Submitted:
10 May 2023
Posted:
12 May 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
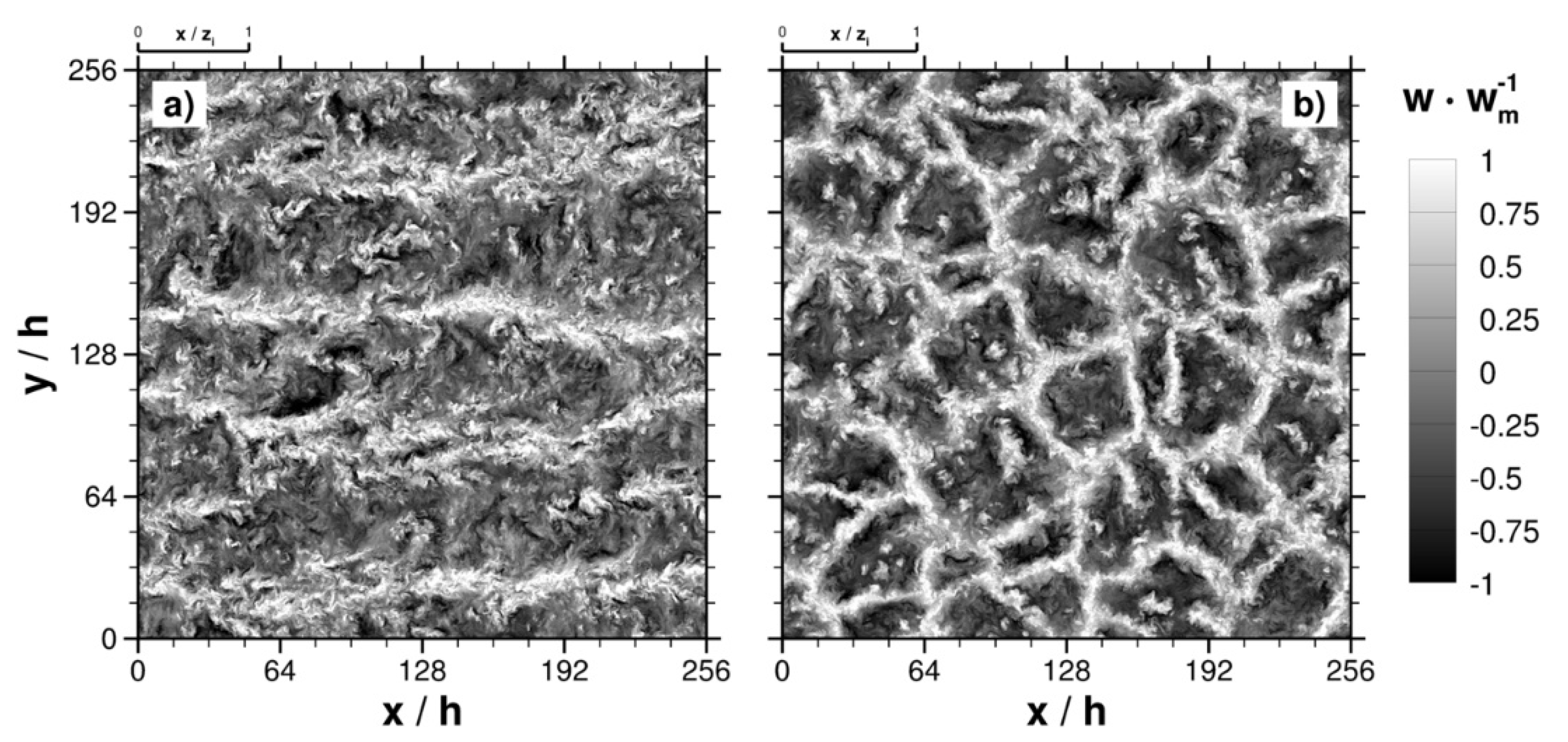
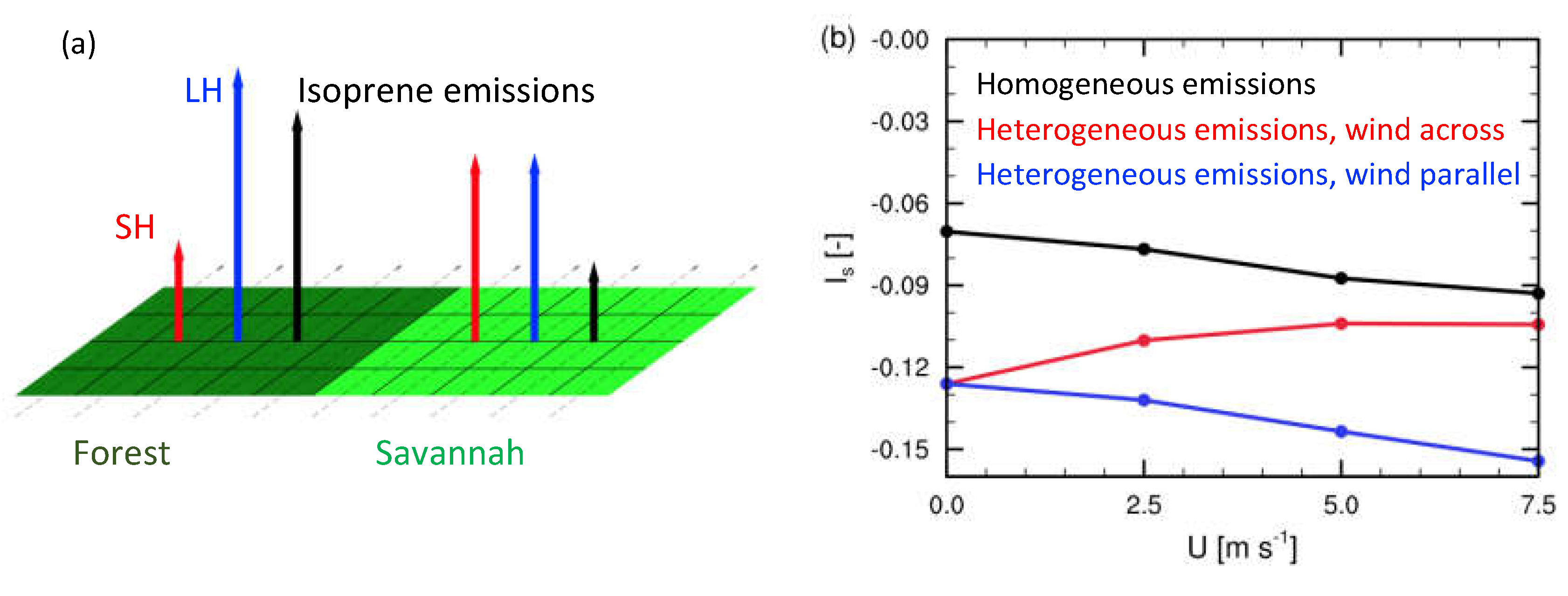
2. Boundary Layer over a Forest
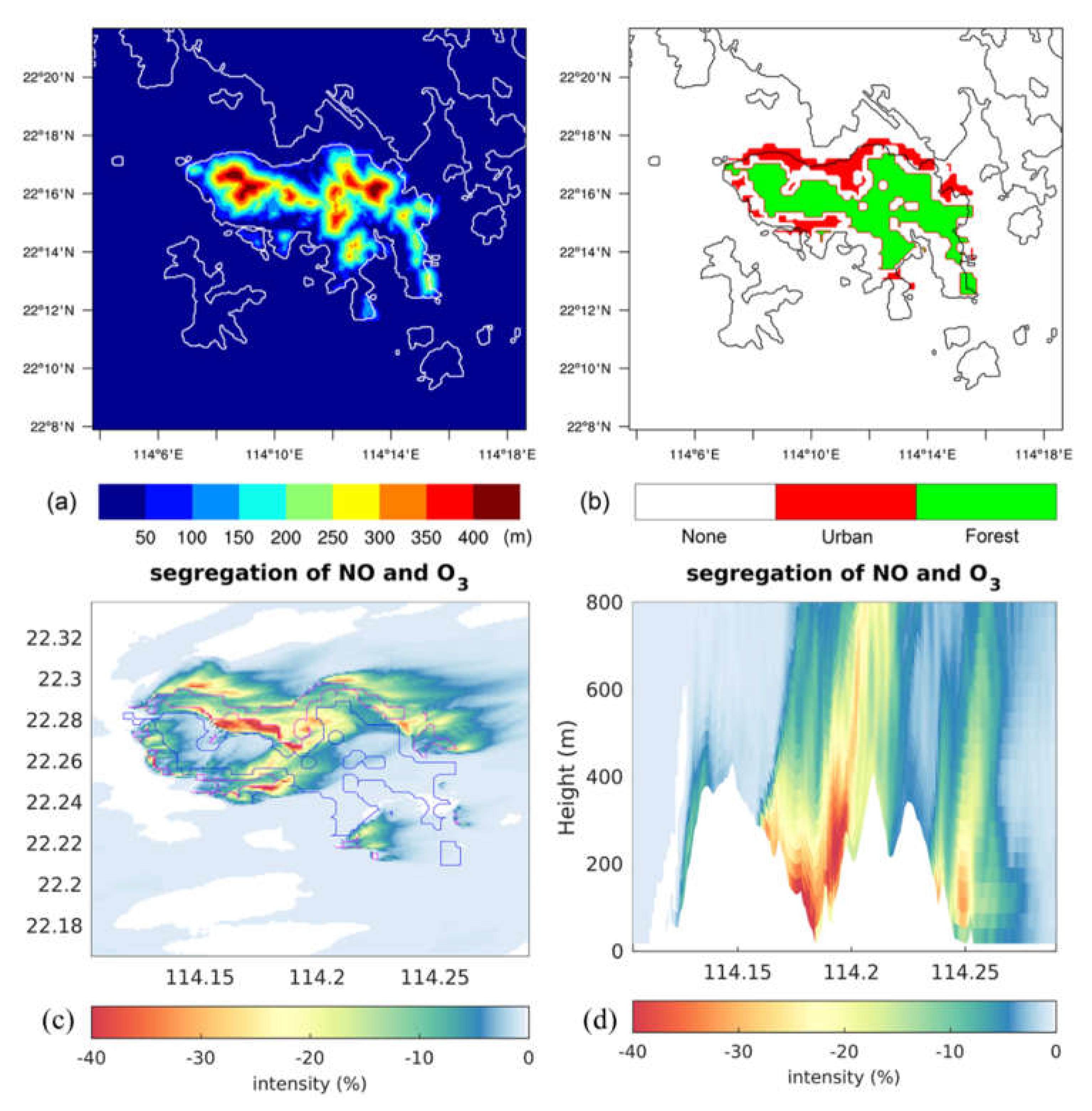
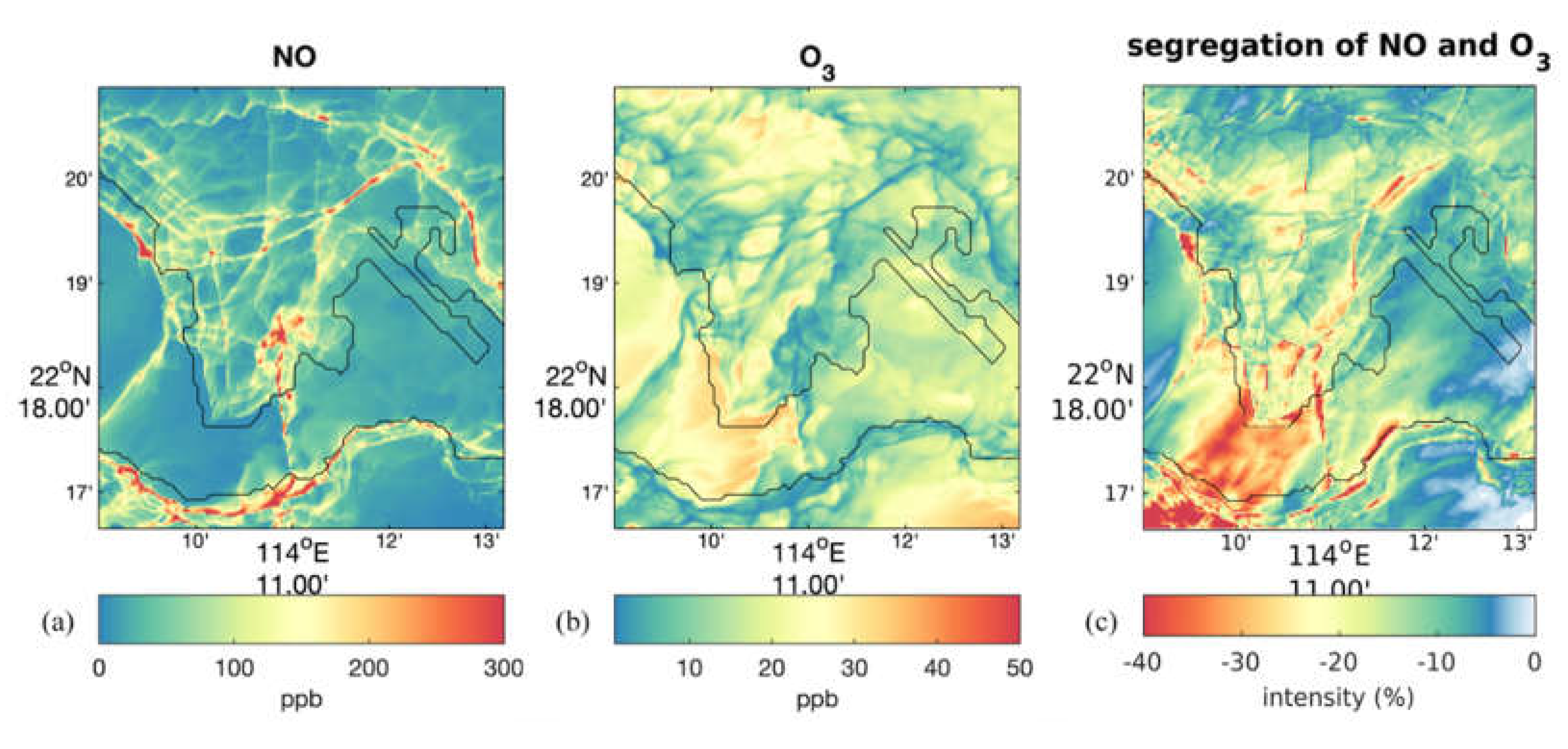
3. Urban Boundary Layer
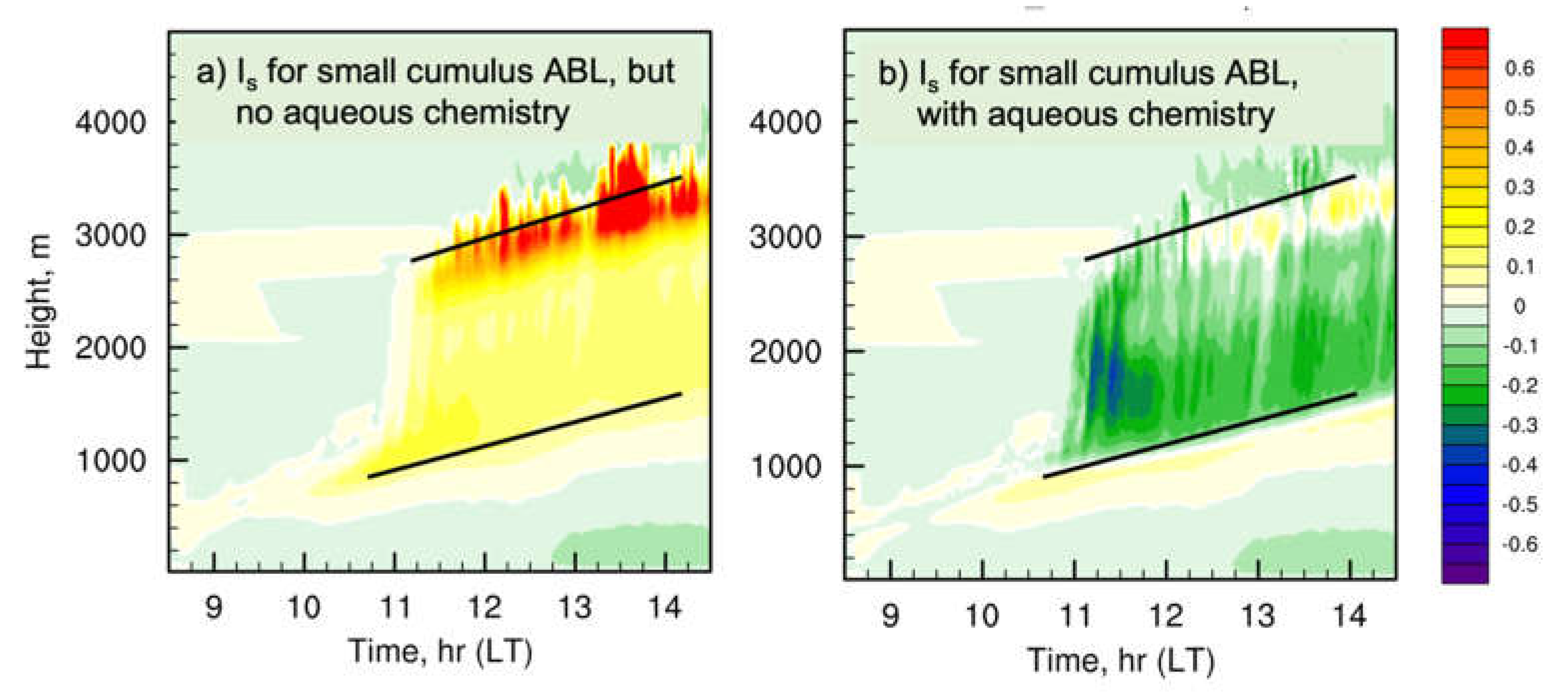
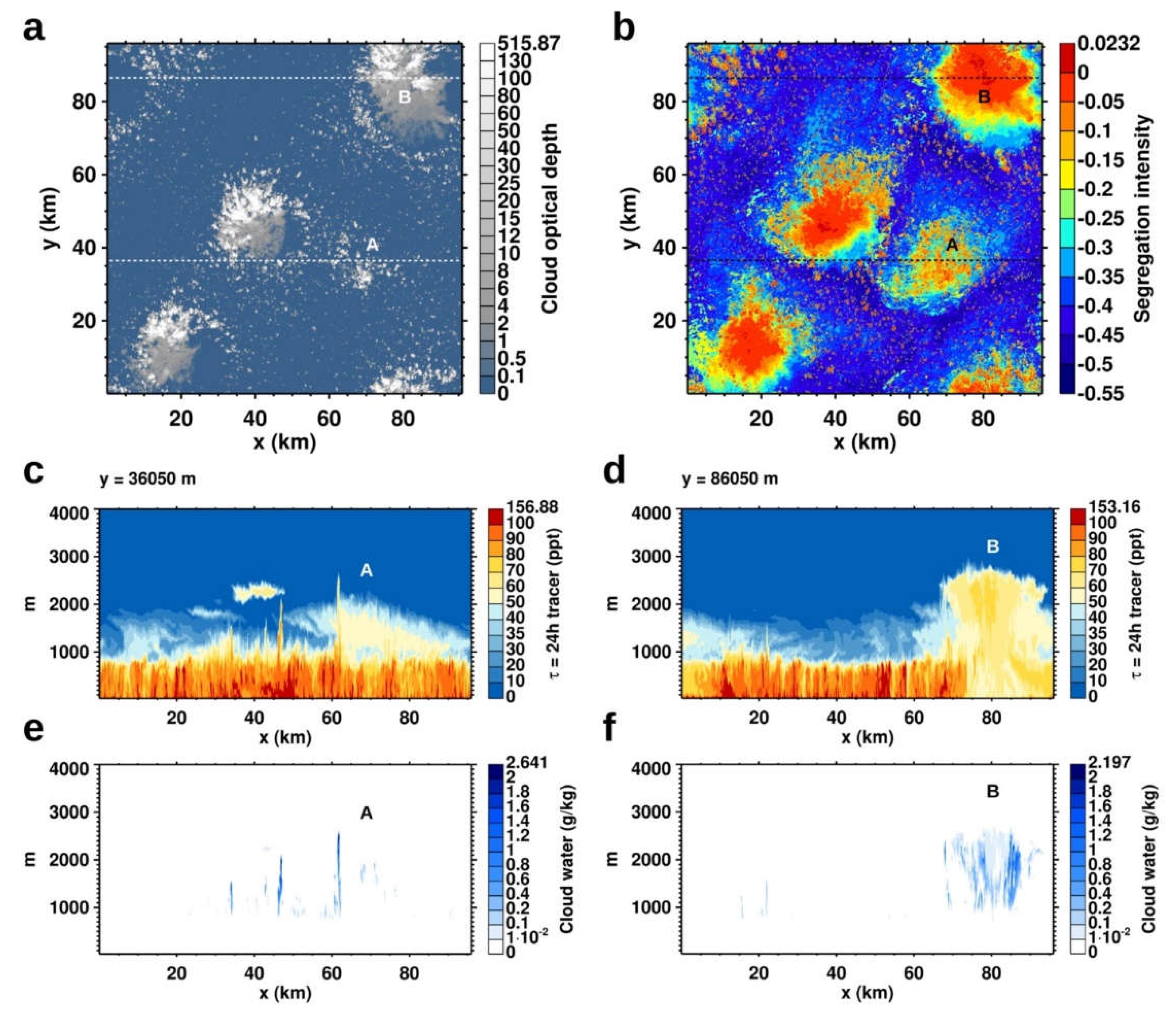
4. Clouds, Turbulence, and Biogenic Sulfur in the Marine Boundary Layer
5. Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Herring, J.R.; Wyngaard, J.C. Convection with a first-order chemically reactive passive scalar. In Proceedings of the Fifth International Symposium on Turbulent Shear Flows, Cornell University, Ithaca, New York, USA, 7–9 August 1987; pp. 324–336. [Google Scholar]
- Patton, E.G.; Sullivan, P.P.; Shaw, R.H.; Finnigan, J.J.; Weil, J.C. Atmospheric stability influences on coupled boundary layer and canopy turbulence. J. Atmos. Sci. 2016, 73, 1621–1647. [Google Scholar] [CrossRef]
- Danckwerts, P.V. The definition and measurement of some characteristics of mixtures. Appl. Sci. Res. 1952, 3, 279–296. [Google Scholar] [CrossRef]
- Damköhler, G. Influence of turbulence on the velocity flames in gas mixtures. Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie 1940, 46, 601–626. [Google Scholar] [CrossRef]
- Mousavi, M.; Soltanieh, M.; Badakhshan, A. Influence of turbulence and atmospheric chemistry on grid size with respect to location in modeling and simulation of photochemical smog formation and transport. Environ. Model. Softw. 1999, 14, 657–663. [Google Scholar] [CrossRef]
- Krol, M.C.; Molemaker, M.J.; de Arellano, J.V.G. Effects of turbulence and heterogeneous emissions on photochemically active species in the convective boundary layer. J. Geophys. Res. Atmos. 2000, 105, 6871–6884. [Google Scholar] [CrossRef]
- Vinuesa, J.-F.; Arellano, J.V.-g.D. Fluxes and (co-)variances of reacting scalars in the convective boundary layer. Tellus B: Chem. Phys. Meteorol. 2003, 55, 935–949. [Google Scholar] [CrossRef]
- Smagorinsky, J. General circulation experiments with the primitive equations: I. The basic experiment. Mon. Weather Rev. 1963, 91, 99–164. [Google Scholar] [CrossRef]
- Lilly, D.K. On the application of eddy viscosity concept in the inertial sub-range of turbulence. NCAR Tech. Note 1966, 123, 19. [Google Scholar] [CrossRef]
- Lilly, D.K. The representation of small-scale turbulence in numerical simulation experiments. Proceedings of the IBM Scientific Computing Symposium on Environmental Sciences 1967, 23. [Google Scholar] [CrossRef]
- Deardorff, J.W. A numerical study of three-dimensional turbulent channel flow at large Reynolds numbers. J. Fluid Mech. 1970, 41, 453–480. [Google Scholar] [CrossRef]
- Schumann, U. Large-eddy simulation of turbulent diffusion with chemical reactions in the convective boundary layer. Atmos. Environ. 1989, 23, 1713–1727. [Google Scholar] [CrossRef]
- Sykes, R.I.; Parker, S.F.; Henn, D.S.; Lewellen, W.S. Turbulent mixing with chemical reaction in the planetary boundary layer. J. Appl. Meteorol. Climatol. 1994, 33, 825–834. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Main Report; 978-92-5-132974-0; Food and Agriculture Organization of the United Nations: Rome, 2020; p. 184. [Google Scholar]
- Olivier, J.G.J.; Berdowski, J.J.M. Global Emissions Sources and Sinks. In The Climate System; Berdowski, J., Guicherit, R., Heij, B.J., Eds.; AA Balkema Publishers Lisse: The Netherlands, 2001; pp. 33–77. [Google Scholar]
- Chameides, W.L.; Lindsay, R.W.; Richardson, J.; Kiang, C.S. The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 1988, 241, 1473–1475. [Google Scholar] [CrossRef] [PubMed]
- Trainer, M.; Williams, E.J.; Parrish, D.D.; Buhr, M.P.; Allwine, E.J.; Westberg, H.H.; Fehsenfeld, F.C.; Liu, S.C. Models and observations of the impact of natural hydrocarbons on rural ozone. Nature 1987, 329, 705–707. [Google Scholar] [CrossRef]
- Carlton, A.G.; Wiedinmyer, C.; Kroll, J.H. A review of Secondary Organic Aerosol (SOA) formation from isoprene. Atmos. Chem. Phys. 2009, 9, 4987–5005. [Google Scholar] [CrossRef]
- Griffin, R.J.; Cocker III, D.R.; Flagan, R.C.; Seinfeld, J.H. Organic aerosol formation from the oxidation of biogenic hydrocarbons. J. Geophys. Res. Atmos. 1999, 104, 3555–3567. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Pandis, S.N.; Paulson, S.E.; Seinfeld, J.H.; Flagan, R.C. Aerosol formation in the photooxidation of isoprene and β-pinene. Atmos. Environ. 1991, 25, 997–1008. [Google Scholar] [CrossRef]
- Butler, T.M.; Taraborrelli, D.; Brühl, C.; Fischer, H.; Harder, H.; Martinez, M.; Williams, J.; Lawrence, M.G.; Lelieveld, J. Improved simulation of isoprene oxidation chemistry with the ECHAM5/MESSy chemistry-climate model: lessons from the GABRIEL airborne field campaign. Atmos. Chem. Phys. 2008, 8, 4529–4546. [Google Scholar] [CrossRef]
- Karl, T.G.; Christian, T.J.; Yokelson, R.J.; Artaxo, P.; Hao, W.M.; Guenther, A. The Tropical Forest and Fire Emissions Experiment: method evaluation of volatile organic compound emissions measured by PTR-MS, FTIR, and GC from tropical biomass burning. Atmos. Chem. Phys. 2007, 7, 5883–5897. [Google Scholar] [CrossRef]
- Ouwersloot, H.G.; Vilà-Guerau de Arellano, J.; Van Heerwaarden, C.C.; Ganzeveld, L.N.; Krol, M.C.; Lelieveld, J. On the segregation of chemical species in a clear boundary layer over heterogeneous land surfaces. Atmos. Chem. Phys. 2011, 11, 10681–10704. [Google Scholar] [CrossRef]
- Kaser, L.; Karl, T.; Yuan, B.; Mauldin III, R.; Cantrell, C.; Guenther, A.B.; Patton, E.; Weinheimer, A.J.; Knote, C.; Orlando, J.; et al. Chemistry-turbulence interactions and mesoscale variability influence the cleansing efficiency of the atmosphere. Geophys. Res. Lett. 2015, 42, 10894–10903. [Google Scholar] [CrossRef]
- Koppmann, R.; Kesselmeier, J.; Meixner, F.; Warnke, J.; Bandur, R.; Hoffmann, T.; Aubrun, S.; Leitl, B.; Schatzmann, M.; Dlugi, R.; et al. Emission and chemical transformation of biogenic volatile organic compounds investigations in and above a mixed forest stand (ECHO): An overview. In Proceedings of the Integrated Land Ecosystem–Atmosphere Processes Study (ILEAPS) International Open Science Conference 2003, Helsinki, Finland, 29 September - 3 October 2003; pp. 198–201. [Google Scholar]
- Dlugi, R.; Berger, M.; Zelger, M.; Hofzumahaus, A.; Siese, M.; Holland, F.; Wisthaler, A.; Grabmer, W.; Hansel, A.; Koppmann, R.; et al. Turbulent exchange and segregation of HOx radicals and volatile organic compounds above a deciduous forest. Atmos. Chem. Phys. 2010, 10, 6215–6235. [Google Scholar] [CrossRef]
- Dlugi, R.; Berger, M.; Zelger, M.; Hofzumahaus, A.; Rohrer, F.; Holland, F.; Lu, K.; Kramm, G. The balances of mixing ratios and segregation intensity: A case study from the field (ECHO 2003). Atmos. Chem. Phys. 2014, 14, 10333–10362. [Google Scholar] [CrossRef]
- Vilà-Guerau de Arellano, J.; Kim, S.-W.; Barth, M.C.; Patton, E.G. Transport and chemical transformations influenced by shallow cumulus over land. Atmos. Chem. Phys. 2005, 5, 3219–3231. [Google Scholar] [CrossRef]
- Kim, S.W.; Barth, M.C.; Trainer, M. Influence of fair-weather cumulus clouds on isoprene chemistry. J. Geophys. Res. Atmos. 2012, 117, D10302. [Google Scholar] [CrossRef]
- Li, Y.; Barth, M.C.; Patton, E.G.; Steiner, A.L. Impact of in-cloud aqueous processes on the chemistry and transport of biogenic volatile organic compounds. J. Geophys. Res. Atmos. 2017, 122, 11131–11153. [Google Scholar] [CrossRef]
- Kim, S.W.; Barth, M.C.; Trainer, M. Impact of turbulent mixing on isoprene chemistry. Geophys. Res. Lett. 2016, 43, 7701–7708. [Google Scholar] [CrossRef]
- Li, Y.; Barth, M.C.; Chen, G.; Patton, E.G.; Kim, S.W.; Wisthaler, A.; Mikoviny, T.; Fried, A.; Clark, R.; Steiner, A.L. Large-eddy simulation of biogenic VOC chemistry during the DISCOVER-AQ 2011 campaign. J. Geophys. Res. Atmos. 2016, 121, 8083–8105. [Google Scholar] [CrossRef]
- Finnigan, J.J.; Shaw, R.H.; Patton, E.G. Turbulence structure above a vegetation canopy. J. Fluid Mech. 2009, 637, 387–424. [Google Scholar] [CrossRef]
- Raupach, M.R.; Finnigan, J.J.; Brunet, Y. Coherent eddies and turbulence in vegetation canopies: The mixing-layer analogy. Boundary-Layer Meteorology 1996, 78, 351–382. [Google Scholar] [CrossRef]
- Gao, W.; Shaw, R.H.; Paw U, K.T. Observation of organized structure in turbulent flow within and above a forest canopy. Bound.-Layer Meteorol. 1989, 47, 349–377. [Google Scholar] [CrossRef]
- Gao, W.; Shaw, R.H.; U Paw, K.T. Conditional analysis of temperature and humidity microfronts and ejection/sweep motions within and above a deciduous forest. Bound.-Layer Meteorol. 1992, 59, 35–57. [Google Scholar] [CrossRef]
- Brunet, Y. Turbulent flow in plant canopies: Historical perspective and overview. Bound.-Layer Meteorol. 2020, 177, 315–364. [Google Scholar] [CrossRef]
- Dupont, S.; Patton, E.G. Influence of stability and seasonal canopy changes on micrometeorology within and above an orchard canopy: The CHATS experiment. Agric. For. Meteorol. 2012, 157, 11–29. [Google Scholar] [CrossRef]
- Clifton, O.E.; Patton, E.G.; Wang, S.; Barth, M.; Orlando, J.; Schwantes, R.H. Large eddy simulation for investigating coupled forest canopy and turbulence influences on atmospheric chemistry. J. Adv. Model. Earth Syst. 2022, 14, e2022MS003078. [Google Scholar] [CrossRef]
- Barbaro, E.; Vilà-Guerau de Arellano, J.; Krol, M.C.; Holtslag, A.A.M. Impacts of aerosol shortwave radiation absorption on the dynamics of an idealized convective atmospheric boundary layer. Bound.-Layer Meteorol. 2013, 148, 31–49. [Google Scholar] [CrossRef]
- Barbaro, E.; de Arellano, J.V.G.; Ouwersloot, H.G.; Schröter, J.S.; Donovan, D.P.; Krol, M.C. Aerosols in the convective boundary layer: Shortwave radiation effects on the coupled land-atmosphere system. J. Geophys. Res. Atmos. 2014, 119, 5845–5863. [Google Scholar] [CrossRef]
- Patton, E.G.; Davis, K.J.; Barth, M.C.; Sullivan, P.P. Decaying scalars emitted by a forest canopy: A numerical study. Bound.-Layer Meteorol. 2001, 100, 91–129. [Google Scholar] [CrossRef]
- Li, C.W.Y.; Brasseur, G.P.; Schmidt, H.; Mellado, J.P. Error induced by neglecting subgrid chemical segregation due to inefficient turbulent mixing in regional chemical-transport models in urban environments. Atmos. Chem. Phys. 2021, 21, 483–503. [Google Scholar] [CrossRef]
- Molemaker, M.J.; de Arellano, J.V.-G. Control of chemical reactions by convective turbulence in the boundary layer. J. Atmos. Sci. 1998, 55, 568–579. [Google Scholar] [CrossRef]
- Baker, J.; Walker, H.L.; Cai, X. A study of the dispersion and transport of reactive pollutants in and above street canyons—A large eddy simulation. Atmos. Environ. 2004, 38, 6883–6892. [Google Scholar] [CrossRef]
- Auger, L.; Legras, B. Chemical segregation by heterogeneous emissions. Atmos. Environ. 2007, 41, 2303–2318. [Google Scholar] [CrossRef]
- Bright, V.B.; Bloss, W.J.; Cai, X. Urban street canyons: Coupling dynamics, chemistry and within-canyon chemical processing of emissions. Atmos. Environ. 2013, 68, 127–142. [Google Scholar] [CrossRef]
- Zhong, J.; Cai, X.-M.; Bloss, W.J. Modelling the dispersion and transport of reactive pollutants in a deep urban street canyon: Using large-eddy simulation. Environ. Pollut. 2015, 200, 42–52. [Google Scholar] [CrossRef]
- Zhong, J.; Cai, X.-M.; Bloss, W.J. Large eddy simulation of reactive pollutants in a deep urban street canyon: Coupling dynamics with O3-NOx-VOC chemistry. Environ. Pollut. 2017, 224, 171–184. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.-F.; Muñoz-Esparza, D.; Li, C.W.Y.; Barth, M.; Wang, T.; Brasseur, G.P. The impact of inhomogeneous emissions and topography on ozone photochemistry in the vicinity of Hong Kong Island. Atmos. Chem. Phys. 2021, 21, 3531–3553. [Google Scholar] [CrossRef]
- Wang, Y.; Brasseur, G.P.; Wang, T. Segregation of atmospheric oxidants in turbulent urban environments. Atmosphere 2022, 13, 315. [Google Scholar] [CrossRef]
- Khan, B.; Banzhaf, S.; Chan, E.C.; Forkel, R.; Kanani-Sühring, F.; Ketelsen, K.; Kurppa, M.; Maronga, B.; Mauder, M.; Raasch, S.; et al. Development of an atmospheric chemistry model coupled to the PALM model system 6.0: Implementation and first applications. Geosci. Model Dev. 2021, 14, 1171–1193. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.-F.; Muñoz-Esparza, D.; Dai, J.; Li, C.W.; Lichtig, P.; Tsang, R.C.; Liu, C.-H.; Wang, T.; Brasseur, G.P. Coupled mesoscale-LES modeling of air quality in a polluted city using WRF-LES-Chem. EGUsphere 2022, 1–31. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Z.; Zhang, D.; Chen, B. Marine boundary layer structure as observed by A-train satellites. Atmos. Chem. Phys. 2016, 16, 5891–5903. [Google Scholar] [CrossRef]
- Krueger, S.K.; McLean, G.T.; Fu, Q. Numerical simulation of the stratus-to-cumulus transition in the subtropical marine boundary layer. Part II: Boundary-layer circulation. J. Atmos. Sci. 1995, 52, 2851–2868. [Google Scholar] [CrossRef]
- Wyant, M.C.; Bretherton, C.S.; Rand, H.A.; Stevens, D.E. Numerical simulations and a conceptual model of the stratocumulus to trade cumulus transition. J. Atmos. Sci. 1997, 54, 168–192. [Google Scholar] [CrossRef]
- Savic-Jovcic, V.; Stevens, B. The structure and mesoscale organization of precipitating stratocumulus. J. Atmos. Sci. 2008, 65, 1587–1605. [Google Scholar] [CrossRef]
- Feingold, G.; Koren, I.; Wang, H.; Xue, H.; Brewer, W.A. Precipitation-generated oscillations in open cellular cloud fields. Nature 2010, 466, 849–852. [Google Scholar] [CrossRef]
- Kazil, J.; Yamaguchi, T.; Feingold, G. Mesoscale organization, entrainment, and the properties of a closed-cell stratocumulus cloud. J. Adv. Model. Earth Syst. 2017, 9, 2214–2229. [Google Scholar] [CrossRef]
- Bretherton, C.S.; Blossey, P.N. Understanding mesoscale aggregation of shallow cumulus convection using large-eddy simulation. J. Adv. Model. Earth Syst. 2017, 9, 2798–2821. [Google Scholar] [CrossRef]
- Narenpitak, P.; Kazil, J.; Yamaguchi, T.; Quinn, P.; Feingold, G. From sugar to flowers: A transition of shallow cumulus organization during ATOMIC. J. Adv. Model. Earth Syst. 2021, 13, e2021MS002619. [Google Scholar] [CrossRef]
- Lu, M.-L.; Seinfeld, J.H. Study of the aerosol indirect effect by large-eddy simulation of marine stratocumulus. J. Atmos. Sci. 2005, 62, 3909–3932. [Google Scholar] [CrossRef]
- Xue, H.; Feingold, G. Large-eddy simulations of trade wind cumuli: Investigation of aerosol indirect effects. J. Atmos. Sci. 2006, 63, 1605–1622. [Google Scholar] [CrossRef]
- Wang, H.; Rasch, P.J.; Feingold, G. Manipulating marine stratocumulus cloud amount and albedo: A process-modelling study of aerosol-cloud-precipitation interactions in response to injection of cloud condensation nuclei. Atmos. Chem. Phys. 2011, 11, 4237–4249. [Google Scholar] [CrossRef]
- Petters, J.L.; Jiang, H.; Feingold, G.; Rossiter, D.L.; Khelif, D.; Sloan, L.C.; Chuang, P.Y. A comparative study of the response of modeled non-drizzling stratocumulus to meteorological and aerosol perturbations. Atmos. Chem. Phys. 2013, 13, 2507–2529. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Feingold, G.; Kazil, J.; McComiskey, A. Stratocumulus to cumulus transition in the presence of elevated smoke layers. Geophys. Res. Lett. 2015, 42, 10478–10485. [Google Scholar] [CrossRef]
- Feingold, G.; Kreidenweis, S.M. Cloud processing of aerosol as modeled by a large eddy simulation with coupled microphysics and aqueous chemistry. J. Geophys. Res. Atmos. 2002, 107, AAC–6. [Google Scholar] [CrossRef]
- Kazil, J.; Wang, H.; Feingold, G.; Clarke, A.D.; Snider, J.R.; Bandy, A. Modeling chemical and aerosol processes in the transition from closed to open cells during VOCALS-REx. Atmos. Chem. Phys. 2011, 11, 7491–7514. [Google Scholar] [CrossRef]
- Andreae, M.O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 1990, 30, 1–29. [Google Scholar] [CrossRef]
- Boucher, O. Clouds and Aerosols. In Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge Univ Press: Cambridge, UK, 2013; pp. 571–657. [Google Scholar]
- Veres, P.R.; Neuman, J.A.; Bertram, T.H.; Assaf, E.; Wolfe, G.M.; Williamson, C.J.; Weinzierl, B.; Tilmes, S.; Thompson, C.R.; Thames, A.B.; et al. Global airborne sampling reveals a previously unobserved dimethyl sulfide oxidation mechanism in the marine atmosphere. Proc. Natl. Acad. Sci. U.S.A. 2020, 117, 4505–4510. [Google Scholar] [CrossRef] [PubMed]
- Novak, G.A.; Fite, C.H.; Holmes, C.D.; Veres, P.R.; Neuman, J.A.; Faloona, I.; Thornton, J.A.; Wolfe, G.M.; Vermeuel, M.P.; Jernigan, C.M.; et al. Rapid cloud removal of dimethyl sulfide oxidation products limits SO2 and cloud condensation nuclei production in the marine atmosphere. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2110472118. [Google Scholar] [CrossRef]
- Novak, G.A.; Kilgour, D.B.; Jernigan, C.M.; Vermeuel, M.P.; Bertram, T.H. Oceanic emissions of dimethyl sulfide and methanethiol and their contribution to sulfur dioxide production in the marine atmosphere. Atmos. Chem. Phys. 2022, 22, 6309–6325. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Quinn, P.K.; Bates, T.S. The case against climate regulation via oceanic phytoplankton sulphur emissions. Nature 2011, 480, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.E. Production of condensation nuclei in clean air by nucleation of H2SO4. Atmos. Environ. 1989, 23, 2841–2846. [Google Scholar] [CrossRef]
- Hoppel, W.A.; Frick, G.M. Submicron aerosol size distributions measured over the tropical and South Pacific. Atmos. Environ. Part A. General Topics 1990, 24, 645–659. [Google Scholar] [CrossRef]
- Covert, D.S.; Kapustin, V.N.; Quinn, P.K.; Bates, T.S. New particle formation in the marine boundary layer. J. Geophys. Res. Atmos. 1992, 97, 20581–20589. [Google Scholar] [CrossRef]
- Hoppel, W.A.; Frick, G.M.; Fitzgerald, J.W.; Larson, R.E. Marine boundary layer measurements of new particle formation and the effects nonprecipitating clouds have on aerosol size distribution. J. Geophys. Res. Atmos. 1994, 99, 14443–14459. [Google Scholar] [CrossRef]
- Wehner, B.; Werner, F.; Ditas, F.; Shaw, R.A.; Kulmala, M.; Siebert, H. Observations of new particle formation in enhanced UV irradiance zones near cumulus clouds. Atmos. Chem. Phys. 2015, 15, 11701–11711. [Google Scholar] [CrossRef]
- Stevens, B.; Vali, G.; Comstock, K.; Wood, R.; Van Zanten, M.C.; Austin, P.H.; Bretherton, C.S.; Lenschow, D.H. Pockets of open cells and drizzle in marine stratocumulus. Bull. Amer. Meteor. Soc. 2005, 86, 51–58. [Google Scholar] [CrossRef]
- Petters, M.D.; Snider, J.R.; Stevens, B.; Vali, G.; Faloona, I.; Russell, L.M. Accumulation mode aerosol, pockets of open cells, and particle nucleation in the remote subtropical Pacific marine boundary layer. J. Geophys. Res. Atmos. 2006, 111, D02206. [Google Scholar] [CrossRef]
- Tomlinson, J.M.; Li, R.; Collins, D.R. Physical and chemical properties of the aerosol within the southeastern Pacific marine boundary layer. J. Geophys. Res. Atmos. 2007, 112, D12211. [Google Scholar] [CrossRef]
- O'Dowd, C.; Monahan, C.; Dall'Osto, M. On the occurrence of open ocean particle production and growth events. Geophys. Res. Lett. 2010, 37, L19805. [Google Scholar] [CrossRef]
- Bates, T.S.; Kapustin, V.N.; Quinn, P.K.; Covert, D.S.; Coffman, D.J.; Mari, C.; Durkee, P.A.; De Bruyn, W.J.; Saltzman, E.S. Processes controlling the distribution of aerosol particles in the lower marine boundary layer during the First Aerosol Characterization Experiment (ACE 1). J. Geophys. Res. Atmos. 1998, 103, 16369–16383. [Google Scholar] [CrossRef]
- Jimi, S.I.; Siems, S.T.; McGregor, J.L.; Gras, J.L.; Katzfey, J.J. An investigation into the origin of aerosol nucleation events observed in the Southern Ocean boundary layer. Aust. Meteorol. Mag. 2008, 57, 85–93. [Google Scholar]
- Zheng, G.; Wang, Y.; Wood, R.; Jensen, M.P.; Kuang, C.; McCoy, I.L.; Matthews, A.; Mei, F.; Tomlinson, J.M.; Shilling, J.E.; et al. New particle formation in the remote marine boundary layer. Nat. Commun. 2021, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Peltola, M.; Rose, C.; Trueblood, J.V.; Gray, S.; Harvey, M.; Sellegri, K. New particle formation in coastal New Zealand with a focus on open-ocean air masses. Atmos. Chem. Phys. 2022, 22, 6231–6254. [Google Scholar] [CrossRef]
- Stevens, B.; Bony, S.; Brogniez, H.; Hentgen, L.; Hohenegger, C.; Kiemle, C.; L'Ecuyer, T.S.; Naumann, A.K.; Schulz, H.; Siebesma, P.A.; et al. Sugar, gravel, fish and flowers: Mesoscale cloud patterns in the trade winds. Q. J. R. Meteorol. Soc. 2020, 146, 141–152. [Google Scholar] [CrossRef]
- Vilà-Guerau de Arellano, J.; Van den Dries, K.; Pino, D. On inferring isoprene emission surface flux from atmospheric boundary layer concentration measurements. Atmos. Chem. Phys. 2009, 9, 3629–3640. [Google Scholar] [CrossRef]
- Kaser, L.; Patton, E.G.; Pfister, G.G.; Weinheimer, A.J.; Montzka, D.D.; Flocke, F.; Thompson, A.M.; Stauffer, R.M.; Halliday, H.S. The effect of entrainment through atmospheric boundary layer growth on observed and modeled surface ozone in the Colorado Front Range. J. Geophys. Res. Atmos. 2017, 122, 6075–6093. [Google Scholar] [CrossRef]
- Lenschow, D.H.; Gurarie, D.; Patton, E.G. Modeling the diurnal cycle of conserved and reactive species in the convective boundary layer using SOMCRUS. Geosci. Model Dev. 2016, 9, 979–996. [Google Scholar] [CrossRef]
- Vilà-Guerau De Arellano, J.; Duynkerke, P.G. Influence of chemistry on the flux-gradient relationships for the NO-O3-NO2 system. Bound.-Layer Meteorol. 1992, 61, 375–387. [Google Scholar] [CrossRef]
- Hamba, F. A modified K model for chemically reactive species in the planetary boundary layer. J. Geophys. Res. Atmos. 1993, 98, 5173–5182. [Google Scholar] [CrossRef]
- Cook, A.W.; Riley, J.J. A subgrid model for equilibrium chemistry in turbulent flows. Phys. Fluids. 1994, 6, 2868–2870. [Google Scholar] [CrossRef]
- Cook, A.W.; Riley, J.J. Subgrid-scale modeling for turbulent reacting flows. Combust. Flame. 1998, 112, 593–606. [Google Scholar] [CrossRef]
- Tong, C.; Wyngaard, J.C.; Khanna, S.; Brasseur, J.G. Resolvable-and subgrid-scale measurement in the atmospheric surface layer: Technique and issues. J. Atmos. Sci. 1998, 55, 3114–3126. [Google Scholar] [CrossRef]
- Tong, C.; Wyngaard, J.C.; Brasseur, J.G. Experimental study of the subgrid-scale stresses in the atmospheric surface layer. J. Atmos. Sci. 1999, 56, 2277–2292. [Google Scholar] [CrossRef]
- Horst, T.W.; Kleissl, J.; Lenschow, D.H.; Meneveau, C.; Moeng, C.-H.; Parlange, M.B.; Sullivan, P.P.; Weil, J.C. HATS: Field observations to obtain spatially filtered turbulence fields from crosswind arrays of sonic anemometers in the atmospheric surface layer. J. Atmos. Sci. 2004, 61, 1566–1581. [Google Scholar] [CrossRef]
- Sullivan, P.P.; Horst, T.W.; Lenschow, D.H.; Moeng, C.-H.; Weil, J.C. Structure of subfilter-scale fluxes in the atmospheric surface layer with application to large-eddy simulation modelling. J. Fluid Mech. 2003, 482, 101–139. [Google Scholar] [CrossRef]
- Patton, E.G.; Horst, T.W.; Sullivan, P.P.; Lenschow, D.H.; Oncley, S.P.; Brown, W.O.; Burns, S.P.; Guenther, A.B.; Held, A.; Karl, T.; et al. The canopy horizontal array turbulence study. Bull. Amer. Meteor. Soc. 2011, 92, 593–611. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




