Submitted:
12 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
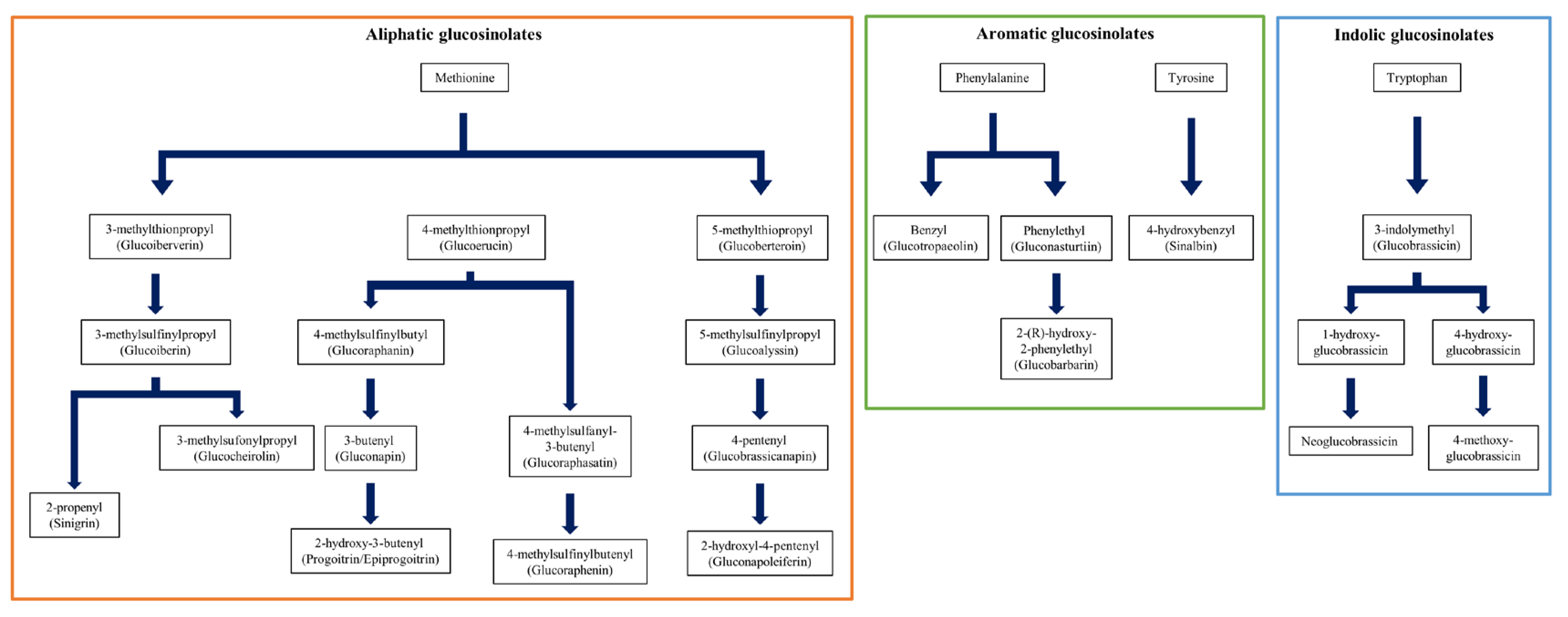
2.1. GSLs Standards Used in This Experiment
2.2. Choy Sum Genetic Materials and Cultivation Condition
| Malaysia | Thailand | Taiwan | Vietnam | China | Mauritius | India | Laos | Bangladesh | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar | 2 | 1 | 1 | 1 | 1 | 6 | ||||
| Landrace | 3 | 4 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 17 |
| Total | 5 | 5 | 4 | 2 | 2 | 2 | 1 | 1 | 1 | 23 |
2.3. Sample Preparation: Pretreatment and Extraction
2.4. Identification and Quantification of GSLs Using UPLC-MS/MS
| Name | Abbreviation | Class | RT (min) | MRM Transition |
CID (ev) | Dwell Time (sec) | Calibration Curve Parameters |
|---|---|---|---|---|---|---|---|
| Progoitrin | PRO | Aliphatic | 5.94 | 387.77 >194.85 | 25 | 0.029 | Y = 8.2526X + 28.1501(r2= 0.961) |
| Sinigrin | SIN | Aliphatic | 6.56 | 357.75 >161.84 | 25 | 0.029 | Y = 12.7878X -11.1181 (r2= 0.999) |
| Gluconapin | GNA | Aliphatic | 7.78 | 371.74 >258.74 | 25 | 0.029 | Y = 8.36216X +29.5397(r2= 0.994) |
| Glucoiberin | GIB | Aliphatic | 7.98 | 421.62 >357.73 | 25 | 0.029 | Y = 33.6632X +446.334(r2= 0.997) |
| Epiprogoitrin | EPI | Aliphatic | 8.06 | 387.7 > 258.74 | 25 | 0.029 | Y = 7.4939X -6.76519(r2= 0.999) |
| Glucocheirolin | GCR | Aliphatic | 8.38 | 437.71 >258.74 | 25 | 0.029 | Y =20.7762X +39.3608(r2= 0.986) |
| Glucoraphanin | GRA | Aliphatic | 8.39 | 435.59 >177.78 | 25 | 0.029 | Y = 25.0808X +60.584(r2= 0.983) |
| Glucoraphenin | GRE | Aliphatic | 8.53 | 433.66 >258.81 | 25 | 0.029 | Y = 15.2565X +3.62242(r2= 0.988) |
| Glucobrassicanapin | GBN | Aliphatic | 8.60 | 385.71 >258.87 | 25 | 0.029 | Y = 7.2514X +47.2841(r2= 0.992) |
| Glucobarbarin | GBB | Aromatic | 8.64 | 437.71 >274.75 | 25 | 0.029 | Y = 9.29915X -0.454779(r2= 0.999) |
| Glucoerucin | GER | Aliphatic | 8.73 | 419.69 >258.74 | 25 | 0.029 | Y = 6.77393X +73.6679(r2= 0.984) |
| Glucotropaeolin | GTL | Aromatic | 8.88 | 407.72 >258.87 | 25 | 0.029 | Y = 18.2122X -3.93949(r2= 0.999) |
| Sinalbin | SNB | Aromatic | 9.10 | 423.62 >258.74 | 25 | 0.029 | Y = 49.7228X -33.0636(r2= 0.999) |
| Glucoberteroin | GBE | Aliphatic | 9.18 | 433.72 >275.06 | 25 | 0.029 | Y = 6.09397X +63.1212(r2= 0.997) |
| Glucobrassicin | GBC | Indolyl | 9.31 | 446.69 >204.94 | 25 | 0.029 | Y = 6.39827X +2.6232(r2= 0.997) |
| Gluconasturtiin | GNS | Aromatic | 9.34 | 421.69 >274.87 | 25 | 0.029 | Y = 4.36109X –90.233(r2= 0.961) |
| Glucoraphasatin | GRH | Aromatic | 9.62 | 417.63 >258.81 | 25 | 0.029 | Y = 15.5149X -5.95281(r2= 0.997) |
2.5. Statistical Analysis
3. Results
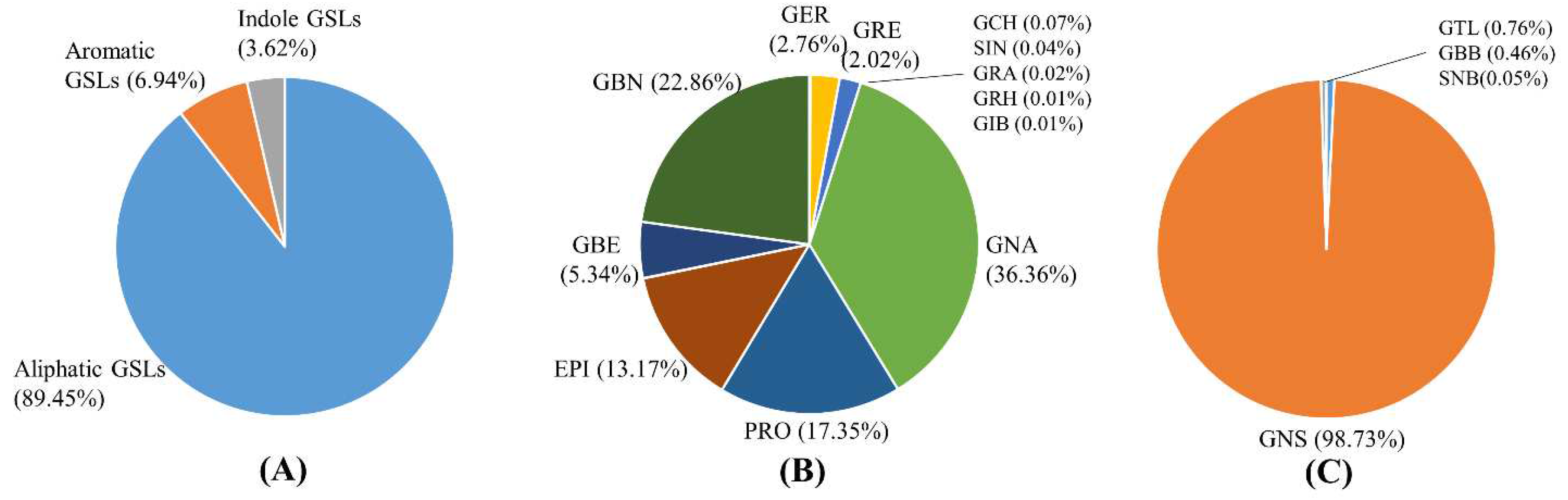
3.1. Quantification of GSLs and Selection of Candidate Germplasm for Breeding Materials
| Variable | Range | Mean | Std. deviation | |
| Aliphatic GSLs | Glucoiberin | 0~1.48 | 0.39 | 0.46 |
| Sinigrin | 0.16~17.69 | 3.69 | 4.21 | |
| Glucocheirolin | 0.08~19.91 | 5.48 | 6.07 | |
| Glucoerucin | 0.64~1,983.01 | 227.83 | 562.36 | |
| Glucoraphanin | 2.29~569.16 | 166.77 | 179.00 | |
| Gluconapin | 117.38~13,111.41 | 2,997.62 | 3,406.77 | |
| Progoitrin | 120.20~3,172.65 | 1,430.06 | 899.82 | |
| Epiprogoitrin | 72.56~2,728.20 | 1,085.29 | 711.35 | |
| Glucoraphasatin | 0.03~9.89 | 0.70 | 2.02 | |
| Glucoraphenin | 0.11~9.18 | 1.35 | 2.10 | |
| Glucoberteroin | 6.02~3,491.34 | 440.22 | 899.73 | |
| Glucobrassicanapin | 148.87~6,830.64 | 1,884.15 | 1,457.35 | |
| Total aliphatic | 8,243~18,110.85 | 8,243.54 | 4,557.95 | |
| Aromatic GSLs | Glucotropaeolin | 1.83~9.58 | 4.87 | 2.08 |
| Gluconasturtiin | 74.28~2,379.24 | 631.28 | 575.41 | |
| Glucobarbarin | 0.97~8.04 | 2.94 | 1.71 | |
| Sinalbin | 0.04~2.96 | 0.34 | 0.69 | |
| Total aromatic | 84.98~2,389.73 | 639.42 | 576.14 | |
| Indolic GSLs | Glucobrassicin | 85.15~908.09 | 333.30 | 203.01 |
| Total GSLs | 9,216~20,023.79 | 9,216.26 | 4,905.73 | |
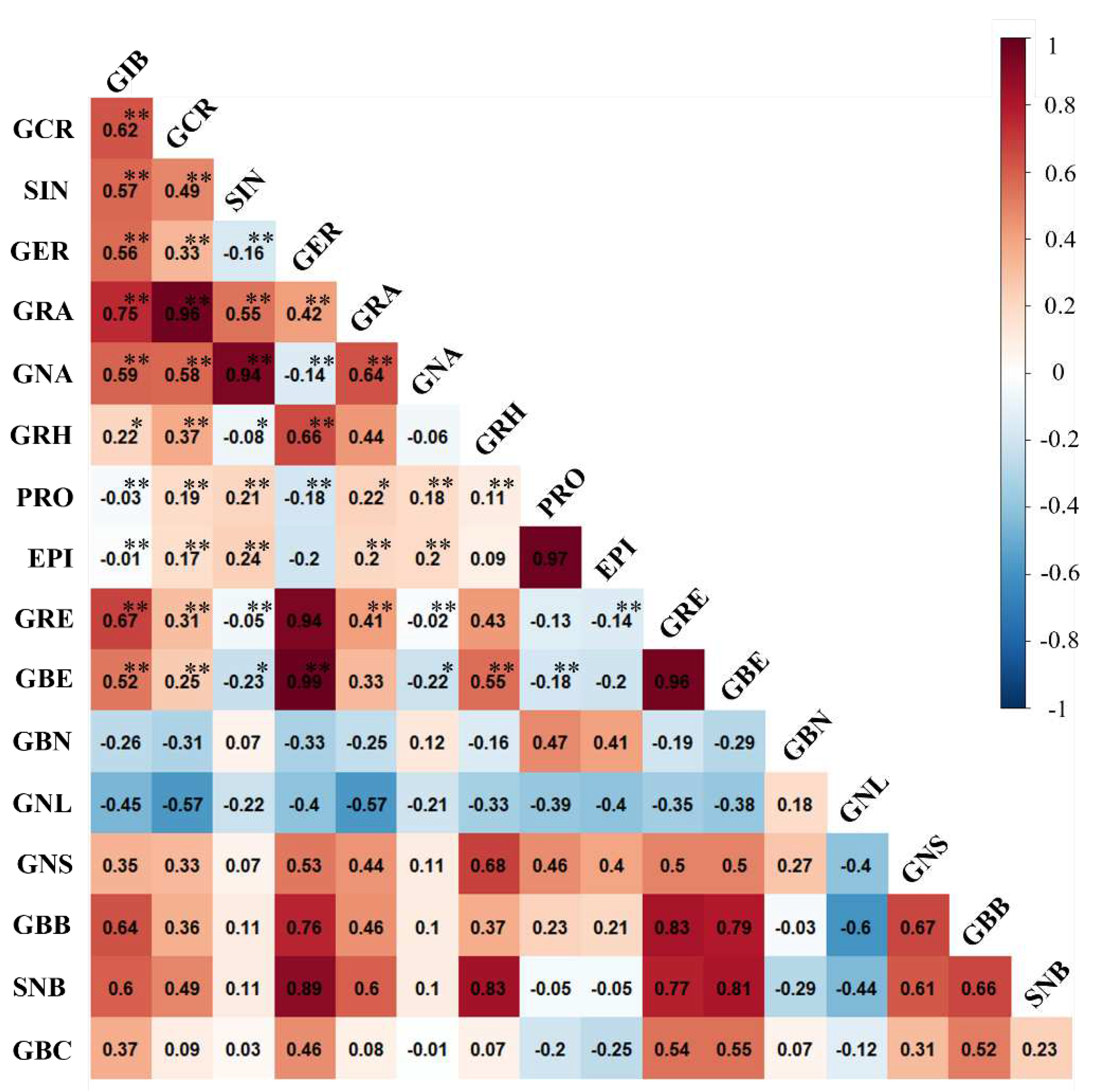
3.2. Correlation Analysis
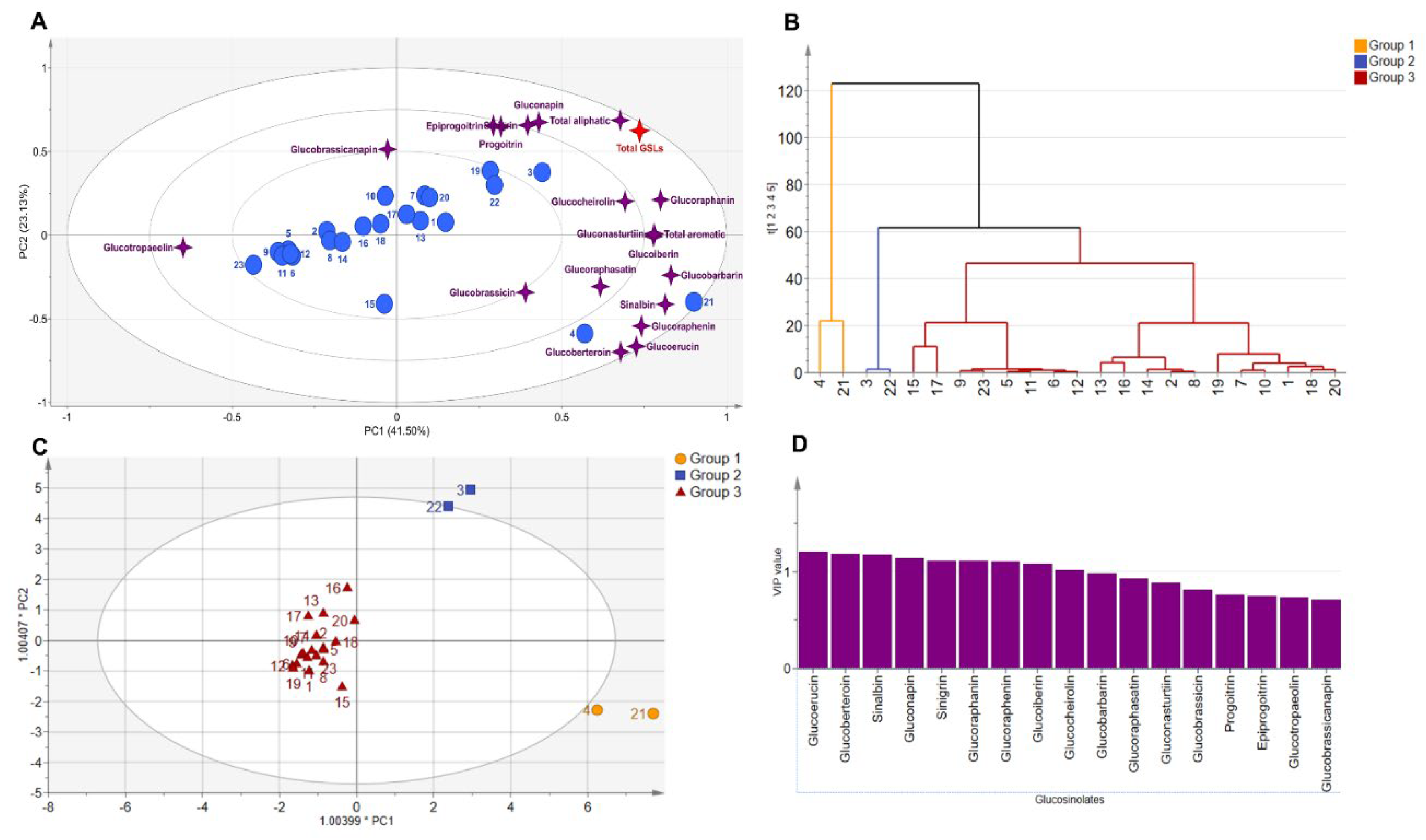
3.3. Diversity Analysis and Clustering
3.4. Nutritional Value of Glucosinolates
| Chemical Compounds | Class | Hydrolysis Products | Functions |
| Gluconapin | Aliphatic | 1-cyano-3,4-etithiobutane |
In Mice ∙ Prevent postprandial hypertriglyceridemia and decrease plasma triglyceride gain [27] In Human ∙ Increase NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione S -transferase A3 and the glutamate–cysteine ligase subunit; (CETP) in Hep G2 Cell [36] |
| Glucobrassicanapin | Aliphatic | 4-pentenyl isothiocyanate |
In Gram negative bacteria ∙ Increase antibacterial activity against Aeromonas hydrophila [23] In rat [24] ∙ Decreas release of leukotriene B4 (LTB4) and release of leukotriene B4 (LTB4) from RBL- |
| Progoitrin | Aliphatic | Nitrile Crambene (1-cyano-2-hydroxy-3-butene) |
IN human Hep Gsub2 cell; mouse Hepa 1c1c7 cells and mouse H4IIEC3 cells [25] ∙ Increase the activity of quinone reductase resulting in cell cycle arrest In Swiss mice In Swiss mice protect against acute pancreatitis by inducing pancreatic acinar cell apoptosis by activating anti-inflammatory and mitochondrial pathways [25,29] ∙ Decerase acute pancreatitis and activate anti-inflammatory pathway [25] ∙ Activate mitochondrial pathways [29] |
| Gluconasturtiin | Aromatic | 2-phenylethyl isothiocyante | The anticancer activity of phenylethyl isothiocyanate, a hydrolyzed product obtained from gluconasturtiin, is excellent as it induces cyto-protective genes mediated by Nrf2 and AhR transcription factors, represses NF-κB, and inhibits both cytochrome P450 and histone deacetylase [37] |
| Glucobrassicin | Indolic | Indole-3-carbinol |
In human ∙ Inhibit breast and ovarian cancer [38] ∙ Inhibit apoptosis of osteoporosis and ROS-mediated Nrf2 pathway [30] In rat ∙ Decrease portal hypertension, the severity of mesenteric angiogenesis, and portosystemic collaterals in cirrhosis [39] In mice ∙ Decrease Citrobacter rodentium growth causing acute intestinal inflammation and increase T cell activity [40] |
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Foo, J.T.S. A Guide to Common Vegetables; Singapore Science Centre: 1996.
- Edward, E. Modelling the Growth of Choy Sum (Brassica Chinensis Var. Parachinensis), at Different Nitrogen Fertilizer Rates. 2008.
- Huang, J.J.; D’Souza, C.; Tan, M.Q.; Zhou, W. Light intensity plays contrasting roles in regulating metabolite compositions in choy sum (Brassica rapa var. parachinensis). Journal of Agricultural and Food Chemistry 2021, 69, 5318-5331.
- Zou, L.; Tan, W.K.; Du, Y.; Lee, H.W.; Liang, X.; Lei, J.; Striegel, L.; Weber, N.; Rychlik, M.; Ong, C.N. Nutritional metabolites in Brassica rapa subsp. chinensis var. parachinensis (choy sum) at three different growth stages: Microgreen, seedling and adult plant. Food Chemistry 2021, 357, 129535. [CrossRef]
- Wang, D.; Shi, Q.; Wang, X.; Wei, M.; Hu, J.; Liu, J.; Yang, F. Influence of cow manure vermicompost on the growth, metabolite contents, and antioxidant activities of Chinese cabbage (Brassica campestris ssp. chinensis). Biology and fertility of soils 2010, 46, 689-696. [CrossRef]
- Domínguez-Perles*, R.; Mena*, P.; Garcia-Viguera, C.; Moreno, D. Brassica foods as a dietary source of vitamin C: A review. Critical reviews in food science and nutrition 2014, 54, 1076-1091.
- Wang, W.; Wang, J.; Wei, Q.; Li, B.; Zhong, X.; Hu, T.; Hu, H.; Bao, C. Transcriptome-wide identification and characterization of circular RNAs in leaves of Chinese cabbage (Brassica rapa L. ssp. pekinensis) in response to calcium deficiency-induced tip-burn. Scientific reports 2019, 9, 1-9. [CrossRef]
- Wu, X.; Zhou, Q.-h.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacologica Sinica 2009, 30, 501-512.
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The roles of cruciferae glucosinolates in disease and pest resistance. Plants 2021, 10, 1097. [CrossRef]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the Arabidopsis era. Trends in plant science 2002, 7, 263-270. [CrossRef]
- Halkier, B.A.; Du, L. The biosynthesis of glucosinolates. Trends in plant science 1997, 2, 425-431. [CrossRef]
- Bouranis, J.A.; Beaver, L.M.; Choi, J.; Wong, C.P.; Jiang, D.; Sharpton, T.J.; Stevens, J.F.; Ho, E. Composition of the gut microbiome influences production of sulforaphane-nitrile and iberin-nitrile from glucosinolates in broccoli sprouts. Nutrients 2021, 13, 3013. [CrossRef]
- Park, S.; Son, H.-K.; Chang, H.-C.; Lee, J.-J. Effects of cabbage-apple juice fermented by Lactobacillus plantarum EM on lipid profile improvement and obesity amelioration in rats. Nutrients 2020, 12, 1135. [CrossRef]
- Mandrich, L.; Caputo, E. Brassicaceae-derived anticancer agents: Towards a green approach to beat cancer. Nutrients 2020, 12, 868. [CrossRef]
- Takasugi, M.; Katsui, N.; Shirata, A. Isolation of three novel sulphur-containing phytoalexins from the chinese cabbage Brassica campestris L. ssp. pekinensis (cruciferae). Journal of the Chemical Society, Chemical Communications 1986, 1077-1078. [CrossRef]
- Le, T.N.; Chiu, C.-H.; Hsieh, P.-C. Bioactive compounds and bioactivities of Brassica oleracea L. var. italica sprouts and microgreens: An updated overview from a nutraceutical perspective. Plants 2020, 9, 946.
- Lim, W.F.; Mohamad Yusof, M.I.; Teh, L.K.; Salleh, M.Z. Significant decreased expressions of CaN, VEGF, SLC39A6 and SFRP1 in MDA-MB-231 xenograft breast tumor mice treated with Moringa oleifera leaves and seed residue (MOLSr) extracts. Nutrients 2020, 12, 2993.
- RDA-Genebank. RDA-Genebank passport data (http://genebank.rda.go.kr/). Available online: (accessed on Mar 23).
- Kim, S.-H.; Lee, G.-A.; Subramanian, P.; Hahn, B.-S. Quantification and Diversity Analyses of Major Glucosinolates in Conserved Chinese Cabbage (Brassica rapa L. ssp. pekinensis) Germplasms. Foods 2023, 12, 1243.
- Tobias, S.; Carlson, J.E. Brief report: Bartlett's test of sphericity and chance findings in factor analysis. Multivariate behavioral research 1969, 4, 375-377.
- Kim, S.-H.; Subramanian, P.; Hahn, B.-S.; Ha, B.-K. High-Throughput Phenotypic Characterization and Diversity Analysis of Soybean Roots (Glycine max L.). Plants 2022, 11, 2017. [CrossRef]
- Kim, S.-H.; Jo, J.W.; Wang, X.; Shin, M.-J.; Hur, O.S.; Ha, B.-K.; Hahn, B.-S. Diversity characterization of soybean germplasm seeds using image analysis. Agronomy 2022, 12, 1004. [CrossRef]
- Jang, M.; Hong, E.; Kim, G.H. Evaluation of antibacterial activity of 3-butenyl, 4-pentenyl, 2-phenylethyl, and benzyl isothiocyanate in Brassica vegetables. Journal of food science 2010, 75, M412-M416.
- Yamada-Kato, T.; Nagai, M.; Ohnishi, M.; Yoshida, K. Inhibitory effects of wasabi isothiocyanates on chemical mediator release in RBL-2H3 rat basophilic leukemia cells. Journal of nutritional science and vitaminology 2012, 58, 303-307. [CrossRef]
- Keck, A.-S.; Staack, R.; Jeffery, E.H. The cruciferous nitrile crambene has bioactivity similar to sulforaphane when administered to Fischer 344 rats but is far less potent in cell culture. Nutrition and cancer 2002, 42, 233-240. [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food chemistry 2012, 133, 735-741. [CrossRef]
- Washida, K.; Miyata, M.; Koyama, T.; Yazawa, K.; Nomoto, K. Suppressive effect of Yamato-mana (Brassica rapa L. Oleifera Group) constituent 3-butenyl glucosinolate (gluconapin) on postprandial hypertriglyceridemia in mice. Bioscience, biotechnology, and biochemistry 2010, 74, 1286-1289. [CrossRef]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754-779.
- Cao, Y.; Adhikari, S.; Clément, M.V.; Wallig, M.; Bhatia, M. Induction of apoptosis by crambene protects mice against acute pancreatitis via anti-inflammatory pathways. The American journal of pathology 2007, 170, 1521-1534. [CrossRef]
- Lin, H.; Gao, X.; Chen, G.; Sun, J.; Chu, J.; Jing, K.; Li, P.; Zeng, R.; Wei, B. Indole-3-carbinol as inhibitors of glucocorticoid-induced apoptosis in osteoblastic cells through blocking ROS-mediated Nrf2 pathway. Biochemical and biophysical research communications 2015, 460, 422-427. [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O'Neill, C.M.; Bernuzzi, F. Transcriptional changes in prostate of men on active surveillance after a 12-mo glucoraphanin-rich broccoli intervention—results from the Effect of Sulforaphane on prostate CAncer PrEvention (ESCAPE) randomized controlled trial. The American journal of clinical nutrition 2019, 109, 1133-1144. [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochemistry Reviews 2009, 8, 283-298. [CrossRef]
- Zasada, I.; Ferris, H. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathology 2003, 93, 747-750. [CrossRef]
- Zhang, Y.; Talalay, P. Anticarcinogenic activities of organic isothiocyanates: chemistry and mechanisms. Cancer research 1994, 54, 1976s-1981s.
- Staack, R.; Kingston, S.; Wallig, M.; Jeffery, E. A comparison of the individual and collective effects of four glucosinolate breakdown products from brussels sprouts on induction of detoxification enzymes. Toxicology and applied pharmacology 1998, 149, 17-23. [CrossRef]
- Kelleher, M.O.; McMahon, M.; Eggleston, I.M.; Dixon, M.J.; Taguchi, K.; Yamamoto, M.; Hayes, J.D. 1-Cyano-2, 3-epithiopropane is a novel plant-derived chemopreventive agent which induces cytoprotective genes that afford resistance against the genotoxic α, β-unsaturated aldehyde acrolein. Carcinogenesis 2009, 30, 1754-1762.
- Hayes, J.D.; Kelleher, M.O.; Eggleston, I.M. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. European journal of nutrition 2008, 47, 73-88. [CrossRef]
- Stoewsand, G. Bioactive organosulfur phytochemicals in Brassica oleracea vegetables—a review. Food and chemical toxicology 1995, 33, 537-543. [CrossRef]
- Chang, T.; Ho, H.-L.; Hsu, S.-J.; Chang, C.-C.; Tsai, M.-H.; Huo, T.-I.; Huang, H.-C.; Lee, F.-Y.; Hou, M.-C.; Lee, S.-D. Glucobrassicin metabolites ameliorate the development of portal hypertension and cirrhosis in bile duct-ligated rats. International Journal of Molecular Sciences 2019, 20, 4161. [CrossRef]
- Wu, Y.; He, Q.; Yu, L.; Pham, Q.; Cheung, L.; Kim, Y.S.; Wang, T.T.; Smith, A.D. Indole-3-carbinol inhibits Citrobacter rodentium infection through multiple pathways including reduction of bacterial adhesion and enhancement of cytotoxic T cell activity. Nutrients 2020, 12, 917. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





