1. Introduction
Annexin A1 (ANXA1) is a member of the annexin superfamily of calcium- and phospholipid-binding proteins [
1]. The protein core comprises 346 amino acids (37 kDa) with C- and N-terminal domains, the latter of which confers specificity and physiological activity to each annexin [
2]. Besides being characterized as a glucocorticoid-regulated anti-inflammatory protein [
3], ANXA1 has also been reported to be involved in critical pathophysiological processes, including cell proliferation, differentiation, and apoptosis of epithelial and cancer cells, which implicates this protein in tissue repair and cancer metastasis [
4,
5,
6].
ANXA1 is found in three distinct subcellular locations—cytoplasm, nucleus, and plasma membrane. Although none of the annexins contains nuclear-targeting sequences, nuclear localization of ANXA1 has been reported under certain conditions [
7]. The translocation of ANXA1 from the cytoplasm to the nucleus is believed to start with mitogenic/proliferative signals or after a DNA-damaging stimulus, such as oxidative stress [
7]. In breast cancer cells, nuclear localization of ANXA1 was associated with protection against heat-induced DNA damage [
8].
ANXA1 contains a DNA- and/or RNA-binding sequence [
9] and has been proposed to perform helicase activity in the nucleus [
10,
11]. Because helicase activity is required in DNA replication and repair, nuclear ANXA1 may participate in tumorigenesis [
6]. DNA lesions, such as double-strand breaks and oxidation of DNA bases, especially guanine, are also induced by hyperglycemia-mediated oxidative stress [
12,
13,
14,
15]. We previously demonstrated that maternal hyperglycemia levels are directly proportional to DNA damage and inversely proportional to the expression of base excision repair (BER) enzymes in peripheral blood cells [
15]. However, this relationship was not observed in the same cell types in newborns because of the regulation of expression of BER enzymes [
15]. BER is the most efficient mechanism for repairing endogenous DNA damage, in which DNA glycosylase (8-oxo guanine DNA glycosylase [OGG1]) removes the damaged base, resulting in an apurinic/apyrimidinic (AP) site. The AP endonuclease (AP endonuclease 1 [APE1]) cleaves the AP site, allowing DNA polymerase to synthesize the repair patch. The latter is re-ligated using the DNA ligase III activity [
16].
As a determinant condition of hyperglycemia, GDM, defined as any degree of glucose intolerance with onset or first recognition during pregnancy, is among the most common complications associated with pregnancy [
17]. Maternal metabolic changes can, directly and indirectly, be reflected in the placenta, interposed between the maternal and fetal circulation [
18], and can lead to impaired embryonic and fetal development [
19,
20,
21]. Cell death [
22], inflammation [
23,
24], and oxidative stress [
25] are important hallmarks of placental cell damage in mothers with GDM.
Because nuclear ANXA1 is apparently involved in DNA replication and repair, we tested the hypothesis that nuclear ANXA1 is associated with the placental cellular response induced by oxidative DNA damage. To this end, we used placental samples from AnxA1 knockout animals (AnxA1-/-) and from mothers with GDM, a classical condition of oxidative stress and inflammation during pregnancy.
3. Discussion
ANXA1 is synthesized by immune, epithelial, and cancer cells via the action of different chemical mediators, such as glucocorticoids and cytokines [
27,
28]. ANXA1, stored in cytoplasmic granules and released into the extracellular compartment, binds to membrane formyl peptides for downstream intracellular signaling of anti-inflammatory, proliferative, apoptosis, and migration processes [
27,
29,
30]. Moreover, ANXA1 is also found in the nucleus, where a protective action against DNA damage has recently been proposed [
31]. This study also highlights the nuclear localization of ANXA1. Here, the translational data showed that the expression of ANXA1 in the placenta was associated with increased apoptosis, which may reflect a failure to protect DNA from oxidative damage. In this context, modulation of BER enzymes is a possible underlying mechanism.
Placentas deficient in AnxA1 showed increased oxidative DNA damage, reduced expression of nuclear BER enzymes with DNA double-strand breaks, and apoptosis induction. Analysis of placentas exposed to an adverse environment, such as hyperglycemia, showed similar outcomes associated with ANXA1 deficiency in the nucleus and cytoplasm of GDM samples.
It is important to emphasize that pregnant women in the GDM group, although treated with a combination of insulin and diet, showed increased HbA1c levels and weight of newborn compared with those in the ND group. The exaggerated supply of glucose from the mother to the fetus explains the weight of the newborns observed in this study [
32]. The degree of maternal glucose tolerance is not reflected in clinical aspects. Changes in placental morphology and physiology have been observed in different degrees of hyperglycemic disorders, including GDM [
33]. Previous studies have demonstrated that GDM placentas show a higher expression of inflammasome pathway components [
24], with an exacerbation in the production of inflammatory cytokines, oxidative stress, DNA damage response, and apoptotic cells [
15,
22,
24,
25]. As these effects were detected in the placenta samples from AnxA1
-/- mice, our data strongly suggest a protective role for ANXA1 in placental physiology, which can be mitigated in certain adverse conditions, such as hyperglycemia.
An imbalance in the expression of ANXA1 in the placenta of high-risk pregnancies has been previously described. ANXA1 showed protective activity against
Toxoplasma gondii infection, based on the observation that the third-trimester placentas expressing lesser ANXA1 were more permissive to this infection than the first-trimester placentas expressing more ANXA1. In the third-trimester placental villous explant culture, parasite reduction was observed after treatment with an ANXA1 peptide mimetic (Ac2-26) [
34]. Reduced expression of placental AnxA1 was observed in mothers post ZIKA infection. The placentas of these mothers showed an increased inflammatory response and impaired tissue repair [
35]. This data may be related to a deficiency in ANXA1, which consolidates the importance of ANXA1 in the placental response to aggression.
As discussed in a review by Boudhraa [
6], ANXA1 can translocate to the nucleus in response to mitogenic/proliferative and DNA-damaging stimuli, such as hydrogen peroxide. In the nuclear compartment, ANXA1 binds to DNA, where helicase activity has been proposed, allowing it to include ANXA1 in DNA synthesis and repair mechanisms [
10].
The DNA damage response related to ANXA1 is tissue dependent. In MCF-7 breast cancer cells, the loss of ANXA1 upon stress led to an increased susceptibility to DNA damage and mutation [
8]. However, experiments performed in nasopharyngeal carcinoma cells, suggest that knockdown of ANXA1 inhibits DNA damage by decreasing the generation of intracellular reactive oxygen species and the formation of γ-H2AX and promotes DNA repair by increasing DNA-dependent protein kinase activity [
36]. Moreover, prevention of nuclear translocation of ANXA1 using the small peptide Tat-NTS inhibited cellular proliferation (G2/M phase arrest), migration, and invasion of glioblastoma cells [
37].
Our data indicate crucial functions of ANXA1 in placental cells, especially in the nuclear compartment. Under hyperglycemic conditions (a classical oxidative stress condition), we observed a reduction in nuclear expression of ANXA1 in GDM placentas. As observed in MCF7 breast adenocarcinoma cells, our data indicate that the absence or reduction of ANXA1 in placental cells might underlie the cell death response and impair placental function.
Overall, our translational data indicate that differential expression of ANXA1 in the placenta alters the cellular response to oxidative DNA damage, leading to apoptosis. We focused on the reduction in BER enzymes in the nuclear compartment to explain apoptotic signaling. These findings demonstrate the relevance of ANXA1 in placental cell responses and shed light on the mechanisms that regulate the functions of this critical protein in placental biology.
4. Materials and Methods
4.1. Ethical Statement
This cross-sectional study included placental samples from AnxA1 knockout (AnxA1-/-) mice and mothers with GDM. All animal procedures were performed according to the Brazilian Society for the Science of Laboratory Animals (SBCAL) and were approved by the Institutional Animal Care and Use Committee of the Faculty of Pharmaceutical Sciences at the University of Sao Paulo (Protocol 521). Placental samples from pregnant women were obtained from the Diabetes and Pregnancy Service of Botucatu Medical School/UNESP, Brazil, with the approval of the Research Ethics Committee (protocol # 48609715.0.0000.5505). Written informed consent was obtained from all participants according to the principles of the Declaration of Helsinki.
4.2. AnxA1 Knockout (AnxA1−/−) Placental Samples
Male and female wild type (WT) and AnxA1
−/− BALB/c mice, aged 5–6 weeks, were maintained and reproduced at the animal house of the Faculty of Pharmaceutical Sciences, University of Sao Paulo (Brazil). Animals were provided chow (Nuvilab) and water were ad libitum. All animals were housed in a temperature-controlled room (22–25 °C and 70% relative humidity) with a 12 h light-dark cycle. Female mice were caged overnight with males (3:1) and successful mating was verified the following morning by the presence of a vaginal plug (day 0.5 of pregnancy). On day 18.5 of pregnancy, mice were euthanized by cervical dislocation or were anesthetized with xylazine and ketamine (i.p., 7 and 77 mg/kg, respectively; Vetbrands, Jacarei, SP, Brazil) [
26] for collection of placenta samples (six from each group: WT and AnxA1
−/−). The placenta samples were processed for morphological (hematoxylin-eosin staining) and immunohistochemical analyses.
4.3. Population Characterization and Collection of Human Placenta Samples
GDM was diagnosed using a 75 g glucose tolerance test (75 g-GTT), as recommended by the American Diabetes Association [
17], between the 24
th and 28
th gestational weeks. Twenty placenta samples were used in this study, ten each from the nondiabetic (ND; normal 75 g-GTT) and GDM (abnormal 75 g-GTT) groups.
Population characteristics included age, body mass index (BMI) in the third trimester of pregnancy, weight gain during pregnancy, gestational age at delivery, glycemic mean (GM), and glycated hemoglobin (HbA1c) levels. GM was calculated from the arithmetic mean of plasma glucose levels measured in all glucose profiles obtained during treatment (diet or diet + insulin). Plasma glucose levels were measured using the oxidase method (Glucose Analyzer II Beckman, Fullerton, CA, USA) and HbA1c levels were measured using high-performance liquid chromatography (D10™ Hemoglobin Testing System, Bio-Rad Laboratories, Hercules, CA, USA). Placental and fetal weights were also included.
The inclusion criteria were as follows: (i) hyperglycemia defined at a minimum gestational age of 28–30 weeks; (ii) prenatal and delivery care at the service; (iii) absence of clinically diagnosed infections and negative serology for HIV and syphilis, absence of multiple pregnancies, overt diabetes, fetal malformations, fetal death, alcohol consumption, or illicit drug habits; and (iv) deliveries before the 36th week of gestation.
Placentas were collected immediately after delivery (cesarean section) and cut into smaller fragments from different cotyledons. Decidual and villous areas were dissected and rinsed in phosphate-buffered saline. A part of the placental villous areas was subjected to routine morphological and immunohistochemical procedures, and the remaining part was frozen and stored at −80 °C for western blotting.
4.4. Preparation of Placenta Samples for Histological Assessment
Placenta samples from pregnant women with GDM and from AnxA1−/− mice were fixed in 4% buffered paraformaldehyde for 24 h, dehydrated in a graded ethanol series, and embedded in paraffin (Merck, Darmstadt, Germany). Three-micrometer thick sections were obtained for hematoxylin-eosin staining and immunohistochemistry.
4.5. Morphological and Morphometric Analyses of Placenta Samples from AnxA1−/− Mice
Morphometric analysis was performed on formalin-fixed placental samples stained with hematoxylin and eosin and scanned using the Image-Pro Plus version 4.5 for Windows software - Zeiss-Jenaval (Zeiss-Jenaval, Jena, Germany). Placental slides from six dams in each group were analyzed using the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The thickest point of the labyrinth or junctional zone was first identified to measure the thickness of the placental layer. The thickness of each layer was measured and the ratio of the thickness of each layer to the total thickness of the placenta was calculated.
4.6. Immunohistochemical Detection of Oxidative DNA Damage, DNA-Double Strand Breaks, DNA Repair Enzymes, and Apoptosis in GDM and AnxA1−/− Placenta Samples
The immunohistochemical detection of 8 hydroxyguanosine (oxidative DNA damage marker), gamma H2AX (DNA-double strand breaks), APE-1 and OGG1 (DNA repair enzymes), and cleaved caspase-3 (apoptosis) in placental sections from GDM and AnxA1−/− mice was performed. Endogenous peroxide activity was blocked using 3% hydrogen peroxide for 30 min. The tissue sections were then incubated with the following primary antibodies overnight at 4 °C: 8 Hydroxyguanosine (Abcam, Cambridge, U.K.), gamma H2AX (Novus Biologicals, Littleton, CO, USA), OGG1 (Novus Biologicals), APE-1 (Novus Biologicals), polyclonal rabbit anti-caspase-3 (Abcam). For negative control, sections were incubated with 10% TBS-BSA (Sigma–Aldrich, St. Louis, MO, USA) instead of the primary antibody. After washing with TBS, the sections were incubated with horseradish peroxidase-conjugated secondary antibodies (Abcam). Staining was visualized using 3,3′-diaminobenzidine substrate (Invitrogen). Finally, the sections were counterstained with hematoxylin (Inlab Confiança, São Paulo, Brazil) and mounted using Entellan (Merck, Germany).
4.7. Nuclear OGG1, APE-1, and Caspase-3 Analysis in Placenta from AnxA1−/− Mice
Cells with positive nuclear staining for OGG1 and APE-1 in the immunohistochemical analysis were counted in ten fields (×40) in each placental zone (labyrinthine or junctional) for all placenta samples. The ImageJ software (NIH) was used to quantify the area and number of positive nuclei to calculate the ratio of positive nuclei per placental zone.
Data are presented as the number of positive cells per 104 µm2 area. Cleaved caspase-3 analysis was performed similarly, considering the number of positive cells. Images were obtained using an Axioskop2-Mot Plus Microscope (Carl Zeiss, Jena, Germany) with the AxioVision software.
4.8. Morphological Analysis of Placenta from Pregnant Women with GDM
Morphological analysis was performed on formalin-fixed placenta samples stained with hematoxylin-eosin and cytokeratin 7. The expression of cytokeratin 7 (a specific marker for trophoblast cells) was detected immunohistochemically using polyclonal rabbit IgG anti-cytokeratin 7 (Abcam; 1:50). Images were obtained using an Axioskop 2-Mot Plus Microscope (Carl Zeiss) with the AxioVision software.
4.9. Analysis of Nuclear OGG1, APE-1, and Caspase-3 in Placenta from Pregnant Women with GDM
Cells with positive nuclear staining for OGG1 and APE-1 in the villous area were counted in ten fields (×40) of each placenta. As described for mouse placentas, the ImageJ software (NIH) was used to quantify the villous area, and the ratio between the number of positive nuclei and villous area was calculated. Data are presented as the number of positive cells per 104 µm2 area. Cleaved caspase-3 analysis was performed considering positive cells.
4.10. Detection and Analysis of Cytoplasmic and Nuclear ANXA1 in GDM Placentas
The expression of ANXA1 was determined by immunohistochemical staining as described above using the following primary antibody: polyclonal rabbit anti-AnxA1 (Zymed Laboratories, Cambridge, UK) at 1:5000 for 1 h.
The intensity of cytoplasmic ANXA1 staining in villous cells was measured densitometrically using 103 random points from ten fields (×40) from each placenta on an arbitrary scale from 0 to 255.
Cells with positive nuclear staining for ANXA1 in the villous area were counted in ten fields (×40) of each placenta. The ImageJ software (NIH) was used to quantify the villous area and the ratio of positive nuclei to the villous area was calculated. Data are presented as the number of positive cells per 104 µm2 area. Images were obtained using an Axioskop 2-Mot Plus Microscope (Carl Zeiss, Jena, Germany) with the AxioVision software.
4.11. Western Blot Analysis of ANXA1 using Placental Extracts from Patients with GDM
Villous fragments of frozen human placental tissues were transferred to propylene tubes containing the lysis buffer (Merck, Darmstadt, Germany) and a cocktail of protease inhibitors (Complete Mini, EDTA-free protease inhibitor cocktail tablets, Roche, Switzerland). Samples were homogenized on ice using an electric homogenizer. The homogenates were centrifuged at 12,000 rpm for 15 min at 4 °C. The protein concentration in the supernatant was measured using a BCA protein assay (PierceTM BCA Protein Assay Kit, Thermo Scientific, USA) and then stored at −80°C for western blot analysis.
Equal amounts of total protein from each group were separated electrophoretically on a 15% SDS-polyacrylamide gel and then transferred to a 0.45 μm nitrocellulose membrane (Millipore, Massachusetts, USA). The transfer of proteins was confirmed by staining the membranes with 10% Ponceau S solution (Sigma Aldrich). The blotted membranes were blocked with 3% TBS-T-milk for 1 h, washed three times with TBS buffer, and then incubated overnight at 4 °C with anti-β-actin (1:2000; Novus Biologicals, Centennial, USA) and anti-ANXA1 (1:5000; Invitrogen, California, USA) antibodies in 3% TBS-T-milk and washed three times with TBS buffer. The membranes were exposed to a horseradish peroxidase-conjugated secondary antibody (1:1000; Abcam) in 3% TBS-T-milk for 1 h and washed three times with TBS buffer. Immunoreactive bands (indicative of peroxidase activity) were detected using the enhanced chemiluminescence method. Quantitative analysis of ANXA1 was performed by densitometry using the ImageJ software (NIH, Maryland, USA). β-Actin was used as a loading control.
4.12. Statistical Analysis
Data are presented as mean ± standard deviation (SD). Statistical analyses were performed using the GraphPad software version 6.00. First, we performed the Kolmogorov–Smirnov normality test to determine whether the data distribution was parametric or nonparametric. Student’s t-test or the Mann–Whitney U test was used for group comparisons. Statistical significance was set at p < 0.05.
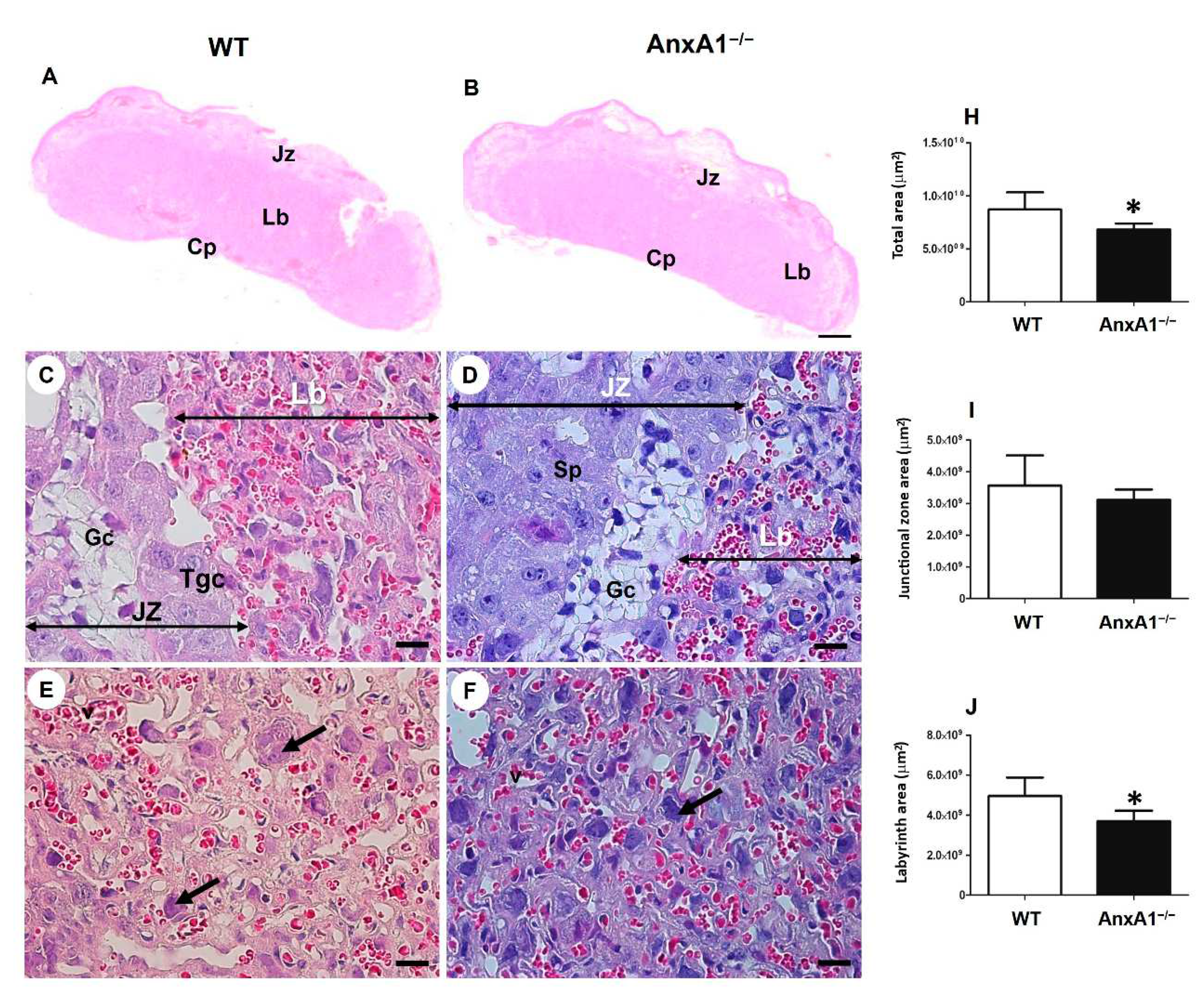
Figure 1.
Representative images of placental sections from wild-type (WT) (A, C, E) and AnxA1 knockout animals (AnxA1−/−, B, D, F). The sections were stained with hematoxylin and eosin (A-F). The histologic sections show the labyrinth (Lb) and junctional (Jz) placental zones. Scale bars = 20 μm. Jz: junctional zone; Lb: labyrinth; Cp: chorionic plate; Gc: glycogen cells; Sp: spongiotrophoblast; Gtc: junctional zone giant trophoblast cells; arrows: labyrinthine giant trophoblast cells. (H-J) Morphometric analyses of the labyrinth and junctional zone of placentas from WT and AnxA1−/− animals. Values are shown as mean ± SD. *p < 0.05.
Figure 1.
Representative images of placental sections from wild-type (WT) (A, C, E) and AnxA1 knockout animals (AnxA1−/−, B, D, F). The sections were stained with hematoxylin and eosin (A-F). The histologic sections show the labyrinth (Lb) and junctional (Jz) placental zones. Scale bars = 20 μm. Jz: junctional zone; Lb: labyrinth; Cp: chorionic plate; Gc: glycogen cells; Sp: spongiotrophoblast; Gtc: junctional zone giant trophoblast cells; arrows: labyrinthine giant trophoblast cells. (H-J) Morphometric analyses of the labyrinth and junctional zone of placentas from WT and AnxA1−/− animals. Values are shown as mean ± SD. *p < 0.05.
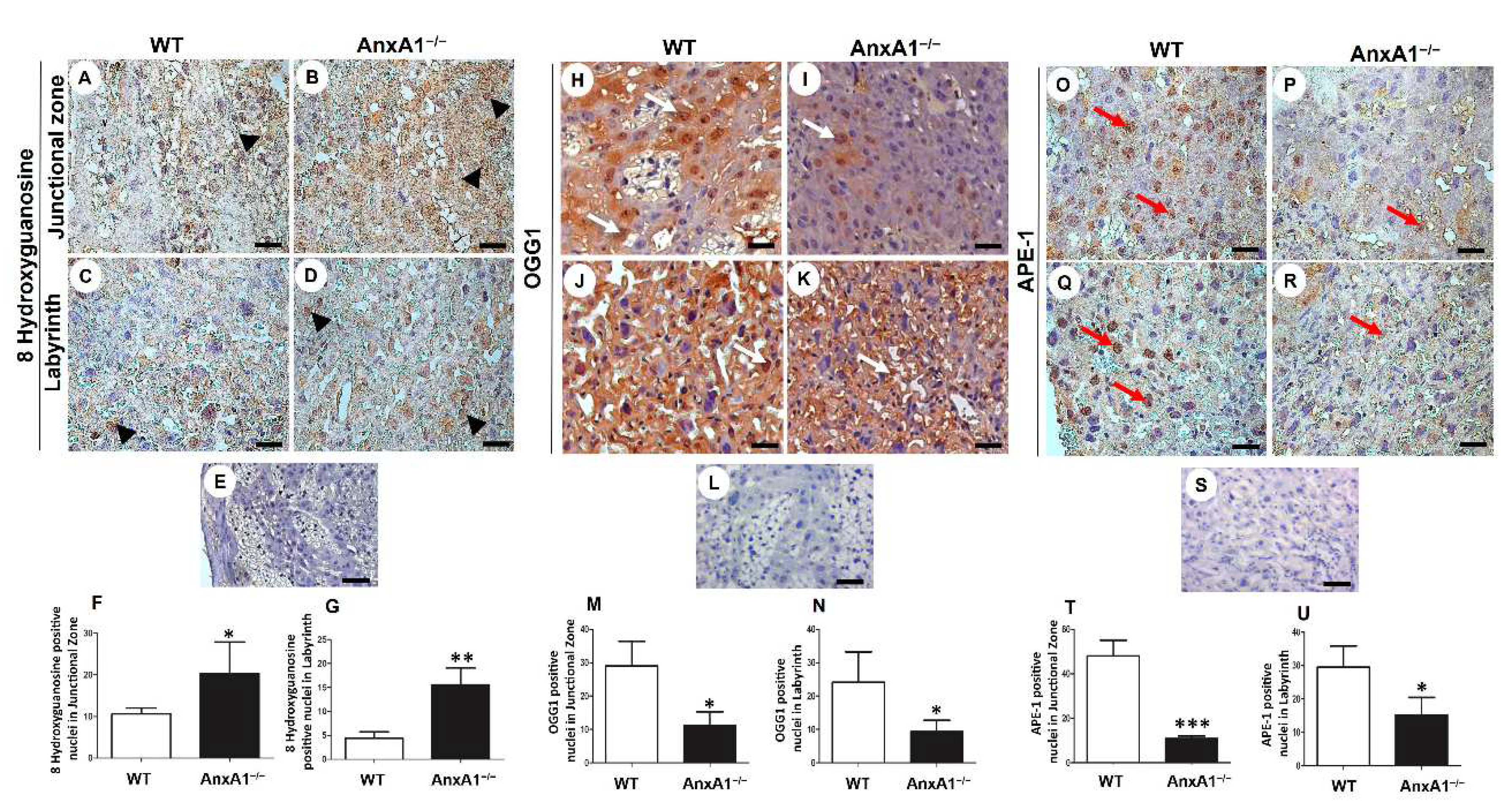
Figure 2.
Immunolocalization of 8 hydroxyguanosine (oxidative DNA damage, A-G) and OGG1 and APE-1 (DNA repair enzymes, H-U) in placenta sections from WT (A, C, H, J, O, Q) and AnxA1−/− (B, D, I, K, P, R) animals. (A-D, H-K, O-R) Placental cells reactive to 8 hydroxyguanosine (arrowheads; A-D), OGG-1 (arrows; H-K), and APE-1 (red arrows; O-R) are found in both junctional and labyrinthine placental zones with a similar pattern of immunoreactivity. Buffer was used instead of the primary antibody as a negative control, in panels E, L, and S. Bars = 50 μm. Immunoperoxidase and hematoxylin counterstaining. (F-G, M-N, T-U) Quantification of 8 hydroxyguanosine- (F-G), OGG-1- (M-N), and APE-1- (T-U) positive nuclei per 10.000 μm² of placental area. Values are shown as mean ± SD; * p < 0.05; **p < 0.01.
Figure 2.
Immunolocalization of 8 hydroxyguanosine (oxidative DNA damage, A-G) and OGG1 and APE-1 (DNA repair enzymes, H-U) in placenta sections from WT (A, C, H, J, O, Q) and AnxA1−/− (B, D, I, K, P, R) animals. (A-D, H-K, O-R) Placental cells reactive to 8 hydroxyguanosine (arrowheads; A-D), OGG-1 (arrows; H-K), and APE-1 (red arrows; O-R) are found in both junctional and labyrinthine placental zones with a similar pattern of immunoreactivity. Buffer was used instead of the primary antibody as a negative control, in panels E, L, and S. Bars = 50 μm. Immunoperoxidase and hematoxylin counterstaining. (F-G, M-N, T-U) Quantification of 8 hydroxyguanosine- (F-G), OGG-1- (M-N), and APE-1- (T-U) positive nuclei per 10.000 μm² of placental area. Values are shown as mean ± SD; * p < 0.05; **p < 0.01.
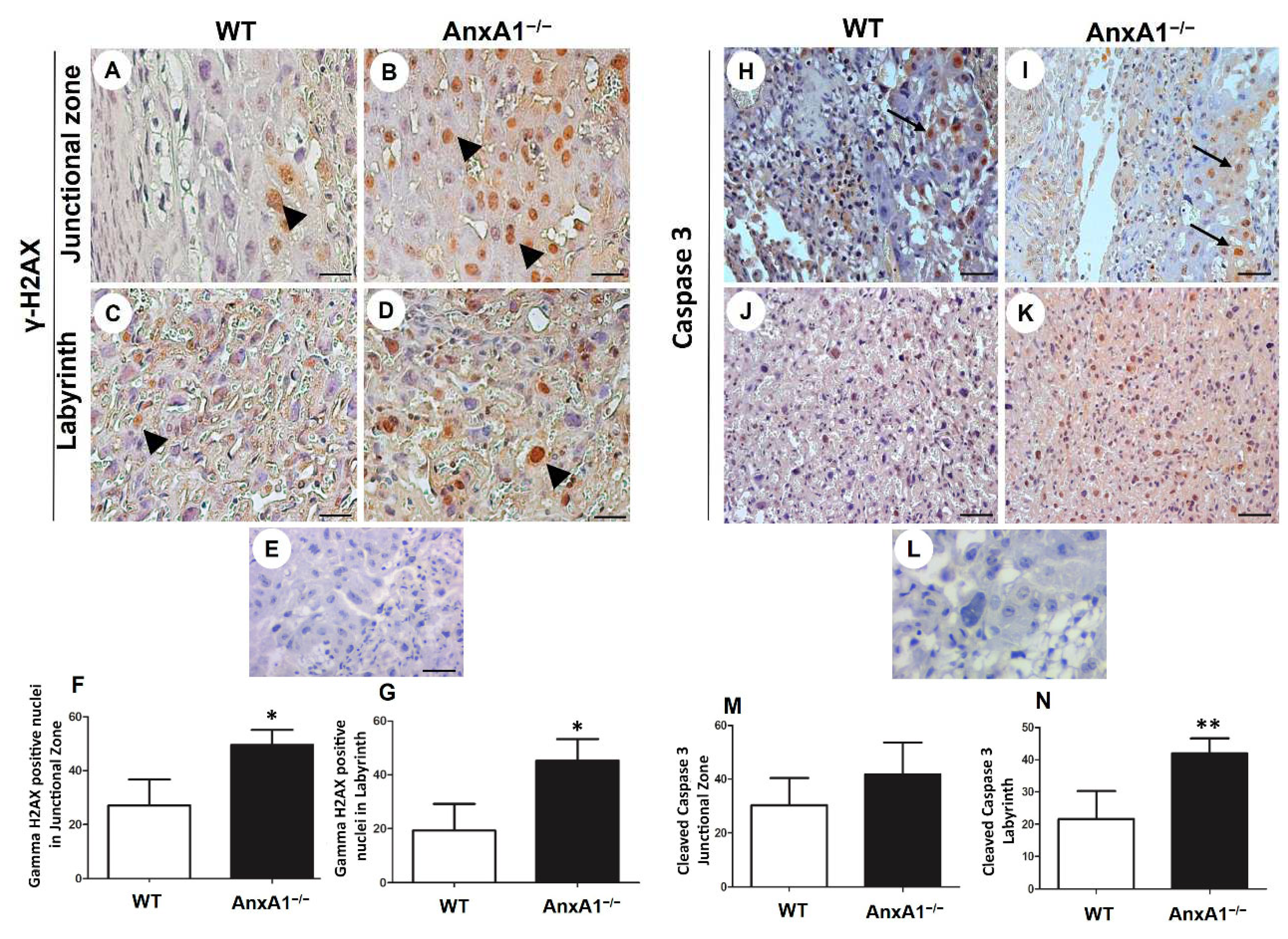
Figure 3.
Immunolocalization of γ-H2AX (DNA double-strand breaks, A-G) and cleaved caspase-3 (apoptosis, H-N) in placenta sections from WT (A, C, H, J) and AnxA1−/− animals (B, D, I, K). (A-E; H-L) Placental cells reactive to γ-H2AX (arrowheads; A-E) and caspase-3 (arrows; H-L) are found in both junctional and labyrinthine placental zones. (E, L) Negative control for immunohistochemical analysis. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (F-G; M-N) Quantification of γ-H2AX-stained nuclei (F- G) and caspase-3-positive cells (M-N) per 10.000 μm² of placental area. Values are shown as mean ± SD; *p < 0.05; **p < 0.01.
Figure 3.
Immunolocalization of γ-H2AX (DNA double-strand breaks, A-G) and cleaved caspase-3 (apoptosis, H-N) in placenta sections from WT (A, C, H, J) and AnxA1−/− animals (B, D, I, K). (A-E; H-L) Placental cells reactive to γ-H2AX (arrowheads; A-E) and caspase-3 (arrows; H-L) are found in both junctional and labyrinthine placental zones. (E, L) Negative control for immunohistochemical analysis. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (F-G; M-N) Quantification of γ-H2AX-stained nuclei (F- G) and caspase-3-positive cells (M-N) per 10.000 μm² of placental area. Values are shown as mean ± SD; *p < 0.05; **p < 0.01.
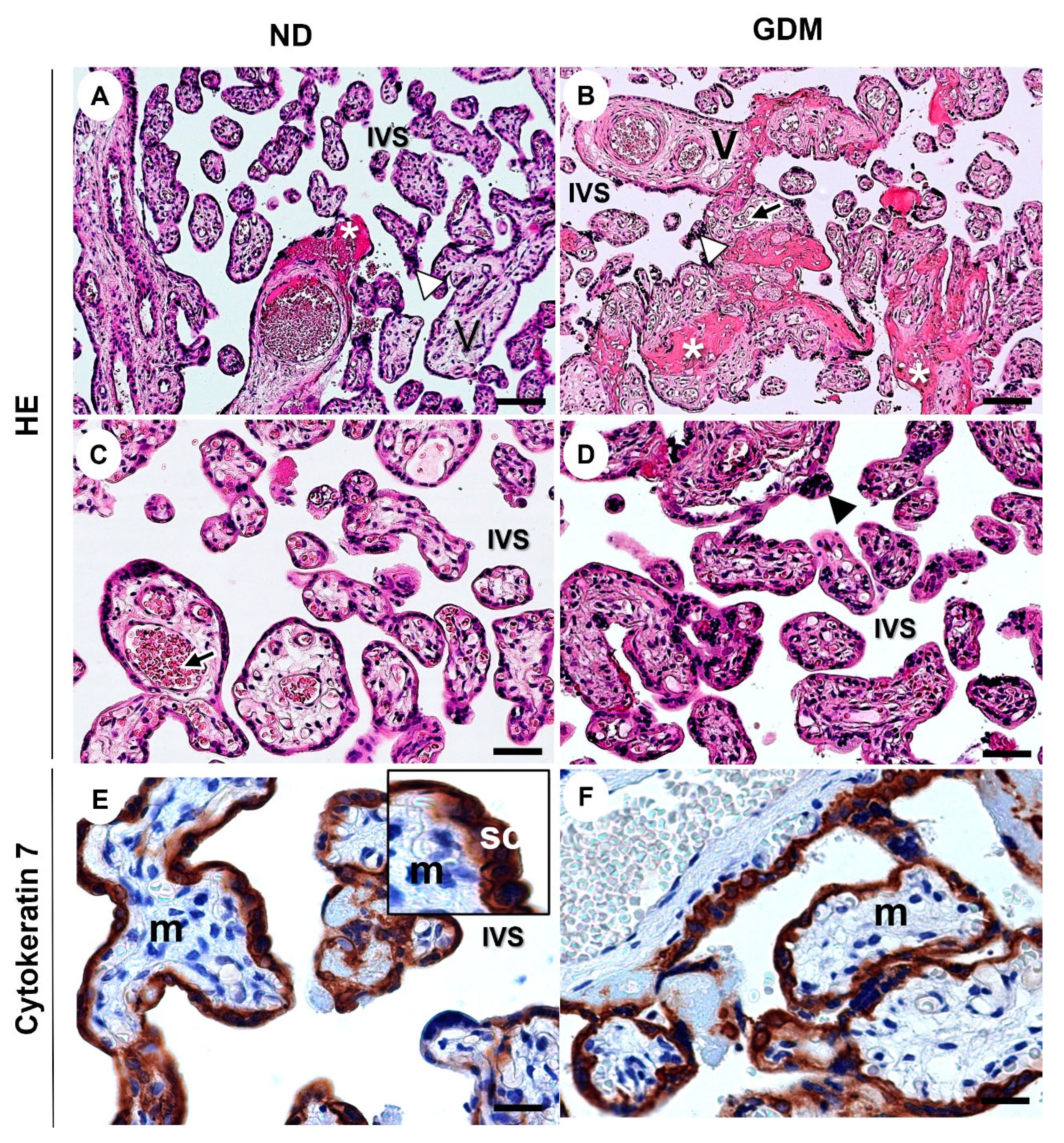
Figure 4.
Representative images of villous compartment of term placentas from pregnant women with no diabetes (ND; A, C, E) or gestational diabetes mellitus (GDM; B, D, F). (A-C) Note the aggregations of syncytiotrophoblast nuclei (arrowhead) and increased intramural fibrinoid (asterisk) mainly in GDM villi (B). V: chorionic villous; arrows: fetal vessels; IVS: intervillous space. The sections were stained with hematoxylin and eosin. (E-F) Immunolabeling of cytokeratin 7 for identification of the syncytiotrophoblast layer (SC) around the mesenchyme (m). Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm.
Figure 4.
Representative images of villous compartment of term placentas from pregnant women with no diabetes (ND; A, C, E) or gestational diabetes mellitus (GDM; B, D, F). (A-C) Note the aggregations of syncytiotrophoblast nuclei (arrowhead) and increased intramural fibrinoid (asterisk) mainly in GDM villi (B). V: chorionic villous; arrows: fetal vessels; IVS: intervillous space. The sections were stained with hematoxylin and eosin. (E-F) Immunolabeling of cytokeratin 7 for identification of the syncytiotrophoblast layer (SC) around the mesenchyme (m). Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm.
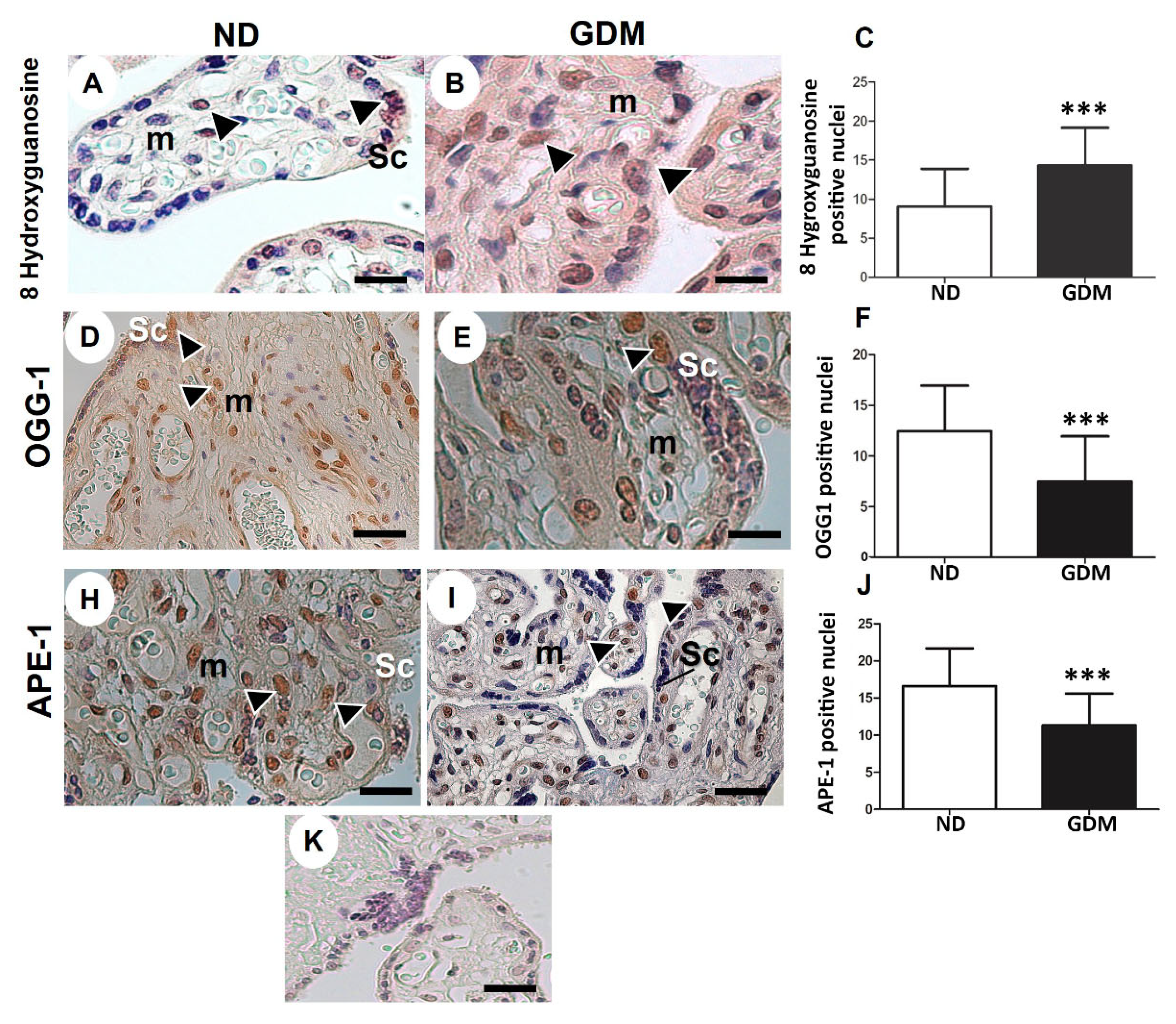
Figure 5.
Immunolocalization of oxidative DNA damage (A-C) and DNA repair enzymes (D-I) in placentas from pregnant women with no diabetes (ND; A, D, H) or gestational diabetes mellitus (GDM; B, E, I). Nuclei of the syncytiotrophoblasts (arrowheads) and mesenchyme cells (m) were reactive for 8 hydroxyguanosine (A-B), OGG-1 (D-E) and APE-1 (H-I) in ND and GDM placentas. (K) Buffer was used instead of the primary antibody as a negative control. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (C, F, J) Quantification of 8 hydroxyguanosine (C), OGG-1 (F), and APE-1 (J) reactive nuclei per 10.000 μm² of placental area. Values are shown as mean ± SD; *** p < 0.001.
Figure 5.
Immunolocalization of oxidative DNA damage (A-C) and DNA repair enzymes (D-I) in placentas from pregnant women with no diabetes (ND; A, D, H) or gestational diabetes mellitus (GDM; B, E, I). Nuclei of the syncytiotrophoblasts (arrowheads) and mesenchyme cells (m) were reactive for 8 hydroxyguanosine (A-B), OGG-1 (D-E) and APE-1 (H-I) in ND and GDM placentas. (K) Buffer was used instead of the primary antibody as a negative control. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (C, F, J) Quantification of 8 hydroxyguanosine (C), OGG-1 (F), and APE-1 (J) reactive nuclei per 10.000 μm² of placental area. Values are shown as mean ± SD; *** p < 0.001.
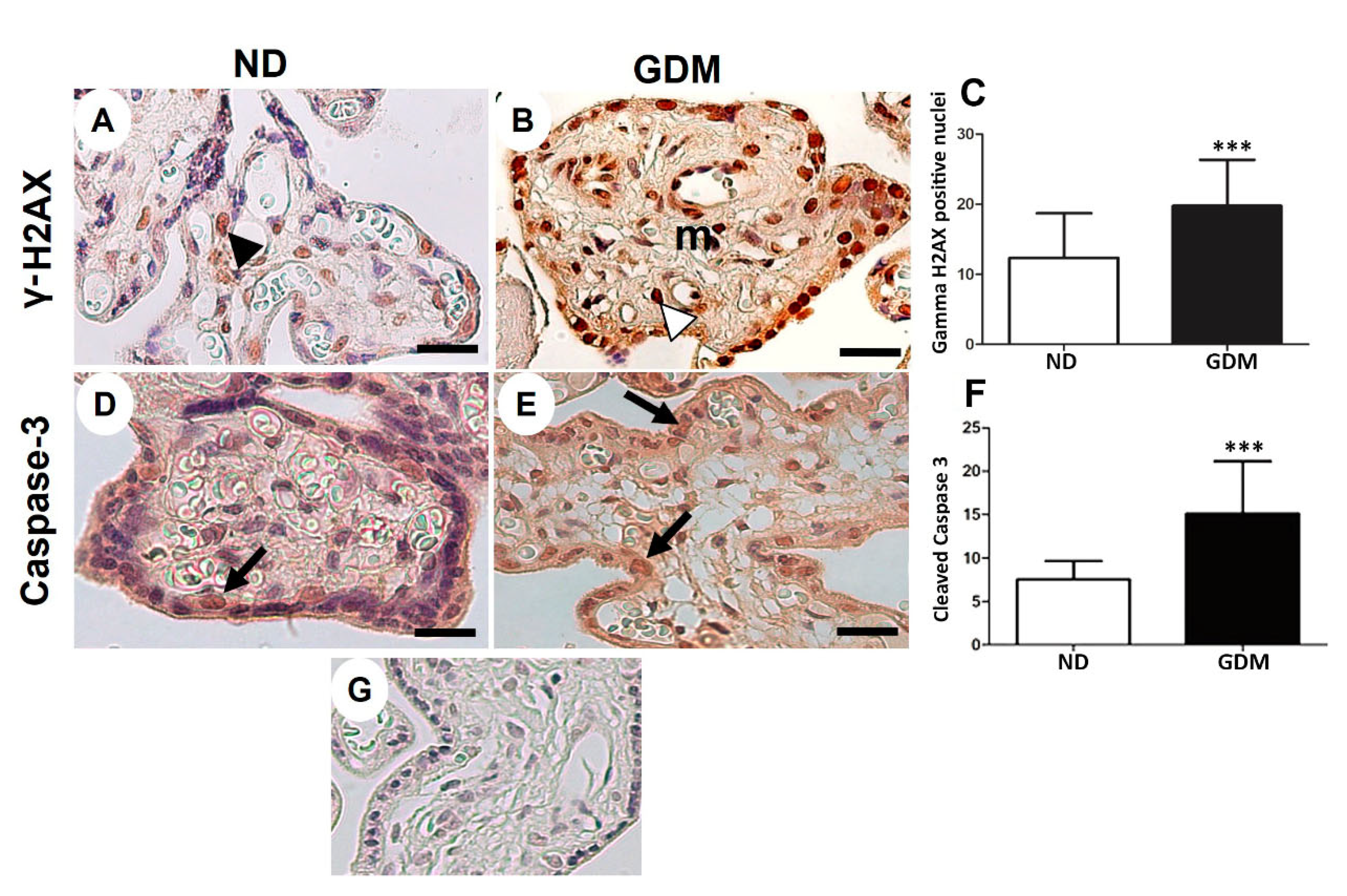
Figure 6.
Immunolocalization of γ-H2AX (DNA double-strand breaks, A-C) and cleaved caspase-3 (apoptosis, D-F) in placenta from pregnant women with no diabetes (ND; A, D) or gestational diabetes mellitus (GDM; B, E). (A-B, D-E) Nuclei of the syncytiotrophoblast and mesenchymal cells (m) are reactive to γ-H2AX (arrowheads). Caspase-3 reactive cells follow a similar pattern of γ-H2AX immunoreactivity (arrows). (G) Negative control used in immunohistochemical analysis. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (C, F) Quantification of γ-H2AX-positive nuclei (C) and caspase-3-reactive cells (F) per 10.000 μm² of placental area. Values are shown as mean ± SD; *** p < 0.001.
Figure 6.
Immunolocalization of γ-H2AX (DNA double-strand breaks, A-C) and cleaved caspase-3 (apoptosis, D-F) in placenta from pregnant women with no diabetes (ND; A, D) or gestational diabetes mellitus (GDM; B, E). (A-B, D-E) Nuclei of the syncytiotrophoblast and mesenchymal cells (m) are reactive to γ-H2AX (arrowheads). Caspase-3 reactive cells follow a similar pattern of γ-H2AX immunoreactivity (arrows). (G) Negative control used in immunohistochemical analysis. Immunoperoxidase and hematoxylin counterstaining. Bars = 50 μm. (C, F) Quantification of γ-H2AX-positive nuclei (C) and caspase-3-reactive cells (F) per 10.000 μm² of placental area. Values are shown as mean ± SD; *** p < 0.001.
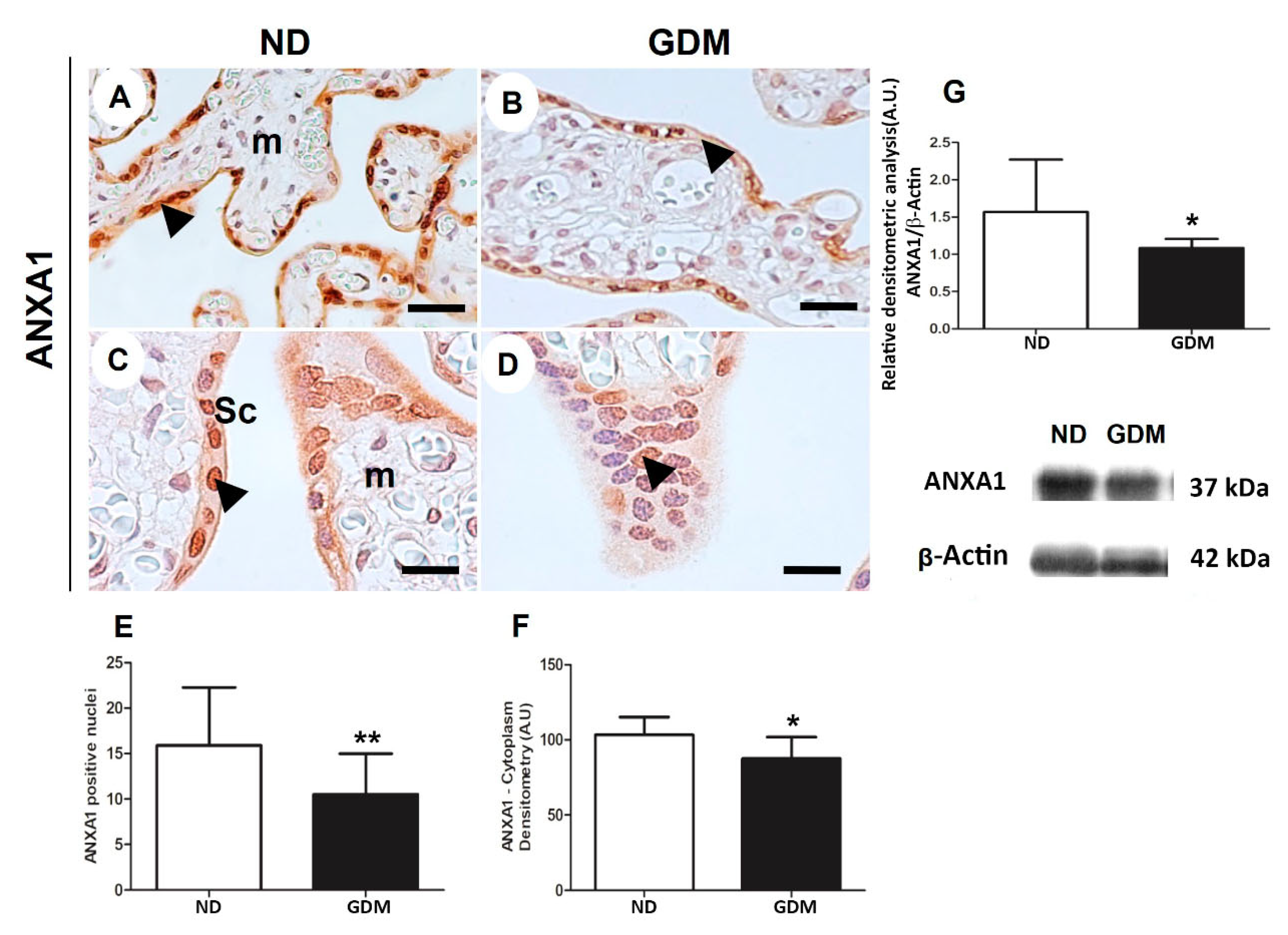
Figure 7.
ANXA1 expression in placenta from pregnant women with no diabetes (ND; A,C, G) or gestational diabetes mellitus (GDM; B, D, G) evaluated by immunohistochemistry (A-F) and western blot (G). Placental reactive components (arrowheads) are mainly nuclei and cytoplasm of syncytiotrophoblast (sc) and some cells found in mesenchyme (m). The sample were counter-stained with hematoxylin. Bars = 50 μm. 40x (A,B); 100x (C,D) Quantification of positive nuclei in 10.000μ² (E) and cytoplasm densitometry (F). The relative band intensities from western blot experiments were normalized to β-actin and analyzed with Image J software (G). Values as mean ± SD; *p < 0.05; ** p < 0.01.
Figure 7.
ANXA1 expression in placenta from pregnant women with no diabetes (ND; A,C, G) or gestational diabetes mellitus (GDM; B, D, G) evaluated by immunohistochemistry (A-F) and western blot (G). Placental reactive components (arrowheads) are mainly nuclei and cytoplasm of syncytiotrophoblast (sc) and some cells found in mesenchyme (m). The sample were counter-stained with hematoxylin. Bars = 50 μm. 40x (A,B); 100x (C,D) Quantification of positive nuclei in 10.000μ² (E) and cytoplasm densitometry (F). The relative band intensities from western blot experiments were normalized to β-actin and analyzed with Image J software (G). Values as mean ± SD; *p < 0.05; ** p < 0.01.
Table 1.
Clinical data.
| |
ND (n = 10) |
GDM (n = 10) |
| Maternal age (years) |
30.85 ± 6.11 |
29.43 ± 5.36 |
| BMI (Kg/m2) |
34.88 ± 8.44 |
38.05 ± 4.37 |
| Pregnancy weight gain (Kg) |
12.83 ± 7.50 |
12.34 ± 6.94 |
| HbA1c (%) |
5.36 ± 0.63 |
6.32 ± 0.82* |
| Placental weight (g) |
623.10 ± 78.38 |
179.80 ± 63.5 |
| Newborn weight (g) |
3062.00 ± 276.60 |
3540.00 ± 574.50* |