1. Introduction
Transglutaminase 2 (TG2) is a multifunctional enzyme that has GTPase, ATPase, protein kinase, protein disulfide isomerize, and transglutaminase activities. It is expressed in several different cell types and organelles, where it carries out various posttranslational modifications, such as protein crosslinking, amine incorporation, acylation, and deamidation [
1]. TG2 can also serve as a scaffold protein that connects protooncogenes, the tumor suppressor PTEN, integrins, and growth factor receptors, both intracellularly and extracellularly [
2]. TG2 is involved in a number of cellular processes, such as differentiation, apoptosis, phagocytosis, signal transduction, adhesion, and wound healing [
3]. Growing evidence now indicates an association between increased TG2 expression and several human cancers in which TG2 can function as a fundamental cancer cell survival factor. In patients with lung, breast, colon, cervical, leukemia, lymphoma, and pancreatic cancers, an elevated expression of TG2 is associated with poor prognosis, metastasis, and disease recurrence [
4,
5,
6,
7,
8].
Acute promyelocytic leukemia (APL) is a bone marrow cancer that is characterized by the presence of a chromosomal translocation between the retinoic acid receptor alpha (RARα) gene on chromosome 17 and the promyelocytic leukemia protein (PML) gene on chromosome 15. The PML-RARα oncoprotein acts as a corepressor of transcription by blocking myeloid cell differentiation. Treatment of APL cells with all-trans retinoic acid (ATRA) triggers a conformational change in the oncoprotein and subsequent proteolysis of PML-RARα fusion protein, thereby shifting leukemic cells from the transcriptionally repressed state to the activated state and resulting in their maturation, while several genes upregulated [
9,
10,
11]. To some extent, ATRA-induced APL differentiation can be modeled using NB4 WT APL cells. To produce functioning neutrophil granulocytes, hundreds of genes are modulated during the differentiation process, among which TG2 is the most strongly up-regulated gene in ATRA-activated NB4 cell maturation [
9,
10,
11,
12,
13].
In the 2000s, the US Food and Drug Administration (FDA) authorized arsenic trioxide (As
2O
3/ATO) as a treatment for APL. Several clinical studies have shown that ATO, either as a single agent or combined with ATRA, generates an exceptionally better result than ATRA alone [
14,
15,
16], making ATO a first-choice option for the treatment of newly diagnosed and relapsed APL patients. Arsenic treatment can induce complete remission in patients and provide a 5-year overall survival rate of 90% when it is combined with ATRA and chemotherapy [
17]. In addition, ATO is an outstanding monotherapy, as it eliminates residual leukemia-initiating cells (LICs), a result that cannot be achieved by ATRA treatment alone. The cytotoxic effects of ATO are concentration-dependent: at low concentrations (<0.5 μM), ATO can induce partial differentiation of APL cells, and at high concentrations (>0.5 μM), it initiates apoptosis. At the cellular and molecular levels, ATO can provoke the degradation of the PML-RARα fusion protein by targeting the PML part and inducing sumoylation, ubiquitination, and proteasomal degradation of the chimeric protein [
18,
19,
20]. In addition, ATO generates both oxidative stress and damage to cellular biomolecules, including DNA, lipids, and proteins, to cause cell death [
20]. Ample evidence also indicates that some transcription factors and signal transduction pathways are redox-sensitized by arsenic [
21].
One group of proteins that might be sensitive to ATO is the calpain family, whose members are ubiquitously expressed in almost all eukaryotes. Calpains are Ca
2+-dependent cysteine proteases that display proteolytic activity and hydrolyze various substrate proteins at membranes or in the cytosol. Although the physiological role of calpains is still poorly understood, they are active participants in cellular processes such as cell apoptosis [
22]. Here, we report that ATO-induced ROS induce both the transcription factor NRF2 (nuclear factor erythroid 2-related factor 2) protein and the proteolytic enzyme calpain, whose activation ultimately leads to the proteolysis of TG2.
2. Results
Transglutaminase 2 impedes ROS production to attenuate ATO-induced cell death in ATRA differentiated NB4 cells
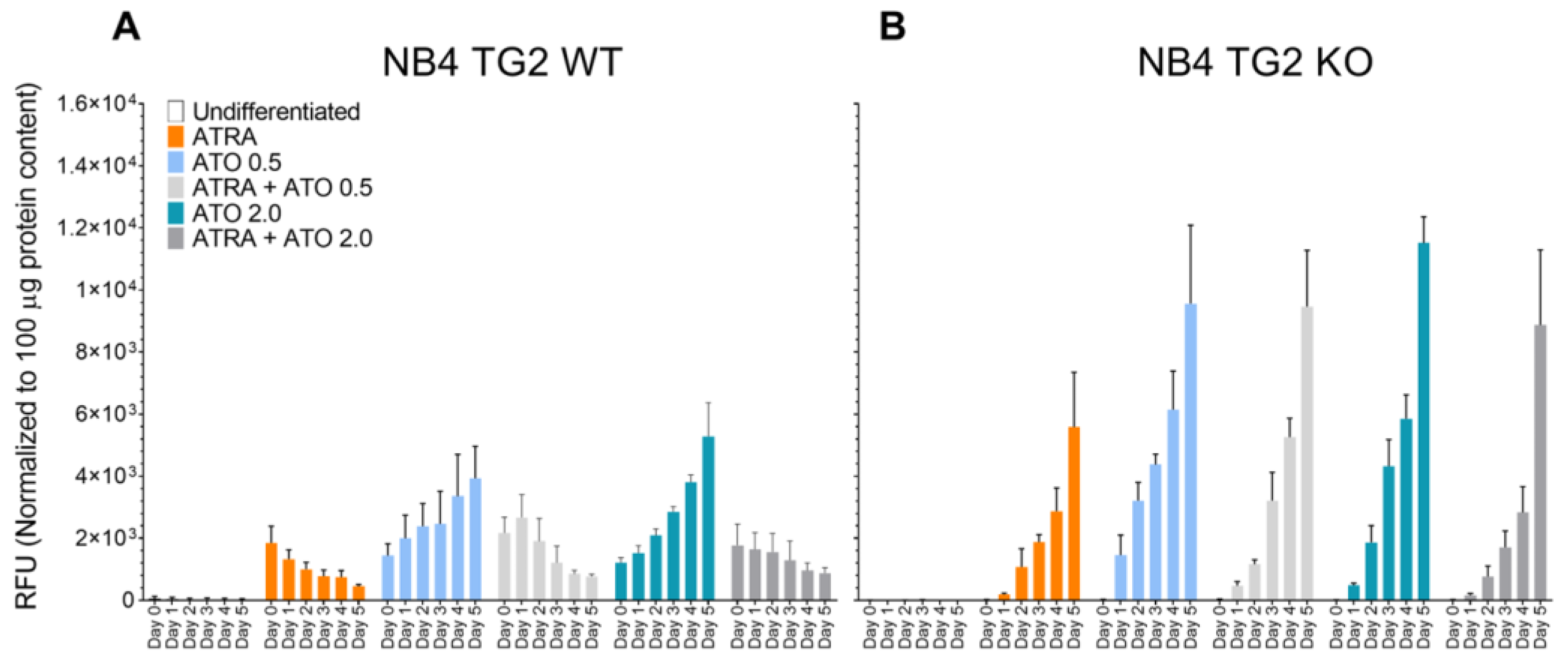
ATO induces the production of reactive oxygen species (ROS) as one of its anti-tumor effects. The ROS levels in NB4 WT cells gradually decreased after the first day of the ATRA and ATRA+ATO treatments, but they increased following ATO treatment alone (
Figure 1A). Similar endogenous ROS production patterns were seen in NB4 TG2-KO cells (
Figure 1B).
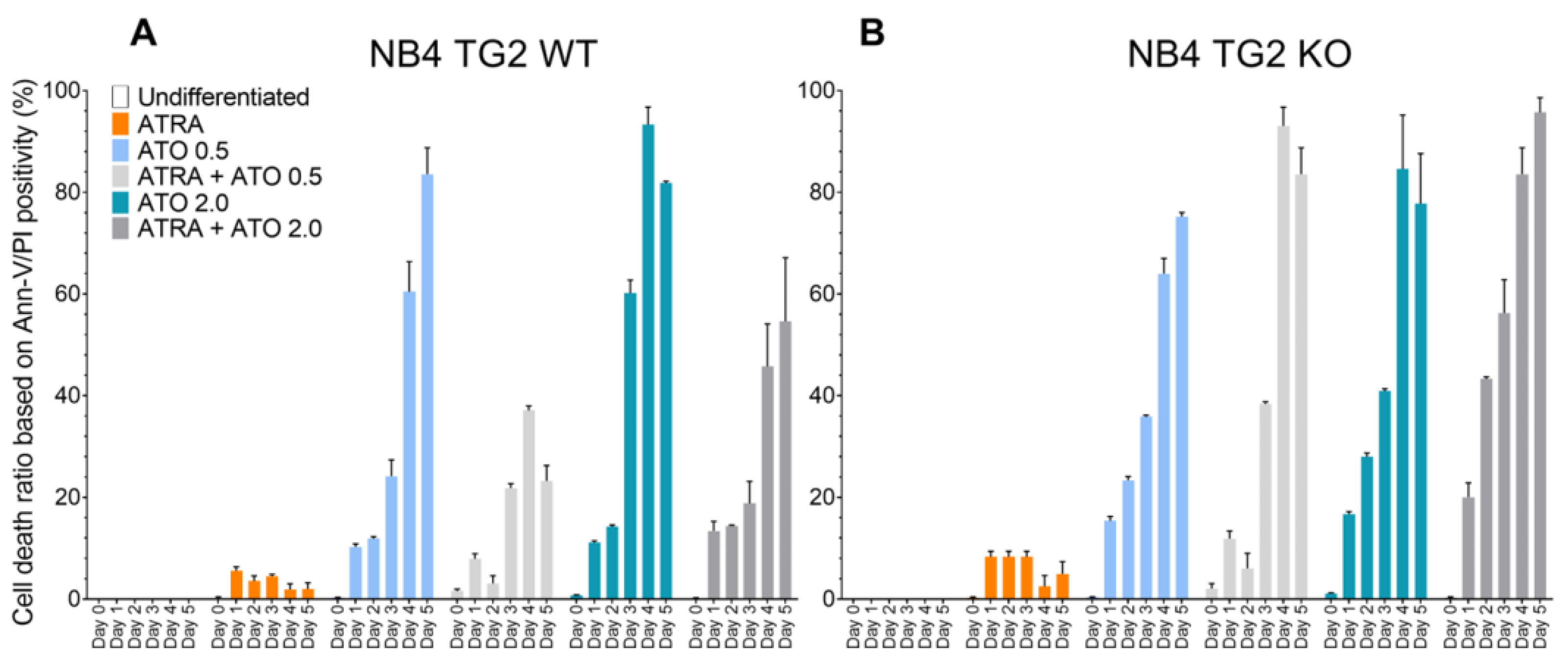
Increased ROS production was associated with an elevated apoptosis ratio. In NB4 WT cells, TG2 expression was induced by ATRA and was associated with a lower rate of cell death (
Figure 2A, orange and gray bars compared to blue bars) [
12]. By contrast, ATO resulted in higher apoptosis rates in the absence of TG2 induction in NB4 TG2-KO cells than in NB4 cells (
Figure 2B).
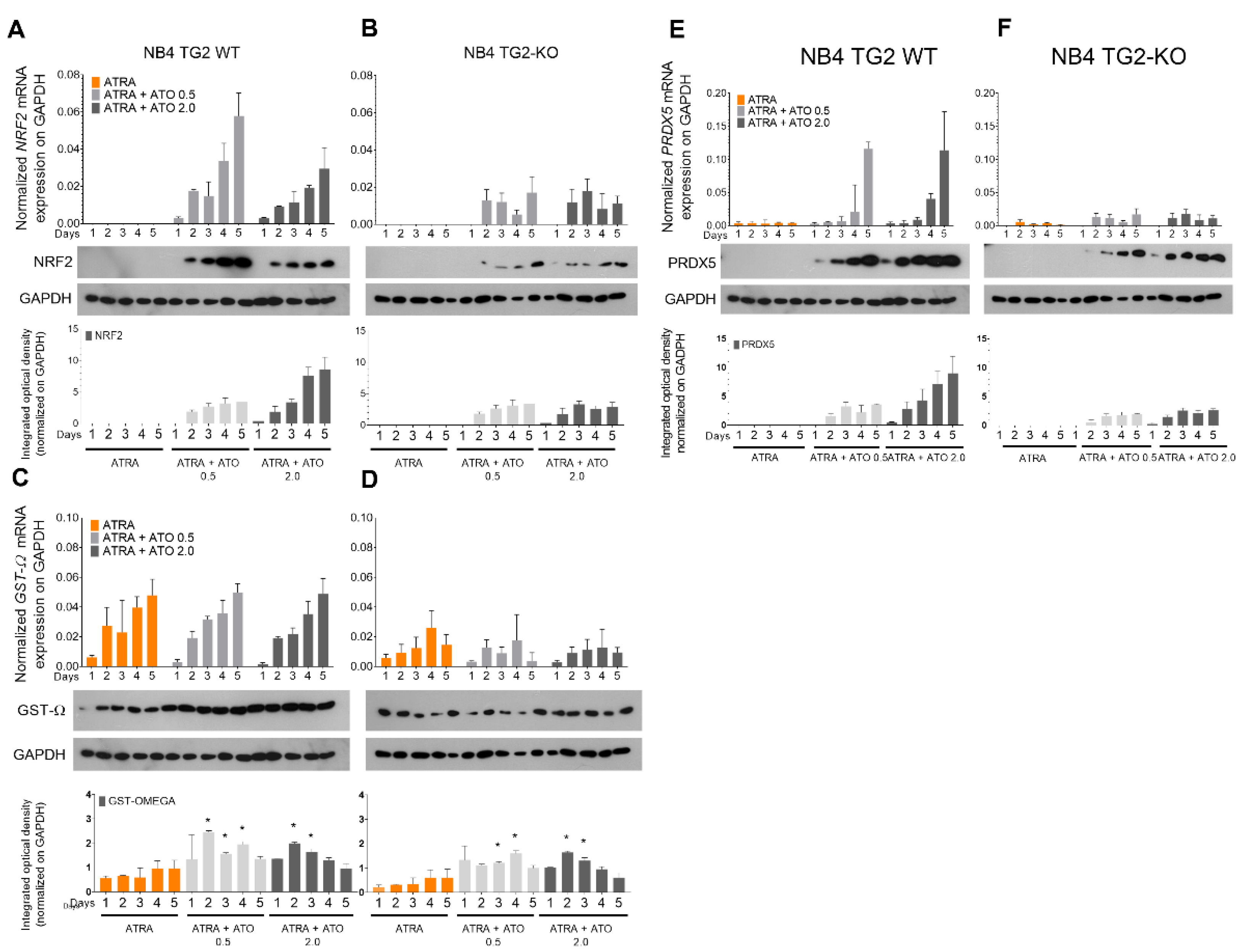
ROS production through the nuclear factor Erythroid 2-related factor 2 (NRF2) transcription factor regulates the expression of antioxidant genes in a TG2 dependent manner
The NRF2 transcription factor plays a crucial role in the adaptive cellular oxidative stress response by strengthening the antioxidant and detoxification systems. The ATRA+ATO treatment increased NRF2 mRNA and protein expression in NB4 WT cells, but this induction was blunted in TG2 KO cells (
Figure 3A,B). Glutathione S-transferase omega-1 (GSTO1-1) plays an essential role in the arsenic biotransformation of the arsenic methylation pathway. Peroxiredoxin 5 (PRDX5) is an antioxidant enzyme with a cytoprotective function during oxidative stress. GSTO1 mRNA was accumulated equally following all treatments in NB4 WT cells, but not in the absence of TG2 (
Figure 3C,D). While the amount of GSTO1 protein in ATRA-treated cells did not change significantly from day 2, the combined treatment of ATRA + ATO gradually increased GSTO1 protein levels (
Figure 3C). The only treatment that caused a change in the PRDX5 mRNA levels was the combination of ATRA+ATO, and the induction was abolished in the absence of TG2, just as it was with GSTO1 (
Figure 3D,F). The protein levels of PRDX5 correlated with the transcript levels (
Figure 3 E,F).
ROS production elicits robust calpain expression and activation, resulting in increased proteolysis of transglutaminase 2
Calpains are ubiquitous non-lysosomal calcium–dependent cysteine proteases that are activated during various pathological and physiological processes (e.g., apoptosis and cell proliferation). ATRA treatment was associated with constant calpain expression, but the ATRA+ATO treatment markedly increased the levels of calpain starting from day 2 and showed ATO concentration dependence (
Figure 4A).
Fluorimetry determinations showed increased calpain enzymatic activity following ATRA or ATRA+ATO treatment in both the total cell population (
Figure 4B, grey bars) and after sorting the Annexin-V-negative (green) and Annexin-V-positive (pink) cells. By day three, calpain activity in the total cell population was 6.28-fold higher in the ATRA+ATO-treated cells than in the ATRA-treated cells. When cells were separated by apoptotic features, the calpain activity was 3.25-fold higher by day 3 and 24.4-fold higher by day 5 in Annexin-V–positive cells than in Annexin-V–negative cells (
Figure 4B). Calpain protein expressions followed the measured activity increases in Annexin-V-positive cells (
Figure 4C).
Treatment of NB4 WT cells with ATRA or ATRA+ATO in the presence of 1 and 2 μM calpain inhibitor for five days resulted in only a minimal fraction of ATRA-treated cells showing signs of apoptosis, whereas the effect of the ATRA+ATO treatment, which induced cell death in close to 60% of the cells, was reduced by about 15 and 20% by the presence of 1 and 2 μM calpain inhibitor, respectively (
Figure 4D). In parallel, treatment with a 2 μM calpain inhibitor reversed the ATO-induced calpain-mediated TG2 degradation, as indicated by increases in TG2 to levels comparable to those seen with ATRA alone (
Figure 4E).
ATO could suppress both transglutaminase 2 mRNA and protein levels in NB4 WT, MCF-7 cells and in MCSF treated monocytes
The NB4 WT cell line was differentiated with ATRA with or without ATO at 0.5 or 2.0 μM for 5 days. ATRA alone induced continuously increasing TG2 expression, which was constantly mitigated at both the mRNA and protein levels by the presence of ATO. The amount of TG2 protein showed a dose-dependent, but not statistically significant, increasing trend (
Figure 5A,B).
We tested whether this response was cell line specific by examining doxycycline-induced TG2 expression in terms of mRNA and protein expression in the MCF-7 breast carcinoma cell line. In comparison to the ATRA-treated samples, the ATRA+ATO treatment reduced TG2 mRNA expression by more than 50% and protein expression by more than 75%. As a positive control for TG2 expression, the NB4 WT cells showed the same reduction in TG2 expression as was observed in MCF-7 breast carcinoma cells (
Figure 5C, blue and black bars, D). Finally, human monocytes were isolated from three healthy donors and treated for 5 days with MCSF, ATO, calpain inhibitor, or combinations ex vivo to validate the ATO-induced TG2 degradation seen in the commercial cell lines. Upon MCSF treatment, the monocytes expressed gradually increasing amounts of TG2 mRNA at days 3 and 5, and this expression was significantly decreased by ATO-induced calpain activation (
Figure 5E). Use of the calpain inhibitor preserved the TG2 protein levels at days 3 and 5 (
Figure 5F). TG2 protein expression showed a similar pattern to that of TG2 mRNA, but the ATO-induced TG2 degradation was more moderate and was completely inhibited by the calpain inhibitor treatment. In samples treated with both MCSF and a calpain inhibitor, the inhibitor stopped the natural breakdown of TG2, resulting in the highest TG2 expression (
Figure 5F,G).
3. Discussion
The American Cancer Society estimated that 1.9 million new cancer cases would be diagnosed in the United States in 2022, and 609,360 cancer deaths would occur. Despite significant advances in cancer treatment in recent years, tumor recurrence, drug resistance, and metastasis remain significant issues. Consequently, new treatment options are still desperately required.
For individuals with acute promyelocytic leukemia, a combination of arsenic trioxide and all-trans retinoic acid has been demonstrated to be an effective therapy choice. ATRA is a drug that can stimulate the differentiation of promyelocytes, whereas ATO can trigger programmed cell death in APL cells, particularly those with the PML-RARalpha fusion gene [
23]. When applied to patients with APL illness, the combination of ATR and ATO therapy has the potential to enhance overall survival rates in addition to increasing the likelihood of reaching full remission [
24]. This combination medication has been shown to be successful in a number of clinical trials, particularly in individuals who have just been diagnosed with APL [
25].
Our findings showed that TG2 expression was inversely correlated with the elevated apoptosis ratio induced by increased ROS production in response to ATRA+ATO treatment (
Figure 1A,B,
Figure 2A,B and
Figure 3A,B). The standard first-line treatment for APL has been a combination of all-trans retinoic acid (ATRA) plus chemotherapy. ATO exerts its cytotoxic effects in several ways, including oxidative stress, genotoxicity, alteration of the signal transduction cascade, and epigenetic modification. In addition, TG2 promotes the expression of genes responsible for the elimination of reactive oxygen and nitrogen species through increased transcription and translation of the NRF2 transcription factor that coordinates stress-inducible activation of cytoprotective genes [
26]. Consequently, the absence of TG2 expression greatly increases ATO-induced oxidative stress (
Figure 3A–F).
Here, we demonstrated that the combination of ATRA and ATO, but not ATRA treatment alone, could increase the amount and proteolytic activity of calpain, thereby driving both the degradation of TG2 and the decreased survival of leukemic cells (
Figure 4A–E). Finally, we showed that degradation of TG2 is not cell-lineage specific, by showing that induced TG2 protein expression can be broken down in myeloid-derived ATRA-differentiated NB4 WT cells, in the MCF-7 breast cancer cell line, and in primary peripheral blood mononuclear cells, thereby sensitizing each of these cell types to apoptotic signals (
Figure 5A–G).
Analyzing the expression of TG2 in nearly 3,000 solid tumors lead to the conclusion that in tumors with elevated TG2 levels the prognosis was worse in terms of overall survival (OS); disease-free (DFS) and recurrence-free survival (RFS) was shorter, with an almost double hazard ratio (HR). These findings suggest that ATO treatment might improve the prognosis and decrease the likelihood of metastasis and recurrence in a variety of cancers after surgery [
6,
18,
27,
28,
29].
Finally, these data could imply that ATO treatment might improve the prognosis and decrease the likelihood of cancer metastasis in a variety of cancers, and so treatment with arsenic trioxide could be a new option for tumor therapies with atypical TG2 expression, including colon, breast, stomach, leukemia, lymphoma, esophagus, pancreatic, pulmonary, and cervical cancers [
30].
4. Materials and Methods
Cell culture and treatment
NB4 cell lines were cultured in RPMI 1640 (HEPES-containing) medium (Sigma-Aldrich), while MCF-7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% (v/v) fetal bovine serum (Gibco, Paisley, Scotland), 2 mM L-glutamine, and 1% (v/v) 100 U/mL penicillin-streptomycin, and 1% sodium pyruvate solution (10 mg/mL) (Sigma-Aldrich) at 37°C in 5% CO2. All cell lines were periodically tested for mycoplasma contamination.
The NB4 cells were treated with 1 μM all-trans retinoic acid (ATRA) (Sigma-Aldrich) alone or in combination with 0.5 μM or 2.0 μM arsenic trioxide (ATO) for 5 days.
MCF-7 cells were treated with 1 µg/mL doxorubicin (Sigma Aldrich), 1 μM ATRA, 1 μM ATRA+ 1 µg/mL doxorubicin, or 1 µg/mL doxorubicin + 2.0 μM ATO for 3 and 5 days.
Human monocytes were treated with 2.0 μM ATO, 2.0 μM calpain inhibitor, 5 nM macrophage colony-stimulating factor (MCSF), 5 nM MCSF + 2.0 μM ATO, 5 nM MCSF + 2.0 μM ATO + 2.0 μM calpain inhibitor, or 5 nM MCSF + 2.0 μM calpain inhibitor for 3 and 5 days.
Human monocyte isolation
Ficoll-gradient centrifugation was used to separate CD14+ human monocytes from healthy donors, and then anti-CD14-conjugated microbeads were used to separate immune cells. The supernatant was thrown away after blood samples were spun at 700 g for 15 minutes at room temperature. The remaining samples were diluted two-fold with physiological saline (Sigma Aldrich). The diluted samples were layered on 10 mL Ficoll reagent and centrifuged at 700 g for 20 minutes at room temperature. The resulting Ficoll layer containing monocytes (approximately 30 mL) was transferred into a new tube, diluted with 20 mL physiological saline, and centrifuged for 10 min at room temperature at 350 g. After centrifugation, the pellet was diluted and resuspended in 9 mL physiological saline, and then filtered through a column prefilter. The filtered cells were centrifuged for 7 min at room temperature at 350 g. The pellet was resuspended in 800 mL of 1 × phosphate buffered saline (PBS) supplemented with 200 μL CD14+ microbeads (R&D). The cells were incubated for 20 min at 4°C. The labeled cells were removed by centrifugation for 7 min at 700 g. The pelleted cells were resuspended, counted, and plated for further experiments. Monocytes were cultured in multi-well culture plates in Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% human AB serum (Invitrogen). For macrophage differentiation, freshly plated monocytes were treated with 5 nM MCSF (0.5 μL/mL). Cells were harvested at 0, 3, and 5 h after treatment.
Inhibition of calpain
Untreated, ATRA-differentiated, and ATRA + 2.0 μM ATO treated NB4 wild-type (WT) cells were treated with 1 μM or 2 μM calpain inhibitor (Bio-Rad) for 3 and 5 days.
Endogenous reactive superoxide production
A total of 200 μL reaction mixture containing 1×105 cells and 2 μL dichloro-fluorescein diacetate (DHCFDA) (200 μM) were combined and incubated for 10 min at room temperature, followed by the relative luminescent unit (RLU) measurement with a Synergy Multi-Mode microplate reader (BioTek Instruments, Inc.) at 10 second intervals for 2 h. Production of endogenous ROS was recorded in relative luminescence units and normalized to 100 μg protein of the samples. Hydrogen peroxide (100 μM) and DHCFDA were used as positive controls.
Annexin-V labeling and live/dead cell sorting
Approximately 2×106 cells were collected, washed with 1× PBS, and centrifuged at 100 g for 3 min at 4°C. All subsequent steps were performed at 4°C. Cells were labeled with FITC-conjugated Annexin-V (Biolegend) for 15 min in the dark, centrifuged, and resuspended in 1× PBS. The cells were analyzed and sorted on a BD FACSAria™ III flow cytometer (B.D. Biosciences, San Jose, CA). Apoptotic features were evaluated by the size and granularity of the NB4 cells, followed by gating out the FITC-positive cell population. A minimum of 1×105 cells was sorted out for further experiments.
Fluorometric measurement of calpain activity
A minimum of 1×105 cells was sorted out by flow cytometry based on the FITC-Annexin-V positivity. Cells were harvested and measured with a Calpain Activity Fluorometric Assay Kit (Sigma Aldrich, MAK228-1KT) following the manufacturer’s protocol.
Gene expression analyses by real-time qPCR
RT-qPCR was conducted with TaqMan probes (ABI, Applied Biosystems) for NRF2, GST-Ω, PRDX5, TG2, and GAPDH on a Roche Light Cycler® 480 II (Roche Molecular Systems, Inc.).
SDS-PAGE and western blotting
A total of 1–2×106 cells were collected and lysed in lysis buffer (50 mM Tris, 1 mM EDTA, MEA, 0.5% Triton X-100, 1 mM PMSF) containing a protease inhibitor cocktail (PIC) (Sigma-Aldrich) at a 1:100 dilution ratio, homogenized with 5–7 strokes of a sonicator at 40% cycle intensity (Branson Sonifer® 450), and centrifuged at a maximum of 13700 g at 4°C for 15 min. Protein concentrations were measured in triplicate in 96-well flat-bottom plates at 595 nm with a Synergy Multi-Mode Microplate Reader (BioTek Instruments, Inc.) and the Bradford assay (Bio-Rad). Samples were diluted to 2 mg/mL, mixed with equal volumes of 2× SDS denaturation buffer (0.125 M Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 10% MEA, and 0.02% bromophenol blue), and incubated at 99°C for 10 min. Proteins were separated on 8–10% SDS-polyacrylamide gels, blotted onto a PVDF membrane (Millipore), blocked with 5% non-fat dry milk in 1× Tris-buffered saline containing Tween 20 (TTBS) for 1 h at room temperature, and incubated overnight at 4°C with primary antibodies diluted in 0.5% milk in TTBS (1:1000–1:5000). After three 15 min washes with TTBS, membranes were incubated for 1 h with horseradish peroxidase–labeled affinity-purified secondary antibody (Advansta; 1:10,000–1:20,000). The protein bands were visualized with an ECL kit (Advansta) and quantified using ImageJ software version 1.09.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism version 9.0.1. with a two-way ANOVA (Bonferroni post hoc test; *p<0.05, **p<0.01, and ***p<0.001).
Author Contributions
Study conceptualization, Z.B.; methodology, K.J.; performed data curation, Sz.P. and B.S.; writing - original draft, Z.B. and K.J.; writing - review and editing, I.P.U., Z.B., K.J. and L.F.; visualization, K.J.; supervision, Z.B.; funding acquisition, L.F and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Research Found OTKA NKFIH K129139. The APC was funded by National Research Found OTKA NKFIH K129139.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank László Fésus for critical reading of the manuscript. The authors would like to thank Jennifer Nagy for assistance with monocyte isolation and experiments and Ágota Nagyné-Veres for monocytes as a donor.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fesus, L.; Piacentini, M., Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends in biochemical sciences 2002, 27, (10), 534-539. [CrossRef]
- Jambrovics, K.; Botó, P.; Pap, A.; Sarang, Z.; Kolostyák, Z.; Czimmerer, Z.; Szatmari, I.; Fésüs, L.; Uray, I. P.; Balajthy, Z., Transglutaminase 2 associated with PI3K and PTEN in a membrane-bound signalosome platform blunts cell death. Cell Death & Disease 2023, 14, (3), 217.
- Tatsukawa, H.; Hitomi, K., Role of Transglutaminase 2 in Cell Death, Survival, and Fibrosis. Cells 2021, 10, (7). [CrossRef]
- Park, K.-S.; Kim, H.-K.; Lee, J.-H.; Choi, Y.-B.; Park, S.-Y.; Yang, S.-H.; Kim, S.-Y.; Hong, K.-M., Transglutaminase 2 as a cisplatin resistance marker in non-small cell lung cancer. Journal of cancer research and clinical oncology 2010, 136, (4), 493-502. [CrossRef]
- Mehta, K.; Kumar, A.; Im Kim, H., Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochemical pharmacology 2010, 80, (12), 1921-1929. [CrossRef]
- Budillon, A.; Carbone, C.; Di Gennaro, E., Tissue transglutaminase: a new target to reverse cancer drug resistance. Amino acids 2013, 44, (1), 63-72. [CrossRef]
- Adhikary, G.; Grun, D.; Alexander, H. R.; Friedberg, J. S.; Xu, W.; Keillor, J. W.; Kandasamy, S.; Eckert, R. L., Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget 2018, 9, (77), 34495. [CrossRef]
- Gao, J.; Wang, S.; Wan, H.; Lan, J.; Yan, Y.; Yin, D.; Zhou, W.; Hun, S.; He, Q., Prognostic Value of Transglutaminase 2 in Patients with Solid Tumors: A Meta-analysis. Genet Test Mol Biomarkers 2023, 27, (2), 36-43. [CrossRef]
- Grignani, F.; Testa, U.; Rogaia, D.; Ferrucci, P. F.; Samoggia, P.; Pinto, A.; Aldinucci, D.; Gelmetti, V.; Fagioli, M.; Alcalay, M., Effects on differentiation by the promyelocytic leukemia PML/RARalpha protein depend on the fusion of the PML protein dimerization and RARalpha DNA binding domains. The EMBO Journal 1996, 15, (18), 4949-4958.
- Estey, E.; Garcia-Manero, G.; Ferrajoli, A.; Faderl, S.; Verstovsek, S.; Jones, D.; Kantarjian, H., Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 2006, 107, (9), 3469-3473. [CrossRef]
- Abaza, Y.; Kantarjian, H.; Garcia-Manero, G.; Estey, E.; Borthakur, G.; Jabbour, E.; Faderl, S.; O’Brien, S.; Wierda, W.; Pierce, S., Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 2017, 129, (10), 1275-1283. [CrossRef]
- Balajthy, Z.; Csomós, K.; Vámosi, G.; Szántó, A.; Lanotte, M.; Fésüs, L., Tissue-transglutaminase contributes to neutrophil granulocyte differentiation and functions. Blood 2006, 108, (6), 2045-2054. [CrossRef]
- Csomós, K.; Német, I.; Fésüs, L.; Balajthy, Z., Tissue transglutaminase contributes to the all-trans-retinoic acid–induced differentiation syndrome phenotype in the NB4 model of acute promyelocytic leukemia. Blood 2010, 116, (19), 3933-3943. [CrossRef]
- Niu, C.; Yan, H.; Yu, T.; Sun, H.-P.; Liu, J.-X.; Li, X.-S.; Wu, W.; Zhang, F.-Q.; Chen, Y.; Zhou, L., Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood, The Journal of the American Society of Hematology 1999, 94, (10), 3315-3324. [CrossRef]
- Wang, Z.-Y.; Chen, Z., Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 2008, 111, (5), 2505-2515. [CrossRef]
- Huynh, T. T.; Sultan, M.; Vidovic, D.; Dean, C. A.; Cruickshank, B. M.; Lee, K.; Loung, C.-Y.; Holloway, R. W.; Hoskin, D. W.; Waisman, D. M., Retinoic acid and arsenic trioxide induce lasting differentiation and demethylation of target genes in APL cells. Scientific reports 2019, 9, (1), 1-13. [CrossRef]
- Chen, S.-J.; Zhou, G.-B.; Zhang, X.-W.; Mao, J.-H.; de Thé, H.; Chen, Z., From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood, The Journal of the American Society of Hematology 2011, 117, (24), 6425-6437. [CrossRef]
- Chen, F.; Zhang, Y.; Varambally, S.; Creighton, C. J., Molecular correlates of metastasis by systematic pan-cancer analysis across The Cancer Genome Atlas. Molecular Cancer Research 2019, 17, (2), 476-487.
- Dos Santos, G. A.; Kats, L.; Pandolfi, P. P., Synergy against PML-RARa: targeting transcription, proteolysis, differentiation, and self-renewal in acute promyelocytic leukemia. Journal of Experimental Medicine 2013, 210, (13), 2793-2802. [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.; Valko, M., Arsenic: toxicity, oxidative stress and human disease. Journal of Applied Toxicology 2011, 31, (2), 95-107. [CrossRef]
- Sumi, D.; Shinkai, Y.; Kumagai, Y., Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicology and applied pharmacology 2010, 244, (3), 385-392. [CrossRef]
- Karlsson, J.; Pietras, A.; Beckman, S.; Pettersson, H. M.; Larsson, C.; Påhlman, S., Arsenic trioxide-induced neuroblastoma cell death is accompanied by proteolytic activation of nuclear Bax. Oncogene 2007, 26, (42), 6150-9. [CrossRef]
- de Thé, H.; Pandolfi, P. P.; Chen, Z., Acute promyelocytic leukemia: a paradigm for oncoprotein-targeted cure. Cancer Cell 2017, 32, (5), 552-560. [CrossRef]
- Ades, L.; Raffoux, E.; Fenaux, P.; Dombret, H., Treatment of adult acute promyelocytic leukaemia. Oncologie 2008, 10, 290-294.
- Wang, H.-Y.; Gong, S.; Li, G.-H.; Yao, Y.-Z.; Zheng, Y.-S.; Lu, X.-H.; Wei, S.-H.; Qin, W.-W.; Liu, H.-B.; Wang, M.-C., An effective and chemotherapy-free strategy of all-trans retinoic acid and arsenic trioxide for acute promyelocytic leukemia in all risk groups (APL15 trial). Blood Cancer Journal 2022, 12, (11), 158. [CrossRef]
- Transcriptional Regulation by Nrf2. Antioxidants & Redox Signaling 2018, 29, (17), 1727-1745.
- Oh, K.; Ko, E.; Kim, H. S.; Park, A. K.; Moon, H.-G.; Noh, D.-Y.; Lee, D.-S., Transglutaminase 2 facilitates the distant hematogenous metastasis of breast cancer by modulating interleukin-6 in cancer cells. Breast Cancer Research 2011, 13, (5), 1-12. [CrossRef]
- Kumar, A.; Xu, J.; Brady, S.; Gao, H.; Yu, D.; Reuben, J.; Mehta, K., Tissue transglutaminase promotes drug resistance and invasion by inducing mesenchymal transition in mammary epithelial cells. PloS one 2010, 5, (10), e13390. [CrossRef]
- Gupta, S.; Garg, S.; Kumar, V.; Chaturvedi, A.; Misra, S.; Akhtar, N.; Rajan, S.; Kaur, J.; Lakshmanan, M.; Jain, K., Study of tumor transglutaminase 2 expression in gallbladder cancer–Is it a novel predictor of survival? Annals of Hepato-biliary-pancreatic Surgery 2020, 24, (4), 460-468.
- Eckert, R. L., Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target. Molecular carcinogenesis 2019, 58, (6), 837-853. [CrossRef]
Figure 1.
Tissue transglutaminase impairs the capability of ATRA+ATO-differentiated NB4 cells for endogenous ROS production. The production of endogenous ROS was determined for each cell line using a DCFDA-based method and reported in Relative Fluorescence Units (RFU). The graphs are the representation of mean ± SD values normalized to 100 µg protein of total cell lysate content. (A) NB4 TG2 WT cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Endogenous ROS values were measured on each day in triplicates (n=5). (B) NB4 TG2 KO cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Endogenous ROS values were measured on each day in triplicates (n=5).
Figure 1.
Tissue transglutaminase impairs the capability of ATRA+ATO-differentiated NB4 cells for endogenous ROS production. The production of endogenous ROS was determined for each cell line using a DCFDA-based method and reported in Relative Fluorescence Units (RFU). The graphs are the representation of mean ± SD values normalized to 100 µg protein of total cell lysate content. (A) NB4 TG2 WT cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Endogenous ROS values were measured on each day in triplicates (n=5). (B) NB4 TG2 KO cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Endogenous ROS values were measured on each day in triplicates (n=5).
Figure 2.
Transglutaminase 2 attenuates ATO-induced cell death. NB4 cells were treated, harvested, and labeled with FITC/PE-conjugated Annexin-V/PI for 15 minutes. Cells were analyzed on a BD FACSAria™ III. Apoptotic features were evaluated by the size and granularity of the NB4 cells, followed by gating out the FITC/PI-positive cell population. (A) NB4 TG2 WT cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Dead cell ratios were measured each day in triplicate (n=5). (B) NB4 TG2 KO cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Dead cell ratios were measured each day in triplicate (n=5).
Figure 2.
Transglutaminase 2 attenuates ATO-induced cell death. NB4 cells were treated, harvested, and labeled with FITC/PE-conjugated Annexin-V/PI for 15 minutes. Cells were analyzed on a BD FACSAria™ III. Apoptotic features were evaluated by the size and granularity of the NB4 cells, followed by gating out the FITC/PI-positive cell population. (A) NB4 TG2 WT cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Dead cell ratios were measured each day in triplicate (n=5). (B) NB4 TG2 KO cells were treated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Dead cell ratios were measured each day in triplicate (n=5).
Figure 3.
Endogenous ROS production is regulated via the transcription factor NRF2, influencing the expression of antioxidant genes. NB4 TG2 WT and TG2 KO cells were incubated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Relative mRNA expressions of (A-B) NRF2, (C-D) GST-Ω, (E-F) PRDX5 were measured on the indicated days by real-time Q-PCR and were normalized to GAPDH (means ± SD, n = 3). Representative Western blots/densitometry analysis showing protein expression levels upon various treatments over 5 days (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; ATRA vs. ATRA + ATO * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).
Figure 3.
Endogenous ROS production is regulated via the transcription factor NRF2, influencing the expression of antioxidant genes. NB4 TG2 WT and TG2 KO cells were incubated with ATRA 1 µM, ATO 0.5 µM, ATRA 1 µM+ATO 0.5 µM, ATO 2.0 µM, ATRA 1 µM+ATO 2.0 µM for five days. Relative mRNA expressions of (A-B) NRF2, (C-D) GST-Ω, (E-F) PRDX5 were measured on the indicated days by real-time Q-PCR and were normalized to GAPDH (means ± SD, n = 3). Representative Western blots/densitometry analysis showing protein expression levels upon various treatments over 5 days (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; ATRA vs. ATRA + ATO * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).
Figure 4.
ATO induced ROS production elevates calpain expression/activation, resulting in increased proteolysis of transglutaminase 2. NB4 WT cells were incubated with ATRA 1 µM, ATRA 1 µM+ATO 0.5 µM and ATRA 1 µM+ATO 2.0 µM for five days. (A) Representative Western blots/densitometry analysis showing calpain total protein expression levels upon treatment over 5 days (n=3). (B) The graph represents relative normalized calpain activity RFU values on 100 µg protein content of the total, Annexin-V positive/negative sorted cell lysates. Samples’ activity values were determined with a Synergy Multireader fluorometer (400/505 nm). (C) Representative Western blots/densitometry analysis showing calpain protein expression levels in total, and Annexin-V sorted cell lysates (n=3). (D) Untreated, ATRA differentiated, and ATRA+ATO 2.0 μM treated NB4 WT cells were treated with 1 μM or 2 μM calpain inhibitor (C.I.) for 5 days. Flow cytometric analysis shows the cell viability changes upon the treatments. (E) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total cell lysates upon calpain inhibitory treatments (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).
Figure 4.
ATO induced ROS production elevates calpain expression/activation, resulting in increased proteolysis of transglutaminase 2. NB4 WT cells were incubated with ATRA 1 µM, ATRA 1 µM+ATO 0.5 µM and ATRA 1 µM+ATO 2.0 µM for five days. (A) Representative Western blots/densitometry analysis showing calpain total protein expression levels upon treatment over 5 days (n=3). (B) The graph represents relative normalized calpain activity RFU values on 100 µg protein content of the total, Annexin-V positive/negative sorted cell lysates. Samples’ activity values were determined with a Synergy Multireader fluorometer (400/505 nm). (C) Representative Western blots/densitometry analysis showing calpain protein expression levels in total, and Annexin-V sorted cell lysates (n=3). (D) Untreated, ATRA differentiated, and ATRA+ATO 2.0 μM treated NB4 WT cells were treated with 1 μM or 2 μM calpain inhibitor (C.I.) for 5 days. Flow cytometric analysis shows the cell viability changes upon the treatments. (E) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total cell lysates upon calpain inhibitory treatments (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).
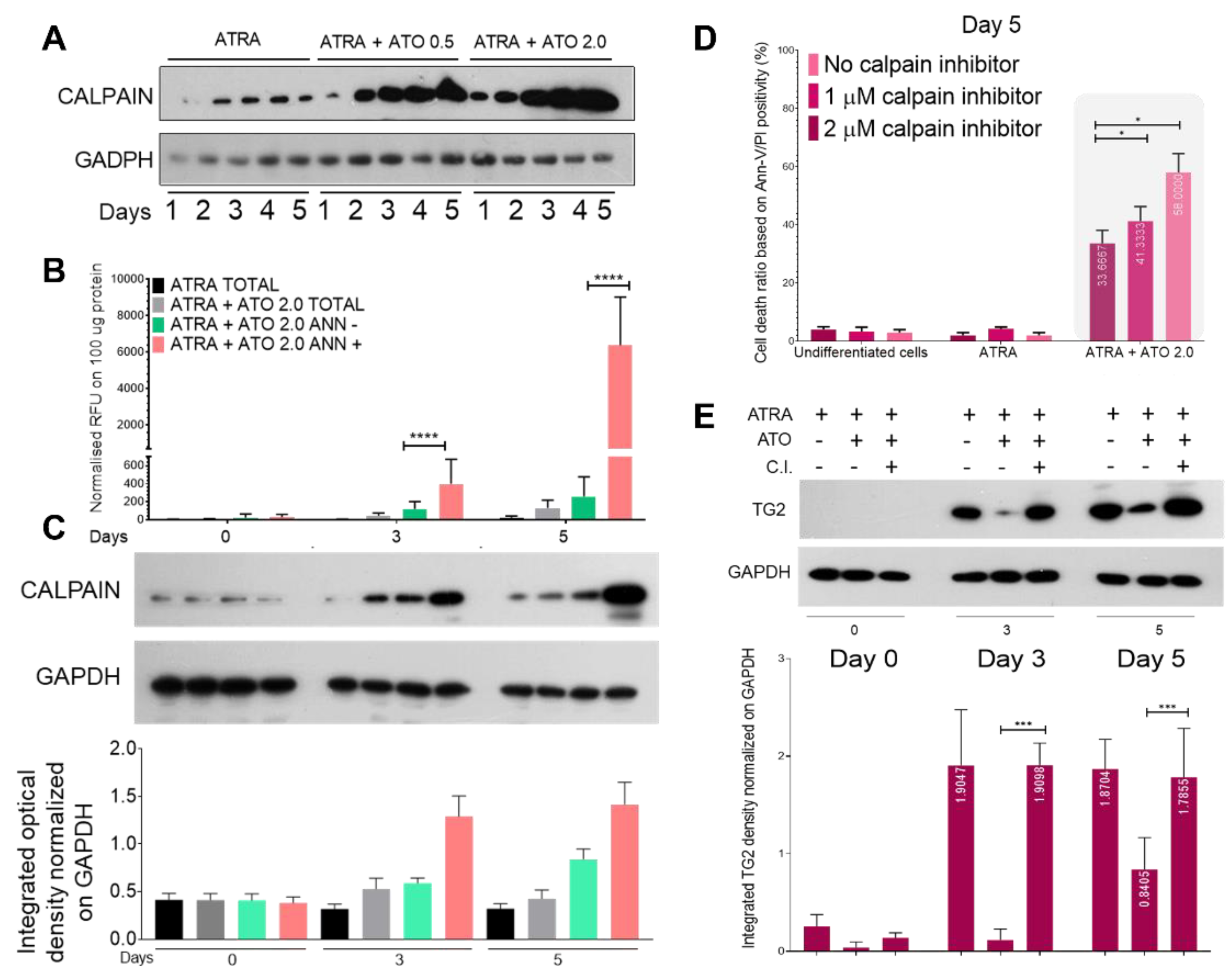
Figure 5.
Transglutaminase 2 levels are reduced by ATO treatment. (A) Relative mRNA expressions of TG2 were measured in NB4 TG2 cells on the indicated days by real-time Q-PCR and normalized to GAPDH. (B) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total cell lysates upon ATRA and ATRA+ATO treatments (n=3). (C) MCF-7 cells were treated with 1 µg/mL doxorubicin (DOX), ATO 2.0 µM or with a combination. As a positive control, ATRA treated NB4 WT cells were used. Relative mRNA expressions of TG2 were measured by real-time Q-PCR and normalized to GAPDH. (D) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total MCF-7 and NB4 WT cell lysates (n=3). (E) Relative mRNA expressions of TG2 were measured in monocytes on the indicated days by real-time Q-PCR and normalized to GAPDH. (F) Representative Western blots and (G) densitometry analysis showing TG2 protein expression levels in total cell lysates upon ATO 2.0 µM, calpain inhibitor 2.0 µM, MCSF, MSCF+ATO 2.0 µM, MSCF+ATO 2.0 µM + calpain inhibitor 2.0 µM and MSCF+calpain inhibitor 2.0 µM treatments for 3 and 5 days (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; MCSF vs. combination treatments; * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).
Figure 5.
Transglutaminase 2 levels are reduced by ATO treatment. (A) Relative mRNA expressions of TG2 were measured in NB4 TG2 cells on the indicated days by real-time Q-PCR and normalized to GAPDH. (B) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total cell lysates upon ATRA and ATRA+ATO treatments (n=3). (C) MCF-7 cells were treated with 1 µg/mL doxorubicin (DOX), ATO 2.0 µM or with a combination. As a positive control, ATRA treated NB4 WT cells were used. Relative mRNA expressions of TG2 were measured by real-time Q-PCR and normalized to GAPDH. (D) Representative Western blots/densitometry analysis showing TG2 protein expression levels in total MCF-7 and NB4 WT cell lysates (n=3). (E) Relative mRNA expressions of TG2 were measured in monocytes on the indicated days by real-time Q-PCR and normalized to GAPDH. (F) Representative Western blots and (G) densitometry analysis showing TG2 protein expression levels in total cell lysates upon ATO 2.0 µM, calpain inhibitor 2.0 µM, MCSF, MSCF+ATO 2.0 µM, MSCF+ATO 2.0 µM + calpain inhibitor 2.0 µM and MSCF+calpain inhibitor 2.0 µM treatments for 3 and 5 days (n=3). Statistical significance was determined via two-way analysis of variance (ANOVA; Bonferroni post-hoc test; MCSF vs. combination treatments; * p < 0.05, ** p< 0.01 and *** p < 0.001, **** p < 0.0001).

|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).