1. Introduction
Obstructive sleep apnea syndrome (OSAS) is one of the most important disorders, which was discovered in the course of the last 50 years because of particular impact on the all organs and systems, as well as reduced quality of life [
1]. Repeated desaturation of oxyhemoglobin, micro-arousals, which are typical in obstructive sleep apnea syndrome (OSAS), have negative impact on the health of patients, leading to complications from the cardiovascular system: arterial hypertension, pulmonary hypertension, chronic heart failure, arrhythmias, myocardial infarction, strokes, insulin resistance, diabetes mellitus, metabolic syndrome, chronic renal failure and neuropsychiatric complications (depression, irritability, low level of attention or loss of short-term memory etc.), which affect familial, occupational and social life, as well as increasing the risk of road traffic accidents or accidents at workplace [
1]. Particular importance of OSAS is associated with an increase of its incidence on 14-55% depending on age and gender [
1]. Such an increase has an great importance for healthcare system and points to the need for timely and effective screening [
2].
Significant impact in the development of complications provides obesity, which is a major risk factor. Influenced by the hypoxia adipocytes leads to changes of adipocytokines secretion, which contribute to insulin resistance and metabolic syndrome in patients with OSAS. Intermittent hypoxia is also causes the decline and necrosis of pancreatic beta cells because of oxidative stress [
1].
Adipose tissue inflammation and local hypoxia contribute to increased cytokine levels, oxygen free radicals, tumor necrosis factor alpha, pre-atherogenic chemokines, and proangiogenic peptides, some of them lead to activation of the sympathetic nervous system with endothelial dysfunction, arterial rigidity and atherosclerosis [
3]. Sympathetic afferents generate renin-angiotensin system activation and
hydrosaline metabolism modification, that, in combination with the reduction of baroreceptor sensitivity, results in development of arterial hypertension(AH) [
4]. Studies demonstrate an association of OSAS with hypercoagulation and decreased fibrinolytic activity, that results in a prothrombotic status with increased risk of thrombotic complications [
5]. Men with apnea/hypopnea index (AHI) more than 19 and women with AHI more than 25 are significantly higher risk of stroke than in a healthy person [
6]. Equally important effect of the hypoxia is proinflammatory state, which is associated with systemic inflammatory response syndrome and oxidative stress [
7]. On the basis of the above, special importance in the diagnosis of OSAS an important role played by comorbidities. The late detection and treatment of comorbidities can lead to severe and dangerous complications.
2. Results
2.1. Cardiovascular diseases
At the moment, there are not only consequential evidence which indicate the role of the OSAS on the etiology and progression of cardiovascular diseases , especially HBP, but also direct evidence that have involved in the recent decade [
1]. One of the reason of this phenomenon may be neglect of the OSAS evaluation in many previously epidemiological studies [
8]. To a certain extent, it was attributable to significant costs, that are required for OSAS detection on the large-scale group of population. Additionally, OSAS patients often suffer from concomitant diseases such as obesity, HBP, diabetes mellitus, chronic obstructive pulmonary disease (COPD), asthma, glucose intolerance, therefore, any independent effect of OSAS on cardiovascular risk could be masked under comorbidities.
However, some prospective researches aimed at investigating the incidence of cardiovascular diseases, as well as assessment studies of therapeutical effect of CPAP, have provided accurate and indisputable evidence, confirming closely cause-effect relationship between OSAS and cardiovascular pathology [
1].
2.2. Hypertension
The most conclusive evidence, confirming the role of OSAS in the HBP occurrence are derived from well-known studies Wisconsin Sleep Cohort [
9]. In the studied population re-evaluated in 4 years after initial investigation was shown, that apnea-hypopnea index rates higher 15 events per hour, regardless of other factors, was associated with increased by 3 times risk of HBP development [
10]. HBP incidence at the OSAS patients is approximately 30-70% [
11]. Typically, rate of incidence of HBP and resistant hypertension is increasing with OSAS aggravation [
1].
These data suggest that significant proportion of cases that were previously considered essential hypertension may reflect consequences of undiagnosed and, as a result, untreated OSAS. Consensus guides on the management of hypertension reflect increasing number of evidence of OSAS involvement in HBP etiopathogenesis. In the 1997, in the sixth “Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure” was first time reported critically important role of OSAS, has also been recommended exclusion of this pathology in course of source determination of HBP cause [
12]. Further, guides published in 2003, were placed the OSAS in the top of the identified causes of resistant hypertension [
13]. Also, other guides published between 2017-2018, confirmed the importance of OSAS in HBP management [
14].
CPAP therapy of obstructive sleep apnea syndrome significantly reduces diurnal blood pressure not only among the patients with resistant hypertension, [
1], but also for the patients with relatively mild form of arterial hypertension [
1].
Even if the effect of the blood pressure reduction is not obvious in the normotensive patients with OSAS on long-term CPAP treatment [
15], two placebo-controlled randomized trials, in which placebo was CPAP at sub-therapeutic dose, have demonstrated that extended duration of therapy has resulted in slight decreases of diurnal blood pressure, but statistically significant, from 1,3 to 5,3 mm Hg [
1]. Therefore, there are ample evidence reaffirming the role of untreated OSAS in etiopathogenesis of HBP developing, also studies demonstrate significant reduction in diurnal blood pressure of CPAP on patients [
16].
2.3. Myocardial ischemia
OSAS induces different types of stress, chronic and acute, that may predispose to sleep-related myocardial ischemia. In condition of pronounced acute hypoxemia and CO2 retention, activation of sympathetic nervous system and dramatic blood pressure elevation can trigger myocardial ischemia. In the long term, establishing of diurnal hypertension, increased production of vasoactive and trophic substances (such as endothelin), along with proinflammatory and procoagulant mechanisms activation, can also contribute to development and progression of ischemic heart disease (IHD). Actually, in
Sleep Heart Health Study cohort, OSAS was recognized as independent risk factor for IHD [
17]. Nocturnal change of ST segment, confirming myocardial ischemia was found in the patients with OSAS with no clinical signs of IHD [
18]. ST segment depression, occurs more frequently in patients with severe form of OSAS with history of nocturnal angina pectoris symptoms and depends of arterial oxygen saturation [
19]. CPAP treatment considerably reduces overall duration of ST segment depression in patients with sleep apnea [
20]. Besides, some epidemiological studies confirm the association between OSAS or snoring with myocardial infarction (MI) [
21].
Obstructive sleep apnea is common in patients with MI in history [
22]. Postinfarction modifications in cardiac function may predispose to sleep apnea development or impairment of previously diagnosed OSAS. At the same time, patients with IHD, obstructive sleep apnea may constitute an prognostic predictor. Monitoring of 62 patients with detected IHD for a duration of 5 years was identified high mortality rate (38%) in the group of OSAS in comparison with non-OSAS patient group, taken into consideration other influential factors [
23].
2.4. Cardiac rhythm disorders
Heart rhythm disorders occurs in approximately 18-48% OSAS patients, although it is difficult to evaluate the real prevalence because of limited number of groups included into researches and considerable number of different types of arrhythmias [
1]. Presence of complicated tachyarrhythmias and bradyarrhythmias increases the risk of cardiovascular complications, reduces the quality of life and increases the risk of unfavorable outcome [
24]. Nocturnal oxygen desaturation is independent risk factor for atrial fibrillation development [
1]. Presence of OSAS is also risk factor for atrial fibrillation recurrence after successful cardioversion [
1]. However, in one randomized trial, that compared the patients on CPAP and non CPAP therapy, no significant difference was observed in the frequencies of arrhythmias between the groups [
1]. Prevalence of bradyarrhythmias is about 8% int patients with AHI less than 60 in comparison with 20% in patients with AHI more than 60 [
25].
Ventricular arrhythmias occurs in about 5 % of the general population, whereas in patients with OSAS has been found in 14-74% [
26]. The prevalence depends on AHI and desaturation below 90% [
27]. Also, it should be noted that 60% patients with ventricular arrhythmias, hospitalized for the catheter ablation or cardioverter-defibrillator implantation had AHI more than 5, and 34% of them had moderate to severe stages of OSAS [
1].
Considering to the diversity of arrhythmias that can occur in OSAS patients, it is difficult to evaluate their impact on the patient. Most probably, that short episodes of bradycardia may not significant importance, whereas atrial fibrillation or ventricular rhythm disorder are the serious risk factor for thromboembolic events and sudden death [
26].
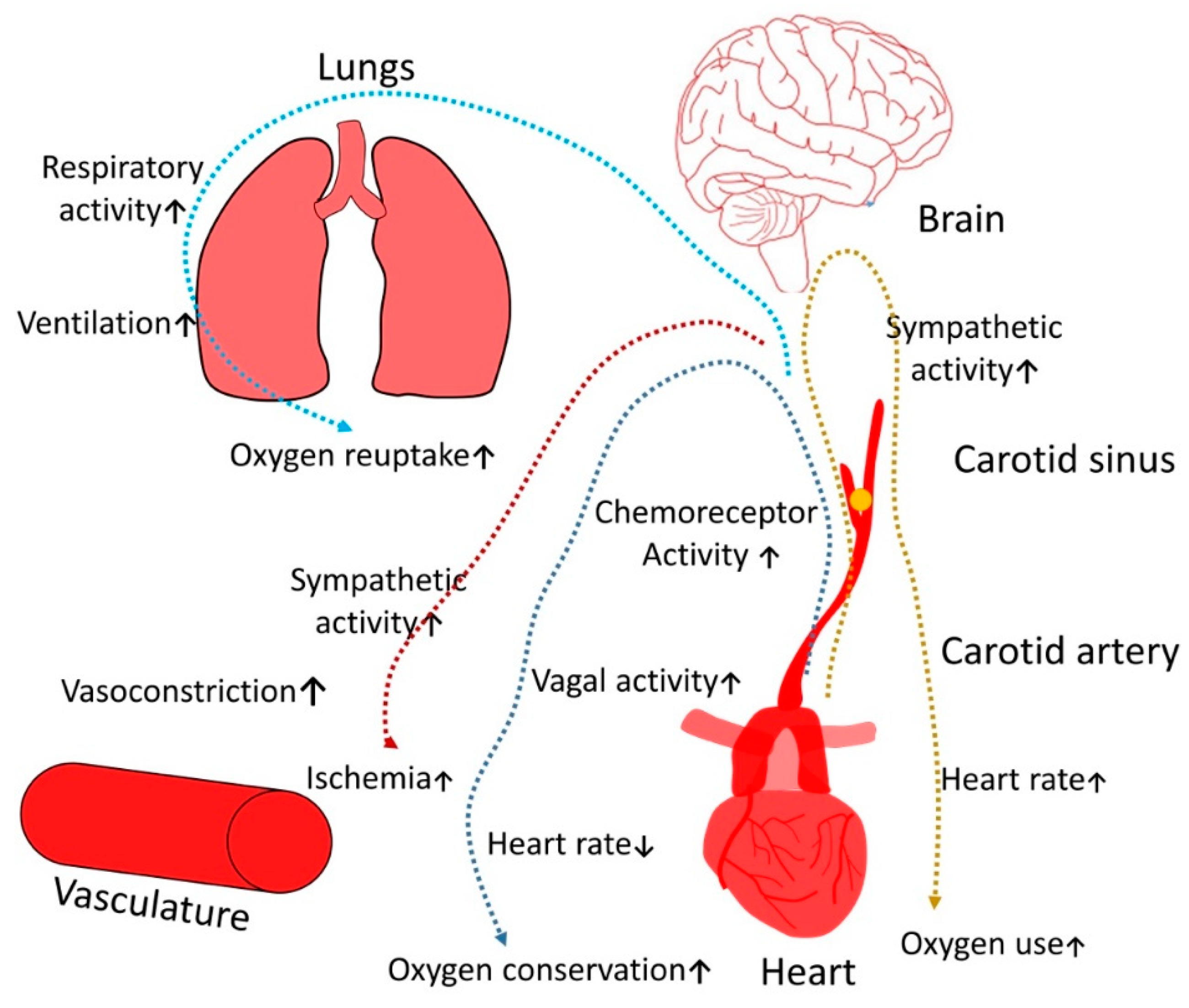
Figure 1.
Pathogenetic role of OSAS on symphato-vagal balance.
Figure 1.
Pathogenetic role of OSAS on symphato-vagal balance.
Hypercapnia is one of the most important trigger for respiratory brain center, it also causes increases sympathetic activity, enhancing oxygen intake which ultimately leads to ischemia; another crucial factor is that hypoxemia provides stimulatory effect on vagal tone that significantly increases risk of conduction rhythm disorders and bradycardia.
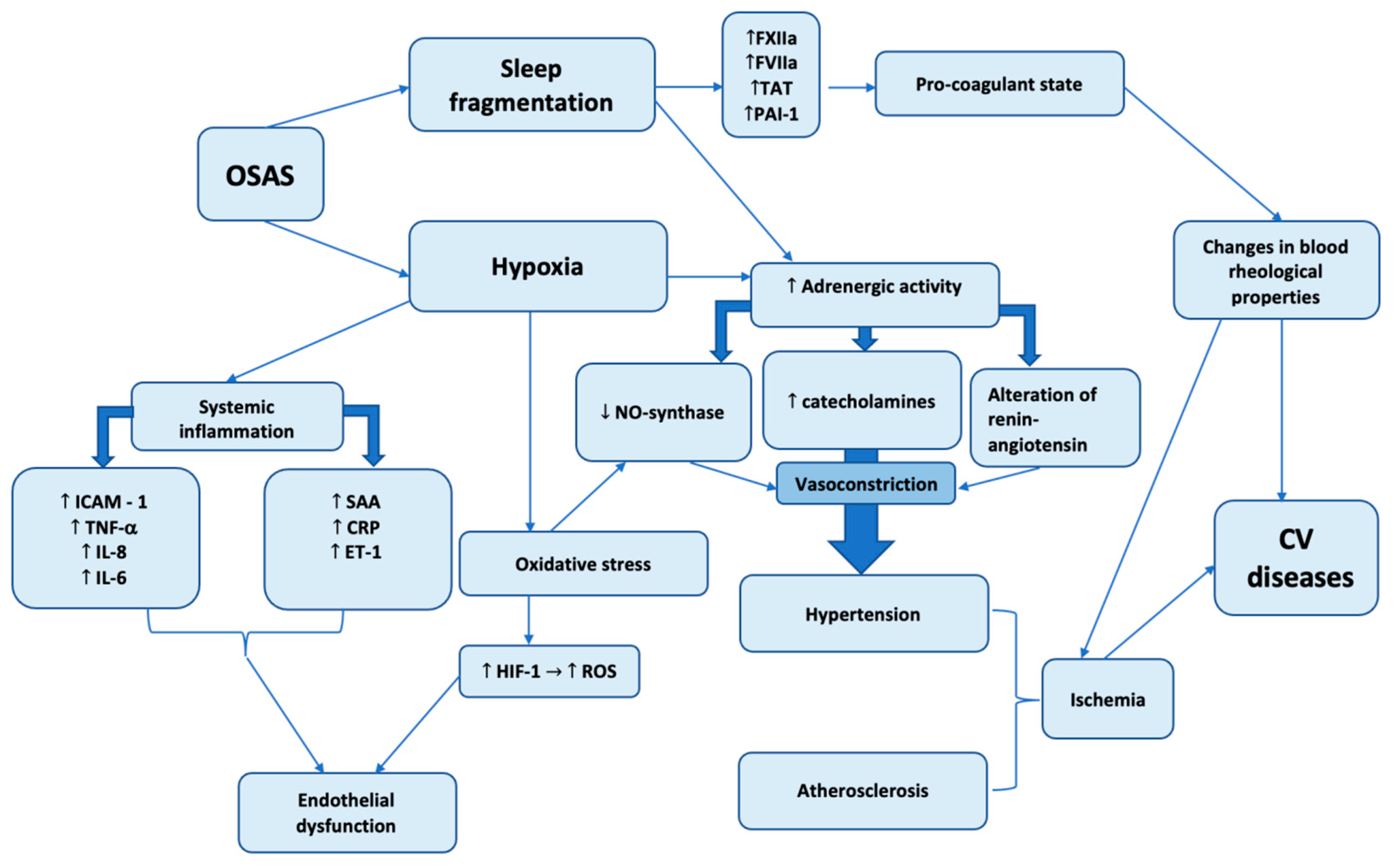
Figure 2.
Pathogenetic mechanisms of OSAS on cardiovascular diseases development.
Figure 2.
Pathogenetic mechanisms of OSAS on cardiovascular diseases development.
The presented scheme demonstrates two most important pathogenetic mechanisms caused by OSAS: hypoxia and sleep fragmentation. The role of hypoxia in initiation and progression of diverse pathological conditions cannot be underestimated. Cardiovascular system is affected by systemic inflammation, oxidative stress and adrenergic activity, both systemic inflammation and oxidative stress induces synthesis of different factors, which leads to endothelial dysfunction which is closely related with atheromatosis – an important element of coronary artery disease.
Hypoxia and sleep fragmentation action are associated with increased adrenergic activity which mediated vasoconstriction and development of hypertension.
At the same time, sleep fragmentation is correlated with increased synthesis of blood clotting factors and deterioration of hemorheological properties. The total action of these factors induce development and progresson of diverse cardiovascular diseases.
2.5. Neurophyschiatric deviations
Neurocognitive disorders associated with OSAS includes daytime sleepiness, poor concentration, depression and even dementia [
28].
Alzheimer’s disease (AD) is the most common form of dementia and its prevalence increases with age. Several studies have demonstrated that cognitive disorders occur more frequently in OSAS patients [
29].
There is a lot of evidence that suggest OSAS influence on AD progression, sleep fragmentation, intermittent hypoxia and hemodynamic changes may possessing a cumulative effect on Alzheimer’s disease development, and this suggests that timely and sufficient CPAP therapy may help to prevent or to reduce cognitive decline and dementia [
30].
Studies in neurodegenerative disorders demonstrate that OSAS has also been associated with increased risk of Parkinson disease development [30,31].
2.6. Cerebrovascular pathology
The presence of snoring predisposes to increased risk of stroke, regardless of other cardiovascular risk factors. Also was found that sleep apnea has increased prevalence in patients with stroke [
32], but it is still unknown, whether the sleep apnea is independent risk factor of cerebrovascular diseases. Hemodynamics, vascular, inflammatory and thrombocytes pathogenetic factors which are activated in OSAS can lead to increased risk of cerebrovascular diseases development, regardless of the circumstances. Acute episodes of apnea leading to dramatic falls in cerebral blood flow [
33]. Ischemia induced by repeated episodes of sleep apnea intensifying by associated hypoxia, as well as any pre-existing modifications of autoregulation or vasodilator reserves. Thus, OSAS directly or indirectly by concomitant diseases increase the risk of stroke. At the same time, stroke can trigger respiratory disorder during the sleep, central and obstructive apnea [
34]. An important role in the therapy of OSAS comorbidities played CPAP therapy. Significant improvement of collateral cerebral blood flow was observed at the OSAS patients on long term CPAP therapy [
35].
Some authors indicated on increased frequency of OSAS occurrence among patients with stroke. Among the patients from stroke department 73,7-86% had AHI over 5, and about a third over 30 [
36,
37].
However, relation between embolic stroke and OSAS is still indirect. Further long term works are needed to establish whether the OSAS an important cause of cerebrovascular diseases independent of other factors Considering that the OSAS is modifiable risk factor for cerebrovascular diseases, specialists needs to paid particular attention on this [
38].
2.7. Peripheral neuropathy
Chronic oxygen deprivation can lead to both central and peripheral nerve injury [
39]. Patients with OSAS often have nerve dysfunction whose severity is partly related to the level of nocturnal hypoxemia. Current studies demonstrate that abnormal nerve conduction suggests axonal lesions and demyelinating neuropathies [
40]. Clinical signs of polyneuropathy can be seen in up to 71% of patients with OSAS and the severity of axonal damage tends to correlate with the percentage of the night time with an O
2 saturation below 90% [
41]. Moreover, the risk of polyneuropathy is increased in case of other comorbidities such as diabetes [
42]. This damage at least to some degree can be reversible with proper CPAP treatment for sleep apnoea [
43].
2.8. Depression
Among patients with OSAS depression was found in 5-63% of cases. At the same time, it should be noted that many symptoms of these pathologies are similar. Sleep disorders are rarely studied in patients with depressive disorder as well as depression is rarely evaluated in patients with OSAS. The bidirectional interaction of these two conditions are complicated and should be closely studied in the future [
44]. Early screening of depressive disorder in patients with OSAS leads to timely psychological and social rehabilitation of the patient [
45].
Nevertheless, a large cohort study, which was held from 1991 to 2015 and were included 10149 of patients over a median follow-up of 9,7 years, has shown no correlation between OSAS and depression [
46]. A part of depression cases in patients with OSAS may be result of other factors as biological (other diseases), as social (unemployment, family conflicts and other) It is important to note that gravity of OSAS, obesity and gender are significant factors that need to be considered for precise determination of the real cause of depressive disorder [
47].
Awareness, timely screening of both depression and OSAS , as well as consideration of possible interaction between these two disorders- is an important step in combating of both illnesses [
48]. It also highlights, that these diseases are characterized by “masks”, a correct diagnosis and treatment requires multidisciplinary team of specialists including a clinical psychologist or psychiatrist.
2.9. Obesity
Weight gain is a slow and multifactorial process which is associated by lifestyle factors such as short sleep duration, sedentary lifestyle, excessive caloric intake, and genetics. Short sleep duration and higher caloric intake can cause hormonal imbalances. One such imbalance is a reduced level of melatonin, which leads to changes in the metabolic circadian rhythm which predisposes to weight gain and metabolic alterations [
49].
There are also leptin and insulin modifications, was demonstrated that obese person develop resistance of both leptin and insulin, leptin, which physiologically reduce appetite and accelerate energy metabolism, was decreased in patients with short sleep duration, that increases appetite and leads to weight gain, but more than that was shown that ghrelin, which stimulate appetite was elevated in short sleep duration person [
50].
Furthermore, positive impact on OSAS has also been demonstrated in patients after bariatric procedures and sleeve gastrectomy which was characterized by resolution or improvement in OSAS [
51,
52].
2.10. Gastrointestinal disease
Recent studies demonstrated that sleep deprivation and impaired sleep quality are associated with a wide range of gastrointestinal disorders. The true nature of these changes is complicated but is tightly linked to metabolic changes, proinflammatory cytokines and gut microbiota. Altogether this can cause a systemic reaction on the organism not limited only to the gastrointestinal tract [
53,
54]. Approximately 10% of patients with snoring or OSA revealed that functional dyspepsia is associated with more severe daytime sleepiness and apnea-hypopnea index compared to those without functional dyspepsia [
55]
In a cross-sectional study of 5792 subjects that surveyed a community-based cohort subjects provided information regarding the quality of sleep according to Pittsburgh Sleep Quality Index (PSQI), and digestive symptoms as assessed by the Gastrointestinal Symptom Rating Scale (GSRS). The results revealed that sleep disturbances were associated with digestive symptoms (aOR = 1.29, 95% CI = 1.22–1.36), especially abdominal pains (aOR = 1.63, 95% CI = 1.19–2.25), acid regurgitation (aOR = 1.48, 95% CI = 1.17–1.86), abdominal distension (aOR = 1.80, 95% CI = 1.42–2.28), and eructation (aOR = 1.59, 95% CI = 1.24–2.03) [
56]. This demonstrated a tight link between sleep quality and gastrointestinal diseases. Similar studies demonstrated an increased risk for inflammatory bowel disease. The odds ratio of IBS in positive sleep apnea group versus negative sleep apnea group was 3.92 (95% confidence interval = 1.58–9.77,
P = 0.003) [
57].
2.11. Nonalcoholic fatty liver disease
NAFLD is characterized by excessive accumulation of lipid in hepatocytes which results in lipotoxicity and inflammatory damage of hepatocytes.
Intermittent hypoxia leads to tissue hypoxia and can result in oxidative stress, mitochondrial dysfunction, inflammation, and increased activity of the sympathetic nervous system. In such studies, intermittent hypoxia has been associated with insulin resistance – one of the key factor of hepatic lipid metabolism dysfunction, hepatic steatosis and fibrosis, each of which is involved to the development and/or progression of NAFLD [
52,
58].
In a study Pretta et al. found an independent association between nocturnal oxygen saturation values and significant liver fibrosis in adult patients biopsy results, severe NAFLD was spotted at low prevalence of morbid obesity [
59]. Moreover, several studies have reported significant improvement of AST, ALT and ALP levels in patients after 6-months CPAP therapy [
60,
61,
62].
2.12. Diabetes mellitus
Prevalence of diabetes mellitus among OSAS patients is about 23-48% [
63,
64]. Experimental studies show that sleep restriction to 4 hours per night for 6 nights is associated with impaired glucose tolerance [
65]. Another important mechanism of diabetes development in OSAS patients is decreased secretion of insulin which leads to short-term or long-term hyperglycemia [
66]. Moreover, OSAS is associated with low adiponectin level, insulin tolerance, elevation of cortisol and catecholamines [
67,
68]. It was observed that fasting glycated hemoglobin and blood glucose levels are correlated with AHI , sleep duration and oxygen saturation lower than 90% [
69,
70].
In condition of proinflammatory state and oxidative stress special attention should be paid to the methods with potential to improve metabolism and to reduce the negative impact of hypoxia. After 6 months of CPAP treatment was observed decrease of endothelial dysfunction, inflammatory mediators and lipid peroxides [
71,
72]. Concerning the CPAP influence on glucose metabolism and insulin resistance the results are still controversial. A number of researchers observed improvements in glycated hemoglobin levels and insulin sensitivity in nondiabetic patients [
73]. Similar results was observed by J. F. Guest et al in patients with two type diabetes and OSAS [
74]. Furthermore, was demonstrated that the incidence of 2 type diabetes is reduced in OSAS patients on regular CPAP therapy in comparison with non-CPAP patients [
75]. However, there are studies that not confirmed the positive effect on glycemic profile in patients on CPAP therapy [
76].
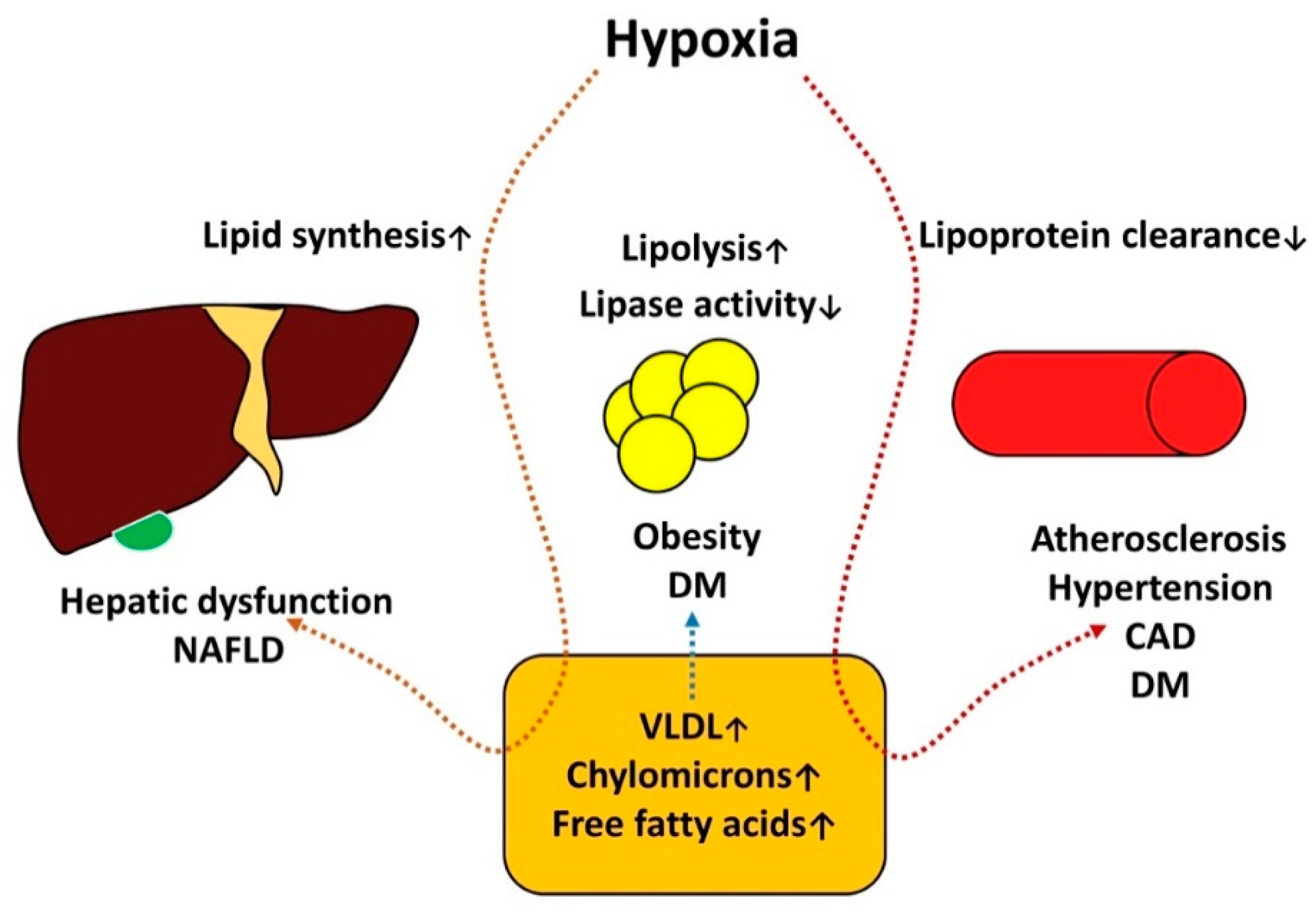
The pathogenesis of metabolic disorders and OSAS is summarized in Figure 3.
Figure 3.
The pathogenesis of metabolic disorders and OSAS.
Figure 3.
The pathogenesis of metabolic disorders and OSAS.
Diabetes mellitus, obesity and non-alcoholic fatty liver disease are constituted components of metabolic syndrome. Insulin and leptin resistance, elevated levels of ghrelin was associated with sleep restrictions, increased catecholamines and cortisol levels mediated by short sleep duration and hypoxia can amplify hyperglycemia negatively affecting this processes, here is an important role of insulin resistance and non-alcoholic fatty liver disease , in this conditions regulatory capacity of insulin on hepatic lipase is compromised this situation becomes complicated by hypoxia. Hypoxia induce hepatocite injury with hepatic lipid metabolism alteration, specifically it increases lipid synthesis and causing build-up of fat in the liver, decreased lipase activity and increased lipid synthesis leads to alterations of lipid profile which is essential for endothelial dysfunction- crucial factor of atherogenesis.
2.13. Chronic kidney disease
End-stage renal failure affects 57% of patients with OSAS [
77,
78]. Hypoxia, fluid retention, rennin-angiotensin system activation are the key elements of the OSAS and kidney failure interconnection, aggravating of both conditions [
79,
80]. Nevertheless, not only OSAS can lead to kidney failure installation - there is an inverse variant [
81]. Due to the fact, that OSAS patients often have comorbidities as AH, advanced atherosclerosis and diabetes mellitus, there is a perception that chronic renal failure appears on the background of these illnesses. Nevertheless, in patient with CKD and diabetes OSAS seems to be a factor that results in higher urinary albumin–creatinine ratio and lower estimated glomerular filtration rate [
82]. OSAS is also an important risk factor for mortality in dialysis patients and by itself linked to metabolic disturbances, proteinuria, arterial disease [
83,
84]. Preliminary data indicated that CPAP therapy contributes to the kidney hypoxia injury protection, however further large-scale randomized trials are needed for estimating of this effect [
85]. Furthermore, up to 73% of patients with OSAS have kidney dysfunction, which is revealed during screening and brings up the importance of multidisciplinary of this difficult group of multimorbid patients [
86].
2.14. Malignant neoplasms
As a result of intermittent hypoxia, OSAS may be involved with cancer progression and, probably, with cancerogenesis. Intermittent hypoxia can also precipitate tumor growth and aggression, by: 1) Hypoxia-inducible factor 1 (HIF-1) activation with angiogenesis and tumour growth stimulation as well as metastatic rate [
87]; 2) immune response changes, specifically, by
tumour-associated macrophages activation [
88]. Involvement of IH in cancerogenesis can be explained by oxidative stress induction and DNA oxidation with the gene mutation creation and cell malignancy [
89]. Sleep fragmentation, that occurs in OSAS can be associated with high risk of cancer genesis [
90,
91]. However, such data mainly were received from studies on animals and cell cultures, where is relatively easy to take into account such cofactors as age, obesity and sleep time. All of these factors are independent increase the risk of oncological diseases development and, at the same time are traditionally associated with OSAS [
92].
Several major studies have found relationship between OSAS and elevated risk of cancer development. The overall time with oxygen saturation less than 90% were associated with 2,33-increased risk of cancer development [
93]. Similar results were reported in patients from the prospective 20 year follow up research [
94]. Nevertheless, age is one of the risk factors for the cancer development. Another important point is correlation of OSAS with different oncologic diseases. For instance, patients with OSAS have increased by 1,5 times risk of CNS neoplasm in comparison with patients without apnea [
95]. A large multicentric study that included 33 711 patients demonstrated that while controlling for confounders, severe OSA was associated with a 15% increased hazard of developing cancer compared with no OSAS (HR = 1.15, 1.02-1.30; ARD = 1.28%, 0.20-2.37; and NNH = 78) and severe hypoxemia was associated with about 30% increased hazard (HR = 1.32, 1.08-1.61; ARD = 2.38%, 0.47-4.31; and NNH = 42) [
96]. This relationship seems to depend on the type of cancer and severity of OSAS. Some cancers are not encountered as frequently in OSAS [
97]. The differences are presented in table. It seems logical that different tumours tend to react differently to oxygen deprivation. The results on some types of cancer are perplexed and it is early to say whether there is a relationship.
Table 1.
Association between OSAS and some types of malignant neoplasms.
Table 1.
Association between OSAS and some types of malignant neoplasms.
| Malignant neoplasia |
Possible risk |
| CNS neoplasms |
The overall risk for developing primary CNS cancers was significantly higher in the OSAS group (aHR, 1.54; P=0.046) after adjusting for age, gender, and obesity, among other variables. Subgroup analysis revealed a significantly higher risk for primary brain cancers but not primary spinal cord cancers [95] |
| Lung cancer |
The data on lung cancer differs from study to study. Kendzerska and coworkers report a higher risk of developing lung cancer in subgroup of OSAS patients with AHI Q4 vs. Q1 (1.78 [1.03–3.10] [96]. Sillah and coworkers report a protective effect of OSAS on lungs (SIR 0.66, 95% CI 0.54-0.79) [98] |
| Melanoma |
The risk of melanoma tends to increase with more severe AHI 2.49 (1.03–6.05) AHI: Q4 vs. Q1 [96]. Other studies also demonstrate and increased risk of melanoma (HR = 1.13, CI = 1.09–1.18 and SIR 1.71, 95% CI 1.42-2.03) [99] |
| Breast cancer |
The aHR of breast cancer in patients with OSAS was higher [HR, 2.09; 95% confidence interval (CI), 1.06-4.12; P < 0.05] than that of the controls during the 5-year follow-up. Despite not meeting statistical significance, authors report an increases in the risk of breast cancer in women aged 30-59 years (HR, 2.06; 95% CI, 0.90-4.70) and ≥60 years (HR, 3.05; 95% CI, 0.90-10.32) compared with those aged 0-29 years [97]. |
| Colorectal cancer |
Patients with OSAS tend to have a higher risk of colorectal cancer (1.63 [1.12–2.38]) [96]. Another study demonstrates similar results, after adjusting for potential confounders, patients with OSAS were associated with a significantly higher risk of without OSAS (aHR, 1.80; 95% CI, 1.28-2.52). Moreover, the cumulative incidence of colorectal cancer was significantly higher in the OSAS cohort than in the comparison cohort [100].Nevertheless several other studies demonstrate a decreased risk of colorectal cancer [98] |
| Pancreatic cancer |
Patients with OSAS tend to have an increased risk of pancreatic cancer (HR = 1.14, CI = 1.06–1.23) [99] |
| Kidney cancer |
The risk of kidney cancer is debatable. Kendzerska and coworkers found no association between kidney cancer and OSAS [96]. Other studies demonstrate and increased risk (HR = 1.30, CI = 1.23–1.37) and (SIR 2.24, 95% CI 1.82-2.72) [99] |
| Prostate cancer |
One of the studies demonstrated and increased risk of prostate cancer 1.63 (1.06–2.51) [96] while another a protective effect (HR = 0.93, CI = 0.90–0.96 in both) [99] |
| Urinary cancer |
Severe OSAS tends to increase urinary cancer 1.72 (1.08–2.75) [96] |
| Uterus |
Uterus cancer is more frequent in OSAS (SIR 2.80, 95% CI 2.24-2.47) [98,99] |
Some types of cancers are reported more frequently, particularly: melanoma, bladder, lung, liver, cervix and kidney, and pancreas. Moreover, it early to say whether the presence of OSAS can be related to an increased risk for metastasis or death [
99].
Research into different oncological diseases in the context of hypoxia is especially important for the better understanding of both, mechanisms of cancer development and for the selection of patients groups, who need timely screening and detection of OSAS.
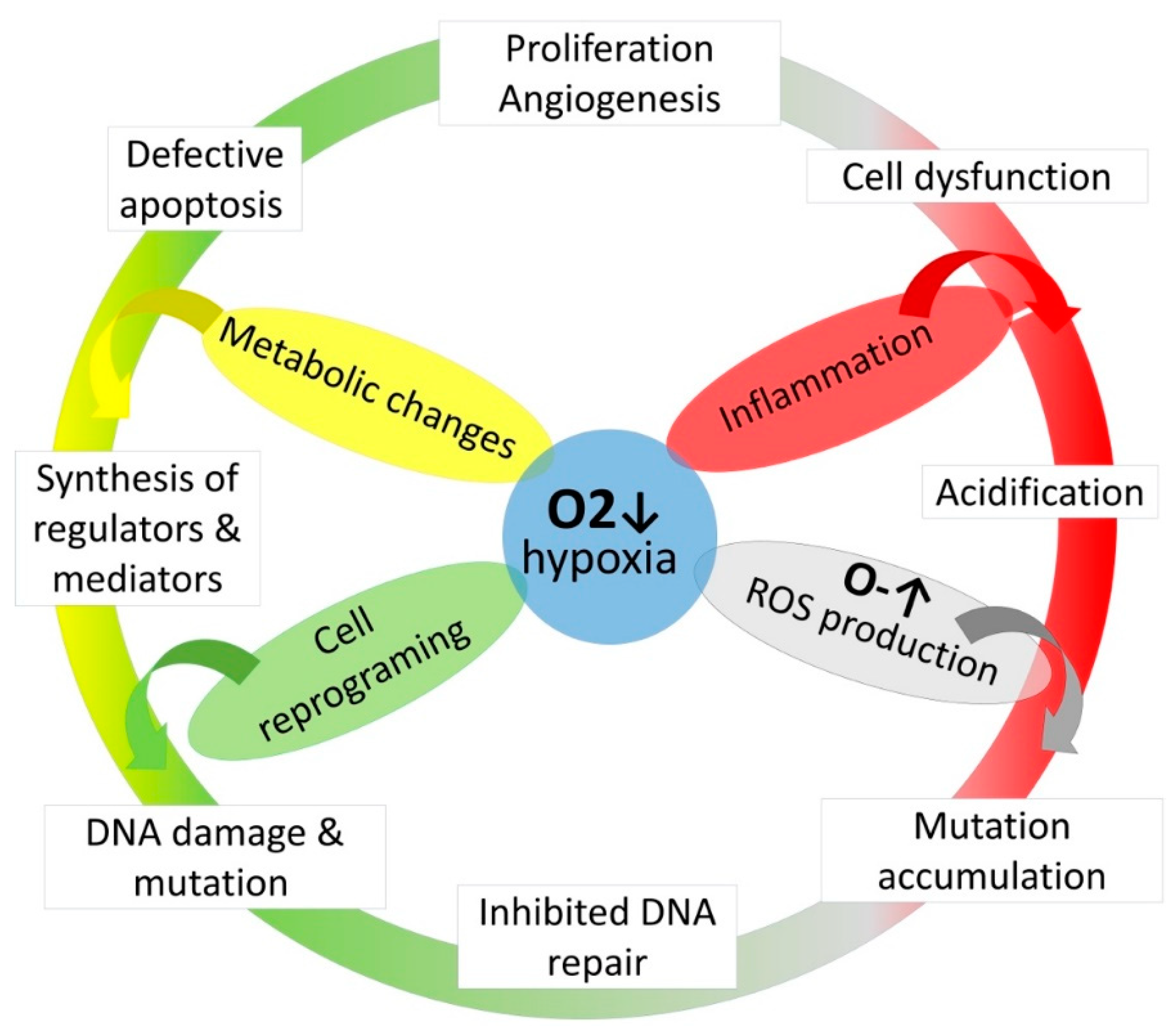
Figure 4.
The role of hypoxia on cancerogenesis.
Figure 4.
The role of hypoxia on cancerogenesis.
Metabolic changes induce synthesis of diverse regulators and mediators synthesis which resulted in inflammation, cell dysfunction and defective apoptosis, it also provides DNA oxidation leads to inhibition of DNA repair and mutation accumulation mediates the malignant cells transformation.
5. Conclusions
OSAS is one of the most important diseases discovered in the last 50 years. The accumulated knowledge helps to understand that this pathology is associated with a marked function disorder not only of respiratory system, but also of many others systems. Due to hypoxia, pro-inflammatory syndrome, oxidative stress and other processes has been observed dysfunction of cardiovascular, nervous, endocrine, excretory and other systems. Timely screening of OSAS and CPAP therapy administration contribute to reparation and in some cases marked deceleration of comorbidities progression. The modern approach of OSAS requires multidisciplinary team, which is able not only to reach a correct diagnosis and treatment plan, but also to make adjustments according to comorbidities.
Author Contributions
Conceptualization, A.C. and S.Cov.; methodology, A.C.; writing—original draft preparation, V.S. and S.C.; writing—review and editing, A.C., C.P., O.C., A.S. and S.Cov.; visualization, V.S. and S.C.; supervision, A.C. and S.Cov. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pack, A.I. Advances in Sleep-disordered Breathing. Am. J. Respir. Crit. Care Med. 2006, 173, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Corlateanu, A.; Covantev, S.; Botnaru, V.; Sircu, V.; Nenna, R. To sleep, or not to sleep - that is the question, for polysomnography. Breathe 2017, 13, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Parati, G.; Lombardi, C.; Hedner, J.; Bonsignore, M.R.; Grote, L.; Tkacova, R.; Lévy, P.; Riha, R.; Bassetti, C.; Narkiewicz, K.; et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur. Respir. J. 2013, 41, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.H.; Liao, W.C.; Chung, W.S.; Muo, C.H.; Chu, C.C.; Liu, C.J.; Kao, C.H. Association between obstructive sleep apnea and deep vein thrombosis/pulmonary embolism: A population-based retrospective cohort study. Thromb. Res. 2014, 134, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.-T.; Huang, C.-C.; Chen, Y.-M.; Su, K.-C.; Shiao, G.-M.; Lee, Y.-C.; Chan, W.-L.; Leu, H.-B. Sleep Apnea and Risk of Deep Vein Thrombosis: A Non-randomized, Pair-matched Cohort Study. Am. J. Med. 2012, 125, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Kukes, V.G.; Corlateanu, A. Coenzyme Q10 in COPD: An Unexplored Opportunity? COPD J. Chronic Obstr. Pulm. Dis. 2021, 18, 114–122. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.M.; Gersh, B.J.; Somers, V.K. Obstructive Sleep ApneaImplications for Cardiac and Vascular Disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. New Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef]
- Fletcher, E.C.; DeBEHNKE, R.D.; Lovoi, M.S.; Gorin, A.B. Undiagnosed Sleep Apnea in Patients with Essential Hypertension. Annals of internal medicine 1985, 103, 190–195. [Google Scholar] [CrossRef] [PubMed]
- The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA Intern. Med. 1997, 157, 2413–2446. [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Williams, B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: More Similar Than DifferentComparison of the 2018 ESC/ESH and 2017 ACC/AHA Hypertension GuidelinesComparison of the 2018 ESC/ESH and 2017 ACC/AHA Hypertension Guidelines. JAMA 2018, 320, 1749–1750. [Google Scholar] [PubMed]
- Narkiewicz, K.; Kato, M.; Phillips, B.G.; Pesek, C.A.; Davison, D.E.; Somers, V.K. Nocturnal Continuous Positive Airway Pressure Decreases Daytime Sympathetic Traffic in Obstructive Sleep Apnea. Circ. 1999, 100, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Marin-Oto, M.; Vicente, E.E.; Marin, J.M. Long term management of obstructive sleep apnea and its comorbidities. Multidiscip. Respir. Med. 2019, 14, 21. [Google Scholar] [CrossRef]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O'Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered Breathing and Cardiovascular Disease. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef]
- Hanly, P.; Sasson, Z.; Zuberi, N.; Lunn, K. ST-segment depression during sleep in obstructive sleep apnea. Am. J. Cardiol. 1993, 71, 1341–1345. [Google Scholar] [CrossRef]
- Franklin, K.; Sahlin, C.; Nilsson, J.; Näslund, U. Sleep apnoea and nocturnal angina. Lancet 1995, 345, 1085–1087. [Google Scholar] [CrossRef]
- Olafiranye, O.; Reis, S.; Patrick, J.; Strollo, J. Sleep Apnea and Subclinical Myocardial Injury: Where Do We Stand? Am J Respir Crit Care Med 2013, 188, 1394–1395. [Google Scholar] [CrossRef]
- Porto, F.; Sakamoto, Y.S.; Salles, C. Association between Obstructive Sleep Apnea and Myocardial Infarction: A Systematic Review. Arq. Bras. de Cardiol. 2017, 108, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O'Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Peker, Y.; Hedner, J.; Kraiczi, H.; Löth, S. Respiratory Disturbance Index. Am. J. Respir. Crit. Care Med. 2000, 162, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hersi, A.S. Obstructive sleep apnea and cardiac arrhythmias. Ann. Thorac. Med. 2010, 5, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.F.; Koehler, U.; Stammnitz, A.; Peter, J.H. Heart block in patients with sleep apnoea. Thorax 1998, 53, S29–S32. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.A.; Stradling, J.R.; Kohler, M. Effects of obstructive sleep apnoea on heart rhythm. Eur. Respir. J. 2012, 41, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Hoffstein, V.; Mateika, S. Cardiac arrhythmias, snoring, and sleep apnea. CHEST 1994, 106, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Djonlagic, I.; Guo, M.; Matteis, P.; Carusona, A.; Stickgold, R.; Malhotra, A. Untreated Sleep-Disordered Breathing: Links to Aging-Related Decline in Sleep-Dependent Memory Consolidation. PLoS ONE 2014, 9, e85918. [Google Scholar] [CrossRef]
- Emamian, F.; Khazaie, H.; Tahmasian, M.; Leschziner, G.D.; Morrell, M.J.; Hsiung, G.Y.R.; Rosenzweig, I.; Sepehry, A.A. The Association Between Obstructive Sleep Apnea and Alzheimer's Disease: A Meta-Analysis Perspective. Front Aging Neurosci 2016, 8, 78. [Google Scholar] [CrossRef]
- Andrade, A.G.; Bubu, O.M.; Varga, A.W.; Osorio, R.S. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J. Alzheimer's Dis. 2018, 64, S255–S270. [Google Scholar] [CrossRef]
- Yeh, N.C.; Tien, K.J.; Yang, C.M.; Wang, J.J.; Weng, S.F. Increased Risk of Parkinson's Disease in Patients With Obstructive Sleep Apnea: A Population-Based, Propensity Score-Matched, Longitudinal Follow-Up Study. Medicine 2016, 95, e2293. [Google Scholar] [CrossRef] [PubMed]
- Good, D.C.; Henkle, J.Q.; Gelber, D.; Welsh, J.; Verhulst, S. Sleep-Disordered Breathing and Poor Functional Outcome After Stroke. Stroke 1996, 27, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.; Werner, P.; Jochums, I.; Lehmann, M.; Strohl, K.P.; Leslie, W.D.; Wali, S.; Kryger, M.; Mohsenin, V.; Wolk, R.; et al. Blood Flow of the Middle Cerebral Artery With Sleep-Disordered Breathing. Stroke 1998, 29, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Alexiev, F.; Brill, A.K.; Ott, S.R.; Duss, S.; Schmidt, M.; Bassetti, C.L. Sleep-disordered breathing and stroke: Chicken or egg? J. Thorac. Dis. 2018, 10, S4244–S4252. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Jiang, Z.-H.; Zhang, L.-G. Therapeutic effects of long-term continuous positive airway pressure treatment on improving leptomeningeal collateral circulation in obstructive sleep apnea syndrome patients. European Review for Medical & Pharmacological Sciences 2018, 22, 4261–4267. [Google Scholar]
- Ifergane, G.; Ovanyan, A.; Toledano, R.; Goldbart, A.; Abu-Salame, I.; Tal, A.; Stavsky, M.; Novack, V. Obstructive Sleep Apnea in Acute Stroke. Stroke 2016, 47, 1207–1212. [Google Scholar] [CrossRef]
- Tosun, A.; Köktürk, O.; Karataş, G.K.; Çiftçi, T.U.; Sepici, V. Obstructive Sleep Apnea in Ischemic Stroke patients. Clinics 2008, 63, 625–630. [Google Scholar] [CrossRef]
- Sharma, S.; Culebras, A. Sleep apnoea and stroke. Stroke Vasc. Neurol. 2016, 1, 185–191. [Google Scholar] [CrossRef]
- Odajiu, I.; Covantsev, S.; Sivapalan, P.; Mathioudakis, A.G.; Jensen, J.-U.S.; Davidescu, E.I.; Chatzimavridou-Grigoriadou, V.; Corlateanu, A. Peripheral neuropathy: A neglected cause of disability in COPD – A narrative review. Respir. Med. 2022, 201, 106952. [Google Scholar] [CrossRef]
- Mayer, P.; Dematteis, M.; Pepin, J.L.; Wuyam, B.; Veale, D.A.N.; Vila, A.; Levy, P. Peripheral neuropathy in sleep apnea. A tissue marker of the severity of nocturnal desaturation. Am J Respir Crit Care Med 1999, 159, 213–219. [Google Scholar] [CrossRef]
- Ludemann, P.; Dziewas, R.; Sörös, P.; Happe, S.; Frese, A. Axonal polyneuropathy in obstructive sleep apnoea. J. Neurol. Neurosurg. Psychiatry 2001, 70, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Altintas, N.; Tutar, U.; Sariaydin, M.; Tiras, R. A novel connection: Obstructive sleep apnoea and diabetic neuropathy. Eur. Respir. J. 2013, 42 (Suppl. S57), P2539. [Google Scholar]
- Dziewas, R.; Schilling, M.; Engel, P.; Boentert, M.; Hor, H.; Okegwo, A.; Lüdemann, P.; Ringelstein, E.B.; Young, P. Treatment for obstructive sleep apnoea: Effect on peripheral nerve function. J. Neurol. Neurosurg. Psychiatry 2006, 78, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, S.M.; Khawaja, I.S.; Bhatia, S.; Hurwitz, T.D. Obstructive sleep apnea and depression: A review. Innovations in clinical neuroscience 2011, 8, 17–25. [Google Scholar]
- Shoib, S.; Malik, J.A.; Masoodi, S. Depression as a manifestation of obstructive sleep apnea. J. Neurosci. Rural. Pr. 2017, 08, 346–351. [Google Scholar] [CrossRef]
- Kendzerska, T.; Gershon, A.S.; Hawker, G.A.; Tomlinson, G.A.; Leung, R.S. Obstructive sleep apnoea is not a risk factor for incident hospitalised depression: A historical cohort study. Eur. Respir. J. 2017, 49, 1601361. [Google Scholar] [CrossRef]
- Aloia, M.S.; Arnedt, J.T.; Smith, L.; Skrekas, J.; Stanchina, M.; Millman, R.P. Examining the construct of depression in obstructive sleep apnea syndrome. Sleep Med. 2005, 6, 115–121. [Google Scholar] [CrossRef]
- Schröder, C.M.; O'Hara, R. Depression and Obstructive Sleep Apnea (OSA). Ann. Gen. Psychiatry 2005, 4, 13–13. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J.; Kim, T.; Van Horn, L.; Attarian, H.; Wolfe, L.; Siddique, J.; Santostasi, G.; Zee, P.C. Circadian timing and alignment in healthy adults: Associations with BMI, body fat, caloric intake and physical activity. Int. J. Obes. 2016, 41, 203–209. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J Endocrinol 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Tan, M.M.; Jin, X.; Taylor, C.; Low, A.K.; Le Page, P.; Martin, D.; Li, A.; Joseph, D.; Kormas, N. Long-Term Trajectories in Weight and Health Outcomes Following Multidisciplinary Publicly Funded Bariatric Surgery in Patients with Clinically Severe Obesity (≥ 3 Associated Comorbidities): A Nine-Year Prospective Cohort Study in Australia. J. Clin. Med. 2022, 11, 4466. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLOS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Khanijow, V.; Prakash, P.; Emsellem, H.A.; Borum, M.L.; Doman, D.B. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol Hepatol 2015, 11, 817–825. [Google Scholar]
- Li, Q.; Xu, T.; Shao, C.; Gao, W.; Wang, M.; Dong, Y.; Wang, X.; Lu, F.; Li, D.; Tan, H.; et al. Obstructive sleep apnea is related to alterations in fecal microbiome and impaired intestinal barrier function. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef]
- Koo, D.L.; Nam, H. The Relationship between Obstructive Sleep Apnea and Functional Dyspepsia. J. Sleep Med. 2020, 17, 73–77. [Google Scholar] [CrossRef]
- Hyun, M.K.; Baek, Y.; Lee, S. Association between digestive symptoms and sleep disturbance: A cross-sectional community-based study. BMC Gastroenterol. 2019, 19, 1–6. [Google Scholar] [CrossRef]
- Azimian, F.; Ghiasi, F.; Amra, B.; Sebghatollahi, V. Association of irritable bowel syndrome and sleep apnea in patients referred to sleep laboratory. J. Res. Med Sci. 2017, 22, 72. [Google Scholar] [CrossRef]
- Mesarwi, O.A.; Loomba, R.; Malhotra, A. Obstructive Sleep Apnea, Hypoxia, and Nonalcoholic Fatty Liver Disease. Am J Respir Crit Care Med 2019, 199, 830–841. [Google Scholar] [CrossRef]
- Petta, S.; Marrone, O.; Torres, D.; Buttacavoli, M.; Cammà, C.; Di Marco, V.; Licata, A.; Bue, A.L.; Parrinello, G.; Pinto, A.; et al. Obstructive Sleep Apnea Is Associated with Liver Damage and Atherosclerosis in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0142210. [Google Scholar] [CrossRef]
- Hirono, H.; Watanabe, K.; Hasegawa, K.; Kohno, M.; Terai, S.; Ohkoshi, S. Impact of continuous positive airway pressure therapy for nonalcoholic fatty liver disease in patients with obstructive sleep apnea. World J. Clin. Cases 2021, 9, 5112–5125. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Zhang, L.; Zeng, H.; Wu, Q.; Hu, M.; Xie, J.; Liu, J. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin. Respir. J. 2016, 12, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Hobzova, M.; Ludka, O.; Stepanova, R.; Sova, M.; Sovova, E. The positive effect of CPAP therapy on the level of liver enzymes in obstructive sleep apnea patients. European Respiratory Journal 2015, 46 (Suppl. S 59), PA2332. [Google Scholar]
- Einhorn, D.; Stewart, D.A.; Erman, M.K.; Gordon, N.; Philis-Tsimikas, A.; Casal, E. Prevalence of Sleep Apnea in a Population of Adults with Type 2 Diabetes Mellitus. Endocr. Pr. 2007, 13, 355–362. [Google Scholar] [CrossRef] [PubMed]
- West, S.D.; Nicoll, D.J.; Stradling, J.R. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax 2006, 61, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Nieto, F.J. Association of Sleep Time With Diabetes Mellitus and Impaired Glucose Tolerance. Arch. Intern. Med. 2005, 165, 863–867. [Google Scholar] [CrossRef]
- Koren, D.; Katz, L.E.L.; Brar, P.C.; Gallagher, P.R.; Berkowitz, R.I.; Brooks, L.J. Sleep Architecture and Glucose and Insulin Homeostasis in Obese Adolescents. Diabetes Care 2011, 34, 2442–2447. [Google Scholar] [CrossRef]
- Leproult, R.; Copinschi, G.; Buxton, O.; Van Cauter, E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997, 20, 865–870. [Google Scholar] [CrossRef]
- Kelly, A.; Dougherty, S.; Cucchiara, A.; Marcus, C.L.; Brooks, L.J. Catecholamines, Adiponectin, and Insulin Resistance as Measured by HOMA in Children with Obstructive Sleep Apnea. Sleep 2010, 33, 1185–1191. [Google Scholar] [CrossRef]
- Papanas, N.; Steiropoulos, P.; Nena, E.; Tzouvelekis, A.; Maltezos, E.; Trakada, G.; Bouros, D. HbA1c is associated with severity of obstructive sleep apnea hypopnea syndrome in nondiabetic men. Vasc. Health Risk Manag. 2009, 5, 751–756. [Google Scholar] [CrossRef]
- Tatti, P.; Strollo, F.; Passali, D. Sleep Apnea, Sleep Disturbance, and Fasting Glucose Variability: A Pilot Study. J. Diabetes Sci. Technol. 2013, 7, 743–748. [Google Scholar] [CrossRef]
- Yagihara, F.; Lucchesi, L.M.; D'Almeida, V.; de Mello, M.T.; Tufik, S.; Bittencourt, L.R.A. Oxidative stress and quality of life in elderly patients with obstructive sleep apnea syndrome: Are there differences after six months of Continuous Positive Airway Pressure treatment? Clinics 2012, 67, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, M.; Nakano, H.; Maekawa, J.; Okamoto, Y.; Ohnishi, Y.; Suzuki, T.; Kimura, H. Oxidative Stress in Obstructive Sleep Apnea. Chest 2005, 127, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.C.M.; Lam, B.; Yao, T.J.; Lai, A.Y.K.; Ooi, C.G.; Tam, S.; Lam, K.S.L.; Ip, M.S.M. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur. Respir. J. 2009, 35, 138–145. [Google Scholar] [CrossRef]
- Guest, J.F.; Panca, M.; Sladkevicius, E.; Taheri, S.; Stradling, J. Clinical Outcomes and Cost-effectiveness of Continuous Positive Airway Pressure to Manage Obstructive Sleep Apnea in Patients With Type 2 Diabetes in the U.K. Diabetes Care 2014, 37, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.H.; Hui, C.K.; Lui, M.M.; Lam, D.C.; Fong, D.Y.; Ip, M.S. Incident Type 2 Diabetes in OSA and Effect of CPAP Treatment: A Retrospective Clinic Cohort Study. CHEST 2019, 156, 743–753. [Google Scholar] [CrossRef] [PubMed]
- West, S.D.; Nicoll, D.J.; Wallace, T.M.; Matthews, D.R.; Stradling, J.R. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax 2007, 62, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Nicholl, D.D.M.; Ahmed, S.B.; Loewen, A.H.S.; Hemmelgarn, B.R.; Sola, D.Y.; Beecroft, J.M.; Turin, T.C.; Hanly, P.J. Declining Kidney Function Increases the Prevalence of Sleep Apnea and Nocturnal Hypoxia. Chest 2012, 141, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Hanly, P.J. ; Md; Faasm Consider the Kidney when Managing Obstructive Sleep Apnea. J. Clin. Sleep Med. 2015, 11, 845–846. [Google Scholar] [CrossRef]
- Lin, C.-H.; Perger, E.; Lyons, O.D. Obstructive sleep apnea and chronic kidney disease. Curr. Opin. Pulm. Med. 2018, 24, 549–554. [Google Scholar] [CrossRef]
- Marrone, O.; Bonsignore, M.R. Sleep Apnea and the Kidney. Curr. Sleep Med. Rep. 2020, 6, 85–93. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lurie, R.C.; Lyons, O.D. Sleep Apnea and Chronic Kidney Disease: A State-of-the-Art Review. CHEST 2020, 157, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Zamarrón, E.; Jaureguizar, A.; García-Sánchez, A.; Díaz-Cambriles, T.; Alonso-Fernández, A.; Lores, V.; Mediano, O.; Rodríguez-Rodríguez, P.; Cabello-Pelegrín, S.; Morales-Ruíz, E.; et al. Obstructive sleep apnea is associated with impaired renal function in patients with diabetic kidney disease. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Murata, M.; Honma, S.; Iwazu, Y.; Sasaki, N.; Ogura, M.; Onishi, A.; Ando, Y.; Muto, S.; Shimada, K.; et al. Sleep-disordered breathing predicts cardiovascular events and mortality in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Ozkok, A.; Kanbay, A.; Odabas, A.R.; Covic, A.; Kanbay, M. Obstructive sleep apnea syndrome and chronic kidney disease: A new cardiorenal risk factor. Clin. Exp. Hypertens. 2014, 36, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Digby, J.W.; Mathioudakis, A.; Heartshorne, R.; Mohammad, M.; Tewkesbury, D.; Needham, M. CPAP appears to protect kidney function of patients with OSA. Eur. Respir. J. 2017, 50 (Suppl. S61). [Google Scholar]
- Beaudin, A.E.; Raneri, J.K.; Ahmed, S.B.; Allen, A.J.M.H.; Nocon, A.; Gomes, T.; Gakwaya, S.; Series, F.; Kimoff, J.; Skomro, R.P.; et al. Risk of chronic kidney disease in patients with obstructive sleep apnea. Sleep 2021, 45. [Google Scholar] [CrossRef] [PubMed]
- Rofstad, E.K.; Gaustad, J.; Egeland, T.A.; Mathiesen, B.; Galappathi, K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int. J. Cancer 2010, 127, 1535–1546. [Google Scholar] [CrossRef]
- Gozal, D.; Almendros, I.; Hakim, F. Sleep apnea awakens cancer. OncoImmunology 2014, 3, e28326. [Google Scholar] [CrossRef]
- Kontogianni, K.; Messini-Nikolaki, N.; Christou, K.; Gourgoulianis, K.; Tsilimigaki, S.; Piperakis, S.M. DNA damage and repair capacity in lymphocytes from obstructive sleep apnea patients. Environ. Mol. Mutagen. 2007, 48, 722–727. [Google Scholar] [CrossRef]
- McELROY, J.A.; Newcomb, P.A.; Titus-Ernstoff, L.; Trentham-Dietz, A.; Hampton, J.M.; Egan, K.M. Duration of sleep and breast cancer risk in a large population-based case–control study. J. Sleep Res. 2006, 15, 241–249. [Google Scholar] [CrossRef]
- Luo, J.; Sands, M.; Wactawski-Wende, J.; Song, Y.; Margolis, K.L. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women's Health Initiative. Am J Epidemiol 2013, 177, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farré, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef] [PubMed]
- nbsp; Campos-Rodriguez, F. ; Martinez-Garcia, M.; Martinez, M.; Duran-Cantolla, J.; Peña, M.L.; Masdeu, M.; Gonzalez, M.; Campo, F.; Gallego, I.; Marin, J.; et al. Association between Obstructive Sleep Apnea and Cancer Incidence in a Large Multicenter Spanish Cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.S.; Wong, K.K.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. Journal of Clinical Sleep Medicine. JCSM Official Publication of the American Academy of Sleep Medicine 2014, 10, 355–362. [Google Scholar]
- Chen, J.-C.; Hwang, J.-H. Sleep apnea increased incidence of primary central nervous system cancers: A nationwide cohort study. Sleep Med. 2014, 15, 749–754. [Google Scholar] [CrossRef]
- Kendzerska, T.; Povitz, M.; Leung, R.S.; Boulos, M.I.; McIsaac, D.I.; Murray, B.J.; Bryson, G.L.; Talarico, R.; Hilton, J.F.; Malhotra, A.; et al. Obstructive Sleep Apnea and Incident Cancer: A Large Retrospective Multicenter Clinical Cohort Study. Cancer Epidemiology Biomarkers Prev. 2020, 30, 295–304. [Google Scholar] [CrossRef]
- Chang, W.-P.; Liu, M.-E.; Yang, A.C.; Ku, Y.-C.; Pai, J.-T.; Lin, Y.-W.; Tsai, S.-J. Sleep apnea and the subsequent risk of breast cancer in women: A nationwide population-based cohort study. Sleep Med. 2014, 15, 1016–1020. [Google Scholar] [CrossRef]
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018, 29, 987–994. [Google Scholar] [CrossRef]
- Gozal, D.; Ham, S.A.; Mokhlesi, B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hu, J.-M.; Shen, C.-J.; Chou, Y.-C.; Tian, Y.-F.; Chen, Y.-C.; You, S.-L.; Hung, C.-F.; Lin, T.-C.; Hsiao, C.-W.; et al. Increased incidence of colorectal cancer with obstructive sleep apnea: A nationwide population-based cohort study. Sleep Med. 2019, 66, 15–20. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).