Submitted:
04 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Objectives/Hypothesis
3. Methods
4. Statistics
5. Results
6. Discussion
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nature Reviews Disease Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- de Graaf G, Buckley F & Skotko BG Birth and population prevalence of Down syndrome in European countries. 2018.
- Dierssen, M. Down syndrome: the brain in trisomic mode. Nat. Rev. Neurosci. 2012, 13, 844–858. [Google Scholar] [CrossRef] [PubMed]
- https://gateway.euro.who. 7120.
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.; Evenson, A.; Luckey, T.; McCoy, E.; Elvehjem, C.; Hart, E. USE OF SULFASUXIDINE, STREPTOTHRICIN, AND STREPTOMYCIN IN NUTRITIONAL STUDIES WITH THE CHICK. J. Biol. Chem. 1946, 165, 437–441. [Google Scholar] [CrossRef] [PubMed]
- E Carpenter, L. Effect of aureomycin on the growth of weaned pigs. . 1950, 27, 469–71. [Google Scholar]
- Coates, M.E. , Fuller, R., Harrison, G.F., Lev, M. and Suffolk, S.F. A comparision of the growth of chicks in the Gustafsson germ-free apparatus and in a conventional environment, with and without dietary supplements of penicillin. British journal of nutrition 1963, 17, 141–150. [Google Scholar] [CrossRef]
- Wegener, H.C. Antibiotics in animal feed and their role in resistance development. Curr. Opin. Microbiol. 2003, 6, 439–445. [Google Scholar] [CrossRef]
- Browne, H.P.; Shao, Y.; Lawley, T.D. Mother–infant transmission of human microbiota. Curr. Opin. Microbiol. 2022, 69, 102173. [Google Scholar] [CrossRef]
- Wright, M.L.; Starkweather, A.R. Antenatal microbiome: potential contributor to fetal programming and establishment of the microbiome in offspring. Nursing Research 2015, 64, 306–319. [Google Scholar] [CrossRef]
- Grech, A.; E Collins, C.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal exposures and the infant gut microbiome: a systematic review with meta-analysis. Gut Microbes 2021, 13, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol Mol Biol Rev. 2017, 8;81[4]:e00036-17.
- I McBurney, M.; Davis, C.; Fraser, C.M.; O Schneeman, B.; Huttenhower, C.; Verbeke, K.; Walter, J.; E Latulippe, M. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS biology 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed]
- Poretsky, R.; Rodriguez-R, L.M.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T. Strengths and Limitations of 16S rRNA Gene Amplicon Sequencing in Revealing Temporal Microbial Community Dynamics. PLOS ONE 2014, 9, e93827. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi-Man, O.; Davenport, E.R.; Gilad, Y. Taxonomic Classification of Bacterial 16S rRNA Genes Using Short Sequencing Reads: Evaluation of Effective Study Designs. PLOS ONE 2013, 8, e53608. [Google Scholar] [CrossRef]
- Human Microbiome Jumpstart Reference Strains Consortium; Nelson, K. E.; Weinstock, G.M.; Highlander, S.K.; Worley, K.C.; Creasy, H.H.; Wortman, J.R.; Rusch, D.B.; Mitreva, M.; Sodergren, E.; Chinwalla, A.T. A catalog of reference genomes from the human microbiome. Science 2010, 328, 994–999. [Google Scholar]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; Bertalan, M. Enterotypes of the human gut microbiome. nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Shetty, S.A.; Hugenholtz, F.; Lahti, L.; Smidt, H.; de Vos, W.M. Intestinal microbiome landscaping: insight in community assemblage and implications for microbial modulation strategies. FEMS Microbiol. Rev. 2017, 41, 182–199. [Google Scholar] [CrossRef]
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51. [Google Scholar] [CrossRef]
- Tiffany, C.R.; Bäumler, A.J. Dysbiosis: from fiction to function. American Journal of Physiology-Gastrointestinal and Liver Physiology 2019, 317, G602–G608. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The gut–brain axis. Annual Review of Medicine 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Pequegnat, B.; Sagermann, M.; Valliani, M.; Toh, M.; Chow, H.; Allen-Vercoe, E.; Monteiro, M.A. A vaccine and diagnostic target for Clostridium bolteae, an autism-associated bacterium. Vaccine 2013, 31, 2787–2790. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses 2011, 77, 270–274. [Google Scholar] [CrossRef]
- Heberling, C.A.; Dhurjati, P.S.; Sasser, M. Hypothesis for a systems connectivity model of autism spectrum disorder pathogenesis: Links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses 2013, 80, 264–270. [Google Scholar] [CrossRef]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Lubomski, M.; Tan, A.H.; Lim, S.-Y.; Holmes, A.J.; Davis, R.L.; Sue, C.M. Parkinson’s disease and the gastrointestinal microbiome. J. Neurol. 2019, 267, 2507–2523. [Google Scholar] [CrossRef]
- Cryan, J.F.; O'Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A.J.; Wang, B.; Sun, X.; Kennedy, E.A.; Hernandez-Leyva, A.; Ndao, I.M.; Tarr, P.I.; Warner, B.B.; Dantas, G. Persistent metagenomic signatures of early-life hospitalization and antibiotic treatment in the infant gut microbiota and resistome. Nat. Microbiol. 2019, 4, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Ryan, C.A.; Dempsey, E.; Ross, R.P.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Velasco, M. The human microbiome in sickness and in health. Revista Clínica Española (English Edition) 2021, 221, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ternák, G. 1: A new discovery: The prevalence/incidence of different non-contagious diseases are associated with the antibiotic consumption patterns Paperback –2022 Lambert Academic Press, ISBN-13978-6204984452 pages, 2022.
- Biagi, E.; Candela, M.; Centanni, M.; Consolandi, C.; Rampelli, S.; Turroni, S.; Severgnini, M.; Peano, C.; Ghezzo, A.; Scurti, M.; et al. Gut Microbiome in Down Syndrome. PLOS ONE 2014, 9, e112023–e112023. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, X.; Qin, J.; Mu, Q.; Ye, S.; Zhang, Y.; Yu, W.; Guo, J. Altered gut microbiota correlates with cognitive impairment in Chinese children with Down’s syndrome. Eur. Child Adolesc. Psychiatry 2021, 31, 189–202. [Google Scholar] [CrossRef]
- Hursitoglu, M.; Kural, A.; Kuras, S.; Akdeniz, E.; Sezer, S.; Caypinar, S.S.; Kazezoglu, C.; Yaprak, B.; Karandere, F.; Guven, H.Z. Maternal Gut Microbiota in Pregnancies Resulting in Down Syndrome Newborns – a Pilot Study. Acta Clin. Croat. 2021, 60., 722–730. [Google Scholar] [CrossRef]
- Quality Indicators for Antibiotic Consumption in the Community. Available online: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/quality-indicators (accessed on 10 March 2021).
- https://ourworldindata.
- Coppedè, F. Risk factors for Down syndrome. Archives of toxicology 2016, 90, 2917–2929. [Google Scholar] [CrossRef]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nature Reviews Disease Primers 2020, 6, 9. [Google Scholar] [CrossRef]
- Mikwar, M.; MacFarlane, A.J.; Marchetti, F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat. Res. Mutat. Res. 2020, 785, 108320. [Google Scholar] [CrossRef] [PubMed]
- Halder, P.; Pal, U.; Ganguly, A.; Ghosh, P.; Ray, A.; Sarkar, S.; Ghosh, S. Understanding etiology of chromosome 21 nondisjunction from gene × environment models. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yannan Liu, Dongjie Hu, Chunfang Dai et al. Characteristics of Intestinal Microbiota and Mother's Reproductive Tract Flora in Children With Down Syndrome, 2021, PREPRINT [Version 1] available at Research Square, 2021. [CrossRef]
- Maier, L.; Goemans, C.V.; Wirbel, J.; Kuhn, M.; Eberl, C.; Pruteanu, M.; Müller, P.; Garcia-Santamarina, S.; Cacace, E.; Zhang, B.; et al. Unravelling the collateral damage of antibiotics on gut bacteria. Nature 2021, 599, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Ternák, G.; Németh, M.; Rozanovic, M.; Márovics, G.; Bogár, L. Antibiotic Consumption Patterns in European Countries Are Associated with the Prevalence of Parkinson’s Disease; the Possible Augmenting Role of the Narrow-Spectrum Penicillin. Antibiotics 2022, 11, 1145. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, M.; Nielsen, R.B.; Jacobsen, J.B.; Gradus, J.L.; Stenager, E.; Koch-Henriksen, N.; Lash, T.L.; Sørensen, H.T. Use of Penicillin and Other Antibiotics and Risk of Multiple Sclerosis: A Population-based Case-Control Study. Am. J. Epidemiology 2011, 174, 945–948. [Google Scholar] [CrossRef]
- Ternák, G.; Berényi, K.; Márovics, G.; Sümegi, A.; Fodor, B.; Németh, B.; Kiss, I. Dominant Antibiotic Consumption Patterns Might Be Associated With the Prevalence of Multiple Sclerosis in European Countries. Vivo 2020, 34, 3467–3472. [Google Scholar] [CrossRef]
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in down syndrome: molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome medicine 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Manor, O.; Dai, C.L.; Kornilov, S.A.; Smith, B.; Price, N.D.; Lovejoy, J.C.; Gibbons, S.M.; Magis, A.T. Health and disease markers correlate with gut microbiome composition across thousands of people. Nat. Commun. 2020, 11, 5206. [Google Scholar] [CrossRef]
- Durack, J.; Lynch, S.V. The gut microbiome: Relationships with disease and opportunities for therapy. J. Exp. Med. 2019, 216, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Keshteli, A.H.; Millan, B.; Madsen, K.L. Pretreatment with antibiotics may enhance the efficacy of fecal microbiota transplantation in ulcerative colitis: a meta-analysis. Mucosal Immunol. 2017, 10, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Markulin, I.; Matasin, M.; Turk, V.E.; Salković-Petrisic, M. Challenges of repurposing tetracyclines for the treatment of Alzheimer’s and Parkinson’s disease. Journal of Neural Transmission 2022, 129, 773–804. [Google Scholar] [CrossRef] [PubMed]
- Cankaya, S.; Cankaya, B.; Kilic, U.; Kilic, E.; Yulug, B. The therapeutic role of minocycline in Parkinson’s disease. Drugs Context 2019, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sorboni, S.G.; Moghaddam, H.S.; Jafarzadeh-Esfehani, R.; Soleimanpour, S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022, 35, e0033820. [Google Scholar] [CrossRef]
- Fattorusso, A.; Di Genova, L.; Dell’Isola, G.B.; Mencaroni, E.; Esposito, S. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Sun, Y.; Baptista, L.C.; Roberts, L.M.; Jumbo-Lucioni, P.; McMahon, L.L.; Buford, T.W.; Carter, C.S. The Gut Microbiome as a Therapeutic Target for Cognitive Impairment. Journals Gerontol. Ser. A 2020, 75, 1242–1250. [Google Scholar] [CrossRef]
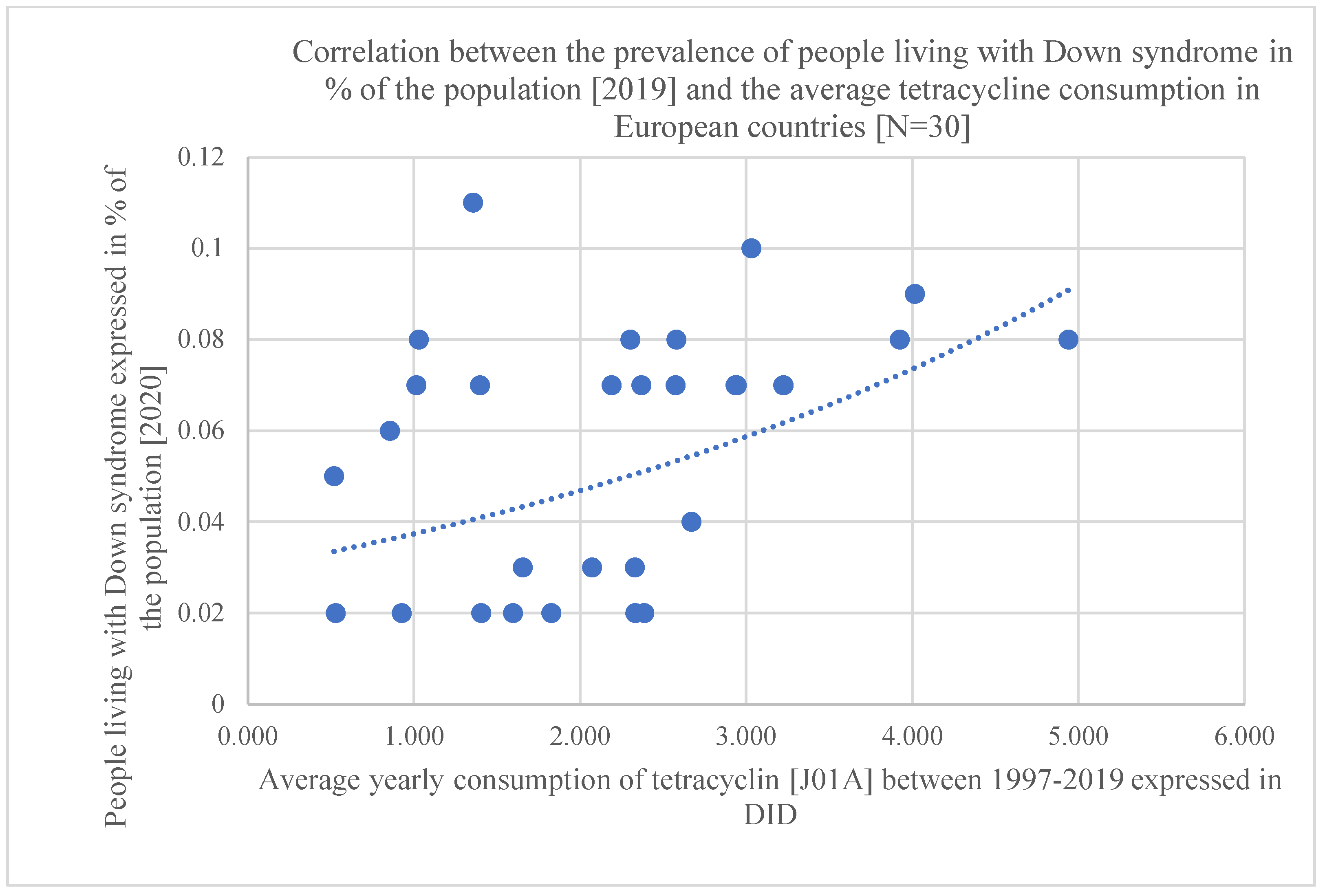
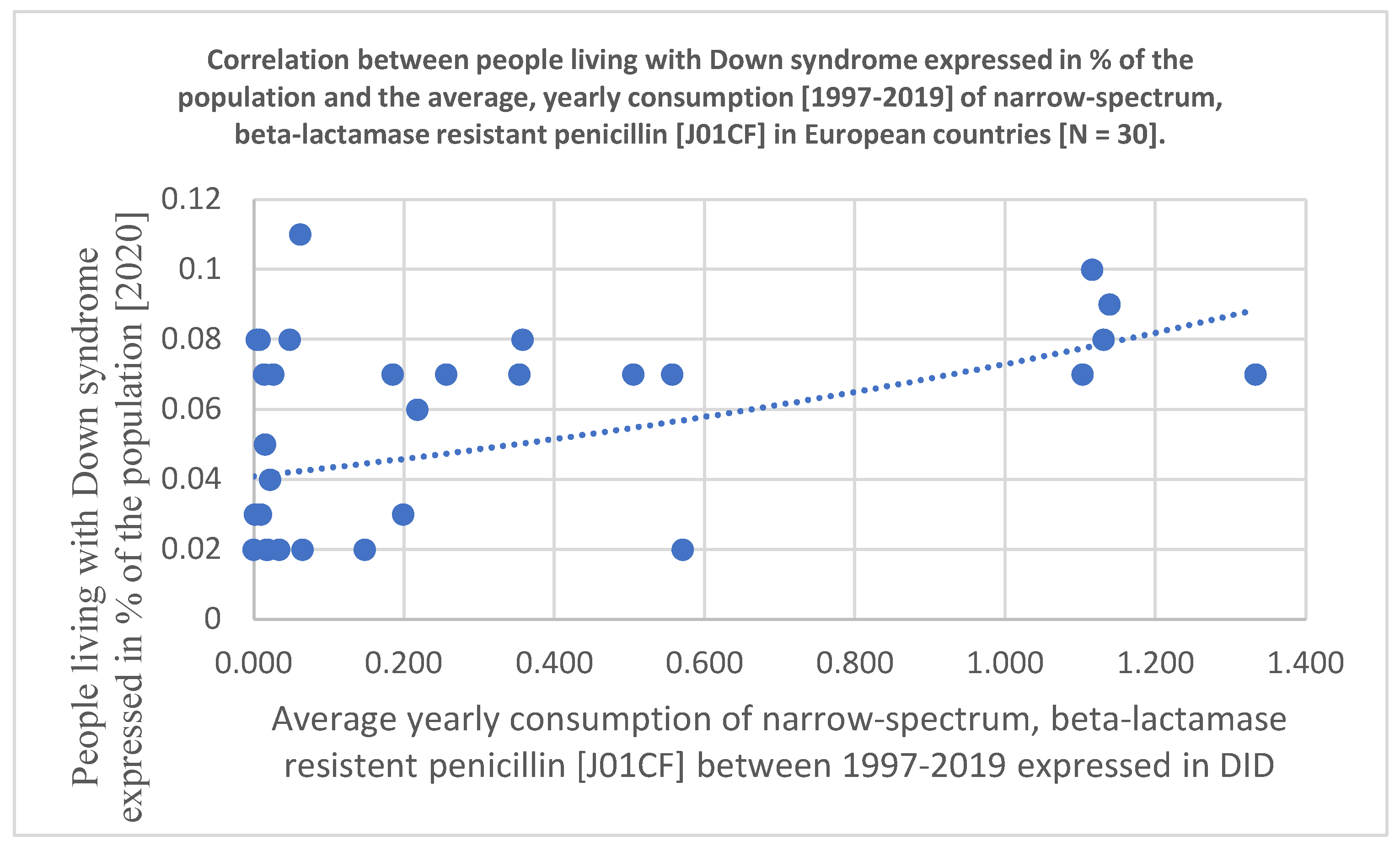
| Antibiotic consumption 1997-2020 DID | J01 | J01A | J01C | J01CA | J01CE | J01CF | J01CR | J01D | J01F | J01M | People living with DS in % of the population. 2019 | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 11.683 | 1.03 | 4.251 | 0.819 | 0.946 | 0.008 | 2.474 | 1.565 | 3.076 | 1.311 | Austria | 0.08 |
| Belgium | 21.427 | 2.368 | 8.725 | 3.796 | 0.091 | 0.256 | 4.578 | 2.387 | 3.205 | 2.206 | Belgium | 0.07 |
| Bulgaria | 17.828 | 2.386 | 6.55 | 4.039 | 0.953 | 0.019 | 1.56 | 2.672 | 2.589 | 1.991 | Bulgaria | 0.02 |
| Croatia | 17.911 | 1.404 | 7.581 | 2.467 | 1.007 | 0.034 | 4.074 | 3.172 | 2.641 | 1.496 | Croatia | 0.02 |
| Cyprus | 27.338 | 3.226 | 9.454 | 3.118 | 0.101 | 0.026 | 6.234 | 5.923 | 3.126 | 4.623 | Cyprus | 0.07 |
| Czech Rep. | 14.685 | 2.333 | 5.539 | 1.355 | 1.877 | 0.065 | 2.222 | 1.55 | 3.028 | 1.03 | Czech Rep. | 0.02 |
| Denmark | 14.068 | 1.398 | 8.782 | 2.732 | 4.575 | 1.103 | 0.378 | 0.03 | 2.031 | 0.455 | Denmark | 0.07 |
| Estonia | 10.947 | 2.072 | 3.701 | 2.422 | 0.264 | 0.01 | 1.007 | 0.892 | 1.947 | 0.83 | Estonia | 0.03 |
| Finland | 16.157 | 3.925 | 4.559 | 2.349 | 1.514 | 0.048 | 0.65 | 2.094 | 1.474 | 0.799 | Finland | 0.08 |
| France | 24.478 | 3.224 | 11.423 | 6.804 | 0.18 | 0.354 | 4.067 | 2.818 | 4.087 | 1.923 | France | 0.07 |
| Germany | 12.61 | 2.574 | 3.419 | 2.033 | 1.064 | 0.014 | 0.302 | 1.908 | 2.293 | 1.205 | Germany | 0.07 |
| Greece | 30.474 | 2.581 | 8.579 | 4.088 | 0.441 | 0.004 | 4.018 | 7.183 | 8.026 | 2.686 | Greece | 0.08 |
| Hungary | 14.711 | 1.595 | 5.269 | 1.467 | 0.634 | 0 | 3.164 | 2.162 | 3.054 | 1.843 | Hungary | 0.02 |
| Iceland | 19.148 | 4.941 | 9.219 | 3.392 | 2.446 | 1.131 | 2.232 | 0.567 | 1.56 | 0.797 | Iceland | 0.08 |
| Ireland | 18.256 | 3.032 | 8.219 | 2.816 | 0.93 | 1.116 | 3.374 | 1.57 | 3.413 | 0.858 | Ireland | 0.1 |
| Italy | 21.524 | 0.518 | 9.092 | 3.509 | 0.013 | 0.015 | 5.56 | 2.835 | 4.726 | 3.073 | Italy | 0.05 |
| Latvia | 10.653 | 2.329 | 4.04 | 2.717 | 0.096 | 0.002 | 1.226 | 0.544 | 1.435 | 0.982 | Latvia | 0.03 |
| Lithuania | 16.714 | 1.656 | 8.744 | 5.103 | 2.225 | 0.199 | 1.227 | 1.301 | 1.747 | 0.983 | Lithuania | 0.03 |
| Luxembourg | 22.41 | 2.189 | 7.974 | 3.09 | 0.088 | 0.185 | 4.618 | 4.087 | 3.998 | 2.413 | Luxembourg | 0.07 |
| Malta | 18.465 | 1.356 | 6.265 | 0.537 | 0.092 | 0.062 | 5.572 | 4.034 | 3.771 | 2.1 | Malta | 0.11 |
| Netherlands | 9.218 | 2.304 | 2.954 | 1.266 | 0.357 | 0.358 | 0.972 | 0.089 | 1.383 | 0.836 | Netherlands | 0.08 |
| Norway | 15.046 | 2.944 | 6.114 | 1.919 | 3.681 | 0.505 | 0.009 | 0.144 | 1.567 | 0.468 | Norway | 0.07 |
| Poland | 18.773 | 2.671 | 6.186 | 3.653 | 0.421 | 0.022 | 2.092 | 2.315 | 3.346 | 1.276 | Poland | 0.04 |
| Portugal | 18.094 | 1.015 | 7.836 | 2.006 | 0.028 | 0.557 | 5.248 | 2.308 | 3.179 | 2.496 | Portugal | 0.07 |
| Romania | 22.897 | 0.926 | 10.725 | 4.063 | 0.695 | 0.571 | 5.478 | 4.281 | 2.866 | 2.954 | Romania | 0.02 |
| Slovakia | 21.098 | 1.827 | 8.279 | 2.232 | 2.82 | 0.017 | 3.218 | 3.604 | 4.632 | 1.842 | Slovakia | 0.02 |
| Slovenia | 12.873 | 0.529 | 7.15 | 2.148 | 1.982 | 0.148 | 2.881 | 0.56 | 2.462 | 1.265 | Slovenia | 0.02 |
| Spain | 17.697 | 0.854 | 9.166 | 3.611 | 0.101 | 0.218 | 5.134 | 2.089 | 2.538 | 2.359 | Spain | 0.06 |
| Sweden | 13.37 | 2.936 | 6.387 | 1.047 | 3.821 | 1.333 | 0.182 | 0.28 | 0.73 | 0.825 | Sweden | 0.07 |
| UK | 15.294 | 4.016 | 5.851 | 3.257 | 0.746 | 1.139 | 0.734 | 0.546 | 2.627 | 0.534 | UK | 0.09 |
| 2019 R [Pearson] | 0.174 | 0.479 | -0.040 | -0.139 | -0.043 | 0.328 | 0.005 | 0.072 | 0.109 | -0.009 | ||
| 2019 p [Pearson] | 0.358 | 0.007 | 0.835 | 0.464 | 0.821 | 0.077 | 0.981 | 0.706 | 0.567 | 0.963 | ||
| 2019 OR | 1.074 | 3.896 | 0.861 | 0.741 | 0.497 | 14.491 | 0.942 | 0.510 | 2.031 | 2.737 | ||
| 2019 CI95% | 0.932-1.246 | 1.632-11.626 | 0.560-1.304 | 0.418-1.262 | 0.193-1.117 | 1.764-186.274 | 0.572-1.508 | 0.139-1.578 | 0.784-5.969 | 0.537-16.944 | ||
| 2019 OR p | 0.324 | 0.005 | 0.478 | 0.274 | 0.109 | 0.021 | 0.806 | 0.263 | 0.160 | 0.239 | ||
| 2019 Kruskal-Wallis p | 0.431 | 0.034 | 0.348 | 0.49 | 0.237 | 0.152 | 0.842 | 0.897 | 0.517 | 0.362 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





