1. Introduction
Aortic valve disease is common, affecting 1 in 100 adults in the United States and some 6 million or so individuals worldwide [
1,
2,
3]. A heterogenous spectrum of conditions with different aetiologies and pathophysiology can lead to valve stenosis, regurgitation and endocarditis. The natural history of these conditions is progressive, with symptom progression from angina, breathless or syncope eventually culminating in heart failure and death. In the western world, senile degenerative stenosis predominates, whilst elsewhere rheumatic disease is more prevalent. The onset of symptoms is thought to mark an inflection point where compensatory mechanisms are exhausted and prognosis worsens quickly. Most patients with severe aortic stenosis will develop symptoms within 5 years and event-free survival may be as low as 21% at two years [
4,
5]. Even with moderate aortic stenosis, progression leads to poor prognostic disease in 38% of patients within five years [
6]. No medical treatments influence the natural history of aortic stenosis [
7].
Treatment for aortic valve conditions, therefore, is invariably replacement of the diseased valve in 99% of cases [
8]. While repair procedures have gained traction in some cases, replacement of the valve has remained the principle strategy since its inception in 1958. Outcomes have been excellent, with mortality from isolated, uncomplicated and conventional aortic valve replacement consistently less than 1% [
9].
Despite excellent outcomes for surgery, a 33% rate of surgical turn-down for patients over the age of 75y in the Euro Heart Survey prompted a search for less invasive means of treatment of aortic valve disease [
10]. The first percutaneous transcatheter aortic valve implantation (TAVI) in man was performed in 2002 [
11]. Over the last two decades, the technology has matured and increased in efficacy and scope, leading to increasingly liberal recommendations for its use in the guidelines [
12]. Conversely, the 2017 European Society of Cardiology / European Association of Cardio-Thoracic Surgery guidelines did not reference minimal access approaches for valve surgery at all, and when refreshed in 2021, these were still not discussed [
13]. Transcatheter techniques, with a compellingly short recovery time even in highly comorbid patients, have been a driver to reduce the invasiveness of conventional surgical approaches. Complications associated with TAVI such as cerebrovascular embolic events, vascular complications, conduction disorders and prosthetic valve dysfunction are gradually being addressed, but provide good rationale to consider the known excellent pedigree of surgical valve replacement as a first line treatment for the majority of patients requiring intervention.
Having both approaches available in the armamentarium of the Heart Team serves the needs of patients well. Nevertheless, there remains an appetite to marry the reduced invasiveness of TAVI with the meticulous decalcification and directly visualized seating of uncrimped valves. At the turn of the millennium, Chitwood predicted that 21
st Century cardiac surgery procedures would be performed minimally invasive as day-cases, with patients returning to normal activity within one or two weeks [
14]. Nearly 25 years later, this is true of percutaneous interventions, but remains an elusive goal in surgery.
In this state-of-the-art review, we describe advances in surgical technique that have improved quality and outcomes in minimal access aortic valve surgery and present a vision for future direction.
2. Minimal Access Options
While the terms
minimally invasive and
minimal access are often used interchangeably, these overlapping philosophies can be disparate. In particular, the relative technical challenges of performing surgery through reduced access incisions can increase cardiopulmonary bypass and ischaemic surgical time, paradoxically increasing the invasiveness of the procedure. The invasiveness of cardiac surgery, some posit, is as much contributed to by the deleterious effects of cardiopulmonary bypass, as it is the trauma of sternotomy, which is generally well tolerated [
15,
16]. Efforts to mitigate for both, in order to make incremental gains, include a plethora of techniques and approaches, each with various advantages, disadvantages and prerequisites. The most common are summarized here and it is worth noting that there is a paucity of evidence to support preferring one approach over others [
17].
2.1. Approach
2.1.1. Hemi-sternotomy
The hemi-sternotomy (also known as partial sternotomy or limited sternotomy) approach that is now the most common approach to minimally invasive aortic valve surgery was developed after initial work using mini-thoracotomy [
18,
19]. The rationale for this was to avoid the subcostal neurovascular bundles that can cause post-thoracotomy pain if retracted and also to improve access to the great vessels for central cannulation.
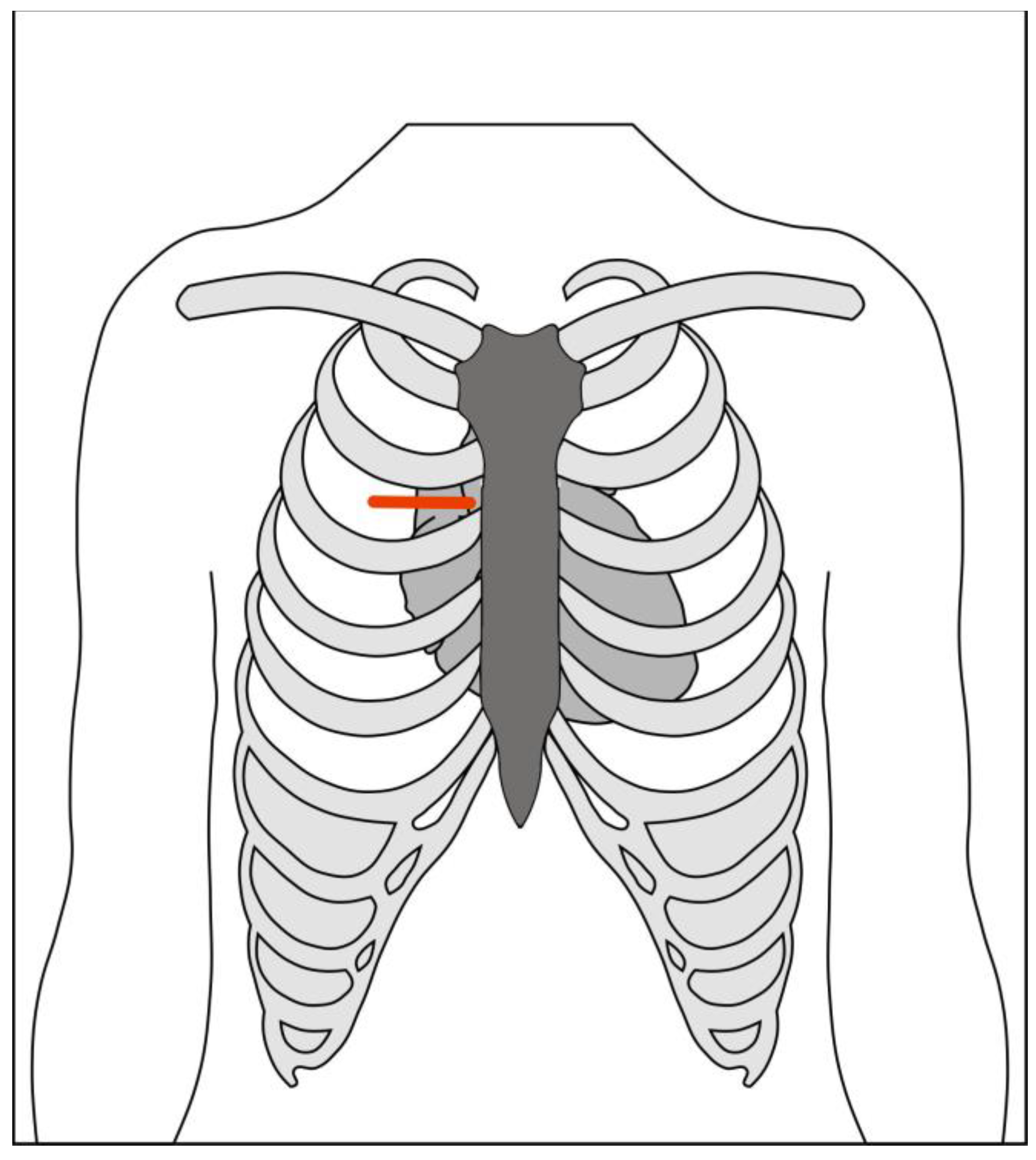
The skin incision (
Figure 1) is made over the upper midline in order to access the aorta, with J-shaped, L-shaped, or chevron sternotomy into the right, left or bilateral intercostal spaces (
Figure 2). The length of the sternotomy can vary and may be into third or fourth intercostal space. Accordingly, the length of the incision can also vary, with 5cm being a typical lower limit. This modifiable access can be utilized to undertake not only aortic valve replacement but also surgery of the aortic root, ascending aorta and hemi-arch [
20].
The upper hemi-sternotomy approach can provide sufficient access to perform complete central cannulation with conventional instruments and cannulae, if desired, although modifications and training to facilitate surgery are well described [
21,
22]. A 2017 Cochrane review of randomised controlled trials comparing full sternotomy to hemi-sternotomy found that across 10 included studies the evidence was of low certainty due to biases and small sample sizes [
23]. For most major outcomes, including peri-operative mortality, pain or quality of life, there was no significant difference. Blood loss was slightly lower with hemi-sternotomy compared to full sternotomy (mean difference 153ml lower compared to 400ml), and index admission costs were also higher (a mean difference of £1190 (~
$1470) more for hemi-sternotomy). Following the publication of the Cochrane review, the publication of two well designed trials performed in the UK, MAVRIC and Mini-Stern prompted a revised review of the literature.
The MAVRIC Trial
The Manubrium-limited Mini-sternotomy versus Conventional Sternotomy for Aortic Valve Replacement (MAVRIC) trial was a single centre, randomised controlled trial comparing patients undergoing AVR via manubrium-limited mini-sternotomy group and AVR via conventional sternotomy group [
24,
25].
Patients in the intervention arm received a manubrium-limited mini-sternotomy, performed using a 5-7cm midline skin incision dividing the manubrium from the sternal notch to 1cm below the manubrium-sternal junction. Cardiopulmonary bypass (CPB) was established with an ascending aortic cannula and percutaneous femoral venous cannulation. Those in the usual care arm received conventional median sternotomy.
The primary outcome was the proportion of patients who received a red cell transfusion post-operatively and within 7 days of AVR surgery.
The trial reported that mini-sternotomy was not found to be superior to conventional sternotomy with respect to red cell transfusion requirements within 7 days of surgery. The proportion of patients receiving red cell transfusion transfusions was 23 of 135 in both groups, Odds ratio 1.0 (95% CI: 0.5, 2.0) and risk difference of 0.0 (95% CI: -0.1, 0.1). However, secondary endpoints showed that there was a statistically significant difference with respect to transfusion volumes of non-red cell blood components. Mini-sternotomy also resulted in a relative reduction in chest drain losses, however, higher blood loss in the conventional group did not translate into red cell transfusions. Patients in the mini-sternotomy group had significantly longer bypass and cross clamp times and worse lung function at 4 days post-surgery. Lung function at twelve weeks, and adverse event rates were otherwise not different between groups.
Mini-Stern Trial
Mini-Stern was a multi-centre, open-label, pragmatic randomized controlled trial with a primary end-point of postoperative length of hospital stay and time to fitness for discharge. In total, 222 patients were randomised, finding that mini-sternotomy patients had no difference in length of stay (median 7 (interquartile range [IQR] 6 – 10) vs 7 (IQR 6 – 10), p=0.692) and no difference in time to fitness for discharge (median 5 (IQR 5 – 10) vs median 6 (IQR 5 – 9), p=0.560). Mini-sternotomy was £1719 more expensive per patient compared to full sternotomy in the first year following surgery. There was no significant difference in EQ-5D based quality adjusted life years (QALYs) and therefore at a willingness to pay threshold of £20,000 per QALY, there was only a 3.7% chance that mini-sternomy was cost-effective.
Current Meta-Analysis
As of August 2021, there were fourteen published randomised controlled trials of 1395 participants comparing hemisternotomy with full median sternotomy, due to be published shortly as an update of the previous Cochrane review. Countries of origin included Austria, Czech Republic, Spain, Italy, Germany, France, Egypt, Russia, Sweden, Serbia and the United Kingdom [
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39]. All but one were single-centre studies of elective, isolated aortic valve replacements, typically excluding patients with poor left ventricular function. The European studies were predominantly focused on patients with degenerative heart valve disease, and therefore an older population, whereas the study from Egypt included younger patients, presumably with rheumatic heart disease. Surgical strategy was broadly similar between studies, with aortic arterial cannulation and either central or femoral venous cannulation. Venting strategies – often considered a source of concern in minimally invasive aortic valve replacement due to potential compromises in bloodless field, de-airing or left ventricular decompression – were variable.
The risk ratio for peri-operative mortality was 0.93 (95% confidence interval [CI] 0.45 – 1.94, in 10 studies, but with low certainty). The mean difference in cardiopulmonary bypass time was 10.7 minutes longer with ministernotomy (95% CI 3.3 – 18.0 in 10 studies, but with very low certainty). The mean difference in aortic cross-clamp time was 6.1 minutes longer with ministernotomy (95% CI 0.8 – 11.3 across 12 studies, but also with very low certainty). Even in the context of the uncertainty, these mean differences in extra-corporeal circulation and ischaemic times are likely to have very little clinical impact. Exclusion of one trial [
31] where rapid deployment valves were used to facilitate expedient surgery in the minimally invasive arm only, and not in the full sternotomy arm, did not impact the findings of the small increase in bypass and cross-clamp times.
There was a modest reduction in length of stay in patients undergoing mini-sternotomy (mean difference 1.1 days less (95% CI -1.9 to -0.3 days across 11 studies with very low certainty). It is important to note that for most of these studies, trial protocols were not published a priori or at all. Outcome measures such as hospital length of stay, which might be contingent on different criteria between studies and which were considered at high risk of bias from blinding, were not directly comparable. Similar issues arose with intensive care length of stay which, in studies at low risk of bias, was marginally lower with minimally invasive surgery (-0.45 days, 95% CI -0.84 to -0.06).
In-hospital pain assessments were also no different between minimally invasive or full sternotomy (standardized mean difference -0.19 for minimally invasive, 95% CI -0.43 to 0.04 with low certainty). Equally, there was no difference in quality of life measures after discharge from hospital (mean difference 0.03, 95% CI 0.00 to 0.06 across 4 studies with low certainty). Finally, pulmonary function tests – considered a surrogate marker for comfort and capability following disruption of the thoracic skeleton – were also minimally different between the two approaches (mean difference 2.1% higher with mini-sternotomy, 95% CI 0.74 to 3.41).
This new meta-analysis would appear to demonstrate that ministernotomy aortic valve replacement is as safe as full-sternotomy surgery, but with few of the anticipated advantages to pain, quality of life or breathing that are cited as reasons to have minimally invasive surgery. Length of stay in hospital and on the intensive care unit were modestly better with minimally invasive surgery, but at an increased cost over standard of care.
2.1.2. Right Anterior Mini-thoracotomy
The Right Anterior mini-Thoracotomy (RAT) approach, using central cannulation, was first described by Rao and Kumar in 1993 [
40]. Cosgrove and Sabik first applied the term “minimally invasive” to a 10cm thoracotomy with excision of two costal cartilages, utilising femoral bypass [
41]. The main advantage of this approach is the absolute preservation of sternal integrity and the absence of lateral traction, similar to the parasternal approach described by Cohn [
42]. The corollary to these benefits is the need for femoral cannulation because of the limited access, necessitating a second incision in the groin and retrograde perfusion, which may offset the cosmetic advantage, quality of life and satisfaction with minimally invasive surgery [
43]. A rate of 1% non-union of the transected ribs may also cause intractable post-thoracotomy pain.
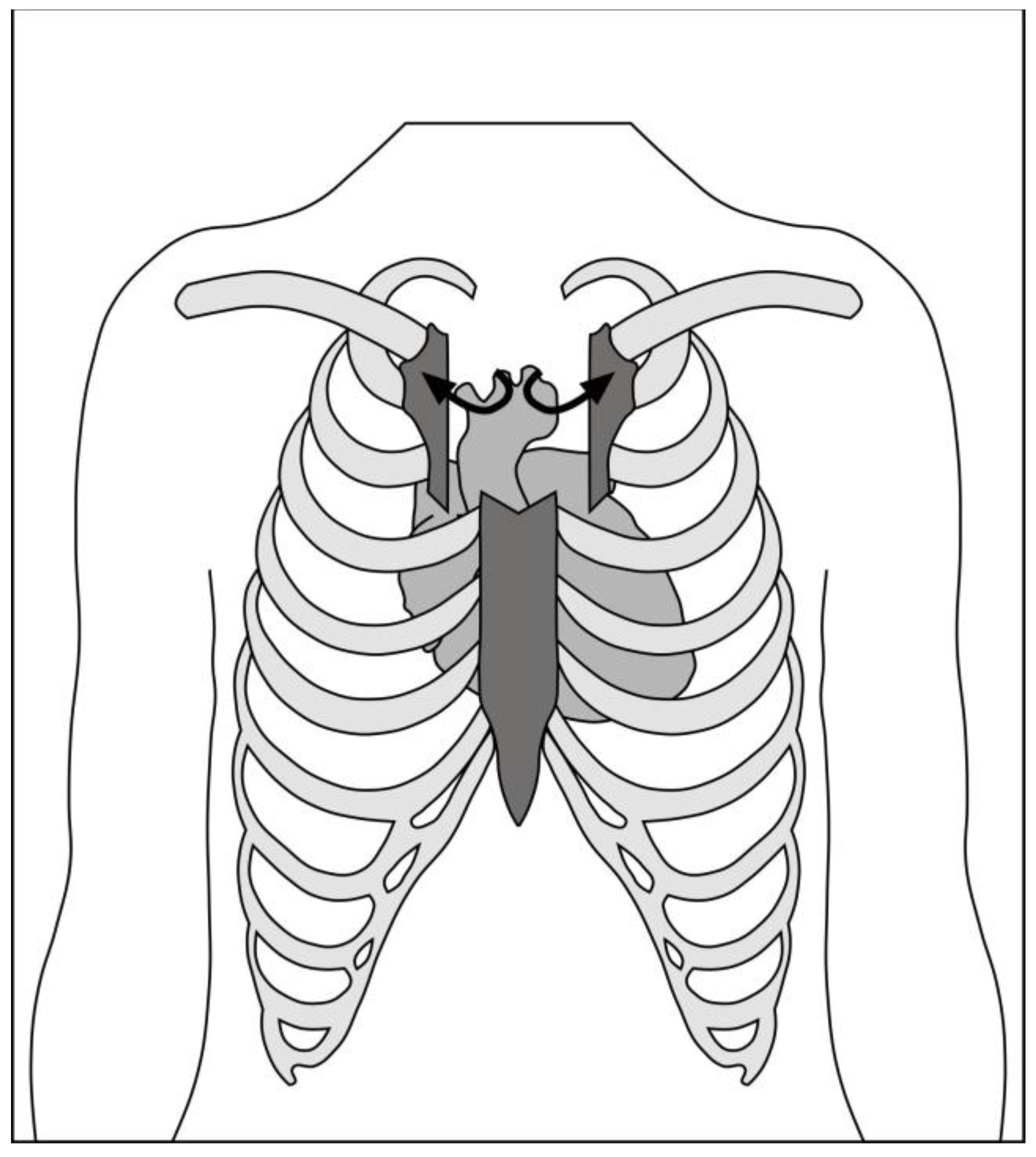
Figure 3.
Schematic of right anterior minithoracotomy through second intercostal space.
Figure 3.
Schematic of right anterior minithoracotomy through second intercostal space.
Initially, meta-analyses found RAT to have shorter hospital stays compared to hemi-sternotomy [
44,
45], despite higher rates of bleeding, transfusion and conversion to full sternotomy. A more recent comprehensive network meta-analysis of propensity matched and randomised studies compared full sternotomy, hemi sternotomy and right anterior mini-thoracotomy [
46]. This found no difference in surgical time, hospital length of stay or ventilation time between the two minimally invasive approaches. As with previous meta-analyses, return to theatre for bleeding was more common with right anterior mini-thoracotomy compared to hemi-sternotomy (RR 1.65, 95% CI 1.18 – 2.30, p=0.003).
The network meta-analysis included 42 studies, including 29 propensity matched studies and 13 randomised controlled trials. The majority compared median sternotomy to hemisternotomy, with 9 comparing RAT to full sternotomy and 2 comparing RAT to hemisternotomy. Of note, only one randomized study evaluated RAT, so much of the evidence was observational, albeit adjusted with propensity matched techniques. Again, the majority of the included studies were European in origin, and methodological quality was fraught with uncertainty. The fact that this study identified a significant peri-operative mortality advantage of hemi-sternotomy over median sternotomy (relative risk (RR) 0.60, 95% CI 0.41 – 0.90) or RAT (RR 0.50, 95% CI 0.27 – 0.97) should be interpreted with caution. A meta-analaysis of randomised trials with didactic scrutiny of methodology and quality found no difference in mortality between hemi-sternotomy and full-sternotomy [
23]. There exists, too, little rationale to explain why hemi-sternotomy should reduce mortality; indeed, all plausible mechanisms for a mortality difference between minimally invasive aortic valve replacement might suggest that minimal access approaches are at
higher risk than conventional surgery for peri-operative mortality. It is our interpretation, therefore, that while the network meta-analysis favours hemi-sternotomy as the access of choice for aortic valve surgery (over both full sternotomy and right anterior mini-thoracotomy), the evidence to support this is scant.
The authors of a recent consensus statement [
47] acknowledged the increased technical challenge of a right anterior mini-thoracotomy approach. Additional equipment such as thoracosopes, long-handled instruments and soft tissue retractors require specific training. Cost comparisons vary across publications. In some, the need for additional equipment and consumables increases the costs of RAT up to US
$4209 higher than full sternotomy, compared to US
$290 more for hemisternotomy [
48,
49]. In others, the costs of RAT is between US
$1891 to US
$3887 lower when propensity matched across real-world registry data [
50,
51].
The right anterolateral mini-thoracotomy (in the third intercostal space) can be used to perform multiple valve replacements, including the aortic, mitral and tricuspid valves [
52,
53,
54].
Table 1.
Advantages and disadvantages of aortic valve surgery approaches.
Table 1.
Advantages and disadvantages of aortic valve surgery approaches.
| |
Full sternotomy |
Hemi-sternotomy |
Right anterior minithoracotomy |
| Access |
Unfettered view to whole mediastinum and whole heart |
Good access to aortic root, limited to whole heart |
Most challenging view |
| Sternal disruption |
Whole sternum |
To 2nd – 4th intercostal space unilaterally or bilaterally |
None, although costal cartilages sometimes divided (may include right mammary artery ligation) |
| Cannulation |
Full central |
Variable – from full central to aortic arterial only |
Typically requires peripheral cannulation |
| Instruments |
Standard cardiac |
Variable – can be standard or long-handled |
Typically requires long-handled |
| Technical difficulty |
Baseline |
Learning curve easily traversed, including for trainee surgeons |
Accepted to be technically challenging |
| Adjuncts Required |
None |
Variable – possible with standard equipment. Facilitated by rapid deployment valves, suture placement devices and knot tying devices |
Facilitated by rapid deployment valves, suture placement devices and knot tying devices.
Light source advantageous.
Single lung ventilation. |
| Benefits (from most recent meta-analyses) * |
|
Reduced intensive care and hospital length of stay;
Reduced ventilation time |
Reduced hospital length of stay;
Reduced ventilation time;
Lower stroke rate;
Lower pacemaker rate |
| Risks * |
|
Increased operative time;
Increased costs |
Increased operative time;
Increased costs (including vs ministernotomy) |
2.1.4. Hybrid approach (Sternotomy + Transcatheter Aortic Valve Implantation)
While strictly not minimal access, and ostensibly not minimally invasive, patients not fit for conventional aortic valve replacement who also require coronary artery revascularization may benefit from lower invasiveness using a hybrid approach. Sternotomy may be performed in order to undertake off-pump coronary artery bypass grafting, with concomitant trans-aortic or trans-femoral TAVI. The avoids the sequelae of cardiopulmonary bypass in patients who may not tolerate it, while still allowing complete revascularization and management of aortic stenosis [
55,
56,
57].
2.2. Cannulation and Cardiopulmonary Bypass
2.2.1. Central
Hemi-sternotomy to the third or fourth space usually provides sufficient access to the aorta and right atrium to cannulate for arterial inflow and venous drainage centrally. The right superior pulmonary vein may or may not be accessible with this approach. Standard cannulae may be used, although to aid retraction of the pipes out of the field of view, some surgeons prefer angled venous pipes or a flat/low-profile cannula. Vacuum-assist can also help to improve the venous drainage.
2.2.2. Peripheral
Where the incision limits the access to the aorta, there may only be space in the surgical field for the cross clamp, cardioplegia site and aortotomy. In this case, peripheral cannulation, typically at the femorals, is utilized. Percutaneous methods are possible, but direct surgical access is the more commonly performed approach. Complications arising from groin cannulation may occur in 10.8% of cases, with seromas in up to 5% [
48].
2.2.3. Hypothermia and systemic hyperkalaemia
In the context of re-operative minimal access surgery, systemic hypothermia to 20
oC along with systemic hyperkalaemia at 7mmol/L can support myocardial protection in the presence of patent left internal mammary bypass grafts [
58]. This requires ultrafiltration on cardiopulmonary bypass following cardiac reperfusion. Retrograde flooding of the field from the left coronary ostium in the presence of an unclamped left internal mammary graft can be mitigated for by use of intermittent circulatory arrest to facilitate suture placement, or with peri-operative percutaneous balloon occlusion of the left internal mammary by cardiology.
2.2.4. Venting (and imaging)
Reduced access to the left ventricle for inspection or manual decompression mandates the use of a trans-oesophageal echo during minimally invasive aortic valve surgery. Where this may be omitted in special circumstances in conventional sternotomy, such as oesophagectomy or oesophageal stricture, the risks are much greater in minimal access surgery where the left ventricle cannot be directly assessed.
Dependent on the level of access, left ventricular venting can be achieved in different ways including:
Access to the pulmonary veins is typically not possible except in larger partial sternotomy approaches (i.e. fourth intercostal space). The pulmonary artery is usually easily accessible, although the visualization reduces considerably once the cross-clamp is removed. Access further deteriorates off cardiopulmonary bypass and therefore it is imperative to achieve haemostasis of the vent site early. The trans-aortic vent approach is usually sufficient to provide a bloodless field for surgery, but is limited should venting be required during reperfusion as the left ventricle cannot be reached manually.
2.3. Adjuncts
2.3.1. Rapid deployment valves
Sutureless and rapid deployment valves have been described as obvious companions for minimal access aortic valve replacement, to mitigate for the increased cardiopulmonary bypass and cross-clamp times that are otherwise seen [
31,
59].
2.3.2. Automatic suture / knotting devices
Automatic suture placement or knotting devices reduce invasiveness in minimal access valve surgery by expediting valve implanatation and therefore reducing cross-clamp and cardiopulmonary bypass time [
60]. The use of the Cor-Knot ® device (LSI Solutions, Victor, NY) for valve surgery was noted to also lead to more reproducible valve implanation with lower rates of paravalular leak [
61]. The same manufacturer has also released an automated annular suturing device to expedite this stage of valve implantation, but there are no published series to show that this is effective yet.
2.3.3. Transvenous pacing, cannulation and venting
A variety of options exist for percutaneous support of cardiopulmonary bypass from the right jugular. These include:
Transvenous pacing can be floated using an inflatable balloon tip into the right ventricle for endocardial pacing if the anterior right ventricle is not accessible
Coronary sinus cannulation via the right internal jugular was previously possible using the Proplege device (Edwards LifeSciences, Irvin, CA) but as the strategies for antegrade cardioplegia alone showed good efficacy, this is no longer available
Pulmonary artery venting through a percutaneously floated catheter has again lost favour as trans-aortic and direct pulmonary artery or pulmonary vein venting have been shown to be efficacious and safe
2.3.4. Thoracoscopes
Direct visualization of the aorta (sufficient to apply a cross-clamp), aortic valve and aortic annulus is possible through both hemi-sternotomy and right anterior mini-thoracotomy approaches. Thoracoscopes can also be utilized to improve light and view for areas that might be more difficult to see due to overhanging thoracic cage. Use of scopes for lighting and/or transmitted video images may, however, limit access for instruments in some cases. This can in part be mitigated for by use of other adjuncts such as automatic suture placement or knotting devices [
47].
2.3.5. Robot assistance
Robotic aortic valve replacement has been attempted since 2004 [
62], with a renewed interest in recent years [
63]. In place of either hemi-sternotomy or right anterior mini-thoractomy, a right
lateral mini-thoracotomy is utilized as the working port, along with 4 arms, which spares division of the costal cartilage or sacrifice of the right internal mammary artery. This has the advantage of an intact thoracic skeleton, superior visualization and virtually unrestricted range of movement in the working space, but with a steep learning curve, high capital investment costs and ongoing consumable expenditure.
2.4. Special circumstances
2.4.1. Concomitant procedures
As minimal access incisions have gained favour, indications for the procedures amenable to this approach have expanded. The hemi-sternotomy approach has been successfully utilized for aortovascular procedures including valve sparing root replacement [
64]
2.4.2. Re-do procedures
Previous sternotomy, even in the presence of patent coronary artery bypass grafts, is not a contraindication to a minimally invasive approach. These can be performed with low-conversion rates of 2.6% [
58]. Minimisation of the dissection and mobilization of the heart mean that bleeding complications are low. Technical challeges from the presence of patent grafts may require alternative strategies for cardioplegic arrest (including systemic hyperkalaemia, deeper hypothermia and brief periods of circulatory arrest).
2. Minimal Access Pre-operative planning / setup
Additional assessment and preparation is required for minimal access aortic valve surgery. These requirements vary depending on the approach adopted and not all practitioners employ all these steps.
CT scan pre-operatively can allow assessment of the position of the aorta relative to the incision planned. If peripheral cannulation is intended, this can also determine if the femoral vessels are adequate calibre and the descending aorta free of mobile atheroma that may preclude retrograde perfusion.
Short acting anaesthetic drugs should be considered to facilitate early extubation and enhanced recovery.
A sheath in the right internal jugular vein can be introduced at the time of induction of anaesthesia if the usual access limits epicardial pacing wires.
A bag of saline behind the shoulder blades can elevate and expand the chest, providing an improved approach to the mediastinum.
External defibrillator pads are required to cardiovert ventricular fibrillation as internal paddles cannot be applied to the heart
Trans-oesophageal echocardiography is mandated for minimally invasive aortic valve surgery as the direct visualization of the right and left ventricular function is impaired
Double lumen tube or bronchial blocker for selective ventilation of the right lung can facilitate the early learning curve [
65].
Carbon dioxide field flooding can aid de-airing at the end of the case where cardiac massage is not possible and venting is limited. Passive and limited active de-airing can therefore be supplemented with displacement of air in the cardiac chambers with highly soluble CO2.
3. Outcomes
It is unlikely that minimally invasive methods of aortic valve surgery would have been permitted to develop if peri-operative mortality was not equivalent to conventional full sternotomy. While the excellent current outcomes for isolated aortic valve replacement mean that most series do not have sufficient power to demonstrate a difference, it is apparent that there is no excess mortality.
Some credence has been placed on minimal access surgery to therefore also improve outcomes in other areas in order to justify the technical challenege and increased costs.
3.1. Pain
For studies that examined post-operative pain scores between minimal-access and full-sternotomy surgery, there was considerable bias in the reporting [
23]. Protocolised analgesic pathways were seldom cited and blinding was only utilised in one trial. The timing of pain score assessments also varied considerably between studies. The overall assessment following review was that minimal access aortic valve surgery did not reduce pain compared to sternotomy.
3.2. Respiratory Mechanics
The impact of full median sternotomy on respiratory function following surgery is often cited as a reason to favour minimal access approaches. While there might be small differences in peri-operative lung function parameters between non-sternotomy and full-sternotomy approaches, the main advantage to respiratory mechanics came in time to return-to-baseline. For partial sternotomy, this was one month, whereas for full sternotomy this was up to three months [
66].
3.3. Quality of Life
Five randomised controlled trials studied quality of life following surgery. Between 6-12 weeks following operation, there was no difference in the quality of life scores between hemi-sternotomy and full-sternotomy [
23].
4. Discussion
Chitwood’s prediction (or aspiration) that cardiac surgery would culminate in routine minimally invasive approaches that would emulate general surgery’s day-case model still seem very distant a quarter of century on. The benefits of minimal access surgery have not been realized as predicted and in the intervening decades, transcatheter techniques have improved and expanded their remit to low-risk patients [
67]. Cost-effectiveness of percutaneous methods has also improved, such that TAVI procedures are now competitive against surgical valves [
68].
In the absence of demonstrable superiority, there is therefore more incentive than ever to propagate minimal access approaches to aortic valve replacement. Where these procedures can be done with minimal or no additional equipment, at similar cost and with no increase in complications, the new standard should surely be the procedure with the same outcomes, same costs… and a smaller incision?
The current state of the art in minimal access aortic valve surgery is advanced. Even in groups such as octogenarians, re-do operations and root/ascending aorta replacement, the results for minimal access aortic surgery can be excellent [
69]. The procedure is reproducible and can be undertaken by surgeons in training without compromise to patient safety [
70]. Peri-operative and one-year mortality are no different in a real-world setting and length of stay is typically reduced using a minimal-access approach [
71].
As transcatheter aortic valve implantation also gathers momentum, gaining approval for increasingly lower-risk patient groups, there is a need to ensure that patients are provided with procedures of good pedigree. Surgeons looking to mitigate for the increased operative times of minimally invasive aortic valve replacement may turn to sutureless aortic valves, which draw parallels with transcatheter valves. Where TAVI valves have higher rates of paravalvular leak, pacemaker implantation and vascular complications [
72], however, sutureless valves can offer the advantage of full decalcification and placement under direct vision. Whether these benefits offset the need for thoracic cage disruption and cardiopulmonary bypass and cross-clamping remains to be seen, particularly in the small or calcified root.
Further research is also still required to elucidate the differences between hemi-sternotomy and right anterior minithoracotomy. The former remains the more accessible minimally invasive approach, with familiar setup, angles and equipment to those trained in conventional full sternotomy. Proponents of the right anterior mini-thoractomy, however, will argue that if a partial-sternotomy is superior to a full-sternotomy, it stands to reasons that no sternotomy should be better still. However, it remains to be compellingly proven that the additional challenges of RAT do not neutralize the benefits provided of maintaining the sternum fully intact. Current syntheses of the existing literature are disparate in their conclusions.
Future directions for this procedure involve ensuring that it is, indeed, developed as the new standard of care for aortic valve procedures. Once this is accepted universally, the process of reducing
invasiveness as well as
access can be developed. Enhanced Recovery After Surgery (ERAS) have been slow to be adopted in cardiac surgery in general and much less in minimal access cardiac surgery. Protocols are needed for patients with reduced incisions that differ to those used for conventional surgery, and might meaningfully engage and utilize the operative advantages to expedite the patient journey more effectively [
73].
5. Conclusions
Minimal access aortic valve surgery is at a watershed moment where it could plausibly become the new standard of care for aortic valve disease, as laparoscopic procedures have become for general surgery. Economic viability will need to be demonstrated in order for this to happen, but moreover, a surgical appetite must grow for non-inferior procedures where benefits are still being developed and demonstrated.
Author Contributions
All authors contributed to the writing and editing and have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charge for this review was waived by Journal of Cardiovascular Development and Disease
Informed Consent Statement
Not applicable for this review.
Data Availability Statement
No new research data was generated for this review.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest.” Authors must identify and declare any personal circumstances or interest that may be perceived as inappropriately influencing the representation or interpretation of reported research results. Any role of the funders in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results must be declared in this section. If there is no role, please state “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results”.
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: a population-based study. The Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Tandon, R. Rheumatic fever & rheumatic heart disease: The last 50 years. Indian J Med Res 2013, 137, 643–658. [Google Scholar] [PubMed]
- Manjunath, C.N.; Srinivas, P.; Ravindranath, K.S.; Dhanalakshmi, C. Incidence and patterns of valvular heart disease in a tertiary care high-volume cardiac center: A single center experience. Indian Heart J 2014, 66, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Burwash, I.G.; Legget, M.E.; Munt, B.I.; Fujioka, M.; Healy, N.L. , et al. Prospective Study of Asymptomatic Valvular Aortic Stenosis Clinical, Echocardiographic, and Exercise Predictors of Outcome. Circulation 1997, 95, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G. , et al. Outcome of 622 Adults With Asymptomatic, Hemodynamically Significant Aortic Stenosis During Prolonged Follow-Up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef]
- Gohlke-Bärwolf, C.; Minners, J.; Jander, N.; Gerdts, E.; Wachtell, K.; Ray, S. , et al. Natural History of Mild and of Moderate Aortic Stenosis—New Insights From a Large Prospective European Study. Current Problems in Cardiology 2013, 38, 365–409. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Vahanian, A. Degenerative calcific aortic stenosis: A natural history. Heart [Internet] 2012, 98. Available from: http://heart.bmj.com/content/98/Suppl_4/iv7.full.pdf+html. /: Available from: http.
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W. , et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European Heart Journal 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Carabello, B.A. Introduction to Aortic Stenosis. Circulation Research 2013, 113, 179–185. [Google Scholar] [CrossRef]
- Iung, B.; Cachier, A.; Baron, G.; Messika-Zeitoun, D.; Delahaye, F.; Tornos, P. , et al. Decision-making in elderly patients with severe aortic stenosis: why are so many denied surgery? Eur Heart J 2005, 26, 2714–2720. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F. , et al. Percutaneous Transcatheter Implantation of an Aortic Valve Prosthesis for Calcific Aortic Stenosis. Circulation 2002, 106, 3006–3008. [Google Scholar] [CrossRef]
- Bourantas, C.V.; Serruys, P.W. Evolution of Transcatheter Aortic Valve Replacement. Circulation Research 2014, 114, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J. , et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Elbeery, J.R.; Chitwood, W.R. Minimally invasive cardiac surgery. Heart surgery for the 21st century. N C Med J 1997, 58, 374–377. [Google Scholar] [PubMed]
- Westaby, S.; Benetti, F.J. Less Invasive Coronary Surgery: Consensus From the Oxford Meeting. The Annals of Thoracic Surgery 1996, 62, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Gleason, T.G.; Sonnad, S.S. Quality of life after aortic valve replacement. Expert Review of Pharmacoeconomics & Outcomes Research 2004, 4, 265–275. [Google Scholar]
- Mikus, E.; Calvi, S.; Campo, G.; Pavasini, R.; Paris, M.; Raviola, E. , et al. Full Sternotomy, Hemisternotomy, and Minithoracotomy for Aortic Valve Surgery: Is There a Difference? The Annals of thoracic surgery 2018, 106, 1782–1788. [Google Scholar] [CrossRef]
- Liu, J.; Sidiropoulos, A.; Konertz, W. Minimally invasive aortic valve replacement (AVR) compared to standard AVR. Eur J Cardiothorac Surg 1999, 16 Suppl 2, S80–3. [Google Scholar]
- Autschbach, R.; Walther, T.; Falk, V.; Diegeler, A.; Metz, S.; Mohr, F.W. S-shaped in comparison to L-shaped partial sternotomy for less invasive aortic valve replacement. Eur J Cardiothorac Surg 1998, 14 Suppl 1, S117–S121. [Google Scholar] [CrossRef]
- Perrotta, S.; Lentini, S. Ministernotomy approach for surgery of the aortic root and ascending aorta. Interactive CardioVascular and Thoracic Surgery 2009, 9, 849–858. [Google Scholar] [CrossRef]
- Malaisrie, S.C.; Barnhart, G.R.; Farivar, R.S.; Mehall, J.; Hummel, B.; Rodriguez, E. , et al. Current era minimally invasive aortic valve replacement: techniques and practice. J. Thorac. Cardiovasc. Surg. 2014, 147, 6–14. [Google Scholar] [CrossRef]
- Walther, T.; Falk, V.; Mohr, F.W. Minimally Invasive Surgery for Valve Disease. Current Problems in Cardiology 2006, 31, 399–437. [Google Scholar] [CrossRef] [PubMed]
- Kirmani, B.H.; Jones, S.G.; Malaisrie, S.C.; Chung, D.A.; Williams, R.J. Limited versus full sternotomy for aortic valve replacement. Cochrane Database Syst Rev 2017, 4, CD011793. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, E.; Goodwin, A.T.; Owens, W.A.; Hancock, H.C.; Maier, R.; Kasim, A. , et al. Manubrium-limited ministernotomy versus conventional sternotomy for aortic valve replacement (MAVRIC): study protocol for a randomised controlled trial. Trials 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Hancock, H.C.; Maier, R.H.; Kasim, A.; Mason, J.; Murphy, G.; Goodwin, A. , et al. Mini-sternotomy versus conventional sternotomy for aortic valve replacement: a randomised controlled trial. BMJ open 2021, 11, e041398. [Google Scholar] [CrossRef] [PubMed]
- Machler, H.E.; Bergmann, P.; Anelli-Monti, M.; Dacar, D.; Rehak, P.; Knez, I. , et al. Minimally invasive versus conventional aortic valve operations: a prospective study in 120 patients. Ann Thorac Surg 1999, 67, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Gofus, J.; Vobornik, M.; Koblizek, V.; Smolak, P.; Myjavec, A.; Vojacek, J. , et al. Pulmonary function and quality of life after aortic valve replacement through ministernotomy: a prospective randomized study. Kardiologia polska 2020, 78, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Aris, A.; Cámara, M.L.; Montiel, J.; Delgado, L.J.; Galán, J.; Litvan, H. Ministernotomy versus median sternotomy for aortic valve replacement: a prospective, randomized study. Ann. Thorac. Surg. 1999, 67, 1583–1587. [Google Scholar] [CrossRef]
- Rodriguez-Caulo, E.A.; Guijarro-Contreras, A.; Otero-Forero, J.; Mataro, M.J.; Sanchez-Espin, G.; Guzon, A. , et al. Quality of life, satisfaction and outcomes after ministernotomy versus full sternotomy isolated aortic valve replacement (QUALITY-AVR): study protocol for a randomised controlled trial. Trials 2018, 19, 114. [Google Scholar] [CrossRef]
- Bonacchi, M.; Prifti, E.; Giunti, G.; Frati, G.; Sani, G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann. Thorac. Surg. 2002, 73, 460–465. [Google Scholar] [CrossRef]
- Borger, M.A.; Moustafine, V.; Conradi, L.; Knosalla, C.; Richter, M.; Merk, D.R. , et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015, 99, 17–25. [Google Scholar] [CrossRef]
- Dogan, S.; Dzemali, O.; Wimmer-Greinecker, G.; Derra, P.; Doss, M.; Khan, M.F. , et al. Minimally invasive versus conventional aortic valve replacement: a prospective randomized trial. J. Heart Valve Dis. 2003, 12, 76–80. [Google Scholar] [PubMed]
- Calderon, J.; Richebe, P.; Guibaud, J.P.; Coiffic, A.; Branchard, O.; Asselineau, J. , et al. Prospective randomized study of early pulmonary evaluation of patients scheduled for aortic valve surgery performed by ministernotomy or total median sternotomy. J. Cardiothorac. Vasc. Anesth. 2009, 23, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.; Abdelsamad, A.A.; Zakaria, G.; Omarah, M.M. Minimal vs Median Sternotomy for Aortic Valve Replacement. Asian Cardiovascular and Thoracic Annals 2007, 15, 472–475. [Google Scholar] [CrossRef]
- Shneider, Y.u.A.; Tsoi, M.D.; Fomenko, M.S.; Pavlov, A.A.; Shilenko, P.A. Aortic valve replacement via J-shaped partial upper sternotomy: randomized trial, mid-term results. Khir. Z. im. N.I. Pirogova 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Dalen, M.; Oliveira Da Silva, C.; Sartipy, U.; Winter, R.; Franco-Cereceda, A.; Barimani, J. , et al. Comparison of right ventricular function after ministernotomy and full sternotomy aortic valve replacement: a randomized study. Interactive cardiovascular and thoracic surgery 2018, 26, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, P.M.; Milojevic, P.; Stojanovic, I.; Micovic, S.; Zivkovic, I.; Peric, M. , et al. The role of ministernotomy in aortic valve surgery-A prospective randomized study. Journal of cardiac surgery 2019, 34, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Hancock, H.C.; Maier, R.H.; Kasim, A.S.; Mason, J.M.; Murphy, G.J.; Goodwin, A.T. , et al. Mini-Sternotomy Versus Conventional Sternotomy for Aortic Valve Replacement. J. Am. Coll. Cardiol. 2019, 73, 2491–2492. [Google Scholar] [CrossRef]
- Nair, S.K.; Sudarshan, C.D.; Thorpe, B.S.; Singh, J.; Pillay, T.; Catarino, P. , et al. Mini-Stern Trial: A randomized trial comparing mini-sternotomy to full median sternotomy for aortic valve replacement. J Thorac Cardiovasc Surg 2018, 156, 2124–2132. [Google Scholar] [CrossRef]
- Rao, P.N.; Kumar, A.S. Aortic valve replacement through right thoracotomy. Tex Heart Inst J 1993, 20, 307–308. [Google Scholar]
- Cosgrove, D.M. , 3rd.; Sabik, J.F. Minimally invasive approach for aortic valve operations. Ann. Thorac. Surg. 1996, 62, 596–597. [Google Scholar] [CrossRef]
- Cohn, L.H. Minimally invasive aortic valve surgery: technical considerations and results with the parasternal approach. J Card Surg 1998, 13, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Detter, C.; Deuse, T.; Boehm, D.H.; Reichenspurner, H.; Reichart, B. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thorac Cardiovasc Surg 2002, 50, 337–341. [Google Scholar] [PubMed]
- Chang, C.; Raza, S.; Altarabsheh, S.E.; Delozier, S.; Sharma, U.M.; Zia, A. , et al. Minimally Invasive Approaches to Surgical Aortic Valve Replacement: A Meta-Analysis. The Annals of Thoracic Surgery 2018, 106, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Yousuf Salmasi, M.; Hamilton, H.; Rahman, I.; Chien, L.; Rival, P.; Benedetto, U. , et al. Mini-sternotomy vs right anterior thoracotomy for aortic valve replacement. Journal of Cardiac Surgery 2020, 35, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Ogami, T.; Yokoyama, Y.; Takagi, H.; Serna-Gallegos, D.; Ferdinand, F.D.; Sultan, I. , et al. Minimally invasive versus conventional aortic valve replacement: The network meta-analysis. J Card Surg 2022, 37, 4868–4874. [Google Scholar] [CrossRef] [PubMed]
- Vohra, H.A.; Salmasi, M.Y.; Mohamed, F.; Shehata, M.; Bahrami, B.; Caputo, M. , et al. Consensus statement on aortic valve replacement via an anterior right minithoracotomy in the UK healthcare setting. Open Heart 2023, 10, e002194. [Google Scholar] [CrossRef] [PubMed]
- Balmforth, D.; Harky, A.; Lall, K.; Uppal, R. Is ministernotomy superior to right anterior minithoracotomy in minimally invasive aortic valve replacement? Interactive cardiovascular and thoracic surgery 2017, 25, 818–821. [Google Scholar] [CrossRef]
- Hassan, M.; Miao, Y.; Maraey, A.; Lincoln, J.; Brown, S.; Windsor, J. , et al. Minimally Invasive Aortic Valve Replacement: Cost-Benefit Analysis of Ministernotomy Versus Minithoracotomy Approach. J Heart Valve Dis 2015, 24, 531–539. [Google Scholar]
- Ghanta, R.K.; Lapar, D.J.; Kern, J.A.; Kron, I.L.; Speir, A.M.; Fonner, E., Jr. , et al. Minimally invasive aortic valve replacement provides equivalent outcomes at reduced cost compared with conventional aortic valve replacement: A real-world multi-institutional analysis. J Thorac Cardiovasc Surg 2015, 149, 1060–1065. [Google Scholar] [CrossRef]
- Rodriguez, E.; Malaisrie, S.C.; Mehall, J.R.; Moore, M.; Salemi, A.; Ailawadi, G. , et al. Right anterior thoracotomy aortic valve replacement is associated with less cost than sternotomy-based approaches: a multi-institution analysis of ‘real world’ data. J Med Econ 2014, 17, 846–852. [Google Scholar] [CrossRef]
- Gumus, F.; Hasde, A.I.; Bermede, O.; Kilickap, M.; Durdu, M.S. Multiple Valve Implantation Through a Minimally Invasive Approach: Comparison of Standard Median Sternotomy and Right Anterior Thoracotomy. Heart Lung Circ 2020, 29, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, F.; Lio, A.; Montalto, A.; Bergonzini, M.; Cammardella, A.G.; Comisso, M. , et al. Minimally invasive treatment of multiple valve disease: A modified approach through a right lateral minithoracotomy. J Card Surg 2020, 35, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wei, L.; Zhu, S.; Zhang, Z.; Liu, H.; Yang, Y. , et al. Combined Mitral and Aortic Valve Procedure via Right Mini-Thoracotomy versus Full Median Sternotomy. Int Heart J 2019, 60, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Manoly, I.; Hasan, R.; Brazier, A.; Farooq, V.; Thompson, T.; Karunaratne, D. , et al. Feasibility of hybrid off pump artery bypass grafting and transaortic transcatheter aortic valve implantation: A case series. Catheter Cardiovasc Interv 2017, 89, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Zubarevich, A.; Zhigalov, K.; Szczechowicz, M.; Thielmann, M.; Rabis, M.; Van den Eynde, J. , et al. Simultaneous transaortic transcatheter aortic valve implantation and off-pump coronary artery bypass: An effective hybrid approach. J Card Surg 2021, 36, 1226–1231. [Google Scholar] [CrossRef]
- Mayr, B.; Firschke, C.; Erlebach, M.; Bleiziffer, S.; Krane, M.; Joner, M. , et al. Transcatheter aortic valve implantation and off-pump coronary artery bypass surgery: an effective hybrid procedure in selected patients. Interact Cardiovasc Thorac Surg 2018, 27, 102–107. [Google Scholar] [CrossRef]
- Tada, N.; Khalpey, Z.; Shekar, P.; Cohn, L.H. Reoperative minimal access aortic valve surgery: Minimal mediastinal dissection and minimal injury risk. The Journal of Thoracic and Cardiovascular Surgery [Internet] 2008 [cited 2023 Apr 15];136. [CrossRef]
- Santarpino, G.; Pfeiffer, S.; Concistre, G.; Fischlein, T. Perceval S aortic valve implantation in mini-invasive surgery: the simple sutureless solution. Interact Cardiovasc Thorac Surg 2012, 15, 357–360. [Google Scholar] [CrossRef]
- Morgant, M.C.; Malapert, G.; Petrosyan, A.; Pujos, C.; Jazayeri, S.; Bouchot, O. Comparison of automated fastener device Cor-Knot versus manually-tied knot in minimally-invasive isolated aortic valve replacement surgery. The Journal of cardiovascular surgery 2020, 61, 123–128. [Google Scholar] [CrossRef]
- Sazzad, F.; Ler, A.; Kuzemczak, M.; Ng, S.; Choong, A.M.T.L.; Kofidis, T. Automated Fastener vs Hand-tied Knots in Heart Valve Surgery: A Systematic Review and Meta-analysis. Ann Thorac Surg 2021, 112, 970–980. [Google Scholar] [CrossRef]
- Folliguet, T.A.; Vanhuyse, F.; Magnano, D.; Laborde, F. Robotic aortic valve replacement: case report. Heart Surg Forum 2004, 7, E551–E553. [Google Scholar] [CrossRef]
- Badhwar, V.; Wei, L.M.; Cook, C.C.; Hayanga, J.W.A.; Daggubati, R.; Sengupta, P.P. , et al. Robotic aortic valve replacement. The Journal of Thoracic and Cardiovascular Surgery 2021, 161, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Krueger, H.; Umminger, J.; Koigeldiyev, N.; Beckmann, E.; Haverich, A. , et al. Minimally invasive valve sparing aortic root replacement (David procedure) is safe. Ann 2015, 4, 148–153. [Google Scholar]
- Glauber, M.; Miceli, A. Minimally Invasive Aortic Valve Surgery [Internet]. In: Raja SG, editor. Cardiac Surgery: A Complete Guide. Cham: Springer International Publishing; 2020 [cited 2023 Apr 16]. page 421–8. [CrossRef]
- Weissman, C. Pulmonary Complications After Cardiac Surgery. Seminars in Cardiothoracic and Vascular Anesthesia [Internet] 2004 [cited 2023 Apr 14];8. [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M. , et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. New England Journal of Medicine 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Tam, D.Y.; Azizi, P.M.; Fremes, S.E.; Chikwe, J.; Gaudino, M.; Wijeysundera, H.C. The cost-effectiveness of transcatheter aortic valve replacement in low surgical risk patients with severe aortic stenosis. Eur Heart J Qual Care Clin Outcomes 2021, 7, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Tada, N.; Umakanthan, R.; Cohn, L.H.; Bolman, R.M.; Shekar, P.; Chen, F.Y. Early and late outcomes of 1000 minimally invasive aortic valve operations☆. European Journal of Cardio-Thoracic Surgery [Internet] 2008 [cited 2023 Apr 15];33. [CrossRef]
- Soppa, G.; Yates, M.; Viviano, A.; Smelt, J.; Valencia, O.; van Besouw, J.P. , et al. Trainees can learn minimally invasive aortic valve replacement without compromising safety. Interactive CardioVascular and Thoracic Surgery 2015, 20, 458–462. [Google Scholar] [CrossRef]
- Attia, R.Q.; Hickey, G.L.; Grant, S.W.; Bridgewater, B.; Roxburgh, J.C.; Kumar, P. , et al. Minimally Invasive Versus Conventional Aortic Valve Replacement: A Propensity-Matched Study From the UK National Data. Innovations 2016, 11, 15–23. [Google Scholar]
- Bilkhu, R.; Borger, M.A.; Briffa, N.P.; Jahangiri, M. Sutureless aortic valve prostheses. Heart 2019, 105, s16–20. [Google Scholar] [CrossRef]
- Berretta, P.; De Angelis, V.; Alfonsi, J.; Pierri, M.D.; Malvindi, P.G.; Zahedi, H.M. , et al. Enhanced recovery after minimally invasive heart valve surgery: Early and midterm outcomes. Int J Cardiol 2023, 370, 98–104. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).