1. Introduction
As of March 10, 2023, there have been 676,609,955 cases of COVID-19; mortality estimates worldwide and in the U.S. are 6,881,955 and 1,123,836 respectively [
1]. Additionally, a
significant proportion of COVID-19 survivors experience a variety of ongoing symptoms also known as post COVID syndrome, “long-haul” COVID [
2]
or post acute sequelae of COVID-19 (PASC). Fatigue, brain fog, and sleep disturbances are amongst the commonly reported symptoms [
3]
. However, there are relatively few reports of the prevalence, evolution and ultimate outcomes of sleep disturbances in long-haul COVID[
4,
5,
6]
. Even if only 10% of those with long-haul COVID develop a sleep disorder, there would be an enormous social and economic impact. Therefore, documentng the timecourse of COVID-19 and subsequently disturbed sleep may better prepare healthcare providers to manage and possibly mitigate the impact of long-haul COVID.
In this study, we report the incidence and prevalence rates of self-reported disturbed sleep symptoms and change in sleep duration in a cohort of individuals who have had a COVID-19 infection. In addition, we document the persistence of these symptoms over the time period after infection.
2. Methods
The data used for this study was obtained from the Massachusetts General Brigham (MGB) Research Patient Data Registry (RPDR). This centralized clinical registry or data warehouse was established in 1991 and can be queried to identify patients based on clinical and demographic information. Using this repository, we assembled a study cohort of patients who had tested positive for SARS-CoV2 at MGB hospitals between February 2020- March 2021. After institutional review board approval, a RPDR query was run to identify patients above the age of 18 years who were enrolled in the Research Opportunities Direct to You program. This outreach program allows patients to agree to be contacted directly by researchers without involving their clinicians.
The RPDR query included patients irrespective of gender and race with a documented positive COVID-19 test. Patients with severe neurologic disease, patients on chronic ventilatory support, pregnant women or in rehabilitation were excluded from this query. Using the Research Electronic Data Capture (REDCap) HIPAA compliant web-based application, patients meeting the defined criteria were sent a survey (Appendix). The survey was sent in two languages (English and Spanish). A total of 1,090 invitations were sent and 290 responses were received. Participants with incomplete surveys were excluded leaving a total of 245 for final analysis (22.5%).
In addition to demographic information, the survey included questions pertaining to the number of hours of sleep, difficulty with initiating or maintaining sleep, daytime sleepiness, snoring, difficulty breathing while asleep, history of vivid dreams or hypnogogic hallucinations, and use of sleep aids before and after their diagnosis of COVID-19 (Appendix). Information related to co-morbid conditions and medications were obtained through chart review.
3. Statistical Analyses
Data are summarized as mean with standard deviation for continuous variables or frequency with proportions for categorical variables. Group comparisons were examined using Student’s paired sample t-test (before and after COVID-19 testing). Inasmuch as the response categories for self-reported sleep duration were in ordinal categories, changes in the distribution of responses were determined using the Stuart-Maxwell Marginal Homogeneity Test. To examine whether symptoms persisted beyond 12 months the data was stratified in two categories (< 12 months or > 12 months) according to the duration of time after their COVID-19 diagnosis each respondent completed the survey. We utilized Pearson’s χ2 to examine gender stratified sleep duration before and after testing positive for SARS-CoV2. Finally, the effects of hypertension, heart disease, anxiety/depression, diabetes, and smoking on changes in sleep characteristics were assessed with McNemar’s Test. In all analyses, the statistical significance was set as p < 0.05. All statistical testing was performed using STATA v17 (MP-parallel Edition; STATA corp, College Station TX) or IBM SPSS v28 (Armonk, NY).
4. Results
In
Table 1 are shown the baseline characteristics of the 245 participants who responded to the sleep survey. Average age was 53.3 ± 16.3 years, and respondents were predominantly Non-Hispanic White (84.1%) and female (74.3%). Average BMI (kg/m
2) was 29.9 ± 6.9, and a greater proportion was non-smokers (63.2%). College education and currently employed were reported by 47.9% and 63.7% respectively. There were significant proportions of the cohort who reported anxiety/depression (50.0%), hypertension (41.8%), heart disease (13.9%) and diabetes (13.7%).
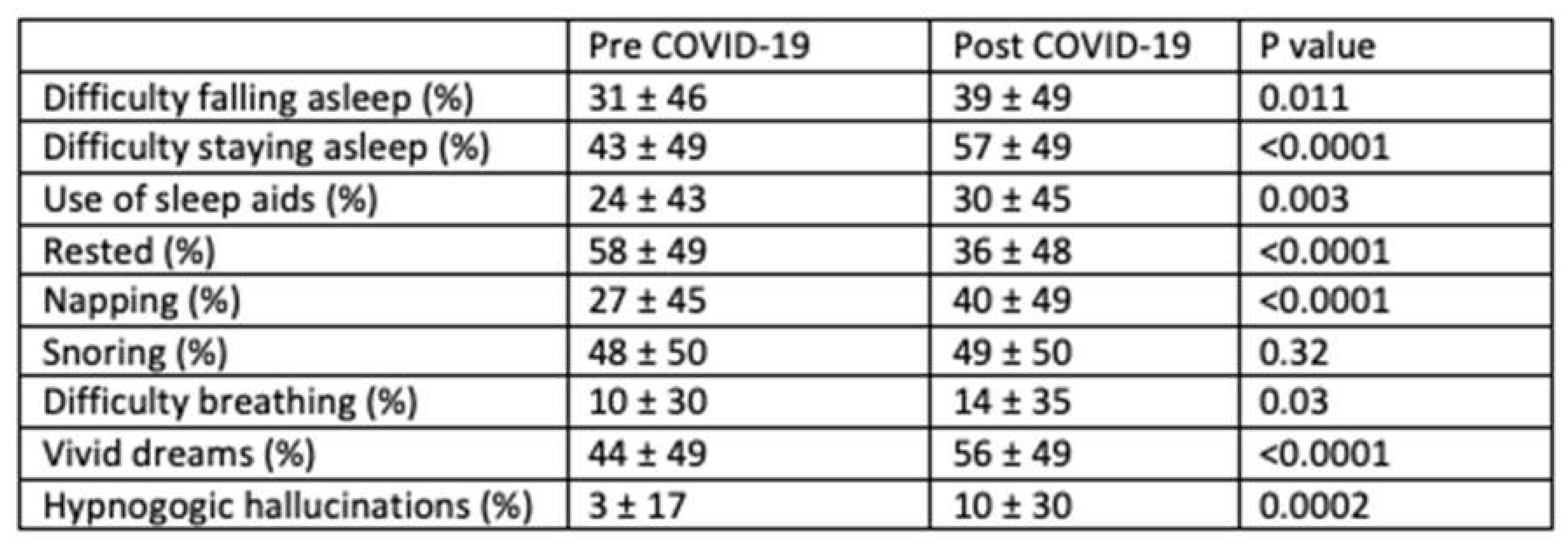
Table 2 displays the effect of COVID-19 diagnosis on various sleep characteristics. After the infection, a significantly greater number of participants reported difficulty initiating (31 ± 46% vs. 39 ± 49%,
P=0.01), and maintaining sleep (43 ± 49% vs. 57 ± 49%,
P<0.001), and increased use of sleep aids (24 ± 43% vs. 30 ± 45% P=0.003) with incidence rates of 24%, 37%, and 12% respectively. In addition, daytime fatigue and the need for napping were greater (58 ± 49% vs. 36 ± 48%,
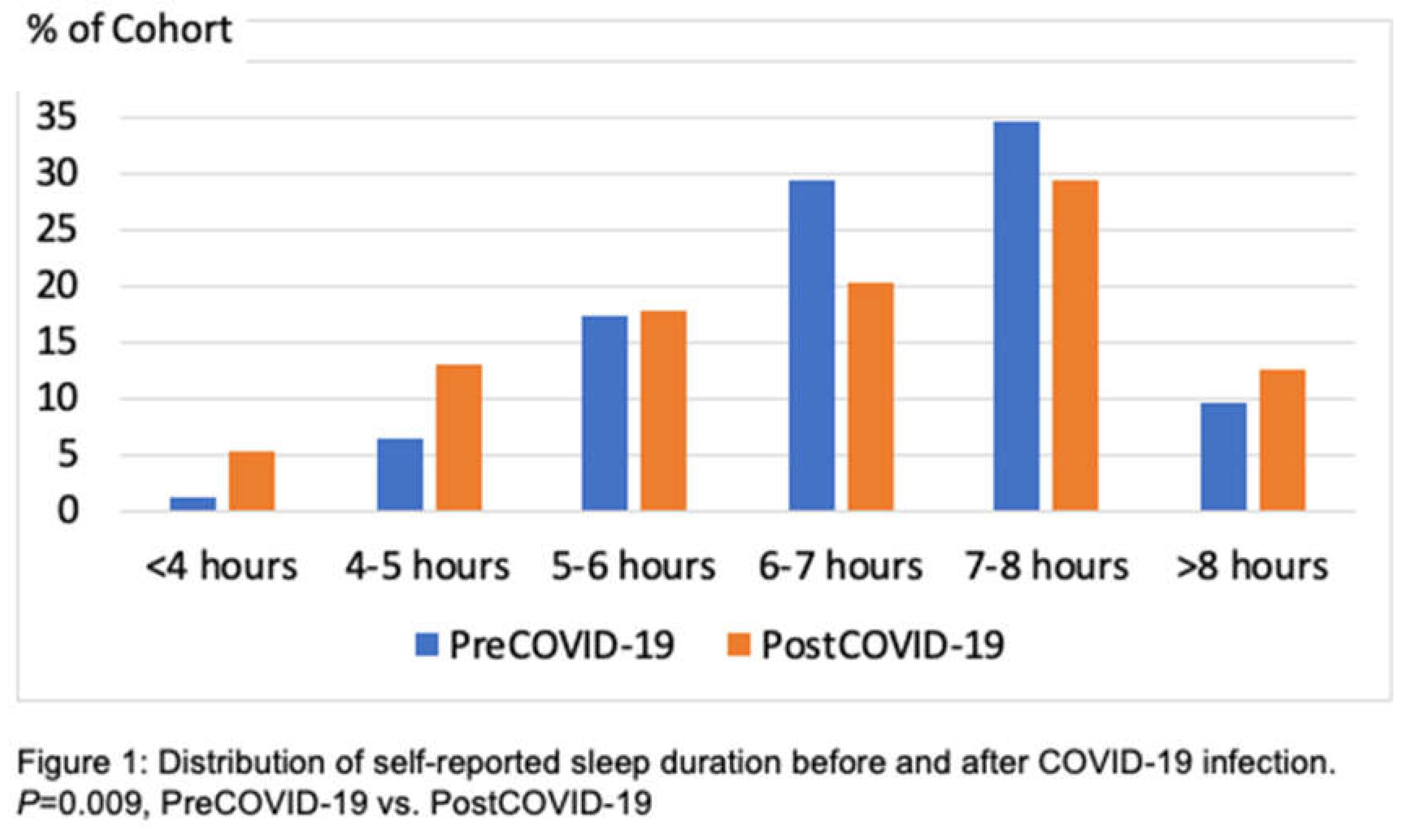
P <0.0001) with an incidence of 7.9% and 22.6% respectively. Interestingly, large number of participants reported having vivid dreams and hypnogogic hallucinations during the post COVID-19 period. The incidence rate of having vivid dreams was 31.1% after recovering from COVID-19 infection. Although shortness of breath while asleep increased slightly, there was no change in snoring prevalence. Figure 1 illustrates the change in distribution of self-reported sleep duration before and after COVID-19. There was a substantial shift in the distribution towards shorter sleep as well as a less perceptible change towards greater sleep (
P=0.009).
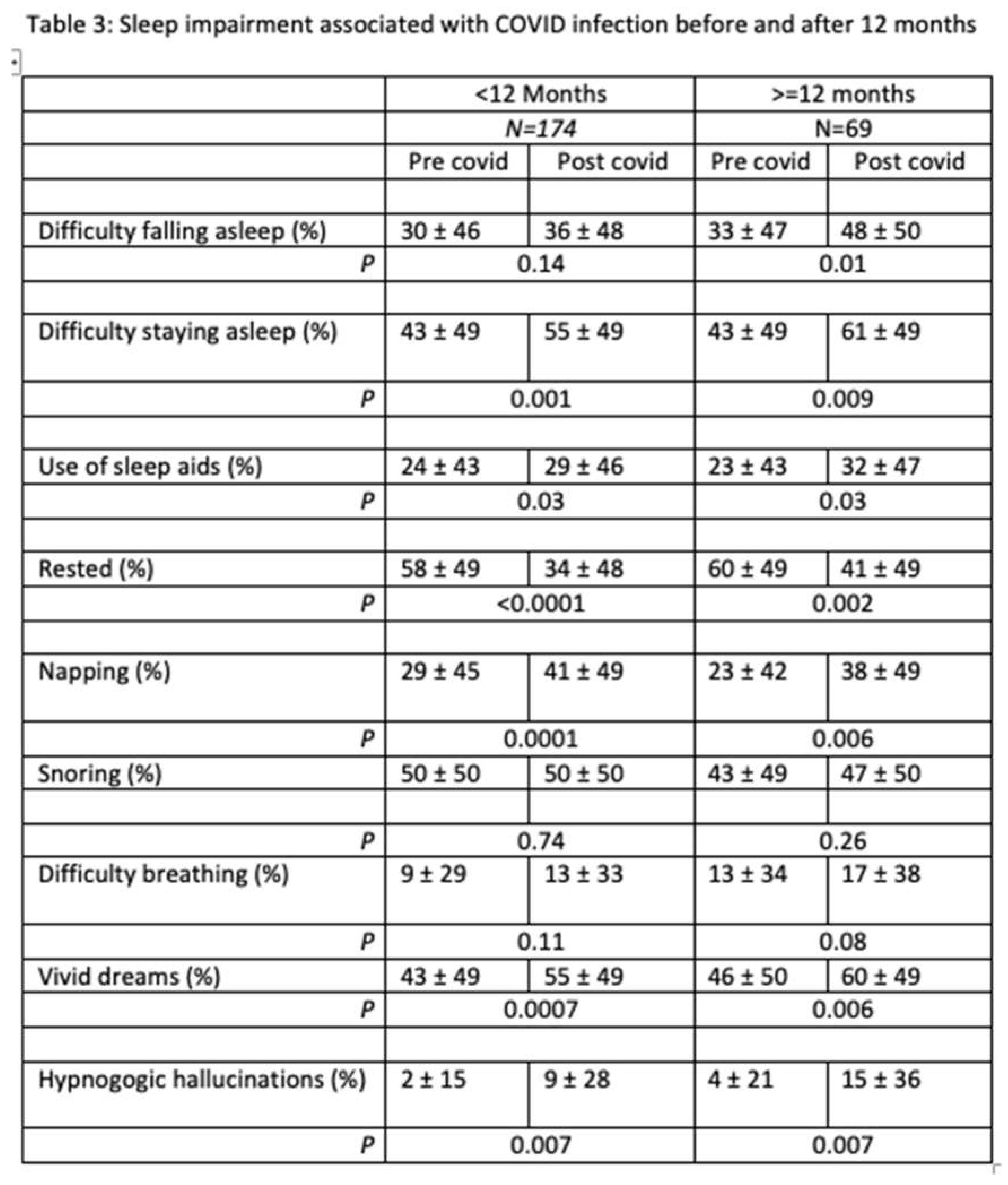
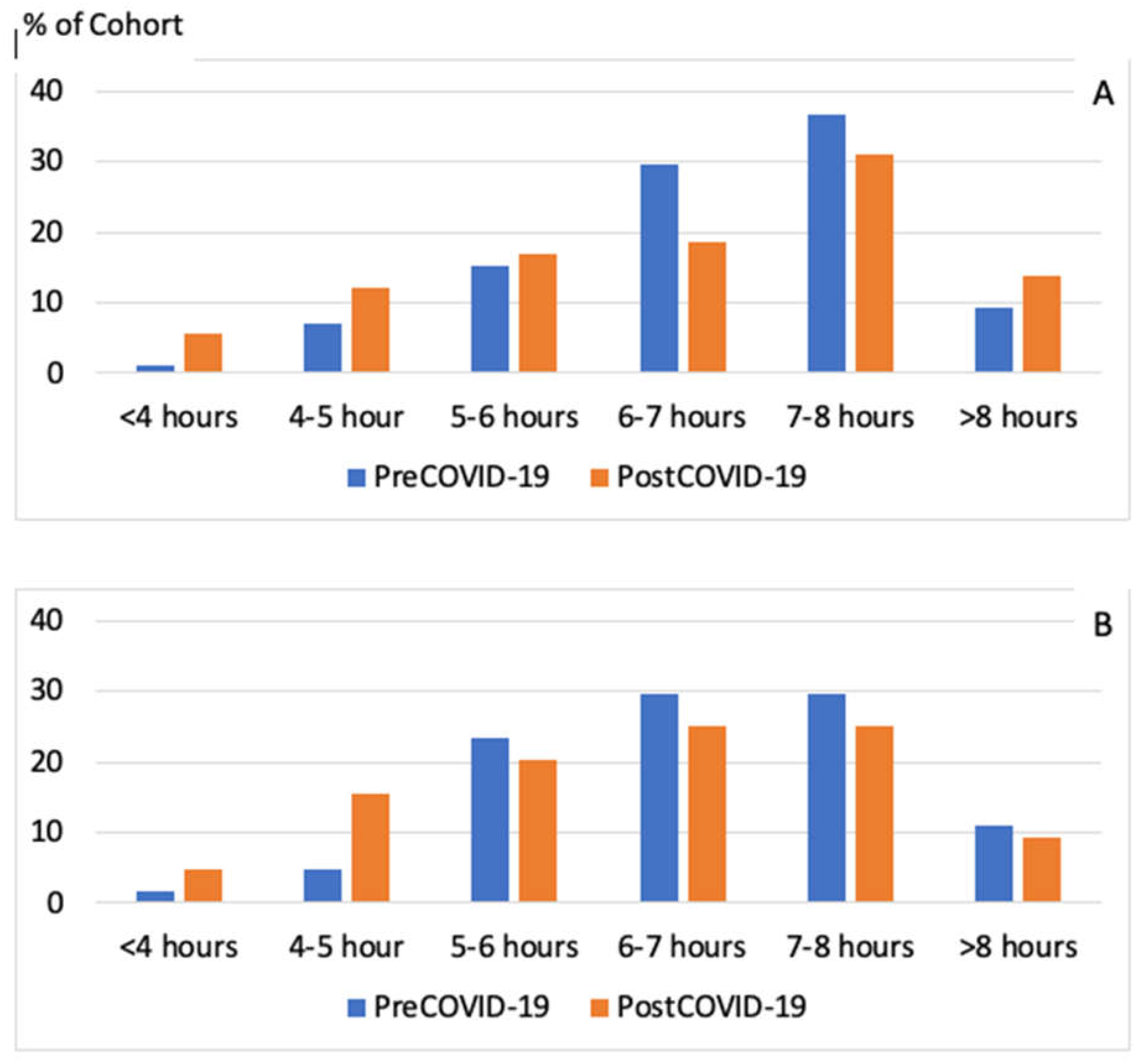
The long-term effects of COVID-19 on sleep are displayed in Table 3. Overall, there were 69 participants (28%) who were experiencing sleep symptoms more than 12 months removed from their COVID-19 infection. Symptom pattern was not different between those with a shorter vs. longer duration of past infection. As displayed in Figure 2, sleep duration decreased similarly in those who were less than 12 months removed from infection and those with 12 months or more removed from infection. However, it appears that an increase in sleep duration was limited only to those less than 12 months after infection.
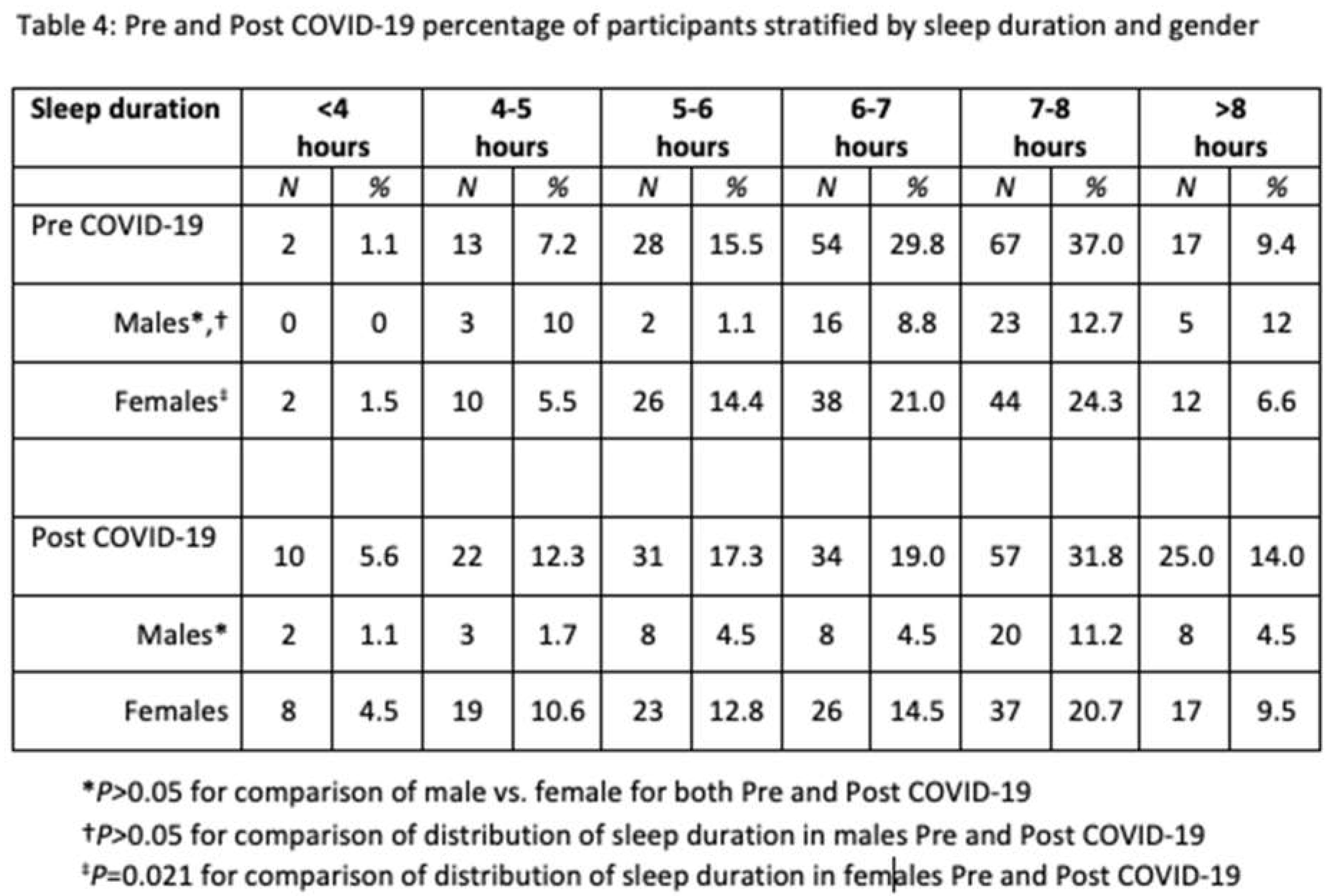
In Table 4 sleep duration is stratified by gender. Although there was a shift towards shorter and to a lesser extent greater sleep duration in both males and females, this was statistically significant only in the latter.
Additional analyses (data not shown) demonstrated that change in sleep characteristics occurred irrespective of the comorbid conditions such as hypertension, heart disease, diabetes, and anxiety/depression.
5. Discussion
In this study, we document that after COVID-19 infection there is a high incidence and prevalence of self-reported symptoms of sleep disturbances. Furthemore, these symptoms persist for over 12 months in many individuals. In addition, self-reported sleep duration changes in many individuals with most experiencing a decline, but with an increase apparent in a few.
A bidirectional relationship exists between sleep and immunity, and sleep disturbances have been reported as consequences of infections, particularly viral infections[
7,
8,
9]. Several sleep symptoms including difficulties with sleep initiation and maintenance, reduced sleep time, prolonged sleep time and daytime napping have been reported. Prior reported neuropsychiatric consequences of COVID-19 showed that the most prevalent symptoms were sleep disturbances (27%) followed by fatigue (24%) [
10] although another study found that fatigue (40%) was more common than sleep disturbances (29.4%) [
11]. However, reports of sleep disturbances in other studies were undifferentiated. Our findings document that symptoms of sleep disturbance associated with insomnia are common and increase after COVID-19 infection. In contrast, symptoms suggestive of sleep disordered breathing such as snoring did not increase. Our results are consistent with previous studies in confirming that disturbed sleep is highly prevalent after COVID 19 and but extend them by demonstrating that it is a result of a high incidence rate of symptoms suggestive of insomnia.
Our cohort of COVID-19 survivors reported insomnia symptoms that persisted beyond 12 months following COVID-19 infection. Insomnia is a fairly-well documented post COVID sequelae [
12,
13,
14,
15] and is one of the symptoms attributed to PASC. The average prevalence of post COVID insomnia is estimated at approximately 24% [
16]. However, there are few studies documenting its time course. One prior study demonstrated persistence of insomnia for up to 30 days after COVID infection [
17]. Our study extends the findings from previous studies by documenting that 28% of individuals with PASC can have symptoms of disturbed sleep longer than 12 months.
We observed that sleep duration decreased in many of our cohort and that it was present for 12 months or more after COVID-19 infection. However, in a small number of individuals, sleep duration actually increased. These changes are similar to what has been observed in uninfected persons in the general population during the COVID-19 pandemic and have been associated with anxiety and depression [
18]. It is possible that a similar relationship exists with individuals who have had a COVID-19 infection inasmuch as there was a high prevalence of anxiety or depression in our cohort.
Fatigue has been shown to be one of the most common persistent symptoms following COVID-19 [
11,
19] and can last up to a year after infection [
20]. It has been shown to occur concurrently with insomnia in some patients [
21]. Post-infection fatigue syndrome (PIFS) has also been reported following infectious mononucleosis [
22,
23,
24] suggesting that fatigue in these two conditions may share the same pathogenetic mechanism. Our analysis also showed increased fatigue and need for daytime naps in COVID survivors confirming previous reports.
Vivid dreaming and hypnagogic hallucinations which can be features of narcolepsy occurred post COVID in our cohort and these again persisted for 12 months or more. Although COVID-19 has not been associated with increase risk of narcolepsy [
25], it has been suggested that infection with SARS-CoV2 may provide an opportunity to investigate mechanisms related to the development of narcolepsy [
26]. In a previous study examining the interaction between COVID-19 and multiple health behaviors (sleep, diet, and physical activity), the authors demonstrated increased vivid dreams and nightmares among 12% of participants and the majority were linked to increased stress and anxiety (75%) [
27]. These symptoms were predominantly reported by women. To our knowledge, this is the first study reporting vivid dreams and hypnagogic hallucinations as part of the neuropsychiatric consequences of COVID-19 infection and persistence of these symptoms beyond12 months.
Study results suggest that women were disproportionately affected in this study. There is conflicting data describing the gender differences in the prevalence of mental health problems. Our findings are consistent with previous research demonstrating increased frequency of insomnia among women [
28]. Compared to men, higher prevalence of mental health symptoms has also been reported among women despite lower levels of inflammatory markers, lower duration of hospitalizations, and lower frequency of ICU admissions [
29]. Another study also demonstrated increased susceptibility to fatigue following infectious mononucleosis among women [
24]. These findings may suggest that women have a greater risk for sleep disturbances and fatigue after viral illness. In contrast a systematic review and meta analysis demonstrated adverse effects of pandemic on mental health regardless of the gender [
16]. As the pandemic has affected the world disproportionately, the gender differences in insomnia prevalence could be related to cultural roles. Several studies from China have found no gender difference [
30,
31] whereas studies from Middle East and the West have demonstrated increased risk of developing mental health problems among women during COVID-19 pandemic [
32,
33]. Further investigation is needed.
To our knowledge this is the first study looking at the long-term effects of COVID-19 on symptoms of disturbed sleep. We do however, acknowledge some limitations. Firstly, the response rate for our study was very low and is concerning for non-response bias. However, the relationship between low response rate and non-response bias has been explored in recent studies and there was very little relationship between the two [
34,
35,
36]. Secondly, the study participants were contacted using the REDCap portal, and it is likely that patients without access to technology are not represented in this study causing sampling bias and skewing the interpretation. Lastly, the cohort was comprised mainly of Caucasians and English-speaking participants despite our efforts at recruiting Spanish speakers. Thus, we acknowledge that our sample is not representative of the US population and the results may not be generalizable.
6. Conclusions
Infection with SARS-CoV2 has negative effect on sleep, and a high proportion of adults experience insomnia and daytime sleepiness beyond 12 months after recovering from the initial infection. Future studies with more diverse population are needed to examine these relationships and to determine whether there are interventions to improve sleep health in persons with PASC.
Author Contributions
Conceptualization, SB, OSF and SFQ; Methodology, SB, OSF, CV and SFQ; Investigation, SB and CV; Formal Analysis, SB and SFQ; Data Curation, SB; Writing—Original Draft Preparation, SB; Writing—Review & Editing, SB and SFQ. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding sources were used in this study.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Mass General Brigham (Protocol #2020P004011, approved on 1/19/2021).
Acknowledgments
The authors thank the Massachusetts General Brigham Research Patient Data Registry (RPDR) team for their support, and also thank the participants for their time and contribution to research.
Conflicts of Interest
SFQ has served as a consultant for Best Doctors, Bryte Foundation, Jazz Pharmaceuticals, and Whispersom. The remaining authors declare no conflict of interest.
References
- Available online:. Available online: https://coronavirus.jhu.edu/map.html. (accessed on 4 October 2023).
- Raveendran A, Jayadevan R, Sashidharan S. Erratum to “Long COVID: An overview”[Diabetes Metabol. Syndr. Clin. Res. Rev.(2021) 869–875]. Diabetes & Metabolic Syndrome 2022.
- HuangC H, RenL G. 6-monthconsequencesofCOVID-19 inpatientsdischargedfrom hospitalacohort study. Lancet 2021; 397: 220-232.
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re'Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef] [PubMed]
- Mahony, L.O.; Buwalda, T.; Blair, M.; Forde, B.; Lunjani, N.; Ambikan, A.; Neogi, U.; Barrett, P.; Geary, E.; O'Connor, N.; et al. Impact of Long COVID on health and quality of life. HRB Open Res. 2022, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Ziauddeen, N.; Gurdasani, D.; O’hara, M.E.; Hastie, C.; Roderick, P.; Yao, G.; Alwan, N.A. Characteristics and impact of Long Covid: Findings from an online survey. PLOS ONE 2022, 17, e0264331. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Coronado EG, Pantaleón-Martínez AM, Velazquéz-Moctezuma J, Prospéro-García O, Méndez-Díaz M, Pérez-Tapia M, Pavón L, Morales-Montor J. The bidirectional relationship between sleep and immunity against infections. Journal of immunology research 2015; 2015.
- Cohen, S.; Doyle, W.J.; Alper, C.M.; Janicki-Deverts, D.; Turner, R.B. Sleep Habits and Susceptibility to the Common Cold. Arch. Intern. Med. 2009, 169, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Tsang HW, Scudds RJ, Chan EY. Psychosocial impact of SARS. 2004.
- Badenoch, J.B.; Rengasamy, E.R.; Watson, C.; Jansen, K.; Chakraborty, S.; Sundaram, R.D.; Hafeez, D.; Burchill, E.; Saini, A.; Thomas, L.; et al. Persistent neuropsychiatric symptoms after COVID-19: a systematic review and meta-analysis. Brain Commun. 2021, 4, fcab297. [Google Scholar] [CrossRef] [PubMed]
- Nasserie T, Hittle M, Goodman SN. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients With COVID-19: A Systematic Review. JAMA Netw Open 2021; 4: e2111417.
- Xu, F.; Wang, X.; Yang, Y.; Zhang, K.; Shi, Y.; Xia, L.; Hu, X.; Liu, H. Depression and insomnia in COVID-19 survivors: a cross-sectional survey from Chinese rehabilitation centers in Anhui province. Sleep Med. 2022, 91, 161–165. [Google Scholar] [CrossRef]
- Badinlou, F.; Lundgren, T.; Jansson-Fröjmark, M. Mental health outcomes following COVID-19 infection: impacts of post-COVID impairments and fatigue on depression, anxiety, and insomnia — a web survey in Sweden. BMC Psychiatry 2022, 22, 1–11. [Google Scholar] [CrossRef]
- Yuan K, Zheng YB, Wang YJ, Sun YK, Gong YM, Huang YT, Chen X, Liu XX, Zhong Y, Su SZ, Gao N, Lu YL, Wang Z, Liu WJ, Que JY, Yang YB, Zhang AY, Jing MN, Yuan CW, Zeng N, Vitiello MV, Patel V, Fazel S, Minas H, Thornicroft G, Fan TT, Lin X, Yan W, Shi L, Shi J, Kosten T, Bao YP, Lu L. A systematic review and meta-analysis on prevalence of and risk factors associated with depression, anxiety and insomnia in infectious diseases, including COVID-19: a call to action. Mol Psychiatry 2022; 27: 3214-3222.
- Kyzar, E.J.; Purpura, L.J.; Shah, J.; Cantos, A.; Nordvig, A.S.; Yin, M.T. Anxiety, depression, insomnia, and trauma-related symptoms following COVID-19 infection at long-term follow-up. Brain, Behav. Immun. - Heal. 2021, 16, 100315. [Google Scholar] [CrossRef]
- Cénat, J.M.; Blais-Rochette, C.; Kokou-Kpolou, C.K.; Noorishad, P.-G.; Mukunzi, J.N.; McIntee, S.-E.; Dalexis, R.D.; Goulet, M.-A.; Labelle, P.R. Prevalence of symptoms of depression, anxiety, insomnia, posttraumatic stress disorder, and psychological distress among populations affected by the COVID-19 pandemic: A systematic review and meta-analysis. Psychiatry Res. 2021, 295, 113599. [Google Scholar] [CrossRef]
- A Choudhry, A.; Shahzeen, F.; A Choudhry, S.; Batool, N.; Murtaza, F.; Dilip, A.; Rani, M.; Chandnani, A. Impact of COVID-19 Infection on Quality of Sleep. Cureus 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Batool-Anwar S, Robbins R, Ali SH, Capasso A, Foreman J, Jones AM, Tozan Y, DiClemente RJ, Quan SF. Examining Changes in Sleep Duration Associated with the Onset of the COVID-19 Pandemic: Who is Sleeping and Who is Not? Behav Med 2021: 1-10.
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2021, 74, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, C.; Zocchi, C.; Tassetti, L.; Silverii, M.V.; Amato, C.; Livi, L.; Giovannoni, L.; Verrillo, F.; Bartoloni, A.; Marcucci, R.; et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur. J. Intern. Med. 2021, 97, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Jarrott B, Head R, Pringle KG, Lumbers ER, Martin JH. “LONG COVID”-A hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol Res Perspect 2022; 10: e00911.
- Fevang, B.; Wyller, V.B.B.; Mollnes, T.E.; Pedersen, M.; Asprusten, T.T.; Michelsen, A.; Ueland, T.; Otterdal, K. Lasting Immunological Imprint of Primary Epstein-Barr Virus Infection With Associations to Chronic Low-Grade Inflammation and Fatigue. Front. Immunol. 2021, 12, 715102. [Google Scholar] [CrossRef] [PubMed]
- A Jason, L.; Cotler, J.; Islam, M.F.; Sunnquist, M.; Katz, B.Z. Risks for Developing Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in College Students Following Infectious Mononucleosis: A Prospective Cohort Study. Clin. Infect. Dis. 2020, 73, e3740–e3746. [Google Scholar] [CrossRef] [PubMed]
- Petersen, I.; Thomas, J.; Hamilton, W.; White, P. Risk and predictors of fatigue after infectious mononucleosis in a large primary-care cohort. Qjm: Int. J. Med. 2005, 99, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Zarifkar, P.; Peinkhofer, C.; Benros, M.E.; Kondziella, D. Frequency of Neurological Diseases After COVID-19, Influenza A/B and Bacterial Pneumonia. Front. Neurol. 2022, 13, 904796. [Google Scholar] [CrossRef]
- Fernandez FX, Flygare J, Grandner MA. Narcolepsy and COVID-19: sleeping on an opportunity? J Clin Sleep Med 2020; 16: 1415.
- Orr, K.; Ta, Z.; Shoaf, K.; Halliday, T.M.; Tobin, S.; Baron, K.G. Sleep, Diet, Physical Activity, and Stress during the COVID-19 Pandemic: A Qualitative Analysis. Behav. Sci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Pedrozo-Pupo, J.C.; Caballero-Domínguez, C.C.; Campo-Arias, A. Prevalence and variables associated with insomnia among COVID-19 survivors in Colombia. . 2022, 93, e2022019. [Google Scholar] [CrossRef]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef]
- Cao, W.; Fang, Z.; Hou, G.; Han, M.; Xu, X.; Dong, J.; Zheng, J. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res. 2020, 287, 112934. [Google Scholar] [CrossRef] [PubMed]
- Huang Y, Zhao N. Mental health burden for the public affected by the COVID-19 outbreak in China: Who will be the high-risk group? Psychology, health & medicine 2021; 26: 23-34.
- Mazza, C.; Ricci, E.; Biondi, S.; Colasanti, M.; Ferracuti, S.; Napoli, C.; Roma, P. A Nationwide Survey of Psychological Distress among Italian People during the COVID-19 Pandemic: Immediate Psychological Responses and Associated Factors. Int. J. Environ. Res. Public Health 2020, 17, 3165. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; Janiri, D.; Pepe, M.; Dattoli, L.; Molinaro, M.; De Martin, V.; Chieffo, D.; Janiri, L.; Fiorillo, A.; Sani, G.; et al. Affective temperament, attachment style, and the psychological impact of the COVID-19 outbreak: an early report on the Italian general population. Brain Behav. Immun. 2020, 87, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Curtin, R.; Presser, S.; Singer, E. The Effects of Response Rate Changes on the Index of Consumer Sentiment. Public Opin. Q. 2000, 64, 413–428. [Google Scholar] [CrossRef]
- Kohut A, Doherty C, Keeter S. Polls face growing resistance, but still representative. Pew Center for Research on People and the Press, Washington, DC 2004.
- Groves, R.M. Nonresponse Rates and Nonresponse Bias in Household Surveys. Public Opin. Q. 2006, 70, 646–675. [Google Scholar] [CrossRef]
Table 1.
Baseline characteristics (N=245).
Table 1.
Baseline characteristics (N=245).
| VARIABLES |
mean |
sd |
| Age (yrs) |
53.3 |
16.3 |
| Gender (M/F %)
|
25.7/74.3 |
|
| Hispanics (%) |
17.0 |
|
| Caucasians (%) |
84.1 |
|
| Employed (%) |
63.7 |
|
| Education (% college) |
47.9 |
|
| BMI |
29.9 |
6.9 |
| Smoking Hx (%): Never |
63.2 |
|
| Current |
6.2 |
|
| Past |
30.6 |
|
| H/o Hypertension (%) |
41.8 |
|
| Anxiety/depression (%) |
50.0 |
|
| Diabetes (%) |
13.7 |
|
| Heart disease (%) |
13.9 |
|
Table 2.
Sleep symptoms before and after COVID -19 infection (N=245).
Table 2.
Sleep symptoms before and after COVID -19 infection (N=245).
 VARIABLES VARIABLES |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




 VARIABLES
VARIABLES




