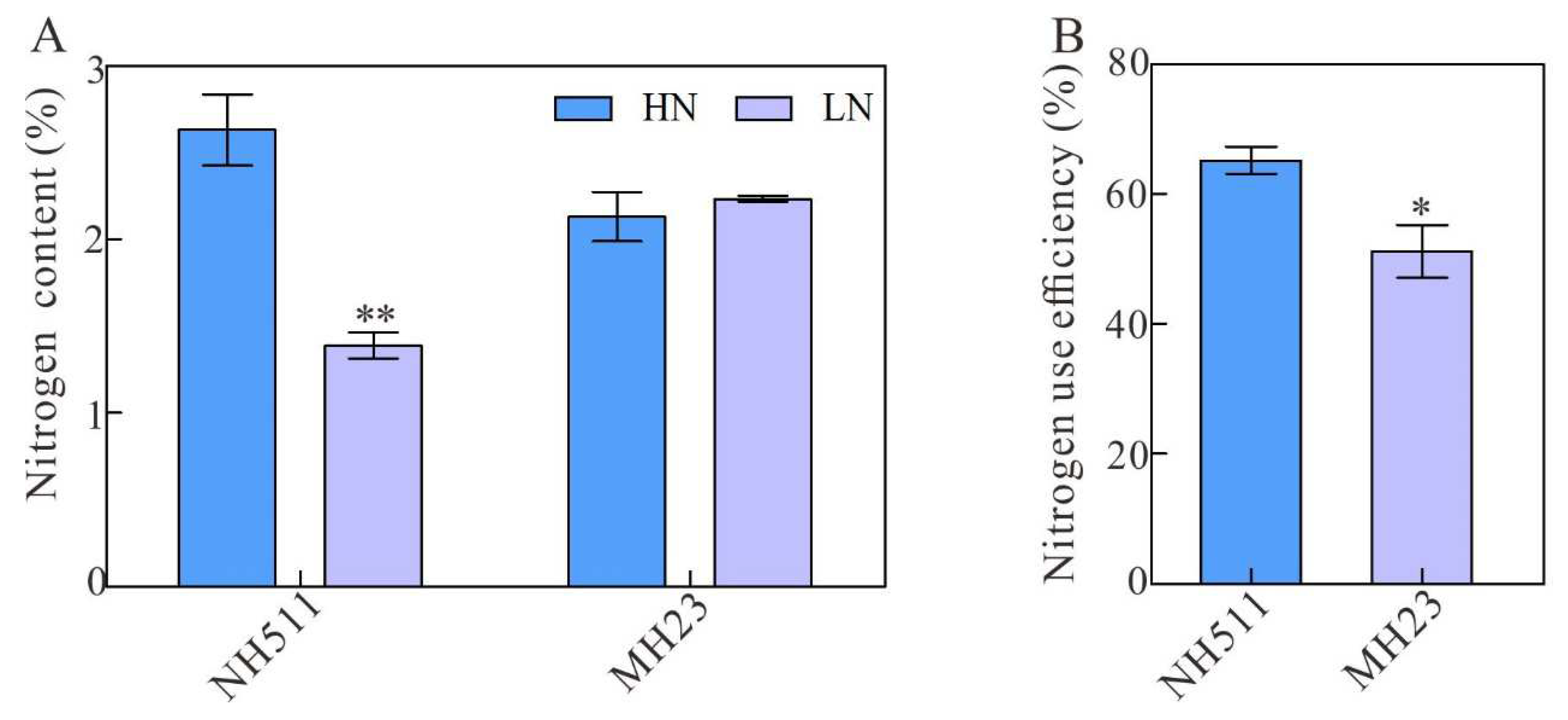
Based on the above results, we concluded that under HN conditions, NH511 is a nitrogen-responsive cultivar, whereas MH23 is not. To investigate whether NH511 accumulates more total nitrogen and exhibits higher NUE when transferred into the HN conditions, the total N content and NUE of seedlings of both cultivars were analyzed. The results showed that NH511 can accumulate more nitrogen than MH23 (
Figure 4A) and exhibits significant difference between these two cultivars, including NH511 showed higher NUE than that of MH23 under the same conditions (
Figure 4B). Similarly, the Nitrogen uptake efficiency of NH511 also exhibits higher than that of MH23 under the same conditions (
Figure S2). Overall, these results further demonstrated that NH511 is sensitive to different N levels and promotes its growth by increasing its nitrogen uptake and NUE and enhancing the activity of its metabolizing enzymes.
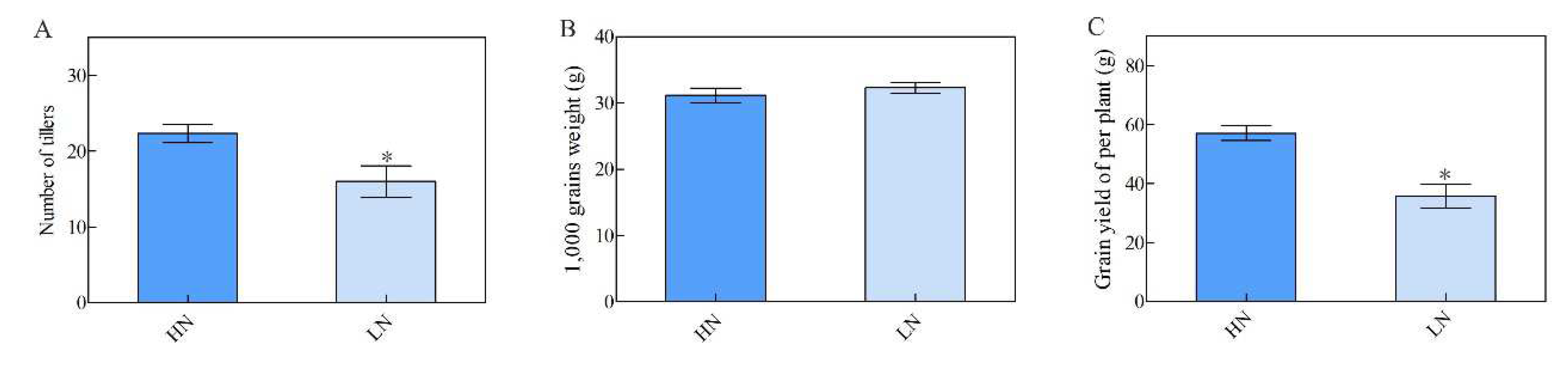
3.5. NH511 improves grain yield by increasing the number of tillers under high N supply
To evaluate the performance of NH511 in the field, we designed a field trial for a period of two years and collected data under HN and LN conditions. Our data showed that the grain yield of NH511 increased under HN conditions, as indicated by an increase in its tiller number (
Figure 5A and C); however, its 1,000-grain weight did not change under HN conditions (
Figure 5B). Furthermore, our field test data indicated that NH511 should be used in rice breeding as it possesses the capacity for high production under high N supply, similar to that of seedlings grown in a hydroponic solution. More importantly, these data provide a foundation for the selection of elite rice restorers and for high NUE rice breeding.
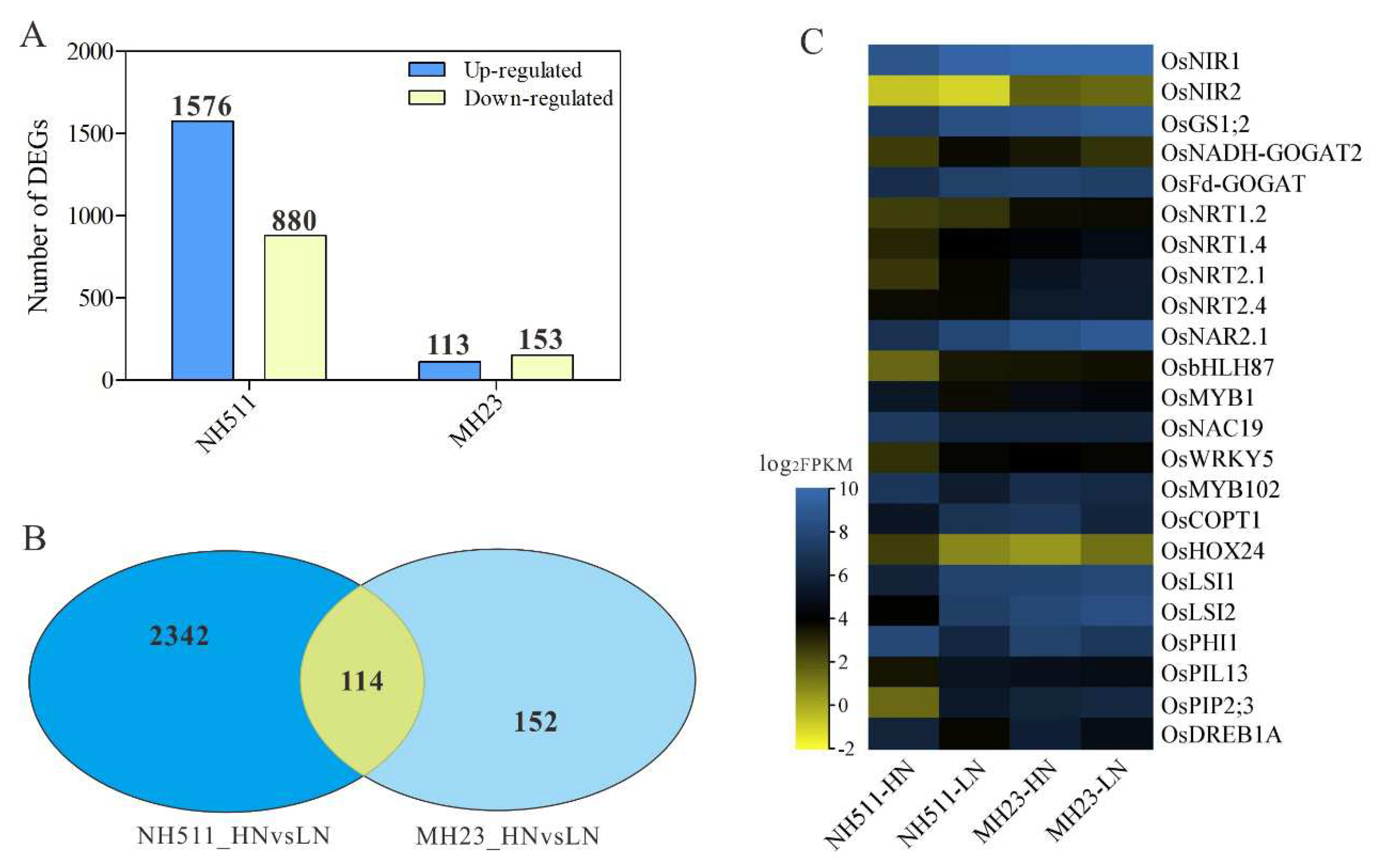
3.6. Differential transcriptome signature underlies the variation in nitrogen metabolism between NH511 and MH23
The observed difference in the performance of NH511 and MH23 under HN and LN conditions prompted us to further investigate the variations between the two cultivars. Based on transcriptomic data, differential expression analyses were performed using the DESeq, which provides statistical routines for determining differential expression in digital gene expression data, using a model based on a negative binomial distribution. The results showed that under HN conditions, a total of 2456 genes were differentially expressed in NH511, including 1576 upregulated and 880 downregulated genes (
Figure 6A and S3A). Notably, under HN conditions, we identified only 266 differentially expressed genes (DEGs) in MH23, including 113 upregulated and 153 downregulated genes (
Figure 6A and S3B). Moreover, 114 DEGs were identified in both NH511 and MH23 under HN conditions (
Figure 6B). All DEGs were categorized into three groups: biological process (BP), cellular component (CC), and molecular function (MF) (
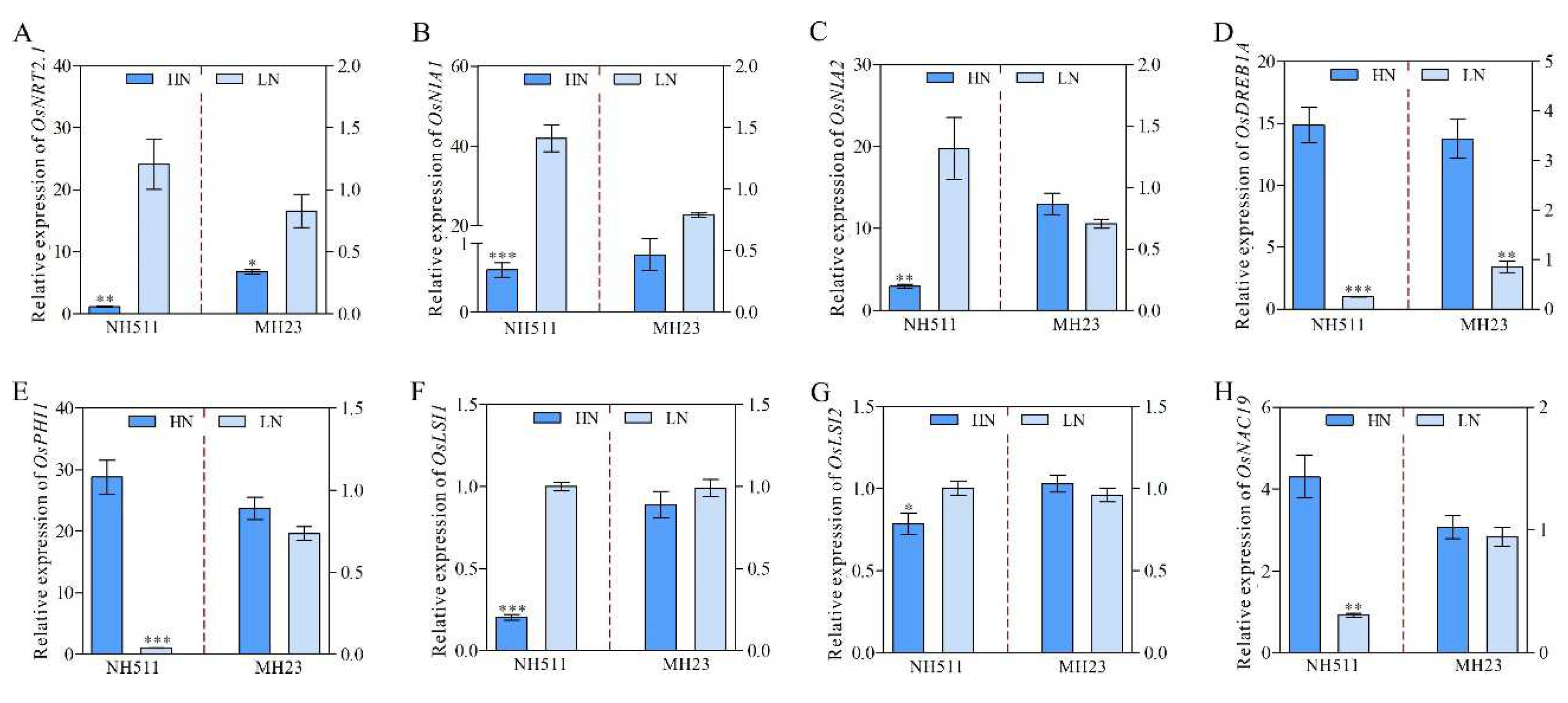
Figure S4A and B). These DEG data showed that most of the DEGs were involved in metabolic activity and stimulus response of biological processes under HN conditions. Additionally, the heat map data revealed that under HN conditions, nitrogen utilization genes and some transport-related genes, such as NRT1.2, NRT1.4, NIA1, NIA2, NAC19 and so on, exhibited obvious differential expression in NH511 but not in MH23 (
Figure 6C). These DEGs were further confirmed by real-time PCR and results showed the significant differential expression variations in NH511 under nitrogen supplies (
Figure 7). Taken together, the transcriptome data further elucidated the difference between NH511 and MH23 in their response to different N supplies, especially their differential gene expression, which provides possibilities for the identification of key NH511 genes exhibiting an N response and high NUE in future rice breeding.
4. Discussion
Nitrogen is an essential mineral nutrient required for plant growth and development, particularly in crops (Hakeem et al. 2011; Kabange et al. 2021). Its deficiency triggers extensive physiological and biochemical changes in plants, and a low nitrogen utilization rate has become one of the main abiotic factors affecting crop growth (Wang et al. 2019; Cai et al. 2012). Excess N is inevitably leached into the underground water system and lost to the atmosphere, resulting in severe environmental problems (Cai et al. 2012). A previous study showed that soil acidification in China is mainly a consequence of high-N fertilizer inputs (Guo et al. 2010). Nevertheless, reducing the use of N fertilizers and cultivating high NUE cultivars are efficient ways to solve environmental problems and ensure adequate crop production.
Rice is one of the most important cereal crops and provides food for half of the world’s population. In China, breeding of semi-dwarf rice varieties led to an increase in rice yield from 2·0 t ha-2 in the 1960s to 3·5 t ha-2 in the 1970s (Cheng et al. 2007). China has been successful in breeding hybrid rice in the past decades but is now also facing challenges in developing new hybrids with high-yielding potential, better grain quality, and tolerance to biotic and abiotic stresses (Cheng et al. 2007; Han et al. 2007; Tian et al. 2007; Chen et al. 2022; Li et al. 2022). Hybrid rice has been successfully developed with a three-line system based on a male sterile line, a maintainer line, and a restorer line, which has greatly contributed to world food production in the past decades (Jiang et al. 2022; Hussain et al. 2022; ElShamey et al. 2022). Nanhui 511 (NH511) was derived from the hybridization between two heavy panicle-type restorer lines, namely Shuhui 881 and Shuhui 527, which exhibit good combining ability and acceptable grain quality (Jie et al. 2005). NH511 has been widely used in hybrid seed production as a restorer line and has been used to breed some hybrid rice cultivars in the past. Minghui23 (MH23) was derived from the hybridization between two restorer lines, namely IRBB23 and Minghui 63, the latter being a well-known indica rice restorer line that has been widely applied to hybrid rice seed production in China (Luo et al. 2003; Zhang et al. 2002).
The results of our previous study revealed that different rice cultivars originating from all over China exhibit diversity in nitrogen utilization, according to large-scale screening and N supply analysis (Xiao et al. 2016). In the present study, we selected two typical indica restorer lines, namely NH511 and MH123, to further investigate this topic and provide new insights into the N utilization of rice restorer lines and high NUE rice breeding. Our analysis of morphological variations and nitrogen-metabolizing enzymes of NH511 and MH23 grown under HN and LN conditions showed that NH511 is a nitrogen-responsive restorer line whose seedling growth is promoted by the regulation of lateral root development and plant height (
Figure 1 and
Figure 3). The chlorate assays and NUE analysis results showed that compared with MH23, NH511 had a high nitrate absorption rate at the seedling stage and showed excellent performance in field tests (
Figure 2,
Figure 4, and
Figure 5). Finally, the transcriptome analysis demonstrated a variation signature between NH511 and MH23 in response to different N supply concentrations, and differential genes were further confirmed by real-time PCR (
Figure 7). Altogether, our results revealed a physiological and differential gene expression variation between the two restorer lines, showing that NH511 is an excellent rice restorer line because of its high NUE, good combining ability, and acceptable grain quality. However, we do not know which genes caused the increase in the number and length of lateral roots in NH511 under HN conditions, which is worthy of further study to uncover the molecular mechanism underlying this lateral root growth. Additionally, the mechanism underlying high NUE in NH511 can be elucidated more specifically using other advanced biotechnologies, particularly those related to gene identification, as well as by further functional analyses. Finally, the high NUE genes identified in NH511 could be used for rice breeding, which would not only help solve certain environmental problems, but also ensure crop production.
5. Conclusions
We investigated the physiological and transcriptomic changes in two indica restorer lines (NH511 and MH23) under high (HN) and low nitrogen (LN) conditions. Based on our findings, we discussed and compared the N-responsive characteristics of the two cultivars and their differentially expressed genes under HN conditions. Compared to MH23, NH511 accumulated more nitrogen and had higher nitrogen use efficiency under the same conditions. We described the physiological mechanisms underlying these differences (namely regulation of lateral development and plant height) and concluded that NH511 is an excellent rice restorer line. We believe that our study makes a significant contribution to rice breeding because we described the regulatory activities involved in the N response in two rice restorer lines, indicating their further uses and perspectives for future studies.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1. Content of soluble proteins under HN and LN conditions. Figure S2. Nitrogen uptake efficiency of NH511 and MH23 under HN supplies. Figure S3. Volcano plot of DEGs identified under HN supplies. Figure S4. Histogram description of Gene Ontology enrichment of DEGs. Table S1. qRT-PCR primers used in this study.
Author Contributions
Xiaojian Qin: Conceptualization, Writing–original draft, Supervision, Funding acquisition. Hanma Zhang: Analyzed the data, Supervision, Funding acquisition. Yongshu Liang: Performed the experiments, Formal analysis. Wenbin Nan: Performed the experiments, Formal analysis. Xiaowei Li: Performed the experiments. Juan Xiao: Performed the experiments. Qian Wu: Performed the experiments. Yuntong Li: Performed the experiments. Cuiping Li: Performed the experiments. Dan Jiang: Performed the experiments. Tingting Tang: Performed the experiments. All the authors read and approved the final manuscript.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31800238), the Natural Science Foundation of Chongqing (cstc2019jcyj-msxmX0224) and the Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJQN201900509 and KJQN202100536).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahrens TD, Lobell DB, Ortiz-Monasterio JI, Li Y, Matson PA (2010) Narrowing the agronomic yield gap with improved nitrogen use efficiency: a modeling approach. Ecol Appl 20 (1):91-100. [CrossRef]
- Beukert U, Li Z, Liu G, Zhao Y, Ramachandra N, Mirdita V, Pita F, Pillen K, Reif JC (2017) Genome-Based Identification of Heterotic Patterns in Rice. Rice (N Y) 10 (1):22. [CrossRef]
- Bi YM, Kant S, Clarke J, Gidda S, Ming F, Xu J, Rochon A, Shelp BJ, Hao L, Zhao R, Mullen RT, Zhu T, Rothstein SJ (2009) Increased nitrogen-use efficiency in transgenic rice plants over-expressing a nitrogen-responsive early nodulin gene identified from rice expression profiling. Plant Cell Environ 32 (12):1749-1760. [CrossRef]
- Cai H, Lu Y, Xie W, Zhu T, Lian X (2012) Transcriptome response to nitrogen starvation in rice. J Biosci 37 (4):731-747. [CrossRef]
- Chen R, Deng Y, Ding Y, Guo J, Qiu J, Wang B, Wang C, Xie Y, Zhang Z, Chen J, Chen L, Chu C, He G, He Z, Huang X, Xing Y, Yang S, Xie D, Liu Y, Li J (2022) Rice functional genomics: decades' efforts and roads ahead. Sci China Life Sci 65 (1):33-92. [CrossRef]
- Chen S, Wang D, Xu C, Ji C, Zhang X, Zhao X, Zhang X, Chauhan BS (2014) Responses of super rice (Oryza sativa L.) to different planting methods for grain yield and nitrogen-use efficiency in the single cropping season. PLoS One 9 (8):e104950. [CrossRef]
- Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY (2007) Progress in research and development on hybrid rice: a super-domesticate in China. Ann Bot 100 (5):959-966. [CrossRef]
- Dobermann A, Cassman KG (2005) Cereal area and nitrogen use efficiency are drivers of future nitrogen fertilizer consumption. Sci China C Life Sci 48 Spec No:745-758.
- ElShamey EAZ, Sakran RM, ElSayed MAA, Aloufi S, Alharthi B, Alqurashi M, Mansour E, Abd El-Moneim D (2022) Heterosis and combining ability for floral and yield characters in rice using cytoplasmic male sterility system. Saudi J Biol Sci 29 (5):3727-3738. [CrossRef]
- Ferrario-Mery S, Valadier MH, Foyer CH (1998) Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol 117 (1):293-302. [CrossRef]
- Guo J, Hu X, Gao L, Xie K, Ling N, Shen Q, Hu S, Guo S (2017) The rice production practices of high yield and high nitrogen use efficiency in Jiangsu, China. Sci Rep 7 (1):2101. [CrossRef]
- Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KW, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327 (5968):1008-1010. [CrossRef]
- Hakeem KR, Ahmad A, Iqbal M, Gucel S, Ozturk M, Neeraja CN, Barbadikar KM, Krishnakanth T, Bej S, Rao IS, Srikanth B, Rao DS, Subrahmanyam D, Rao PR, Voleti SR (2011) Nitrogen-efficient rice cultivars can reduce nitrate pollution Down regulation of transcripts involved in selective metabolic pathways as an acclimation strategy in nitrogen use efficient genotypes of rice under low nitrogen. Environ Sci Pollut Res Int 18 (7):1184-1193. [CrossRef]
- . [CrossRef]
- Han B, Xue Y, Li J, Deng XW, Zhang Q (2007) Rice functional genomics research in China. Philos Trans R Soc Lond B Biol Sci 362 (1482):1009-1021. [CrossRef]
- Hu B, Wang W, Ou S, Tang J, Li H, Che R, Zhang Z, Chai X, Wang H, Wang Y, Liang C, Liu L, Piao Z, Deng Q, Deng K, Xu C, Liang Y, Zhang L, Li L, Chu C (2015) Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat Genet 47 (7):834-838. [CrossRef]
- Hussain I, Ali S, Liu W, Awais M, Li J, Liao Y, Zhu M, Fu C, Liu D, Wang F (2022) Identification of Heterotic Groups and Patterns Based on Genotypic and Phenotypic Characteristics Among Rice Accessions of Diverse Origins. Front Genet 13:811124. [CrossRef]
- 2020; Iqbal A, Qiang D, Zhun W, Xiangru W, Huiping G, Hengheng Z, Nianchang P, Xiling Z, Meizhen S, Zhang X, Li F, Ding Y, Ma Q, Yi Y, Zhu M, Ding J, Li C, Guo W, Zhu X (2020) Growth and nitrogen metabolism are associated with nitrogen-use efficiency in cotton genotypes.
- Transcriptome Analysis of Two Near-Isogenic Lines with Different NUE under Normal Nitrogen Conditions in Wheat. Plant Physiol Biochem 149 (8):61-74. [CrossRef]
- 10.1016/j.plaphy.2020.02.002.
- Jiang H, Lu Q, Qiu S, Yu H, Wang Z, Yu Z, Lu Y, Wang L, Xia F, Wu Y, Li F, Zhang Q, Liu G, Song D, Ma C, Ding Q, Zhang X, Zhang L, Zhang X, Li X, Zhang J, Xiao J, Li X, Wang N, Ouyang Y, Zhou F, Zhang Q (2022) Fujian cytoplasmic male sterility and the fertility restorer gene OsRf19 provide a promising breeding system for hybrid rice. Proc Natl Acad Sci U S A 119 (34):e2208759119. [CrossRef]
- Jie RS, Liu FP, Yang CH, He P, MAO Jin-xiong (2005) Breeding of New Rice Restorer Line Nanhui 511 with High Combining Ability. Hybrid Rice, 2005, 20(5): 15-16.
- Kabange NR, Park SY, Lee JY, Shin D, Lee SM, Kwon Y, Cha JK, Cho JH, Duyen DV, Ko JM, Lee JH (2021) New Insights into the Transcriptional Regulation of Genes Involved in the Nitrogen Use Efficiency under Potassium Chlorate in Rice (Oryza sativa L.). Int J Mol Sci 22 (4). [CrossRef]
- Kim YJ, Zhang D (2018) Molecular Control of Male Fertility for Crop Hybrid Breeding. Trends Plant Sci 23 (1):53-65. [CrossRef]
- Li P, Chen YH, Lu J, Zhang CQ, Liu QQ, Li QF (2022) Genes and Their Molecular Functions Determining Seed Structure, Components, and Quality of Rice. Rice (N Y) 15 (1):18. [CrossRef]
- Luo LG, Xu JF, Zhai HQ, Wan JM. Analysis of photoperiod-sensitivity genes in Minghui63, an restorer line of indica rice (Oryza sativa L.). Yi Chuan Xue Bao. 2003 Sep;30(9):804-10. Chinese. PMID: 14577370.
- Mandal VK, Jangam AP, Chakraborty N, Raghuram N, Jagadhesan B, Sathee L, Meena HS, Jha SK, Chinnusamy V, Kumar A, Kumar S (2022) Nitrate-responsive transcriptome analysis reveals additional genes/processes and associated traits viz. height, tillering, heading date, stomatal density and yield in japonica rice.
- Genome wide analysis of NLP transcription factors reveals their role in nitrogen stress tolerance of rice. Planta 255 (2):42. [CrossRef]
- Ouyang Y, Li X, Zhang Q (2022) Understanding the genetic and molecular constitutions of heterosis for developing hybrid rice. J Genet Genomics 49 (5):385-393. [CrossRef]
- Shen C, Chen K, Cui Y, Chen J, Mi X, Zhu S, Zhu Y, Ali J, Ye G, Li Z, Xu J (2021) QTL Mapping and Favorable Allele Mining of Nitrogen Deficiency Tolerance Using an Interconnected Breeding Population in Rice. Front Genet 12:616428. [CrossRef]
- Shin SY, Jeong JS, Lim JY, Kim T, Park JH, Kim JK, Shin C (2018) Transcriptomic analyses of rice (Oryza sativa) genes and non-coding RNAs under nitrogen starvation using multiple omics technologies. BMC Genomics 19 (1):532. [CrossRef]
- Singh A, Kumar P, Gautam V, Rengasamy B, Adhikari B, Udayakumar M, Sarkar AK (2016) Root transcriptome of two contrasting indica rice cultivars uncovers regulators of root development and physiological responses. Sci Rep 6:39266. [CrossRef]
- Sinha SK, Sevanthi VA, Chaudhary S, Tyagi P, Venkadesan S, Rani M, Mandal PK (2018) Transcriptome Analysis of Two Rice Varieties Contrasting for Nitrogen Use Efficiency under Chronic N Starvation Reveals Differences in Chloroplast and Starch Metabolism-Related Genes. Genes (Basel) 9 (4). [CrossRef]
- Tian XH, Tsutomu M, Li SH, Lin JC (2007) [High temperature stress on rice anthesis: research progress and prospects]. Ying Yong Sheng Tai Xue Bao 18 (11):2632-2636.
- Tu J, Zhang G, Datta K, Xu C, He Y, Zhang Q, Khush GS, Datta SK (2000) Field performance of transgenic elite commercial hybrid rice expressing bacillus thuringiensis delta-endotoxin. Nat Biotechnol 18 (10):1101-1104. [CrossRef]
- Wang J, Song K, Sun L, Qin Q, Sun Y, Pan J, Xue Y (2019) Morphological and Transcriptome Analysis of Wheat Seedlings Response to Low Nitrogen Stress. Plants (Basel) 8 (4). [CrossRef]
- Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10 (1):57-63. [CrossRef]
- Xiao J, Yan HH, Yang YQ, Liang YS, Nan WB, Zhang HM, Qin XJ (2016). Screening and research of different rice (Oryza sativa) varieties based on nitrate absorption and utilization in seedlings. Plant Physiology Journal 2016, 52 (12): 1941-1949. [CrossRef]
- Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153-182. [CrossRef]
- Yang W, Yoon J, Choi H, Fan Y, Chen R, An G (2015) Transcriptome analysis of nitrogen-starvation-responsive genes in rice. BMC Plant Biol 15:31. [CrossRef]
- Yu S, Ali J, Zhou S, Ren G, Xie H, Xu J, Yu X, Zhou F, Peng S, Ma L, Yuan D, Li Z, Chen D, Zheng R, Zhao Z, Chu C, You A, Wei Y, Zhu S, Gu Q, He G, Li S, Liu G, Liu C, Zhang C, Xiao J, Luo L, Li Z, Zhang Q (2022) From Green Super Rice to green agriculture: Reaping the promise of functional genomics research. Mol Plant 15 (1):9-26. [CrossRef]
- Zhang Q (2007) Strategies for developing Green Super Rice. Proc Natl Acad Sci U S A 104 (42):16402-16409. [CrossRef]
- Zhang ZS, Xia JQ, Alfatih A, Song Y, Huang YJ, Sun LQ, Wan GY, Wang SM, Wang YP, Hu BH, Zhang GH, Qin P, Li SG, Yu LH, Wu J, Xiang CB (2022) Rice NIN-LIKE PROTEIN 3 modulates nitrogen use efficiency and grain yield under nitrate-sufficient conditions. Plant Cell Environ 45 (5):1520-1536. [CrossRef]
- Zhang H, Wang Y, Deng C, Zhao S, Zhang P, Feng J, Huang W, Kang S, Qian Q, Xiong G, Chang Y. High-quality genome assembly of Huazhan and Tianfeng, the parents of an elite rice hybrid Tian-you-hua-zhan. Sci China Life Sci. 2022 Feb;65(2):398-411. . Epub 2021 Jun 28. PMID: 34251582. [CrossRef]
- Zhao Y, Li Z, Liu G, Jiang Y, Maurer HP, Wurschum T, Mock HP, Matros A, Ebmeyer E, Schachschneider R, Kazman E, Schacht J, Gowda M, Longin CF, Reif JC (2015) Genome-based establishment of a high-yielding heterotic pattern for hybrid wheat breeding. Proc Natl Acad Sci U S A 112 (51):15624-15629. [CrossRef]
- Zhu S, Liu L, Xu Y, Yang Y, Shi R (2020) Application of controlled release urea improved grain yield and nitrogen use efficiency: A meta-analysis. PLoS One 15 (10):e0241481. [CrossRef]
Figure 1.
Phenotype variations of NH511 and MH23 under HN and LN conditions. A, performance of NH511under HN and LN supplies. Scale bar=3cm. B, performance of MH23 under HN and LN supplies. C, plant height. D, statistical analysis of root length in NH511 and MH23 respectively. E, the number of crown root analysis in NH511 and MH23 respectively. F, the number of lateral roots in NH511 and MH23 respectively. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 1.
Phenotype variations of NH511 and MH23 under HN and LN conditions. A, performance of NH511under HN and LN supplies. Scale bar=3cm. B, performance of MH23 under HN and LN supplies. C, plant height. D, statistical analysis of root length in NH511 and MH23 respectively. E, the number of crown root analysis in NH511 and MH23 respectively. F, the number of lateral roots in NH511 and MH23 respectively. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 2.
Chlorate assays for NH511 and MH23. A, phenotype of NH511under 2mM chlorate treatment. BF, before treatment, RE, recovery, Scale bar=3cm. B, phenotype of NH511under 2mM chlorate treatment. C, Root length analysis of before and after treatment. D, statistical analysis for the number of crown root. E, statistical analysis of survival rate between NH511 and MH23. The statistical analysis of comparing BF with RE was performed by t-tests in NH511 and MH23 respectively **, P<0.01; *, P<0.05.
Figure 2.
Chlorate assays for NH511 and MH23. A, phenotype of NH511under 2mM chlorate treatment. BF, before treatment, RE, recovery, Scale bar=3cm. B, phenotype of NH511under 2mM chlorate treatment. C, Root length analysis of before and after treatment. D, statistical analysis for the number of crown root. E, statistical analysis of survival rate between NH511 and MH23. The statistical analysis of comparing BF with RE was performed by t-tests in NH511 and MH23 respectively **, P<0.01; *, P<0.05.
Figure 3.
Analysis of NR and GS activity under HN and LN conditions respectively. A, NR activity measurement under HN and LN supplies. B, GS activity analysis under HN and LN supplies. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 3.
Analysis of NR and GS activity under HN and LN conditions respectively. A, NR activity measurement under HN and LN supplies. B, GS activity analysis under HN and LN supplies. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 4.
Measurement of nitrogen content and NUE under different N supplies. A, nitrogen content of NH511 and MH23 under HN and LN respectively. B, statistical analysis of NUE between NH511 and MH23 under HN supplies. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 4.
Measurement of nitrogen content and NUE under different N supplies. A, nitrogen content of NH511 and MH23 under HN and LN respectively. B, statistical analysis of NUE between NH511 and MH23 under HN supplies. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. **, P<0.01; *, P<0.05.
Figure 5.
Agronomic traits of NH511 in the field test. A, the number of tillers was analyzed under HN and LN respectively. B, 1,000 grains weight analysis under HN and LN condition respectively. C, statistical analysis of grain yield per plant in NH511. The statistical analysis of comparing HN with LN was performed by t-tests. *, P<0.05.
Figure 5.
Agronomic traits of NH511 in the field test. A, the number of tillers was analyzed under HN and LN respectively. B, 1,000 grains weight analysis under HN and LN condition respectively. C, statistical analysis of grain yield per plant in NH511. The statistical analysis of comparing HN with LN was performed by t-tests. *, P<0.05.
Figure 6.
Comparative transcriptome analysis for NH511 and MH23 under HN supplies. A, DEGs numbers of NH511 and MH23 under HN supplies. B, number of the common differential genes in NH511 and MH23 under HN and LN supplies. C, heat map analysis of significant differential expressed genes that related to N and ion transportation. The heat map represents the relative expression levels based on FPKM values.
Figure 6.
Comparative transcriptome analysis for NH511 and MH23 under HN supplies. A, DEGs numbers of NH511 and MH23 under HN supplies. B, number of the common differential genes in NH511 and MH23 under HN and LN supplies. C, heat map analysis of significant differential expressed genes that related to N and ion transportation. The heat map represents the relative expression levels based on FPKM values.
Figure 7.
Real-time PCR validation of partial differential genes in NH511 and MH23. A, the expression level of OsNRT2.1 under HN and LN supplies. B, the expression level of OsNIA1. C, the expression level of OsNIA2. D, the expression level of OsDREB1A. E, the expression level of OsPHI1. F, the expression level of OsLSI1. G, the expression level of OsLSI2. H, the expression level of OsNAC19. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. ***, P<0.001; **, P<0.01; *, P<0.05.
Figure 7.
Real-time PCR validation of partial differential genes in NH511 and MH23. A, the expression level of OsNRT2.1 under HN and LN supplies. B, the expression level of OsNIA1. C, the expression level of OsNIA2. D, the expression level of OsDREB1A. E, the expression level of OsPHI1. F, the expression level of OsLSI1. G, the expression level of OsLSI2. H, the expression level of OsNAC19. The statistical analysis of comparing HN with LN was performed by t-tests in NH511 and MH23 respectively. ***, P<0.001; **, P<0.01; *, P<0.05.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).