1. Introduction
Along with the rapid development of the internet and e-commerce market, the number of online orders dramatically increases, following the consumption of disposable lunch-boxes [
1]. In 2022, there were over 60 million disposable lunch-boxes discarded on each day [
2], which has inevitably posed challenges to the whole public health system and social environment all over the world [
2,
3]. An urgent need is to replace plastic food packaging materials (
i.
e., disposable lunch-box) using biodegradable, compostable, and environmentally ones such as biodegradable plant resourcing components or wastes [
4,
5,
6].
Sugarcane pulp (SCP) is a compostable environmentally material made from residual bagasse from the sugar industry through homogenizing, molding, shaping, and disinfection. In general, SCP contains 33.5 ~ 55 % glucan, 17 ~ 32% hemicellulose, 17 ~ 32% lignin, and 0.7 ~ 8% ash [
7,
8]. In 2020, the global sugarcane cultivation reached to 187 million tons [
9], while papermaking is still one of the most sustainable ways to use ba
gasse. SCP disposable lunch-box, which has the characteristics of lightness, moderate strength and toughness, biodegradability, and oil/water resistance, has been widely used in Eastern and Western countries for food packaging [
10,
11].
While the heavy metal content in food packaging materials made from plant-based wastes is generally hypothesized to be high [
12,
13,
14,
15]. Research by Ranjan
, et al. [
16]and Liu
, et al. [
17] suggested that the release of microplastics into hot water may also increase the concentration of heavy metals in the liquid, which could pose a health hazard. In ad
dition to (heavy) metals, the released microplastics (the particle size < 5 mm) are also cause for concern [
16,
18]
. Studies have also reported that the microplastics can induce male reproductive toxicity and intestinal microflora imbalance and inflammation in mice [
19,
20], while the microplastics released from polypropylene (PP) with particle diameter < 20 μm can cause cytotoxicity by increasing reactive oxygen species [
21].
Accordingly, similar to PP/polystyrene (PS) lunch-boxes, it is reasonable to assume that the SCP lunch-boxes may also produce microparticles when in contact with foods [
22]
. Fadare, et al. [
18]
determined the average weight of the isolated microparticles to be as high as 38 ± 5.29 mg per plastic container, with various morphological characteristics. While, Du, et al. [
22]
speculated that microplastic intake through containers may be up to 203 items/person/week. At present, there are few studies on the release of microparticles from food contact materials, especially sugarcane pulp lunch-boxes.
In the current study, fifteen brands of commercial SCP lunch-boxes were purchased in China. Fourier Transform-Infrared Spectrometer (FTIR) and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) were used to identify the functional groups and measure the content of (heavy) metal within the lunch-box, respectively. DI water, 4% HAc, and 95% EtOH were used as aqueous, acidic, and fatty foods simulants to analyze the overall migration as well as the migration of microparticles under different conditions (i.e., temperature and time). Laser diffraction (LSD) and scanning electron microscope (SEM) were used to size distribution and quantitative analysis of microparticles. This is the first study to systematically uncover how different foods including aqueous, acidic, and fatty foods influence the release of microparticles under different conditions, which will provide corresponding suggestions and opinions to SCP lunch-box manufacturers and consumers.
2. Materials and Methods
2.1. Chemicals
Ethanol, acetic acid, and Nitric acid were of analytical-reagent grade from Macklin Biochemical Co., Ltd. (Shanghai, China). Water was prepared by a Milli-Q ultrapure water system (Millipore, Woburn, MA, USA).
2.2. Collection of SCP lunch-boxes
In total, fifteen types/brands SCP lunch-boxes from nine provinces in China were purchased in June 2021. The purchased lunch-boxes, named as S-1 ~ S-15, respectively, (
Table 1) were sealed and stored in a dark place. While most of the lunch-boxes can withstand the temperature as high as 100 ºC, the maximum withstand temperature for S-13 is only 80 ℃.
2.3. Overall migration
The overall migration test refers to the total amount of all non-volatile substances that migrate from food contact materials and products to food contact simulators under specific soaking conditions with appropriate food simulators. It is usually expressed as milligrams of non-volatile migrations per kilogram of food simulators (mg/kg), or milligrams of non-volatile migrations per square decimeter of contact area (mg/dm2). As all the SCP lunch-box purchased from different companies showed differences in terms of density and thickness, the immersing area of each lunch-box was thus used.
Referring to the regulations in GB 31604.1-2015 [
23] and combined with the expected use of the lunch-box, the overall migration test conditions were set at 70 °C for 2 h. Food simulants including DI water, 4% HAc (
v/
v) and, 95% EtOH (
v/
v) were used to replace real aqueous, acidic, and fatty foods, so as to eliminate the influence of impurities in real foods. Other test conditions were carried out according to GB5009.156-2016 [
24] and GB 31604.8-2016 [
25].
Namely, for each lunch-box, six pieces of fragments (each with a surface area of 1 cm
2) were strictly selected and then immersed with 20 mL food simulants, to give a volume/contact area ratio of 6. After immersing with food simulants at 70
oC for 2 h, the solution was collected, transferred to a pre-dried glass evaporating dish, and placed in a water bath (HH-4, Juchuang, Qingdao, China) to evaporate for 30 ~ 60 min. The mixture was dried at 105±2 ºC in an oven for another 2 h. After cooling to room temperature in a desiccator, the dish was weighted. For the overall migration tests in contact with all three food simulants, the overall migration value was calculated using
Eq (1):
Here, Xi is the overall migration value of the SCP lunch-box, mg/dm2; M1 is the residue weight of the immersing solution, mg; M2 is the residual mass of the blank (only the food simulants without immersing with the lunch-box pieces), mg; V1 is the total volume of soaking solution, mL; V2 is the evaporated volume of soaking solution, mL. In the current study, V1 = V2; S represents the area of contact between the lunch-box, 12 cm2.
2.4. Collection of the evaporation residue particles (ERP)
As stated in
Section 2.3, for all lunch-boxes, the residues after the overall migration tests when in contact with all the three types food simulants were collected, mixed, and freeze-dried. Freeze-dried residues were named as the evaporation residue particles (ERP).
2.5. Cluster analysis
Based on the overall migration results described in
Section 2.3, cluster analysis was carried out with the overall migration value of each lunch-box into different food simulants setting as variables, the inter-group connection as the clustering method, and the Euclidean distance as the measurement interval. This is aimed to classify all the fifteen lunch-boxes into different groups based on the overall migration results into different food simulants. According to the cluster analysis results, representative lunch-boxes were then selected for the following analysis of the microparticles.
2.6. Fourier Transform Infrared Spectroscopy (FTIR) analysis
Fourier Transform Infrared Spectroscopy (FTIR) (Nicolet iS50+iN10, Thermo Fisher Scientific, Massachusetts, USA) was used to identify the chemical groups of all SCP lunch-boxes and the Evaporation Residue Particles (ERP).
Prior to analysis, all SCP lunch-boxes, except ERP which was already fine powders, were shredded/cut into small pieces, frozen with liquid nitrogen, and then ground into fine powders. Transmission method with a spectral range of 4000 ~ 400 cm-1 and 64 scans were used.
2.7. Inductively Coupled Plasma-Mass Spectrometry analysis (ICP-MS)
The concentration of metallic elements of the lunch-boxes including S-1 ~ S-15 was measured using the ICP-MS (ThermoFisher Scientific, Massachusetts, United States) according to the method of Xie
, et al. [
26] with minor modifications.
In brief, 0.5 g SCP powders was weighted and mixed with 50 mL nitric acid for digestion. The digestion solution was then diluted tenfold for analysis using the ICP-MS. For ERP, only 0.05 g ERP were weighted and mixed with 10 mL nitric acid for digestion and then analyzed using the ICP-MS as well.
2.8. Collection of microparticles
For collecting the microparticles released from the lunch-boxes when in contact with food simulants, the total volume of food simulant was used based on the maximum capacity of the lunch-box as specified by the manufacturer (
Table 1). During the experiment, it was observed that when in contact with 95% EtOH, the liquid leaked significantly (Figure S1). Accordingly, for all food simulants including DI water, 4% HAc, and 95% EtOH, the full immersion method was adopted. That is, each lunch-box was cut into equal quarters and then completely immersed with the food simulants which have been preheated to 70
oC. The solution containing released microparticles after immersing with the food simulants under different temperatures (70 or 100
oC) or times (30 or 120 min), respectively, were obtained and filtered using a vacuum pump (SHZ-DIII, Yuhua, China), sand core filter device (T-50, Jinteng, China) combined with a 0.22 μm nylon filter membrane (Jinteng, China). For the solution immersed with 95% EtOH, polytetrafluoron filter membrane (Jinteng, China) instead of nylon filter membrane was used. The microparticles that remained on the filter paper were rinsed into a glass triangular conical bottle using exactly 15 mL DI water.
For the washed solution containing microparticles, 1 mL was stored at -20 ºC, which was further used to conduct quantitative and morphology analysis of microparticles. The remaining solution was used for diameter or particle size analysis. In order to avoid contamination of the environment, the laboratory doors and windows were closed during the whole experimental process, and three sets of parallel and blank controls were set.
2.9. Measurement of the particle diameters
The particle diameters of the collected microparticles were measured immediately after extraction using the LSD particle diameter distribution apparatus (SALD-2300, Shimadzu, Japan). For Shimadzu SALD-2300, the LSD scattering method was applied to measure the particle diameters of the collected microparticles released when immersing with food simulants (0.17 ~ 2500 μm). The machine automatically adjusts the refractive index so that the diameter of the microparticles could be measured properly. Preliminary experiments showed that when the refractive index was 1.35 - 0.00i. The particle diameter was ranged 1 ~ 1000 μm. In order to reduce accidental errors (i.e., the anisotropy of particle distribution), triplicate measurement was set in parallel and the averaged value was recorded.
2.10. Quantitative analysis and morphological observation of the microparticles
The morphology and the number of the collected microparticles were observed and visualized using the SEM (EVO MA15, Germany).
In brief, a 10 μL solution containing macroparticles as prepared in
Section 2.8 was dropped onto a copper platform covered with Al conductive adhesive, dried in an oven at 30 ºC, and coated with a gold coating for visualization. A fixed and appropriate magnification was chosen so that all microparticles could be captured in one graph. Based on the SEM images, the total number of macroparticles was calculated manually with the help of counting tools in Adobe Photoshop. For each sample, three determinations were performed and the final value was the average of the three determinations. The conversion between the quantity and concentration of microparticles released by the lunch-box when immersed with food simulants was as follows:
Here,C represents the total concentration of microparticles in the solution released from the lunch-box when immersing with different food simulants, items/mL; Qi is the averaged value of the total number of microparticles observed in SEM image with triplicates. 15 is the total volume of the washed liquid, mL; V0 is the volume of food simulants that was used for immersing, mL; The value 100 is the computational multiple of 10 μL to 1 mL.
2.11. Data analysis
Microsoft Excel was used for data processing and image rendering. Cluster analysis and significance analysis were performed by IBM SPSS Statistics 25. Kruskal-Wallis one-way ANOVA test was used to analyze the significance of lunch-boxes in different simulants (P < 0.05). Adobe Photoshop 2020 was used to conduct quantitative and morphological analysis.
3. Results and discussion
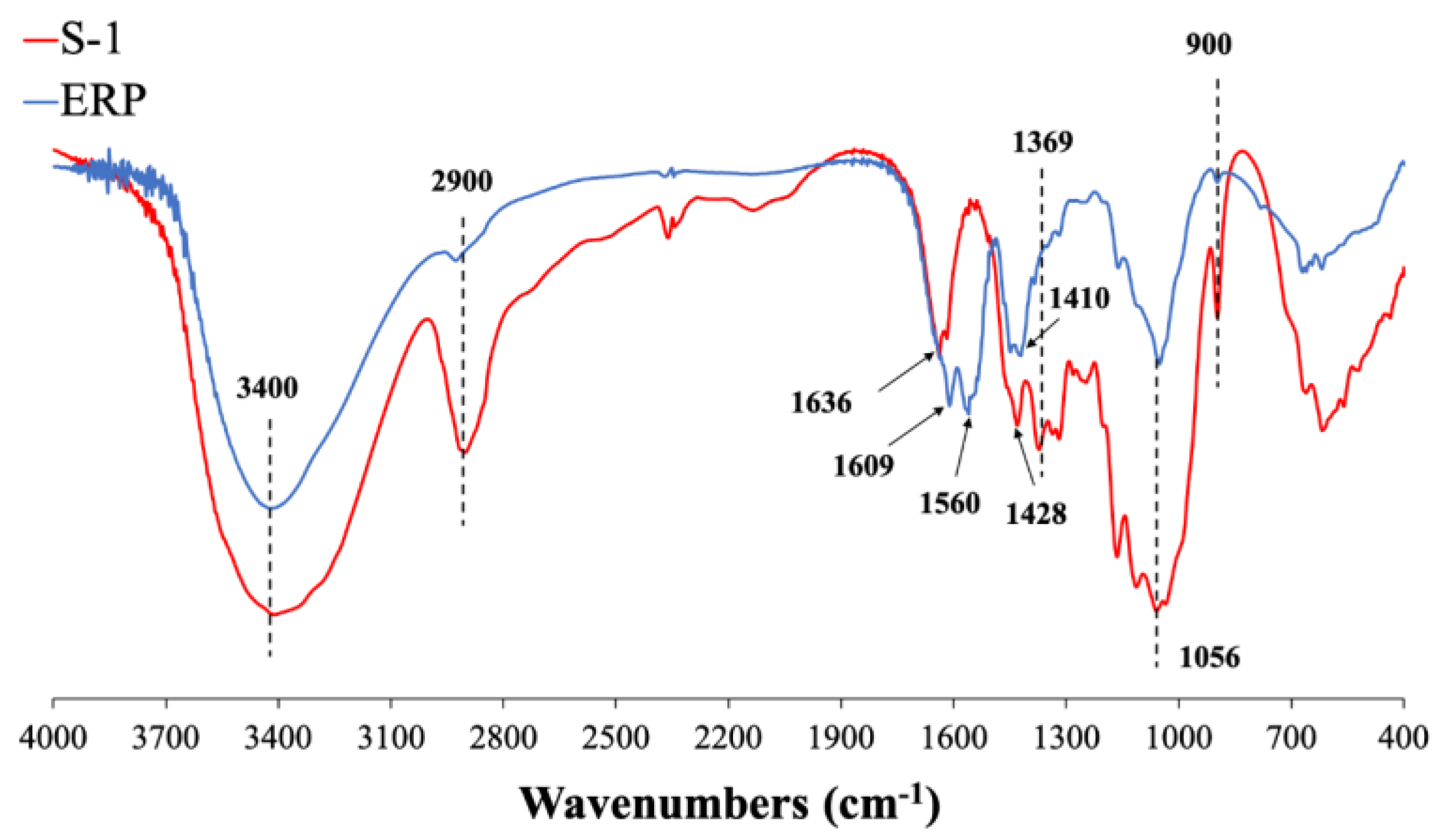
3.1. Characterization of the lunch-boxes
Figure 1 shows typical FTIR spectra of SCP lunch-boxes where S-1 was used as an example, while that of all rest lunch-boxes was provided in Figure S2. Of all SCP lunch-boxes, the infrared spectra were all similar to previous studies [
11,
27]. The peak at ~ 3400 cm
-1 was mainly attributed to the vibration of hydrogen bonds in cellulose and hemicellulose hydroxyl (-OH) groups, the peak near 2900 cm
-1 was caused by the asymmetric stretching vibration of C-H groups in lignin and/or cellulose of the sugarcane fibers.
Compared to that of the lunch-box, certain differences were observed in the FTIR peaks of ERP. Note that ERP was collected as a mixture of all the migrated components from lunch-box to food simulants (DI water, 4% HAc, and 95% EtOH) at 70
oC for 2 h. Compared with S-1, the peaks of ERP at ~ 2900 cm
-1 and 900 cm
-1almost disappeared, representing the stretching vibration of hydroxyl groups (-OH) in cellulose/hemicellulose and C-O-H / C-O-C of sugar rings in hemicellulose and lignin. It was indicated that only trace amounts of cellulose/lignin migrated from SCP disposable lunch-boxes when immersed with these food simulants at 70
oC for 2 h. Of ERP, the peak at ~ 1636 cm
-1 also disappeared, whereas the new peak at ~ 1609 cm
-1 was probably caused by the vibration of the carboxyl group (-COO) in 4% HAc remaining in ERP, as seen in a previous study as well [
28]. Moreover, of ERP, the peaks at ~ 1428 and ~ 1369 cm
-1, respectively, were probably created by the vibrations of aliphatic or aromatic C-H and C-O groups in lignin [
27,
28]. The peaks ranging between 1369 ~ 1316 cm
-1 were mainly attributed to the C-O and/or O-H vibration of hemicellulose and/or lignin groups, and may also be caused by the bending vibration of C - O and C - H bonds in the aromatic ring.
3.2. Metal contents of SCP lunch-box
When grown in contaminated soil, the plant may accumulate metals, which may eventually pass into the processed products (
i.
e., tableware and lunch-box). As shown in
Table 2, of all SCP lunch-box except S-11, Al and Fe are the main metals, ranging from 35.16 to 1244.04 and from 44.71 to 398.52 mg per kg lunch-box, respectively, followed by Pb, Ti, and Sr. For Al, as no such high concentration has been generally detected in either sugarcane fibers or its relevant products, it thus can be speculated that the high content of Al in SCP disposable lunch-boxes might be introduced because of the addition of additives (
i.
e., aluminate coupling agent or titanium dioxide which are generally used as surfactants for disposable lunch-box) [
29,
30].
Sugarcane plants may also contain a relatively high content of Fe, Zn, Sr, Mn, and other micronutrients such as Ce and Ba. Interestingly, unlike other lunch-boxes, the content of Pb in S-11 was the highest, with a number of 136 mg per kg lunch-box (
Table 2). This result has also been observed previously [
31]. During plant growth, Pb may be accumulated in the roots [
32]. The large amount of Pb in S-11 may be either related to the soil or the industrial emissions [
33]. This suggests that notification should be paid to the heavy metal element contamination of the lunch-box made from plant wastes (
i.
e., wheat or sugarcane straw) so that their content could be kept in a safty range.
Lastly, the averaged concentration of metallic elements of all lunch-boxes has also been calculated and was found in an order of Al > Fe > Pb > Ti > Sr > Mn > Zn > Ba > Zr > Cr. As for metal element content in ERP, it was found that the average content of metal elements was significantly higher than that in the original lunch-box counterparts. For example, the average content of Al in ERP was 21779.86 mg per kg lunch-box, which was almost 100 times higher than that of the original lunch-boxes counterpart. This was probably because when immersing with food simulants, the metal elements were more easily migrated into the food simulants, and the adsorption of ERP further increases its content in ERP.
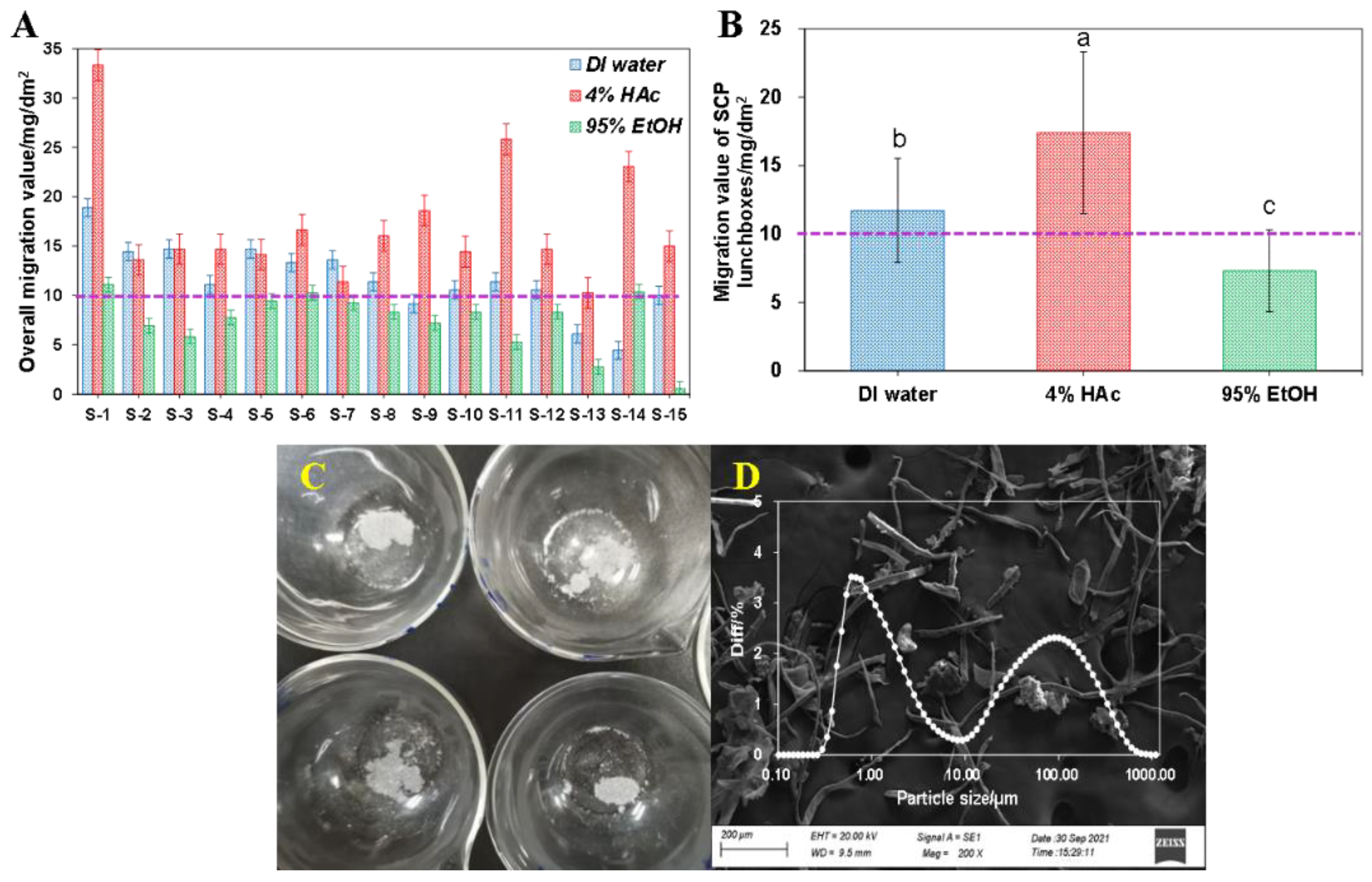
3.3. Overall migration of SCP lunch-boxes
Table 3 and
Figure 2A show the overall migration results of the SCP lunch-boxes after immersing with different food simulants at 70 ºC for 2 h. As showed clearly, when immersing with 4% HAc, the overall migration of all lunch-boxes was ranged between 33.33 ± 1.18 mg/dm
2 for S-1 and 10.28 ± 0.39 mg/dm
2 for S-13, respectively. When immersing with DI water, the overall migration value was between 4.44 ± 0.39 and 14.72 ± 1.42 mg/dm
2, whereas it was ranged between 0.56 ± 0.39 and 11.11 ± 1.04 mg/dm
2 when immersing with 95% EtOH. Moreover, results also showed that compared with either DI water or 95% EtOH, the total weight of migrating components from the lunch-box to 4% HAc food simulant was the highest.
As showing in
Figure 2B, significant differences were observed in terms of the averaged migration value of all lunch-boxes in contact with different food simulants. In detail, after immersing with 4% HAc at 70
oC for 2 h, it was found that the averaged overall migration of all fifteen lunch-boxes was 17.41 mg/dm
2, followed by that with the DI water and then with the 95% EtOH, with an average number of 11.72 and 7.31 mg/dm
2, respectively. This was probably because that under acidic conditions, the lignocellulosic (
i.
e., cellulose and lignin) in the SCP lunch-boxes degrade, and leached out [
34]. Moreover, although compared with DI water, 95% EtOH food simulant has stronger penetration ability and can quickly penetrate into the lunch-boxes. Nevertheless, in this study, our results showed that the overall migration value of lunch-boxes when immersing with DI water was higher than that in 95% EtOH food simulant (
Figure 2B), which may be caused by the filler or surface coating added to the lunch-boxes. Lastly, as shown in
Figure 2C&D, after freezer-drying, the white flocculent precipitates (ERP) are mainly composed of fibers with different lengths, while some spherical particles with much smaller sizes also exist. The diameter of the ERP was found in the range of 0.1 ~ 1000 μm, which belongs to the category of microparticles (particle diameter < 5 mm) [
18,
20,
22].
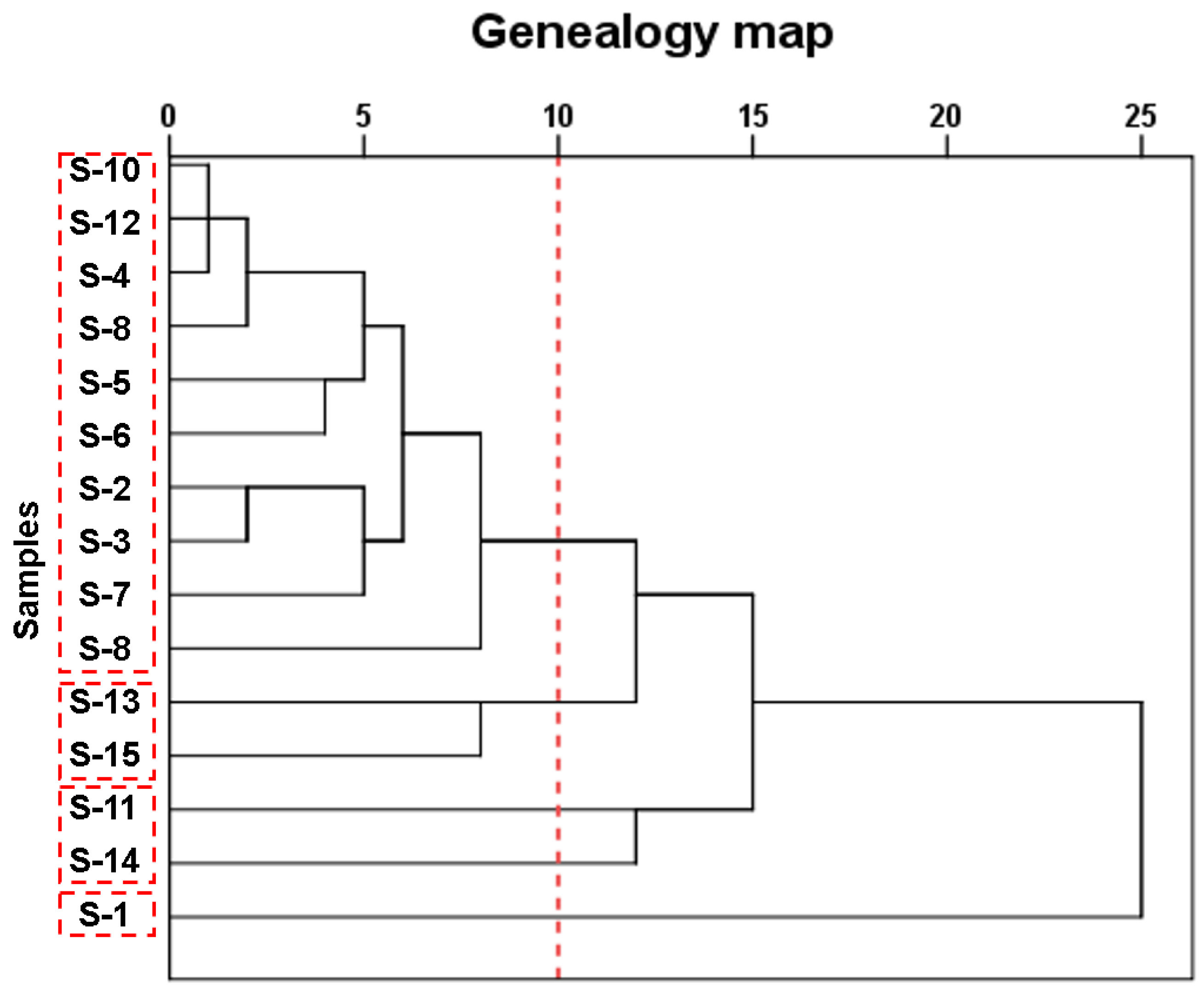
3.4. Cluster analysis
In order to explore how immersing temperature, immersing time, and the type of food simulants including the DI water, 4% HAc, and 95% EtOH impact the diameter of the microparticles released from the SCP lunch-boxes, a fast and simple systematic clustering analysis was conducted using the overall migration value to each food simulant as variables. As shown in
Figure 3, all fifteen lunch-boxes could be classified into four classes. In detail, while S-1 performed completely differently from the rest lunch-boxes, S-11 and S-14 performed similarly and thus could be clustered together. In the meantime, while both S-13 and S-15 performed similarly, the rest types of lunch-boxes all performed similarly, and thus were grouped together.
3.5. Impacts of immersing conditions on the production of microparticles
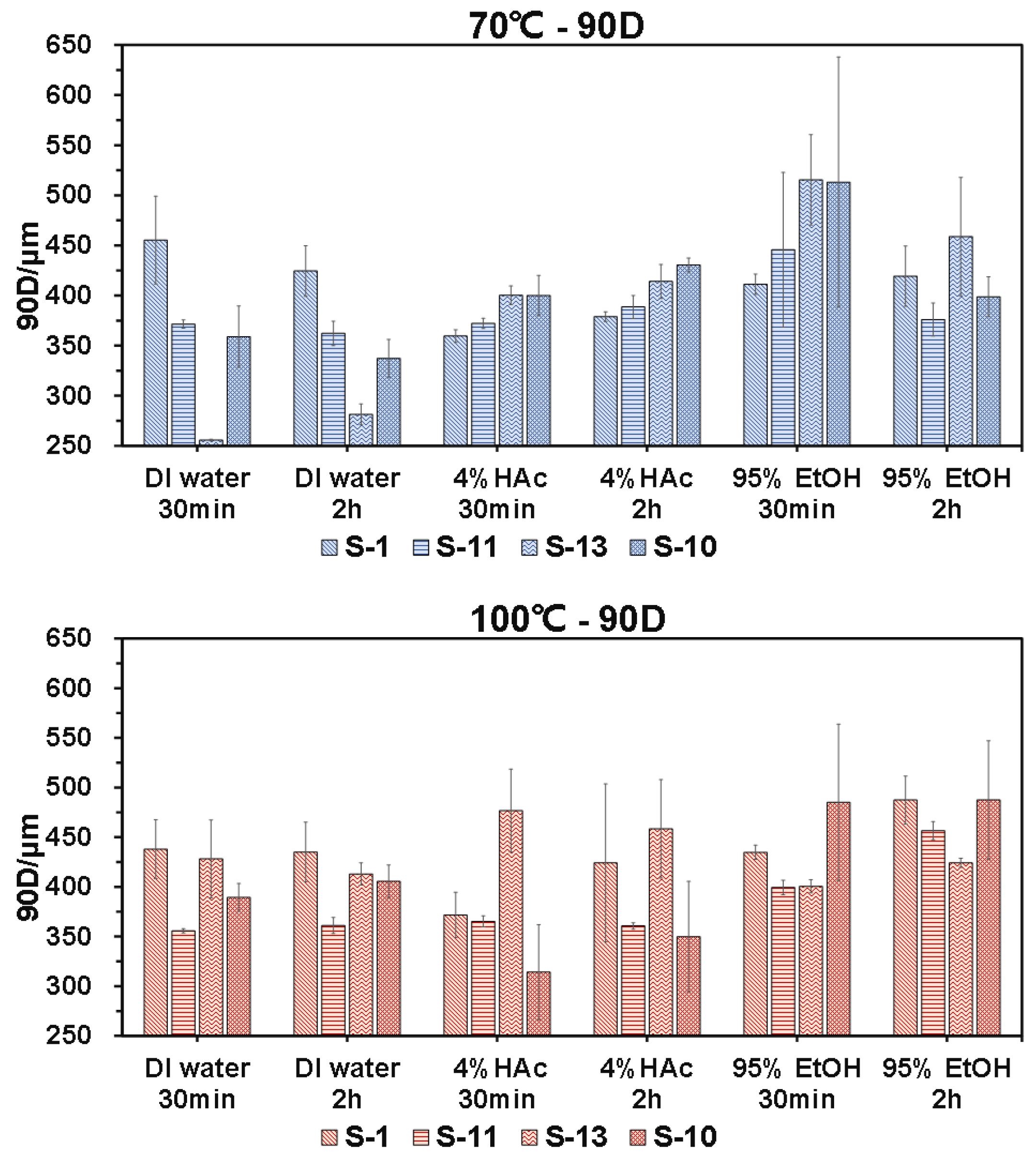
3.5.1 190. D
Figure 4 shows the particle size of the microparticles of the four brands of commercial SCP lunch-boxes after immersing with different food simulants at two temperatures (70 & 100
oC, respectively) and immersing time (30 & 120 min, respectively). As shown on the left, the 90D, representing the particle diameter when the cumulative percentage of the particle diameter distribution of the sample reaches 90%, of the four lunch-boxes was significantly different even when immersing with the same food simulant. For example, when immersing with DI water at 70
oC for either 30 min or 120 min, the 90D of the macroparticle produced by S-13 was significantly smaller than the rest three brands of lunch-boxes, especially the S-1. Nevertheless, when immersing with 4% HAc food simulant, the 90D of the microparticles produced by S-13 was significantly larger than that of the S-1. This thus necessitates future research to be conducted to investigate how the interaction between the SCP lunch-box and food simulant affects the migration properties of the microparticles.
When the immersing temperature increased from 70 to 100
oC, it was found that the particle diameter of the microparticles generated by S-13 showed fewer differences with other lunch-boxes. According to the manufacturer’s introduction, the S-13 was not suitable for the temperature of 80 ºC and above (
Table 1). This suggests that the changes in the temperature of the packaged food could significantly influence the diameter of the released microparticles. This is different from previous study where the author reported that for plastic disposable lunch-boxes (
i.
e., PP/PS), the treatment of hot water or shaking showed no significant influence on microplastics abundance of take-out containers. This is probably because the disposable lunch-box made from plant wastes is more vulnerable to the temperature of the packaged food.
Now if we compare the diameters (90D) of the microparticles produced by S-13 when immersing with DI water and 4% HAc at 70ºC, respectively, it was found that the particle diameter of the microparticles produced by 4% HAc was significantly larger than that of by DI water. This suggests that the nature of the packaged foods (i.e., water-soluble food or oily food) leads to significant variations in the particle size of microparticles released from the lunch-boxes. Compared with water-soluble foods, it was less safe when packing acidic food.
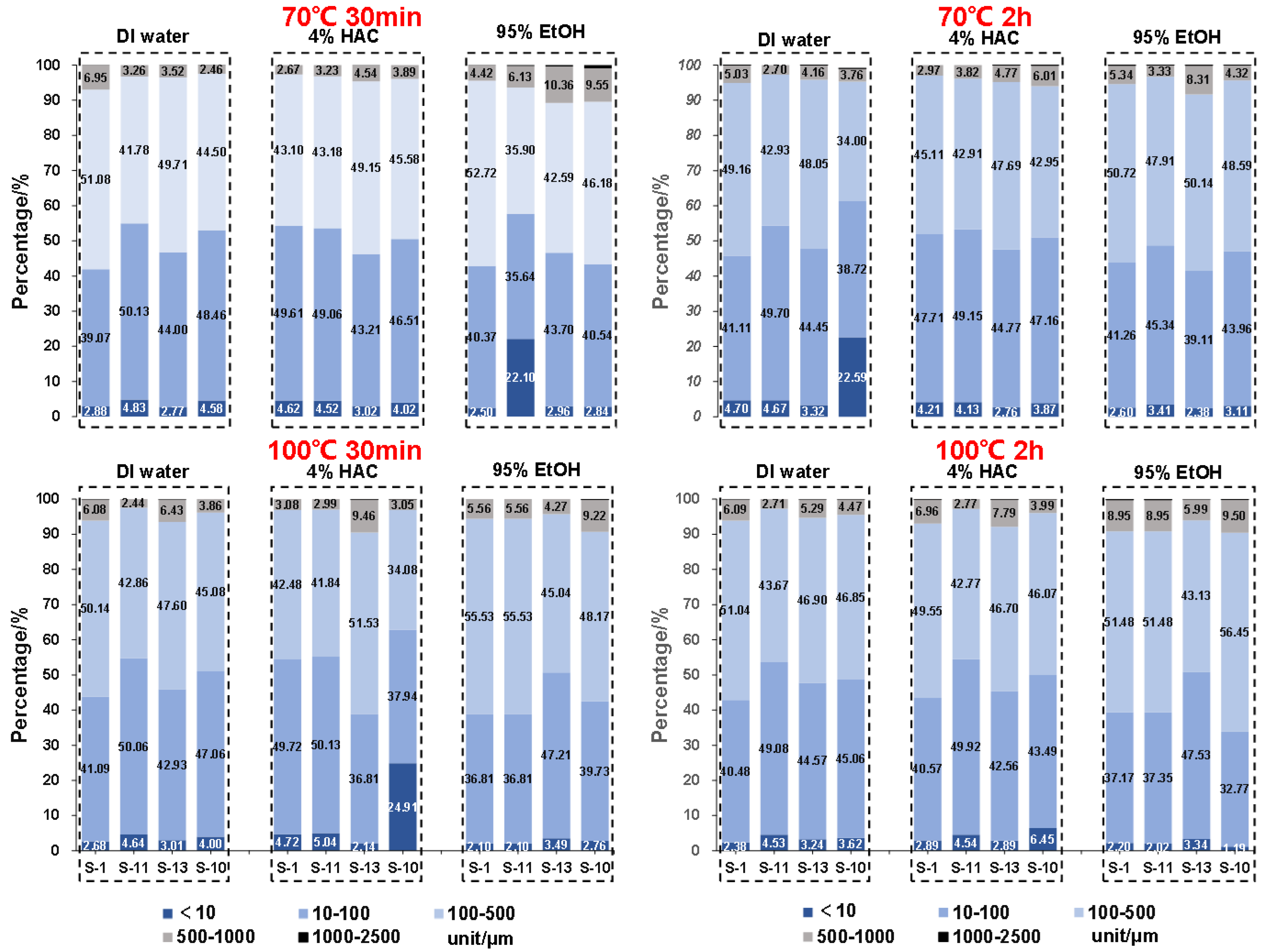
3.5.2. Particle size distributions of microparticles in contact with different food simulants
Based on the cumulative distribution data of the microparticles released from different lunch-boxes when in contact with different food simulants under different conditions, the whole range of microparticles was sub-divided into five fractions including < 10, 10 ~ 100, 100 ~ 500, 500 ~ 1000 and 1000 ~ 2500 μm (Fra I ~ V). In the first place, as shown in
Figure 5, under different conditions (
i.
e., immersing time & temperature), significant differences were observed. It was found that for all lunch-boxes, almost no microparticles with particle size > 1000 μm were released, whereas the largest proportion of microparticles ranged 10 ~ 500 μm including Fra II and Fra III.
(1) DI water
When in contact with DI water at 70 oC for 30 min, of all SCP lunch-boxes, the percentage of Fra I (< 10 μm) ranged between 2.88 ~ 4.58% and was between 4.70 ~ 22.58% when the immersing time increased to 120 min. As noted, for S-10, when immersing with the DI water food simulant at 70 oC for 120 min, the percentage of the microparticles with particle size < 10 μm (Fra I) reached to 22.58%, which was significantly higher than of the rest lunch-boxes. Now if we increased the temperature to 100 oC, the percentage of the microparticles with particle size < 10 μm (Fra I) was between 2.68~4.64% and 2.38 ~ 4.53% when immersing for 30 and 120 min, respectively. These results suggest that with increased temperature, the content of Fra I did not necessarily increase accordingly.
(1) 4% HAc
When in contact with 4% HAc at 70 oC for 30 min, of all SCP lunch-boxes, the percentage of Fra I (< 10 μm) was between 3.02 ~ 4.62%, and was between 43.21 ~ 49.61% for Fra II (10 ~ 100 μm), 43.10 ~ 49.15% for Fra III (100 ~ 500 μm) and 2.67~4.54% for Fra IV (500 ~ 1000 μm), respectively. Except S-10, for the rest three lunch-boxes, no significant differences were observed even though the immersing time and temperature have been changed. Particularly, for S-10, when the temperature increased from 70 to 100 oC, the percentage of microparticles with particle size < 10 μm (Fra I) increased dramatically from 4.02% to 24.91%. However, at 100 oC, when immersing time increased from 30 to 120 min, the percentage of the released microparticles with particle size < 10 μm (Fra I) decreased significantly from 24.91% to 6.45%. This suggests that the conditions, especially the temperature of the food simulant significantly influences the particle size distribution of the microparticles, while it is also easily-understood that different lunch-boxes purchased from different company performed differently.
95% EtOH
Compared with DI water and 4% HAc, oily foods are more frequently consumed in Asian countries, especially in China, which is generally represented by 95% EtOH. As shown in
Figure 5, when immersing with 95% EtOH at 70
oC for 30 min, the particle size distribution of the microparticles released from the four lunch-boxes was ranged between 2.50 ~ 22.10% (Fra I, < 10 μm), 35.64 ~ 43.70% (Fra II, 10 ~ 100 μm), 35.90 ~ 52.72% (Fra III, 100 ~ 500 μm) and 4.42 ~ 10.35% (Fra IV, 500 ~ 1000 μm), respectively. When the temperature reached to 100
oC, the particle size distribution of each fraction was in the range of 2.1~ 3.49% (Fra I), 36.81 ~ 47,21% (Fra II), 45.04 ~ 55.53% (Fra III) and 4.27 ~ 9.22% (Fra IV), respectively.
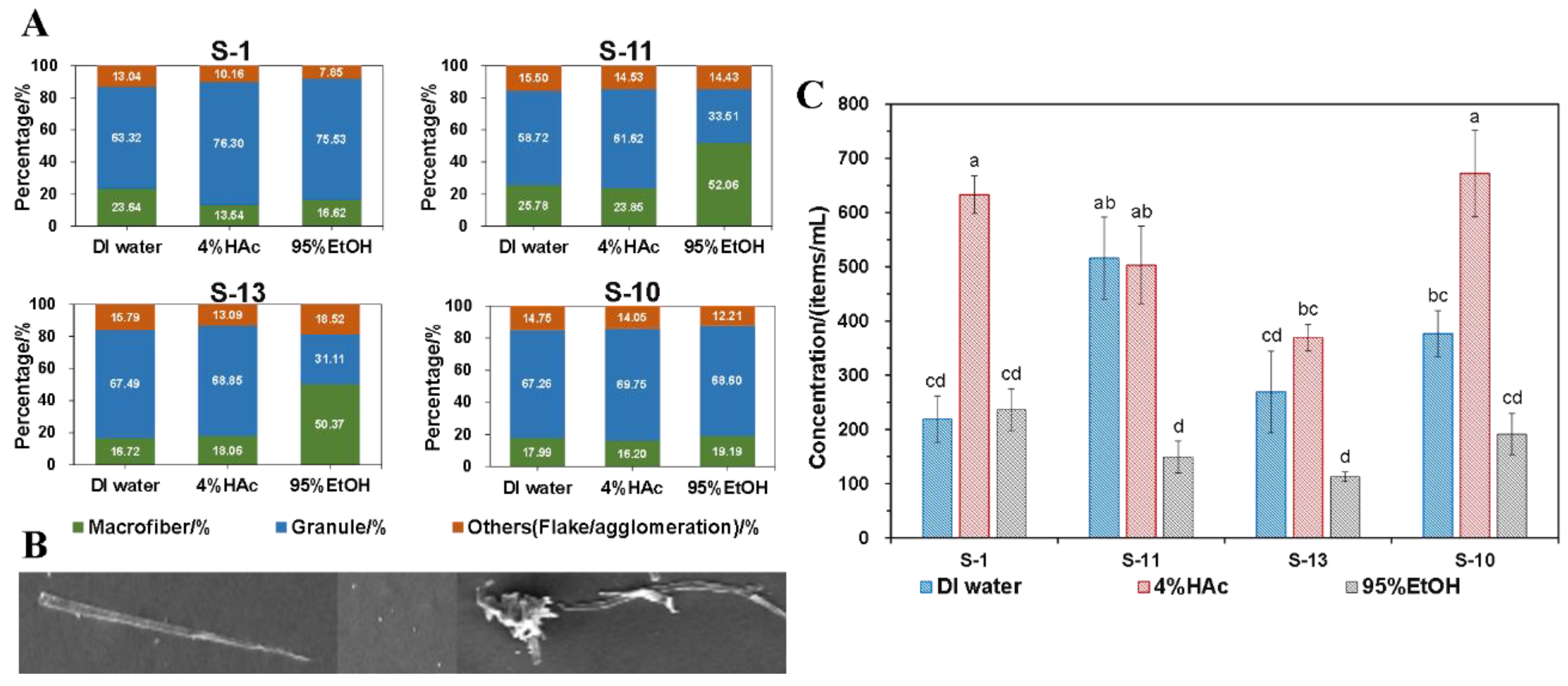
3.6. Quantitative analysis of microparticles
As stated in
Section 2.10, SEM was adopted to observe the influence of different food simulants on the release of microparticles quantitatively. To do this, the immersing temperature was fixed at 70
oC with a total immersing time of 120 min. In the meantime, as shown in
Figure 6B, when calculating the total items of microparticles, the long fiber particles observed were regarded as microfibers particles, while the spherical and punctate particles presented were regarded as granules, as marked in
Figure 6. The interlacing, twining and lamellar particles were all recorded as other categories.
As shown in
Figure 6A, S-1, it produced more fibrous particles when in contact with DI water, while S-11 and S-13 released more fibrous particles when in contact with 95% EtOH, accounting for more than 50% of the total number of microparticles. On the contrary, S-10 showed certain differences, that is, no significant differences in the proportion of microparticles with different morphological characters were observed when in contact with different food simulants. Similar to the overall migration results, the total number of microparticles (item/mL food simulant) produced by immersing with different simulants was the largest in 4% HAc, followed by DI water and 95% EtOH (
Figure 6C). Moreover, although actions have been taken to avoid the impact of the environment on the results, a lower content of microparticles was still detected in the blank sample and thus needs to be deleted. After deducting the blank, results showed that there was still a high concentration of microparticles concentration, and the total number of microparticles released by the SCP lunch-box into different food simulants was in the range of 104 ~ 662 items/mL food and was 52 ~ 331 items/mL food when translated into one-sided contact.
3.7. Estimation of microparticle from SCP lunch-box by humans
In this study, human intake of microparticles via the SCP lunch-box has also been calculated based on the average abundance of microparticles in SCP containers. As above mentioned, the average number of microparticles released by the SCP lunch-box into food simulants was in the range of 104 ~ 662 items/mL food. Based on the overall migration results and the assumption that the internal surface of the SCP lunch-box with a volume of 700 mL is 9 dm2 when in contact with food or food simulants, it can be estimated that the consumer may intake 1.53 ~ 310.59 mg or 36400 ~ 231,700 items microparticles at one time along with the diet, which was far higher than that reported by 's that produced by plastic take-away containers. In addition, we also have estimated the particle diameter distribution of the released microparticles. In detail, the consumer may intake 433 ~ 57,716 items of microparticles with diameter < 10 μm, 11,928 ~ 116,151 items ranging between10 ~ 100 μm, 12,376 ~ 13,0795 items between 100 ~ 500 μm, and 888 ~ 24,004 items in the range of 500 ~ 1000 μm. Moreover, results also showed that the released content of the metal elements from the SCP lunch-boxes into the food or food simulants should also be taken into account.
4. Conclusions
In this study, the production of microparticles from SCP lunch-boxes when in contact with different types of food including DI water, 4% HAc, and 95% EtOH under different contact times and temperatures have been investigated. Our results showed that compared with DI water and 95% EtOH, the 4% HAc, which is usually used as acidic foods, might cause degradation of SCP lunch-boxes, thereby resulting in the highest number of microparticles and the number was significantly greater than the specified limit value. This suggests that, when packaging acid food, the safety of the SCP lunch-box should be considered, especially in terms of the release of microparticles. Moreover, the human body may ingest 36,400 ~ 231,700 items per meal, of which the proportion of microparticles with particle size between 100 ~ 500 μm was the largest, with a number of 12,376 ~ 130,795 items. The accumulation of metal elements or organic matter carried by microparticles in the human body may cause toxicity and should not be ignored either.
Author Contributions
Yi Hu: Experiment execution, Data analysis, Image drawing, Writing-original draft. Chunru Mo: Experiment execution. Wenwen Yu: Project administration, Conceptualization, Funding acquisition. Changying Hu: Conceptualization, Writing-review & Editing, Funding acquisition. Zhiwei Wang: Conceptualization, Writing-review & Editing, Funding acquisition
Acknowledgments
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC1603205/2018YFC1603200) and Key R&D Program of Guangdong province, China (Grant No. 2019B020212002), Guangzhou Basic and Applied Basic Research.
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- Maimaiti, M.; Zhao, X.; Jia, M.; Ru, Y.; Zhu, S. How we eat determines what we become: opportunities and challenges brought by food delivery industry in a changing world in China. European Journal of Clinical Nutrition 2018, 72, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Chen, H.; Huang, B.; Lu, J. Analysis on advances and characteristics of microplastic pollution in China’s lake ecosystems. Ecotoxicology and Environmental Safety 2022, 232, 113254. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, M.; Chen, X.; Hu, L.; Xu, Y.; Fu, W.; Li, C. A comparative review of microplastics in lake systems from different countries and regions. Chemosphere 2022, 286, 131806. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luan, P.; Li, Q.; Cheng, Z.; Sun, X.; Cao, D.; Zhu, H. Biodegradable, hygienic, and compostable tableware from hybrid sugarcane and bamboo fibers as plastic alternative. Matter 2020, 3, 2066–2079. [Google Scholar] [CrossRef]
- Hadidi, M.; Jafarzadeh, S.; Forough, M.; Garavand, F.; Alizadeh, S.; Salehabadi, A.; Khaneghah, A.M.; Jafari, S.M. Plant protein-based food packaging films; recent advances in fabrication, characterization, and applications. Trends in Food Science & Technology 2022, 120, 154–173. [Google Scholar] [CrossRef]
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustainable Production and Consumption 2019, 19, 194–215. [Google Scholar] [CrossRef]
- Szczerbowski, D.; Pitarelo, A.P.; Zandona, A.; Ramos, L.P. Sugarcane biomass for biorefineries: Comparative composition of carbohydrate and non-carbohydrate components of bagasse and straw. Carbohydrate Polymers 2014, 114, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Arshad, M.; Bano, I.; Abbas, M. Biotechnological applications of sugarcane bagasse and sugar beet molasses. Biomass Conversion and Biorefinery 2020. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and livestock products. 2020.
- Becerra, P.; Acevedo, P.; Gonzalez, L.e.a. Sustainability evaluation of sugarcane bagasse valorization alternatives in Valle del Cauca-Colombia. Chemical Engineering Transactions 2018, 65, 817–822. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, X.; Hu, C.; Yu, W. HS-GC-IMS identification of volatile aromatic compounds of freshly-cooked rice packaged with different disposable lunchboxes. J Hazard Mater 2022, 438, 129516. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Rengel, Z.; Qaswar, M.; Amir, M.; Zafar-ul-Hye, M. Arsenic and Heavy Metal (Cadmium, Lead, Mercury and Nickel) Contamination in Plant-Based Foods. In Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects, Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, 2019; pp. 447–490. [Google Scholar]
- Wang, X.F.; Deng, C.B.; Yin, J.; Tang, X. Toxic heavy metal contamination assessment and speciation in sugarcane soil. 2017 3rd International Conference on Environmental Science and Material Application (Esma2017), Vols 1-4 2018, 108. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by Sugarcane Bagasse. Journal of the Taiwan Institute of Chemical Engineers 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Halysh, V.; Sevastyanova, O.; Pikus, S.; Dobele, G.; Pasalskiy, B.; Gun'ko, V.M.; Kartel, M. Sugarcane bagasse and straw as low-cost lignocellulosic sorbents for the removal of dyes and metal ions from water. Cellulose 2020, 27, 8181–8197. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and other harmful substances released from disposable paper cups into hot water. J Hazard Mater 2021, 404. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Wang, M.; Ying, R.; Li, X.; Hu, Z.; Zhang, Y. Disposable plastic materials release microplastics and harmful substances in hot water. Science of The Total Environment 2022, 818, 151685. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Wan, B.; Guo, L.H.; Zhao, L. Microplastics from consumer plastic food containers: Are we consuming it? Chemosphere 2020, 253. [Google Scholar] [CrossRef]
- Jin, H.B.; Ma, T.; Sha, X.X.; Liu, Z.Y.; Zhou, Y.; Meng, X.N.; Chen, Y.B.; Han, X.D.; Ding, J. Polystyrene microplastics induced male reproductive toxicity in mice. J Hazard Mater 2021, 401. [Google Scholar] [CrossRef]
- Li, B.Q.; Ding, Y.F.; Cheng, X.; Sheng, D.D.; Xu, Z.; Rong, Q.Y.; Wu, Y.L.; Zhao, H.L.; Ji, X.F.; Zhang, Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 2020, 244. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Science of the Total Environment 2019, 684, 657–669. [Google Scholar] [CrossRef]
- Du, F.N.; Cai, H.W.; Zhang, Q.; Chen, Q.Q.; Shi, H.H. Microplastics in take-out food containers. J Hazard Mater 2020, 399. [Google Scholar] [CrossRef] [PubMed]
- Commission, N.H.a.F.P. National Food Safety Standard General Rules for Migration Test of Food Contact Materials and Articles. 2015, GB 31604.1-2015.
- Commission, N.H.a.F.P. National Food Safety Standard Food Contact Materials and Articles General rules for pre-experimental processing methods. 2016, GB 5009.156-2016.
- Commission, N.H.a.F.P. National Food Safety Standard Food Contact Materials and Articles Overall Migration Determination. 2016, GB 31604.8-2016.
- Xie, C.H.; Chen, Y.F.; Liu, Y.Y.; Yin, N.; Zhong, H.N.; Li, D. Determination of 42 kinds of inorganic elements in food contact paper articles by microwave digestion-inductively coupled plasma mass spectrometry. Journal of Food Safety & Quality 2021, 12, 4602–4607. [Google Scholar] [CrossRef]
- Gond, R.K.; Gupta, M.K. A novel approach for isolation of nanofibers from sugarcane bagasse and its characterization for packaging applications. Polymer Composites 2020, 41, 5216–5226. [Google Scholar] [CrossRef]
- Afrazeh, M.; Tadayoni, M.; Abbasi, H.; Sheikhi, A. Extraction of dietary fibers from bagasse and date seed, and evaluation of their technological properties and antioxidant and prebiotic activity. Journal of Food Measurement and Characterization 2021, 15, 1949–1959. [Google Scholar] [CrossRef]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent Progress in Silane Coupling Agent with Its Emerging Applications. Journal of Polymers and the Environment 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Winkler, H.C.; Notter, T.; Meyer, U.; Naegeli, H. Critical review of the safety assessment of titanium dioxide additives in food. Journal of Nanobiotechnology 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Deng, C.B.; Sunahara, G.; Yin, J.; Xu, G.P.; Zhu, K.X. Risk Assessments of Heavy Metals to Children Following Non-dietary Exposures and Sugarcane Consumption in a Rural Area in Southern China. Exposure and Health 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Xu, G.P.; Deng, C.B.; Wang, J.; Zhu, H.X.; Sun, Z.; Wang, X.F.; Zhu, K.X.; Yin, J.; Tang, Z.F. Lead bioaccumulation, subcellular distribution and chemical form in sugarcane and its potential for phytoremediation of lead-contaminated soil. Human and Ecological Risk Assessment 2020, 26, 1175–1187. [Google Scholar] [CrossRef]
- Da Silva, F.B.V.; Do Nascimento, C.W.A.; Araujo, P.R.M.; da Silva, L.H.V.; da Silva, R.F. Assessing heavy metal sources in sugarcane Brazilian soils: an approach using multivariate analysis. Environmental Monitoring and Assessment 2016, 188. [Google Scholar] [CrossRef]
- Dupont, A.L.; Tetreault, J. Cellulose Degradation in an Acetic Acid Environment. Studies in Conservation 2000, 45, 201–210. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).