Introduction
There has been a significant increase over the past decade in adult use of amphetamine (common brand name: Adderall) and methylphenidate (common brand name: Ritalin), both central nervous system stimulants, especially among women and the 45+ age group [
1,
2]. Amphetamine and methylphenidate are primarily prescribed for narcolepsy and attention deficit hyperactivity disorder [
3,
4,
5,
6,
7]. Off-label use of amphetamine and methylphenidate has also shown therapeutic potential for late-life, post-stroke depression and rehabilitation, depression in medically ill patients, Parkinson’s disease, age-related cognitive decline (e.g., apathy in dementia), catatonia, fall prevention (e.g., gait and postural instability), and anorexia [
8]. However, amphetamine and methylphenidate can have serious side effects, and clinical trials of amphetamine and methylphenidate showed high attrition rates due to adverse effects [
6,
9,
10]. For example, amphetamine and methylphenidate can increase blood pressure, induce arrhythmias, and contribute to development of cardiomyopathies and sudden death [
11,
12,
13].
Amphetamine and methylphenidate are also classified as Schedule II drugs by the U.S. Controlled Substances Act given their high potential for nonmedical use and misuse/abuse (referred to as misuse hereafter) despite having an accepted medical use [
14,
15]. Intentional misuse (e.g., for performance enhancement, recreational use, and use in amounts or with methods that are harmful) of amphetamine and methylphenidate, including clandestinely produced amphetamine and diverted amphetamine and methylphenidate, has steadily increased over the years [
16,
17,
18]. Negative physiological, cognitive, and behavioral effects on individuals who misuse amphetamine and methylphenidate are serious public health issues [
19]. Between 2008 and 2015, amphetamine-related hospitalizations increased to a greater degree than hospitalizations associated with other substances, and in-hospital mortality was higher for amphetamine-related than other hospitalizations [
20]. A study of the 2012-2017 U.S. National Poison Data System also found that compared to unintentional oral exposure to amphetamine, all nonmedical (including suicide attempt) amphetamine exposure cases via intravenous injection, inhalation, and intentional ingestion were at a greater risk for critical care admissions and adverse medical outcomes, including death [
21].
In all age groups, intentional misuse of amphetamine or methylphenidate has been associated with other substance use and use disorders [
22,
23,
24,
25,
26,
27,
28,
29]. Studies also found that attention deficit and hyperactivity disorder often co-occurs with substance use disorders and is associated with early onset and more severe development of substance use disorders and with reduced treatment effectiveness [30-32]. These previous studies indicate the importance of examining other substance use/misuse associated with suspected suicide attempts and other intentional misuse and medical outcomes among adult amphetamine or methylphenidate users. In particular, despite the high suicide attempt rate among intentional amphetamine misusers [
21], little research has been done on these suicide attempts and co-used other substances in such attempts.
In the present study based on the 2015-2021 U.S. National Poison Data System’s amphetamine and methylphenidate cases age 50+, our primary research questions were to examine associations between co-used other substances and (1) suspected suicide attempt versus other intentional misuse, and (2) major medical outcomes (major effect/death) in suspected suicide attempt and other intentional misuse cases, controlling for demographic variables. Our focus on cases age 50+ is significant since this age group in both sexes have had the highest suicide rates in the U.S. [
33]. A recent study also showed that among female suicide decedents, those age 45+ had higher rates of poisoning as suicide method than younger decedents (37 % and 41% of the 45-64 and 65+ age groups, respectively, vs. 19% and 25% of the 18-24 and 25-44 age groups), and that those who used poisoning had a significantly higher rates of mental and substance use disorders [
34]. The present study’s findings will provide important insights into late-life suicide attempts and other intentional misuse involving amphetamine or methylphenidate and polysubstance use.
Materials and Methods
Data Source
The National Poison Data System (NPDS) includes data from 55 poison control centers in the U.S. (See NPDS website [
https://aapcc.org/data-system] or Gummin et al. [
35] for detailed descriptions.) Although NPDS lists cases, not individuals, the extent to which these cases include duplicate individuals is minimal as poison center specialists are trained to detect duplication and correct it as soon as it is discovered. In this study, we focused on amphetamine and/or methylphenidate exposure (i.e., for all reasons except withdrawal and bites/stings) cases age 50+ that were closed between January 1, 2015 and December 31, 2021. Cases were identified by substance/product ID/generic category codes and involving any number of other substances. The 7,701 cases thus identified included those with all associated medical outcomes, except indirectly reported deaths (n=48 from Arizona and 2 from all other states). Indirectly reported deaths (that poison control centers acquired from medical examiners or media but did not manage [
35]) have been sporadic, and the Arizona’s high number was due to inclusion of deaths from state vital statistics from 2017 through 2021. Cases were from all 50 states, the District of Columbia, Puerto Rico, and unknown/refused geographic areas. Based on the authors’ institutional review board guidelines, institutional review board exemption was assumed for analysis of these de-identified data.
Measures
Exposures involving amphetamine and methylphenidate: Based on the NPDS substance codes, we identified “amphetamine and related compounds” and “methylphenidate” exposure cases. The small number of cases (n=36) that involved both amphetamine and methylphenidate were treated as amphetamine cases based on our preliminary analysis finding that these cases were more similar to amphetamine than methylphenidate cases in terms of exposure reasons and medical outcomes. Although the NPDS provides the substance sequence numbers for each case, we included all amphetamine and methylphenidate cases regardless of the sequence number as these numbers in many cases tend to be nonsystematically assigned and do not signify substance priority.
Exposure reasons: NPDS listed the following: unintentional (therapeutic error, adverse reaction, other unintentional misuse or exposures via environmental/other routes); suspected suicide attempt; intentional misuse (including intentional but unknown reasons); malicious intent by others; and unknown reasons.
Medical outcomes: NPDS has the following medical outcome categories for human exposure: no effect; minor effect; moderate effect; major effect; death; no follow-up, judged to be nontoxic (clinical effects not expected); no follow-up, minimal/no more than minor clinical effects possible; unable to follow, judged to be a potentially toxic exposure; and indirectly reported death. In this study, we combined these outcomes into two categories: major effect/death (n=592 major effects and n=71 deaths) vs. all others (except indirectly reported deaths that were excluded in this study). As opposed to moderate effect referring to “signs or symptoms that were not life-threatening or had no residual disability or disfigurement,” major effect refers to “signs or symptoms that were life-threatening or resulted in significant residual disability or disfigurement” [
35].
Co-used other substances: These included benzodiazepines; antidepressants; any of 23 types of NPDS-coded prescription opioids; drugs for cardiovascular diseases; antipsychotics; antihistamines; muscle relaxants; alcoholic beverages; marijuana; methadone; and illicit drugs (cocaine, methamphetamine, heroin, illicit fentanyl, Phencyclidine, or lysergic acid diethylamide). Codes for illicit fentanyl and analogues were added to NPDS on 10/30/2019.
Covariates in multivariable models included exposure year (2015-2021), U.S. census region, age group (50-59 and 60+ or 50-59, 60-69, and 70+ years), and sex.
For descriptive purposes: We reported route of administration (ingestion, inhalation, injection) management/care site (on site [non-healthcare facility], treated/evaluated/released from healthcare facility, admitted to a psychiatric facility, admitted to a noncritical care unit; admitted to a critical care unit; refused referral/did not arrive at healthcare facility/lost to follow-up/left against medical advice), and the number of all substances involved.
Analysis
All analyses were conducted with Stata 17/MP (Stata Corp, College Station, TX). We first reported the changes in amphetamine and methylphenidate cases by age group (50-59 and 60+) and sex during the study period (2015-2021). Second, we used
χ2 and ANOVA to compare demographic and exposure-related characteristics, medical outcomes, and co-used substances among three exposure reason groups: unintentional exposure, suspected suicide attempt, and intentional misuse. We excluded malicious intent by others (n=17) and unknown reasons (n=282) from this comparison. Third, to examine primary research questions, we fit two generalized linear models for a Poisson distribution with log link function. The first generalized linear model (GLM) focused on co-used substances in suspected suicide attempt cases versus intentional misuse cases, and the second GLM focused on co-used substances in major effect/death among suspected suicide attempt and intentional misuse cases. GLM rather than logistic regression models were used as odds ratios tend to exaggerate the true relative risk when the event (i.e., attempted suicides in study) is a common (i.e., >10%) occurrence [
36]. As a preliminary diagnostic, we used variance inflation factor, using a cut-off of 2.50 [
37], from linear regression models to assess multicollinearity among covariates, which indicated no concerning multicollinearity among covariates. GLM results are reported as incidence rate ratios (IRRs) with 95% CIs. Statistical significance was set at p<0.05.
Results
Amphetamine and Methylphenidate Cases, 2015-2021, by Age Group and Sex
Of the 7,701 cases, 5,416 (70.3%) were amphetamine and 2,285 (29.7%) were methylphenidate cases, and two thirds of both amphetamine (67.3%) and methylphenidate (68.1%) cases were female.
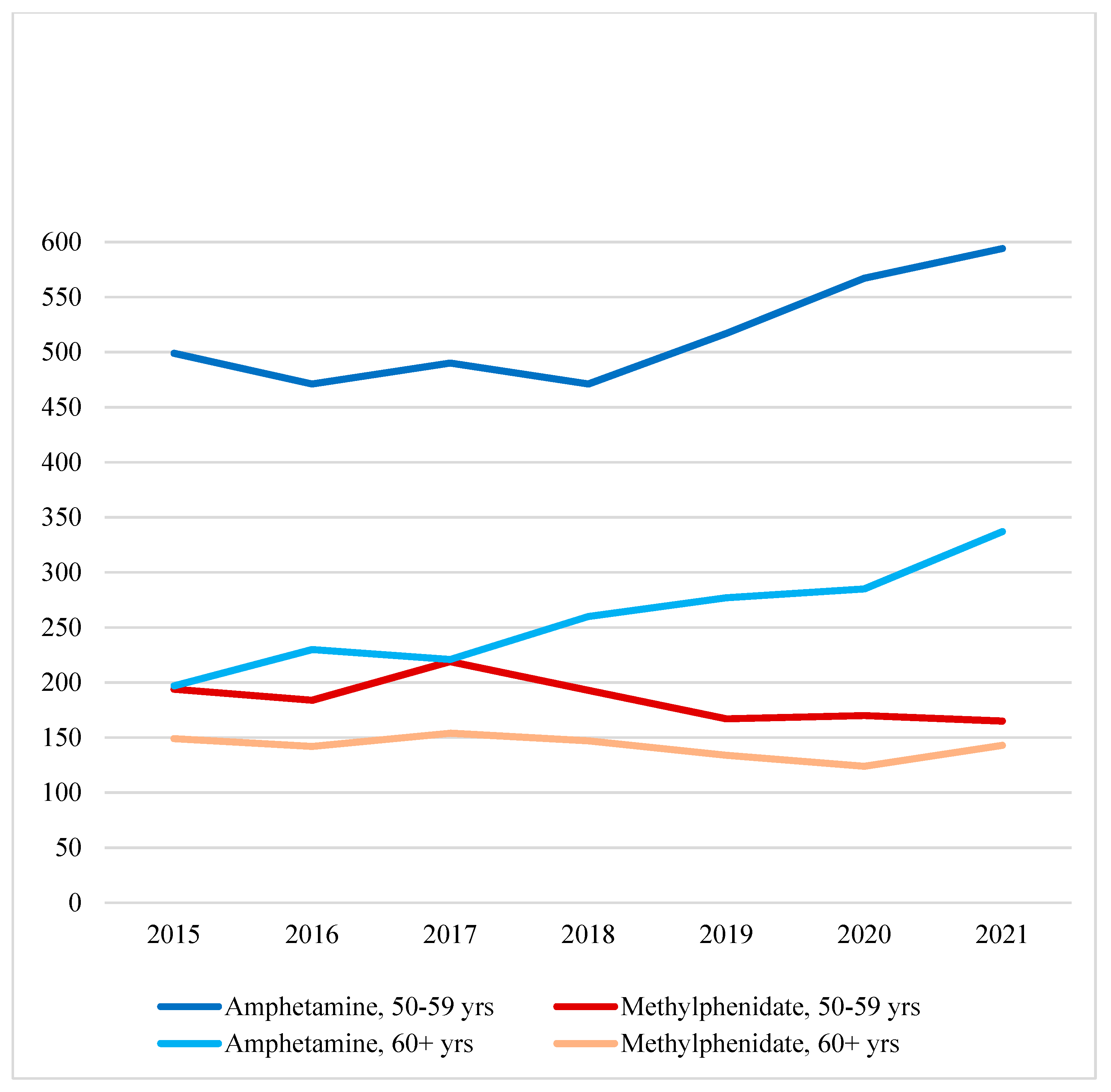
Figure 1 shows that amphetamine cases increased between 2018 and 2021 for the 50-59 age group and between 2017 and 2021 for the 60+ age group. While the 50-59 age group was significantly larger than the 60+ age group all throughout the seven-year study period, the 60+ age group had a proportionally greater increase over the years (Pearson
χ2(df=6)=15.64,
p=.016). On the other hand, methylphenidate cases did not significantly change between 2015 and 2021 for either age group (Pearson
χ2(df=6)=2.16,
p=.904).
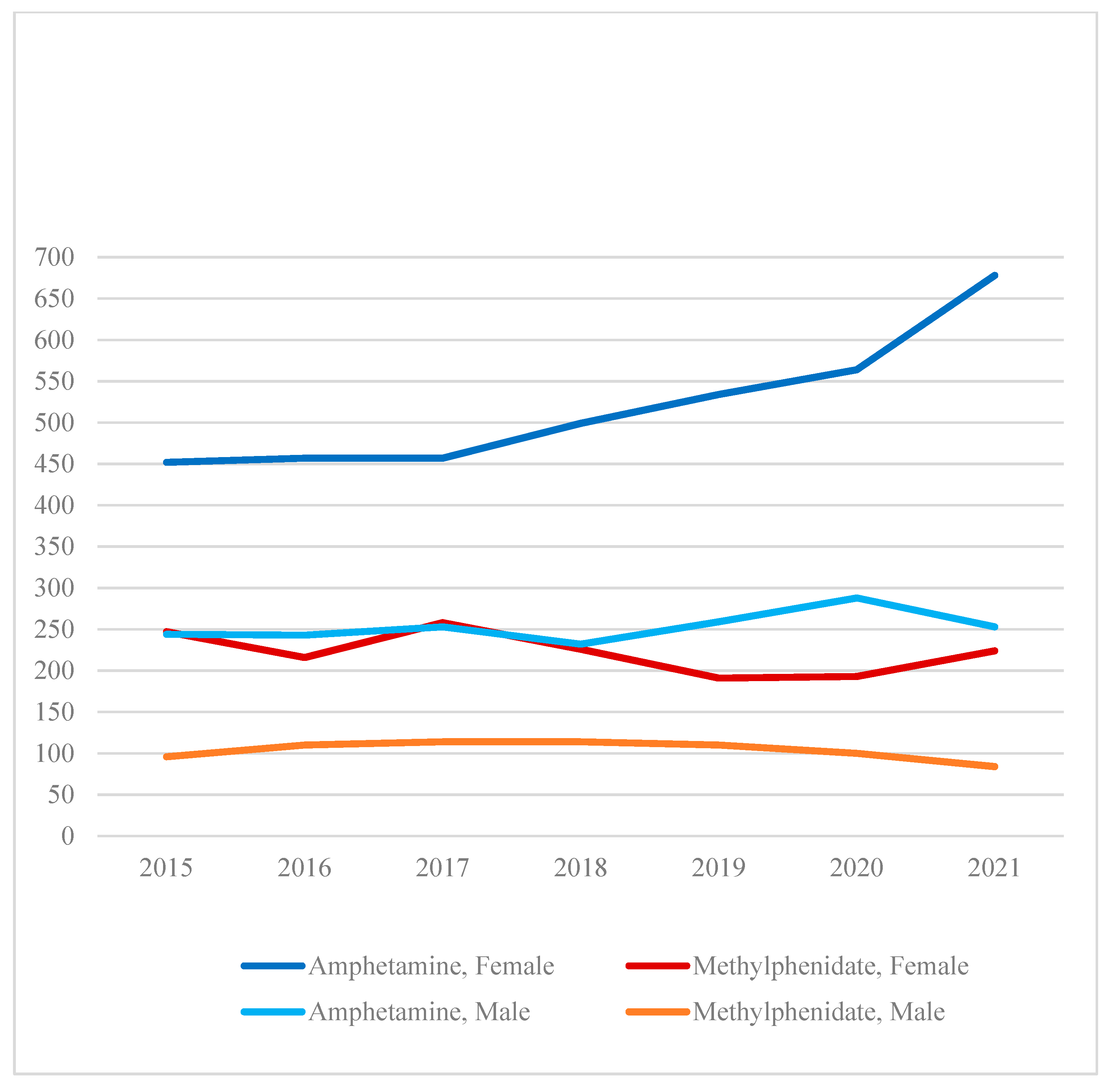
Figure 2 shows that female amphetamine cases increased to a greater extent than male amphetamine cases during the seven years (Pearson
χ2(df=6)=19.50,
p=.003). Male amphetamine cases were stable between 2015 (n=244) and 2021 (n=253). Methylphenidate cases did not significantly change between 2015 and 2021 for both sexes (Pearson
χ2(df=6)=10.31,
p=.113).
Additional analysis showed that one half of amphetamine and 71.1% of methylphenidate cases were unintentional exposures; 30.5% of amphetamine and 19.6% of methylphenidate cases were suspected suicide attempts; and 15.0% of amphetamine and 7.3% of methylphenidate cases were intentional misuse. Major effect/death was reported for 10.2% of amphetamine cases and 4.8% of methylphenidate cases; however, there was no difference between amphetamine and methylphenidate cases in terms of the number of other substances involved (M=2.57 [SD=2.27] for amphetamine cases and M=2.48 [SD=2.35] for methylphenidate cases, t=1.687, p=.092).
Amphetamine and Methylphenidate Cases by Exposure Reason
Table 1 shows that after excluding exposures due to unknown reasons or malicious intent, unintentional exposures, suspected suicide attempts, and intentional misuse were 58.4%, 28.4%, and 13.2%, respectively, of combined amphetamine and methylphenidate cases. Unintentional exposure (e.g., inadvertently took medication twice, took medications too close together, took someone else’s medication) cases significantly differed from suspected suicide attempt and intentional misuse cases on demographic characteristics, route of administration, management/care site, other substance involvement, and medical outcomes (i.e., 0.7% major effect/death vs. 18.8% for suspected suicide attempts and 17.0% for intentional misuse).
Compared to intentional misuse cases, suspected suicide attempt cases included higher proportions of 70+-year olds, women, critical care admissions, and users of benzodiazepines (34.9% vs. 15.4%), antidepressants, antipsychotics, cardiovascular disease drugs, antihistamines, muscle relaxants, and alcohol, but included a lower proportion of marijuana and illicit drug users (3.0% vs. 12.0%). Co-used illicit drugs among intentional misuse cases included cocaine (5.9%), methamphetamine (3.9%), heroin (1.9%), illicit fentanyl (0.6%), phencyclidine (0.6%), and/or lysergic acid diethylamide (0.2%). (Illicit fentanyl was added to the NPDS substance code on October 30, 2019). No difference was found in the proportions that used prescription opioids and methadone and in medical outcomes.
Associations between Co-used Substances and Suspected Suicide Attempts vs. Intentional Misuse
The first column of
Table 2 shows that benzodiazepine use (IRR=1.09, 95% CI=1.06-1.16) was associated with a higher likelihood but any illicit drug use (IRR=0.83, 95% CI=0.73-0.95) was associated with a lower likelihood of suspected suicide attempts compared to intentional misuse. Type of stimulant involved (amphetamine or methylphenidate) was not significant. Of the covariates, only female sex (IRR=1.07, 95% CI=1.01-1.13) was associated with a higher likelihood of suspected suicide attempts.
Associations between Co-used Substances and Major Effect/Death among Suspected Suicide Attempt and Intentional Misuse Cases
The second column of
Table 2 shows that co-use of antidepressants (IRR=1.43, 95% CI=1.16-1.76), prescription opioids (IRR=1.48, 95% CI=1.21-1.82), cardiovascular disease drugs (IRR=1.51, 95% CI=1.20-1.90), antipsychotics (IRR=1.26, 95% CI=1.02-1.55), or illicit drugs (IRR=2.40, 95% CI=1.82-3.15) was associated with a higher likelihood of major effect/death. Whether the exposure was suspected suicide attempt or intentional misuse was not a significant factor. Amphetamine than methylphenidate had a higher but nonsignificant (
p=.064) association with major effect/death. Of the covariates, year 2019, compared to 2015, and the Northeast and Midwest regions, compared to the South region, were associated with higher likelihood of major effect/death.
Discussion
We examined co-used substances in suspected suicide attempts or other intentional misuse in amphetamine or methylphenidate cases age 50+ in NPDS. While nonmedical use and use disorders of prescription stimulants are lower than those of prescription opioids or sedatives [
38,
39], it is important to examine polysubstance use in amphetamine and methylphenidate cases given increases in these cases in recent years. Our findings showed that amphetamine cases increased between 2017/2018 and 2021, especially among the 60+ age group and women. Nearly 42% of amphetamine and methylphenidate cases were suspected suicide attempts or other intentional misuse, and as expected, co-use of other substance and major effect/death were significantly higher among these cases than among unintentional exposure cases.
Multivariable findings show that among suicide attempt and other intentional misuse cases, benzodiazepine co-use was associated with a higher likelihood of suspected suicide attempts and illicit drug co-use was associated with a higher likelihood of other intentional misuse. Multivariable findings also show significant correlates of major effect/death to be prescription opioids, antidepressants, antipsychotics, cardiovascular disease drugs, and illicit drugs, without any significant difference between suspected suicides and intentional misuse or between amphetamine and methylphenidate.
The higher likelihood of benzodiazepine co-use in suspected suicide attempts compared to other intentional misuse appears to be consistent with previous findings. An analysis of censuses of emergency department and inpatient discharges for suicidal intentional overdoses in 11 US states found that 19.6%-22.5% of the suicidal overdoses involved benzodiazepines, and 15.4%-17.3% involved opioids [
40]. However, the study found benzodiazepines were most often involved in nonfatal act, while opioids were most commonly identified in fatal suicide poisonings among adults, followed by barbiturates, antidepressants, antidiabetics, calcium channel blockers, alcohol, and psychostimulants [
40].
The significant associations of major effect/death with co-used prescription opioids and illicit drugs in suspected suicide attempt and intentional misuse cases are also consistent with previous research findings. For example, a study found significantly more 12-month emergency department visits and hospitalizations, controlling for fair/poor health and other characteristics, among older-adult prescription opioid users, compared to nonusers [
41]. Although the rate of illicit drug use and use disorders are, in general, lower among those age 50+ than among younger adults, [
39] the effect of these substances are more severe because of aging-related changes in pharmacokinetics and drug metabolism [
42,
43]. Serious health consequences from illicit drug use include adverse cardiovascular, renal, and cognitive effects, inflammation, and overdose deaths [
44,
45,
46,
47,
48].
The higher likelihood of major effect/death in suspected suicide attempt and intentional misuse cases involving antidepressants, antipsychotics, and cardiovascular disease drugs also shows likely confounding effects of comorbid mental and physical health problems in these cases. Analysis of the 2013-2018 U.S. Medical Expenditure Survey found the marked increase in the risk of amphetamine and methylphenidate misuse in a population often reporting multiple neurological or mental disorders and taking medications for depression and anxiety [
1]. A study also found significant association between stimulant exposure in early life and earlier onset of psychosis [
49]. We also speculate that the co-use of amphetamine or methylphenidate and antipsychotics and cardiovascular disease drugs may have been necessary to treat side effects (e.g., psychotic symptoms and adverse cardiovascular events) of amphetamine or methylphenidate [
50]. Future research is needed to examine this possibility.
Of the covariates, higher likelihood of suspected suicide attempts among female than male amphetamine or methylphenidate users may stem from the fact that poisoning is more often used as a suicide method among women than men [
34]. However, reasons for 44% higher likelihood of major effect/death in 2019 than in 2015 but not in other years is not clear. The reasons for significantly higher rates of major effect/death in the Northeast and Midwest regions than in the South region are not clear, either, although the regional differences are in line with the higher drug overdose death rates in the Northeast and Midwest than in the South [
51].
The study has the following limitations due to data constraints. First, since NPDS contains only exposures that were reported to poison control centers, they do not represent all exposures among the population, limiting the findings’ generalizability. The increase in amphetamine cases among the 60+ age group and women between 2017/2018 and 2021 may reflect a rise in poisoning incidents in these population groups; however, it may also be a result of better reporting practices. Second, deaths among cases are underestimates as not all cases were followed up. Also, it is not clear if all reported deaths were related to substance use or from other causes. Third, data that are telephone-reported to poison centers without medical record validation and toxicological confirmation may compromise validity. This applies to suspected suicide attempts as it is unclear whether or not the exposure reason was self-reported by the individual or verified/reported by any health care provider. Fourth, lack of data on characteristics such as race, pre-existing health conditions, health insurance, and substance use history precluded more detailed analyses of sociodemographic and health-related factors.
Despite these limitations, the study has the following implications. First, with increasing amphetamine exposure cases in older adults, healthcare professionals need to monitor adverse events and unintentional or intentional misuse. They need to pay special attention to increased risks of suicide attempts, especially among older women, and adverse medical outcomes when amphetamine or methylphenidate are co-used with other psychotropic drugs, prescription opioids, or cardiovascular disease drugs. Second, although a small proportion of amphetamine and methylphenidate cases co-used illicit drugs, the significant association between major effect/death and illicit drug use points to the importance of screening these drugs. For cases referred to and/or admitted to a critical care unit, substance use and mental health treatment programs need to be an important treatment component.
Conclusions
Amphetamine and methylphenidate misuse in the 50+ age group may be lower than other prescription drug misuse/abuse; however, the increase in amphetamine cases reported to poison control centers in recent years is a warning sign that preventive measures are needed to stem such increase, especially the cases of suicide attempt and intentional misuse. Careful monitoring of those who were prescribed amphetamine and methylphenidate as well as continued research on nonpharmacological treatment of attention deficit hyperactivity disorder, narcolepsy, and other conditions are needed. Those who misuse amphetamine or methylphenidate also need to be provided easy access to integrated physical and behavioral health services.
Funding
The authors did not receive any funding for the study.
Acknowledgments
The American Association of Poison Control Centers made the National Poison Data System (NPDS) available to the authors for this study. This study’s findings and conclusions are those of the authors alone and do not necessarily represent the official position of the American Association of Poison Control Centers or participating poison control centers.
Conflicts of Interest
The authors declare no conflict of interest.
Declaration
The American Association of Poison Control Centers (AAPCC) maintains the National Poison Data System (NPDS), which houses de-identified case records of self-reported information collected from callers during exposure management and poison information calls managed by the country’s poison control centers (PCCs). NPDS data do not reflect the entire universe of exposures to a particular substance as additional exposures may go unreported to PCCs; accordingly, NPDS data should not be construed to represent the complete incidence of U.S. exposures to any substance(s). Exposures do not necessarily represent a poisoning or overdose and AAPCC is not able to completely verify the accuracy of every report. Findings based on NPDS data do not necessarily reflect the opinions of AAPCC.
References
- Moore, T.J.; Wirtz, P.W.; Kruszewski, S.P.; Alexander, G.C. Changes in medical use of central nervous system stimulants among US adults, 2013 and 2018: A cross-sectional study. BMJ Open. 2021, 11, e048528. [Google Scholar] [CrossRef] [PubMed]
- Schepis, T.S.; McCabe, S.E. Trends in older adult nonmedical prescription drug use prevalence: Results from the 2002-2003 and 2012-2013 National Survey on Drug Use and Health. Addict Behav. 2016, 60, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Cortese, S.; Adamo, N.; Del Giovane, C.; Mohr-Jensen, C.; Hayes, A.J.; Carucci, S.; Atkinson, L.Z.; Tessari, L.; Banaschewski, T.; Coghill, D.; et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. Lancet Psychiatry 2018, 5, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Stuhec, M.; Lukić, P.; Locatelli, I. Efficacy, Acceptability, and tolerability of lisdexamphetamine, mixed amphetamine salts, methylphenidate, and modafinil in the treatment of attention-deficit hyperactivity disorder in adults: A systematic review and meta-analysis. Ann Pharmacother. 2019, 53, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Thorpy, M.J.; Bogan, R.K. Update on the pharmacologic management of narcolepsy: Mechanisms of action and clinical implications. Sleep Med. 2020, 68, 97–109. [Google Scholar] [CrossRef]
- Elliott, J.; Johnston, A.; Husereau, D.; Kelly, S.E.; Eagles, C.; Charach, A.; Hsieh, S.C.; Bai, Z.; Hossain, A.; Skidmore, B.; Tsakonas, E.; Chojecki, D.; Mamdani, M.; Wells, G.A. Pharmacologic treatment of attention deficit hyperactivity disorder in adults: A systematic review and network meta-analysis. PLoS ONE. 2020, 15, e0240584. [Google Scholar] [CrossRef]
- Mechler, K.; Banaschewski, T.; Hohmann, S.; Häge, A. Evidence-based pharmacological treatment options for attention deficit hyperactivity disorder in children and adolescents. Pharmacol Ther. 2022, 230, 107940. [Google Scholar] [CrossRef]
- Sassi, K.L.M.; Rocha, N.P.; Colpo, G.D.; John, V.; Teixeira, A.L. Amphetamine use in the elderly: A systematic review of the literature. Curr Neuropharmacol. 2020, 18, 126–135. [Google Scholar] [CrossRef]
- Castells, X.; Blanco-Silvente, L.; Cunill, R. Amphetamine for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2018, 8, CD007813. [Google Scholar] [CrossRef]
- Cândido, R.C.F.; Menezes de Padua, C.A.; Golder, S.; Junqueira, D.R. Immediate-release methylphenidate for attention deficit hyperactivity disorder (ADHD) in adults. Cochrane Database Syst Rev. 2021, 1, CD013011. [Google Scholar] [CrossRef]
- Steinkellner, T.; Freissmuth, M.; Sitte, H.H.; Montgomery, T. The ugly side of amphetamine: Short- and long-term toxicity of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’), methamphetamine and D-amphetamine. Biol. Chem. 2011, 392, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Torres-Acosta, N.; O'Keefe, J.H.; O'Keefe, C.L.; Lavie, C.J. Cardiovascular effects of ADHD therapies: JACC review topic of the week. J Am Coll Cardiol. 2020, 76, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Tadrous, M.; Shakeri, A.; Chu, C.; Watt, J.; Mamdani, M.M.; Juurlink, D.N.; Gomes, T. Assessment of Stimulant Use and Cardiovascular Event Risks Among Older Adults [published correction appears in JAMA Netw Open. 2021, 4, e2138512]. JAMA Netw Open. 2021, 4, e2130795. [Google Scholar] [CrossRef] [PubMed]
- Liao, S. Why are ADHD medicines controlled substances? 2017. https://www.webmd.com/add-adhd/features/adhd-medicines-controlled-substances#:~:text=The%20majority%20of%20ADHD%20stimulant,risk%20of%20abuse%20and%20dependence.
- Shellenberg, T.P.; Stoops, W.W.; Lile, J.A.; Rush, C.R. An update on the clinical pharmacology of methylphenidate: Therapeutic efficacy, abuse potential and future considerations. Expert Rev Clin Pharmacol. 2020, 13, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Weyandt, L.L.; Oster, D.R.; Marraccini, M.E.; Gudmundsdottir, B.G.; Munro, B.A.; Rathkey, E.S.; McCallum, A. Prescription stimulant medication misuse: Where are we and where do we go from here? Exp Clin Psychopharmacol. 2016, 24, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Department of Justice/Drug Enforcement Agency. Drug fact sheet: Amphetamine. 2020. https://www.dea.gov/sites/default/files/2020-06/Amphetamine-2020_0.pdf.
- Thurn, D.; Riedner, A.; Wolstein, J. Use motives of patients with amphetamine-type stimulants use disorder and attention-deficit/hyperactivity disorder. Eur Addict Res. 2020, 26, 254–262. [Google Scholar] [CrossRef]
- Clemow, D.B.; Walker, D.J. The potential for misuse and abuse of medications in ADHD: A review. Postgrad Med. 2014, 126, 64–81. [Google Scholar] [CrossRef]
- Winkelman, T.N.A.; Admon, L.K.; Jennings, L.; Shippee, N.D.; Richardson, C.R.; Bart, G. Evaluation of amphetamine-related hospitalizations and associated clinical outcomes and costs in the United States. JAMA Netw Open. 2018, 1, e183758. [Google Scholar] [CrossRef]
- Faraone, S.V.; Hess, J.; Wilens, T. Prevalence and consequences of the nonmedical use of amphetamine among persons calling poison control centers. J Atten Disord. 2019, 23, 1219–1228. [Google Scholar] [CrossRef]
- Wong, S.H.M.; Stevens, C.; Liu, C.H.; Chen, J.A. Prevalence and correlates of prescription stimulant misuse among US college students: Results from a national survey. J Clin Psychiatry. 2022, 84, 22m14420. [Google Scholar] [CrossRef]
- Sepúlveda, D.R.; Thomas, L.M.; McCabe, S.E.; Cranford, J.A.; Boyd, C.J.; Teter, C.J. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: Preliminary analysis among college students. J Pharm Pract. 2011, 24, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Clemow, D.B. Misuse of methylphenidate. Curr Top Behav Neurosci. 2017, 34, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Dertien, D.; Bentley, S.I. Prevalence of ADHD symptom malingering, nonmedical use, and drug diversion among college-enrolled adults with a prescription for stimulant medications. J Addict Dis. 2020, 38, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chawarski, M.C.; Hawk, K.; Edelman, E.J.; O'Connor, P.; Owens, P.; Martel, S.; Coupet E., Jr.; Whiteside, L.; Tsui, J.I.; Rothman, R.; Cowan, E.; Richardson, L.; Lyons, M.S.; Fiellin, D.A.; D'Onofrio, G. Use of amphetamine-type stimulants among emergency department patients with untreated opioid use disorder. Ann Emerg Med. 2020, 76, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.E. Emergency department visits involving attention deficit/hyperactivity disorder stimulant medications. In: The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); January 24, 2013.1-8.
- Compton, W.M.; Han, B.; Blanco, C.; Johnson, K.; Jones, C.M. Prevalence and correlates of prescription stimulant use, misuse, use disorders, and motivations for misuse among adults in the United States. Am J Psychiatry. 2018, 175, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Shearer, R.D.; Jones, A.; Howell, B.A.; Segel, J.E.; Winkelman, T.N.A. Associations between prescription and illicit stimulant and opioid use in the United States, 2015-2020. J Subst Abuse Treat. 2022, 143, 108894. [Google Scholar] [CrossRef] [PubMed]
- Crunelle, C.L.; Brink, W.v.D.; Moggi, F.; Konstenius, M.; Franck, J.; Levin, F.R.; van de Glind, G.; Demetrovics, Z.; Coetzee, C.; Luderer, M.; et al. International consensus statement on screening, diagnosis and treatment of substance use disorder patients with comorbid attention deficit/hyperactivity disorder. Eur Addict Res. 2018, 24, 43–51. [Google Scholar] [CrossRef]
- Westover, A.N.; Nakonezny, P.A.; Halm, E.A.; Adinoff, B. Risk of amphetamine use disorder and mortality among incident users of prescribed stimulant medications in the Veterans Administration. Addiction 2018, 113, 857–867. [Google Scholar] [CrossRef]
- Vosburg, S.K.; Robbins, R.S.; Antshel, K.M.; Faraone, S.V.; Green, J.L. Characterizing prescription stimulant nonmedical use (NMU) among adults recruited from Reddit. Addict Behav Rep. 2021, 14, 100376. [Google Scholar] [CrossRef]
- Garnett, M.F.; Curtin, S.C.; Stone, D.M. Suicide mortality in the United States, 2000-2020. NCHS Data Brief. 2022, 1–8. [Google Scholar]
- Choi, N.G.; Marti, C.N.; Choi, B.Y. Three leading suicide methods in the United States, 2017-2019: Associations with decedents' demographic and clinical characteristics. Front Public Health. 2022, 10, 955008. [Google Scholar] [CrossRef] [PubMed]
- Gummin, D.D.; Mowry, J.B.; Beuhler, M.C.; Spyker, D.A.; Bronstein, A.C.; Rivers, L.J.; Pham, N.P.T.; Weber, J. 2020 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 38th annual report. Clin Toxicol 2021, 59, 1282–1501. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Schulz, K.F. Making sense of odds and odds ratios. Obstet Gynecol. 2008, 111 Pt 1, 423–426. [Google Scholar] [CrossRef]
- Allison, P. When can you safely ignore multicollinearity? 2012. https://statisticalhorizons.com/multicollinearity/.
- Cassidy, T.A.; McNaughton, E.C.; Varughese, S.; Russo, L.; Zulueta, M.; Butler, S.F. Nonmedical use of prescription ADHD stimulant medications among adults in a substance abuse treatment population: Early findings from the NAVIPPRO surveillance system. J Atten Disord. 2015, 19, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Substance Abuse and Mental Health Services Administration. (2021). Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available from https://www.samhsa.gov/data/.
- Miller, T.R.; Swedler, D.I.; Lawrence, B.A.; Ali, B.; Rockett, I.R.H.; Carlson, N.N.; Leonardo, J. Incidence and lethality of suicidal overdoses by drug class. JAMA Netw Open. 2020, 3, e200607. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Tian, C.; Wu, L.; Liu, M.; Lu, H. Prescribed opioid use is associated with increased all-purpose emergency department visits and hospitalizations in community-dwelling older adults in the United States. Front Psychiatry 2022, 13, 1092199. [Google Scholar] [CrossRef] [PubMed]
- ElDesoky, E.S. Pharmacokinetic-pharmacodynamic crisis in the elderly. Am J Ther. 2007, 14, 488–498. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011, 12, 601–610. [Google Scholar] [CrossRef]
- Yim, C.L.H.; Wong, E.W.W.; Jellie, L.J.; Lim, A.K.H. Illicit drug use and acute kidney injury in patients admitted to hospital with rhabdomyolysis. Intern Med J. 2019, 49, 1285–1292. [Google Scholar] [CrossRef]
- Thomas, I.C.; Nishimura, M.; Ma, J.; Dickson, S.D.; Alshawabkeh, L.; Adler, E.; Maisel, A.; Criqui, M.H.; Greenberg, B. Clinical characteristics and outcomes of patients with heart failure and methamphetamine abuse. J Card Fail. 2020, 26, 202–209. [Google Scholar] [CrossRef]
- Soder, H.E.; Berumen, A.M.; Gomez, K.E.; Green, C.E.; Suchting, R.; Wardle, M.C.; Vincent, J.; Teixeira, A.L.; Schmitz, J.M.; Lane, S.D. 2020. Elevated neutrophil to lymphocyte ratio in older adults with cocaine use disorder as a marker of chronic inflammation. Clin Psychopharmacol Neurosci. 2020, 18, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Frazer, K.M.; Richards, Q.; Keith, D.R. The long-term effects of cocaine use on cognitive functioning: A systematic critical review. Behav Brain Res. 2018, 348, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Zolopa, C.; Høj, S.B.; Minoyan, N.; Bruneau, J.; Makarenko, I.; Larney, S. Ageing and older people who use illicit opioids, cocaine or methamphetamine: A scoping review and literature map. Addiction 2022, 117, 2168–2188. [Google Scholar] [CrossRef] [PubMed]
- Moran, L.V.; Masters, G.A.; Pingali, S.; Cohen, B.M.; Liebson, E.; Rajarethinam, R.; Ongur, D. Prescription stimulant use is associated with earlier onset of psychosis. J Psychiatr Res. 2015, 71, 41–47. [Google Scholar] [CrossRef]
- Buoli, M.; Serati, M.; Cahn, W. Alternative pharmacological strategies for adult ADHD treatment: A systematic review. Expert Rev Neurother. 2016, 16, 131–144. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Death Rate Maps & Graphs. 2022. https://www.cdc.gov/drugoverdose/deaths/index.html.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).