Submitted:
10 May 2023
Posted:
11 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
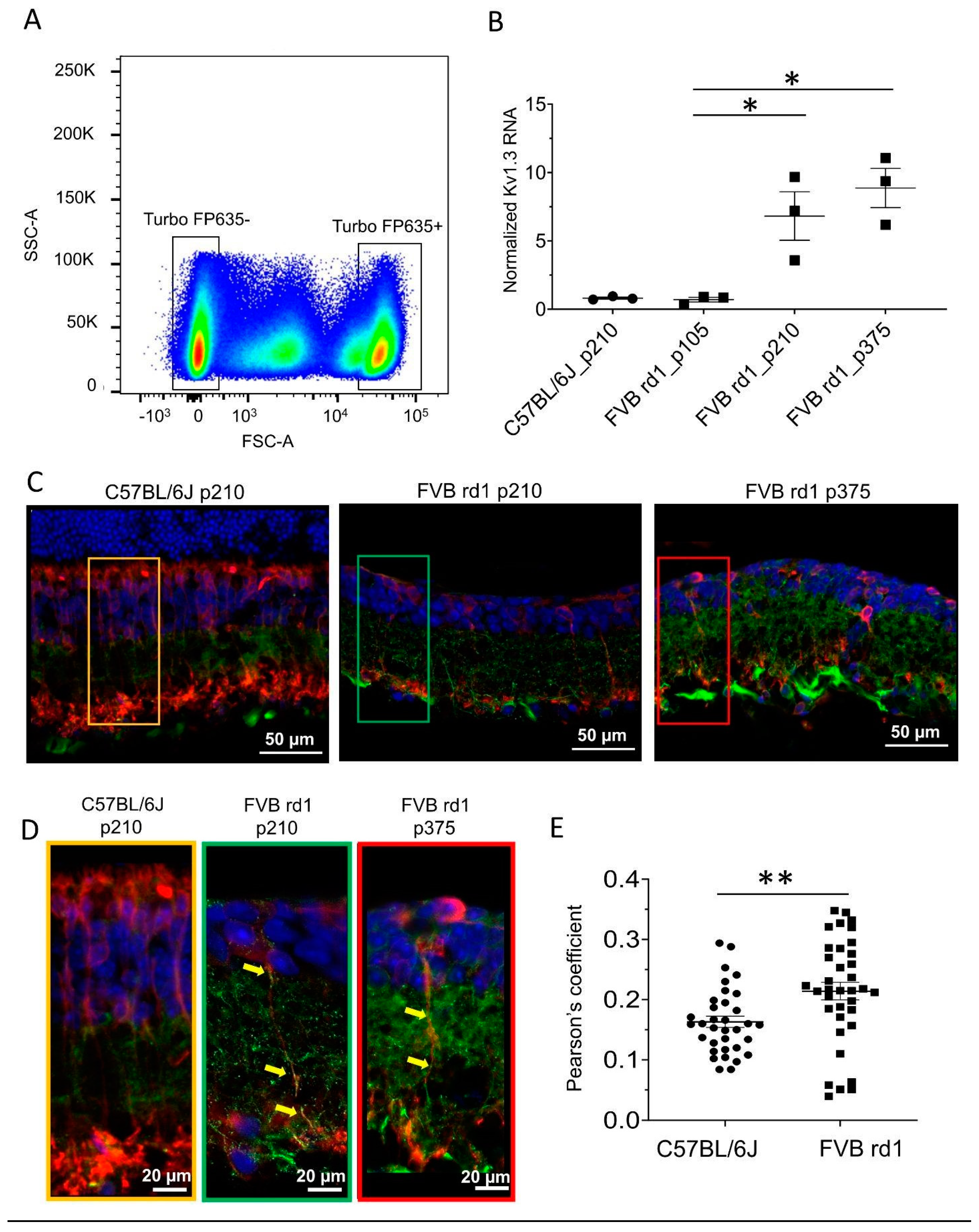
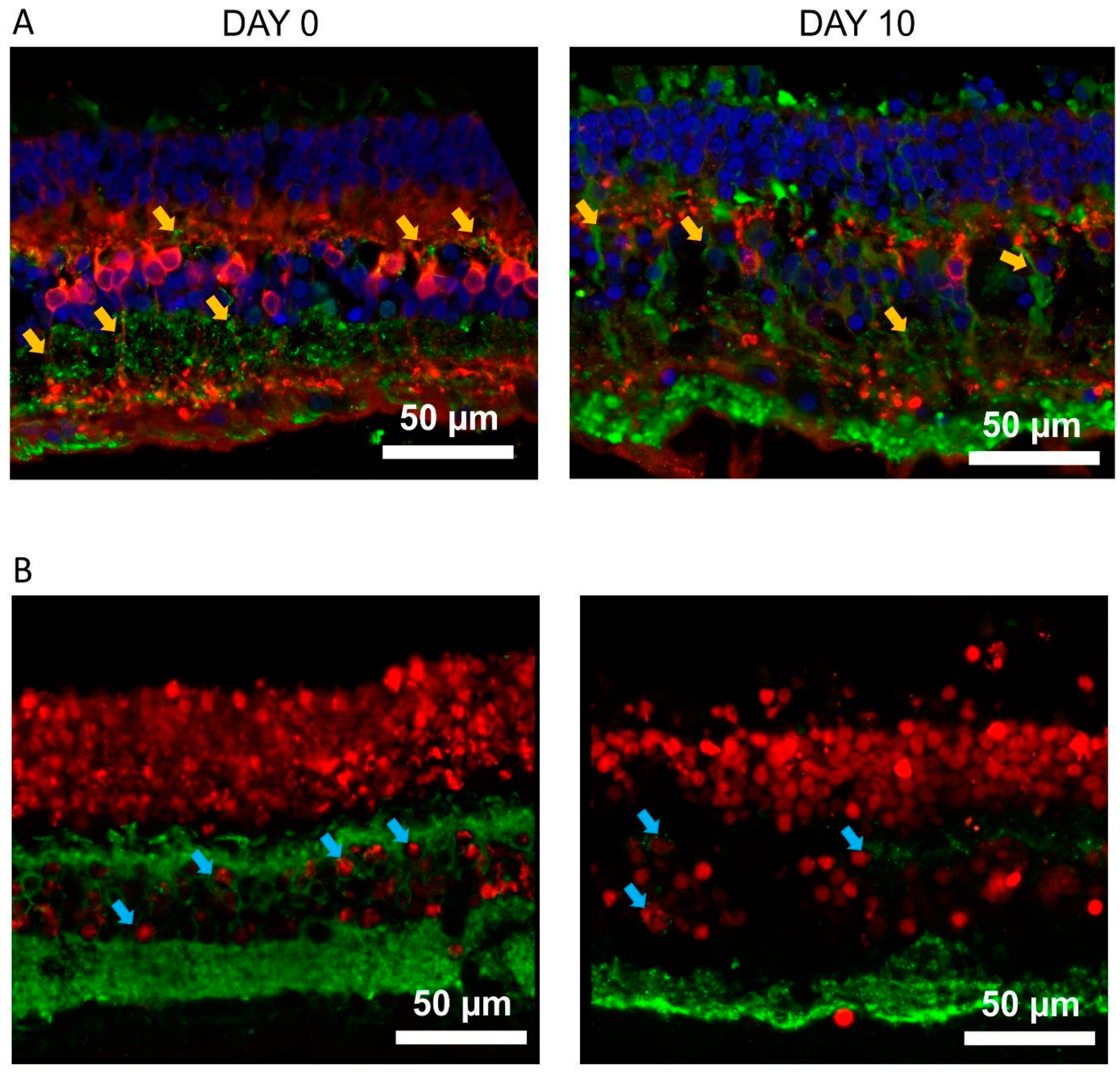
2.1. Kv1.3 is expressed in OBC axons of rd1 (FVB/NCrl_Opto-mGluR6) mouse retina
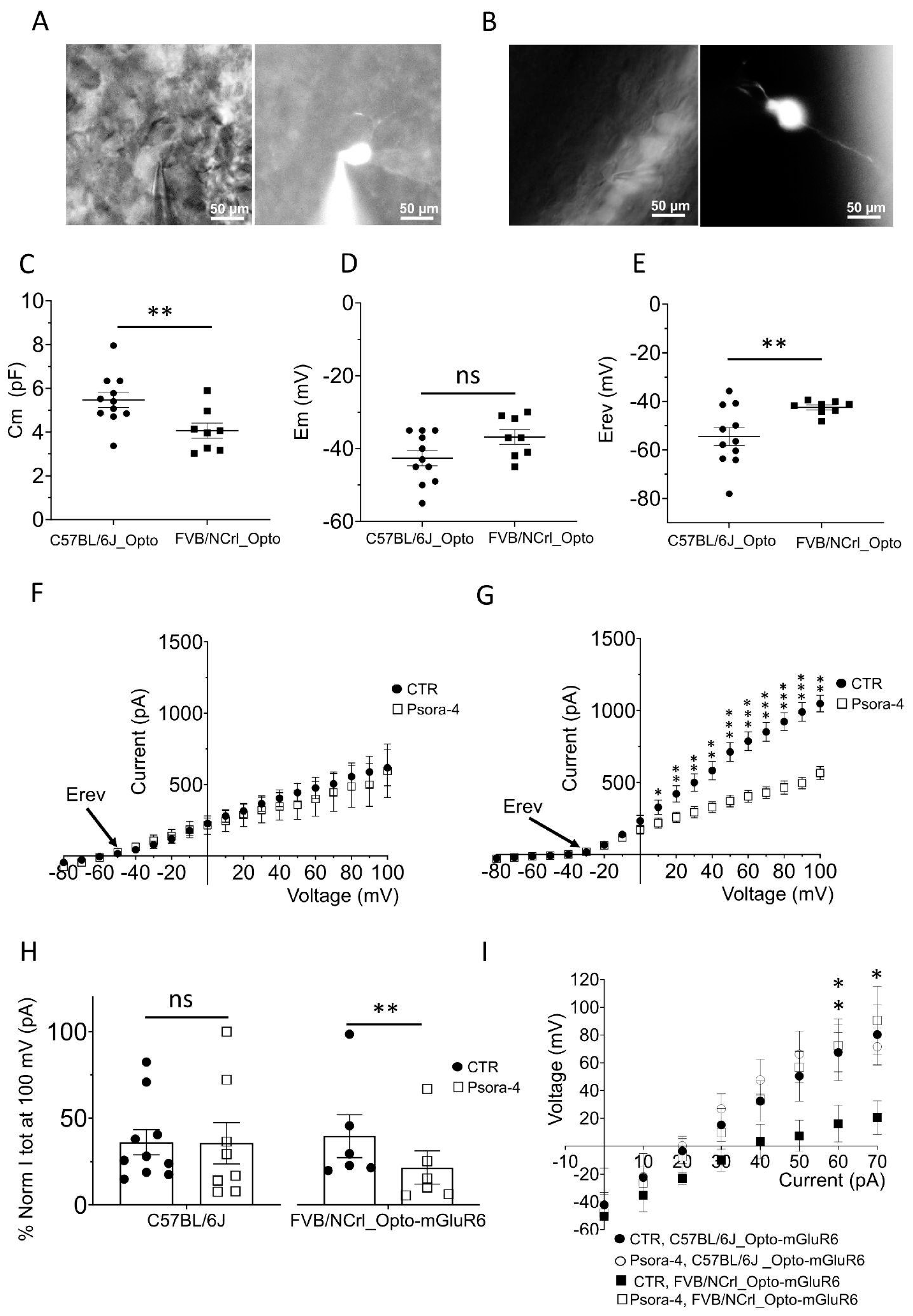
2.2. The delayed rectifier potassium current is driven by Kv1.3 in OBCs of the degenerated FVB rd1 retina
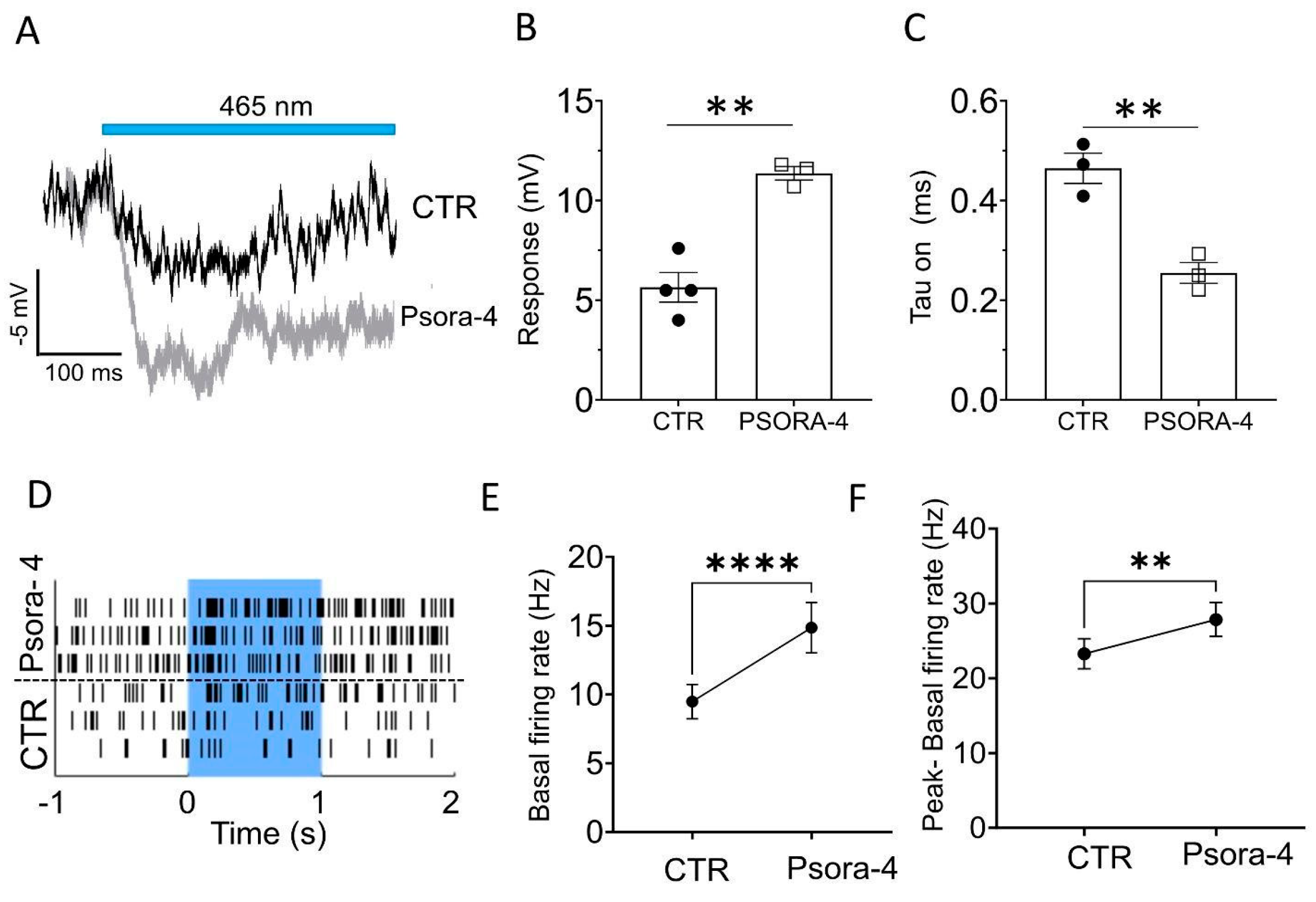
2.3. Kv1.3 inhibition accelerates and enhances the light-induced, Opto-mGluR6-mediated conductance in OBCs of the degenerated retina
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Fluorescence-activated cell sorting (FACS) and qPCR
4.3. Human retinal tissue and culturing
4.4. Immunohistochemistry
4.5. Patch-clamp recordings
4.5.1. Solutions and Drugs
4.5.2. OBC identification
4.5.3. Voltage and Current clamp recordings
4.5.4. Light responses from OBCs in the FVB rd1 retina
4.5.5. Multi electrode array recordings
4.5.6. Data analysis and statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeon, C.-J.; Strettoi, E.; Masland, R.H. The Major Cell Populations of the Mouse Retina. J Neurosci. 1998, 18, 8936–8946. [Google Scholar] [CrossRef] [PubMed]
- Behrens, C.; Schubert, T.; Haverkamp, S.; Euler, T.; Berens, P. Connectivity map of bipolar cells and photoreceptors in the mouse retina. eLife. 2016;5. [CrossRef]
- Ichinose, T.; Fyk-Kolodziej, B.; Cohn, J. Roles of ON Cone Bipolar Cell Subtypes in Temporal Coding in the Mouse Retina. J Neurosci. 2014, 34, 8761–8771. [Google Scholar] [CrossRef] [PubMed]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 2016, 166, 1308. [CrossRef] [PubMed]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. The Lancet. 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C. Retinitis pigmentosa. Orphanet J Rare Dis 2006 11. 2006, 1, 1–12. [Google Scholar] [CrossRef]
- Ferrari, S.; Iorio, E.D.; Barbaro, V.; Ponzin, D.; Sorrentino, F.S.; Parmeggiani, F. Retinitis Pigmentosa: Genes and Disease Mechanisms. Curr Genomics. 2011, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes. 2020, 11, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Leroy BP, Fischer MD, Flannery JG, MacLaren RE, Dalkara D, Scholl HPN, et al. Gene therapy for inherited retinal disease: long-term durability of effect. Ophthalmic Res. 2022. [CrossRef]
- Jang, J.; Kim, H.; Song, Y.M.; Park, J. Implantation of electronic visual prosthesis for blindness restoration. Opt Mater Express. 2019;9. [CrossRef]
- Bloch E, Luo Y, Cruz L da. Advances in retinal prosthesis systems. Ther Adv Ophthalmol. 2019, 11, 251584141881750. [CrossRef]
- Singh MS, Park SS, Albini TA, Canto-Soler MV, Klassen H, MacLaren RE, et al. Retinal stem cell transplantation: Balancing safety and potential. Prog Retin Eye Res. 2020, 75, 100779. [CrossRef]
- Yao K, Qiu S, Wang YV, Park SJH, Mohns EJ, Mehta B, et al. Restoration of vision after de novo genesis of rod photoreceptors in mammalian retinas. Nature. 2018, 560, 484–488. [CrossRef]
- Tochitsky, I.; Kienzler, M.A.; Isacoff, E.; Kramer, R.H. Restoring Vision to the Blind with Chemical Photoswitches. Chem Rev. 2018, 118, 10748–10773. [Google Scholar] [CrossRef] [PubMed]
- Hüll, K.; Benster, T.; Manookin, M.B.; Trauner, D.; Gelder, R.N.V.; Laprell, L. Photopharmacologic Vision Restoration Reduces Pathological Rhythmic Field Potentials in Blind Mouse Retina. Sci Rep. 2019;9. [CrossRef]
- Kralik, J.; Kleinlogel, S. Functional availability of on-bipolar cells in the degenerated retina: Timing and longevity of an optogenetic gene therapy. Int J Mol Sci. 2021;22. [CrossRef]
- Baker, C.K.; Flannery, J.G. Innovative Optogenetic Strategies for Vision Restoration. Front Cell Neurosci. 2018;12. [CrossRef]
- Sahel J-A, Boulanger-Scemama E, Pagot C, Arleo A, Galluppi F, Martel JN, et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 2021 277. 2021, 27, 1223–1229. [CrossRef]
- Gaub, B.M.; Berry, M.H.; Holt, A.E.; Isacoff, E.Y.; Flannery, J.G. Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol Ther. 2015, 23, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Wyk M van, Pielecka-Fortuna J, Löwel S, Kleinlogel S. Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLOS Biol. 2015, 13, e1002143. [CrossRef]
- Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R, et al. Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr Biol. 2015, 25, 2111. [CrossRef]
- Kralik J, Wyk M van, Stocker N, Kleinlogel S. Bipolar cell targeted optogenetic gene therapy restores parallel retinal signaling and high-level vision in the degenerated retina. Commun Biol 2022, 5, 1–15. [CrossRef]
- Strettoi, E.; Pignatelli, V. Modifications of retinal neurons in a mouse model of retinitis pigmentosa. Proc Natl Acad Sci. 2000, 97, 11020–11025. [Google Scholar] [CrossRef]
- Strettoi, E.; Porciatti, V.; Falsini, B.; Pignatelli, V.; Rossi, C. Morphological and Functional Abnormalities in the Inner Retina of the rd/rd Mouse. J Neurosci. 2002, 22, 5492–5504. [Google Scholar] [CrossRef]
- Menzler, J.; Zeck, G. Neurobiology of Disease Network Oscillations in Rod-Degenerated Mouse Retinas. 2011. [CrossRef]
- Dagar, S.; Nagar, S.; Goel, M.; Cherukuri, P.; Dhingra, N.K. Loss of photoreceptors results in upregulation of synaptic proteins in bipolar cells and amacrine cells. PloS One. 2014;9. [CrossRef]
- Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, et al. Neural reprogramming in retinal degenerations. Invest Ophthalmol Vis Sci. 2007, 48, 3364. [CrossRef]
- Soto, F.; Kerschensteiner, D. Synaptic remodeling of neuronal circuits in early retinal degeneration. Front Cell Neurosci. 2015, 9, 395. [Google Scholar] [CrossRef]
- Pfeiffer, R.L.; Marc, R.E.; Jones, B.W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog Retin Eye Res. 2020, 74, 100771. [Google Scholar] [CrossRef] [PubMed]
- Chua, J.; Fletcher, E.L.; Kalloniatis, M. Functional remodeling of glutamate receptors by inner retinal neurons occurs from an early stage of retinal degeneration. J Comp Neurol. 2009, 514, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Schilardi, G.; Kleinlogel, S. Two Functional Classes of Rod Bipolar Cells in the Healthy and Degenerated Optogenetically Treated Murine Retina. Front Cell Neurosci. 2022, 15, 562. [Google Scholar] [CrossRef] [PubMed]
- Ivanova E, Müller f. Retinal bipolar cell types differ in their inventory of ion channels. Vis Neurosci. 2006, 23, 143–154. [CrossRef] [PubMed]
- Hook, M.J.V.; Nawy, S.; Thoreson, W.B. Voltage- and calcium-gated ion channels of neurons in the vertebrate retina. Prog Retin Eye Res. 2019, 72, 100760. [Google Scholar] [CrossRef] [PubMed]
- Wässle, H.; Yamashita, M.; Greferath, U.; Grünert, U.; Müller, F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991, 7, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Klumpp, D.J.; Song, E.J.; Ito, S.; Sheng, M.H.; Jan, L.Y.; Pinto’, L.H. The Shaker-Like Potassium Channels of the Mouse Rod Bipolar Cell and Their Contributions to the Membrane Current. J Neurosci. 1995 pp. 5004–5013. [CrossRef] [PubMed]
- Wyk M van, Schneider S, Kleinlogel S. Variable phenotypic expressivity in inbred retinal degeneration mouse lines: A comparative study of C3H/HeOu and FVB/N rd1 mice. Mol Vis. 2015, 21, 811.
- Chang, B. Mouse models for studies of retinal degeneration and diseases. Methods Mol Biol Clifton NJ. 2013, 935, 27–39. [Google Scholar] [CrossRef]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Xiong, W.-H.; Pang, J.-J.; Pennesi, M.E.; Duvoisin, R.M.; Wu, S.M.; Morgans, C.W. The Effect of PKCα on the Light Response of Rod Bipolar Cells in the Mouse Retina. Invest Ophthalmol Vis Sci. 2015, 56, 4961–4974. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, P.D.; Wang, Y.; Schlichter, L.C. Kv1.1 and Kv1.3 channels contribute to the degeneration of retinal ganglion cells after optic nerve transection in vivo. Cell Death Differ 2010 171. 2009, 17, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, P.D.; Schlichter, L.C. Targeting KV channels rescues retinal ganglion cells in vivo directly and by reducing inflammation. Channels. 2010, 4, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Walston, S.T.; Chow, R.H.; Weiland, J.D. Direct Measurement of Bipolar Cell Responses to Electrical Stimulation in Wholemount Mouse Retina. J Neural Eng. 2018, 15, 046003. [Google Scholar] [CrossRef]
- Euler, T.; Wässle, H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995, 361, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Euler, T.; Masland, R.H. Light-Evoked Responses of Bipolar Cells in a Mammalian Retina. 2000, 83, 1817–1829. [Google Scholar] [CrossRef] [PubMed]
- Gilhooley MJ, Hickey DG, Lindner M, Palumaa T, Hughes S, Peirson SN, et al. ON-bipolar cell gene expression during retinal degeneration: Implications for optogenetic visual restoration. Exp Eye Res. 2021, 207, 108553. [CrossRef] [PubMed]
- Hargrove-Grimes P, Mondal AK, Gumerson J, Nellissery J, Aponte AM, Gieser L, et al. Loss of endocytosis-associated RabGEF1 causes aberrant morphogenesis and altered autophagy in photoreceptors leading to retinal degeneration. PLoS Genet. 2020;16. [CrossRef]
- Bales, K.L.; Gross, A.K. Aberrant protein trafficking in retinal degeneration: The initial phase of retinal remodelling. Exp Eye Res. 2016, 150, 71. [Google Scholar] [CrossRef]
- McLaughlin, T.; Medina, A.; Perkins, J.; Yera, M.; Wang, J.J.; Zhang, S.X. Cellular stress signaling and the unfolded protein response in retinal degeneration: mechanisms and therapeutic implications. Mol Neurodegener 2022 171. 2022, 17, 1–19. [Google Scholar] [CrossRef]
- Gayet-Primo, J.; Puthussery, T. Alterations in kainate receptor and TRPM1 localization in bipolar cells after retinal photoreceptor degeneration. Front Cell Neurosci. 2015, 9, 486. [Google Scholar] [CrossRef]
- Xu Y, Dhingra A, Fina ME, Koike C, Furukawa T, Vardi N. mGluR6 deletion renders the TRPM1 channel in retina inactive. J Neurophysiol. 2012, 107, 948. [CrossRef]
- Carmody, R.J.; Cotter, T.G. Oxidative stress induces caspase-independent retinal apoptosis in vitro. Cell Death Differ. 2000, 7, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Pliego, J.A.F.; Pedroarena, C.M. Kv1 potassium channels control action potential firing of putative GABAergic deep cerebellar nuclear neurons. Sci Rep. 2020;10. [CrossRef]
- Gazula, V.R.; Strumbos, J.G.; Mei, X.; Chen, H.; Rahner, C.; Kaczmarek, L.K. Localization of Kv1. 3 channels in presynaptic terminals of brainstem auditory neurons. J Comp Neurol. 2010, 518, 3205–3220. [Google Scholar] [CrossRef] [PubMed]
- Unique molecular characteristics and microglial origin of Kv1.3 channel–positive brain myeloid cells in Alzheimer’s disease | PNAS. [cited ]. Available: https://www.pnas.org/doi/full/10.1073/pnas. 10 May 2013.
- Pérez-García, M.T.; Cidad, P.; López-López, J.R. The secret life of ion channels: Kv1. 3 potassium channels and proliferation. Am J Physiol Cell Physiol. 2018, 314, 27–42. [Google Scholar] [CrossRef]
- Han, J.; Dinculescu, A.; Dai, X.; Du, W.; Smith, W.C.; Pang, J. Review: The history and role of naturally occurring mouse models with Pde6b mutations. Mol Vis. 2013, 19, 2579. [Google Scholar]
- Hulliger, E.C.; Hostettler, S.M.; Kleinlogel, S. Empowering Retinal Gene Therapy with a Specific Promoter for Human Rod and Cone ON-Bipolar Cells. Mol Ther - Methods Clin Dev. 2020, 17, 505–519. [Google Scholar] [CrossRef]
- Arman, A.C.; Sampath, A.P. Patch Clamp Recordings from Mouse Retinal Neurons in a Dark-adapted Slice Preparation. J Vis Exp JoVE. 2010; 43. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



